Abstract
The present study aimed to determine whether a polysaccharide obtained from Spirulina platensis shows protective effects on dopaminergic neurons. A Parkinson's disease model was established through the intraperitoneal injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in C57BL/6J mice. Prior to the MPTP injection, some mice were pretreated with intraperitoneal injections of a polysaccharide derived from Spirulina platensis once daily for 10 days. The results showed that the immunoreactive staining and mRNA expression of the dopamine transporter and tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, in the substantia nigra, were significantly increased in mice pretreated with 800 mg/kg of the polysaccharide compared with those in MPTP-treated mice. The activities of superoxide dismutase and glutathione peroxidase in the serum and midbrain were also increased significantly in mice injected with MPTP after pretreatment with the polysaccharide from Spirulina platensis. By contrast, the activity of monoamine oxidase B in serum and midbrain maintained unchanged. These experimental findings indicate that the polysaccharide obtained from Spirulina platensis plays a protective role against the MPTP-induced loss of dopaminergic neurons in C57BL/6J mice, and that the antioxidative properties of this polysaccharide likely underlie its neuroprotective effect.
Keywords: nerve regeneration, polysaccharide from Spirulina platensis, Parkinson's disease, MPTP, dopaminergic neurons, antioxidation, neural regeneration
Introduction
Parkinson's disease is a chronic, progressive, neurodegenerative disorder affecting mainly older people. This disease is characterized by massive degeneration of dopaminergic neurons in the substantia nigra pars compacta (Wang et al., 2013; Fan et al., 2014). Subsequently, a reduction of dopamine levels is observed in the striatum, the region receiving the majority of the projections from the substantia nigra, and this is considered to underlie the most overt (motor) symptoms of the disease (Fahn, 2003). Since current therapeutic strategies offer symptomatic improvement only, the development of treatment strategies that delay or prevent the progression of Parkinson's disease has become an important goal.
Polysaccharides are important macromolecular substances in organisms as they provide the main source of energy in many creatures and are involved in almost every process in life. Previous studies examining polysaccharides were mainly focused on those polysaccharides derived from plants, such as astragali and Ganoderma. However, increasingly more attention has become focused on the polysaccharides derived from Spirulina platensis (Joventino et al., 2012). Spirulina platensis, a blue-green alga, was originally used as a source of nutrition in some African areas. The polysaccharide extracted from Spirulina platensis (PSP) is a water-soluble, nontoxic heteropolysaccharide. It consists of rhamnose, xylose, glucose, galactose, and arabopyranose glucuronic acid (Chen et al., 2012). PSP participates in a variety of biological functions, including those related to anti-aging, reducing hypercholesterolemia, facilitating protein synthesis, enhancing immune functions, and anti-radiation (Majdoub et al., 2009; Yang et al., 2012). Some of these biological functions suggest that PSP may play a neuroprotective role in organisms. However, it is currently unknown whether PSP provides a neuroprotective effect in dopamine neurons. Therefore, the aim of the present study was to determine whether PSP protects dopaminergic neurons in a C57BL/6J mouse model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and, if so, to determine the possible mechanisms for this protective action.
Materials and Methods
Animals
Male 10-week-old C57BL/6J mice (specific pathogen-free grade, clean level, 23–28 g, n = 130) were obtained from Vital River Laboratory Animal Research Center, Beijing, China (license No. SCXK (Jing) 2002-2003). Mice were maintained under a 12-hour light/dark cycle with lights on at 7:00 a.m. The temperature was maintained at 19 ± 2°C, and mice were permitted water and food ad libitum. All experimental procedures were approved by the Qingdao University Committee for Animal Care in China.
Grouping and interventions
For the immunohistochemistry assay, mice were randomly divided into the following three groups (n = 10 per group): control, MPTP, and 800 mg/kg PSP. For the reverse transcription polymerase chain reaction (RT-PCR) assay, mice were randomly divided into the following five groups (n = 10 per group): control; MPTP; 200, 400, or 800 mg/kg PSP + MPTP. Mice in the control group were intraperitoneally injected with normal saline. Mice in the MPTP groups were injected with MPTP (15 mg/kg, intraperitoneal; Sigma, St. Louis, MO, USA) four times within 24 hours, at 6-hour intervals (Ye et al., 2014). Mice in the 200, 400 or 800 mg/kg PSP + MPTP groups were given 200, 400 or 800 mg/kg of PSP (Sanya Neptunus Marine Biological Technology Co., Ltd., Sanya, Hainan Province, China) via gastric lavage once daily for 10 days. Following completion of the PSP pretreatment, the mice were injected with MPTP (15 mg/kg, intraperitoneal) four times within 24 hours. All mice were killed 24 hours after the last MPTP treatment.
