Abstract
Rationale
Dysfunctional Parkin-mediated mitophagic culling of senescent or damaged mitochondria is a major pathological process underlying Parkinson disease and a potential genetic mechanism of cardiomyopathy. Despite epidemiological associations between Parkinson disease and heart failure, the role of Parkin and mitophagic quality control in maintaining normal cardiac homeostasis is poorly understood.
Objective
We used germline mutants and cardiac-specific RNA interference to interrogate Parkin regulation of cardiomyocyte mitochondria and examine functional crosstalk between mitophagy and mitochondrial dynamics in Drosophila heart tubes.
Methods and Results
Transcriptional profiling of Parkin knockout mouse hearts revealed compensatory upregulation of multiple related E3 ubiquitin ligases. Because Drosophila lack most of these redundant genes, we examined heart tubes of parkin knockout flies and observed accumulation of enlarged hollow donut mitochondria with dilated cardiomyopathy, which could be rescued by cardiomyocyte-specific Parkin expression. Identical abnormalities were induced by cardiomyocyte-specific Parkin suppression using 2 different inhibitory RNAs. Parkin-deficient cardiomyocyte mitochondria exhibited dysmorphology, depolarization, and reactive oxygen species generation without calcium cycling abnormalities, pointing to a primary mitochondrial defect. Suppressing cardiomyocyte mitochondrial fusion in Parkin-deficient fly heart tubes completely prevented the cardiomyopathy and corrected mitochondrial dysfunction without normalizing mitochondrial dysmorphology, demonstrating a central role for mitochondrial fusion in the cardiomyopathy provoked by impaired mitophagy.
Conclusions
Parkin deficiency and resulting mitophagic disruption produces cardiomyopathy in part by contamination of the cardiomyocyte mitochondrial pool through fusion between improperly retained dysfunctional/senescent and normal mitochondria. Limiting mitochondrial contagion by inhibiting organelle fusion shows promise for minimizing organ dysfunction produced by defective mitophagic signaling.
Keywords: cardiomyopathies, mitochondrial dynamics, mitochondrial degradation
Dysfunction of the PTEN-inducible kinase 1 (PINK1)–Parkin mitochondrial quality control pathway is most widely recognized for its genetic linkage to Parkinson disease1–3; accumulating evidence points to an important role of this pathway in normal heart function.4,5 Parkin is an E3 ubiquitin ligase recruited to dysfunctional mitochondria through the actions of the kinase PINK1.6,7 Mitochondrial levels of PINK1 are chronically suppressed under normal conditions, but mitochondrial senescence or damage that provokes mitochondrial depolarization results in PINK1 accumulation8 and phosphorylation of mitofusin-2 (Mfn2), the receptor for mitochondrial Parkin recruitment.5 Parkin-mediated polyubiquitination of multiple mitochondrial membrane proteins9 then selectively targets damaged organelles for mitophagic elimination.10
The functional links between PINK1, mitofusins, and Parkin were initially revealed by targeting their Drosophila melanogaster gene orthologs and studying skeletal muscle and neuronal mitochondria.6,7 Mechanistic foundations for our growing understanding of mitophagic disruption in human Parkinson disease were also established in large part through the use of fruit fly Parkin gene knockout models.11–14 It is notable that interruption of PINK1 and Parkin in D. melanogaster provokes more severe phenotypes compared with the orthologous genetic manipulation in mice,6,12 likely because fruit flies lack functionally redundant compensatory pathways present in mammals. Thus, both germline ablation and system-wide mutation of the mouse Parkin gene (Park2) fail to evoke more than subtle Parkinson disease phenotypes in mice.15 Likewise, mice systemically lacking Parkin reportedly have no basal cardiac dysfunction.16 These findings either impugn the idea that mitochondrial quality control is essential to the mouse brain and heart, or point to induction of as-yet-unknown compensatory mechanisms in germline parkin null mouse models.
