Abstract
Mesenchymal stem cells are good candidates for the clinical application of bone repair because of their osteogenic differentiation potential, but in vivo osteoinduction potential should be verified for culture expanded cells before clinical application.
This study analyzed in vivo bone formation by MSCs quantitatively after implantation of MSCs planted porous biphasic ceramic cubes into athymic mice. MSCs were divided into osteogenic differentiation-induced and normal groups and also tested in vitro to evaluate the degree of differentiation into osteoblast. The osteogenic induced group showed higher alkaline phosphatase and calcium level in vitro and corresponding higher level of bone formation in vivo compared to control group. Whereas there was no bone formation observed in fibroblast-implanted negative control group.
In critical sized bone defect models, commonly used for evaluation of bone regeneration ability, it is difficult to distinguish between osteoinduction and osteoconduction, and quantitative analysis is not simple. However, this method for evaluating osteoinduction is both accurate and simple.
In conclusion, the analysis of in vivo bone formation using porous ceramic cubes is a powerful and simple method for evaluating the osteoinduction ability of target cells and, furthermore, can be applied for evaluation of scaffolds for their osteoinductive properties.
Keywords: Mesenchymal stem cells, Osteoinduction, Quantitative analysis, Cube score, Porous ceramic cube
1. Introduction
Mesenchymal stem cells (MSCs) in bone marrow support the micro-environment for hematopoiesis but also maintain diverse differentiation potential (Devine and Hoffman, 2000; Majumdar et al., 1998). Culture expanded MSCs differentiate into mesodermal lineages including osteoblasts, adipocytes, chondrocytes and myocytes in vivo after transplantation (Liechty et al., 2000; Mackenzie and Flake, 2001; Shake et al., 2002) and also differentiate into such lineages in vitro with proper supplements such as dexamethasone (Beresford et al., 1992; Gimble et al., 2008).
The diverse flexibility of MSC differentiation potential has driven extensive studies for the recovery of damaged tissues or organs in the field of regenerative medicine and produced many fruitful results, especially in skeletal system (Ankrum and Karp, 2010; Veronesi et al., 2012). Autograft is widely used for reconstruction of damaged bone tissue, but limitations in graft amounts make it difficult or impossible to repair large fracture. Culture expanded MSCs may be used to cover this limitation (Pourebrahim et al., 2013) but evaluation of cell characteristics and osteogenic potential are needed after long-term culture or large scale expansion.
Alkaline phosphatase activity and osteoblast marker genes, such as RUNX2 and osteopontin are widely used for evaluation of osteoblastic differentiation of MSCs in vitro (Chen et al., 1997; Komori, 2010) but genuine, histologically identifiable bone formation is impossible to produce in vitro, so the expression of such markers in vitro cannot guarantee bone formation in vivo.
Transplanted MSCs can be differentiate into osteoblasts and produce bone in isogenic tissue, but this process influenced by the complex in vivo micro-environment as well as by target cell characteristics. Qualitative analysis of ectopic bone formation is essential for meaningful evaluation of osteoinduction ability of transplanted MSCs. To more accurately evaluate the osteoinductive ability of culture expanded MSCs, with or without osteo-induction, we quantified new bone formation by MSCs after implantation of MSCs combined with biphasic ceramic cubes into athymic mice
2. Materials and methods
2.1. Isolation and culture of mesenchymal stem cells
Human bone marrow specimens were obtained from the iliac crest of patients undergoing non-emergency orthopedic surgery through an Internal Review Board approved protocol at Yeungnam University Hospital. An equal volume of Dulbecco's modified Eagle medium with low glucose (DMEM-LG; Sigma, St Louis, MO, USA) was mixed with the specimen and centrifuged at 450 ×g for 10 min. The pellets were suspended in DMEM-LG and fractionated on a density gradient centrifuge with density 1.08 Percoll (Sigma) solution at 480 ×g for 15 min. The low density, upper nucleated cell layer was collected and washed with 10% FBS (FBS; Gibco-BRL, Rokville, MD, USA) supplemented DMEM then plated at 1.8×105 cells per cubic centimeter. When cultures became confluent, cells were trypsinized and replated at 4×103 cells per cubic centimeter for continued passage. Second passage cells were used for experimentation.
Cells were cultivated in DMEM-LG with 10% FBS only for Fibroblast (Fib) and normal MSCs cultures (Con): 100 nM dexamethasone (Sigma), 50 µM ascorbic acid phosphate (Wako Chemical, Osaka, Japan) and 10 mM beta-glycerophospahate (Sigma) were supplemented for osteogenic induction culture (Osteo).