For the biochemical detection assays, mice were divided into the following five groups (n = 10 per group): control; MPTP; PSP + MPTP; PSP; deprenyl + MPTP. Mice in the control group were intraperitoneally injected with normal saline. Mice in the MPTP groups were injected with MPTP (15 mg/kg, intraperitoneal) four times within 24 hours. Mice in the PSP + MPTP group were given 800 mg/kg PSP once daily via intragastric administration for 10 days, and then were injected with MPTP (15 mg/kg, intraperitoneal) four times within 24 hours. Mice in the PSP group were given 800 mg/kg PSP once daily for 10 days via intragastric administration. Mice in the deprenyl + MPTP group, serving as a positive control, were given 0.5 mg/kg deprenyl (Sigma, St. Louis, MO, USA) via intragastric lavage once daily for 10 days (Haji Ghasem Kashani et al., 2013), and then they were injected with MPTP (15 mg/kg, intraperitoneal) four times within 24 hours.
Immunohistochemical determination of tyrosine hydroxylase (TH) and dopamine transporter (DAT) in substantia nigra pars compacta in a mouse model of Parkinson's disease
At 24 hours after the last MPTP injection, mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal) and transcardially perfused with 4% paraformaldehyde in PBS. Brains were removed and dehydrated for 24 hours in 25% sucrose. Horizontal midbrain sections at a thickness of 16 μm were cut through the substantia nigra (−2.92 mm to −3.88 mm from bregma) (Paxinos and Watson, 2005). The sections were processed for TH and DAT immunohistochemistry using the streptavidin-peroxidase method. Specimens were incubated with 0.3% H2O2 formic acid solution at 37°C for 30 minutes, rinsed with PBS, blocked with 10% goat serum at 37°C for 1 hour, and incubated with rabbit anti-DAT polyclonal antibody (1:50; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) and rabbit anti-TH polyclonal antibody (1:5,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) in a humid chamber at 4°C overnight. After another wash in PBS, specimens were incubated with biotin-labeled goat anti-rabbit IgG (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at 37°C for 30 minutes and horseradish peroxidase-conjugated streptavidin solution (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at 37°C for 30 minutes. After another PBS wash, specimens were developed using 3,3′-diaminobenzidine (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.), followed by gradient ethanol dehydration, xylene clearing, and mounting. The sections were observed under a light microscope (Olympus, Tokyo, Japan).
The TH immunostaining was analyzed by counting the number of TH-immunoreactive neurons. The number of TH-immunoreactive neurons in the substantia nigra pars compacta was counted in four fields (40 × 10) within one section. The average number of positive cells in four fields per slide was used to represent TH-immunoreactive neurons.
The DAT immunoreactivity was analyzed with a Simple PCI computerized image analysis system (C-imaging Co, Ltd., Irvine, CA, USA) as described by Andringa et al. (2005). DAT immunoreactivity was determined in the substantia nigra pars compacta to detect the MPTP-induced DAT loss in nigral neuronal cell bodies. Images were captured using a digital camera (Olympus, Tokyo, Japan). In the substantia nigra pars compacta, optical density was analyzed at three rostrocaudal levels, whereas nonspecific labeling was measured in the thalamus. Specific immunoreactivity was calculated by subtracting the nonspecific immunoreactivity assessed in the thalamus from the immunoreactivity assessed in the substantia nigra pars compacta. The DAT binding ratios were expressed as specific immunoreactivity divided by nonspecific immunoreactivity.