Both cardiomyocytes and neurons are amitotic and, therefore, unable to repair mitochondrial damage through cell division.17 Because the brain and heart have a common reliance on mitochondrial-generated ATP for minute-by-minute functioning, it might be expected that genetic defects in Parkin-mediated mitophagic signaling that impair removal of damaged mitochondria would affect both organs. In support of this notion, and unlike Parkin knockout mice, mice deficient in PINK1 develop progressive cardiomyopathy and evidence of mitochondrial dysfunction4 as well as features of Parkinson disease.18 Likewise, we recently showed that mice with cardiomyocyte-restricted ablation of Mfn2, which is the obligate mitochondrial Parkin mitophagy receptor, develop cardiomyocyte respiratory impairment and progressive heart failure.5 These findings may suggest a possible physiological basis for previously described associations between heart failure and Parkinson disease.19,20
Here, we report results of RNA sequencing studies supporting the notion that compensatory upregulation of alternate E3 ubiquitin ligases can contribute to the absence of severe phenotypes after germline park2 ablation. Accordingly, we return to the fruit fly system that has proven use in mechanistic in vivo studies of Parkin effects in neurons and skeletal muscle,21 and in which we recently described cardiomyopathy.5 Because we found that germline parkin gene ablation in Drosophila has multiple adverse confounding systemic consequences, we developed cardiomyocyte-specific Parkin suppression flies in which we observed abnormal mitochondrial structure and function and cardiomyopathy. In this model, we discovered that suppressing mitochondrial fusion (thus preventing fusion-mediated contamination of the normal cardiomyocyte mitochondrial pool by pathologically retained damaged mitochondria) fully rescues mitochondrial dysfunction and cardiomyopathy induced by Parkin deficiency. These findings describe a novel therapeutic strategy for diseases caused by defective mitochondrial quality control: limiting mitochondrial contagion through suppression of organelle fusion.
Methods
w1118 (#6326), UAS-mitoGFP (#8442), UAS-GCaMP3 (#32234), Parkin RNAi (#37509 [park KD1] and #38333 [park KD2]), UAS-Parkin (#34748), UAS-SOD1 (#33605), UAS-SOD2 (#24494), and mef-Gal4 (#27390) were obtained from the Bloomington Stock Center. UAS-Romo1 RNAi (#101353) was obtained from the Vienna Drosophila RNAi Center. The parkin null mutant, Park,11 was from L.J. Pallanck (University of Washington, Seattle, WA), UAS-marf RNAi was from M. Guo (University of California, Los Angeles, CA), and UAS-mito-DsRed was from F. Kawasaki (Pennsylvania State University, University Park, PN). UAS-cb5-GFP was described previously.25 UAS transgenes were expressed in Drosophila cardiomyocytes using the tincΔ4-Gal4 driver from Rolf Bodmer (Sanford-Burnham Medical Research Institute, La Jolla, CA). Studies of Drosophila heart tubes were performed as described.25,26
RNA sequencing of Parkin null hearts22 was performed as previously described.23
An expanded Methods section is in the Online Data Supplement.
Results
Compensatory Upregulation of Multiple E3 Ubiquitin Ligases in Germline Parkin Null Mouse Hearts
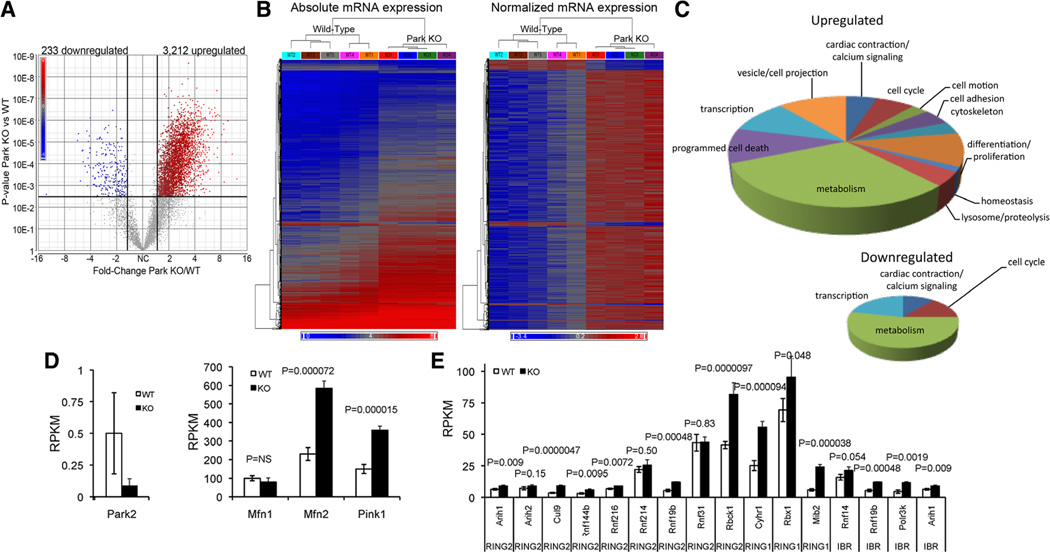
