Abstract
Context
Circadian variation is a fundamental characteristic of plasma glucocorticoids, with a postprandial rise in cortisol an important feature. The diurnal rhythm is presumed to reflect alterations in hypothalamic-pituitary-adrenal axis activity; however, cortisol is produced not only by the adrenal glands but also by regeneration from cortisone by the enzyme 11β-hydroxysteroid dehydrogenase type 1, mainly in liver and adipose tissue.
Objective
We tested the contribution of peripheral cortisol regeneration to macronutrient-induced circadian variation of plasma cortisol in humans.
Design
This was a randomized, single-blinded, crossover study.
Setting
The study was conducted at a hospital research facility.
Participants
Eight normal-weight healthy men participated in the study.
Interventions
Subjects were given isocaloric energy isodense flavor-matched liquid meals composed of carbohydrate, protein, fat, or low-calorie placebo during infusion of the stable isotope tracer 9,11,12,12-[2H]4-cortisol.
Outcome Measures and Results
Plasma cortisol increased similarly after all macronutrient meals (by ~90 nmol/L) compared with placebo. Carbohydrate stimulated adrenal secretion and extra-adrenal regeneration of cortisol to a similar degree. Protein and fat meals stimulated adrenal cortisol secretion to a greater degree than extra-adrenal cortisol regeneration. The increase in cortisol production by 11β-hydroxysteroid dehydrogenase type 1 was in proportion to the increase in insulin. The postprandial cortisol rise was not accounted for by decreased cortisol clearance.
Conclusions
Food-induced circadian variation in plasma cortisol is mediated by adrenal secretion and extra-adrenal regeneration of cortisol. Given that the latter has the more potent effect on tissue cortisol concentrations and that effects on adrenal and extra-adrenal cortisol production are macronutrient specific, this novel mechanism may contribute to the physiological interplay between insulin and glucocorticoids and the contrasting effects of certain diets on postprandial metabolism.
Mechanisms that determine contrasting fuel metabolism in the fasted and fed state are central to our understanding of metabolic (patho)physiology. Although long-established mechanisms, such as insulin signaling, have central and potent effects, the increasing complexity of metabolic control has been recognized in recent decades. For example, dietary constituents induce differential effects, particularly on satiety, and novel mediators have been shown to modulate metabolic responses, notably the incretin hormones (1). Glucocorticoids have long been recognized as permissive regulators of metabolic responses, with medium- and long-term effects on the balance between catabolism and anabolism in different tissues (2). Whether glucocorticoids influence acute responses to fasting and feeding and might contribute to differential effects of dietary constituents has, however, received scant attention.
Adrenal cortisol secretion is controlled by the hypothalamic-pituitary-adrenal axis, with peak plasma cortisol concentrations on wakening and a nadir during sleep. In addition to diurnal variation, it is well known that circulating cortisol concentrations rise following each meal (3, 4). Some reports have suggested protein to be the primary mediator of this effect (5, 6); however, carbohydrate (7) and fat (8) are also implicated. Tissue glucocorticoid levels are further controlled by the 11β-hydroxysteroid dehydrogenase enzymes (9); the type 2 isozyme (11β-HSD2) is expressed primarily in the kidney and converts cortisol to inactive cortisone to prevent glucocorticoid activation of mineralocorticoid receptors, whereas the type 1 isozyme (11β-HSD1) is present mainly in the liver, adipose tissue, and brain and regenerates cortisol from cortisone, amplifying local tissue glucocorticoid levels. Cortisol regenerated by 11β-HSD1 is released into the bloodstream and so impacts on circulating in addition to tissue concentrations (Figure 1). Indeed, using stable isotope tracers, we and others have shown that cortisol production by 11β-HSD1 is similar to the adrenal glands in healthy men at rest, each generating approximately 40 nmol/min (10, 11).
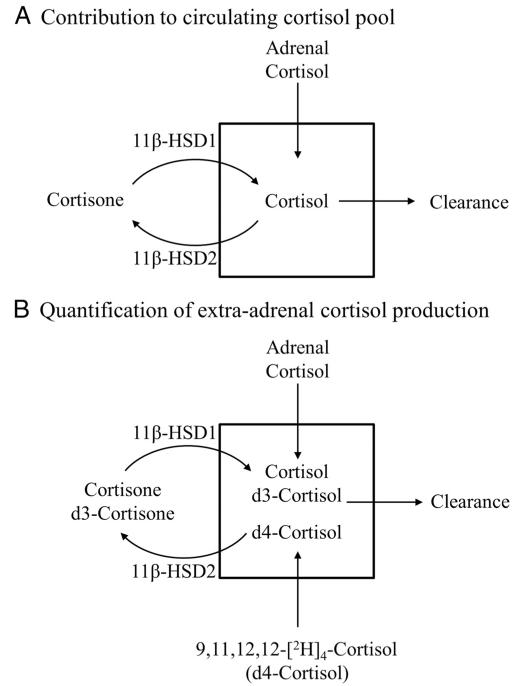
Figure 1.
Use of deuterated cortisol tracer to quantify the contribution of adrenal and extra-adrenal tissues to the circulating cortisol pool. The circulating cortisol pool (A) is determined by three sources: adrenal cortisol production, cortisol regeneration by 11β-HSD1, and cortisol clearance predominantly by the hepatic A-ring reductases and by 11β-HSD2. A rise in circulating cortisol may be due to either increased production by the adrenals or by 11β-HSD1 or to reduced cortisol clearance. The stable isotope tracer d4-cortisol can be used to determine the contribution of these three sources (B). On removal of the deuterium from the 11 position by 11β-HSD2, d4-cortisol is converted to d3-cortisone, which then forms d3-cortisol once regenerated by 11β-HSD1. d3-Cortisol can be formed only by 11β-HSD1 so can be used to quantify cortisol production by 11β-HSD1, whereas d4-cortisol cannot be regenerated and therefore can be used to quantify cortisol clearance.