RT-PCR detection of TH and DAT mRNA expression in substantia nigra in a mouse model of Parkinson's disease
Total RNA was isolated from the substantia nigra using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized from 3 μg of total RNA treated with DNAse by reverse transcription according to the kit instructions (4 μL of MgCl2 (5 mM), 10 × Buffer (2 μL), dNTPs (1 mM, 2 μL), 0.5 μL of AMV Reverse Transcriptase (15 U), 0.5 μL of RNase inhibitor (0.5 U), 1 μL of Oligo(dT) primer (0.5 μg), 5 μL of mRNA, RNase free water, total 20 μL). Total RNA and Oligo(dT) primer were incubated at 70°C for 10 minutes prior to reverse transcription. After incubation for 1 hour at 42°C, the reaction was terminated by incubation with a denaturing enzyme for 5 minutes at 99°C. For PCR amplification, the following primers were used:

All primers were designed to include intron regions using primer 5 (Sangong Biotech Co, Ltd., Shanghai, China). The reaction mix contained 1.0 μL of cDNA, 0.5 μL of 10 μM each primer, 2 μL of 10 mM dNTP mix, 2 U of Taq polymerase, and 2.5 μL of 10 × reaction buffer. Water was added to achieve a final volume of 25 μL. PCR was performed in a DNA Thermal Cycler (PTC-2000). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA and TH cDNA were amplified under conditions of 35 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 45 seconds. DAT cDNA was amplified under conditions of 35 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 60 seconds.
Following RT-PCR, the GAPDH and TH/DAT PCR products were mixed together and loaded on the same agarose gel. The gel was stained with ethidium bromide and photographed under ultraviolet light. Band densities were obtained using Gis-1000 software (Tanon, Shanghai, China). The amount of TH and DAT mRNA in each brain structure from the different groups was estimated as optical density ratio of TH(DAT)/GAPDH.
Biochemical detection of monoamine oxidase B (MAO-B), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) in the serum and midbrain in a mouse model of Parkinson's disease
At 24 hours after the last injection of MPTP, blood samples were collected by retro-orbital puncture from anesthetized mice, placed in heparin anticoagulation EP tubes, and centrifuged at 4°C and 6,500 r/min for 10 minutes. The supernatant was collected for analysis. The midbrains were rinsed in chilled normal saline, blotted dry on ash-free filter paper, and weighed. After nine washes with normal saline and ultrasonic homogenization in an ice bath for 10 seconds, 10% brain tissue homogenates were prepared and centrifuged at 4°C at 2,500 r/min for 10 minutes. The supernatant was aspirated for analysis. The protein concentration of the brain tissue was detected according to the assay kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). The activities of MAO-B at a wavelength of 242 nm, SOD at 550 nm, and GSH-Px at 412 nm were detected with an ultraviolet spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Data are expressed as the mean ± SD. One-way analysis of variance and the Student-Newman-Keuls multiple comparisons test were used for statistical analysis. Values of P ≤ 0.05 were considered statistically significant.
Results
Effect of PSP on TH and DAT immunoreactivities in the substantia nigra pars compacta of mice with MPTP-induced Parkinson's disease
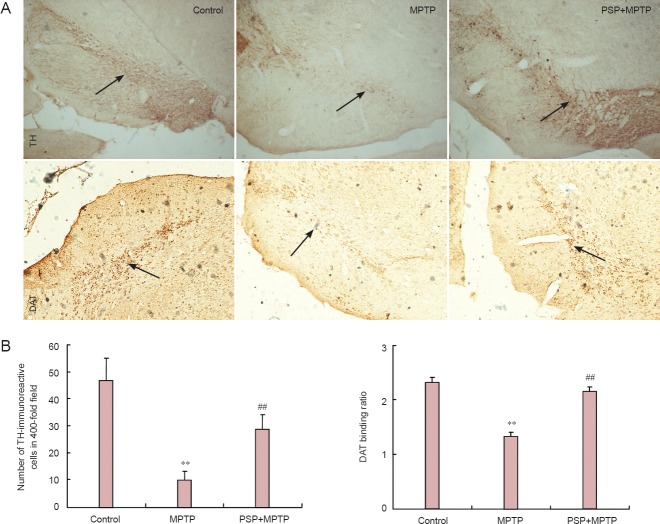
The results of the immunohistochemical analysis showed that mice in the MPTP group had significantly fewer TH-immunoreactive neurons and a lower DAT binding ratio in the substantia nigra pars compacta than mice in the control group (P < 0.01). After PSP pretreatment, the mean number of TH-immunoreactive neurons and the DAT binding ratio in the substantia nigra pars compacta were significantly increased compared with those for mice in the MPTP group (P < 0.01; Figure 1).
