Abstract
BACKGROUND
Sturge Weber Syndrome (SWS) is characterized by a facial port-wine birthmark, vascular eye abnormalities, and a leptomeningeal angioma. Attention and behavioral issues are common in SWS; however, literature evidence for stimulant treatment is minimal. This study evaluates stimulant medication safety and efficacy in SWS patients.
METHODS
The research database of the Hunter Nelson Sturge-Weber Center (n = 210 subjects with SWS brain involvement) was reviewed for stimulant use. Twelve subjects (mean age 10.5 years, age range 4 to 21 years) on stimulants were seen between 2003 and 2012. A retrospective chart review obtained co-morbid diagnoses, stimulant type and dosage, medication side effects, vital signs, and medication efficacy.
RESULTS
All twelve subjects had brain involvement (unilateral - nine; bilateral – three). Additional co-morbidities included epilepsy (twelve), hemi-paresis (eight), headaches (eight), and vision deficits (seven). Eight subjects reported side effects, primarily appetite suppression (four) and headaches (three). There were no statistically significant changes in weight or blood pressure six months after medication initiation. Medication efficacy was subjectively reported in eleven subjects. Seven patients remained on stimulants at their most recent follow up visit.
CONCLUSIONS
This study preliminarily evaluates stimulant medication use in a small group of SWS patients. Stimulants were tolerated and effective in most subjects. Side effects were mostly minor and medication did not negatively impact growth or vital signs. Stimulant medication may be a safe and effective intervention for SWS children with attention issues/attention deficit hyperactivity disorder (ADHD). Further studies with larger sample sizes are needed.
Keywords: Sturge Weber Syndrome, ADHD, stimulant medication, neurocutaneous syndromes
INTRODUCTION
Major clinical features of Sturge Weber Syndrome (SWS) include seizures, stroke like episodes, and glaucoma due to vascular malformations involving the skin, brain, and eyes. Several patients also have cognitive issues as well as difficulties with attention and behavior. Attention deficit hyperactivity disorder (ADHD) was observed in 42% of SWS subjects over one forty year chart review1. Chapieski et al reported 22% of SWS patients with ADHD in a group of 79 subjects2. While Turin et al reported a 90% rate of attention problems in ten children with SWS, none of those subjects met DSM IV TR criteria for ADHD after psychiatric evaluation3. A recent survey from our center noted 10% of patients with self-reported ADHD4. Clinicians’ reluctance to use stimulant medications in SWS patients likely involves concerns for worsening seizure activity and possible neurovascular interactions. Current research is focused on aspirin as a potential neurological disease treatment option as well5.
Patients with SWS commonly have epilepsy and strokes like episodes. Interestingly, ADHD is quite common in epilepsy; one population based study reported a 12.8% prevalence in pediatric epilepsy patients6. Dunn et al also found a gender ratio of 1:1 in comparison to a 2:1 male/female ratio in the general ADHD population, as well as a higher overall prevalence of inattentive type ADHD7. In addition, one study showed higher rates of ADHD in children with a history of stroke than in the general population8. SWS patients are typically treated with a combination of medication titration and behavioral therapy to safely optimize seizure control, behavioral issues, and school performance. In addition, multiple studies have shown that stimulants do not increase seizures in epilepsy9,10.
While stimulant use is common in the typical child with a diagnosis of ADHD, medication safety and efficacy in children with neurovascular disease has not been well established. This study is the first to review stimulant use in a cohort of patients with SWS. The objective of the study is to describe efficacy and side effects of stimulant medications in a retrospective cohort of SWS patients.
