Abstract
Objective
To establish whether daily mechanical stimulation improves functional recovery of whisking after facial nerve transection injury/ repair in the rodent.
Methods
Forty rats underwent facial nerve transection injury/ repair, and subsequent quantitative facial movement testing. Animals were randomized into two experimental groups (n=20 each). Both groups received daily 5-minute manual stimulation of their whiskers, with one group undergoing whisker protraction and the other whisker retraction. Rats were tested on postoperative weeks 1, 4-8, and 15 using a validated, quantitative whisking kinematics apparatus. Whisks were counted and analyzed for whisking amplitude, velocity and acceleration.
Results
Animals receiving manual stimulation by passive protraction of their whiskers demonstrated significantly improved functional recovery at multiple time points during the 15 weeks compared with historical controls (p<.05, one-tailed t-test). Recovery was similar in the protraction and retraction groups, trending towards better whisking recovery in the protraction group.
Conclusions
The present report demonstrates that daily mechanical whisker stimulation significantly improves recovery of whisking after facial nerve transection/ repair in animals undergoing either protraction or retraction. This finding supports the role of early soft tissue manipulation after facial nerve repair, and may have clinical implications for the postoperative management of patients following facial nerve manipulations.
Introduction
Facial paralysis is a clinical disorder that carries significant adverse social and functional consequences, including decreased ability to communicate using facial expression, incomplete eye closure, external nasal valve collapse, and oral incompetence. Clinically, many studies have demonstrated poor functional recovery after facial nerve transection injury and microsurgical repair1-5. The slow rate of facial nerve regeneration following certain injury scenarios can lead to degeneration of the motor end organ, with permanent loss of function. Recognizing this, many researchers have used animal models to study manipulations that might accelerate recovery, employing qualitative and semiquanitative methods to measure facial recovery after injury6-8.
A variety of pharmacologic agents have been shown to improve motor nerve regeneration in animals, including FK-5068, TJ-23 (Tokishakuyakusan)9, angiotensin II (ANG II)10, nitric oxide (NO)11,12, and brain-derived neurotropic factor (BDNF)13; however, given the side effects and the difficulties with drug delivery and bioavailability none are in clinical use. Likewise, some non-pharmacologic treatments have been shown to be of benefit. Recently, manual mechanical stimulation of paralyzed facial14 and tongue15 musculature has demonstrated promise as a possible treatment option following cranial nerve transection. Despite a wealth of research to date, few treatments are available to accelerate or improve recovery after facial nerve injury, and none prevent the adverse effects of aberrant regeneration and its clinical correlate, synkinesis 16.
Our laboratory has recently adapted a rodent whisker movement monitoring system to quantitatively measure return of facial nerve function after injury17-20. This system automatically measures the amplitude, velocity, and acceleration of whisks, providing a useful tool for precise quantification of the timing and completeness of facial nerve recovery after injury. We have found that transection-injured animals recover poorly; barely achieving measurable recovery after four months14,19. This poor recovery is commensurate with the suboptimal clinical recovery seen in patients after a facial nerve transection and repair or cable grafting21,22. Our objectives in this study were to corroborate the recently reported improved functional recovery of whisking associated with daily mechanical whisker stimulation 14, and to compare mechanical stimulation performed by daily whisker protraction to daily whisker retraction under the hypothesis that different directions of whisker stimulation would lead to different functional levels of recovery.
METHODS
PREPARATION FOR RIGID HEAD FIXATION
Forty female Wistar-Hannover rats (Charles River Laboratories, Wilmington, Massachusetts), weighing 200 to 250g, were handled daily for 2 weeks prior to surgical manipulation to condition them to behavior testing. Subsequently, all rats underwent surgical insertion of a light-weight titanium head implant that provided a set of four external attachment points for rigid head fixation, as previously described19,20. One week after head-fixation device implantation, rats were conditioned to a body restraint apparatus by brief daily placements into a fitted sack. In the third week, head restraint was added to the daily conditioning regimen. After the third week, the rats were sufficiently conditioned to undergo head/body restraint without struggling or signs of stress, and pre-surgical baseline testing was performed. Rats were not food or water deprived prior to testing, and all experimentation was conducted under protocols approved by the Massachusetts Eye and Ear Infirmary Animal Care and Use Committee.
