Abstract
Objectives
This study assessed the hypothesis that smoking strengthens the association of adult arterial stiffness with long-term cumulative burden of BP from childhood to adulthood.
Backgrounds
Tobacco smoking and elevated blood pressures (BP) are important risk factors of vascular stiffness. However, the synergistic effect of these two risk factors is not well established, especially for the long-term burden of elevated BP since childhood.
Methods
The study cohort consisted of 945 adults (661 whites and 284 blacks; ages 24–43 years) who have BP measured 4–15 times since childhood (ages 4–17 years) in Bogalusa, Louisiana. The adult arterial stiffness was measured as aorta-femoral pulse wave velocity (afPWV); the total area under the curve (AUC) and incremental AUC were used as a measure of long-term burden and trends of BP, respectively.
Results
Increased adult afPWV was significantly associated with higher adulthood (p<0.001), total AUC (p<0.001) and incremental AUC (p<0.001) values of systolic and diastolic BP, but not with childhood BP, after adjusting for age, race, gender, body mass index and heart rate. Furthermore, smoking was a significant predictor of increased adult afPWV and BP levels. In the interaction analyses, the increasing trend of afPWV with increasing adult systolic BP (p=0.009) and its incremental AUC (p=0.007) was significantly greater among current smokers than among nonsmokers. Diastolic BP showed a similar pattern regarding the smoking-BP interaction on afPWV.
Conclusions
These results by showing the synergistic effect of tobacco smoking and long-term BP measures from childhood to adulthood on arterial stiffening process underscore the importance of undertaking preventive strategies early in life and smoking behavior control.
Introduction
Arterial stiffness expressed as pulse wave velocity (PWV) is a strong independent predictor of future cardiovascular events and all-cause mortality. Longitudinal studies showed that an increase in aortic PWV by 1 standard deviation was associated with increases of 47%, 47%, and 42% in total cardiovascular events, cardiovascular mortality and all-cause mortality, respectively [1]. Large-artery stiffness has become increasingly important for the assessment of cardiovascular risk [2].
Blood pressure (BP) is the strongest risk factor of arterial stiffening during the aging process. A systematic review of 77 cross-sectional studies on risk factors of aortic PWV in adults has shown that age and BP are consistently and independently associated with aortic PWV [3]. Tobacco smoking has long been established as a powerful risk factor of cardiovascular disease and hypertension and is also strongly associated with arterial stiffness [4–6]. However, it is not well understood whether smoking exerts an independent effect on arterial stiffening or it has a joint effect with other risk factors like elevated BP. The data in this regard are limited to date, especially for the long-term cumulative burden of elevated BP [7–9].
Alterations in arterial structure and function and essential hypertension are considered growth-related disorders with their origin in childhood [10–12]. Further, BP levels tend to “track” or “persist” over time, and increases in childhood BP levels are a strong predictor of adulthood hypertension [13,14] and arterial stiffness [15–17]. However, no studies have focused on the influence of long-term burden and increasing trends of BP from childhood to adulthood and its synergistic effect with smoking on arterial stiffening later in life. Although the interaction effect of smoking with hypertension on the arterial stiffening process has been reported in previous studies [7–9], the data are lacking regarding the joint effect of smoking and longitudinal changes of BP in this regard. The objective of the present study is to examine the effect of longitudinal measures of BP from childhood on adult arterial stiffness and whether this relationship is independent of smoking status utilizing a longitudinal cohort from the Bogalusa Heart Study.
Methods
Study Cohort
The Bogalusa Heart Study is a biracial (65% white and 35% black) community-based long-term investigation of the early natural history of cardiovascular disease beginning in childhood since 1973 [18]. Between 1973 and 2002, nine cross-sectional surveys of children aged 4–17 years and ten cross-sectional surveys of adults aged 18–43 years, who had been previously examined as children, were conducted in Bogalusa, Louisiana. This panel design of repeated cross-sectional examinations has resulted in serial observations from childhood to adulthood every 2–3 years. In this longitudinal cohort, 1084 adults were examined for cardiovascular risk factors and aorta-femoral pulse wave velocity (afPWV) during 2000–2002. After exclusion of 3 subjects who were examined <4 times for cardiovascular risk factors and 136 hypertensive patients who were under treatment, 945 adult subjects (661 whites and 284 blacks; 45.2% males; mean age=36.5 years; range=23.8–43.3 years) who had been examined for afPWV one time in adulthood, body weight and BP 4–15 times (at least 2 times each in childhood and adulthood) formed the longitudinal study cohort for this report. The average follow-up period was 26.7 years. In this longitudinal cohort, 733 subjects had lipid, insulin resistance and physical activity variables measured in adulthood. Individuals (n=212) who did not have data on lipid, insulin resistance and physical activity were 0.6 years younger as adults and had fewer withes; however, other study variables did not show significant differences between the two subgroups in terms of BMI and BP measures, smoking status and afPWV after adjusting for race, gender and age.
All subjects in this study gave informed consent at each examination, and for those under 18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
General Examination
Replicate measurements of height and weight were made, and the mean values were used for analysis. Body mass index (BMI, weight in kilograms divided by the square of the height in meters) was used as a measure of obesity. BP levels were measured between 8:00 AM and 10:00 AM on the right arm of subjects in a relaxed, sitting position by 2 trained observers (3 replicates each). Systolic (SBP) and diastolic (DBP) blood pressure was recorded using a mercury sphygmomanometer. The fourth Korotkoff phase was used for DBP for children and adults because the fourth phase is more reliably measured in childhood and more predictive of adult hypertension [19]. The mean values of the 6 readings were used for analysis. Resting heart rate was counted at the radial pulse in a relaxed, sitting position. Three measurements were made, separated by brief intervals, by each of two trained observers, and average values were used. Information on health, medication history, and behavioral lifestyles were obtained by questionnaires. Information on smoking status and duration was collected; current smokers were identified as smoking at least one cigarette per day during the past 12 months; years of smoking were added up for episodic smokers. Participants were asked to provide a subjective rating of their physical activity level outside of work; the rating was coded as 1 (physically inactive) to 5 (very active).