METHODS
The study population was drawn from a group of patients seen at the Kennedy Krieger Institute Hunter Nelson Sturge-Weber Center, a multi-disciplinary clinic dedicated to the patient care and research involving SWS. All subjects signed informed consent forms and the research was approved by the Johns Hopkins Institutional Review Board. The Sturge-Weber Center research database contains records of 210 subjects seen during January 2000 to December 2011. The medical records of subjects with confirmed SWS brain involvement were evaluated for study involvement if their medication list included a stimulant medication (methylphenidate or dextroamphetamine). Charts were reviewed systematically and the following information was collected for each subject: gender, side of brain involvement (left, right, or bilateral), age at time of stimulant treatment initiation, stimulant medication efficacy and side effects as reported by neurologist in medical record, anthropomorphic measures at medication initiation, six weeks, and six months into treatment, additional medications, and previously diagnosed SWS comorbidities. Parents were asked about the child's ability to complete homework, teacher reports about their ability to focus and pay attention in class, social functioning, and grades in school. Medication was only continued if parents reported a positive response in these areas. Prospectively assigned Sturge Weber Syndrome – Neurological Rating Scores (SWS – NRS) based on disease severity were also collected from each patient over multiple visits as well. SWS – NRS are clinical measures of certain SWS disease characteristics, specifically seizure frequency, cognitive level, motor disability, and visual field deficits (Figure 1). These scores have been clinically validated through correlation with perfusion deficits on neuroimaging, decreased power on quantitative EEG, and adaptive function scores and are available for review in previous studies11,12,13. MRIs were available for nine subjects and were scored by two researchers based on a previously described scoring system rating atrophy and leptomeningeal enhancement11.
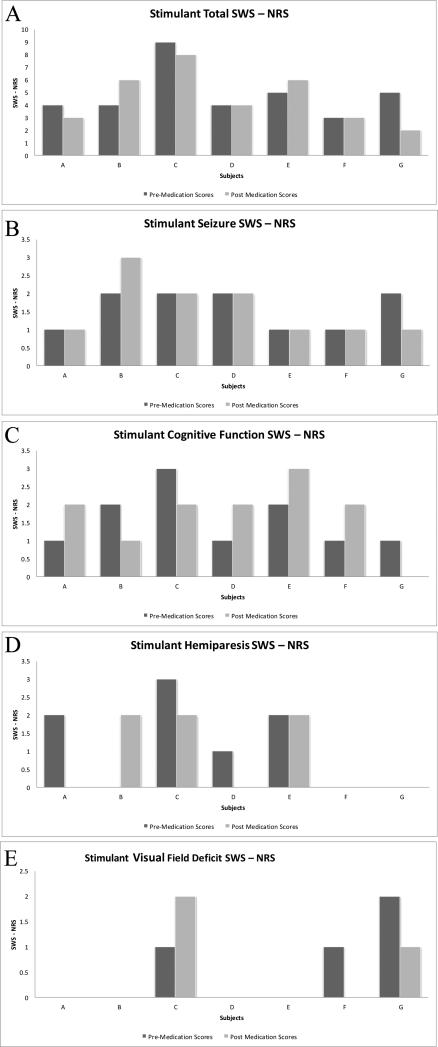
Figure 1.
Sturge Weber Syndrome - Neurological Rating Scores (SWS-NRS) noted for the 7 out of 12 subjects with data from before and after starting stimulant medication.Panel A shows the Total SWS-NRS pre and post medication. Panel B shows the Seizure SWS-NRS pre and post medication. Panel C shows the Cognitive Function SWS-NRS pre and post medication. Panel D shows the Hemiparesis SWS-NRS pre and post medication. Panel E shows the Visual Field Deficit SWS-NRS pre and post medication.
All data were analyzed using Spearman correlations, chi square analysis, Fisher's exact tests, t-tests, Mann Whitney U, and Kruskal–Wallis as appropriate using SSPS (Statistical Package for Social Sciences) Versions 18 and 19 (SPSS Inc., Chicago, IL). The significance level for all analyses was p < 0.05.
RESULTS
Ninety-eight SWS subjects with diagnosed brain involvement were seen at the Hunter Nelson Sturge-Weber Center between January 2000 and December 2011. Of the 98 subjects with brain involvement seen during that period, 13 were treated with a stimulant medication. One patient was not included in the study group due to overlapping treatment of symptoms with Atomoxetine. The remaining twelve patients were on stimulants between 2003 and the present.
Demographics
The twelve subjects were comprised of 7 males and 5 females. Subjects also had varying degrees of co-morbid diagnoses, including epilepsy, hemiparesis, headaches, and visual field deficits. Eleven patients were on epilepsy medications concomitantly. One patient had a remote history of epilepsy and had been seizure free for many years and was no longer on an anti-convulsant regimen. See Table 1 for additional information regarding the subjects.
Table 1.