SURGICAL PROCEDURE
Rats were anesthetized with an intramuscular injection of ketamine (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, Iowa) and medetomidine hydrochloride (0.5 mg/kg) (Orion Corporation, Espoo, Finland) in saline. The left infraauricular area was shaved and sterilely prepared. Left facial nerve exposure involved a pre-auricular incision, reflection of the parotid gland, and visual identification of the main trunk of the facial nerve. The common trunk was electrically stimulated with a nerve stimulator (Montgomery Nerve Stimulator; Boston Medical Products, Westford, Massachusetts) at a setting of 1mV to verify complete hemifacial movement. The nerve was then transected and the cut ends were microsurgically reconnected with two 9-0 nylon sutures (Ethilon, Somerville, New Jersey). The wound was then closed in a single layer with absorbable suture, and the anesthetic was reversed with a subcutaneous injection of 0.05mg/kg of atipamezole hydodrochloride. Rats were allowed to recover on a warming pad, and were monitored post-operatively for signs of discomfort including changes in grooming, social interaction and maintenance of normal body weight.
MECHANICAL STIMULATION
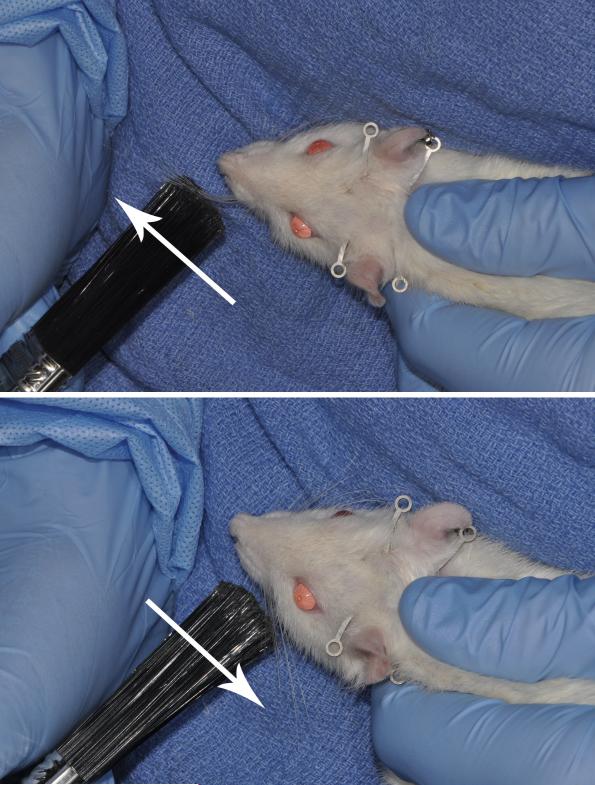
All experimental animals received systematic mechanical stimulation to the whiskers five days per week, starting on the first postoperative day. Over a five minute period, with the animal held securely against the body of the handler to limit head movement, approximately forty-five stokes per minute were delivered to the vibrissae in either a posterior- anterior direction (protraction group) or in and anterior-posterior direction (retraction group), using a soft-bristled paint brush (Figure 1). Care was taken to avoid stroking the whisker pad during stimulation. Animals rapidly habituated to the procedure and did not show signs of stress during or after manipulation.
Figure 1.
Postoperative mechanical stimulation technique. (Top) Manual protraction of the left vibrissae. (Bottom) Manual retraction of the left vibrissae. Care was taken in both groups not to directly stimulate the whisker pad with the soft bristled brush.