2.2. Flow cytometry
Cultured MSCs were analyzed for stem cell marks. The cells were trypsinized and incubated in primary antibodies at room temperature for 30 min. Mouse anti-human CD105 (Abcam, Cambridge, UK), mouse anti-human CD90 (Abcam), mouse anti-human CD73 (Abcam), mouse anti-human CD45 (Abcam), were used for primary antibodies. After incubation with secondary FITC-labeled anti-mouse IgG (Sigma) in the dark for 30 min, cells were analyzed using FACScane fluorescence-activated cell sorter (Becton Dickinson, Franklin Lakes, NJ, USA) equipped with Cell Quest software (Becton Dickinson).
2.3. Scanning electron microscopy
Samples were fixed in 2.5% glutaraldehyde (Polysciences, Niles, IL, USA), followed by further fixation in 1% osmium tetroxide (Polysciences) in 0.1 M PBS and dehydration in graded series of ethanol. After dehydration, samples were critical-point dried, mounted on aluminum stubs with conductive silver paint, and then sputtered with Pt-Pd in ion sputter (Hitachi, Naka, Japan). The samples were observed using a scanning electron microscope (Hitachi) at 25 keV.
2.4. In vivo analysis of bone formation
Biphasic porous tricalcium phosphate-hydroxyapitite (60:40) ceramic with mean pore size of 200 µm donated from Zimmer (Warsaw, IN, USA) were cut into 3×3×3 mm and sonicated in distilled water to remove dust particles. Autoclaved sterile ceramic cubes were immersed in 100 µg/ml human fibronectin (Sigma) and dried overnight in a laminar flow hood. Human dermal fibroblasts and MSCs were trypsinized, rinsed, counted and suspended in DMEM-LG without serum at 5×106 cells/ml. Fibronectin-coated ceramic cubes were added to a tube containing cell suspensions and incubated at 37°C for 2 hr with mixing every 20 min, and then implanted into mice for standard MSC bone assay. For osteogenic inducing studies, cell-loaded cubes were incubated for 3 weeks in osteogenic induction or normal culture to examine the bone formation ability of different cell groups. Cubes were transferred to a 24-well culture dish and switched to each media. After 3 week’s culture, cubes (n=8 in each groups) were implanted subcutaneously on dorsum of the mice. After 6 weeks the ceramic cubes were harvested (preliminary test showed increase of bone formation reached plateau after 6 weeks), fixed for 24 hr with 10% neutral buffered formalin phosphate, washed overnight in tap water, decalcified in RDO (Apex Engineering, Plainfield, IL, USA), paraffin embedded and serial sectioned at 5 µm thickness. Hematoxylin-eosin or toluidine blue stained samples were analyzed for bone formation
Every 10th section was collected and evaluated a score of 0–5 based on a visual estimate of the percentage of bone positive pores. Briefly, a score of 0 means only trace or no bone was formed; a score of 1 means bone formed in more than one pore; a score of 2 means bone formed in more than two separated regions; a score of 3 means great than two regions but less than 30% of the pores contain bone; a score of 4 means 30–50% of pores contained bone, and a score 5 means 50–100% pores contain bone. The cumulative scores of a cube were divided by the number of sections studied.
2.5. In vitro Assay
Osteogenic differentiation levels of MSCs cultured in ordinary or osteogenic induction media were assayed to evaluate osteoinduction potential of MSCs before in vivo transplantation. Alkaline Phosphatase (APase) activity was measured in quadriplicate cultures in 12 well plates. PBS washed cells were incubated for 5–10 min in 500 µl of 5 mM p-nitrophenyl phosphate (Sigma) in 50 mM glycine and 1 mM MgCl2 solution adjusted to pH 10.5. An equal volume of 0.1 M NaOH was added to stop the reaction after sufficient color development was detected. APase activity was measured by absorbance at 405 nm on a plate reader (Molecular Device, CA, USA) and absolute amounts of enzyme activity were calculated from a p-nitrophenol standard (Sigma). The enzyme activity per unit cells was expressed as nM/min/mg by dividing ALP activity per min by the DNA content of the well. After APase assays, all the culture plates were placed in −70°C after washing with PBS. After all samples were collected, the plates were thawed and 500 µl 0.1 M NaOH was added to each well. The samples were then refrozen, thawed and an equal volume of neutralization buffer (5 M NaCl, 100 mM Na2HPO4, 2 mM EDTA, 0.1 N HCl) was added. Aliquots of 100 µl were loaded into 96 well plates and an equal volume of 1 µM/ml Hoechst dye (Sigma) was added, and DNA content was measured in a Spectra® MAX Gemine EM fluorescent measurement plate reader (Molecular Device) equipped with Soft Max PRO software (Molecular Device) at 360 nm excitation, 460 nm emission. The absolute amount of DNA was calculated from a calf thymus DNA (Amersham Bioaciences, Piscataway, NJ, USA) standard curve.
