Abstract
Ultraviolet (UV) radiation results in a significant loss in years of healthy life, approximately 1.5 million disability-adjusted life years, and is associated with greater than 60,000 deaths annually worldwide that are attributed to melanoma and other skin cancers. Currently, there are no standardized biomarkers or assay panels to assess oxidative stress skin injury patterns in human skin exposed to ionizing radiation. Using biopsy specimens from chronic solar UV-exposed and UV-protected skin, we demonstrate that UV radiation-induced oxidative skin injury can be evaluated by an immunohistochemical panel that stains 8-hydroxydeoxyguanosine (8-OH-dG) to assess DNA adducts, 4-hydroxy-2-nonenal (HNE) to assess lipid peroxidation, and advanced glycation end products (AGEs) to assess protein damage. We believe this panel contains the necessary cellular biomarkers to evaluate topical agents, such as sunscreens and anti-oxidants that are designed to prevent oxidative skin damage and may reduce UV-associated skin aging, carcinogenesis, and inflammatory skin diseases. We envision that this panel will become an important tool for researchers developing topical agents to protect against UV radiation and other oxidants and ultimately lead to reductions in lost years of healthy life, DALYs, and annual deaths associated with UV radiation.
Keywords: oxidative stress, immunohistochemistry, 8-hydroxydeoxyguanosine, 4-hydroxy-2-nonenal, advanced glycation end products
INTRODUCTION
Ultraviolet (UV) radiation, a form of ionizing radiation, results in approximately 1.5 million disability-adjusted life years (DALYs) and is associated with greater than 60,000 deaths annually worldwide attributed to melanoma and other skin cancers.1 There are no standardized biomarker panels to assess UV-radiation oxidative stress injury patterns in skin. Unless assessing erythema after UV exposure, it is difficult to clinically determine the efficacy of UV-protective agents.2-6Therefore, developing a biomarker panel to evaluate UV radiation oxidative damage would facilitate rapid evaluation of skin injury due to UV radiation and enable the development and evaluation of novel oxidation-protective agents, such as sunscreens and topical antioxidants, to treat or prevent UV radiation-induced oxidation resulting in a decrease in lost years of healthy life, DALYs, and annual deaths that are attributed to UV radiation.7
UV and other forms of ionizing radiation cause skin injury through the generation of free radicals, reactive aldehydes and other oxidative byproducts.8 These free radicals and oxidative byproducts damage DNA, lipids, and proteins.9-11 Oxidative damage to DNA, lipids, and proteins disrupts normal cellular functions and is thought to play a major role in the pathogenesis of aging, carcinogenesis, and inflammatory skin diseases through the generation of gene mutations and post-translational modifications of essential cancer-related proteins.8 These mutations and modifications alter the cellular pathways involved in cell growth, apoptosis, DNA repair, cell cycle regulation, and gene expression leading to either inactivation of tumor-suppressor genes or enhancement of proto-oncogenes.8,12
Our results demonstrate that UV radiation-induced oxidative damage can be evaluated by an immunohistochemical panel that includes 8-hydroxydeoxyguanosine (8-OH-dG) to assess DNA adducts, 4-hydroxy-2-nonenal (HNE) to assess lipid peroxidation, and advanced glycation end products (AGEs) to assess protein damage (Table 1). Together these markers serve as a comprehensive panel to evaluate UV radiation damage to critical skin components. To demonstrate this panel's utility, we conducted a study to characterize and quantify the aforementioned oxidative damage byproducts in chronic solar UV-exposed human skin compared with UV-protected human skin.
Table 1.
Location of oxidative stress biomarkers (AGEs, HNE, 8-OH-dG)
MATERIALS AND METHODS
Ethics
This study was approved by Western Institutional Review Board. Biopsy specimens were obtained under an IRB-approved waiver of consent due to use of de-identified samples of residual tissue not needed for clinical purposes.