Laboratory Analysis
Serum lipoprotein cholesterols and triglycerides were analyzed by using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures. The triglycerides/high-density lipoprotein cholesterol ratio (TG/HDL-C) was included in the current analysis because TG and HDL-C have been commonly used to define the dyslipidemia component of metabolic syndrome (20), and the TG/HDL-C ratio as a conjoint trait has shown a stronger association with other cardiovascula risk factors (21). Plasma glucose was measured enzymatically as part of a multichemistry (SMA20) profile. Plasma immunoreactive insulin levels were measured by a commercial radioimmunoassay kit (Phadebas, Pharmacia Diagnostics, and Piscataway, NJ). An index of insulin resistance was calculated according to the homeostasis model assessment (HOMA) of insulin resistance formula: HOMA=fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5.
Aorta-femoral Pulse Wave Velocity (afPWV)
The afPWV was measured using a Toshiba digital ultrasound instrument (Xario, SSA-660A, Toshiba America Medical Systems, Tustin CA). A non-directional transcutaneous Doppler flow probe (Toshiba PSK25AT, 2.5 MHz) was positioned at suprasternal notch and another probe (Toshiba PCK703AT, 7.5 MHz) at left femoral artery in a supine position. A computer system displayed and recorded output from the electrocardiography and the two Doppler probes. The arterial flow waves from the two arterial sites were recorded, and the output was captured and stored in a computer system for subsequent scoring. After the collection of the waveform data, the distance between the aorta and femoral arteries was measured with a caliper instrument to reduce the influence of body contours on the distance measured. The software averages the selected waveforms and determines the time from the R-wave of the ECG to the foot of each waveform. The difference in timing between the two waves represents the time component of the velocity equation. afPWV was then calculated by dividing the distance traveled by the time differential between the two waveforms. Results from 3 data collection runs for each participant were averaged for analysis. Re-screenees (n=46) were re-measured for afPWV for reproducibility analysis. The correlation between the two measurements was 0.91 on the same day and 0.68 on different days. The day-to-day variations were influenced by both measurement errors and physiologic fluctuations.
Statistical Methods
Analyses of covariance were performed using general linear models to test differences in study variables between males and females as well as between blacks and whites, adjusting for age.
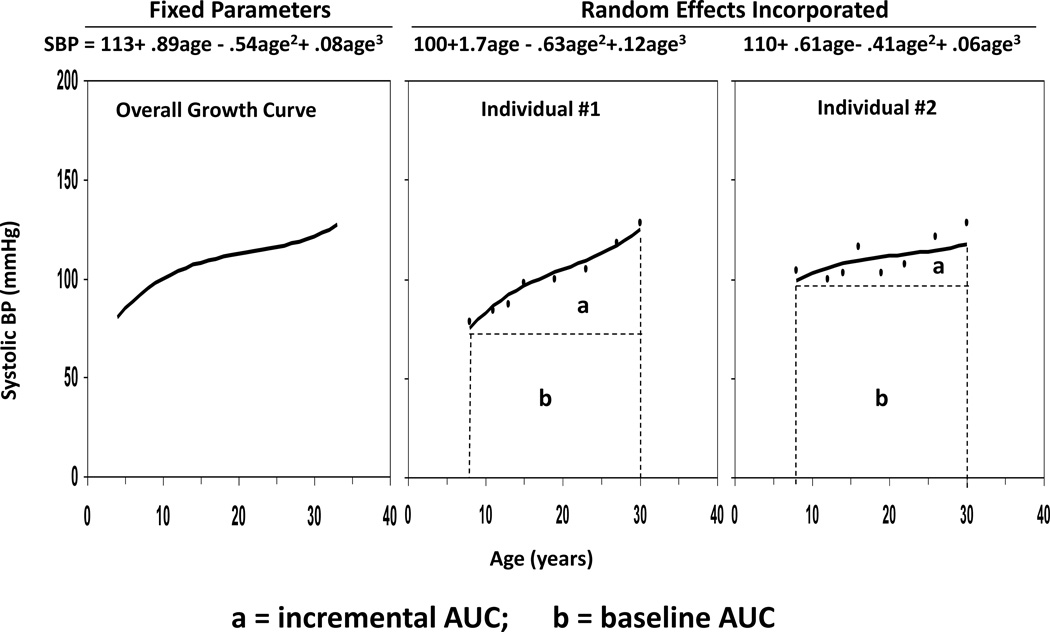
Long-term burden and trends of BMI and BP were measured as the area under the curve (AUC) calculated using the statistical models we have previously developed [20–23]. Growth curves of BMI and BP measured repeatedly at multiple time points from childhood to adulthood were constructed using a random effects model by SAS proc MIXED. A quadratic curve was fit for BMI, and a cubic curve for SBP and DBP in race-gender groups. As shown in Figure 1, using SBP as an example, the AUC was calculated as the integral of the curve parameters during the follow-up period for each individual. Since individuals had different follow-up periods, the AUC values were divided by the number of follow-up years. The AUC measures have advantages over other longitudinal analysis models in that they measure both long-term burden and trends. As shown in Figure 1, total AUC (a+b) can be considered a measure of a long-term cumulative burden; incremental AUC (a) determined by within-individual variability represents a combination of linear and nonlinear longitudinal trends as we used in our previous studies [20–23].