Figure 1.
Effect of PSP (800 mg/kg) on TH and DAT immunoreactivities in the substantia nigra pars compacta of mice with MPTP-induced Parkinson's disease.
(A) Microphotographs of TH immunostaining in the substantia nigra pars compacta and DAT immunostaining in substantia nigra of MPTP-injected mice (immunohistochemical staining, × 4). Arrows show TH or DAT positive expressin. (B) Effect of PSP on the number of TH-immunoreactive cells and DAT binding ratio in the substantia nigra of mice with MPTP-induced Parkinson's disease. Data are expressed as the mean ± SD. One-way analysis of variance followed by Student-Newman-Keuls multiple comparisons test was used for statistical analysis. **P < 0.01, vs. control group; ##P < 0.01, vs. MPTP group. The experiments were repeated three times. PSP: Polysaccharide from Spirulina platensis; TH: tyrosine hydroxylase; DAT: dopamine transporter; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Effect of PSP dose on TH and DAT mRNA expression in the substantia nigra of mice with MPTP-induced Parkinson's disease
The results from the RT-PCR analysis showed that the levels of TH and DAT mRNA expression in the substantia nigra of MPTP-treated mice were significantly decreased compared with those in the control group (P < 0.01). However, PSP (800 mg/kg) pretreatment significantly increased the mRNA expression of TH and DAT in the substantia nigra compared with that in mice treated with only MPTP. By contrast, neither the 200 nor the 400 mg/kg PSP dose significantly affected the mRNA expression of TH or DAT compared with that in mice treated with only MPTP (P < 0.01; Figure 2).
Figure 2.
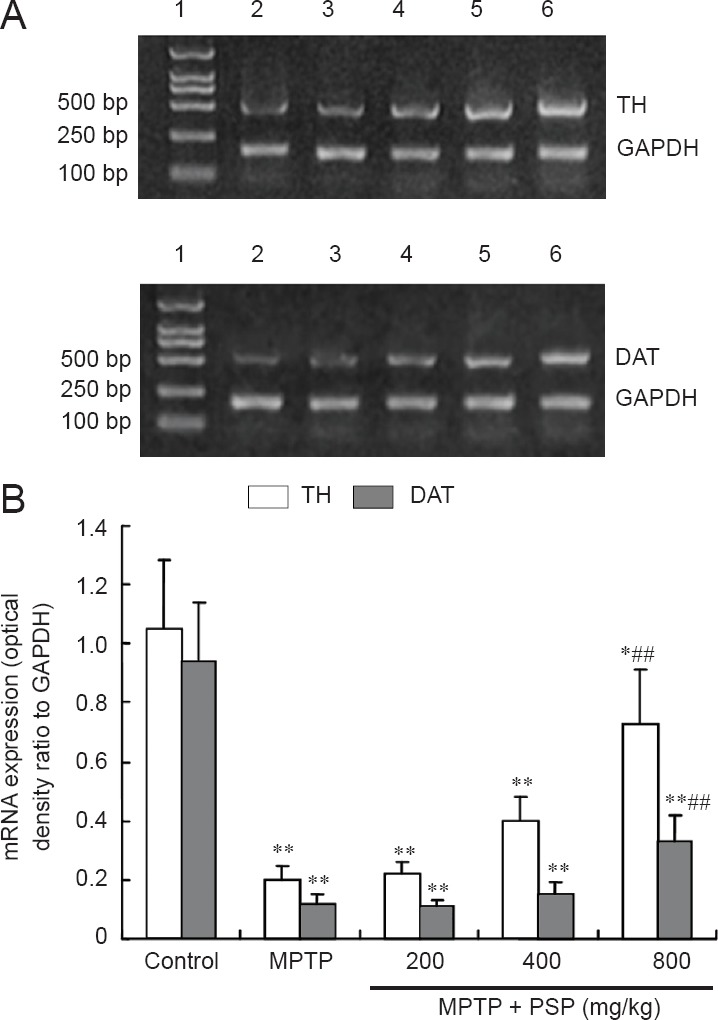
Effect of PSP dose on TH and DAT mRNA expression in substantia nigra of mice with MPTP-induced Parkinson's disease.