An additional component regulating circulating cortisol concentrations is the rate of metabolism and clearance of cortisol, primarily by the hepatic A-ring reductases (5α- and 5β-reductases). Cortisol production by 11β-HSD1 and metabolism by 5α- and 5β-reductases are altered during weight-loss diets, with differential effects according to dietary macronutrient content (12–15). Moreover, cortisol production by 11β-HSD1 acutely increases after a mixed meal (16) and after iv insulin/glucose infusion (17). However, no previous studies have examined the relative contribution of adrenal secretion, extra-adrenal regeneration, and clearance to the rise in circulating cortisol after a meal or tested whether the effects differ between dietary constituents.
We hypothesized that individual dietary macronutrients differentially regulate postprandial adrenal and extra-adrenal cortisol production. To test this, we fed lean healthy men three different isocaloric single-macronutrient meals during a deuterated cortisol tracer infusion (18) to determine how specific macronutrients regulate adrenal and extra-adrenal cortisol production and to quantify the relative contribution of the adrenal, 11β-HSD1 and cortisol metabolism to the postprandial rise in circulating cortisol.
Materials and Methods
Eight healthy men were recruited to a randomized, single-blind, crossover study. Inclusion criteria were as follows: aged 18–70 years; body mass index 20–25 kg/m2; no significant medical conditions; on no regular medications; alcohol intake less than 21 U/wk; no exposure to glucocorticoid therapy within the previous 6 months; and normal screening blood tests (full blood count, glucose, kidney, liver, and thyroid function). Local ethical approval was obtained as was informed consent from each participant. Subjects attended the clinical research facility after an overnight fast on four occasions, each separated by 1 week. Subjects were instructed to follow a weight-maintenance diet containing 50% carbohydrate (percentage total energy intake), 20% protein, and 30% fat from 3 days prior to their first visit until the end of their participation in the study and to avoid alcohol and exercise for 24 hours prior to each study visit. Subjects completed food diaries for the study duration.
At each visit, measurements were performed of body weight, height, blood pressure (BP), and body fat mass (using an Omron BF-302 bioimpedance monitor). Two 21-gauge cannulae were inserted into each antecubital fossa for tracer infusion and blood sampling, respectively, and blood was collected for fasting glucose, insulin, lipids, and cortisol. An infusion of 9,11,12,12-[2H]4-cortisol (d4-cortisol) (Cambridge Isotopes) was commenced at 0.35 mg/h for 390 minutes at 8:30 am, after a 0.7 mg priming bolus. Blood was sampled frequently during steady state (t + 180–210 min), and then subjects received one of four similarly flavored liquid meals in random order: either one of three isocaloric energy isodense meals (561 kcal in 500 mL) with different macronutrient content (high carbohydrate, high protein, or high fat) or alternatively a placebo drink with minimal caloric content (Table 1). All the test meals were prepared using dietetic products normally used to supplement feeding. The high-carbohydrate meal consisted of concentrated carbohydrate supplement drink (Polycal Liquid; Nutricia Clinical Care). The high-protein meal contained powdered milk protein and soy lecithin (Vitapro; Vitaflo International Ltd), but due to palatability did not contain pure protein. The high-fat meal consisted of a fat emulsion made from vegetable oil (Calogen Liquid; Nutricia Clinical Care). Raspberry flavoring (Flavor Pac; Vitaflo International Ltd) was added to each meal, and none of the subjects was able to discriminate between the test meals. The meal was consumed within 10 minutes (12:00–12:10 pm). After the meals, blood sampling continued until t + 390 minutes (Figure 2). Subjects were given 100 mL water hourly to aid bladder voiding, and urine samples were collected at baseline and then at t + 60, t + 120, t + 210, t + 270, t + 330, and t + 390 minutes.
Table 1.
Composition of Test Meals
| Test Meal | Meal Composition | Carbohydrate, g | Protein, g | Fat, g | Total Energy, kcal |
|---|---|---|---|---|---|
| High carbohydrate | Polycal liquid, 215 mL | 132.6 | 0 | 0 | 531 |
| Water, 285 ml | 0 | 0 | 0 | 0 | |
| Flavor Pac × 2, 8 g | 7.5 | 0 | 0 | 30 | |
| Total content, g (% total calories) | 140.5 (100) | 0 (0) | 0 (0) | 561 (100) | |
| High protein | Vitapro, 136 g | 12.2 | 102.0 | 8.2 | 531 |
| Water, 500 mL | 0 | 0 | 0 | 0 | |
| Flavor Pac × 2, 8 g | 7.5 | 0 | 0 | 30 | |
| Total content, g (% total calories) | 19.7 (14) | 102.0 (73) | 8.2 (13) | 561 (100) | |
| High fat | Calogen, 118 mL | 0 | 0 | 59.0 | 531 |
| Water, 382 mL | 0 | 0 | 0 | 0 | |
| Flavor Pac × 2, 8 g | 7.5 | 0 | 0 | 30 | |
| Total content, g (% total calories) | 7.5 (5) | 0 (0) | 59 (95) | 561 (100) | |
| Placebo | Water, 500 mL | 0 | 0 | 0 | 0 |
| Flavor Pac × 2, 8 g | 7.5 | 0 | 0 | 30 | |
| Total content, g (% total calories) | 7.5 (100) | 0 (0) | 0 (0) | 30 (100) |
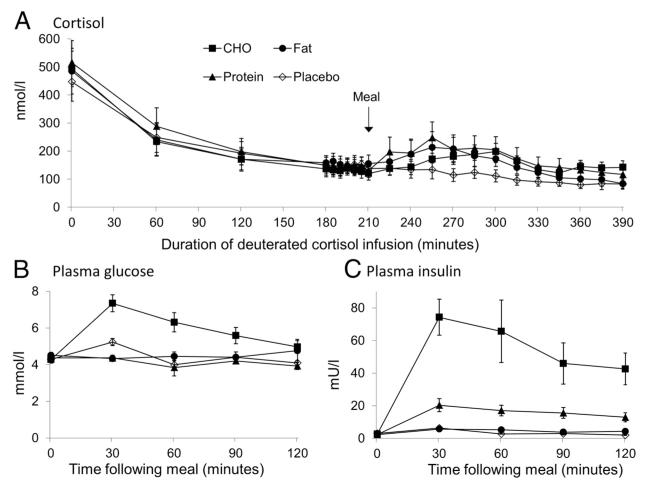
Figure 2.
