SUMMARY
In general, pig embryos established by somatic cell nuclear transfer (SCNT) are transferred at the one-cell stage because of suboptimal embryo culture conditions. Improvements in embryo culture can increase the practical application of late embryo transfer. The goal of this study was to evaluate embryos cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) in vitro, and to track the in vivo developmental competency of SCNT-derived blastocysts from these GM-CSF embryos. The receptor for GM-CSF was up-regulated in in vitro-produced embryos when compared to in vivo-produced cohorts, but the level decreased when GM-CSF was present. In vitro fertilized (IVF) embryos, supplemented with GM-CSF (2 or 10 ng/ml), showed a higher frequency of development to the blastocyst stage compared to controls. The total cell numbers of the blastocysts also increased with supplementation of GM-CSF. Molecular analysis demonstrates that IVF-derived blastocysts cultured with GM-CSF exhibit less apoptotic activity. Similarly, an increase in development to the blastocyst stage and an increase in the average total-cell number in the blastocysts were observed when SCNT-derived embryos were cultured with either concentration of GM-CSF (2 or 10 ng/ml). When SCNT-derived embryos, cultured with 10 ng/ml GM-CSF, were transferred into six surrogates at Day 6, five of the surrogates became pregnant and delivered healthy piglets. Our findings suggest that supplementation of GM-CSF can provide better culture conditions for IVF- and SCNT-derived embryos, and pig SCNT-derived embryos cultured with GM-CSF in vitro can successfully produce piglets when transferred into surrogates at the blastocyst stage. Thus, it may be practical to begin performing SCNT-derived embryo transfer at the blastocyst stage.
INTRODUCTION
The first report of cloned pigs by somatic cell nuclear transfer (SCNT) was published in 2000 (Onishi et al., 2000; Polejaeva et al., 2000). The development of SCNT technology allowed for the production of transgenic pigs with specific genetic modifications. Since pigs share a similar physiology with humans, these transgenic pigs have wide applications as biomedical models for human diseases (Dai et al., 2002; Lai et al., 2002; Rogers et al., 2008). Even though the potential applications of transgenic pigs are well recognized, the application is limited due to the low efficiency in their production. One of the major obstacles that leads to the low production is low efficiency in SCNT; only ~1% of SCNT-derived embryos make it to term.
Although most SCNT-derived embryos, such as from ruminants, are typically cultured in vitro and transferred at the blastocyst stage, pig SCNT-derived embryos are typically transferred into the oviduct of surrogates at the one-cell stage. Early transfer in pigs is generally practiced because of the poor quality of SCNT-derived embryos combined with suboptimal in vitro culture conditions for pig embryos. Early transfer provides an in vivo environment for the transferred embryos as soon as possible, but transferring embryos at an early stage has its own disadvantages. One major disadvantage is that the developmental potential of transferred embryos cannot be evaluated prior to the transfer. Developmental potential of SCNT-derived embryos can vary due to oocyte quality, type of donor cells, manipulation process, etc. Thus, when performing early stage embryo transfer, it is hard to predict the number of viable embryos that are transferred into the surrogates. In other words, only a portion of the embryos transferred may be viable. At the other end of the spectrum, there is a report suggesting that transferring more blastocysts can actually reduce term development (Berthelot et al., 2007). These reports suggest that transfer of proper number of viable embryos is important for term development after embryo transfer in pigs. Term development from transferring in vitro-cultured blastocysts has been reported in pigs when using multiple culture media (Rath et al., 1995; Kikuchi et al., 2002; Yoshioka et al., 2002). References on the transfer of SCNT-derived blastocysts are more limited because SCNT-derived embryos are considered more fragile compared to in vitro fertilized (IVF) embryos. Hence, embryos are generally transferred early to provide the best conditions for development. A few reports indicate that blastocysts resulting from SCNT can be transferred to produce piglets (Lagutina et al., 2006; Li et al., 2009; Schmidt et al., 2010), although there are still limited live birth data from such experiments.
Improvements to in vitro culture can assist in the production of developmentally competent SCNT-derived embryos, and can thus increase the practical application of blastocyst transfers. Because pig embryo culture is suboptimal, in vitro-cultured embryos are developmentally inferior to in vivo-derived embryos (Machaty et al., 1998). Moreover, our molecular data suggests that in vitro-cultured embryos have different patterns of gene expression compared to in vivo-derived embryos (Whitworth et al., 2005; Bauer et al., 2010). From our deep sequencing data, we found that message for a subunit of the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor (CSFR) was more abundant in in vitro-cultured blastocysts when compared to in vivo-derived blastocysts.
GM-CSF is expressed in the female reproductive tract, and the level of its expression is high between conception and implantation (Robertson, 2007). Previous studies show that supplementing GM-CSF in embryo culture promotes embryonic development in both human and bovine embryos (Sjoblom et al., 1999; Loureiro et al., 2009). There are only limited reports on the effect of GM-CSF on pig embryos, however. Introducing mouse GM-CSF into embryo culture increased in vitro development of parthenotes in a protein free culture condition (Cui et al., 2004). Pig GM-CSF had a positive effect on the in vitro development of IVF- and SCNT-derived embryos (Kwak et al., 2012a,b). While positive effects of GM-CSF on embryos has been reported, all results are restricted only to in vitro studies, thus limiting its application in vivo. However, GM-CSF is thought to also have a major effect during implantation of transferred embryos. Since treatments that improve the in vitro quality of blastocysts do not always result in piglet production (Spate et al., 2012), we deemed it is important to determine if GM-CSF treated embryos would be capable of producing piglets.