APase staining was accomplished with an ELF97®Endogenous Phosphatase Detection Kit from Molecular Probes (Eugene, OR, USA). Briefly, cells grown on coverslips were fixed in 4% neutral buffered formalin for 10 min and treated 10 min in Tween 20. Enzyme staining was done by incubating in substrate solution for 90 sec followed by stopping buffer and counter staining the nuclei with 100 nM DAPI (Sigma) in PBS for 5 min.
Measurement of calcium deposition was done with a Calcium Assay Kit from Biotron Diagnostics (Hemet, CA, USA) in triplicated culture in 12 well plates. Cultures were fixed in 10% neutral buffered formalin for 30 min and washed three times with distilled water, air dried and stored at 4°C. When all samples were collected, calcium in each well was extracted by shaking overnight in 1 ml 0.6 of N HCl. After centrifugation at 2000 ×g for 10 min, the supernatant was used for calcium detection according to the manufacturer’s instructions and read on a microplate reader at 570 nm absorbance. DNA content was measured with parallel cultured MSCs and relative calcium deposition per cells was expressed as µg/mg by dividing calcium content of the well by DNA amount of the corresponding well.
Von Kossa staining was used to detect calcium phosphate deposits. Cell cultures were fixed with 10% neutral buffered formalin for 30 min. After rinsing with distilled water, the cultures were incubated in 2% silver nitrate (Sigma) for 10 min in the dark and exposed to bright light for 15 min. After washing thoroughly with distilled water, the samples were dehydrated with 100% ethanol and air dried.
2.6. Statistical analysis
