Abstract
The transplantation of embryonic stem cells can effectively improve the creeping strength of nerves near an injury site in animals. Amniotic epithelial cells have similar biological properties as embryonic stem cells; therefore, we hypothesized that transplantation of amniotic epithelial cells can repair peripheral nerve injury and recover the creeping strength of the brachial plexus nerve. In the present study, a brachial plexus injury model was established in rabbits using the C6 root avulsion method. A suspension of human amniotic epithelial cells was repeatedly injected over an area 4.0 mm lateral to the cephal and caudal ends of the C6 brachial plexus injury site (1 × 106 cells/mL, 3 μL/injection, 25 injections) immediately after the injury. The results showed that the decrease in stress and increase in strain at 7,200 seconds in the injured rabbit C6 brachial plexus nerve were mitigated by the cell transplantation, restoring the viscoelastic stress relaxation and creep properties of the brachial plexus nerve. The forepaw functions were also significantly improved at 26 weeks after injury. These data indicate that transplantation of human amniotic epithelial cells can effectively restore the mechanical properties of the brachial plexus nerve after injury in rabbits and that viscoelasticity may be an important index for the evaluation of brachial plexus injury in animals.
Keywords: nerve regeneration, brachial plexus injury, human amniotic epithelial cells, forepaw function, stress relaxation, creep, viscoelasticity, neural regeneration
Introduction
Stem cell transplantation has been widely used in the treatment of central and peripheral nerve injuries. Amniotic epithelial cells contain a large number of stem cell- or embryonic stem cell-like components (Alviano et al., 2007; Ilancheran et al., 2007; Kobayashi et al., 2008; Marcus et al., 2008; Simat et al., 2008; Wolbank et al., 2010). Therefore, human amniotic epithelial cells (hAECs) have been used to treat central and peripheral nerve injuries in animal experiments and clinical studies (Guo et al., 2013). Sankar and Muthusamy (2003) reported that transplanted AECs survived in an injured spinal cord, promoted the growth of host neurons, prevented scar formation at the cut-off point and neuronal death after axotomy, guided regenerative sprouting, provided a micro-environment for the growth of host neurons, rescued apoptotic neurons, and protected the damaged spinal cord neurons from demyelination. Zhang et al. (2013) demonstrated that the forepaw functions of rabbits with brachial plexus injury were recovered at 6 weeks after hAEC transplantation, and AECs were found at the injury after 18 weeks. Xia et al. (2011) found that hAECs promoted the recovery of sensory and motor functions after complete resection of the ulnar nerve. Guo (2011) found that hAEC transplantation was clearly effective for treating paraplegic patients. However, the majority of published studies have examined the functions, electrophysiology, and morphology of damaged nerves (Sankar and Muthusamy, 2003; Guo, 2011; Mokarram and Bellamkonda, 2011; Xia et al., 2011; He and Ye, 2012; He et al., 2013a, b; Li et al., 2013a, b; Xiupeng et al., 2013; Yao et al., 2013; Zhang et al., 2013; Lian et al., 2014). Similar studies assessing the mechanical properties of nerve have only examined normal nerves from humans and animals (Xu et al., 2013b), and the stress relaxation and creep properties of the standard nerve injury models remain poorly understood. Therefore, it is crucial to quantitatively analyze and compare the viscoelastic properties of the brachial plexus nerve in rabbits with brachial plexus injury and after hAEC transplantation. The aim of the present study was to determine the viscoelasticity of the brachial plexus nerve and evaluate the nerve repair after hAEC transplantation in an experimental rabbit model of brachial plexus injury to provide mechanical evidence for the use of hAECs in the clinical treatment of brachial plexus injury.
Materials and Methods
Animals
Sixty-six healthy, clean, male Japanese white rabbits (aged 5 months, weighing 2.5–2.8 kg) were obtained from the Changchun High-Tech Experimental Animal Center (Changchun, Jilin Province, China; license No. SCXK (Ji) 2003-0004). The rabbits were housed at 24–25°C in a ventilated environment with natural light and humidity of 65–70%. The rabbits were fed with a complete forage and allowed free access to food and water. The animal experiments were approved by the Animal Ethics Committee of Jinan Maternity and Child Care Hospital (Jinan, Shandong Province, China). The 66 rabbits were randomly divided into normal control, model injury, and hAEC intervention groups, with each group containing 22 rabbits.