Improvement of in vitro embryo culture is desired for the practical application of blastocyst transfers in pigs. The blastocyst transfers would result in an increase in the developmental potential of transferred embryos and, ultimately, higher efficiency in the production of transgenic pigs. The aim of this study was to investigate the effect of GM-CSF on the culture of pig embryos derived from IVF and SCNT. Based on the in vitro experiments, in vivo developmental potential of SCNT-derived embryos cultured with GM-CSF was examined. Finally, we compared in vivo development efficiency of SCNT-derived embryos cultured with GM-CSF to SCNT-derived embryos transferred at the one-cell stage.
RESULTS
CSFR in Pig Blastocysts
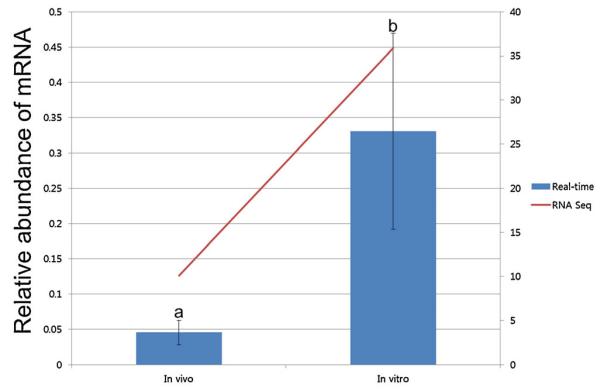
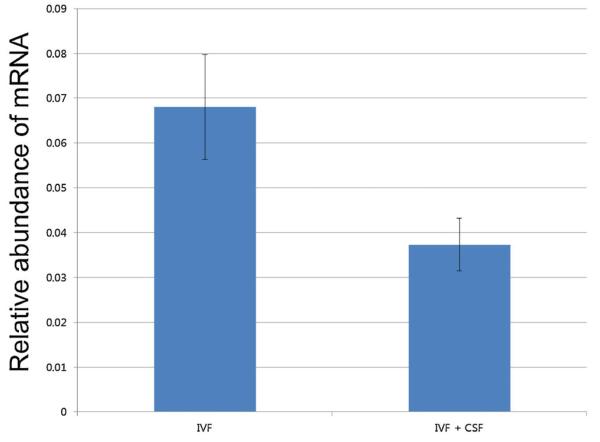
Both subunits of CSFR are expressed in pig blastocysts. The expression of the two subunits, CSFRα and CSFRβ, was confirmed by RT-PCR, although expression of CSFRβ was extremely low (Fig. 1). Previous deep sequencing results indicated that CSFRα was three times more abundant in in vitro-produced blastocysts when compared to those from in vivo development (Bauer et al., 2010). When this was verified by real-time PCR, the difference was even more dramatic, at 7.28-times higher in in vitro-derived blastocysts (P < 0.05, Fig. 2). Expression of CSFRβ was not detected by real-time analysis. When 10 ng/ml GM-CSF was introduced into culture media, the transcript level of CSFRα had a tendency to decrease in the treated blastocysts when compared to the control blastocysts (P = 0.09, Fig. 3).
Figure 1.
The presence of CSF receptor subunit transcript in pig IVF-derived blastocysts (+, with DNA; −, negative control).
Figure 2.
The level of CSFRα in in vivo (a) versus in vitro- (b) cultured blastocysts. Different letters indicate a statistically significant difference (P < 0.05). RNA-Seq data was obtained from a previous deep sequencing analysis (Bauer et al., 2010). [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Figure 3.
Expression of CSFRα after culture in the presence of GM-CSF. The level of CSFRα transcript tended to be lower when embryos were cultured with 10 ng/ml GM-CSF (P = 0.09).
Effect of GM-CSF on the In Vitro Developmental Potential of IVF- and SCNT-Derived Embryos
IVF-derived embryos cultured with two different concentrations of GM-CSF showed a significant increase in their developmental potential in vitro compared to the control group (Table 1). Development to the blastocyst stage was increased from 50.6% to ~64.7% in response to GM-CSF, and the total number of nuclei increased from 31.2 to ~37.0 (P < 0.05). Similar results were observed when SCNT-derived embryos were cultured with GM-CSF. SCNT-derived embryos showed an increase in in vitro developmental potential similar to the IVF study (Table 2). While incubation of SCNT-derived embryos with GM-CSF did not significantly affect the frequency of cleaved embryos, it did increase the percent blastocyst from 22.4% to ~32.2% (P < 0.05). A significant increase in the total number of nuclei from 30.8 to ~33.9 in blastocysts was observed when GM-CSF was introduced into the embryo culture (P < 0.05).
TABLE 1.
Effect of GM-CSF on the Development of IVF Embryos In Vitro
| Group | Number of cleaved embryos |
Frequency of Day 6 blastocysts (% ± SEM) |
Total cell number in blastocysts (mean ± SEM) |
|---|---|---|---|
| Control | 330 | 50.6 ± 3.8a | 31.2 ± 1.0a (n = 29) |
| 2 ng/ml GM-CSF | 255 | 64.7 ± 5.1b | 36.3 ± 1.2b (n = 64) |
| 10ng/ml GM-CSF | 225 | 63.6 ± 4.0b | 37.0 ± 1.0b (n = 48) |
Different letters indicate a significant difference (P< 0.05).
TABLE 2. Effect of GM-CSF on the Development of SCNT Embryos In Vitro.
| Group | Total | Frequency of cleaved embryos (% ± SEM) |
Frequency of Day 6 blastocysts (% ± SEM) |
Total cell number in blastocysts (mean ± SEM) |
|---|---|---|---|---|
| Control | 201 | 66.2 ± 2.8 | 22.4 ± 2.7a | 30.8 ± 0.8a (n = 44) |
| 2 ng/ml GM-CSF | 185 | 73.5 ± 3.9 | 30.8 ± 2.3b | 33.9 ± 1.0b (n = 55) |
| 10ng/ml GM-CSF | 177 | 76.8 ± 2.8 | 32.2 ± 2.1b | 33.7 ± 1.1b (n = 56) |
Different letters indicate a significant difference (P < 0.05).