Subject Demographics
| Stimulant Group | |
|---|---|
| Total subjects | 12 |
| Gender | |
| Male/Female | 7/5 |
| Mean Age when stimulant started (range) | 10.5 years (59 - 255 months) |
| Median Duration of time on stimulants (range) | 24.5 months (2 month - 90 months) |
| Brain Involvement | |
| Left/Right/Bilateral | 7/2/3 |
| Symptoms | |
| Seizures | 12 (100%) |
| Hemiparesis | 8 (67%) |
| Headaches | 8 (67%) |
| Visual Field Deficit | 6 (50%) |
| Additional Medications | |
| Aspirin | 9 (75%) |
| Oxcarbazepine | 6 (50%) |
| Carbamezipine | 3 (25%) |
| Valproic Acid | 3 (25%) |
| Levitiracetam | 2 (17%) |
| Lamotrigine | 1 (8%) |
| Topiramate | 1 (8%) |
| Felbamate | 1 (8%) |
Medication Management
Stimulant medication was initiated in subjects primarily for ADHD (11 subjects) and impaired alertness (1 subject). The patient with impaired alertness would fall asleep multiple times during the day and have brief seizures during these times. Stimulant medication was started and increased his alertness during the day, decreased the frequency of his seizures, and improved sleep. Six subjects were diagnosed with ADHD clinically by the neurologist due to impaired inattention and hyperactivity by history, exam and parent report; four subjects were diagnosed with ADHD by neuropsychological testing; two subjects had prior diagnoses of ADHD before their clinic visit. One patient was started on stimulants prior to his first visit with the clinic and it was unknown what age medication was started. There was a trend towards significance in patients with bilateral brain involvement being started on stimulants at a younger age than those with unilateral brain involvement (6.8 years – 82 months vs 11.3 years – 136 months, p = 0.052). Eleven subjects were started on stimulant medication specifically by the Kennedy Krieger Institute Sturge Weber Center. Patients here were typically started on low dose methylphenidate preparations (5 mg) and titrated up to higher dosages and then transitioned to long acting dextroamphetamines and methylphenidates preparations over time based on responsiveness and side effects. Seven subjects were still on stimulants at the time of their last visit.There were no significant differences between the groups currently on and currently off stimulants currently in terms of gender, brain involvement, comorbid conditions, age started on stimulants, duration of medication treatment, and total and subgroup (cognitive function, seizures, hemiparesis, visual field deficit) pre and post medication SWS – NRS.
Side Effects
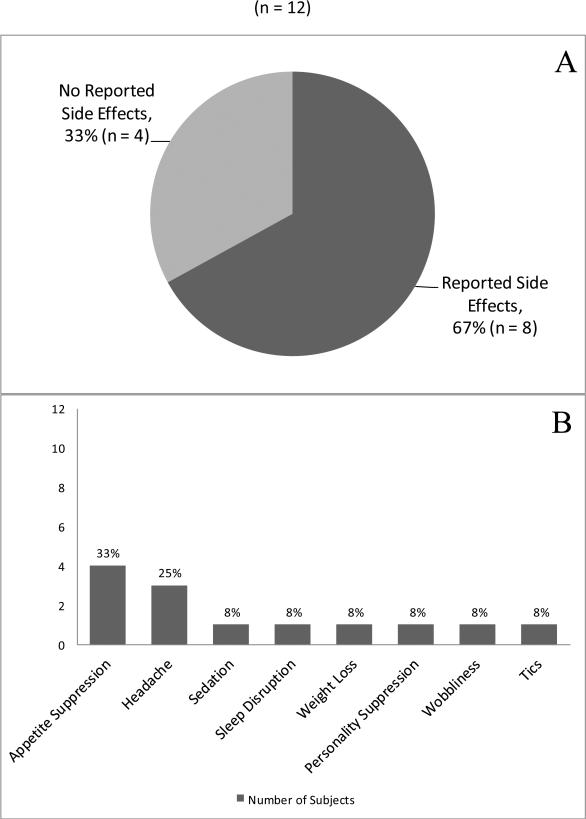
Eight subjects (67%) reported side effects from the medication (Figure 2, Panel A). No patients reported experiencing tremor, GI upset, chest palpitations, or anxiety while on stimulant medication. One patient, an adult, did have impaired sleep while on stimulant treatment and had a seizure and subsequent stroke like episode. Stimulant medication was stopped immediately in this case, and the sleep cycle was returned, neurologic deficits resolved, and seizure control was promptly regained. See Figure 2, Panel B for additional information regarding these side effects.
Figure 2.