Establishing the rabbit models of brachial plexus injury
The model of brachial plexus injury was established as previously described (Zhang et al., 2013). In brief, the rabbits in the injury and hAEC intervention groups were fixed in a prone position on the operating table and anesthetized with 6% chloral hydrate (6 mL/kg) via intraperitoneal injection. After the rabbits were sufficiently anesthetized, the skin was disinfected and the surgery was performed. The C5–8 vertebral plate was resected on the left side through a semi-laminectomy, exposing the dura mater. Along the superior margin of the scapula, the brachial plexus nerve was exposed down to the nerve root holes, and the C5–8 brachial plexus was determined. Because the C5 and C6 roots are reported to be prone to brachial plexus injury (Feng et al., 2010), we used the C6 brachial plexus root to establish the injury model. The C6 brachial plexus nerve root on the left side was avulsed with a type KL-0.25 spring dynamometer (Beijing Tianchuang Shangbang Instrument and Equipment Co., Ltd., Beijing, China) with 0.9 N of tensile force for 1 minute and then sutured, after removing the spring dynamometer. The normal control group received no treatment.
hAEC transplantation
In the hAEC intervention group, the rabbits were injected with an hAECs suspension (Bioleaf Biotech Co., Ltd., Shanghai, China) over an area of 4.0 mm lateral to the cephal and caudal ends of the C6 brachial plexus injury site via a glass spinning needle on a microsyringe (Shanghai GaoGe Industrial and Trading Co., Ltd., Shanghai, China). The injections to a depth of 1.25 mm were made immediately after the injury model was established, as previously described (Zhang et al., 2013). The injection consisted of 3 μL of the hAEC suspension at each point (1 × 106 cells/mL, 25 points, 75,000 cells). After the injection, the needles were kept in place for 5 minutes to prevent reflux.
Evaluation of rabbit motor function
The motor function of the rabbits in the model injury and hAEC intervention groups was assessed with grooming criteria (Bertelli and Mira, 1993) at 1 day and 2, 8, 14, 20, and 26 weeks after brachial plexus injury. The animals were scored as 0: forepaw paralysis; 1: forepaws could reach the mouth; 2: forepaws could reach the mouth and eyes; 3: forepaws could touch the eyes; 4: forepaws could touch the preauricular area; or 5: forepaws could touch the postauricular area.
Slice preparation
Twenty-two, 30-mm long brachial plexus nerve specimens were prepared in each group at 26 weeks after injury and preserved in saline. Among the 22 specimens in each group, 10 were randomly selected for stress relaxation testing, 10 for creep testing, and the remaining two specimens were used for morphological observation. The samples were cut using a type S-5 sterile scalpel (HuaiAn Uniecom Medical Supplies Co., Ltd., Xuyi County, Jiangsu Province, China). The length and diameter of the samples were measured with a type CGH-3 reading microscope (Changchun Third Optics Instrument Factory, Changchun, Jilin Province, China). After preparation, the samples were 25 mm in length and 0.98–1.06 mm in diameter.
Brachial plexus stress relaxation testing
The stress relaxation tests were performed using an automatic electronic universal testing machine (MODEL55100, Changchun Research Institute for Mechanical Science Co., Ltd., Changchun, Jilin Province, China) with an incubator that could control the temperature within a range of −30°C to 250°C. Each sample was prepared as previously described (Yu et al., 2010; Li et al., 2013c). The test was performed at 36.5 ± 1°C. The samples in each group were secured in the clamp of the testing machine and were strained at 50% per minute. After the strain of the brachial plexus samples was maintained at 8.63% in the control group, 8.17% in the model injury group, and 8.61% in the hAEC intervention group, the stress applied to each group was set as 2.51 MPa and the experiment was run for 7,200 seconds. The samples were sprayed with saline during the experiments to maintain tissue hydration. When the test was completed, the stress relaxation data and curve were automatically output to a computer. The stress relaxation data from the brachial plexus nerve samples are shown in Figure 1.