Chronic Ultraviolet-Exposed and Ultraviolet-Protected Skin Biopsy Selection
Five patient-matched skin biopsy specimens from chronic solar UV-exposed (lower eyelid, malar cheek, mandible) and UV-protected (upper thigh, hip, mid abdomen, mid back) skin were processed to evaluate the immunohistochemical staining patterns of 8-OH-dG, HNE, and AGEs. The slides were sectioned from archived formalin-fixed and paraffin-embedded tissue blocks of skin sections.
Immunohistochemistry
Immunohistochemistry was carried out using “R.T.U Vectastain Universal ABC kit” (PK-7200, Vector laboratories, Burlingame, CA) instructions. Briefly, the sections of 4 μm were deparaffinized in xylene and rehydrated. Endogenous peroxidase activity was inactivated by incubation for 10 minutes in 3% hydrogen peroxide (H2O2)/methanol. After blocking the non-specific sites with prediluted normal horse serum from “R.T.U Vectastain Universal ABC kit” (PK-7200, Vector laboratories, Burlingame, CA) for 20 minutes, the primary antibodies were incubated for 30 min at room temperature. Anti-8-OH-dG mouse monoclonal antibody (ab62623, Abcam, Cambridge, MA) was diluted at 1:100 in antibody diluent (S3022, Dako, Carpinteria, CA). Anti-4-HNE rabbit polyclonal antibody (HNE11-S, Alpha Diagnostics, San Antonio, TX) was reconstituted in PBS to the concentration of 1mg/ml and diluted at 1:1000 in antibody diluent (S3022, Dako, Carpinteria, CA). Anti-AGE rabbit polyclonal antibody (ab23722, Abcam, Cambridge, MA) was diluted at 1:2000 in antibody diluent (S3022, Dako, Carpinteria, CA). The sections were incubated with prediluted biotinylated horse antibody from “R.T.U Vectastain Universal ABC kit” (PK-7200, Vector laboratories, Burlingame, CA) for 30 minutes. The peroxidase reaction was revealed using a diaminobenzidine substrate (SK-4105, ImmPACT DAB Peroxidase Substrate, Vector Laboratories, Burlingame, CA). The sections were counter stained with Harris haematoxylin. Positive and negative controls were also run simultaneously and gave appropriate results.
Dermatopathologist Grading of Staining Intensity and Nuclei
The primary outcome measure assessed was the overall staining intensity grades of the stratum corneum, epidermis, and dermis. The secondary outcome measure was the relative number of positively stained nuclei. Three independent blinded dermatopathologists characterized and quantified the intensity of 8-OH-dG, HNE, and AGEs staining in the stratum corneum, epidermis, and dermis of these skin biopsies using the method previously described.13 Staining intensity was evaluated individually for each marker and graded using the following scale: (0) no staining; (1) weak staining; (2) moderate staining; (3) strong staining; (4) very strong staining. The percentage of cells with nuclear staining of the biomarkers was calculated by assessing the number of positively stained nuclei compared to the number of total nuclei per high power field.
Statistical Analysis
Statistical analysis was carried out using the paired two-tailed Student's t test, where p < 0.05 is significant. All data are represented as mean ± the standard error of the mean (SEM).
RESULTS
UV-protected and UV-exposed immunohistochemically stained skin specimens were graded by three independent and blinded dermatopathologists as previously described.14 Staining intensity and percent positive nuclei were assessed. In all specimens, UV-exposed skin demonstrated strong and consistent staining of 8-OH-dG, HNE, and AGEs compared to matched UV-protected skin specimens (Figs. 1, 3, 4). Increased nuclear staining was demonstrated in UV-exposed skin specimens stained with 8-OH-dG and AGEs.