Panel A shows that of the 12 subjects on stimulants, 8 (67%) had side effects. Panel B shows the occurrence of each side effect.
There was no statistically significant difference between mean weight and mean blood pressure before and after stimulant medication initiation at 6 weeks and 6 months. There was a statistically significant decrease between the initial mean heart rate and the mean heart rate 6 weeks into stimulant medication management (93 beats per minute, 85 beats per minute; p = 0.02). There was no statistically significant difference between initial mean heart rate and mean heart rate at 6 months or between mean heart rate at 6 weeks and 6 months. See Table 2 for additional information regarding these anthropomorphic measures.
Table 2.
Anthropomorphic measures
| Mean Values on Stimulants | |||
|---|---|---|---|
| Measurement | Initial | 6 weeks (n = 5) | 6 months (n = 7) |
| Weight | 33 kg (Range 16.8 - 64.2, SD 17) | 33.6 kg (Range 16.9 - 62.1, SD 17.6) | 32.9 (Range 17.6 - 64.8, SD 15.5) |
| Systolic BP | 103 mmHg (SD - 10.3) | 104 mmHg (SD - 12.5) | 107 mmHg (SD - 19.1) |
| Diastolic BP | 62 mmHg (SD - 7.4) | 62 mmHg (SD - 11.8) | 60 mmHg (SD - 6.7) |
| Heart Rate | 93* beats per minute (SD - 8.4) | 85* beats per minute (SD - 9.5) | 96 beats per minute (SD - 10.1) |
Significant difference using paired samples T-test, p = 0.02
Clinical Effects
Eleven of the twelve patients were found to have efficacy from stimulant medication by their neurologist per clinical notes (92%) (i.e. parent reported improved attention in school and improved ability to focus in school and carry out homework). There were no statistically significant differences between males and females and subjects with unilateral and bilateral brain involvement in terms of demographic characteristics, side effects, or SWS – NRS. MRI scores did correlate positively with final total SWS – NRS on stimulants (Spearmans rho 0.898, p = 0.006).
SWS – NRS were collected from charts at the visit prior to stimulant initiation and for 3 subsequent visits after medication initiation when available. Median SWS – NRS Total and Subscores are displayed in Table 3 for the seven subjects who had both pre and post scores. There was no significant difference in pre and post median stimulant total and subscale SWS – NRS. Figure 1 shows the pre and post medication total and subscores for each subject. A majority of patients had stable or only slight changes in their post medication total SWS NRS when compared to their pre medication total SWS NRS (Figure 1, Panel A). A majority of the seizures subscores (n = 5) remained stable and did not change with medication (Figure 1, Panel B). In terms of cognition, all of the subjects stayed relatively stable, increasing or decreasing by no more than one point after medication initiation (Figure 1, Panel C).
Table 3.
Median Sturge Weber Syndrome - Neurological Rating Scores (SWS - NRS)
| Mean Age (Range) | Mean Time Since Visit 1 (Range) | Total Score | Seizure Subscore | Cognitive Function Subscore | Hemiparesis Subscore | Visual Field Deficit Subscore | |
|---|---|---|---|---|---|---|---|
| Visit 1 Pre-stimulant (n = 7) | 8.4 years - 101 months (59 - 181) | n/a | 4 | 2 | 1 | 1 | 0 |
| Visit 2 1st post stimulant visit (n = 7) | 8.8 years - 106 months (60 - 185) | 4 months (1 - 13) | 5 | 1 | 1 | 2 | 0 |
| Visit 3 2nd post stimulant visit (n = 6) | 9.3 years - 112 months (61 - 192) | 7 months (2 - 14) | 3 | 1 | 1 | 0.5 | 0 |
| Visit 4 Final post stimulant visit (n = 7) | 10.4 years - 125 months (65 - 192) | 23 months (6 - 49) | 4 | 1 | 2 | 0 | 0 |
CONCLUSIONS
This study reviews stimulant use in a specialty clinic for patients with SWS. 11% (11/98) of the total subjects were diagnosed with ADHD in this study. This percentage is lower than many of the reported studies on SWS patients, but higher than survey data reported from our same patient population and the general population1,2,3,4. Gender ratios were equal between males and females in our group, similar to the gender breakdown in the epilepsy population7. Diagnoses made in our clinic were primarily based on clinical neurological diagnosis or neuropsychological testing.