Figure 1.

Stress relaxation testing of the brachial plexus nerve.
The brachial plexus nerve samples were secured in the clamp of an electronic universal testing machine. Arrow 1: the clamp; arrow 2: the sample.
Brachial plexus creep testing
The sample fixation, testing temperature, data collection, and sample mounting methods for the creep test were the same as those used in the stress relaxation test. The stress was increased at 0.5 GPa/min. After the strain of the brachial plexus samples was maintained at 8.62% in the control group, 8.16% in the model injury group, and 8.61% in the hAEC intervention group, the stress applied to each group was set as 2.51 MPa and the experiment was run for 7,200 seconds. When the test was completed, the creep data and curve were automatically output to a computer.
Brachial plexus morphology
The injured brachial plexus nerves in each group were prepared as frozen sections and cut to a thickness of 5 μm. The sections were fixed with 4% paraformaldehyde, rinsed with tap water, and stained with hematoxylin. After washing, the color was separated with hydrochloric acid and ethanol and then returned to blue using an alkaline solution. Next, the sections were counterstained with eosin, dehydrated through a gradient of ethanol, cleared with xylene, and mounted. The sections were rinsed with tap water between each step. Finally, the sections were observed with a BX51 optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS for Windows, version 16.0 (SPSS, Chicago, IL, USA). Differences between groups were compared using one-way analysis of variance and Sceffe's method. P-values less than 0.05 were considered statistically significant. The normalized stress relaxation function equation and normalized creep function equation for specimens in each group were calculated.
Results
hAEC transplantation improved forepaw function in rabbits with brachial plexus injury
The rabbits in the hAEC intervention group showed some restoration of forepaw function at 8 weeks after brachial plexus injury, and forepaw function was significantly improved at 26 weeks. In the model injury group, forepaw function had slightly recovered at 26 weeks (Table 1).
Table 1.
Effects of human amniotic epithelial cell (hAEC) transplantation on forepaw function in rabbits with brachial plexus injury
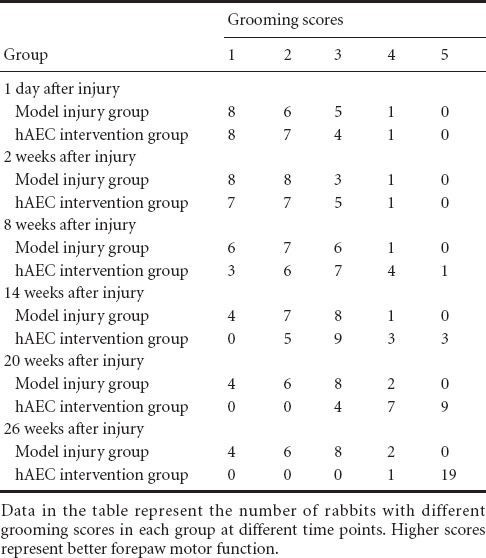
Effect of hAEC transplantation on the stress relaxation of injured brachial plexus nerve in rabbits
The stress relaxation curves from each brachial plexus nerve sample in all three groups decreased over time. The stress decreased sharply within the initial 1,200 seconds and then continued to slowly decrease, becoming nearly horizontal by 7,200 seconds. The stress relaxation curves of the brachial plexus nerve samples in each group showed a logarithmic correlation. The decrease in stress from 2.51 MPa at 7,200 seconds of the brachial plexus nerve samples in the model injury group was lower than that found in the control group (P < 0.05). Compared with the model injury group, the stress at 7,200 seconds of the brachial plexus nerve samples in the hAEC intervention group was significantly decreased (P < 0.05). The decrease in stress at 7,200 seconds of the brachial plexus nerve samples was similar in the normal control and hAEC intervention groups (P > 0.05; Figure 2).