To gain insight into why systemic ablation of Parkin has minimal effects on mice,15,22 we performed deep mRNA sequencing23 of parkin null and wild-type control mouse hearts. Parkin ablation produced >50% change (false discovery rate, 0.01; P<0.005) in steady-state levels of 3445 of the ≈8000 cardiac mRNAs expressed at levels ≥1 mRNA copy per cell (3212 increased; 233 decreased; Figure 1A and 1B). Gene ontology analysis revealed dominant effects on metabolic/mitochondrial genes (Figure 1C). Parkin mRNA was absent in knockout hearts, but transcript levels of upstream Parkin activators PINK1 and Mfn2 increased (Figure 1D). Of 15 Parkin-like (ie, RING1, RING2, and in-between RING domain) E3 ubiquitinligases expressed in hearts, 11 were significantly upregulated in Parkin deficiency (Figure 1E). These results add to accumulating evidence for functional compensation induced by germline murine Parkin insufficiency,24 suggest how ablation of the parkin gene produces cardiomyocyte mitochondrial enlargement without basal cardiac dysfunction,16 and support a re- evaluation of cardiac Parkin biology.
Figure 1. Cardiac RNA transcript levels of Parkin pathway factors and Parkin-related E3 ubiquitin ligases in Parkin knockout (KO) mice.
A and B, Volcano plot (A) and heat maps (B) of cardiac transcriptional changes induced by germline Parkin gene ablation in mice. C, Gene ontology analysis of up- (top) and downregulated mRNAs in Parkin KO mouse hearts. D, mRNA levels of Parkin and upstream Parkin signaling factors PINK1 and Mfn2; Mfn1 is shown for comparison. E, Transcript levels of Parkin-related E3 ubiquitin ligases of RING2, RING1, and in-between ring families. Gene names are: Arih1, ariadne homolog, ubiquitin-conjugating enzyme E2 binding protein 1; Arih2, ariadne homolog 2; Cul9, cullin 9; Rnf, ring finger protein; Rbck1, RanBP-type and C3HC4-type zinc finger containing 1; Cyhr1, cysteine/histidine-rich 1; Rbx1, ring-box 1; Mib2, mindbomb homolog 2; Polr3k, polymerase (RNA) III (DNA directed) polypeptide K, 12.3 kDa. Values are mean±SEM of RNA sequencing reads per million bases (RPKM) for n=5 wild type (WT) and n=4 germline Parkin null (KO) mice.
Dilated Cardiomyopathy and Mitochondrial Abnormalities in Parkin Null Flies
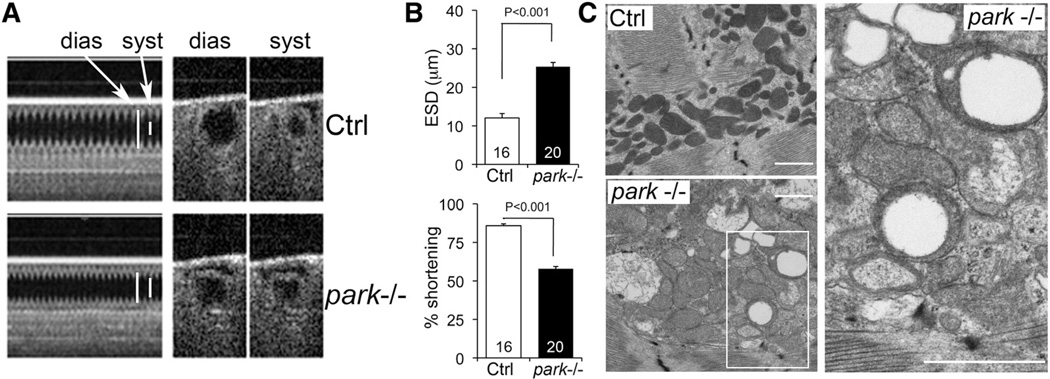
Drosophila melanogaster Parkin null mutants (park−/−) exhibit defective mitochondrial quality control in skeletal muscle.11 We recently described heart tube contractile and respiratory impairment in the same line of park−/− flies.5 Here, we demonstrate that the cardiomyopathy of Drosophila Parkin deficiency is sustained and we define the ultrastructrual consequences of Parkin ablation on cardiomyocyte mitochondria. One and 4 weeks after eclosure, heart tubes of parkin−/− Drosophila exhibited reduced fractional shortening (Figure 2A and 2B; Online Figure IA). Ultrastructural examination of parkin−/− heart tubes revealed abnormally enlarged cardiomyocyte mitochondria, many of which had disorganized cristae or a characteristic hollow donut morphology (Figure 2C). However, germline parkin deletion adversely impacted fly size (Online Figure IB) and markedly reduced longevity (log-rank P<0.001; Online Figure IC) as previously reported.11 Because the systemic effects and premature lethality of parkin−/− Drosophila confound detailed mechanistic assessments of the cardiac consequences of Parkin deficiency, we developed cardiac-specific Parkin-deficient flies.