Hormone concentrations before and after meals. Data are mean ± SEM for plasma cortisol (A), glucose (B) and insulin (C) concentrations prior to and after carbohydrate (CHO, filled squares), fat (filled circles), protein (filled triangles), and placebo (open diamonds) meals (n = 8 for all meals). A deuterated cortisol infusion was commenced at t = 0 minutes, with the meals each given at t + 210 minutes. Cortisol, glucose, and insulin were all similar prior to the meal. Plasma cortisol followed its usual circadian pattern and fell during the morning and then increased after all meals compared with placebo (all P < .05). Glucose and insulin significantly increased after CHO compared with the three other meals (both P < .001), and insulin levels were increased after protein compared with fat and placebo meals (P < .05).
Laboratory analyses
Plasma and urine samples were frozen at −80°C until analysis. Plasma cortisol, d4-cortisol, d3-cortisol, cortisone, and d3-cortisone were measured by liquid chromatography-tandem mass spectrometry as previously described (19). Serum lipids and plasma glucose were measured on an Olympus Diagnostics analyzer using enzymatic colorimetric methods. Plasma insulin was measured by ELISA (DRG Diagnostics).
For measurement of urinary steroids by gas chromatography triple quadrupole mass spectrometry, derivatization of steroids was performed as described previously (18). Analysis was performed on a Quantum gas chromatography triple quadrupole mass spectrometer (Thermoelectro) using a DB17MS column (30 m, 0.25 mm, 0.25 μm; Agilent Technologies Ltd). Samples were injected by programmed temperature vaporizing injection at 120°C, and the injector temperature increased to 270°C at a rate of 14.5°C/min. The oven temperature (120°C initially) was increased (by 30°C/min) to 200°C and then increased (by 5°C/min) to 280°C and then by 2°C/min to achieve a final temperature of 300°C, which was sustained for 1 minute. The auxiliary line and source temperatures were both 280°C. The mass spectrometry was operated in electron impact (70 eV), using argon as a collision gas. The following derivatized steroids were analyzed using multiple reaction monitoring (precursor → product ions, collision energy): 5β-tetrahydrocortisol (β-THF), 5α-tetrahydrocortisol (α-THF), and epi-tetrahydrocortisol (652 → 562; 15 V), 5β-tetrahydrocortisone (THE) (578 → 488; 15 V), cortisol (F) and epi-cortisol (605 → 515; 20 V), cortisone (531 → 441; 15 V), d8-cortisone (539 → 449; 15 V); and the remainder by selected ion monitoring: d4-β-THF and d4-α-THF (656), d3-β-THF and d3-α-THF (655), d3–5β-THE (581), d4-F (609), d3-F (608), and d3-cortisone (534). Quantitation was performed by interpolation on to a calibration curve of the relevant peak area ratio (of analyte vs internal standards) plotted against an amount of standard.
Kinetic calculations
Kinetic analysis in each subject was performed for steady-state measurements using the mean of seven samples obtained from t + 180–210 minutes. After the three isocaloric meals, the change in cortisol kinetics was calculated by subtracting the values obtained after placebo from those at each individual time point. The rate of appearance of cortisol (Ra cortisol) was calculated using equation 1, where [cortisolt] is the cortisol concentration at time t.
| (Equation 1) |
Results obtained using nonsteady state equations did not significantly alter the results. The rate of appearance of d3-cortisol (Ra d3-cortisol, a specific measure of cortisol production by 11β-HSD1) was calculated using equation 2.
| (Equation 2) |
Clearance of d4-cortisol was determined using equation 3.
| (Equation 3) |
The contribution of 11β-HSD1 to total Ra cortisol at steady state (ss) was calculated using equation 4 and to the peak rise in Ra cortisol after the meal using equation 5 by extrapolating from the rise in Ra d3-cortisol. Assuming that cortisol production by 11β-HSD1 is linearly correlated with cortisone substrate availability and that d3-cortisone has a similar affinity for 11β-HSD1 as endogenous cortisone, Ra cortisol secondary to 11β-HSD1 reductase activity can be calculated from Ra d3-cortisol by adjusting for the relative concentrations of the substrates cortisone and d3-cortisone.
| (Equation 4) |
| (Equation 5) |
The contribution of the adrenal glands was calculated by subtracting the contribution from 11β-HSD1 from the total Ra cortisol.