Figure 1.
The area under the curve (AUC) of systolic blood pressure (SBP) with two examples
In addition to AUC analyses, the association analyses of afPWV with BMI and BP values were also performed in both childhood (first measurement) and adulthood (last measurement) using linear regression models. Prior to regression analyses, the total and incremental AUC values of BMI and BP were adjusted for average age by regression residual analyses and then standardized with Z-transformation (mean=0, SD=1) by race-gender groups in order to avoid colinearity of multiple ages in the regression model. Since BMI and BP levels tend to “track” or “persist” over time, childhood BMI and BP values were adjusted for their corresponding adulthood values in the same manner to remove the effect of tracking correlation form childhood to adulthood on adult afPWV. In addition, for analyses of incremental AUC, baseline values of BP and BMI were also included in the model to control the regression-to-the-mean bias. The differences in slopes of afPWV with respect to increasing BP values between current smokers and nonsmokers were examined in interaction regression models (homogeneity-of-slopes models), adjusting for covariates. A p-value<0.05 associated with the interaction term indicated a significant difference in slopes between current smokers and nonsmokers. Different slopes of afPWV among smokers versus nonsmokers were plotted using separate-slopes models.
Results
Table 1 summarizes the study variables in childhood and adulthood and as AUC values by race and gender. In childhood, gender and race differences in all study variables were not significant. In adulthood, black females had significantly higher BMI than white females; BMI showed a significant gender difference in an opposite direction in whites (males>females) versus blacks (males<females). Adulthood values of SBP and DBP showed significant race (blacks>whites) and gender (males>females) differences. Adulthood physical activity had a significant race (blacks>whites) difference in males and a gender (males>females) in whites. Adulthood TG/HDL-C ratio and HOMA index showed significant race and gender differences except for the gender difference in in blacks and race difference in males for HOMA index. AUC values of BMI, SBP and DBP showed significant race and gender differences except gender difference in total AUC of BMI among blacks, and race difference in incremental and total AUC of BMI among males. Significant gender differences in adulthood heart rate (females>males) and afPWV (males>females) were seen among whites, but not among blacks. Blacks had significantly higher afPWV values than whites for both males and females. Black males had a significantly higher prevalence of smokers than white males.
Table 1.
Characteristics (mean (SD), %) of study variables by race and gender
| Whites | Blacks | P for Race Difference |
Total (n=945) |
||||
|---|---|---|---|---|---|---|---|
| Males (n=295) |
Females (n=366) |
Males (n=102) |
Females (n=182) |
Males | Females | ||
| Childhood (First exam) | |||||||
| Age (year) | 10.0 (3.1) | 9.7 (3.1) | 10.1 (2.9) | 9.5 (2.8) | 0.762 | 0.392 | 9.7(3.0) |
| BMI | 17.8 (3.5) | 17.6 (3.5) | 17.8 (3.8) | 17.4 (3.5) | 0.793 | 0.812 | 17.6(3.4) |
| SBP (mmHg) | 101.0 (9.8) | 99.5 (9.5) | 100.5 (11.6) | 98.7 (10.0) | 0.532 | 0.727 | 99.8(9.9) |
| DBP (mmHg) | 61.7 (8.2) | 61.9 (8.5) | 63.3 (8.7) | 61.4 (8.8) | 0.098 | 0.793 | 61.9(8.5) |
| Adulthood (Last exam) | |||||||
| Age (year) | 36.9 (4.1) | 36.4 (4.3) | 37.1 (4.2) | 35.7 (4.6)* | 0.639 | 0.084 | 36.5(4.3) |
| BMI | 29.3 (5.7) | 28.2(7.1)* | 29.9 (7.6) | 32.2 (9.1)* | 0.362 | <0.001 | 29.4(7.2) |
| SBP (mmHg) | 119.4 (11.9) | 111.5(11.4)** | 130.1 (18.5) | 121.4 (16.9)** | <0.001 | <0.001 | 117.8(14.9) |
| DBP (mmHg) | 81.0 (8.2) | 75.3(8.6)** | 87.7 (13.5) | 81.4 (11.3)** | <0.001 | <0.001 | 79.6(10.4) |
| AUC measures | |||||||
| Total AUC of BMI | 24.8 (4.4) | 23.5 (4.8)** | 24.9 (5.4) | 25.9 (5.8) | 0.806 | <0.001 | 24.4(4.9) |
| Total AUC of SBP | 113.4 (7.3) | 107.3 (6.3)** | 116.4 (9.3) | 111.1 (7.7)** | <0.001 | <0.001 | 110.9(7.9) |
| Total AUC of DBP | 72.5 (5.6) | 69.8 (4.5)** | 73.9 (7.3) | 71.8 (5.9)* | 0.016 | <0.001 | 71.4(5.7) |
| Incremental AUC of BMI | 6.9 (2.6) | 6.0 (3.6)** | 7.0 (2.9) | 8.3 (4.1)* | 0.874 | <0.001 | 6.8(3.4) |