(A) Lanes represent the results from the following groups: 1: DNA marker; 2: MPTP group; 3: MPTP + PSP 200 mg/kg group; 4: MPTP + PSP 400 mg/kg group; 5: MPTP + PSP 800 mg/kg group; 6: control group. (B) Data are expressed as the mean ± SD (n = 10 mice in each group). One-way analysis of variance followed by the Student-Newman-Keuls multiple comparisons test was used for statistical analysis. *P < 0.05, **P < 0.01, vs. control group; ##P < 0.01, vs. MPTP group. PSP: Polysaccharide from Spirulina platensis; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH: tyrosine hydroxylase; DAT: dopamine transporter.
Effect of PSP on activities of MAO-B, SOD, and GSH-Px in the serum and midbrains of mice with MPTP-induced Parkinson's disease
The activity of MAO-B in the serum and midbrains of mice in control, MPTP, PSP, and PSP + MPTP groups were similar (P > 0.05), but the MAO-B activity in the serum and midbrains of mice in the deprenyl + MPTP group was significantly decreased compared with that for mice in the MPTP group (P < 0.01; Figure 3).
Figure 3.
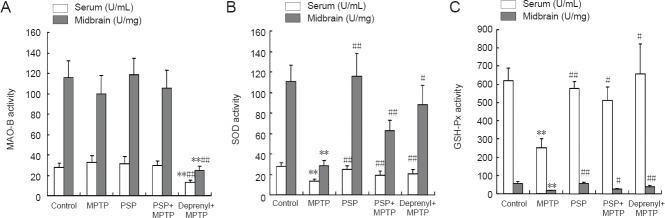
Effect of PSP on the activities of MAO-B (A), SOD (B), and GSH-Px (C) in the serum and midbrains of mice with MPTP-induced Parkinson's disease.
Data are expressed as the mean ± SD (n = 10 mice in each group). One-way analysis of variance followed by Student-Newman-Keuls multiple comparisons test was used for statistical analysis. **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. MPTP group. PSP: Polysaccharide from Spirulina platensis; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MAO-B: monoamine oxidase B; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase.
The activity of SOD in the serum and midbrains of mice in the MPTP group was significantly decreased compared with that in the control group (P < 0.01). However, pretreatment with 800 mg/kg PSP or with 0.5 mg/kg deprenyl significantly increased the activity of SOD compared with that in mice treated with only MPTP. In addition, there was no significant difference in SOD activity between mice in the 800 mg/k PSP group and those in the control group (Figure 3).
The activity of GSH-Px in the serum and midbrains of mice in the MPTP group was significantly decreased compared with that in the control group (P < 0.01). However, pretreatment with 800 mg/kg PSP or 0.5 mg/kg deprenyl significantly increased the activity of GSH-Px compared with that in mice treated with only MPTP (P < 0.01). In addition, there was no significant difference in GSH-Px activity between mice in the 800 mg/kg PSP group and those in the control group (Figure 3).
Discussion
The present study investigated the neuroprotective effect of PSP on MPTP-induced neurotoxicity. Our findings indicated that the highest dose of PSP effectively protected against the MPTP-induced loss of TH-positive neurons in the substantia nigra. PSP also attenuated the reduction in TH and DAT expression induced by MPTP. Furthermore, PSP attenuated the decrease in dopamine levels and the increase in dopamine metabolism rates in MPTP-treated mice. We also found that PSP protected against the reduction in SOD and GSH-px caused by MPTP.