Figure 2.
Effects of human amniotic epithelial cell (hAEC) transplantation on the stress relaxation of brachial plexus nerves in rabbits.
Data are expressed as the mean ± SD of ten rabbits in each group. The differences between groups were compared using one-way analysis of variance and Sceffe's tests. *P < 0.05, vs. normal control group; #P < 0.05, vs. model injury group.
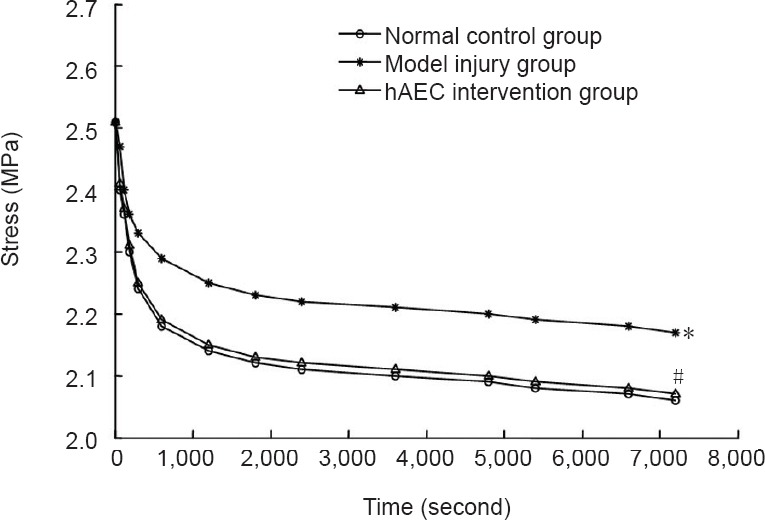
The stress relaxation function G(t) values for the brachial plexus specimens in each group were calculated from the stress relaxation test data, and the stress relaxation function curves were plotted using curve fitting (Figure 3).
Figure 3.
Stress relaxation function curves for the brachial plexus nerve specimens in each group.
Data are expressed as the mean ± SD of ten rabbits in each group. The differences between groups were compared using one-way analysis of variance and Sceffe's tests. *P < 0.05, vs. normal control group; #P < 0.05, vs. model injury group. hAECs: Human amniotic epithelial cells.
As shown in Figure 4, the stress relaxation curve appeared logarithmic. Based on a previous study (Ma et al., 2004), we propose that:
Figure 4.
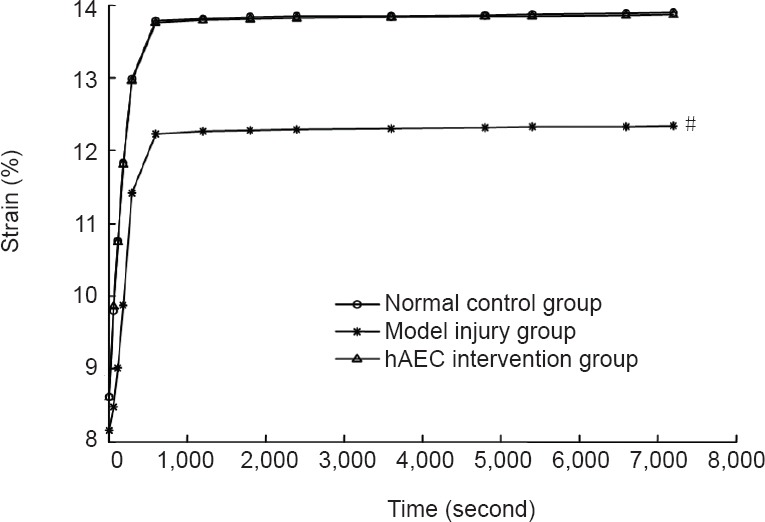
Effects of human amniotic epithelial cell (hAEC) transplantation on the creep properties of injured brachial plexus nerve in rabbits.
Data are expressed as the mean ± SD of ten rabbits in each group. *P < 0.05, vs. normal control group; #P < 0.05, vs. model injury group.