Figure 2. Cardiomyopathy and mitochondrial abnormalities in parkin null (−/−) flies.
A, Optical coherence tomography (OCT) of heart tube contractions in tincΔ4-Gal4 (Ctrl) and parkin−/− flies 7 days after eclosure. dias indicates diastolic; and syst, systolic. B, Bar graphs showing group mean OCT data for end-systolic dimension (ESD) and percent fractional shortening. C, Transmission electron micrographs showing enlarged mitochondria with disrupted or absent cristae in parkin−/− cardiomyocytes. Note the characteristic hollow donut mitochondrial morphology. White scale bars are 1 µm.
Cardiac-Specific Parkin Suppression Causes Symptomatic Heart Failure in Flies
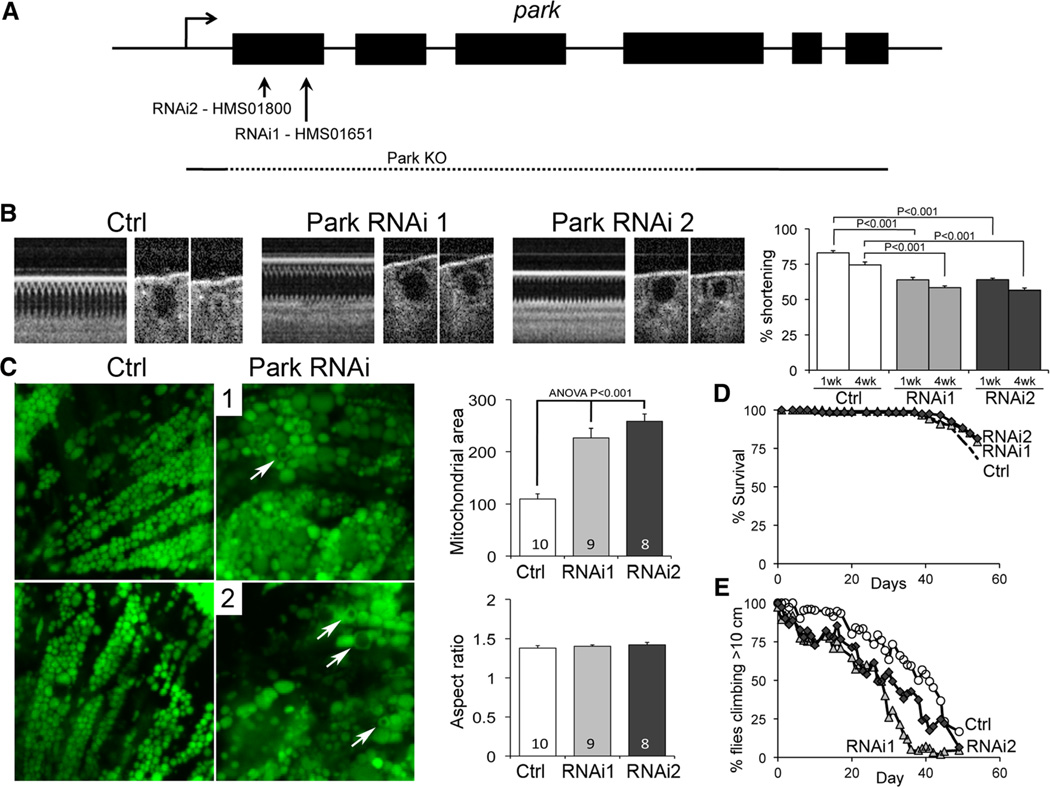
We used the tincΔ4-Gal4 driver to express Parkin inhibitory RNAs and suppress Parkin exclusively in Drosophila cardiomyocytes (UAS-Park RNAi; Figure 3A). As with germline parkin ablation, cardiomyocyte-specific Parkin suppression evoked heart tube hypocontractility 1 and 4 weeks after eclosure (Figure 3B) and increased mitochondrial size imaged with mito-GFP25 (Figure 3C). Because longevity was normal in cardiac-specific Parkin-deficient flies (log-rank P=NS; Figure 3D), we determined the impact of cardiomyocyte-autonomous Parkin deficiency on systemic cardiorespiratory function using the negative geotaxis Drosophila stress test.26 Both lines of cardiac-specific Parkin-deficient flies displayed accelerated age-related declines in climbing performance (log-rank P=6.6E–16 for RNAi1 and P=1.7E–13 for RNAi2 versus tincΔ4-Gal4 controls; Figure 3E).