| Incremental AUC of SBP | 12.7 (5.4) | 7.9 (5.9)** | 16.5 (7.1) | 12.8 (6.7)** | <0.001 | <0.001 | 11.3(6.7) |
| Incremental AUC of DBP | 11.3 (3.1) | 8.5 (4.1)** | 12.1 (4.1) | 10.5 (4.5)** | 0.050 | <0.001 | 10.1(4.2) |
| Adulthood (Last exam) | |||||||
| Heart rate (beat/min) | 67.7 (8.1) | 69.9 (9.2)** | 68.3 (9.4) | 70.6 (9.5) | 0.560 | 0.433 | 69.1 (9.0) |
| Physical activity (n=733) | 3.3 (1.0) | 3.0 (1.1)** | 3.5 (1.1) | 3.5 (1.0) | 0.113 | <0.001 | 3.3(1.1) |
| TG/HDL-C (n=733) | 4.5 (4.5) | 2.7 (2.2)** | 3.2 (3.7) | 1.9 (1.1)** | 0.007 | <0.001 | 3.2(3.3) |
| HOMA (n=733) | 3.3 (3.3) | 2.5 (2.9)** | 3.0 (2.6) | 3.6 (3.6) | 0.414 | <0.001 | 3.0(3.2) |
| Current Smoker (%) | 28.1 | 30.1 | 39.2 | 32.4 | 0.037 | 0.573 | 30.9 |
| Years of smoking† | 18.9 (6.7) | 17.7 (5.6) | 18.6 (7.9) | 15.2 (7.2)* | 0.941 | 0.004 | 17.9(6.7) |
| afPWV (m/sec) | 5.4 (0.9) | 5.2 (0.8)* | 5.6 (1.0) | 5.5 (1.3) | 0.036 | <0.001 | 5.3(1.0) |
Gender difference within racial groups:
p<0.05;
p<0.01
BMI=body mass index; S(D)BP=systolic (diastolic) blood pressure; AUC=area under the curve; TG/HDL-C=triglycerides/high-density lipoprotein cholesterol; HOMA=homeostasis model assessment of insulin resistance; afPWV=aorta-femoral pulse wave velocity
, years of smoking of current smokers and ex-smokers
Tables 2 and 3 present standardized regression coefficients (β) derived from 4 separate linear regression models, with adult afPWV as a dependent variable and SBP and DBP values as independent variables, adjusting for covariates. As shown in Table 2, adult afPWV was significantly and positively associated with SBP levels in in terms of adulthood, total and incremental AUC values, but not with childhood SBP. BMI in childhood (model I) and in adulthood (model II) was not significantly associated with adult afPWV, whereas total (model III) and incremental (model IV) AUC values of BMI were significantly and positively associated with adult afPWV. Smoking was significantly associated with higher adult afPWV in all the 4 models. All values of DBP showed a very similar association pattern to SBP as shown in Table 3. The association strength of afPWV with adulthood and AUC values of BP did not differ significantly between blacks and whites; the p-values for race-BP interaction were 0.551–0.816 for SBP and 0.140–0.952 for DBP. The race-smoking interaction on afPWV was not significant either (p=0.709). In addition, smoking was significantly and positively associated with adulthood BP (β=0.117, P<0.001 for SBP; β=0.069, P=0.014 for DBP) and incremental AUC (β=0.146, P<0.001 for SBP; β=0.130, P<0.001 for DBP), but not with childhood BP and total AUC of BP. With respect to duration of smoking, years of smoking were significantly and positively associated with adult afPWV (β=0.067, P=0.027), adult SBP (β=0.109, P<0.001) and adult DBP (β=0.067, P=0.016).
Table 2.
Standardized regression coefficients (β) of SBP and smoking for adult aorta-femoral pulse wave velocity
| Independent Variable | Model I | Model II | Model III | Model IV | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Childhood BMIa | 0.011 | 0.749 | --- | --- | 0.062 | 0.092 | ||
| Childhood SBPa | 0.012 | 0.713 | --- | --- | 0.018 | 0.589 | ||
| Adulthood BMIb | --- | 0.056 | 0.084 | --- | --- | |||
| Adulthood SBPb | --- | 0.350 | <0.001 | --- | --- | |||
| Total AUC of BMIc | --- | --- | 0.104 | 0.002 | --- | |||
| Total AUC of SBPc | --- | --- | 0.201 | <0.001 | --- | |||
| Incremental AUC of BMId | --- | --- | --- | 0.132 | 0.001 | |||
| Incremental AUC of SBPd | --- | --- | --- | 0.173 | <0.001 | |||
| Adulthood age | 0.188 | <0.001 | 0.171 | <0.001 | 0.215 | <0.001 | 0.240 | <0.001 |
| Females | −0.090 | 0.005 | 0.010 | 0.757 | −0.083 | 0.006 | −0.081 | 0.008 |
| Blacks | 0.115 | <0.001 | 0.0003 | 0.991 | 0.120 | <0.001 | 0.122 | <0.001 |
| Heart rate | 0.124 | <0.001 | 0.101 | 0.001 | 0.110 | <0.001 | 0.112 | <0.001 |
| Current smoking | 0.098 | 0.002 | 0.076 | 0.011 | 0.092 | 0.002 | 0.080 | 0.010 |
BMI=body mass index; SBP=systolic blood pressure; AUC=area under the curve
, adjusted for childhood age and adulthood values and Z-transformed;
, Z-transformed;
, adjusted for average age and Z-transformed;
, adjusted for average age and Z-transformed
Table 3.