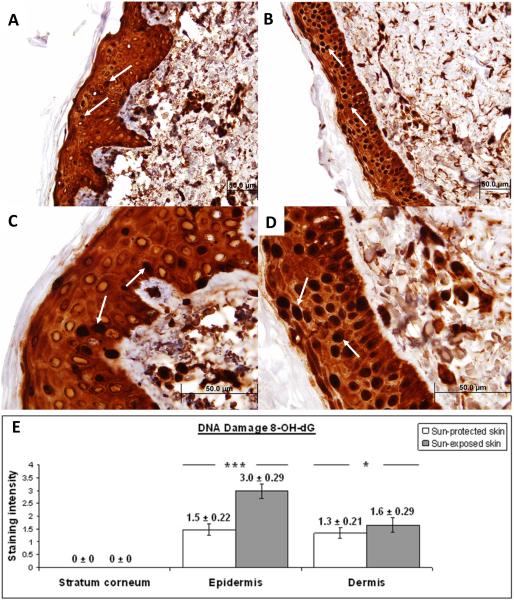
Figure 1. Immunohistochemical characterization and quantification of 8-OH-dG in UV-protected and UV-exposed human skin biopsy specimens.
A), C) UV-protected human skin sections stained with antibodies against 8-OH-dG. Strong nuclear staining is observed in approximately 15% of epidermal cells (arrows).
B), D) UV-exposed human skin sections stained with antibodies against 8-OH-dG. Strong nuclear staining is observed in approximately 90% of epidermal cells (arrows).
Moderate staining of intracelular material is observed equally in the epidermis of UV-protected as well as UV-exposed skin specimens.
Original magnification A, B × 40 and C, D × 100.
E) Analysis of staining intensity scores for 8-OH-dG. No difference was observed in 8-OH-dG staining of the stratum corneum of UV-exposed and UV-protected skin (0 ± 0 versus 0 ± 0). 8-OH-dG staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (3.0 ± 0.29 versus 1.5 ± 0.22; p = <0.01). 8-OH-dG staining was significantly increased in the dermis of UV-exposed versus UV-protected skin (1.6 ± 0.29 versus 1.3 ± 0.21; p = <0.05). *p < 0.05, ***p < 0.01. Data are shown as mean ± SEM; n=5.
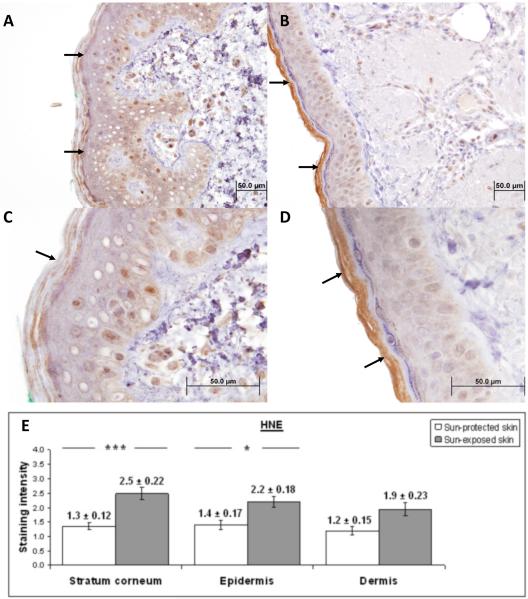
Figure 3. Immunohistochemical characterization and quantification of HNE in UV-protected and UV-exposed human skin biopsy specimens.
A), C) UV-protected human skin sections stained with antibodies against HNE-adducts.
B), D) UV-exposed human skin sections stained with antibodies against HNE-adducts.
In UV-exposed skin samples there was a stronger staining for HNE in stratum corneum compared to the UV-protected skin (brown color, arrows). There is nuclear staining evenly observed in epidermis and dermis of both skin samples. Immunostaining is defined as low (A, C) and strong (B, D).
Original magnification A, B × 40 and C, D × 100.
E) Analysis of staining intensity scores for HNE. HNE staining of the stratum corneum was significantly increased in UV-exposed versus UV-protected skin (2.5 ± 0.22 versus 1.3 ± 0.12; p = <0.01). HNE staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (2.2 ± 0.18 versus 1.4 ± 0.17; p = <0.05). HNE staining was not significantly increased in the dermis of UV-exposed and UV-protected skin (1.9 ± 0.19 versus 1.2 ± 0.15). * p < 0.05, ***p < 0.01. Student t test. Data are shown as mean ± SEM; n=5.