Effect of GM-CSF on Gene Expression Profiles in IVF-Derived Embryos
To investigate the molecular effect of GM-CSF on pig IVF-derived embryos, quantitative real-time PCR was used to identify the molecular targets of GM-CSF. Expression levels of genes known to be affected by GM-CSF were analyzed (Fig. 4). When genes involved with apoptosis (BCL2, BAD, TP53, and CASP3) were analyzed, we found that the anti-apoptotic gene, BCL2, was not affected by culture with GM-CSF, whereas the pro-apoptotic gene, BAD, was significantly decreased (P < 0.05) with addition of GM-CSF. TP53 was also significantly decreased upon GM-CSF treatment (P < 0.05). The signaling genes JAK1, STAT3, cRAF, and PPP3CC were not affected by culture with GM-CSF.
Figure 4.
The effect of GM-CSF on genes involved with apoptosis and cellular signaling. Different letters indicate a statistically significant difference (P < 0.05).
Effect of GM-CSF on the In Vivo Developmental Potential of SCNT-Derived Embryos
To determine if culture from the one-cell stage to the blastocyst stage in the presence of GM-CSF is compatible with development to term, six embryo transfers were performed with five different donor cell lines. Standard embryo transfer results with SCNT-derived embryos constructed with the same donor cell lines were analyzed to compare the efficiency. Five of the six surrogates receiving embryos on Day 6 were pregnant at Day 40 while 63.6% of the controls were also pregnant. Piglets were produced from all five different donor cells examined (Table 3). Using donor E, all SCNT-derived embryos were generated simultaneously and divided into three groups, for two Day 1 transfers and one Day 6 transfer, after culturing with GM-CSF. Efficiency of term development from the Day 6 transfer was significantly higher using donor E (P < 0.05), and tended to be higher using donor B (P = 0.15) compared to Day 1 transfers. When overall cloning efficiency of the five different donor cells were analyzed, SCNT-derived embryos cultured with GM-CSF showed higher developmental potential compared to the control transfer results (Day 1 transfer). Average birth weights from the piglets were similar. There was no developmental abnormality from piglets from GM-CSF cultured embryos. An image of two piglets from SCNT-derived embryos cultured with GM-CSF (donor A) is shown in Figure 5.
TABLE 3. In Vivo Developmental Potential of Transferred SCNT Embryos Cultured With GM-CSF.
| Group | GM-CSF | Total number of embryos |
Number of transfers |
Number of embryos transferred on Day 6 |
Number of live piglets |
Cloning efficiency (%) |
Average birth weight of piglets (kg) |
|---|---|---|---|---|---|---|---|
| Donor A | + | 180 | 1 | 35 | 2 | 1.1 | 1.03 |
| − | 777 | 4 | N/A | 7 | 0.9 | 0.99 | |
| Donor B | + | 152 | 1 | 40 | 5 | 3.3 | 1.16 |
| − | 1,829 | 9 | N/A | 28 | 1.5 | 1.20 | |
| Donor C | + | 140 | 1 | 38 | 1 | 0.7 | 1.41 |
| − | 792 | 4 | N/A | 6 | 0.7 | 1.07 | |
| Donor D | + | 140 | 2 | 58 | 1 | 0.7 | 0.44 |
| − | 728 | 3 | N/A | 7 | 1.0 | 0.39 | |
| Donor E | + | 135 | 1 | 46 | 7 | 5.0a | 0.58 |
| − | 440 | 2 | N/A | 3 | 0.7b | 0.49 | |
| Total | + | 747 | 6 | 187 | 16 | 2.1a | |
| − | 4,566 | 22 | N/A | 51 | 1.2b |
Developmental potential was compatible with previous transfers performed at the one-cell stage. Different letters indicate statistically significant difference (P < 0.05).
Figure 5.
First pigs born from SCNT-derived embryos cultured with 10 ng/ml GM-CSF (donor A, Ossabaw pigs born on Oct 3rd 2011). No abnormal development or health issue was observed.
DISCUSSION
GM-CSF is known to interact with the low-affinity alpha subunit of its receptors located on the embryos. The presence of the alpha subunit has been confirmed in mouse and human embryos (Robertson et al., 2001; Sjoblom et al., 2002), and the expression of the CSF alpha subunit receptor in pig blastocysts has been identified in deep sequencing data sets (unpublished data; Redel et al., 2012). In this study, the presence of message for CSFR was detected by RT-PCR. Higher expression of the α-subunit suggests that this is the main subunit of CSFR in pig blastocysts; this is consistent with reports in other species as well. The pig blastocyst may need GM-CSF since the CSFR was highly expressed in blastocysts cultured in vitro. Similarly, the transcript level of CSFRα tended to be lower in the GM-CSF-treated group than in the control group. Thus supplementation with GM-CSF (10 ng/ml) may be meeting the needs of the embryos. Based on this, we can assume that the effect of GM-CSF on embryos is through its receptor; however, the specific mechanisms by which GM-CSF and CSFRα promote improved development still needs to be elucidated.