Standardized regression coefficients (β) of DBP and smoking for adult aorta-femoral pulse wave velocity
| Independent Variable | Model I | Model II | Model III | Model IV | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Childhood BMIa | 0.018 | 0.575 | --- | --- | 0.049 | 0.178 | ||
| Childhood DBPa | 0.045 | 0.166 | --- | --- | 0.045 | 0.154 | ||
| Adulthood BMIb | --- | 0.085 | 0.010 | --- | --- | |||
| Adulthood DBPb | --- | 0.284 | <0.001 | --- | --- | |||
| Total AUC of BMIc | --- | --- | 0.128 | <0.001 | --- | |||
| Total AUC of DBPc | --- | --- | 0.174 | <0.001 | --- | |||
| Incremental AUC of BMId | --- | --- | --- | 0.138 | <0.001 | |||
| Incremental AUC of DBPd | --- | --- | --- | 0.158 | <0.001 | |||
| Adulthood age | 0.188 | <0.001 | 0.171 | <0.001 | 0.214 | <0.001 | 0.233 | <0.001 |
| Females | −0.090 | 0.005 | −0.006 | 0.842 | −0.082 | 0.007 | −0.081 | 0.008 |
| Blacks | 0.115 | <0.001 | 0.025 | 0.423 | 0.120 | <0.001 | 0.121 | <0.001 |
| Heart rate | 0.123 | <0.001 | 0.101 | <0.001 | 0.105 | 0.001 | 0.110 | <0.001 |
| Current smoking | 0.100 | 0.002 | 0.098 | 0.001 | 0.103 | 0.001 | 0.090 | 0.004 |
BMI=body mass index; DBP=diastolic blood pressure; AUC=area under the curve
, adjusted for childhood age and adulthood values and Z-transformed;
, Z-transformed;
, adjusted for average age and Z-transformed;
, adjusted for average age and Z-transformed
Table 4 presents standardized regression coefficients (β) of SBP and smoking for afPWV derived from 4 separate linear regression models in a subset of 733 subjects by additionally adjusting for physical activity, TG/HDL-C ratio and HOMA index. Compared with the results in Table 2, the inclusion of these 3 covariates did not considerably change the effects of SBP and smoking on afPWV; however, total and incremental AUC of BM was no longer associated adult afPWV. With respect to DBP, the patterns of change in the regression coefficients were very similar to those for SBP (data not shown).
Table 4.
Standardized regression coefficients (β) of SBP and smoking for adult aorta-femoral pulse wave velocity, adjusting for additional covariates in a subset of 733 subjects
| Independent Variable | Model I | Model II | Model III | Model IV | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Childhood BMIa | 0.006 | 0.855 | --- | --- | 0.027 | 0.479 | ||
| Childhood SBPa | 0.013 | 0.703 | --- | --- | 0.026 | 0.436 | ||
| Adulthood BMIb | --- | −0.032 | 0.403 | --- | --- | |||
| Adulthood SBPb | --- | 0.354 | <0.001 | --- | --- | |||
| Total AUC of BMIc | --- | --- | 0.012 | 0.753 | --- | |||
| Total AUC of SBPc | --- | --- | 0.225 | <0.001 | --- | |||
| Incremental AUC of BMId | --- | --- | --- | 0.038 | 0.356 | |||
| Incremental AUC of SBPd | --- | --- | --- | 0.189 | <0.001 | |||
| Adulthood age | 0.217 | <0.001 | 0.160 | <0.001 | 0.203 | <0.001 | 0.226 | <0.001 |
| Females | −0.069 | 0.042 | 0.018 | 0.591 | −0.071 | 0.029 | −0.071 | 0.031 |
| Blacks | 0.098 | 0.003 | −0.001 | 0.972 | 0.100 | 0.002 | 0.102 | 0.002 |
| Heart rate | 0.107 | 0.001 | 0.092 | 0.003 | 0.097 | 0.002 | 0.099 | 0.002 |
| Physical activity | −0.039 | 0.243 | −0.022 | 0.487 | −0.021 | 0.518 | −0.024 | 0.456 |
| TG/HDL-C ratio | 0.060 | 0.090 | 0.040 | 0.234 | 0.055 | 0.106 | 0.053 | 0.121 |
| HOMA | 0.157 | <0.001 | 0.097 | 0.007 | 0.113 | 0.002 | 0.120 | <0.001 |
| Current smoking | 0.127 | <0.001 | 0.094 | 0.003 | 0.122 | <0.001 | 0.107 | 0.001 |
BMI=body mass index; SBP=systolic blood pressure; AUC=area under the curve; TG/HDL-C=triglycerides/high-density lipoprotein cholesterol; HOMA=homeostasis model assessment of insulin resistance
, adjusted for childhood age and adulthood values and Z-transformed;
, Z-transformed;
, adjusted for average age and Z-transformed;
, adjusted for average age and Z-transformed
Figures 2 and 3 illustrate differences in relationships between adult afPWV and SBP in terms of childhood and adulthood values (Figure 2) and total and incremental AUC values (Figure 3) among current smokers versus nonsmokers. The unstandardized regression coefficients (slopes) of afPWV on various SBP measures were derived from separate-slopes models among smokers and nonsmokers, adjusting for race, gender, age (average age for AUC values), BMI (total and incremental AUC of BMI for Figure 3, A and 3, B, respectively) and heart rate. The slope of afPWV with increasing values of adult SBP (Figure 2, B) was significantly greater among current smokers than among nonsmokers. The slope of afPWV with increasing incremental AUC of SBP (Figure 3, B) was significantly greater among current smokers than among nonsmokers, while the slope of afPWV with total AUC of SBP (Figure 3, A) showed a similar but nonsignificant trend (p=0.091). All the values of DBP showed a very similar pattern to SBP regarding the difference in slopes of afPWV, with slightly different slopes and p-values (data not shown). Furthermore, the inclusion of physical activity, TG/HDL-C ratio and HOMA index in the interaction models on 733 subjects did not considerably change the difference in slopes between smokers and nonsmokers (p=0.375 for childhood SBP; p<0.001 for adulthood SBP; p=0.037 for total AUC of SBP; p<0.001 for incremental AUC of SBP). Similar patterns of change in p-values were obtained for DBP in the interaction models by additionally adjusting for these 3 covariates (data not shown).
Figure 2.