Figure 3. Cardiomyocyte-specific Parkin suppression induces heart failure and mitochondrial abnormalities in Drosophila.
A, Parkin targeting by RNAi constructs. B, Optical coherence tomography studies showing hypocontractile heart tubes in tincΔ4-Gal4 UAS-Parkin RNAi fly lines. Bar graphs show group mean fractional shortening data 7 and 28 days after eclosure (n=15–20 per group). C, Cardiomyocyte mitochondria visualized by tincΔ4-Gal4-mitoGFP show organelle enlargement and hollow donut dysmorphology (arrows). Group mean data are to the right (>100 mitochondria analyzed per heart). D, Survival analysis of tincΔ4-Gal4 UAS-Parkin RNAi fly lines (n=150 per group). E, Climbing performance in negative geotaxis test in tincΔ4-Gal4 UAS-Parkin RNAi fly lines (n=150 per group).
Parkin Insufficiency Causes Cardiomyocyte-Autonomous Cardiomyopathy Without Altering Sarcoplasmic Reticulum Calcium Cycling
The results of cardiac Parkin suppression indicate that even partial cardiomyocyte Parkin deficiency (Figure 6G) can adversely impact cardiac function. We confirmed this finding in germline Parkin haploinsufficient flies (park+/−), which also developed a cardiomyopathy with mitochondrial abnormalities, despite longer survival compared with parkin−/− flies and normal body size (Online Figure IIA–IID). We proved that the cardiomyopathy provoked by germline Parkin haploinsufficiency results from cardiomyocyte Parkin deficiency by crossing park+/− flies with tincΔ4-Gal4–driven UAS-Parkin transgenic flies that express Parkin exclusively in cardiomyocytes, which completely normalized cardiac dysfunction (Online Figure IIE).
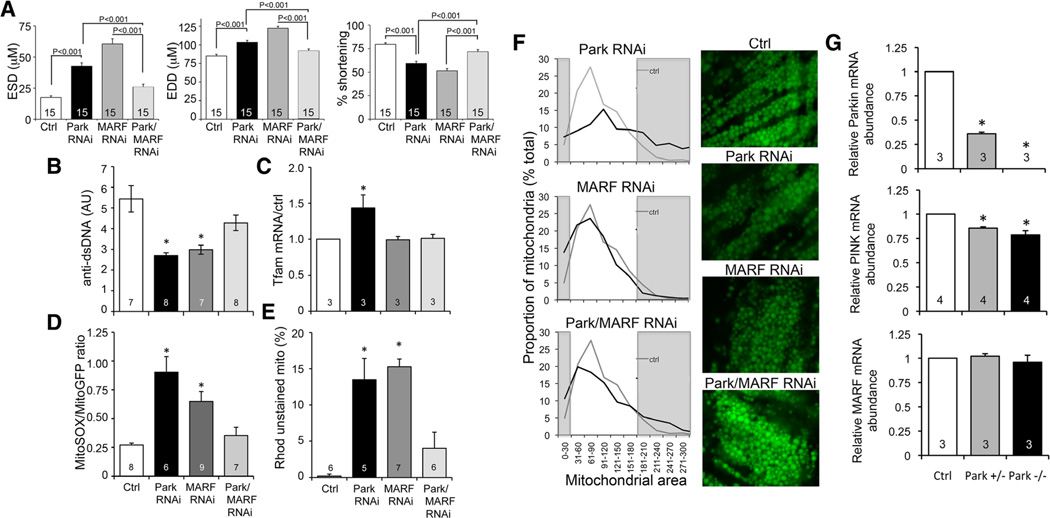
Figure 6. Interrupting mitochondrial fusion rescues mitochondrial and cardiac dysfunction evoked by Parkin deficiency.