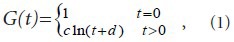
where c and d are undetermined coefficients.
With 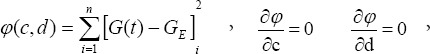
and 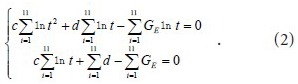
The experimental data were used in equation (2) to calculate the c and d coefficient values of the brachial plexus specimens. Then, the c and d coefficients were used in equation (1) to obtain the normalized stress relaxation function equations for the brachial plexus specimens, as follows:
Normal control group: 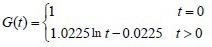
Model injury group: 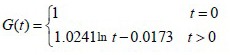
hAEC intervention group: 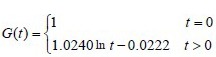
Effects of hAEC transplantation on the creep properties of injured brachial plexus nerve in rabbits
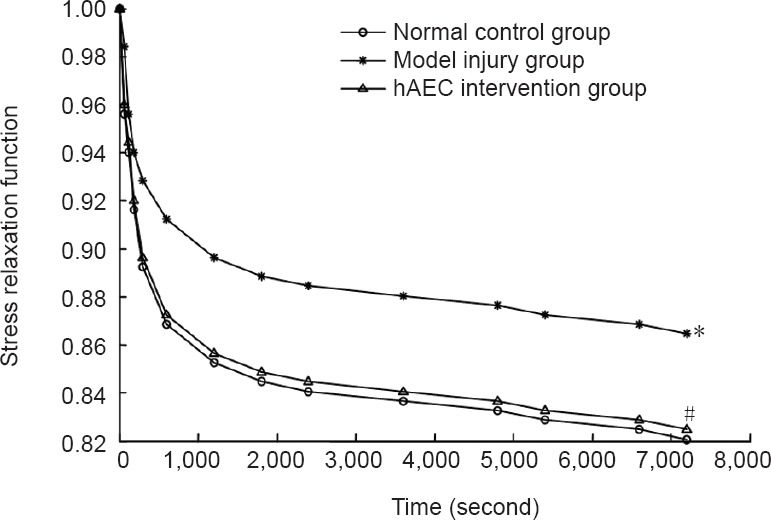
The results of the creep test showed that the strain sharply increased within the initial 600 seconds and then slowly increased over time, becoming nearly horizontal by 7,200 seconds. This shape correlated with an exponential function. The strain at 7,200 seconds of the brachial plexus nerve samples in the model injury group was lower than that in the normal control group (P < 0.05). Compared with the model injury group, the strain at 7,200 seconds of the brachial plexus nerve samples in the hAEC intervention group was significantly larger (P < 0.05), and this level was similar to that of the normal control group (P > 0.05; Figure 4).
The creep function J(t) values from the brachial plexus specimens in each group were calculated from the stress relaxation test data, and the creep function curves were plotted using curve fitting (Figure 5).
Figure 5.
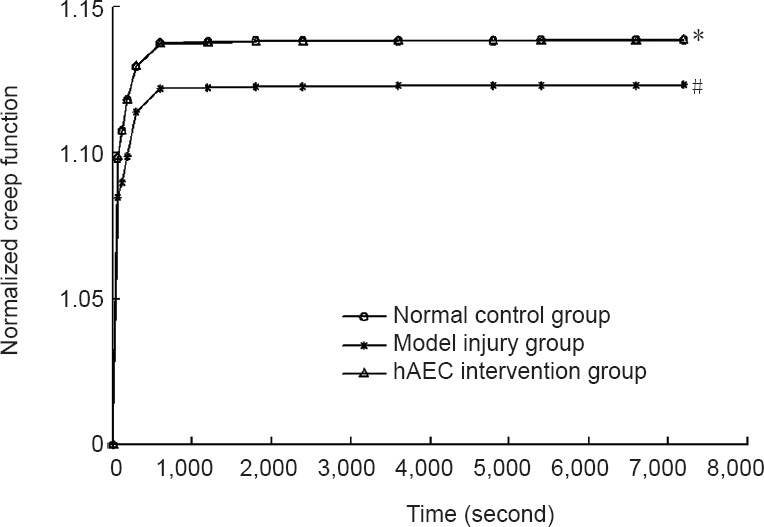
Creep function curves for the brachial plexus nerve specimens in each group.