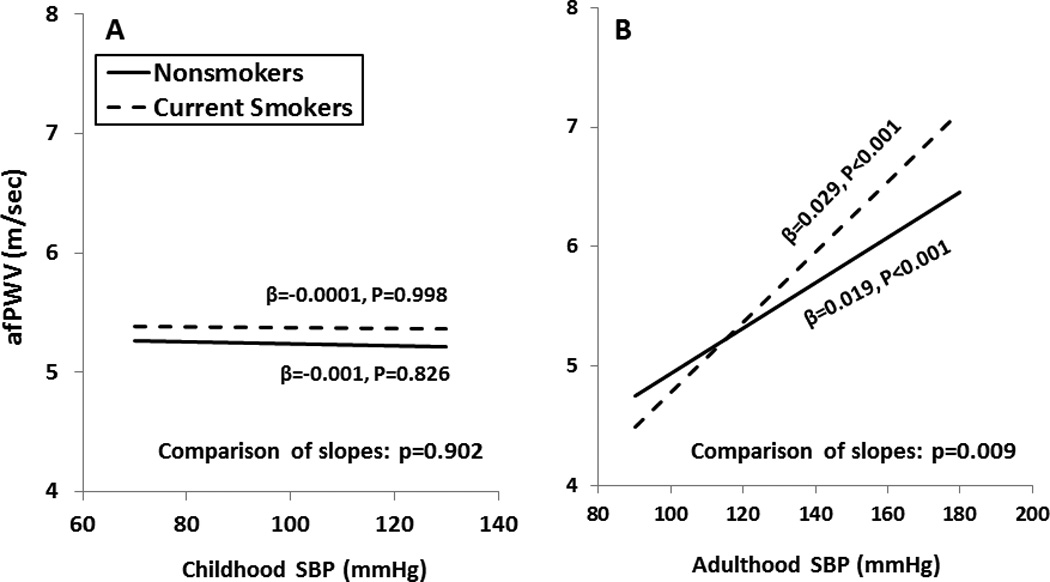
Relationship of adult afPWV with SBP in childhood and adulthood among current smokers and nonsmokers, adjusted for covariates as shown in Table 2
afPWV=aorta-femoral pulse wave velocity; SBP=systolic blood pressure
Figure 3.
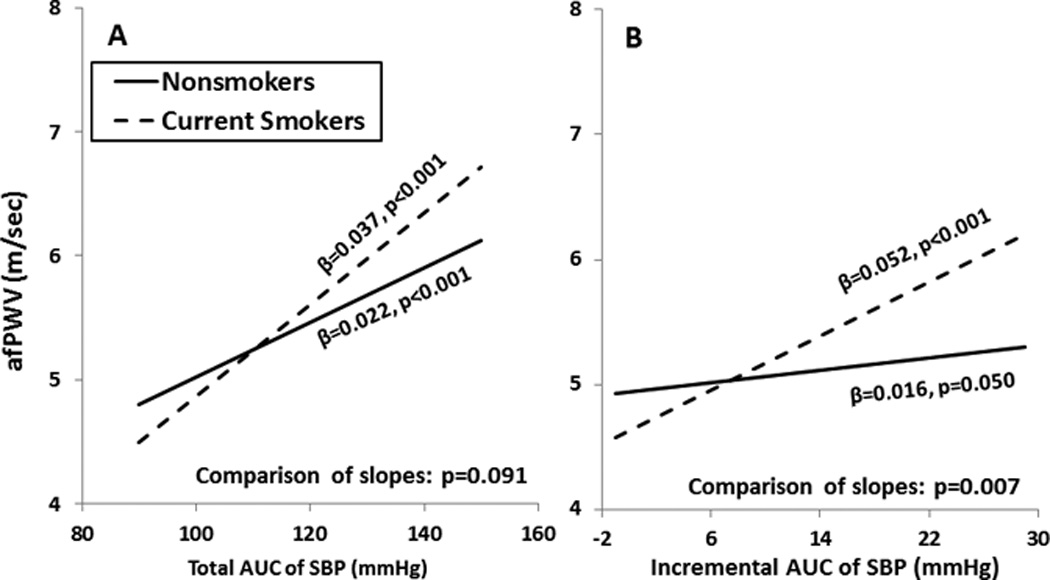
Relationship of adult afPWV with total and incremental AUC of SBP among current smokers and nonsmokers, adjusted for covariates as shown in Table 2
afPWV=aorta-femoral pulse wave velocity; AUC= the area under the curve; SBP=systolic blood pressure
Discussion
Arterial compliance and BP levels are hypertension-related traits and are influenced by the aging process and cardiovascular risk factors [3,24–27], which may in part explain the high inter-correlation between these two traits. Among traditional cardiovascular risk factors, age and BP were found to be consistently and independently associated with aortic PWV in 91% and 90% of studies, respectively, in a systematic review of 77 cross-sectional studies on risk factors of aortic PWV in adults [3]. Chronic smoking as a risk factor for increasing arterial stiffness has long been identified in the majority of previous studies; however, the influence of chronic smoking on arterial stiffness is not as strong as the effect of elevated BP [3,6]. The slightly inconsistent observations on the smoking-PWV association from previous studies point to the need of effort focusing on the joint effect of smoking and BP on increasing arterial stiffness. The strong associations of BP and smoking with increased adult afPWV noted in the current study are consistent with observations from previous studies [3,6]. Furthermore, childhood and adult BP as well as long-term cumulative burden and trends of BP from childhood were all significant predictors of adult afPWV. Importantly, the BP-afPWV association was stronger among current smokers than among nonsmokers. The findings of this study support the notion that the adverse long-term influence of BP levels on arterial stiffness from childhood to adulthood, and tobacco smoking accelerates this process.