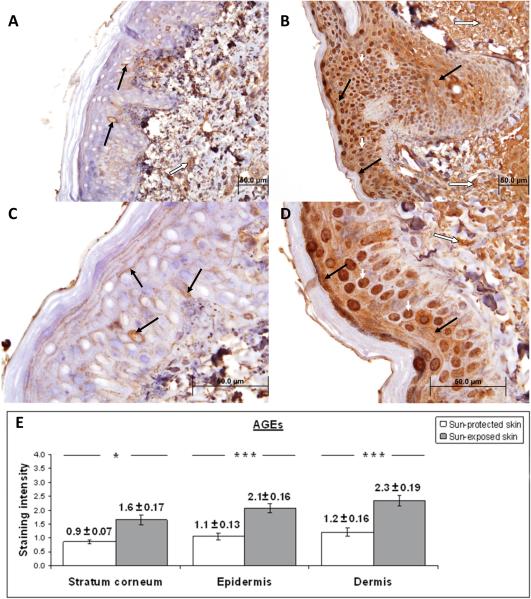
Figure 4. Immunohistochemical characterization and quantification of AGEs in UV-protected and UV-exposed human skin biopsy specimens.
A), C) UV-protected human skin sections stained with antibodies against AGEs. Weak staining is observed in epidermis (brown color staining, solid black arrow) and dermis (brown color staining, white arrow). Nuclear staining is demonstrated in approximately 10% epidermal cells.
B), D) UV-exposed human skin sections stained with antibodies against AGEs. Moderate staining is observed in the epidermis (brown color, black arrows) and dermis (brown color, long white arrow). Strong nuclear staining is noted in approximately 70% of epidermal cells (brown color, short white arrows).
The increased staining intensity in UV-exposed skin samples is determined to be 191% for stratum corneum, 196% for epidermis and 193% for dermis as compared to UV-protected skin samples. Original magnification A, B × 40 and C, D × 100.
E) Analysis of staining intensity scores for AGEs. AGEs staining of the stratum corneum was significantly increased in UV-exposed versus UV-protected skin (1.6 ± 0.17 versus 0.9 ± 0.07; p = <0.05). AGEs staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (2.1 ± 0.16 versus 1.1 ± 0.13; p = <0.01). HNE staining was significantly increased in the dermis of UV-exposed and UV-protected skin (2.3 ± 0.19 versus 1.2 ± 0.16; p = <0.01). * p < 0.05, ***p < 0.01. Student t test. Data are shown as mean ± SEM; n=5.
8-OH-dG staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (3.0 ± 0.29 versus 1.5 ± 0.22; p = <0.01). In the UV-exposed specimens, 8-OH-dG demonstrated strong nuclear staining in approximately 90% of epidermal cells versus 15% in UV-protected skin. 8-OH-dG staining was significantly increased in the dermis of UV-exposed versus UV-protected skin (1.6 ± 0.29 versus 1.3 ± 0.21; p = <0.05). No difference was observed in 8-OH-dG staining of the stratum corneum of UV-exposed and UV-protected skin (0 ± 0 versus 0 ± 0).
HNE staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (2.2 ± 0.18 versus 1.4 ± 0.17; p = <0.05). No difference in nuclear staining was observed between UV-exposed and UV-protected specimens. HNE staining of the stratum corneum was significantly increased in UV-exposed versus UV-protected skin (2.5 ± 0.22 versus 1.3 ± 0.12; p = <0.01). HNE staining was not significantly increased in the dermis of UV-exposed versus UV-protected skin (1.9 ± 0.19 versus 1.2 ± 0.15).