TH is the rate-limiting enzyme for dopamine synthesis in dopaminergic neurons (Bézard et al., 2013). The physiological function of DAT is to reuptake the released dopamine from the synaptic cleft into vesicles, which is important for the transient function and salvage of dopamine (Eriksen et al., 2010). DAT is synthesized in the perikarya, dendrites, and axons of dopaminergic neurons and then transported to the membrane. High transcriptional levels of TH and DAT are reported in the midbrain and ventral tegmental area (Elsinga et al., 2006). Consistent with another report, the results of the present study showed that the immunoreactivity for TH and DAT was weaker and the expression of TH and DAT mRNA was decreased in MPTP-treated mice, suggesting that the majority of dopaminergic neurons were lost in this mouse model of Parkinson's disease (Meissner et al., 2003). Dopamine is synthesized in the substantia nigra pars compacta and released in the striatum to exert its physiological functions (Messripour and Mesripour, 2013). Thus, the death of the dopaminergic neurons in the substantia nigra pars compacta would be expected to lead to a decrease in levels of striatal dopamine. However, the results of the present study showed that pretreatment with 800 mg/kg PSP inhibited the MPTP-induced decrease in the immunoreactivity and mRNA expression of TH and DAT. This evidence indicated that PSP has a neuroprotective effect on dopaminergic neurons and dopamine levels in mice with MPTP-induced Parkinson's disease.
The neurotoxin MPTP recreates a Parkinson's disease-like model in both rodents and primates (Kolata, 1983). MPTP neurotoxicity depends on the MAO-B-catalyzed production of MPP+, which is taken up selectively by dopaminergic neurons and concentrated in mitochondria (Dorszewska et al., 2013; Kim et al., 2014). Since MPTP neurotoxicity depends on MAO-B, the activity of MAO-B was examined in the present study to elucidate whether the protective effect of PSP was secondary to the inhibition of MAO-B. Deprenyl-treated mice served as the positive control for this experiment. The results showed that deprenyl significantly inhibited the activity of MAO-B. By contrast, PSP showed no apparent effect on the activity of MAO-B in either control or MPTP-treated mice, suggesting that mechanisms other than the inhibition of MAO-B are involved in the protective effect of PSP on dopaminergic neurons.
MPP+ inhibits the activity of complex I in the electron transport chain (Gluck et al., 1994), leading to the production of reactive oxygen species, loss of the mitochondrial membrane potential and ATP production, and neuronal death (Cassarino et al., 1999). MPP+ also induces DA efflux (Zhang et al., 2014), increasing dopamine autoxidation and oxidative damage (Rollema et al., 1988; Chiueh et al., 1993). The activities of SOD and GSH-px, important endogenous antioxidants, were analyzed in the present study to determine whether enhancing antioxidation was the mechanism underlying the protective effect of PSP. The results showed that the activities of SOD and GSH-px were significantly decreased in MPTP-treated groups, indicating that the excessive reactive oxygen species in these mice consumed endogenous antioxidants. However, mice pretreated with 800 mg/kg PSP showed an increase in the activities of SOD and GSH-px compared with those in the MPTP-treated groups. This evidence suggested that antioxidation is one of the mechanisms involved in the protective effect of PSP on dopaminergic neurons.
Footnotes
Funding: This study was financially supported by grants from the Natural Science Foundation of Shandong Province of China, No. ZR2011HQ008, ZR2011HM044.
Conflicts of interest: None declared.
Copyedited by Smith T, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Andringa G, Drukarch B, Bol JG, de Bruin K, Sorman K, Habraken JB, Booij J. Pinhole SPECT imaging of dopamine transporters correlates with dopamine transporter immunohistochemical analysis in the MPTP mouse model of Parkinson's disease. Neuroimage. 2005;26:1150–1158. doi: 10.1016/j.neuroimage.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Bézard E, Olanow CW, Obeso JA. Levodopa-induced dyskinesias in the absence of nigrostriatal degeneration. Mov Disord. 2013;28:1023–1024. doi: 10.1002/mds.25533. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker WD, Bennett JP. The parkinsonian neurotoxin MPP + opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Chen HW, Yang TS, Chen MJ, Chang YC, Lin CY, Wang EI, Ho CL, Huang KM, Yu CC, Yang FL, Wu SH, Lu YC, Chao LK. Application of power plant flue gas in a photobioreactor to grow Spirulina algae, and a bioactivity analysis of the algal water-soluble polysaccharides. Bioresour Technol. 2012;120:256–263. doi: 10.1016/j.biortech.2012.04.106. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Miyake H, Peng MT. Role of dopamine autoxidation, hydroxyl radical generation, and calcium overload in underlying mechanisms involved in MPTP-induced parkinsonism. Adv Neurol. 1993;60:251–258. [PubMed] [Google Scholar]