FUNCTIONAL RECOVERY TESTING
Baseline whisking testing was performed pre-operatively, and initial post-surgical testing was performed one week after facial nerve manipulation. Weekly post-operative testing of the animals continued across postoperative weeks 4-8, and was conducted one final time at week 15. Whisking recovery was monitored by our previously validated testing apparatus19,20. Briefly, on the day of testing, animals were placed in the body restraint device, right and left C-1 whiskers were marked using polyimide tubes (SWPT-045, SWPT-008, Small Parts Inc.) to increase their detectability, and then rats were placed into the monitoring apparatus. The horizontal movement of the marked C-1 whiskers was independently tracked using commercial laser micrometers (MetraLight, Santa Mateo CA) and a data acquisition computer20. A computer-controlled air valve was used to deliver 10 second sustained flows of scented air toward the snout in order to elicit whisking behavior at two random time points during each 5-minute data recording session per animal.
DATA ANALYSIS
The three largest amplitude whisks were detected and analyzed in automated fashion for each rat on each day of recording using software adapted from Bermejo et al., 1998, 2002. The data were normalized for each animal, across the two sides of the face by dividing the whisking amplitude on the injured side by the amplitude on the uninjured side, giving the relative recovery. This is based on prior observations that right/left whisking is generally symmetrical, and to account for behavioral changes in whisking effort17,18. A group average for relative recovery of amplitude was calculated. Independent, two sample, one tailed t-tests were then performed for weeks 4, 7, and 15 between the experimental groups (protraction and retraction) and our normative data set (in press). An independent, two sample, two tailed t-test was performed between the protraction and retraction groups under the hypothesis that mechanical stimulation of the whiskers would affect recovery. The same data analysis was performed for the three whisks with the largest velocity and the three whisks with the greatest acceleration.
We further analyzed the top three performers in each group for post-operative weeks 4, 7, and 15. The top three amplitude values were then normalized to the uninjured side and the average was calculated. This was repeated for velocity and acceleration.
RESULTS
Rats were housed in groups of 2-3 animals per cage, and demonstrated normal social, grooming and feeding behavior in the 15 weeks of post-operative observation. There were no post-operative wound infections after either the head fixation device placement or the facial nerve transection procedure. There was an attrition of 20% of the rats across the 15 week post-surgical survival period, due to head fixation device failure or generalized poor adaptation to the testing apparatus. Two rats in the retraction group and three rats in the protraction group were not included at any time point due to early implant failure. Three additional rats in the protraction group were euthanized after postoperative weeks 4, 7, and 8 secondary to implant failure. Therefore, at the 15 week post-surgical time-point there were 18 rats in the retraction group and 14 rats in the protraction group.
WHISKING RECOVERY
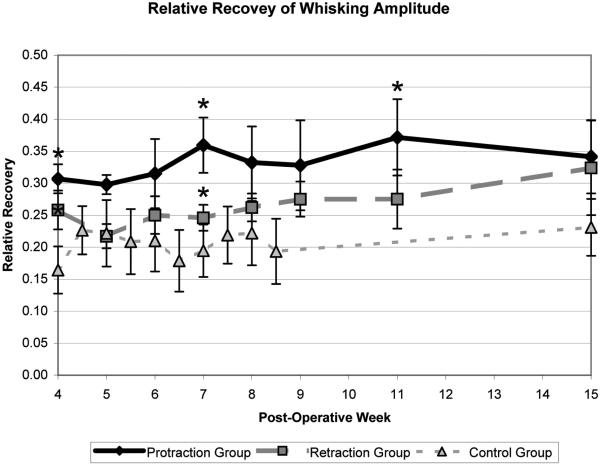
Pre-operative testing reveled that protraction, retraction, and control animals demonstrated symmetric right and left whisking, with a relative amplitude of 1.0 (standard error .023) and 1.0 (standard error .030) and .9 (standard error .191), respectively. Animals had complete absence of whisking function on post-operative week 1, and measureable whisking recovery by post-operative week 4, in both the protraction and retraction groups. This was significantly greater than the recovery observed without mechanical stimulation, in our normative data set (in press). There were statistically significant differences in relative amplitude between the protraction group and our normative data set (p<.05, one-tailed t-test), and the retraction group and our normative data set (p<.05, one-tailed t-test) at multiple time points (Figure 2). In addition, there were statistically significant differences in relative velocity and acceleration between the protraction and the retraction groups and our normative data at multiple time points (Table 1). At 7 weeks the protraction group demonstrated a statistically significant improvement in acceleration as compared to the retraction group. For all other time points and kinematic measures the protraction group (n= 15-18) trended toward improved functional recovery as compared to the retraction group (n=18); however, no statistical significance was demonstrated between the experimental groups (p>.05, two tailed t-test) (Table 1).