AGEs staining was significantly increased in the epidermis of UV-exposed versus UV-protected skin (2.1 ± 0.16 versus 1.1 ± 0.13; p = <0.01). AGEs demonstrated strong nuclear staining in approximately 70% of epidermal cells and UV-protected demonstrated strong nuclear staining in approximately 10% of epidermal cells. AGE staining was significantly increased in the dermis of UV-exposed versus UV-protected skin (2.3 ± 0.19 versus 1.2 ± 0.16; p = <0.01). AGEs staining of the stratum corneum was significantly increased in UV-exposed versus UV-protected skin (1.6 ± 0.17 versus 0.9 ± 0.07; p = <0.05).
DISCUSSION AND FUTURE DIRECTIONS
Our data demonstrates that an immunohistochemical panel assaying 8-OH-dG, HNE, and AGEs can be used to evaluate oxidative damage in UV-protected and UV-unprotected human skin. Given that 8-OH-dG is a DNA adduct that may contribute to carcinogenesis, it is not surprising that we observed increased nuclear staining in epidermis, including the basal layer, as these cells propagate future generations of keratinocytes. This reinforces the view that 8-OH-dG may be a biologically significant marker to assess when developing and evaluating topical agents to prevent UV oxidative skin injury as actinic keratosis, squamous cell skin cancer, and basal cell skin cancer arise from keratinocytes with DNA damage. The lack of 8-OH-dG staining in the stratum corneum is consistent with and due to lack of nuclei in the stratum corneum.
Our data demonstrates strong staining of the oxidative lipid byproduct HNE in the stratum corneum in UV-exposed skin versus UV-protected skin. Lipids located in the stratum corneum play an important role in skin appearance, health, and barrier function. Oxidative damage to lipids may decrease skin barrier function and contribute to photoaged skin, xerosis, and eczema. Thus, the increase in stratum corneum HNE levels may represent an important marker of lipid-associated oxidative skin injury.
AGEs are oxidative byproducts that accumulate in extracellular matrix (ECM) proteins collagen and elastin. AGEs may contribute to the pathogenesis of photoaging and cancer by changing the structure and function of key proteins and causing protein cross-links that result in skin stiffening due to loss of elasticity.10 We found a significant increase in AGE staining in the UV-protected versus UV-exposed specimens, confirming recent findings of increased AGEs in UV-exposed skin.10
We envision that this panel will become an important tool for researchers developing topical agents to protect against UV radiation. Further study is needed to confirm if this panel of biomarkers may be utilized to evaluate UV and other forms of ionizing radiation and potential anti-oxidant agents to prevent and treat oxidative skin injury.
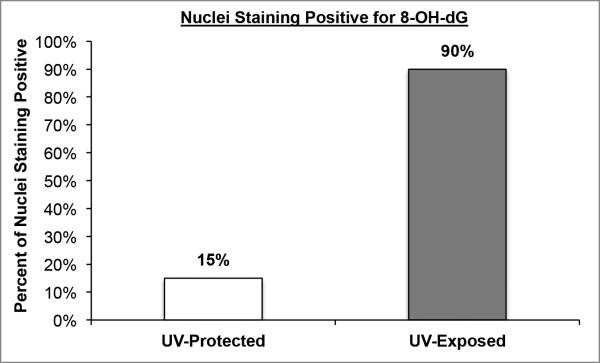
Figure 2. Nuclear staining of 8-OH-dG in UV-protected and UV-exposed human skin biopsy specimens.
Nuclear staining of 8-OH-dG in UV-protected and UV-exposexposed human skin biopsy specimens. UV-exposed human skin sections stained with antibodies against 8-OH-dG demonstrated strong nuclear staining in approximately 90% of epidermal cells and UV-protected demonstrated nuclear staining in approximately 15% of epidermal cells.