Data are expressed as the mean ± SD of ten rabbits in each group. *P < 0.05, vs. normal control group; #P < 0.05, vs. model injury group. hAECs: Human amniotic epithelial cells.
As shown in Figure 5, the stress relaxation curve appears exponential. Based on a previous study (Ma et al., 2004), we propose that:

with 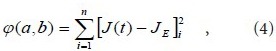 and the canonical equation
and the canonical equation 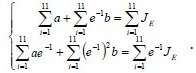
The experimental data were used in equation (4) to calculate values for the coefficients a and b of the brachial plexus specimens. Then, the a and b coefficient values were used in equation (3) to obtain the normalized creep function equations for the brachial plexus specimens, as follows:
Normal control group: 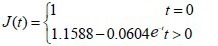
Model injury group: 
hAEC intervention group: 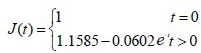
Effects of hAEC transplantation on the pathological morphology of the injured brachial plexus nerve in rabbits
Hematoxylin-eosin staining showed that the brachial plexus nerve axons were surrounded by myelin in the normal control group, and the axons and other components were clearly visible. The fibers were arranged neatly, and the myelin was wrapped with endoneurium, which consisted of connective tissue at the surface of the nerve fibers. In the model injury group, the brachial plexus nerve axons and myelin appeared fractured, blocking the basal membrane lumen, and the morphology of the endometrium, myelin, axons, and other components was changed. In the hAEC transplantation group, the majority of the brachial plexus nerve axons were surrounded by myelin, which was wrapped with endoneurium that consisted of connective tissue at the surface of the nerve fibers. The axons and other components were clearly visible, and the nerve fibers were arranged neatly (Figure 6).
Figure 6.
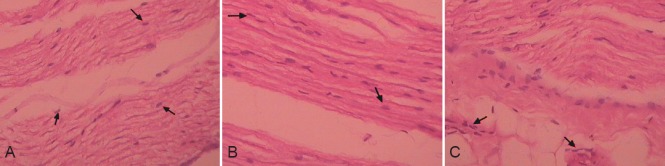
Effect of human amniotic epithelial cell (hAEC) transplantation on the pathological morphology of the brachial plexus nerve in rabbits (hematoxylin-eosin staining, × 400).
(A) In the normal control group, the axons and other components were clearly visible, and the nerve fibers (arrows) were arranged tightly and neatly. (B) In the hAEC transplantation group, the axons and other components were clearly visible, and the nerve fibers (arrows) were arranged tightly and neatly. (C) In the model injury group, the endometrium (arrows), myelin, axons, and other components were changed.
Discussion
Amniotic epithelial cells are derived from the amniotic sac and have stem cell-like properties, including the ability to secrete a variety of cytokines, such as brain-derived neurotrophic factor, neurotrophin-3, and nerve growth factor (Sakuragawa et al., 1996, 2001; Uchida et al., 2000; Xu et al., 2013a). Amniotic epithelial cells do not express HLA antigens, such as HLA-ABCDR protein and β2-microglobulin, and therefore do not induce immunological rejection after transplantation (Adinolfi et al., 1982). In addition, amniotic epithelial cells do not express telomerase, which reduces their tumorigenicity (Miki et al., 2005). Amniotic epithelial cells are often obtained from the afterbirth tissues after parturition and do not raise any ethical issues, making them a good choice for cell therapy (Navarro et al., 2007).