Figure 2.
Relative recovery of amplitude during the period of maximal recovery. Asterisks indicate the time points that were significantly different from the normative data set (p<.05, one-tailed t-test). Stand error bars are shown.
Table 1.
Relative Whisking Kinematic Data for the Transected Side.
| Protraction | Retraction | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Amplitude | Velocity | Acceleration | Amplitude | Velocity | Acceleration | Amplitude | Velocity | Acceleration |
| 4 | 0.307* (SD 0.202) | 0.488* (SD 0.314) | 0.545 (SD 0.329) | 0 258* (SD 0.129) | 0.400 (SD 0.180) | 0.514 (SD 0.212) | 0.187 (SD 0.109) | 0.313 (SD 0.149) | 0.422 (SD 0.176) |
| 7 | 0.362* (SD 0.278) | 0.517+* (SD 0.302) | 0.652* (SD 0.316) | 0.246* (SD 0.086) | 0.394* (SD 0.144) | 0.302 (SD 0.124) | 0.194 (SD 0.089) | 0.292 (SD 0.129) | 0.329 (SD 0.142) |
| 15 | 0.341* (SD 0.194) | 0.409 (SD 0.210) | 0.524* (SD 0.278) | 0 324 (SD 0.314) | 0.508 (SD 0.306) | 0.595* (SD 0.381) | 0.231 (SD 0.122) | 0.378 (SD 0.192) | 0.348 (SD 0.169) |
An indicates a significant improvement as compared to the control group (p<.05, one-tailed t-test)
indicates a significant improvement as compared to the retraction group(p<.05, two-tailed t-test).
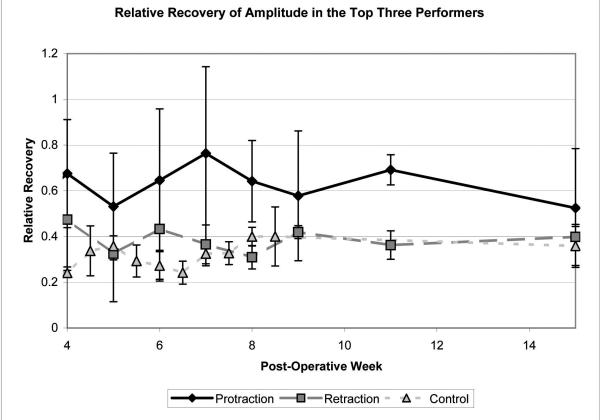
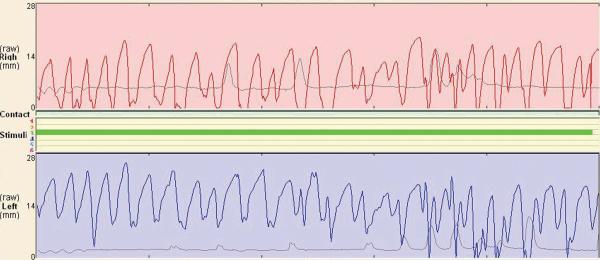
Examination of the top three performers in each group during post-operative weeks 4, 7, and 15 revealed a dramatic improvement in the protraction group as compared with the retraction and the control groups (Figure 3). One animal in the protraction group obtained nearly symmetric whisking by week 7 (Figure 4).
Figure 3.
Average relative recovery of amplitude for the top three performers in each group. Note the improvement in the protraction group as compared with the retraction and control groups. Standard error bars are shown.
Figure 4.
Representative whisking tracing during olfactory stimulus for one animal in the protraction group on postoperative week 7. Note the symmetry of the whisking patterns for the uninjured side (right -side, top tracing) and the manipulated side (left side, bottom tracing).