Different patients were treated with a combination of anti-convulsants, aspirin, and low dose short acting methylphenidate, which were eventually titrated up to long acting methylphenidate and/or long acting dextoamphetamine. A majority of subjects experienced typical side effects for stimulants, including appetite suppression and headache, but remained on stimulants at their last clinic visit. There was no statistically significant increase in heart rate or blood pressure during stimulant medication treatment, and weight remained stable. Subjective improvement in stimulants was reported in clinical notes for a majority of patients. A majority of patients ended up with good SWS – NRS with regards to seizures, cognitive function, and visual field deficits. Seizure scores remained stable in a majority of patients; however, cognitive function worsened over time in some patients. It is important to note that most of these subjects were young children when started on stimulant medication. Their worsening in cognitive scores likely represents underlying cognitive impairment revealed as academic demands increased, requiring further school support and services.
Stimulants are widely used in the typical pediatric ADHD population. The MTA Cooperative Group study showed significant improvement in ADHD and oppositional defiant disorder symptoms in school-age children on stimulants in comparison to behavioral modification therapy after 24 months14. There has been particular concern for stimulant use in SWS because of potential harmful effects on seizure frequency as well their pharmacological vasoactive effects on the brain. Stimulants do not appear to increase seizures in the typical population, children with refractory epilepsy, nor our subset of SWS subjects15,16. While stimulants are widely used in other pediatric chronic diseases, efficacy and safety data of stimulants has not be comprehensively studied in cancer, traumatic brain injury, or other neurovascular disorders such as sickle cell disease17. One recent crossover controlled trial in 14 subjects with sickle cell disease and a history of stroke showed improved attention ratings; more important, in this subset of patients with known cerebrovascular disease, no serious side effects were noted18. In addition, a recent cohort study of children and young adults on stimulants medications noted no increased risk of cardiovascular events in these patients, despite mandatory labeling and blackbox warnings to the contrary19. Our study is a preliminary look at stimulant use in a chronic pediatric neurocutaneous disease population with comorbid epilepsy and stroke.
Though this study has a small total number, SWS is a rare neurovascular disorder. Regular neuropsychological testing is useful for diagnosis of comorbid conditions that can affect cognition and learning. One weakness of our study is that diagnoses were not standardized in all patients through ADHD patient questionnaires and symptoms were not tracked quantitatively before, during, and after stimulant treatment in all patients. Our clinic plans to continue neuropsychological testing in SWS patients with neurodevelopmental issues and implement regular screening of patients with ADHD parent and teacher symptom checklists to monitor response to treatment. Future studies may benefit from quantitative tracking of common symptoms in SWS, such as seizure frequency, stroke like episodes, and headaches. Quantitative data in patients over time may allow better comparison of treatment effects once the natural history of seizures and cognition in SWS is established. Preliminarily in a small subset of SWS patients, stimulants appear to improve attention and behavior in SWS without severe side effects, neurovascular effects, or worsening seizures. Stimulants may be a valid and safe treatment option in patients with attention and behavioral issues and SWS, but further study is needed with a larger sample size to validate these results.
ACKNOWLEDGEMENTS
Source of Funding: Authors Lanier and Comi received grant funding from Hunter's Dream For A Cure. Authors Lance, Lanier, and Comi have received grant funding from the Brain Vascular Malformation Consortium (BVMC; U54NS065705) which is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the NIH Office of Rare Diseases Research (ORDR), and the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: For the remaining authors, none were declared.