Our results illustrate that supplementing GM-CSF in embryo culture media can increase the developmental potential of pig embryos. An increase in the frequency of blastocyst formation and total cell number in IVF-derived blastocysts suggests that GM-CSF can be used to produce better-quality IVF-derived embryos in vitro. This positive effect was also observed when SCNT-derived embryos were cultured with GM-CSF, as both increase in blastocyst formation and total cell number were found. Similar results can be found in different species. Supplementation of GM-CSF in culture increased developmental potential in mouse, human, and bovine embryos (Sjoblom et al., 1999; Robertson et al., 2001; Loureiro et al., 2009), but only a few studies have been reported on the effect of GM-CSF on the culture of pig embryos. When mouse GM-CSF was introduced in pig embryo culture, limited effects were observed; supplementation of GM-CSF increased the developmental potential of parthenotes only when the embryos were cultured in protein-free culture medium (Cui et al., 2004). On the other hand, an increase in development was reported when pig GM-CSF was introduced into culture media of IVF- and SCNT-derived embryos (Kwak et al., 2012a,b). This suggests the activity of GM-CSF is species-specific, and the source of GM-CSF is important to support embryo development. Our results confirm these previous studies on IVF-derived embryos, and further indicate that the positive effect of GM-CSF is not confined to IVF-derived embryos but can be found for the culture of SCNT-derived embryos as well.
Our in vivo results suggest SCNT-derived embryos cultured with GM-CSF (10 ng/ml) can fully support the term development of pigs. Bovine embryos cultured with GM-CSF for 2 days showed an increase in pregnancy rate and term development (Loureiro et al., 2009). In our study, the term development of embryos cultured with GM-CSF was compatible to conventional embryo transfers performed at the one-cell stage, a protocol typically used to transfer pig embryos into surrogates because of suboptimal culture conditions and the inefficiency of non-surgical embryo transfer. While there are reports with IVF-derived blastocyst transfers pigs (Nakazawa et al., 2008; Zijlstra et al., 2008; Akaki et al., 2009; Beebe et al., 2011), there are few reports of SCNT-derived blastocyst transfers, likely because SCNT-derived embryos are more sensitive than IVF-derived embryos to in vitro culture conditions (Yamanaka et al., 2009).
A previous report showed that cloned piglets are born when 50 or more SCNT-derived blastocysts are transferred into surrogates (Lagutina et al., 2006) whereas another report indicates the efficiency of piglet production is higher when over 60 embryos are transferred (Schmidt et al., 2010). Here, we successfully produced cloned piglets when only 28–46 SCNT-derived embryos were transferred. In addition, embryo transfer resulting from donor E indicates at least one out of seven embryos transferred developed to term (7/46; 15.2%). This efficiency is compatible to or higher than the efficiency of piglets from IVF-derived blastocysts that were cultured in vitro (Kikuchi et al., 2002), an average of 12.7%. SCNT-derived embryos from donor E were generated all at once, and then divided into three surrogates (two Day 1 transfers and one Day 6 transfer with GM-CSF); we saw a significant increase in term development from Day 6 transfer. This clearly shows that the late transfer of blastocysts cultured with GM-CSF is beneficial. In addition, considering SCNT-derived embryos are difficult to generate, transferring GM-CSF-cultured, SCNT-derived embryos could be a solution when the supply of pig embryos are limited. In some cases, cloned pigs show abnormalities after birth (Estrada et al., 2007); however, no pigs with abnormal development were detected during this study. Also, the birth weights from the piglets were compatible to ones from early transfers. This suggests that SCNT-derived embryos cultured with GM-CSF can fully support in vivo development without any complications to their health. Moreover, piglets from five different donor cells were produced after culturing with GM-CSF. This indicates the effect of GM-CSF is not limited to a particular cell line, but can be applied in general.
The importance of GM-CSF on pregnancy is well demonstrated (Seymour et al., 1997; Robertson et al., 1999), although specific mechanisms of GM-CSF on embryos are still under investigation. It is thought that one of the mechanisms by which GM-CSF improves development is that it can protect embryos against apoptosis. Mouse embryos treated with GM-CSF showed higher expression of the anti-apoptotic gene Bcl-2 (Behr et al., 2005). In humans, a 50% reduction in apoptotic nuclei was observed when embryos were cultured with GM-CSF (Sjoblom et al., 2002). This protective effect against apoptosis could be a reason for the increase in in vitro developmental potential of IVF- and SCNT-derived embryos in this study. In fact, our results show that embryos cultured with GM-CSF express lower level of BAD, a pro-apoptotic gene. Conversely, the level of PPP3CC, which can stimulate BAD activity by dephosphorylation (Wang et al., 1999), was not changed. PPP3CC is regulated by Ca2+ rise, thus we speculate that the decrease of BAD in GM-CSF-cultured embryos is not because of the Ca2+ signal. TP53, which is activated by DNA breaks or oxidative stress (Liu and Xu, 2011), was decreased in embryos cultured with GM-CSF. This supports an anti-apoptotic effect of GM-CSF. Previous reports suggest that GM-CSF can stimulate the JAK1/STAT pathway (Martinez-Moczygemba and Huston, 2003) when introduced into cell culture experiments, but was not seen here. There was no change in the level of cRAF, which was previously found to activate the MAPK signaling pathway (Kyriakis et al., 1992). Based on these results, we argue that the effect of GM-CSF on pig embryos is mainly from its anti-apoptotic effect, similar to reports in other species.
Only one pregnancy was lost during this study when embryos, cultured with GM-CSF, were transferred. In cows, pregnancy loss was dramatically decreased when embryos were cultured with GM-CSF prior to transfers (Loureiro et al., 2009). In addition, a higher level of interferin tau, an essential signaling molecule for the recognition of pregnancy, from conceptuses was detected in embryos cultured with GM-CSF compared to the control group (Loureiro et al., 2011). These results from cow studies suggest that embryos cultured with GM-CSF are better able to implant. High pregnancy rates in our study also suggest that embryos cultured with GM-CSF may have a better chance to attach to the uterus and for subsequent establishment of a pregnancy.