A, Results of optical coherence tomography studies of Parkin RNAi-expressing fly heart tubes without and with concomitant RNAi-mediated suppression of cardiomyocyte dMfn/MARF (Parkin/MARF RNAi). EDD indicates end-diastolic dimension; ESD, end-systolic dimension; and MARF, mitochondrial assembly regulatory factor. Normal Ctrl and tincΔ4-Gal4 MARF RNAi results are shown for comparison. B to E, Mitochondrial function studies of the same groups as A; B, mitoDNA content performed as in Figure 4A; C, Tfam mRNA abundance by RT-qPCR; D, Reactive oxygen species production performed as in Figure 4D; E, mitochondrial depolarization assayed as in Figure 4B and 4C. F, Mitochondrial morphology assessed by mito-GFP confocal analysis of the same groups. Group histogram data for mitochondrial area are on the left, with representative micrographs on the right. The grey areas depict abnormally small (left) and abnormally large (right) mitochondria defined as the bottom and top decile of the normal control data. The same control data (grey lines) are shown in all 3 histograms. G, RT-qPCR of Parkin (top), PTEN-inducible kinase 1 (PINK; middle), and mitochondrial assembly regulatory factor (MARF; bottom) mRNA. *Significantly different from Ctrl by ANOVA and Bonferroni test.
Parkin may also modulate endoplasmic reticular calcium release.27,28 Although sarcoplasmic reticulum (SR) dysfunction is implicated in many cardiomyopathies, cardiomyocyte SR architecture and SR calcium handling were normal in Parkin haploinsufficient Drosophila hearts (Online Figure III).
Parkin Insufficiency Causes Accumulation of Dysfunctional Cardiomyocyte Mitochondria
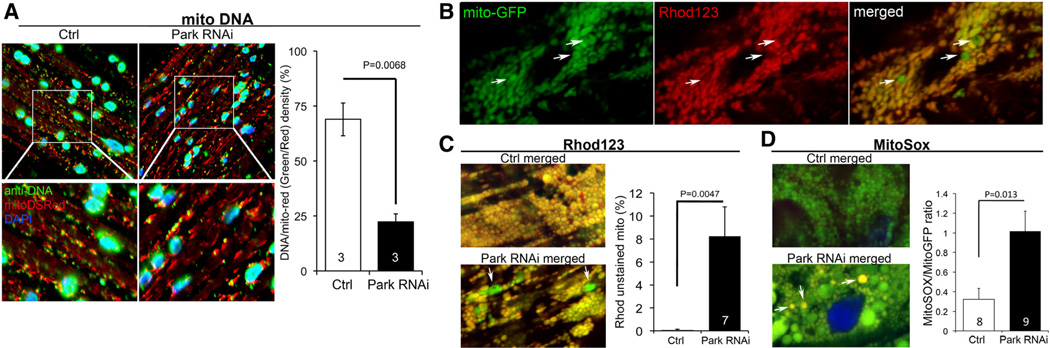
The essential function of Parkin is mediating mitophagic mitochondrial culling.29,30 Consistent with interruption of normal mitophagic quality control caused by Drosophila cardiac-specific Parkin deficiency, we observed increases in dysmorphic cardiomyocyte mitochondria and loss of cardiomyocyte mitochondrial nucleoids (see above and Figure 4A). We, therefore, further interrogated mitochondrial function in Parkin RNAi-expressing hearts and observed a marked increase in the number of depolarized cardiomyocyte mitochondria (Figure 4B and 4C) and greater numbers of mitochondria producing reactive oxygen species (ROS; Figure 4D). Evidence for mitochondrial depolarization and ROS production was more common in abnormally large or dysmorphic mitochondria (Figures 4C and 4D), linking altered structure to dysfunction. Collectively, these data reveal widespread mitochondrial dysfunction in the cardiomyopathy induced by Parkin deficiency.
Figure 4. Cardiomyocyte mitochondrial dysfunction induced by Parkin suppression.