A plateau of recovery was achieved for whisking amplitude, velocity, and acceleration on the operated sides of all animals between postoperative weeks 7 and 15. On post-operative week 15, animals continued to exhibit a significant difference for amplitude, velocity, and acceleration between the operated and non-operated sides (p<.005, one tailed t-test), indicating that complete recovery of whisking function was not achieved at the 15 week post-operative period for either mechanically stimulated group.
COMMENT
Facial nerve transection and epineural suture repair typically results in poor whisker function14,19, even after 16 weeks of recovery or longer19 (in press). Because the results of most surgical nerve repairs remain generally disappointing, the discovery by Angelov et al in 2007 that mechanical stimulation improves functional recovery of whisking compared with surgical repair of nerve transection alone was remarkable14. The current effort endeavored to verify that interesting finding, further quantify the whisking kinematics during the recovery process, and to examine whether the particular nature of mechanical stimulation (protraction vs. retraction) is important in enhancing functional recovery.
Recent evidence shows ipsilateral mechanical stimulation of the whisker pad and protraction of the vibrissae leads to recovery of normal whisking function14 and manual stimulation of the orbicularis oculi muscle improves blink responses 14,23. Normal whisking motion is also recovered after facial nerve transection and repair in blind rat strains, where functional demands on whisking and whisker sensory feedback are heightened15,24. In addition, mechanical stimulation under the mandible (submental region) has been shown to improve functional recovery and reduce polyneuronal reinnervation of tongue musculature after hypoglossal nerve transection and repair15. In these situations, although polyneuronal reinnervation is reduced compared to non-stimulated or visually intact rats, aberrant peripheral reinnervation is not, suggesting that the return of normal whisking function is attributable to central neuromotor reorganization14,15. Integrity of the sensory trigeminal system has been shown to influence the recovery of the facial nucleus in rodents25. Recent evidence shows that in a mixed nerve, using a median nerve transection model, manual stimulation after transection and repair does not improve functional outcome, degree of axonal sprouting, or the extent of motor endplate polyinnervation26. Evidence also suggests decreased muscle fibrosis with whisker pad massage, perhaps allowing superior functional behavior after neuroregeneration occurs14.
The fact that we were able to find a significant improvement in whisking function with mechanical stimulation in our automated apparatus should draw the attention of facial re-animation specialists, because of the pressing need for more effective treatments for patients with facial paralysis16. Neuromuscular retraining has been shown in a few small evidence-based clinical studies to improve facial function27-29. However, there is lack of consensus regarding which manipulations and modes of sensory feedback are most effective. Current treatments include elecromyographic and visual feedback27,29, physiotherapy30, and the motor rehabilitation method of Kabat28,31; however, the underlying neural and physiological mechanisms for improved functional outcomes are not known. This points to the importance of a quantitative animal model in which to study the mechanisms of improved recovery.
Our study was designed to reproduce and further elucidate the actions responsible for the positive effects of mechanical stimulation found by Angelov et al, 14 by altering the technique used to deliver mechanical stimulation. In their study14, the whiskers and whisker pad were simultaneously stimulated in the direction of whisker protraction (with passive retraction) by finger massage. In the current report, we compared whisker protraction with retraction, and attempted to avoid direct mechanical stimulation of the whisker pad to isolate the stimulatory effects to the whiskers alone. We found that massage of the whisker pad along with the whiskers is not necessary for enhanced whisking recovery (compared to historical controls), and that whisker protraction stimulation appears to produce better functional recovery compared to whisker retraction, though recovery was modest compared with that found by Angelov et al.14. However, when the top performers in each group were evaluated there was dramatic functional improvement in the protraction group compared to the control group, with one animal in the protraction group regaining symmetric whisking function.