Success of embryo transfers can also depend on the competency of surrogates. GM-CSF is produced from uterine epithelial cells in mice (Robertson et al., 1992). Because GM-CSF is essential for proper pregnancy, a pregnancy could be compromised regardless of the quality of embryos if the uterine epithelial cells from surrogates do not produce enough GM-CSF. As GM-CSF-cultured embryos would have received the signal in vitro, they would be somewhat protected if transferred to less-than-competent surrogates.
In conclusion, supplementing GM-CSF into the culture of IVF- and SCNT-derived embryos increased the in vitro developmental potential of the embryos. Because pig embryo culture conditions are suboptimal, SCNT-derived embryos are generally transferred into surrogates at an early stage. Here, we showed that transferring SCNT-derived blastocyst stage embryos, cultured with GM-CSF, can produce cloned pigs more efficiently compared to SCNT-derived embryos that are transferred at the one-cell stage. This is the first study to show full in vivo developmental potential of embryos cultured with GM-CSF in pigs. This embryo transfer approach can potentially replace conventional transfer methods because it can provide the benefit of evaluating embryo quality before the transfer. Further studies that elucidate the specific mechanism of GM-CSF on pig embryos and that follow up studies on the piglets produced would help to identify any long-term effect(s) of GM-CSF.
MATERIALS AND METHODS
Chemicals
All chemicals in the experiments were purchased from Sigma–Aldrich Chemical Company (St. Louis, MO), unless indicated otherwise.
RT-PCR
Ten to 15 IVF-derived blastocysts were collected as a group to identify the abundance of message for CSFR (α and β). The embryos were rinsed in phosphate-buffered saline (PBS) containing 0.1 mg/ml polyvinyl alcohol (PVA) and 0.1 mg/ml diethylpyrocarbonate (DEPC), and then transferred into 100 μl lysis/binding buffer consisting of 100 mM Tris-HCl, 500 mM LiCl, 10 mM EDTA, 1% LiDS, and 5 mM dithiothereitol (DTT). Messenger RNA was then extracted from the lysed embryos with the Dynabeads mRNA direct kit (Invitrogen Corporation, Carlsbad, CA), according to the manufacturer’s instructions. Magnetic beads, with mRNA attached, were re-suspended in buffer of the SuperScript™ III First-Strand Synthesis System (Invitrogen) for reverse transcriptase reaction. The synthesized cDNA was used for RT-PCR. GAPDH was used as internal control, and the primer sequences are shown in Table 4. The program used for the RT-PCR had an initial denaturation of 95°C for 2 min followed by 28 cycles of 30 sec at 94°C, 30 sec at 58°C, and 30 sec at 72°C. After the PCR reaction, the products were loaded on 2% agarose gel for visualization.
TABLE 4. Primer Sequences Used for RT-PCR and Real-Time PCR.
| Gene | Primer sequences (5′-3′) | Product size (bp) | GenBank accession number |
|---|---|---|---|
| CSFRα | F: GGGCGTTTCGTTCACGGTTAAA | 141 | XM_003362074 |
| R: GTGCAGTTCATGAAATCCGCGTTG | |||
| CSFRβ | F: AGAACCTGCGCTGCTATAATGACT | 215 | XM_001924779 |
| R: AGCCTTGTAGGGAATGACACATCT | |||
| GAPDH | F: ATGACATCAAGAAGGTGGTGAAGC | 126 | NM_001206359 |
| R:CCAGCATCAAAAGTGGAAGAGTGA | |||
| BCL2 | F: ACTGAATGCCCTCCGGTACC | 80 | NM_214285 |
| R: ATCCCCATGGCTGCAGTGAA | |||
| BAD | F: TTGCCAGCCGAGATTAACCCTAAC | 100 | XM_003122573 |
| R: CACGCGGGCTTTATTAGCACGTTT | |||
| CASP3 | F: ACACGCCATGTCATCTTCAGTCC | 81 | NM_214131 |
| R: TTCATAATTCAGGCCTGCCGAG | |||
| JAK1 | F: CCTTGATGCCAGCTCATTGGAA | 111 | NM_214114 |
| R:TCAATATCATGCCCGTCCTGCT | |||
| STAT3 | F: TGAGAGAGCAGAGATGTGGGAA | 99 | NM_001044580 |
| R: ACACCTCGGTCTCAAAGGTGAT | |||
| PPP3CC | F: CTGCCCTCTTAAACCAGCAGTT | 137 | XM_001928163 |
| R: AGACCAAAGCAGGTCACACACT | |||
| cRAF | F: ACCTACTATGTGTGTGGACTGGAG | 113 | XR_135376 |
| R: GCATCCGACGCATTGTCAAAGA | |||
| TP53 | F: GGAACAGCTTTGAGGTGCGTGTTT | 182 | NM_213824 |
| R: ATACTCGCCATCCAGTGGCTTCTT | |||
| YWHAG | F: TCCATCACTGAGGAAAACTGCTAA | 137 | NM_012479 |
| R: TTTTTCCAACTCCGTGTTTCTCTA |
Quantitative Real-Time PCR
To investigate the expression of CSFR in blastocysts derived from in vitro culture or from in vivo development, cDNA previously generated for deep-sequencing analysis (Bauer et al., 2010) was used. To investigate the effect of GM-CSF on the expression of CSFR, mRNA was extracted and cDNA was synthesized from 10 to 15 IVF-derived blastocysts, as described above. Quantitative real-time PCR was then conducted using IQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Prior to quantification, a validation test was run for each designed primer to verify that the amplification efficiencies were similar for each amplicon. The PCR was performed on a MyiQ single color real-time thermal cycler (Bio-Rad). The program used for amplification included an initial temperature of 94°C for 2 min followed by 40 cycles of 5 sec at 94°C, 30 sec at 60°C, and 30 sec at 72°C. Real-time fluorescence data was collected during the extension time, and transcript levels of CSFRα and CSFRβ were quantified using the relative quantification method based on comparative threshold cycles (Ct) values. The transcript abundance of each gene was determined relative to that of the internal control gene, GAPDH. ΔCt was calculated by subtracting Ct values of each gene from that of the GAPDH. Control group Ct values served as calibrators, and were used subsequently to obtain ΔΔCt values. Fold differences in transcript abundance were calculated for CSFRα and CSFRβ assuming an amplification efficiency of 100% and using the equation 2−ΔΔCt. Three biological and two experimental replications were used for the assay; for each biological replicate, cDNA from a single group of embryos was divided to amplify CSFRα, CSFRβ, and GAPDH during the same PCR assay.