Statistical analysis was conducted using SPSS 20 software (SPSS Inc., Chicago, IL, USA). Cube score was assessed for significant differences via ANOVA analysis with Duncan’s post-hoc analysis but DNA, APase, calcium assay were assessed via general linear model with Duncan’s post-hoc analysis. A P value of less than 0.01 was regarded as statistically significant.
3. Results
3.1. Cell proliferation and characteristics
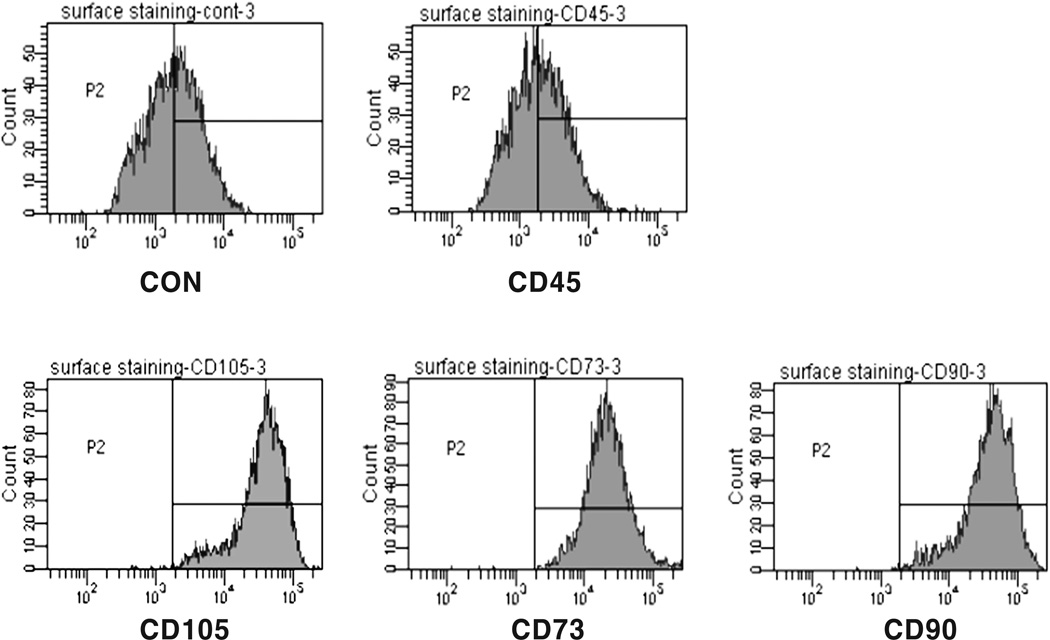
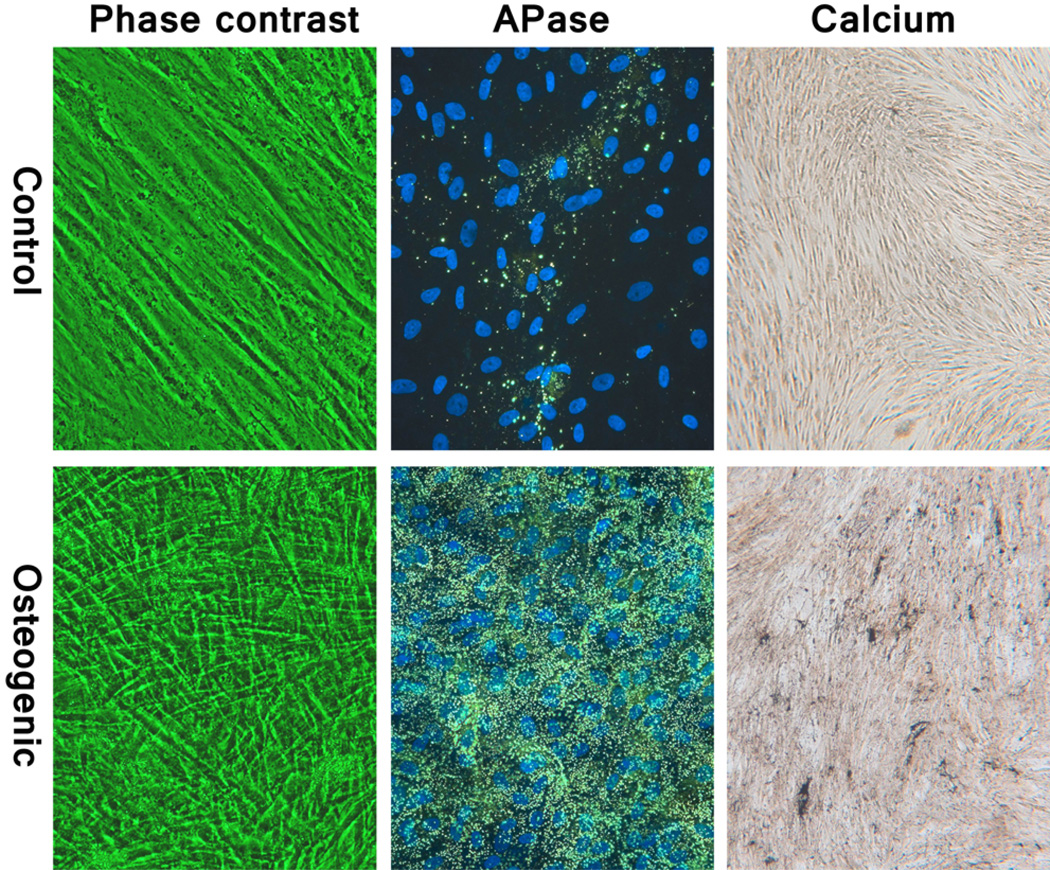
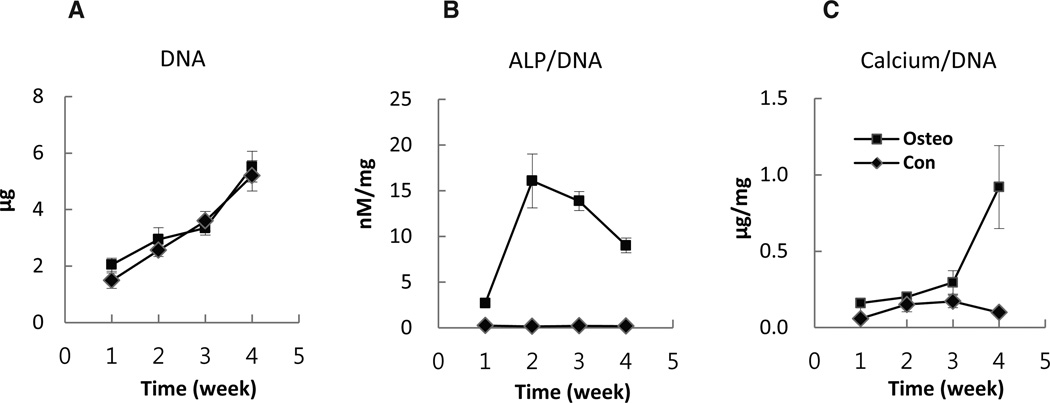
Mesenchymal stem cell markers, CD90, CD73, and CD105, were highly positive while the hematopoietic cell marker, CD 45, was negative (Fig. 1). The fibroblastic-like morphology of the MSCs changed into polygonal shape 1 day after switching to osteogenic medium. When cells became over-confluent, parallel arranged spindle shaped cells became thinner but rarely piled up in Con, whereas disordered polygonal cells became multi-layered in differentiation induced Osteo (Fig. 2). Cell proliferation patterns were similar between Con and Osteo, and increased about three times at 4th weeks (Fig. 7A).
Fig. 1.
Flowcytometric analysis of surface CD markers expression on cultured MSCs. Mesenchymal stem cell markers, CD90, CD73, and CD105, were highly positive while the hematopoietic cell marker, CD 45, was negative.
Fig. 2.
Phase contrast micrographs of 4 week sample (left); fluorescent micrographs of alkaline phosphatase stain (middle); and bright field micrographs of Von Kossa stained samples (right) of control (upper) and osteogenic induced (lower) MSCs. The Con group retained its spindle shape whereas the Osteo group became more polygonal in shape and became multi-layered. The Osteo group also showed strong APase activity and calcium deposition.