A, Merged confocal analysis of cardiomyocyte mitochondria nucleoids visualized using anti-DNA antibody (green) and cardiomyocyte-specific expression of Mito-DSRed (red). Nuclei visualized with DAPI (blue) were digitally masked and mitochondrial DNA content measured using ImageJ. Quantitative results are to the right. B and C, Depolarization of structurally abnormal mitochondria assessed by rhodamine (Rhod) 123 staining. B, Separate tincΔ4-Gal4–driven mito-GFP (green; left) and Rhod 123 (red; middle) and merged images (right) with representative depolarized (less red staining) mitochondria indicated with arrows. C, Representative merged control and Parkin RNAi mito-GFP/Rhod 123 double-stained cardiomyocytes (left) and group quantitative data (right). Two of several depolarized mitochondria are indicated by arrows.
D, Representative merged control and Parkin RNAi mito-GFP/MitoSox double-stained cardiomyocytes (left) and group quantitative data (right). Three of several reactive oxygen species–producing mitochondria are indicated by arrows.
Mitochondrial ROS Inhibition Prevents the Cardiomyopathy Evoked by Cardiac Parkin Insufficiency
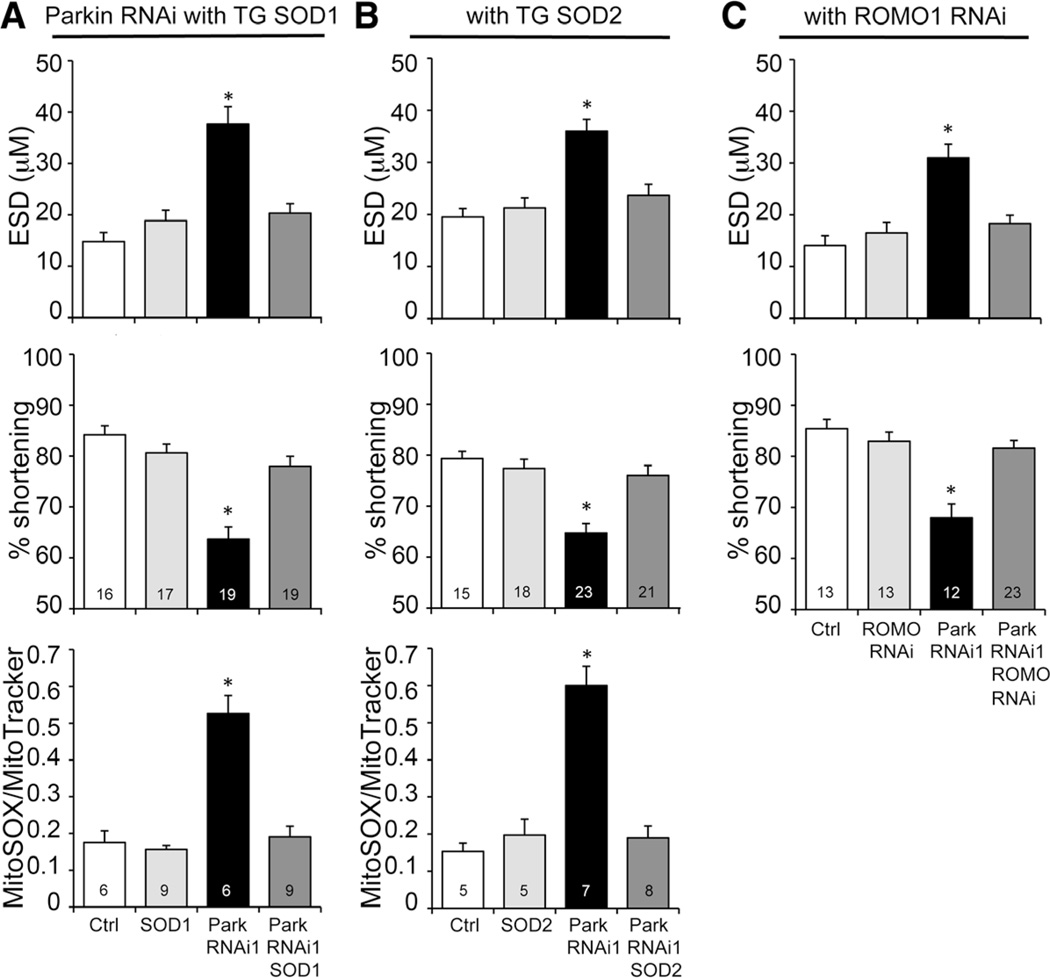
We tested whether the observed increase in mitochondria producing ROS was a cause or consequence of cardiomyopathy in cardiac-specific Parkin-deficient flies by crossing them to flies transgenically expressing either soluble (largely cytosolic) superoxide dismutase 1 (SOD1) or mitochondrial localized SOD2.31 Neither SOD1 nor SOD2 alone affected heart tube contraction measured by optical coherence tomography (Figure 5A and 5B). Strikingly, both SOD1 and SOD2 normalized heart tube contraction and the increase in mitochondrial ROS of cardiomyocyte-specific Parkin-deficient flies (Figure 5A and 5B; Online Figure IV), revealing a role for ROS in cardiac dysfunction that accrues after the dominant Parkin-mediated mitophagic pathway is suppressed.