Statistical analyses
Statistical analysis was performed using R version 2.14.0. The area under the curve was calculated for each subject on each diet using the kulife package. The area under the curve by macronutrient was log transformed as necessary and used as the dependent variable in linear mixed-effects models, with the subject as the grouping factor and the placebo as the dummy variable, in the nlme package. P < .05 was considered significant. Data are presented as mean ± SEM. The study had greater than 90% power to detect a 10% difference in the Ra cortisol and d3-cortisol, based on previous measurements (12).
Results
Characteristics of participants
Subjects were aged 23.5 ± 2.3 years with blood pressure of 123 ± 3/68 ± 2 mm Hg, a body mass index of 22.4 ± 0.4 kg/m2, and total body fat mass of 8.7 ± 1.1 kg. At baseline, fasting plasma glucose was 4.7 ± 0.1 mmol/L and cholesterol and triglycerides were 3.8 ± 0.3 mmol/L and 0.9 ± 0.1 mmol/L, respectively. Body weight, fat mass, fasting glucose, and lipid profile were unaltered between visits.
Plasma glucose and insulin after meals
Glucose and insulin levels were similar prior to each meal (Figure 2). Plasma glucose levels rose significantly after the carbohydrate meal (P < .001 vs other meals) but not after protein, fat, or placebo. Plasma insulin concentrations rose significantly after carbohydrate (P < .001 vs other meals) and to a lesser extent after protein (P < .05 vs placebo and fat).
Cortisol kinetics
Prior to each meal, cortisol concentrations (Figure 2), Ra cortisol, Ra d3-cortisol (Table 2), and d4-cortisol clearance (Figure 3) did not differ between visits. Steady-state enrichments of d4-cortisol with cortisol and d3-cortisol were achieved by 180 minutes of the d4-cortisol infusion.
Table 2.
Relative Contribution of 11β-HSD1 and the Adrenals to Cortisol Appearance Rates
| Carbohydrate |
Protein |
Fat |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol Appearance Rates, nmol/min | Steady State (Before Meal) | Postprandial Peak (t = 285 min) | Placebo-Corrected Peak (t = 285 min) | Steady State (Before Meal) | Postprandial Peak (t = 255 min) | Placebo-Corrected Peak (t = 255 min) | Steady State (Before Meal) | Postprandial Peak (t = 255 min) | Placebo-Corrected Peak (t = 255 min) | Placebo Steady State (Before Meal) |
| Ra cortisol | 34.9 ± 8.2 | 59.5 ± 2.8a | 27.8 ± 10.4b | 36.3 ± 7.9 | 76.5 ± 18.1a | 36.9 ± 17.0b | 38.4 ± 6.5 | 55.9 ± 12.3a | 18.0 ± 8.1b | 34.8 ± 6.6 |
| Ra d3-cortisol | 15.5 ± 1.4 | 22.7 ± 2.3c | 5.5 ± 1.2d | 15.2 ± 1.5 | 19.2 ± 1.7c | 2.3 ± 1.3 | 16.6 ± 1.6 | 19.2 ± 1.6 | 0.8 ± 1.0 | 15.1 ± 1.1 |
| Ra cortisol by 11β-HSD1 | 17.7 ± 2.1 | 26.5 ± 2.8c | 11.9 ± 2.4e | 20.4 ± 3.5 | 28.4 ± 4.6a | 7.4 ± 2.7b | 19.3 ± 2.1 | 23.2 ± 3.6a | 5.1 ± 2.6b | 17.1 2.1 |
| Ra cortisol by adrenals | 17.2 ± 4.7 | 33.0 ± 4.3 | 15.9 ± 9.5 | 15.9 ± 2.7 | 48.1 ± 14.3a | 29.4 ± 16.2b | 19.1 ± 3.2 | 32.7 ± 9.0a | 12.9 ± 9.8 | 17.7 ± 2.9 |
The contribution of 11β-HSD1 to total Ra cortisol was calculated at steady state and at postprandial peak values using equations 4 and 5. There was no rise in Ra cortisol after the placebo meal; hence, only the steady state values are presented. The adrenal contribution to total Ra cortisol was calculated by subtracting the contribution of 11β-HSD1 from Ra cortisol.
P < .05 vs steady state.
P < .05 vs placebo.
P < .01 vs steady state.
P < .001 vs placebo.
P < .01 vs placebo.
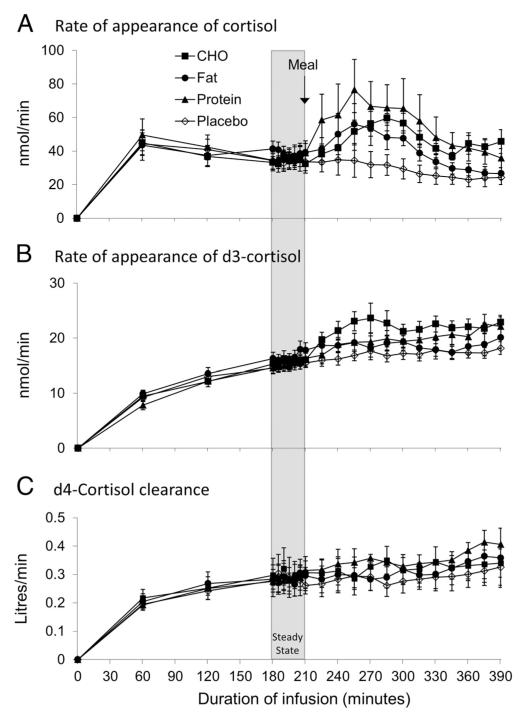
Figure 3.