Fig. 7.
Proliferation of MSCs cultured with osteogenic inducing medium (Osteo) or normal medium (Con) evaluated by DNA content in the wells (A). Alkaline phosphatase (ALP) activity per unit MSCs evaluated by dividing ALP activity of each well into DNA content of the well (B). Osteo groups maintained a significant higher level of activity than Con (P<0.01) and peaked at 2–3 weeks. Calcium deposition per unit MSCs evaluated by dividing calcium content of each well into DNA content of parallel well (C). The Osteo group showed significantly higher level of calcium deposition (P<0.01) and increase rapidly after 3 weeks. Bars represent mean±SD.
3.2. In vivo assay Cube scoring
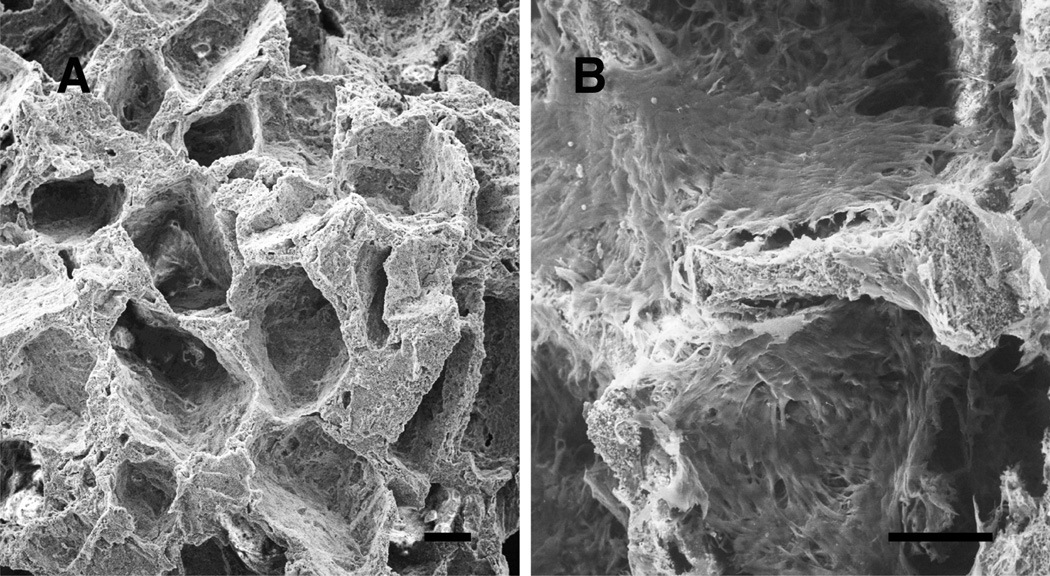
After three weeks of culture, SEM images showed that MSCs in ceramic cubes were healthy and had formed a cell sheet which covered the entire surface of the ceramics but there was no noticeable difference among Fib, Con, and Osteo groups (Fig. 3).
Fig. 3.
Scanning electron micrographs of biphasic ceramic cube (A) and a cube after 3 weeks culture with MSCs (B). Well grown MSCs made a cell sheet on the surface of the ceramic. Bars indicate 100 µm.
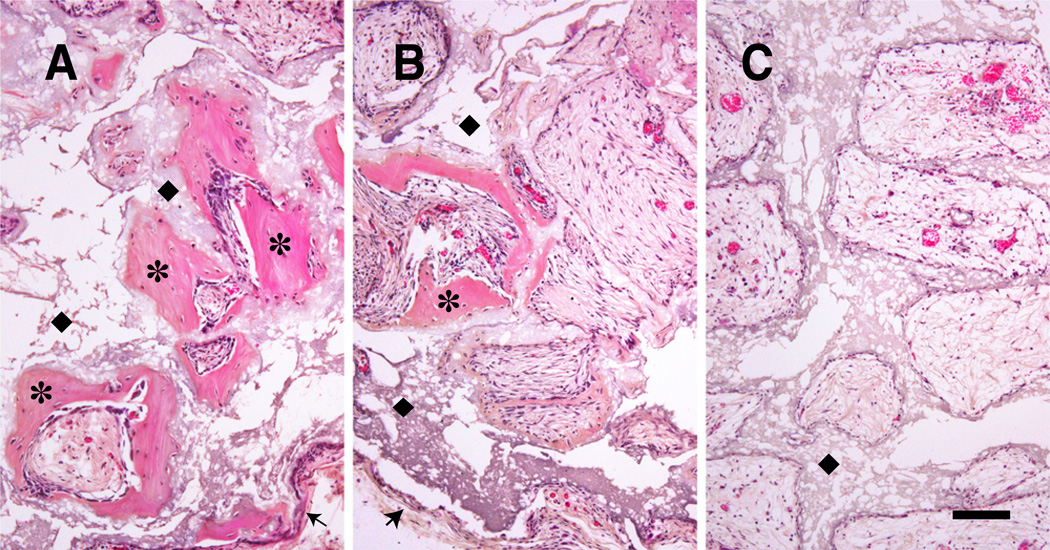
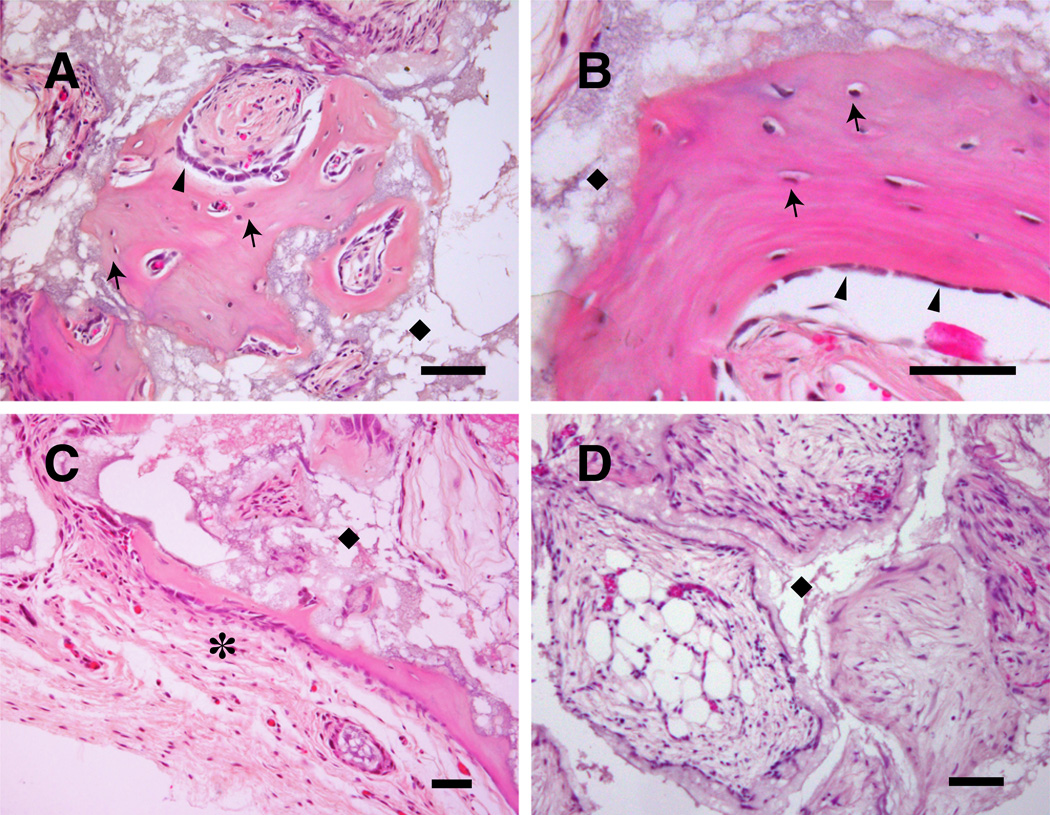
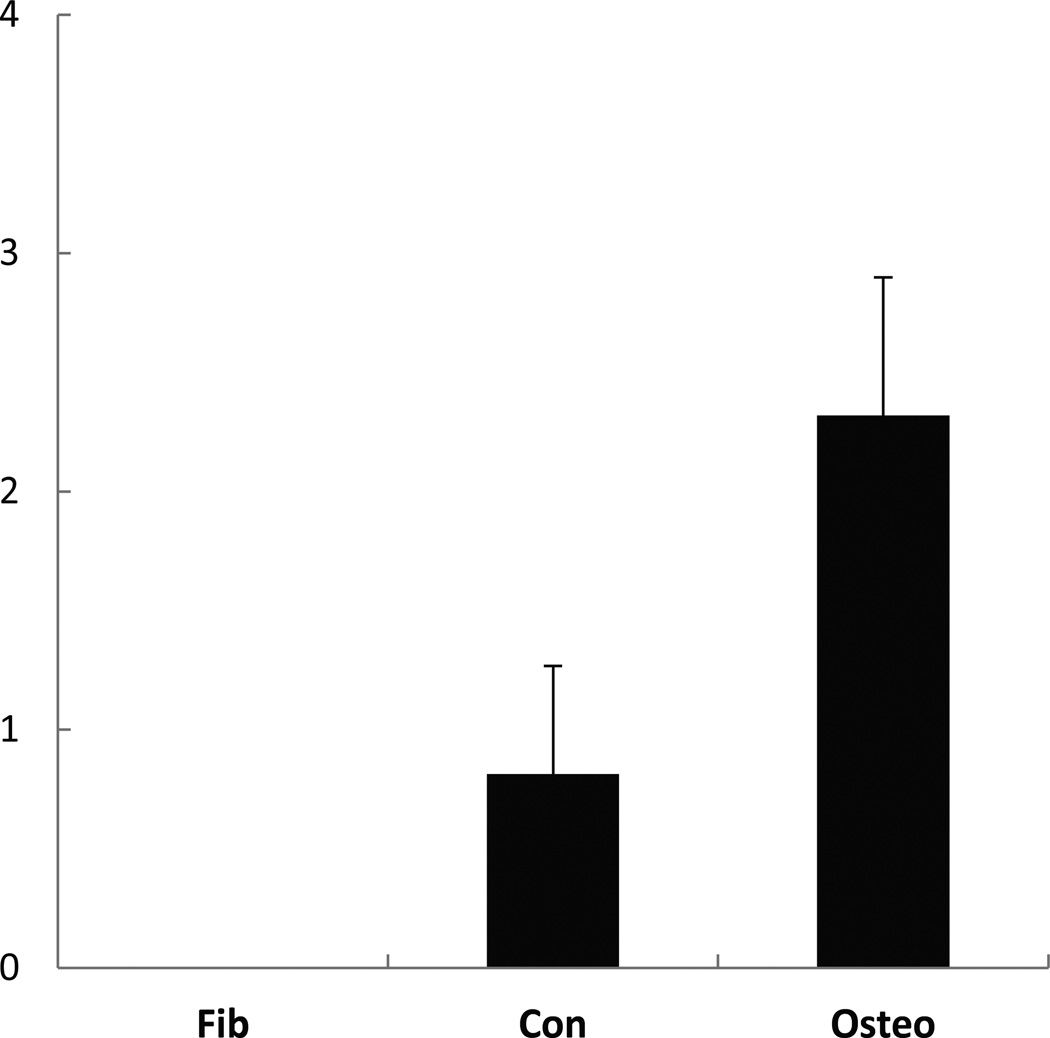
Histologic images of transplanted cubes showed a capsular structure of connective tissue surrounding cubes. Cavities were occupied by loose connective tissue or adipose tissue including vessels, and bone formation was observed in