Several possible explanations exist for the improved functional outcome observed in this study. Both protraction and retraction of rodent vibrissae are under active muscular control32,33. The nasalis initiates protraction, and the intrinsic muscles pivot the vibrissae further forward. Retraction involves relaxation of the nasalis and the intrinsic muscles, and contraction of the caudal extrinsic muscles, nasolabialis and maxillolabialis, pulling the vibrissae backward32,33. Passive mechanical protraction of the vibrissae stretches the caudal extrinsic muscles and involves a large arch of motion. Retraction of the vibrissae stretches the intrinsic muscles; but involves a smaller arch of motion. The fact that both retraction and protraction actions stretch whisking musculature could explain the benefit to recovery that both manipulations demonstrated. The protraction group may have trended towards improved recovery because of the greater degree of movement provided by passive mechanical protraction as compared to retraction, due to the whiskers starting from a relatively retracted position at rest (between strokes). The improved recovery found by Angelov et al 14 may represent an additional benefit of direct whisker pad manipulation, providing greater somatosensory input than by isolated whisker manipulation. In addition, neither daily handling nor an enhanced environment was shown to improve recovery unless daily whisker pad stimulation was also provided. These finding points toward the potential importance of both somatosensory input and proprioceptive input in recovery of the facial nerve function after injury.
CONCLUSION
The present study demonstrates improved functional recovery from daily mechanical retraction or protraction of whiskers after facial nerve transection injury and repair. These results are consistent with prior findings of enhanced facial nerve recovery after mechanical whisker protraction in rats, and further indicate that the benefit of such stimulation may be derived from trigeminal nerve feedback. Future research will be directed toward better understanding the mechanisms of enhanced functional recovery brought about by mechanical stimulation, so that similar interventions can be explored in human facial palsy.
ACKNOWLEDGEMENTS
Dr. Robin Lindsay and Dr. Tessa Hadlock had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by NIH grant number K-08 DEO15665-01 A2.
References
- 1.Angelov DN, Guntinas-Lichius O, Wewetzer K, Neiss WF, Streppel M. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats. Adv Anat Embryol Cell Biol. 2005;180:1–130. [PubMed] [Google Scholar]
- 2.Constantinidis J, Akbarian A, Steinhart H, Iro H, Mautes A. Effects of immediate and delayed facial-facial nerve suture on rat facial muscle. Acta Otolaryngol. 2003;123:998–1003. doi: 10.1080/00016480310001853. [DOI] [PubMed] [Google Scholar]
- 3.Guntinas-Lichius O, Neiss WF, Gunkel A, Stennert E. Differences in glial, synaptic and motoneuron responses in the facial nucleus of the rat brainstem following facial nerve resection and nerve suture reanastomosis. Eur Arch Otorhinolaryngol. 1994;251:410–417. doi: 10.1007/BF00181967. [DOI] [PubMed] [Google Scholar]
- 4.Guntinas-Lichius O, Wewetzer K, Tomov TL, et al. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22:7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streppel M, Angelov DN, Guntinas-Lichius O, et al. Slow axonal regrowth but extreme hyperinnervation of target muscle after suture of the facial nerve in aged rats. Neurobiol Aging. 1998;19:83–88. doi: 10.1016/s0197-4580(97)00163-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferri CC, Moore FA, Bisby MA. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34:1–9. [PubMed] [Google Scholar]
- 7.Most SP. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg. 2004;6:82–87. doi: 10.1001/archfaci.6.2.82. [DOI] [PubMed] [Google Scholar]
- 8.Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007;9:333–339. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Ohbayashi M, Furukawa M, Okoyama S. Neuroprotective effects of TJ-23 (Tokishakuyakusan) on adult rat motoneurons following peripheral facial nerve axotomy. Otolaryngol Head Neck Surg. 2007;136:225–230. doi: 10.1016/j.otohns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. Faseb J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- 11.Hindley S, Juurlink BH, Gysbers JW, Middlemiss PJ, Herman MA, Rathbone MP. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J Neurosci Res. 1997;47:427–439. [PubMed] [Google Scholar]