To investigate specific targets of GM-CSF, isolated total RNA from pools of 10 embryos cultured either in 0 or 10 ng/ml GM-CSF was amplified using the WT-Ovation™ Pico RNA Amplification System (NuGEN Technologies Inc., San Carlos, CA). After amplification, the samples were purified using Micro Bio-Spin P-30 Columns (Bio-Rad Laboratories). Real-time PCR was then conducted for each of eight candidate genes (primers are listed in Table 4). The levels for each transcript will be calculated relative to the internal control gene, YWHAG. Three biological and three experimental replications were used for the assay.
Oocyte Maturation and Fertilization
For the in vitro study with IVF-derived embryos, ovaries from pre-pubertal gilts were obtained from an abattoir (Farmland Foods Inc., Milan, MO). Immature oocytes were aspirated from medium size (3–6 mm) follicles using an 18-gauge hypodermic needle attached to a 10-ml syringe. Oocytes with evenly dark cytoplasm and intact surrounding cumulus cells were then selected for maturation. Around 50 cumulus oocyte complexes were place in a well containing 500 μl of maturation medium, TCM 199 (Invitrogen, Grand Island, NY) with 3.05 mM glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 10 ng/ml epidermal growth factor (EGF), 0.5 μg/ml luteinizing hormone (LH), 0.5 μg/ml follicle stimulating hormone (FSH), 10 ng/ml gentamicin (APP Pharm, Schaumburg, IL), and 0.1% polyvinyl alcohol (PVA) for 42–44 h at 38.5°C, 5% CO2, in humidified air. At the end of the maturation, the surrounding cumulus cells were removed from the oocytes by vortexing for 3 min in the presence of 0.1% hyaluronidase.
Oocytes with a visible polar body were selected in manipulation medium (TCM199 with 0.6 mM NaHCO3, 2.9 mM Hepes, 30 mM NaCl, 10 ng/ml gentamicin, and 3 mg/ml bovine serum albumin [BSA]; and osmolarity of 305) and used for the experiments. For fertilization, groups of 25–30 oocytes were placed in 50-μl droplets of IVF medium (modified Tris-buffered medium with 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2, 11 mM glucose, 20 mM Tris, 2 mM caffeine, 5 mM sodium pyruvate, and 2 mg/ml BSA (A8022)) and covered with mineral oil. One 100 μl frozen semen pellet was thawed in 3 ml of DPBS supplemented with 0.1% BSA and used for fertilization. Semen was washed in 60% Percoll for 20 min at 650 rfc, and once in modified Tris-buffered medium (MTBM) for 10 min by centrifugation. The semen pellet was re-suspended with IVF medium to 0.5 × 106 cells/ml. Then, 50 μl of the semen suspension was introduced into the droplets with oocytes. Finally, the gametes were co-incubated for 5 hr at 38.5°C in an atmosphere of 5% CO2 in air. After, IVF-derived embryos were cultured in PZM3 at 38.5°C, 5% O2, 5% CO2, 90% N2 in humidified air. To investigate the effect of porcine GM-CSF (Genway Biotech Inc., San Diego, CA) on in vitro culture, cleaved IVF-derived embryos were divided into three groups (control, 2 ng/ml GM-CSF, and 10 ng/ml GM-CSF) and cultured for an additional 5 days. Only cleaved embryos were used to exclude a potential variation from fertilization. Embryos were then assayed for the frequency of blastocysts and total cell number in the blastocysts at Day 6.
Somatic Cell Nuclear Transfer (SCNT)
For SCNT experiments, sow-derived oocytes were purchased from ART (Madison, WI). The oocytes were shipped overnight in maturation medium (TCM199 with 2.9 mM Hepes, 5 μg/ml insulin, 10 ng/ml EGF, 0.5 μg/ml p-FSH, 0.91 mM pyruvate, 0.5 mM cysteine, 10% porcine follicular fluid, 25 ng/ml gentamicin) and transferred into fresh medium at 24 hr. After 40–42 hr of maturation, cumulus cells were removed from the oocytes by vortexing for 3 min in the presence of 0.1% hyaluronidase. Oocytes were manipulated in manipulation medium supplemented with 7.0 μg/ml cytochalasin B. The polar body along with a portion of the adjacent cytoplasm was removed, and a donor cell was placed in the perivitelline space (Lai and Prather, 2003). The reconstructed embryos were then fused in fusion medium (0.3 M mannitol, 0.1 mM CaCl2, 0.1 mM MgCl2, and 0.5 mM Hepes) by two direct-current (DC) pulses (1-sec interval) at 1.2 kV/cm for 30 μsec (using BTX Electro Cell Manipulator, Harvard Apparatus, Holliston, MA). After fusion, embryos were fully activated with 200 μM thimerosal for 10 min in the dark and 8 mM dithiothreitol for 30 min (Machaty et al., 1997). Embryos were then incubated in PZM3 (Yoshioka et al., 2002) with 0.5 μM Scriptaid (Sigma, S7817), a histone deacetylase inhibitor, for 14–16 hr. At Day 1, SCNT-derived embryos were divided into three groups (control, 2 ng/ml GM-CSF, and 10 ng/ml GM-CSF), and cultured for an additional 5 days. At the end of the culture, SCNT-derived embryos were collected, and the frequency of cleaved embryos and blastocysts, and total cell number in the blastocysts were obtained.