some cavities. The space occupied by ceramics was remained as “ghost” area as result of decalcification. The fibroblast-implanted negative control group (Fib) showed no bone formation with all cavities occupied by typical loose connective tissue or adipose tissue. Whereas the Con group showed a similar pattern to the negative control but bone formation was observed in some cavities. The Osteo group was characterized by extensive bone formation. Osteocytes occupied in lacunae and bone matrix displayed a typical lamellar pattern with palisading arrangement of osteoblasts was observed. There was more bone formation in peripheral cavities than in the central region, and the bone spicules were variable in size and shape (Fig. 4, 5). The quantified bone formation by cube scores were 0.0: 0.8: 2.3 for Fib: Con: Osteo groups respectively (Fig. 6).
Fig. 4.
Micrographs of porous ceramic cubes 6 weeks after implantation. Cavities were usually occupied by loose connective tissue including vessels. Extensive bone formation observed in osteogenic induced MSCs-planted cube (A) and some bone formation observed in normal MSCs-planted cube (B) but no bone formation is observed in fibroblasts-planted cube (C). ✶: New bone formation by MSCs in the cavities of the cubes.◆; Ghost area as result of decalcification where ceramic was located. Bars indicate 100µm.
Fig. 5.
Magnified histologic images of osteogenic induced group (A–C). Osteocytes occupied in lacunae (arrows) and bone matrix displayed a typical lamellar pattern with palisading arrangement of osteoblasts (arrow heads) was observed. The space occupied by ceramic was remained as “ghost” area after decalcification (◆). Cube was surrounded by capsular structure (✶). All cavities were occupied by loose connective tissue and adipose tissue in fibroblasts-planted cubes (D). Bars indicate 50 µm.
Fig. 6.
Cube score, representing osteoinductive ability of the cells in ceramic cubes 6 weeks after implantation to athymic mice (n=8). Three groups showed bone formation in significant difference (P<0.01). Bars represent mean±SD.
3.3. In vitro Assay
APase activity in osteogenic induced Osteo group was at a high level from first week and showed rapid increase in the 2nd week then diminished thereafter. DNA adjusted APase activity per unit cell peaked at 2 to 3 weeks and decreased but still maintained at a high level compared to the Con group (Fig. 7B). APase stain by ELF showed positivity in only small number of the cells in Con plates, but a majority cells showed strong positive in Osteo. The reaction granules were fine and small in the 1 week samples but became intense and strong after 2 weeks (Fig. 2). Quantitative analysis showed no significant calcium deposition detected in Con at any time point, while the Osteo group showed a slight increase at week 3 and a dramatic increase at 4th week (Fig. 7C). Von Kossa staining for calcium phosphate showed no meaningful positive reaction in Con, but there was a strong positive reaction detected at the 4th week in Osteo (Fig. 2).