- 12.Gonzalez-Hernandez T, Rustioni A. Nitric oxide synthase and growth-associated protein are coexpressed in primary sensory neurons after peripheral injury. J Comp Neurol. 1999;404:64–74. [PubMed] [Google Scholar]
- 13.Lewin SL, Utley DS, Cheng ET, Verity AN, Terris DJ. Simultaneous treatment with BDNF and CNTF after peripheral nerve transection and repair enhances rate of functional recovery compared with BDNF treatment alone. Laryngoscope. 1997;107:992–999. doi: 10.1097/00005537-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis. 2007;26:229–242. doi: 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Evgenieva E, Schweigert P, Guntinas-Lichius O, et al. Manual stimulation of the suprahyoid-sublingual region diminishes polynnervation of the motor endplates and improves recovery of function after hypoglossal nerve injury in rats. Neurorehabil Neural Repair. 2008;22:754–768. doi: 10.1177/1545968308316387. [DOI] [PubMed] [Google Scholar]
- 16.Byrne PJ. Importance of facial expression in facial nerve rehabilitation. Curr Opin Otolaryngol Head Neck Surg. 2004;12:332–335. doi: 10.1097/01.moo.0000134829.61048.64. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking--I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–346. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 18.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 19.Hadlock T, Kowaleski J, Lo D, et al. Functional Assessments of the Rodent Facial Nerve: A Synkinesis Model. Laryngoscope. 2008 doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 20.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton DA, Hirsch BE, Mansour OI. Recovery of facial nerve function after repair or grafting: our experience with 24 patients. Am J Otolaryngol. 2007;28:37–41. doi: 10.1016/j.amjoto.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Gidley PW, Gantz BJ, Rubinstein JT. Facial nerve grafts: from cerebellopontine angle and beyond. Am J Otol. 1999;20:781–788. [PubMed] [Google Scholar]
- 23.Bischoff A, Grosheva M, Irintchev A, et al. Manual stimulation of the orbicularis oculi muscle improves eyelid closure after facial nerve injury in adult rats. Muscle Nerve. 2008 doi: 10.1002/mus.21126. [DOI] [PubMed] [Google Scholar]
- 24.Tomov TL, Guntinas-Lichius O, Grosheva M, et al. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Exp Neurol. 2002;178:207–218. doi: 10.1006/exnr.2002.8040. [DOI] [PubMed] [Google Scholar]
- 25.Streppel M, Popratiloff A, Angelov DN, et al. Significance of trigeminal sensory input on regrowth of hypoglossal and facial motoneurons after hypoglossal facial anastomosis in rats. Acta Otolaryngol. 1998;118:790–796. doi: 10.1080/00016489850182459. [DOI] [PubMed] [Google Scholar]
- 26.Sinis N, Guntinas-Lichius O, Irintchev A, et al. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Exp Brain Res. 2008;185:469–483. doi: 10.1007/s00221-007-1174-y. [DOI] [PubMed] [Google Scholar]
- 27.Balliet R, Shinn JB, Bach-y-Rita P. Facial paralysis rehabilitation: retraining selective muscle control. Int Rehabil Med. 1982;4:67–74. doi: 10.3109/09638288209166880. [DOI] [PubMed] [Google Scholar]
- 28.Barbara M, Monini S, Buffoni A, et al. Early rehabilitation of facial nerve deficit after acoustic neuroma surgery. Acta Otolaryngol. 2003;123:932–935. doi: 10.1080/00016480310000629. [DOI] [PubMed] [Google Scholar]
- 29.Cronin GW, Steenerson RL. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngol Head Neck Surg. 2003;128:534–538. doi: 10.1016/S0194-59980300005-6. [DOI] [PubMed] [Google Scholar]
- 30.Cederwall E, Olsen MF, Hanner P, Fogdestam I. Evaluation of a physiotherapeutic treatment intervention in “Bell's” facial palsy. Physiother Theory Pract. 2006;22:43–52. doi: 10.1080/09593980500422529. [DOI] [PubMed] [Google Scholar]
- 31.Kabat H, Knott M. Proprioceptive facilitation technics for treatment of paralysis. Phys Ther Rev. 1953;33:53–64. doi: 10.1093/ptj/33.2.53. [DOI] [PubMed] [Google Scholar]
- 32.Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- 33.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]