It is generally considered that BP and arterial stiffness influence each other in the development of hypertension during different age periods based on vascular wall biology and hemodynamics. Higher tension in the arterial tree resulted from elevated SBP accelerates arterial stiffening due to an increased “wear and tear” on the artery wall [28,29], which, in turn, increases SBP and decreases DBP leading to widening of pulse pressure due to alterations of “buffering” function of the conduit artery walls in older individuals [30–32]. This artery stiffening-induced elevation of pulse pressure increases the risk of coronary heart disease since coronary artery perfusion occurs mainly during diastole [28,29]. Furthermore, both alterations in arterial structure and function and essential hypertension are considered to originate in childhood [10–12]; BP levels tend to track from childhood to adulthood, and increases in childhood BP levels are a strong predictor of adult hypertension [13,14] and arterial stiffness [15–17]. In the present study, strong and consistent associations were found between adult afPWV and long-term cumulative burden and increasing trends of BP from childhood to adulthood measured as total and incremental AUC, respectively. Childhood BP was not significantly associated with adult afPWV measured 27 years later after adjustment for adulthood BP although our previous [15] and other studies [16,17] reported that the adverse influence of elevated BP on arterial stiffening process began in early life.
The effect of acute, chronic and passive smoking on arterial stiffness has long been documented, and the reversible effect of smoking cessation on arterial stiffness has been reported in some longitudinal studies [5,6]. However, compared with BP, the smoking-PWV association is not so consistent. In a systematic literature review of 21 studies on chronic smoking and arterial stiffness measures, 13 (62%) studies found significant associations of chronic smoking with at least one arterial stiffness measures [6]. In a literature review on risk factors of aortic PWV in adults of 77 cross-sectional studies, 40 studies included smoking as a risk factor, only 5 (13%) studies reported a significant association between smoking and aortic PWV. In contrast, BP was found to be significantly associated with aortic PWV in 69 (90%) published papers among 77 studies [3]. In the present study, smoking was found to be a strong risk factor of afPWV in a community-based young adult cohort, but its effect size was smaller than BP based on comparison of standardized regression coefficients (Tables 2 and 3). These findings are consistent with our previous reports in a biracial cohort from the same community [33,34].
The slightly inconsistent results on the smoking-arterial stiffness association as summarized in the two literature reviews [3,6] may be in part due to several reasons, i.e. relatively smaller cumulative tobacco consumption in younger smokers, and different selection criteria of studies for review. Another important question in this regard still remains to be answered based on previous observations, that is, whether smoking can sensitize the vascular system response to other risk factors, e.g. related to atherosclerosis. In particular, whether smoking interacts with elevated BP, the strongest and most consistent risk factor, on arterial stiffness is not well known. Despite the extensive investigations on the relationships among smoking, BP and arterial stiffness, the data on the synergistic effect of smoking and high BP on arterial stiffness are limited to date, especially for the long-term cumulative burden of elevated BP. In a study involving 679 subjects aged 49 to 82 years (372 smokers and 307 nonsmokers), a significant interaction between smoking and BP was noted on arterial stiffness index [7]. The combined effect of hypertension and smoking was found to raise arterial stiffness more than either solitary factor in a review of four relevant studies [8]. In a study of short term antihypertensive treatment, cigarette smoking blunted the effect of amlodipine on the reduction of arterial stiffness [9]. Furthermore, it was found in this study that adult afPWV increased significantly faster with adult BP and longitudinal rate of increase of BP since childhood among current smokers than among nonsmokers. In addition, smoking was found to be significantly associated with elevated BP levels in adults in this study. Taken all evidence together, it seems that smoking and hypertension synergistically increase arterial stiffness. It is a generally acceptable concept that elevated BP and arterial stiffening influence each other with a bidirectional temporal relationship during the aging process. Thus, hypertension and arterial stiffness forms a vicious cycle [35]. Tobacco smoking, as one of the important risk factors related to both elements, seems to be involved in this cycle by elevating BP and consequently increasing arterial stiffness. In addition, it has been well established that smoking is closely associated with dyslipidemia, insulin resistance, impaired kidney function, chronic inflammation, oxidative stress and endothelial dysfunction, and these pathophysiologic pathways contribute to the arterial stiffening process. Therefore, the interactive effect of smoking and elevation of BP is also potentially through one or more of these mechanisms.
This community-based longitudinal study has certain limitations. First, exclusion of hypertensives under treatment resulted in a loss of information because these patients represented a subgroup with the highest BP levels. Second, physical activity, lipid and insulin resistance data were available only in a subset of 733 subjects, and the physical activity level outside of work was used as a simple indicator in the current study. Further investigation is needed to confirm the results in this regard.
In conclusion, the current study demonstrates that long-term cumulative burden and trends of BP from childhood to adulthood are all powerful predictors of adult arterial stiffness measured as afPWV; smoking was significantly associated with adult elevated BP levels. Importantly, adult afPWV increases faster with adult BP and longitudinal rate of increase of BP among current smokers than among nonsmokers. These results support the notion that the adverse long-term influence of BP levels on arterial stiffness begins in childhood, and tobacco smoking accelerates this process. These findings underscore the importance of undertaking preventive strategies for cardiovascular disease and hypertension early in life and smoking behavior control.
Acknowledgments
This study was supported by grants 5R01ES021724 from National Institute of Environmental Health Science, 2R01AG016592 from the National Institute on Aging and 13SDG14650068 from American Heart Association.
Abbreviations
- afPWV
aorta-femoral pulse wave velocity
- AUC
the area under the curve
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
Footnotes
Authors do not have conflict of interest.