- Dorszewska J, Prendecki M, Oczkowska A, Rozycka A, Lianeri M, Kozubski W. Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in Parkinson's disease. Curr Genomics. 2013;14:518–533. doi: 10.2174/1389202914666131210210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr Med Chem. 2006;13:2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Jørgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fan ZG, Qiao Lei. Dopamine content in the stratum of Parkinson's disease rats after transplantation of tyrosine hydroxylase-modified human umbilical cord blood mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7285–7289. [Google Scholar]
- Gluck MR, Krueger MJ, Ramsay RR, Sablin SO, Singer TP, Nicklas WJ. Characterization of the inhibitory mechanism of 1-methyl-4-phenylpyridinium and 4-phenylpyridine analogs in inner membrane preparations. J Biol Chem. 1994;269:3167–3174. [PubMed] [Google Scholar]
- Haji Ghasem Kashani M, Ghorbanian MT, Hosseinpour L. Transplantation of deprenyl-induced tyrosine hydroxylase-positive cells improves 6-OHDA-lesion rat model of Parkinson's disease: behavioral and immunohistochemical evaluation. Cell J. 2013;15:55–64. [PMC free article] [PubMed] [Google Scholar]
- Joventino IP, Alves HG, Neves LC, Pinheiro-Joventino F, Leal LK, Neves SA, Ferreira FV, Brito GA, Viana GB. The microalga Spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J Complement Integr Med. 2012;9 doi: 10.1515/1553-3840.1534. Article 17. [DOI] [PubMed] [Google Scholar]
- Kim YD, Lantz-McPeak SM, Ali SF, Kleinman MT, Choi YS, Kim H. Effects of ultrafine diesel exhaust particles on oxidative stress generation and dopamine metabolism in PC-12 cells. Environ Toxicol Pharmacol. 2014;37:954–959. doi: 10.1016/j.etap.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Kolata G. Monkey model of Parkinson's disease. Science. 1983;220:705. doi: 10.1126/science.6403987. [DOI] [PubMed] [Google Scholar]
- Majdoub H, Mansour MB, Chaubet F, Roudesli MS, Maaroufi RM. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim Biophys Acta. 2009;1790:1377–1381. doi: 10.1016/j.bbagen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Meissner W, Prunier C, Guilloteau D, Chalon S, Gross C, Bezard E. Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of parkinson's disease. Mol Neurobiol. 2003;28:209–218. doi: 10.1385/MN:28:3:209. [DOI] [PubMed] [Google Scholar]
- Messripour M, Mesripour A. Age related interaction of dopamine and serotonin synthesis in striatal synaptosomes. Biocell. 2013;37:17–21. [PubMed] [Google Scholar]
- Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Rollema H, Kuhr WG, Kranenborg G, De Vries J, Van den Berg C. MPP+-induced efflux of dopamine and lactate from rat striatum have similar time courses as shown by in vivo brain dialysis. J Pharmacol Exp Ther. 1988;245:858–866. [PubMed] [Google Scholar]
- Wang YL, Gao H, Yang XL. Establishment of rat models of Parkinson's disease by unilateral two-point injection with 6-hydroxydopamine. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8030–8035. [Google Scholar]
- Yang LL, Zhou QJ, Wang Y, Gao Y, Wang YQ. Comparison of the therapeutic effects of extracts from Spirulina platensis and amnion membrane on inflammation-associated corneal neovascularization. Int J Ophthalmol. 2012;5:32–37. doi: 10.3980/j.issn.2222-3959.2012.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Lee Sg, Chung ES, Lim SJ, Kim WS, Yoon H, Kim SK, Ahn KS, Jang YP, Bae H. (2014) Neuroprotective effects of cuscutae semen in a mouse model of Parkinson's disease. Evid Based Complement Alternat Med. 2014 doi: 10.1155/2014/150153. 150153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chiu VM, Stoica G, Lungu G, Schenk JO, Hill HH. Metabolic analysis of striatal tissues from Parkinson's disease-like rats by electrospray ionization ion mobility mass spectrometry. Anal Chem. 2014;86:3075–3083. doi: 10.1021/ac4040967. [DOI] [PubMed] [Google Scholar]