The mechanism of brachial plexus avulsion injury was reported to be highly associated with the biomechanical properties of the avulsed nerve (Greening et al., 2005). The brachial plexus nerve is a group of soft biological tissues that are both viscous and elastic (Gu and Pei, 2001). The biomechanical properties of the brachial plexus include resistance to tension, a stress-strain relationship, lagging, stress relaxation, and creep (Sunderland, 1981). Stress relaxation is an adaptive response by biological materials to a constantly applied strain (Xu et al., 2013b). The recovery of stress relaxation in the injured brachial plexus nerve in rabbits from the hAEC intervention group contributed to the attenuation of the tension in the injured nerves. Creep is an adaptive response to the deformity of nerve tissues that plays a crucial role in determining the critical tension point after nerve injury. After rabbits with injured brachial plexus nerves were treated with the hAEC intervention, the creep properties of the brachial plexus nerve were restored, contributing to the restoration of the damaged nerves. In the present study, we established normalized stress relaxation functions and normalized creep functions for brachial plexus nerve specimens from each group, and fit the corresponding curves to theoretical equations. The results contribute to the elucidation of a better understanding of the stress relaxation and creep properties of the brachial plexus nerve in rabbits.
In this study, the ambient temperature, test speed, and brachial plexus preparation methods were consistent among the groups. The brachial plexus nerve tissues were sampled from the same area and the same preset conditions were used in the stress relaxation and creep tests to ensure that the experimental data were as accurate as possible.
Footnotes
Funding: This study was financially supported by grants from the Science and Technology Development Plan Program of Jilin Province of China, No. 20110492.
Conflicts of interest: None declared.
Copyedited by McCarty W, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, Hsi BL, Faulk WP, Travers P, Bodmer WF. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli JA, Mira JC. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J Neurosci Methods. 1993;46:203–208. doi: 10.1016/0165-0270(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Feng TJ, Sun CJ, Luo M, Ma HS. Creep properties of rat brachial plexus. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14:5244–5247. [Google Scholar]
- Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115:248–253. doi: 10.1016/j.pain.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Gu LQ, Pei GX. Beijing: People's Military Medical Press; 2001. Basic and Clinical Research of Peripheral Nerve Injury. [Google Scholar]
- Guo B, Xu JJ. Rat amniotic epithelial cells are induced to differentiate into neural-like stem cells in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:3503–3507. [Google Scholar]
- Guo LH. Pre-clinical research of human amniotic cells: progress on treatment of nerve injury. Zhongguo Xibao Shengwu Xue Xuebao. 2011;35:590–593. [Google Scholar]
- He F, Ye J. In vitro degradation, biocompatibility, and in vivo osteogenesis of poly(lactic-co-glycolic acid)/calcium phosphate cement scaffold with unidirectional lamellar pore structure. J Biomed Mater Res A. 2012;100:3239–3250. doi: 10.1002/jbm.a.34265. [DOI] [PubMed] [Google Scholar]
- He F, Li J, Ye J. Improvement of cell response of the poly(lactic-co-glycolic acid)/calcium phosphate cement composite scaffold with unidirectional pore structure by the surface immobilization of collagen via plasma treatment. Colloids Surf B Biointerfaces. 2013a;103:209–216. doi: 10.1016/j.colsurfb.2012.10.018. [DOI] [PubMed] [Google Scholar]
- He F, Wang X, Maruyama O, Kosaka R, Sogo Y, Ito A, Ye J. Improvement in endothelial cell adhesion and retention under physiological shear stress using a laminin-apatite composite layer on titanium. J R Soc Interface. 2013b;10:20130014. doi: 10.1098/rsif.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yakuwa T, Sasaki K, Sato K, Kikuchi A, Kamo I, Yokoyama Y, Sakuragawa N. Multilineage potential of side population cells from human amnion mesenchymal layer. Cell Transplant. 2008;17:291–301. doi: 10.3727/096368908784153904. [DOI] [PubMed] [Google Scholar]