Embryo Transfer of SCNT-Derived Embryos Cultured With GM-CSF
Based on previous results, SCNT-derived embryos were cultured with 10 ng/ml of GM-CSF, and blastocysts were then transferred into six surrogates. Five different donor cell lines were used to generate SCNT-derived embryos for the experiment. For in vivo study at Day 6, the SCNT-derived embryos were surgically transferred into the ampullary-isthmic junction of a surrogate at 5 or 6 days after observed estrus. Percentage of pregnancy was collected at Day 40 of gestation by ultrasound. To investigate in vivo developmental potential of embryos cultured in GM-CSF, birth weights of piglets and cloning efficiencies were recorded. In vivo developmental potential of GM-CSF-cultured, SCNT-derived embryos were compared to a data set generated in the lab where SCNT-derived embryos, from the same types of cells, were transferred at the one-cell stage. All animals used in the experiments were approved by the Institutional Animal Care and Use Committee of the University of Missouri.
Statistical Analysis
Quantitative real-time results were analyzed by general linear model (PROC GLM) of Statistical Analysis System (SAS Institute, Cary, NC). The values were log-transformed prior to the comparison. Frequency of cleaved and blastocysts were subjected to analysis of variance (ANOVA) using the PROC MIX procedure. Percentage data was transformed by arc sine transformation before the ANOVA analysis. Differences among the treatment means were analyzed using the Tukey test. Student’s t-test was used to compare difference in average total cell number in blastocysts. Difference in in vivo data was analyzed by χ2-test. Differences with P < 0.05 were considered significant in all statistical comparisons.
ACKNOWLEDGMENTS
This work was supported by Woo Jang-Choon project (PJ007849) from the Rural Development Administration (RDA), Republic of Korea, Food for the 21st Century, the National Institutes of Health U42 RR018877 and now U42 OD011140.
Abbreviations
- IVF
in vitro fertilized/fertilization
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- SCNT
somatic cell nuclear transfer
Genes
- BAD
BCL2-associated agonist of cell death
- BCL2
B-cell lymphoma 2
- CASP3
caspase 3, apoptosis-related cysteine peptidase
- cRAF (RAF1)
v-raf-1 murine leukemia viral oncogene homolog 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- JAK1
Janus kinase 1
- PPP3CC
Serine/threonine-protein phosphatase 2B catalytic subunit gamma isoform
- STAT3
signal transducer and activator of transcription 3 (acute-phase response factor)
- TP53
tumor protein p53
- YWHAG
tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, gamma polypeptide
REFERENCES
- Akaki Y, Yoshioka K, Noguchi M, Hoshi H, Funahashi H. Successful piglet production in a chemically defined system for in-vitro production of porcine embryos: Dibutyryl cyclic amp and epidermal growth factor-family peptides support in-vitro maturation of oocytes in the absence of gonadotropins. J Reprod Develop. 2009;55:446–453. doi: 10.1262/jrd.20219. [DOI] [PubMed] [Google Scholar]
- Bauer BK, Isom SC, Spate LD, Whitworth KM, Spollen WG, Blake SM, Springer GK, Murphy CN, Prather RS. Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos. Biol Reprod. 2010;83:791–798. doi: 10.1095/biolreprod.110.085936. [DOI] [PubMed] [Google Scholar]
- Beebe LF, Bouwman EG, McIlfatrick SM, Nottle MB. Piglets produced from in vivo blastocysts vitrified using the cryologic vitrification method (solid surface vitrification) and a sealed storage container. Theriogenology. 2011;75:1453–1458. doi: 10.1016/j.theriogenology.2010.11.043. [DOI] [PubMed] [Google Scholar]
- Behr B, Mooney S, Wen Y, Polan ML, Wang H. Preliminary experience with low concentration of granulocyte-macrophage colony-stimulating factor: A potential regulator in preimplantation mouse embryo development and apoptosis. J Assist Reprod Genet. 2005;22:25–32. doi: 10.1007/s10815-005-0817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot F, Venturi E, Cognie J, Furstoss V, Martinat-Botte F. Development of OPS vitrified pig blastocysts: Effects of size of the collected blastocysts, cryoprotectant concentration used for vitrification and number of blastocysts transferred. Theriogenology. 2007;68:178–185. doi: 10.1016/j.theriogenology.2007.04.050. [DOI] [PubMed] [Google Scholar]
- Cui XS, Lee JY, Choi SH, Kwon MS, Kim T, Kim NH. Mouse granulocyte-macrophage colony-stimulating factor enhances viability of porcine embryos in defined culture conditions. Anim Reprod Sci. 2004;84:169–177. doi: 10.1016/j.anireprosci.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- Estrada J, Sommer J, Collins B, Mir B, Martin A, York A, Petters RM, Piedrahita JA. Swine generated by somatic cell nuclear transfer have increased incidence of intrauterine growth restriction (IUGR) Cloning Stem Cells. 2007;9:229–236. doi: 10.1089/clo.2006.0079. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Onishi A, Kashiwazaki N, Iwamoto M, Noguchi J, Kaneko H, Akita T, Nagai T. Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol Reprod. 2002;66:1033–1041. doi: 10.1095/biolreprod66.4.1033. [DOI] [PubMed] [Google Scholar]