Adrenal and extra-adrenal cortisol production. Data are mean ± SEM for Ra cortisol (A), rate of appearance of d3-cortisol (Ra d3-cortisol) (B), and d4-cortisol clearance (C) after carbohydrate (CHO, filled squares), fat (filled circles), protein (filled triangles), and placebo (open diamonds) meals (n = 8 for all meals). Ra cortisol was increased after all three meals vs placebo (P < .05). Ra d3-cortisol was increased by CHO compared with all other meals (P < .001) and by protein compared with placebo and fat meals (P < .05). d4-cortisol clearance was increased after the high-protein meal (P < .05 vs placebo).
After the placebo meal, plasma cortisol continued to fall, following normal diurnal variation (Figure 2). Ra d3-cortisol (a specific measure of cortisol production by 11β-HSD1) and d4-cortisol clearance were both unchanged after placebo (Figure 3, B and C).
After the carbohydrate meal, plasma cortisol rose compared with placebo (P < .05). Cortisol concentrations peaked 75 minutes after the meal (change from placebo +98 ± 44 nmol/L) and remained increased for the remainder of the study protocol (Figure 2). Similarly, Ra cortisol (a measure of cortisol production from any source) was increased after carbohydrate (change from placebo +27.8 ± 10.4 nmol/min, P < .05) (Figure 3A). In addition, the Ra d3-cortisol was substantially increased after carbohydrate (peak +5.5 ± 1.2 nmol/min vs placebo, P < .001 vs all other meals) (Figure 3B). d4-Cortisol clearance was unchanged compared with placebo (Figure 3C).
After the high-protein meal, plasma cortisol rose compared with placebo (P < .05) and similarly to the rise after carbohydrate (Figure 2). Cortisol concentrations peaked 45 minutes after the meal (change from placebo +97 ± 41 nmol/L), and Ra cortisol was similarly increased (peak change from placebo +36.9 ± 17.0 nmol/min, P < .05) (Figure 3A). Ra d3-cortisol was increased but to a lesser extent than after carbohydrate and peaked 175 minutes after the meal (peak +5.1 ± 1.2 nmol/min vs placebo, P < .01) (Figure 3B). d4-Cortisol clearance was increased after the protein meal compared with placebo (peak +0.08 ± 0.05 L/min, P < .05) (Figure 3C).
After the fat meal, circulating cortisol rose compared with placebo (P < .05) and similarly to the rise after the carbohydrate and protein meals (Figure 2). Cortisol levels peaked 45 minutes after the meal (change from placebo +87 ± 33 nmol/L), while Ra cortisol was also increased (peak change from placebo +18.0 ± 8.1 nmol/min, P < .05) (Figure 3A). However, Ra d3-cortisol was not increased (+0.8 ± 1.0 nmol/min compared with placebo). d4-Cortisol clearance was unchanged compared with placebo (Figure 3C).
Contribution of adrenal and extra-adrenal production to the postprandial rise in plasma cortisol
The ratio of cortisone to d3-cortisone during steady state was approximately 1.2 and did not significantly vary between visits (Supplemental Figure 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). At steady state before meals, cortisol production by 11β-HSD1 and by the adrenals each accounted for approximately 50% of total Ra cortisol (Table 2).
The contribution of 11β-HSD1 reductase activity to the peak postprandial rise in Ra cortisol was calculated using equation 5. The ratio of cortisone to d3-cortisone increased after all three meals from approximately 1.2 to approximately 1.5, whereas it fell, following normal diurnal variation after placebo (Supplemental Figure 1). The rise in Ra cortisol attributable to extra-adrenal production by 11β-HSD1 was 11.9 ± 2.4 nmol/min after carbohydrate (P < .01 vs placebo/fat, P < .05 vs protein), 7.4 ± 2.7 nmol/min after protein (P < .05 vs placebo), and 5.1 ± 2.6 nmol/min after fat (P < .05 vs placebo). Correspondingly, the rise in Ra cortisol attributable to adrenal secretion was 15.9 ± 9.5 nmol/min after carbohydrate (P = .06), 29.4 ± 16.2 nmol/min after protein (P < .05), and 12.9 ± 9.8 nmol/min after fat (P = .27).
Cortisol clearance by A-ring reductases and 11β-HSD2
To determine whether altered 5α-reductase, 5β-reductase, or 11β-HSD2 activity was responsible for the increased cortisol clearance after protein, tetrahydro- and free deuterated cortisol, and cortisone metabolites were quantified in urine sampled during tracer infusion. The sum of each deuterated glucocorticoid metabolite was calculated prior to (comprising baseline/t +60/t +120/t +210 min samples) and after each meal (t +270/t +330/t +390 min samples) (Table 3). The urinary deuterated glucocorticoid metabolites did not vary between diets either before or after meals. Urinary excretion of endogenous glucocorticoid metabolites was also unchanged between diets.
Table 3.