- Li M, Guo W, Zhang P, Li H, Gu X, Yao D. Signal flow and pathways in response to early Wallerian degeneration after rat sciatic nerve injury. Neurosci Lett. 2013a;536:56–63. doi: 10.1016/j.neulet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Li S, Liu Q, Wang Y, Gu Y, Liu D, Wang C, Ding G, Chen J, Liu J, Gu X. Differential gene expression profiling and biological process analysis in proximal nerve segments after sciatic nerve transection. PLoS One. 2013b;8:e57000. doi: 10.1371/journal.pone.0057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Wang YF, Luo M. The experimental study on tracheal cartilage creep. Shengwu Yixue Gongcheng Yanjiu. 2013c;32:91–93. [Google Scholar]
- Lian JC, Liu Y, Liu C, Lv SJ, Guo X, Nan F, Sun GW, He X, Ma XJ. Phenotype and differentiation capacity of human amniotic epithelial cells cultured in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:211–217. [Google Scholar]
- Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130–144. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- Mokarram N, Bellamkonda RV. Overcoming endogenous constraints on neuronal regeneration. IEEE Trans Biomed Eng. 2011;58:1900–1906. doi: 10.1109/TBME.2010.2103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sakuragawa N, Thangavel R, Mizuguchi M, Hirasawa M, Kamo I. Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci Lett. 1996;209:9–12. doi: 10.1016/0304-3940(96)12599-4. [DOI] [PubMed] [Google Scholar]
- Sakuragawa N, Elwan MA, Uchida S, Fujii T, Kawashima K. Non-neuronal neurotransmitters and neurotrophic factors in amniotic epithelial cells: expression and function in humans and monkey. Jpn J Pharmacol. 2001;85:20–23. doi: 10.1254/jjp.85.20. [DOI] [PubMed] [Google Scholar]
- Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118:11–17. doi: 10.1016/s0306-4522(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Simat SF, Chua KH, Abdul Rahman H, Tan AE, Tan GC. The stemness gene expression of cultured human amniotic epithelial cells in serial passages. Med J Malaysia. 2008;63(Suppl A):53–54. [PubMed] [Google Scholar]
- Sunderland S. The anatomic foundation of peripheral nerve repair techniques. Orthop Clin North Am. 1981;12:245–266. [PubMed] [Google Scholar]
- Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62:585–590. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Wolbank S, van Griensven M, Grillari-Voglauer R, Peterbauer-Scherb A. Alternative sources of adult stem cells: human amniotic membrane. Adv Biochem Eng Biotechnol. 2010;123:1–27. doi: 10.1007/10_2010_71. [DOI] [PubMed] [Google Scholar]
- Xia J, Xu YF, Xu YW, Wan AG, Ding J. Repair of human amniotic epithelial cells transplantation in ulnar nerve injury. Shiyong Linchuang Yixue. 2011;12:44–51. [Google Scholar]
- Xiupeng W, Fupo H, Xia L, Atsuo I, Yu S, Osamu M, Ryo K, Jiandong Y. Tissue-engineered endothelial cell layers on surface-modified Ti for inhibiting in vitro platelet adhesion. Sci Technol Adv Mater. 2013;14:035002. doi: 10.1088/1468-6996/14/3/035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Li F, Sun Q, Xu YY, Zhao J, Liang HS, Ma SL, Chen YZ. Tissue-engineered skin construction with amniotic epithelial cells and amniotic mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013a;17:7213–7220. [Google Scholar]
- Xu DH, Zhao CH, Ma HL, Wei J, Li DY. Comparison of viscoelasticity between normal human sciatic nerve and amniotic membrane. Neural Regen Res. 2013b;8:1269–1275. doi: 10.3969/j.issn.1673-5374.2013.14.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Li M, Shen D, Ding F, Lu S, Zhao Q, Gu X. Expression changes and bioinformatic analysis of Wallerian degeneration after sciatic nerve injury in rat. Neurosci Bull. 2013;29:321–332. doi: 10.1007/s12264-013-1340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Sun CJ, Quan TG, Xu DH, Ma HS. Tensile mechanical properties of middle cerebral artery in an atherosclerosis model. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14:4445–4448. [Google Scholar]
- Zhang Y, Liu CX, Wang L, Zhang L, Liu W, Zhao W, Li Z. Human amniotic epithelial cell transplantation for repair of brachial plexus injury in rabbits. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:9157–9163. [Google Scholar]