REFERENCES
- 1.Pascual-Castroviejo I, Pascual-Pascual S, Velazquez-Fragua R, Viano J. Sturge-weber syndrome: study of 55 patients. Can J Neurol Sci. 2008;35:301–307. doi: 10.1017/s0317167100008878. [DOI] [PubMed] [Google Scholar]
- 2.Chapieski L, Friedman A, Lachar D. Psychological functioning in children and adolescents with sturge-weber syndrome. J Child Neurol. 2000;15:660. doi: 10.1177/088307380001501004. [DOI] [PubMed] [Google Scholar]
- 3.Turin E, Grados MA, Tierney E, Ferenc LM, Zabel A, Comi AM. Behavioral and psychiatric features of sturge-weber syndrome. J Nerv Ment Dis. 2010;198(12):905. doi: 10.1097/NMD.0b013e3181fe75ee. [DOI] [PubMed] [Google Scholar]
- 4.Bay MJ, Kossoff EH, Lehmann CU, Zabel TA, Comi AM. Survey of aspirin use in sturgeweber syndrome. J Child Neurol. 2011;26(6):692–702. doi: 10.1177/0883073810388646. [DOI] [PubMed] [Google Scholar]
- 5.Lance EI, Sreenivasan AK, Zabel TA, Kossoff EH, Comi AM. Aspirin use in sturge-weber syndrome: Side effects and clinical outcomes. J Child Neurol. 2013;28(2):213–8. doi: 10.1177/0883073812463607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen R, Senecky Y, Shuper A, et al. Prevalence of epilepsy and attention-deficit hyperactivity (ADHD) disorder: A population-based study. J Child Neurol. 2013;28:120–123. doi: 10.1177/0883073812440327. [DOI] [PubMed] [Google Scholar]
- 7.Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45(01):50. [PubMed] [Google Scholar]
- 8.Everts R, Pavlovic J, Kaufmann F, et al. Cognitive functioning, behavior, and quality of life after stroke in childhood. Child Neuropsychol. 2008;14(4):323–38. doi: 10.1080/09297040701792383. [DOI] [PubMed] [Google Scholar]
- 9.Hemmer SA, Pasternak JF, Zecker SG, Trommer BL. Stimulant therapy and seizure risk in children with ADHD. Pediatr Neurol. 2001;24(2):9–102. doi: 10.1016/s0887-8994(00)00240-x. [DOI] [PubMed] [Google Scholar]
- 10.Gucuyener KF, Erdemoglu AK, Senol SF, Serdaroglu A, Soysal S, Kockar AI. Use of methylphenidate for attention-deficit hyperactivity disorder in patients with epilepsy or electroencephalographic abnormalities. J Child Neurol. 2003;18(2):109–12. doi: 10.1177/08830738030180020601. [DOI] [PubMed] [Google Scholar]
- 11.Hatfield LA, Crone NE, Kossoff EH, et al. Quantitative EEG asymmetry correlates with clinical severity in unilateral sturge-weber syndrome. Epilepsia. 2007;48(1):191–5. doi: 10.1111/j.1528-1167.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin DDM, Barker PB, Kraut MA, Comi A. Early characteristics of sturge-weber syndrome shown by perfusion MR imaging and proton MR spectroscopic imaging. Am J Neuroradiol. 2003;24(9):1912–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Reesman J, Gray R, Suskauer SJ, et al. Hemiparesis is a clinical correlate of general adaptive dysfunction. J Child Neurol. 2009;24(6):701–8. doi: 10.1177/0883073808329529. [DOI] [PubMed] [Google Scholar]
- 14.MTA Cooperative Group National Institute of Mental Health Multimodal Treatment Study of ADHD Follow-up: 24-Month Outcomes of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Pediatrics. 2004;113(4):754–61. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- 15.Gucuyener K, Erdemoglu AK, Senol S, Serdaroglu A, Soysal S, Kockar I. Use of Methylphenidate for Attention-Deficity Hyperactivity Disorder in Patients With Epilepsy of Electroencephalographic Abnormalities. J Child Neurol. 2003;18:109–12. doi: 10.1177/08830738030180020601. [DOI] [PubMed] [Google Scholar]
- 16.Santos K, Palmini A, Radziuk AL, et al. The impact of methylphenidate on seizure frequency and severity in children with attention-deficity-hyperactivity disorder and difficult-to-treat epilepsies. Dev Med Child Neurol. 2013;55(7):654–60. doi: 10.1111/dmcn.12121. [DOI] [PubMed] [Google Scholar]
- 17.Nicholls E, Hildenbrand BS, Aggarwal R, McCarthy L, Daly B. The Use of Stimulant Medication to Treat Neurocognitive Deficits in Patients with Pediatric Cancer, Traumatic Brain Injury, and Sickle Cell Disease: A Review. Postgrad Med. 2012;5:124. doi: 10.3810/pgm.2012.09.2596. [DOI] [PubMed] [Google Scholar]
- 18.Daly B, Kral MC, Brown RT, et al. Ameliorating Attention Problems in Children With Sickle Cell Disease: A Pilot Study of Methylphenidate. J Dev Behav Pediatr. 2012;33:244–51. doi: 10.1097/DBP.0b013e31824ba1b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896–904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]