Urinary Excretion of Deuterated Glucocorticoid Metabolites During d4-cortisol Infusion
| Metabolites, ng | Carbohydrate | Protein | Fat | Placebo |
|---|---|---|---|---|
| d4-5β-THF | 322 ± 67 | 246 ± 51 | 343 ± 68 | 256 ± 50 |
| d3-5β-THF | 253 ± 59 | 198 ± 42 | 272 ± 54 | 205 ± 42 |
| d4-5α-THF | 134 ± 39 | 111 ± 24 | 143 ± 32 | 87 ± 22 |
| d3-5α-THF | 114 ± 35 | 97 ± 23 | 121 ± 28 | 75 ± 20 |
| d3-5β-THE | 345 ± 150 | 218 ± 65 | 318 ± 94 | 216 ± 39 |
| Total deuterated tetrahydrometabolites | 1168 ± 315 | 869 ± 161 | 1197 ± 195 | 839 ± 94 |
| d4-Cortisol | 7 ± 1 | 6 ± 1 | 5 ± 1 | 4 ± 2 |
| d3-Cortisol | 15 ± 2 | 21 ± 8 | 14 ± 3 | 10 ± 2 |
| d3-Cortisone | 109 ± 27 | 74 ± 17 | 100 ± 24 | 132 ± 20 |
Data are mean ± SEM for urinary excretion of deuterated 5β-THF, 5α-THF, 5β-THE, and urinary free deuterated cortisol and cortisone after high-carbohydrate, -protein, -fat, and -placebo meals (n = 8 all groups). Urinary deuterated metabolites were summed from samples collected at t + 270, t + 330, and t + 390 min time points. There were no differences in excretion of any deuterated metabolites between diets either before or after the meals.
Discussion
These data show that circulating cortisol increases post-prandially to a similar degree after meals containing any of the three major macronutrients (carbohydrate, protein, and fat). In these lean healthy men, eating a liquid meal at midday containing approximately 560 kcal increased circulating cortisol by approximately 90 nmol/L. However, the underlying mechanisms differ. Using a stable isotope cortisol tracer, we demonstrated macronutrient-specific effects on adrenal and extra-adrenal cortisol production. Carbohydrate substantially increased cortisol regeneration by 11β-HSD1 for a given d3-cortisone substrate concentration compared with the other three meals, while protein had a lesser effect. d3-Cortisone concentrations were similar between the macronutrient meals. However, cortisone concentrations rose similarly after all three macronutrient meals, most likely due to increased adrenal cortisol production providing a greater substrate for 11β-HSD2. After accounting for the change in cortisone, cortisol regeneration by 11β-HSD1 was significantly increased after the fat meal and similar to that observed after protein. All three meals also substantially increased cortisol secretion from the adrenal glands, which accounted for the majority (70%– 80%) of the cortisol rise after both protein and fat. The adrenal glands and 11β-HSD1 each accounted for approximately 50% of the postprandial cortisol rise after carbohydrate, although the increase in adrenal secretion was not statistically significant.
Most previous relevant studies have given subjects mixed meals with varying proportions of macronutrients (3–5, 8, 20), which makes the effect of single macronutrients difficult to interpret. In this study the carbohydrate and fat meals were composed only of one macronutrient. However, our trial meal, which contained 100% protein, was unpalatable, so we used a high-protein meal in which protein accounted for 73% of energy content, with carbohydrate (14%) and fat (13%) accounting for the remainder. The timing of the cortisol peaks differed between meals (Figure 2); therefore, placebo corrected values were used for the comparisons rather than absolute values to ensure that diurnal variation of cortisol did not influence the results.
Although circulating cortisol concentrations are similar after meals with different macronutrient contents, the changes in 11β-HSD1 predict that tissue cortisol levels are more substantially elevated after meals with a high carbohydrate content. The most likely tissue responsible for this extra-adrenal cortisol regeneration is the liver, which accounts for the vast majority (~95%) of whole-body 11β-HSD1 activity (19, 21). Subcutaneous adipose tissue (19) and skeletal muscle (22) might also contribute but are unlikely to account in full for the rise in Ra d3-cortisol (~6 nmol/min) because this is far greater in magnitude than the baseline d3-cortisol generation in these tissues. As confirmed in animal models (23), increased liver 11β-HSD1 can substantially increase glucocorticoid action and may contribute to metabolic adaptation to a carbohydrate load, for example, by increasing recycling between glucose-6-phosphate and glycogen (24).
Insulin is an obvious candidate mediator of the rise in 11β-HSD1 activity after the meals. First, the increase in cortisol production by 11β-HSD1 paralleled the change in circulating insulin concentrations, which was greatest after carbohydrate with a smaller rise after protein (Figures 2C and 3B). In addition, iv insulin acutely increases cortisol regeneration by 11β-HSD1 in humans (17). Protein ingestion also increases insulin levels (25). The high-protein meal contained some carbohydrate, which could have contributed to elevated 11β-HSD1 activity but is unlikely to be solely responsible because the Ra d3-cortisol after the high protein meal rose to that of the carbohydrate meal by the end of the study protocol (Figure 3B).
The mediator of the rise in cortisol secretion from the adrenals is unclear. We have shown that protein is not the only macronutrient able to stimulate adrenal production, although adrenal release was (nonsignificantly) greater after protein, which may explain why some previous studies concluded this was a specific protein-mediated mechanism. It seems unlikely that particular macronutrients act directly on the adrenal cortex to stimulate cortisol production, given that iv infusion of lipids (17) or amino acids (6) do not produce a demonstrable rise in circulating cortisol. It is more likely to be mediated by an indirect signal from the gut. Incretin hormones such as glucagon like peptide-1 and gastric inhibitory polypeptide are potential candidates because they acutely stimulate the hypothalamic-pituitary-adrenal axis, including in humans (26), and all macronutrients stimulate incretin release when ingested orally (27). However, carbohydrate classically produces the greatest rise in incretins, and yet in our study carbohydrate did not have the largest effect on adrenal cortisol secretion. It is possible that each macronutrient induces a rise in cortisol through distinct mechanisms; for example, carbohydrate could induce adrenal cortisol production through insulin secretion (28). Interestingly, the cortisol peak after carbohydrate was 30 minutes later than the peak after protein or fat. Carbohydrate delays early gastric emptying compared with protein or fat (29), which would slow absorption and cause a later cortisol peak. An alternative explanation is that the cells in the gut responsible for sensing carbohydrate are more distal than those sensing protein and fat (30).