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 4.Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16:2518–2525. doi: 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]
- 5.Takami T, Saito Y. Effects of smoking cessation on central blood pressure and arterial stiffness. Vasc Health Risk Manag. 2011;7:633–638. doi: 10.2147/VHRM.S25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res. 2010;33:398–410. doi: 10.1038/hr.2010.25. [DOI] [PubMed] [Google Scholar]
- 7.Liang YL, Shiel LM, Teede H, Kotsopoulos D, McNeil J, Cameron JD, McGrath BP. Effects of blood pressure, smoking, and their Interaction on carotid artery structure and function. Hypertension. 2001;37:6–11. doi: 10.1161/01.hyp.37.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Scallan C, Doonan RJ, Daskalopoulou SS. The combined effect of hypertension and smoking on arterial stiffness. Clin Exp Hypertens. 2010;32:319–328. doi: 10.3109/10641960903443558. [DOI] [PubMed] [Google Scholar]
- 9.Matsui Y, Kario K, Ishikawa J, Hoshide S, Eguchi K, Shimada K. Smoking and antihypertensive medication: interaction between blood pressure reduction and arterial stiffness. Hypertens Res. 2005;28:631–638. doi: 10.1291/hypres.28.631. [DOI] [PubMed] [Google Scholar]
- 10.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 11.Lever AF, Harrap SB. Essential hypertension: a disorder of growth with origins on childhood. J Hypertens. 1992;10:101–120. doi: 10.1097/00004872-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Voors AW, Berenson GS. Search for the determinants of the early onset of essential hypertension. In: Onesti G, Kim KE, editors. Phasic Pressor Mechanisms: Hypertension in the Young and the Old. New York: Grune and Stratton; 1981. pp. 43–55. [Google Scholar]
- 13.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao W, Threefoot S, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: The Bogalusa Heart Study. Am J Hyperten. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the Bogalusa Heart Study. Hypertension. 2004;43:541–546. doi: 10.1161/01.HYP.0000115922.98155.23. [DOI] [PubMed] [Google Scholar]
- 16.Aatola H, Hutri-Kähönen N, Juonala M, Viikari JS, Hulkkonen J, Laitinen T, et al. Lifetime risk factors and arterial pulse wave velocity in adulthood: the Cardiovascular Risk in Young Finns Study. Hypertension. 2010;55:806–811. doi: 10.1161/HYPERTENSIONAHA.109.145102. [DOI] [PubMed] [Google Scholar]
- 17.Aatola H, Magnussen CG, Koivistoinen T, Hutri-Kähönen N, Juonala M, Viikari JS, et al. Simplified Definitions of Elevated Pediatric Blood Pressure and High Adult Arterial Stiffness. Pediatrics. 2013;132:2012–3426. doi: 10.1542/peds.2012-3426. [DOI] [PubMed] [Google Scholar]
- 18.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC. Cardiovascular Risk Factors in Children. The Early Natural History of Atherosclerosis and Essential Hypertension. Oxford: Oxford University Press; 1980. pp. 47–123. [Google Scholar]
- 19.Elkasabany AM, Urbina EM, Daniels SR, Berenson GS. Prediction of adult blood pressure by K4 and K5 diastolic blood pressure in children: the Bogalusa Heart Study. J Pediatr. 1998;132:687–692. doi: 10.1016/s0022-3476(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 20.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Morrison JA, Barton BA, Biro FM, Sprecher DL. The conjoint trait of low high-density lipoprotein cholesterol and high triglycerides in adolescent black and white males. Metabolism. 1998;47:514–521. doi: 10.1016/s0026-0495(98)90233-5. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Li S, Cook NR, Rosner BA, Srinivasan SR, Boerwinkle E, Berenson GS. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:462–469. doi: 10.1038/sj.ijo.0802610. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Li S, Boerwinkle E, et al. Gender-specific influence of endothelial nitric oxide synthase gene on blood pressure since childhood: The Bogalusa Heart Study. Hypertension. 2004;44:668–673. doi: 10.1161/01.HYP.0000145474.23750.2b. [DOI] [PubMed] [Google Scholar]
- 24.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, et al. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23. doi: 10.1016/s0895-7061(01)02228-2. [DOI] [PubMed] [Google Scholar]
- 25.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, Berenson GS. Correlates of carotid artery stiffness in young adults: The Bogalusa Heart Study. Atherosclerosis. 2004;176:157–164. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 26.McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, et al. Anglo-Cardiff Collaboration Trial Investigators. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III) Hypertension. 2010;56:591–597. doi: 10.1161/HYPERTENSIONAHA.110.156950. [DOI] [PubMed] [Google Scholar]
- 27.Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: establishing normal and reference values. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dart AM, Kingwell BA. Pulse pressure—a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 29.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 30.Izzo JL., Jr Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19:341–352. doi: 10.1097/01.hco.0000126581.89648.10. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises, Part I: Aging Arteries: A “Set Up” for Vascular Disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 32.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Racial (black-white) divergence in the association between adiponectin and arterial stiffness in asymptomatic young adults: the Bogalusa heart study. Am J Hypertens. 2008;21:553–557. doi: 10.1038/ajh.2008.14. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Srinivasan SR, Chen W, Xu JH, Li S, Berenson GS. Vascular abnormalities in asymptomatic, healthy young adult smokers without other major cardiovascular risk factors: the Bogalusa Heart Study. Am J Hypertens. 2005;18:319–324. doi: 10.1016/j.amjhyper.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Tomiyama H, Yamashina A. Arterial stiffness in prehypertension: a possible vicious cycle. J Cardiovasc Transl Res. 2012;5:280–286. doi: 10.1007/s12265-011-9345-4. [DOI] [PubMed] [Google Scholar]