4. Discussion
During the last 10 years, many investigators have improved the technique for isolation and replication of MSCs from bone marrow and other tissues for regeneration of damaged tissue or organs (Bernardo et al., 2011; Park et al., 2011). The tendency for MSCs to easily differentiate into bone and cartilage led to early studies in this field which advanced to clinical applications for bone and cartilage repair. The effects of MSC therapy works through two different mechanisms: directly, by supplying fully differentiated cells from MSCs or, indirectly, by the effects of MSC-derived soluble factors. However, in a majority of cases in the skeletal system, repairs have been accomplished from the direct differentiation into bone or cartilage (Ankrum and Karp, 2010).
Bone matrix proteins osteocalcin, osteopontin, bone sialoprotein or alkaline phosphatase are crucial to the actual process of bone deposition and mineralization and are used widely as indicators of osteogenic differentiation of MSCs in the field of cell biology. Even though these markers cannot guarantee bone formation in vivo, these proteins are important markers for osteogenic differentiation. APase increased to a higher level in Osteo and reached a peak level at 2–3 weeks, which lead to calcium deposition at 4th week. The general progression is that calcium deposition follows the APase peak. The APase peak indicated the initiation of osteoblast differentiation and our previous study also indicated that 3 week’s culture in osteogenic supplements was enough to induce osteogenic differentiation (Song et al., 2009). With the in vitro results, the Osteo group of MSC-seeded cubes had proved to have higher osteoinductive capacity and was predicted be produce great bone formation after transplantation. As expected, no bone formation was observed in the fibroblast-implanted negative control group, but some bone formation observed in Con. Bone formation in Con would be the result of spontaneous differentiation of some MSCs into osteoblasts. There was extensive bone formation observed in the Osteo group. This result showed that the amount of in vivo bone formation correlated with the osteoinductive capacity of transplanted cells.
In these experiments, we cultured cell implanted cubes for 3 weeks to distinguish bone formation ability of different ell groups but in actual application, 2 hours culture is sufficient for cell attachment to cubes before implantation. Harvest time of cubes also can be modified according to experimental goals. In critical size skull defect or segmental long bone fracture studies widely used to evaluate bone repair, it is often difficult to distinguish between osteoinduction from osteoconduction because of endogenous osteogenic cells that migrate in from neighboring bone and periosteum. In addition, quantitative analysis is often complicated and time-consuming. The porous ceramic cubes analysis method of the in vivo osteoinduction by MSCs provides an accurate measure of the osteogenic potential of MSCs.
Conclusively analysis of in vivo bone formation using porous ceramic cubes was determined to be a powerful and simple method for evaluating osteoinductive ability of target cells and, furthermore, can be applied to the evaluation of scaffolds for their osteoinductive properties.
Highlights.
-
-
Quantified in vivo bone formation for MSCs seeded porous ceramic cubes
-
-
Useful method for evaluating the osteoinduction ability of target cells
-
-
Simple and accurate than critical sized bone defect model for analysis of osteoinduction
-
-
Can be applied for evaluation of scaffolds for their osteoinductive properties.
Acknowledgement
This research was supported by a grant of Yeungnam University Medical Center (2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Cometa AM, Pagliara D, Vinti L, Rossi F, Cristantielli R, Palumbo G, Locatelli F. Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol. 2011;24:73–81. doi: 10.1016/j.beha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr. Opin. Hematol. 2000;7:358–363. doi: 10.1097/00062752-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus Med Hemother. 2008;35:228–238. doi: 10.1159/000124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- Mackenzie TC, Flake AW. Multilineage differentiation of human MSC after in utero transplantation. Cytotherapy. 2001;3:403–405. doi: 10.1080/146532401753277571. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell. Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Park DG, Kim KG, Lee TJ, Kim JY, Sung EG, Ahn MW, Song IH. Optimal supplementation of dexamethasone for clinical purposed expansion of mesenchymal stem cells for bone repair. J. Orthop. Sci. 2011;16:606–612. doi: 10.1007/s00776-011-0114-7. [DOI] [PubMed] [Google Scholar]
- Pourebrahim N, Hashemibeni B, Shahnaseri S, Torabinia N, Mousavi B, Adibi S, Heidari F, Alavi MJ. A comparison of tissue-engineered bone from adipose-derived stem cell with autogenous bone repair in maxillary alveolar cleft model in dogs. Int. J. Oral Maxillofac. Surg. 2013;42:562–568. doi: 10.1016/j.ijom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- Song IH, Caplan AI, Dennis JE. In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J. Orthop. Res. 2009;27:916–921. doi: 10.1002/jor.20838. [DOI] [PubMed] [Google Scholar]
- Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2012;22:181–192. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]