- Kwak SS, Cheong SA, Jeon Y, Hyun SH. Porcine granulocyte-macrophage colony-stimulating factor improves the in vitro development of cloned porcine embryos. J Vet Med Sci. 2012a;74:1095–1102. doi: 10.1292/jvms.12-0050. [DOI] [PubMed] [Google Scholar]
- Kwak SS, Jeung SH, Biswas D, Jeon YB, Hyun SH. Effects of porcine granulocyte-macrophage colony-stimulating factor on porcine in vitro-fertilized embryos. Theriogenology. 2012b;77:1186–1197. doi: 10.1016/j.theriogenology.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Lagutina I, Lazzari G, Galli C. Birth of cloned pigs from zona-free nuclear transfer blastocysts developed in vitro before transfer. Cloning Stem Cells. 2006;8:283–293. doi: 10.1089/clo.2006.8.283. [DOI] [PubMed] [Google Scholar]
- Lai L, Prather RS. Production of cloned pigs by using somatic cells as donors. Cloning Stem Cells. 2003;5:233–241. doi: 10.1089/153623003772032754. [DOI] [PubMed] [Google Scholar]
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Li R, Murphy CN, Spate L, Wax D, Isom C, Rieke A, Walters EM, Samuel M, Prather RS. Production of piglets after cryo-preservation of embryos using a centrifugation-based method for delipation without micromanipulation. Biol Reprod. 2009;80:563–571. doi: 10.1095/biolreprod.108.073387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu Y. p53, oxidative stress, and aging. Antioxid Redox Signal. 2011;15:1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro B, Bonilla L, Block J, Fear JM, Bonilla AQ, Hansen PJ. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology. 2009;150:5046–5054. doi: 10.1210/en.2009-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro B, Block J, Favoreto MG, Carambula S, Pennington KA, Ealy AD, Hansen PJ. Consequences of conceptus exposure to colony-stimulating factor 2 on survival, elongation, interferon-tau secretion, and gene expression. Reproduction (Cambridge, England) 2011;141:617–624. doi: 10.1530/REP-10-0511. [DOI] [PubMed] [Google Scholar]
- Machaty Z, Wang WH, Day BN, Prather RS. Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biol Reprod. 1997;57:1123–1127. doi: 10.1095/biolreprod57.5.1123. [DOI] [PubMed] [Google Scholar]
- Machaty Z, Day BN, Prather RS. Development of early porcine embryos in vitro and in vivo. Biol Reprod. 1998;59:451–455. doi: 10.1095/biolreprod59.2.451. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–665. doi: 10.1016/S0091. quiz 666. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Misawa H, Fujino Y, Tajima S, Misumi K, Ueda J, Nakamura Y, Shibata T, Hirayama Y, Kikuchi K. Effect of volume of non-surgical embryo transfer medium on ability of porcine embryos to survive to term. J Reprod Develop. 2008;54:30–34. doi: 10.1262/jrd.19132. [DOI] [PubMed] [Google Scholar]
- Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry AC. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Rath D, Niemann H, Torres CR. In vitro development to blastocysts of early porcine embryos produced in vivo or in vitro. Theriogenology. 1995;43:913–926. doi: 10.1016/0093-691x(95)00042-7. [DOI] [PubMed] [Google Scholar]
- Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg Effect. Mol Reprod Develop. 2012;79:262–271. doi: 10.1002/mrd.22017. [DOI] [PubMed] [Google Scholar]
- Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992;46:1069–1079. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol Reprod. 1999;60:251–261. doi: 10.1095/biolreprod60.2.251. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001;64:1206–1215. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr., Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Kragh PM, Li J, Du Y, Lin L, Liu Y, Bogh IB, Winther KD, Vajta G, Callesen H. Pregnancies and piglets from large white sow recipients after two transfer methods of cloned and transgenic embryos of different pig breeds. Theriogenology. 2010;74:1233–1240. doi: 10.1016/j.theriogenology.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–3049. [PubMed] [Google Scholar]
- Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–3076. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67:1817–1823. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- Spate LD, Redel BK, Brown AN, Murphy CN, Prather RS. Replacement of bovine serum albumin with N-methyl-d-aspartic acid and homocysteine improves development, but not live birth. Mol Reprod Develop. 2012;79:310. doi: 10.1002/mrd.22032. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, Agca C, Kim JG, Patel RV, Springer GK, Bivens NJ, Forrester LJ, Mathialagan N, Green JA, Prather RS. Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos. Biol Reprod. 2005;72:1437–1451. doi: 10.1095/biolreprod.104.037952. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Sugimura S, Wakai T, Kawahara M, Sato E. Difference in sensitivity to culture condition between in vitro fertilized and somatic cell nuclear transfer embryos in pigs. J Reprod Develop. 2009;55:299–304. doi: 10.1262/jrd.20174. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
- Zijlstra C, Kidson A, Schoevers EJ, Daemen AJ, Tharasanit T, Kuijk EW, Hazeleger W, Ducro-Steverink DW, Colenbrander B, Roelen BA. Blastocyst morphology, actin cytoskeleton quality and chromosome content are correlated with embryo quality in the pig. Theriogenology. 2008;70:923–935. doi: 10.1016/j.theriogenology.2008.05.055. [DOI] [PubMed] [Google Scholar]