Cortisol clearance, calculated using the d4-cortisol tracer, was not decreased by any of the meals and so does not contribute to postprandial elevations in plasma cortisol. Indeed, somewhat unexpectedly, the protein meal increased d4-cortisol clearance. Analysis of urinary deuterated metabolite excretion did not establish whether this is due to increased 11β-dehydrogenase, 5α-reductase, and/or 5β-reductase activity. We have not explored whether the type of carbohydrate influences the postprandial cortisol rise. We used maltodextrin, which has a high glycemic index (GI) (approximately 105). Other studies showing a cortisol rise after carbohydrate have used similarly high GI foods (7, 31), whereas some studies that did not demonstrate a cortisol rise have used carbohydrate with a lower GI (5). Intriguingly, high-GI foods increase hepatic insulin resistance (32), a poorly understood effect that our data suggest might be due to increased intrahepatic postprandial cortisol levels.
The duration and magnitude of the postprandial cortisol rise is potentially important. Circulating cortisol levels increased substantially (~90 nmol/L) and remained elevated compared with placebo for at least 3 hours after both the carbohydrate and protein meals and for 2 hours after the fat meal. Chronic excess glucocorticoid exposure causes adverse metabolic effects (33); therefore, meals that raise cortisol levels intermittently could potentially lead to adverse cardiometabolic outcomes, although meals at times other than midday may induce a lesser rise in cortisol (4). The ingestion of large infrequent meals increases cortisol production to a greater extent than more frequent smaller meals, despite identical total daily caloric intake (34). In addition, higher cortisol levels after glucose tolerance testing (35, 36) and after a standardized meal in men exhibiting dysregulated hypothalamic-pituitary-adrenal axis activity have been associated with features of the metabolic syndrome (37). Although each single macronutrient meal increased circulating cortisol levels equally, our findings suggest that higher carbohydrate intake might increase intracellular cortisol, particularly in the liver and thus have a disproportionate effect on metabolism. Further work is required to determine whether, under conditions of chronic feeding using mixed meals prepared from whole foods as reflective of normal living conditions, differences in the macronutrient content of meals results in sustained and substantial differences in cortisol appearance and whether this contributes to the differential effects on energy expenditure (38) and cardiometabolic risk factors (39, 40) in overweight and obese subjects.
To conclude, we have shown that all categories of macronutrient stimulate a postprandial rise in circulating cortisol; however, each macronutrient has specific effects on adrenal and extra-adrenal cortisol production. These data predict that intrahepatic glucocorticoid levels are particularly amplified by meals with a high carbohydrate content. If these results are sustained under chronic conditions involving mixed meals more representative of real-world conditions, then the resulting changes in glucocorticoid action may be important in mediating the contrasting metabolic effects of diets with different macronutrient content previously reported in isocaloric feeding studies. These observations provide a novel insight into the complexity of circadian control of glucocorticoid secretion.
Supplementary Material
Acknowledgments
We thank Alison Rutter, Sanjaykumar Kothiya, Lynne Ramage, Carolynn Cairns, Natalie Homer, Sam Donaldson, and the staff at the Wellcome Trust Clinical Research Facility for their help with this work.
This work was supported by the British Heart Foundation and a PhD studentship from the Malaysian Ministry of Higher Education (to N.A.M.-S.).
Abbreviations
- d4-cortisol
9,11,12,12-[2H]4-cortisol
- F
cortisol
- GI
glycemic index
- 11β-HSD1
type 1 isozyme of 11β-hydroxysteroid dehydrogenase
- 11β-HSD2
type 2 isozyme of 11β-hydroxysteroid dehydrogenase
- Ra cortisol
rate of appearance of cortisol
- α-THF
5α-tetrahydrocortisol
- β-THF
5β-THF
Footnotes
Disclosure Summary: B.R.W. is an inventor of relevant patents held by the University of Edinburgh. The other authors have nothing to disclose.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- 3.Quigley ME, Yen SS. A mid-day surge in cortisol levels. J Clin Endocrinol Metab. 1979;49:945–947. doi: 10.1210/jcem-49-6-945. [DOI] [PubMed] [Google Scholar]
- 4.Follenius M, Brandenberger G, Hietter B. Diurnal cortisol peaks and their relationships to meals. J Clin Endocrinol Metab. 1982;55:757–761. doi: 10.1210/jcem-55-4-757. [DOI] [PubMed] [Google Scholar]
- 5.Slag MF, Ahmad M, Gannon MC, Nuttall FQ. Meal stimulation of cortisol secretion: a protein induced effect. Metabolism. 1981;30:1104–1108. doi: 10.1016/0026-0495(81)90055-x. [DOI] [PubMed] [Google Scholar]
- 6.Benedict C, Hallschmid M, Scheibner J, et al. Gut protein uptake and mechanisms of meal-induced cortisol release. J Clin Endocrinol Metab. 2005;90:1692–1696. doi: 10.1210/jc.2004-1792. [DOI] [PubMed] [Google Scholar]
- 7.Martens MJ, Rutters F, Lemmens SG, Born JM, Westerterp-Plant-enga MS. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol Behav. 2010;101:563–567. doi: 10.1016/j.physbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Volek JS, Gomez AL, Love DM, Avery NG, Sharman MJ, Kraemer WJ. Effects of a high-fat diet on postabsorptive and postprandial testosterone responses to a fat-rich meal. Metabolism. 2001;50:1351–1355. doi: 10.1053/meta.2001.25648. [DOI] [PubMed] [Google Scholar]