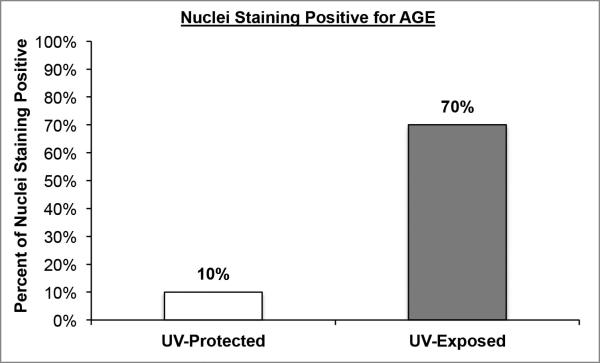
Figure 5. Nuclear staining of AGEs in UV-protected and UV-exposed human skin biopsy specimens.
UV-exposed human skin sections stained with antibodies against AGE demonstrated strong nuclear staining in approximately 70% of epidermal cells and UV-protected demonstrated strong nuclear staining in approximately 10% of epidermal cells.
Acknowledgments
The authors would like to thank Mengru Li, MB, clinical lab technician, pathology department, SUNY Downstate Medical Center for her outstanding technical contribution to immunohistochemical staining; Dr. Michl J, associate professor of pathology, cell and molecular biology, microbiology and immunology, SUNY Downstate Medical Center for the use of his laboratory and technical assistance.
Funding: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award TL1 TR000133.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award KL2 TR000134.
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R33AI080604.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests:
The authors declare that they have no competing interests.
Disclosures: None declared
References
- 1.American Cancer Society Cancer Facts & Figures. 2013 [Google Scholar]
- 2.Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. Journal of drugs in dermatology : JDD. 2010 Dec;9(12):1523–1526. [PubMed] [Google Scholar]
- 3.Jagdeo J, Brody N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. Journal of drugs in dermatology : JDD. 2011 Jul;10(7):753–761. [PubMed] [Google Scholar]
- 4.Silverberg JI, Jagdeo J, Patel M, Siegel D, Brody N. Green tea extract protects human skin fibroblasts from reactive oxygen species induced necrosis. Journal of drugs in dermatology : JDD. 2011 Oct;10(10):1096–1101. [PubMed] [Google Scholar]
- 5.Silverberg JI, Patel M, Brody N, Jagdeo J. Caffeine protects human skin fibroblasts from acute reactive oxygen species-induced necrosis. Journal of drugs in dermatology : JDD. 2012 Nov;11(11):1342–1346. [PubMed] [Google Scholar]
- 6.Mamalis A, Nguyen DH, Brody N, Jagdeo J. The active natural anti-oxidant properties of chamomile, milk thistle, and halophilic bacterial components in human skin in vitro. Journal of drugs in dermatology : JDD. 2013 Jul 1;12(7):780–784. [PubMed] [Google Scholar]
- 7.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. The Journal of clinical and aesthetic dermatology. 2011 Sep;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Witz G. Active oxygen species as factors in multistage carcinogenesis. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1991 Nov;198(2):675–682. doi: 10.3181/00379727-198-43306. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Progress in lipid research. 2003 Jul;42(4):318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 10.Gkogkolou P, Bohm M. Advanced glycation end products: Key players in skin aging? Dermato-endocrinology. 2012 Jul 1;4(3):259–270. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. The Journal of biological chemistry. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- 12.Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert reviews in molecular medicine. 2002 Dec;4(26):1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- 13.Angele S, Treilleux I, Bremond A, Taniere P, Hall J. Altered expression of DNA double-strand break detection and repair proteins in breast carcinomas. Histopathology. 2003 Oct;43(4):347–353. doi: 10.1046/j.1365-2559.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 14.Crisan M, Taulescu M, Crisan D, Cosgarea R, Parvu A, Cãtoi C, Drugan T. Expression of Advanced Glycation End-Products on Sun-Exposed and Non-Exposed Cutaneous Sites during the Ageing Process in Humans. PLoS One. 2013 Aug;8(10):e75003. doi: 10.1371/journal.pone.0075003. [DOI] [PMC free article] [PubMed] [Google Scholar]