- 9.Seckl JR, Walker BR. 11β-Hydroxysteroid dehydrogenase type 1—a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 10.Basu R, Singh RJ, Basu A, et al. Splanchnic cortisol production occurs in humans: evidence for conversion of cortisone to cortisol via the 11-β hydroxysteroid dehydrogenase (11β-hsd) type 1 pathway. Diabetes. 2004;53:2051–2059. doi: 10.2337/diabetes.53.8.2051. [DOI] [PubMed] [Google Scholar]
- 11.Andrew R, Westerbacka J, Wahren J, Yki-Jarvinen H, Walker BR. The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes. 2005;54:1364–1370. doi: 10.2337/diabetes.54.5.1364. [DOI] [PubMed] [Google Scholar]
- 12.Stimson RH, Johnstone AM, Homer NZ, et al. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab. 2007;92:4480–4484. doi: 10.1210/jc.2007-0692. [DOI] [PubMed] [Google Scholar]
- 13.Johnstone AM, Faber P, Andrew R, et al. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur J Endocrinol. 2004;150:185–194. doi: 10.1530/eje.0.1500185. [DOI] [PubMed] [Google Scholar]
- 14.Engeli S, Bohnke J, Feldpausch M, et al. Regulation of 11β-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res. 2004;12:9–17. doi: 10.1038/oby.2004.3. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson JW, Moore JS, Clark PM, Holder G, Shakespeare L, Stewart PM. Weight loss increases 11β-hydroxysteroid dehydrogenase type 1 expression in human adipose tissue. J Clin Endocrinol Metab. 2004;89:2711–2716. doi: 10.1210/jc.2003-031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu R, Singh R, Basu A, Johnson CM, Rizza RA. Effect of nutrient ingestion on total-body and splanchnic cortisol production in humans. Diabetes. 2006;55:667–674. doi: 10.2337/diabetes.55.03.06.db05-1335. [DOI] [PubMed] [Google Scholar]
- 17.Wake DJ, Homer NZ, Andrew R, Walker BR. Acute in vivo regulation of 11β-hydroxysteroid dehydrogenase type 1 activity by insulin and Intralipid infusions in humans. J Clin Endocrinol Metab. 2006;91:4682–4688. doi: 10.1210/jc.2006-0819. [DOI] [PubMed] [Google Scholar]
- 18.Andrew R, Smith K, Jones GC, Walker BR. Distinguishing the activities of 11β-hydroxysteroid dehydrogenases in vivo using isotopically labelled cortisol. J Clin Endocrinol Metab. 2002;87:277–285. doi: 10.1210/jcem.87.1.8157. [DOI] [PubMed] [Google Scholar]
- 19.Stimson RH, Andersson J, Andrew R, et al. Cortisol release from adipose tissue by 11β-hydroxysteroid dehydrogenase type 1 in humans. Diabetes. 2009;58:46–53. doi: 10.2337/db08-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R. Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes. J Clin Endocrinol Metab. 2002;87:3984–3988. doi: 10.1210/jcem.87.8.8718. [DOI] [PubMed] [Google Scholar]
- 21.Basu R, Basu A, Grudzien M, et al. Liver is the site of splanchnic cortisol production in obese nondiabetic humans. Diabetes. 2009;58:39–45. doi: 10.2337/db08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes KA, Manolopoulos KN, Iqbal J, et al. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes. 2012;61:1357–1364. doi: 10.2337/db11-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson JM, Morton NM, Fievet C, et al. Metabolic syndrome without obesity: hepatic overexpression of 11β-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA. 2004;101:7088–7093. doi: 10.1073/pnas.0305524101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 25.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465–470. doi: 10.2337/diacare.7.5.465. [DOI] [PubMed] [Google Scholar]
- 26.Gil-Lozano M, Perez-Tilve D, Alvarez-Crespo M, et al. GLP-1(7–36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology. 2010;151:2629–2640. doi: 10.1210/en.2009-0915. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 28.Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hyper-insulinemia causes activation of the hypothalamus-pituitary-adrenal axis in humans. Int J Obes Relat Metab Disord. 2001;25(suppl 1):S38–S40. doi: 10.1038/sj.ijo.0801695. [DOI] [PubMed] [Google Scholar]
- 29.Goetze O, Steingoetter A, Menne D, et al. The effect of macronutrients on gastric volume responses and gastric emptying in humans: a magnetic resonance imaging study. Am J Physiol Gastrointest Liver Physiol. 2007;292:G11–G17. doi: 10.1152/ajpgi.00498.2005. [DOI] [PubMed] [Google Scholar]
- 30.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 31.Iranmanesh A, Lawson D, Dunn B, Veldhuis JD. Glucose ingestion selectively amplifies ACTH and cortisol secretory-burst mass and enhances their joint synchrony in healthy men. J Clin Endocrinol Metab. 2011;96:2882–2888. doi: 10.1210/jc.2011-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haus JM, Solomon TP, Lu L, et al. Intramyocellular lipid content and insulin sensitivity are increased following a short-term lowglycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab. 2011;301:E511–E516. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins DJ, Wolever TM, Vuksan V, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–934. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 35.Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds RM, Walker BR, Syddall HE, Whorwood CB, Wood PJ, Phillips DI. Elevated plasma cortisol in glucose-intolerant men: differences in responses to glucose and habituation to venepuncture. J Clin Endocrinol Metab. 2001;86:1149–1153. doi: 10.1210/jcem.86.3.7300. [DOI] [PubMed] [Google Scholar]
- 37.Rosmond R, Holm G, Bjorntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. Int J Obes Relat Metab Disord. 2000;24:416–422. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- 38.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 40.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.