Figure 5. Mitochondrial reactive oxygen species (ROS) production contributes to the cardiomyopathy evoked by Parkin suppression.
A and B, Results of optical coherence tomography (OCT) and MitoSOX studies of cardiomyocyte-specific Parkin RNAi flies without (black) and with (dark grey) concomitant cardiomyocyte-specific expression of superoxide dismutase (SOD) 1 (A) or SOD2 (B). End-systolic dimension (ESD) is on top, percent fractional shortening in middle, mitoSOX on bottom. C, OCT studies of cardiac Parkin-deficient flies without (black) and with (dark grey) concomitant RNAi-mediated suppression of mitochondrial ROS modulator 1 (Romo1), a major mitochondrial ROS-producing enzyme.
*Significantly different from Ctrl by ANOVA and Bonferroni test.
Because SOD2 is localized to the mitochondrial matrix, its efficacy at preventing the cardiomyopathy implicates mitochondria as the source of toxic ROS under conditions of Parkin insufficiency. Recently, ROS modulator 1 (Romo1) was identified as a major source of ROS originating from the mitochondrial electron transport chain, and Romo1-derived ROS were implicated in cellular senescence.32 We considered that Romo1 may be both physically and functionally positioned as the source of ROS produced by abnormal mitochondria that accumulate after suppression of Parkin. Consistent with this notion, cardiomyocyte-specific RNAi-mediated Romo1 suppression exhibited the same protective effects on heart tube function in cardiomyocyte-specific Parkin-deficient flies as did SOD expression (Figure 5C). These data further support interruption of normal mitophagic mitochondrial culling and accumulation of toxic damaged organelles as the primary mechanism leading to cardiomyopathy in Parkin-deficient heart tubes.
Suppressing Mitochondrial Fusion in Cardiomyocytes Rescues the Cardiomyopathy Induced by Parkin Deficiency Without Normalizing Mitochondrial Dysmorphology
We considered that the increase in ROS-producing and depolarized mitochondria in cardiomyopathic, Parkin-deficient cardiomyocytes seems disproportionate because of the unusually low rate of mitochondrial turnover reported for cardiomyocytes in Drosophila and mice.25,33,34 Previous studies suggested a functional interaction between mitochondrial quality control and dynamics pathways.12 Therefore, we postulated that ongoing mitochondrial fusion might directly contribute to cardiac dysfunction in Parkin insufficiency by promoting contamination of the normal mitochondrial pool with components of damaged mitochondria that escape mitophagic culling. If this mechanism were to be correct, then inhibiting mitochondrial fusion would improve both mitochondrial health (by preventing transorganelle contagion) and heart function (by limiting disproportionate increase in dysfunctional mitochondria) in Parkin deficiency. We tested this hypothesis by suppressing Drosophila cardiomyocyte mitochondrial fusion using cardiac-specific inhibition of MARF (mitochondrial assembly regulatory factor, the Drosophila mitofusin ortholog) on the Parkin RNAi background. Cardiomyocyte-specific Parkin suppression increased heart tube end-systolic and end-diastolic dimensions as expected (Figure 6A, black) and produced mitochondria with decreased mitochondrial DNA (Figure 6B, black), evidence for increased mitochondrial biogenesis (Figure 6C, black), increased mitochondrial ROS (Figure 6D, black), and increased numbers of depolarized organelles (Figure 6E, black). As previously reported,25 MARF suppression likewise caused a dilated cardiomyopathy (Figure 6A, grey) characterized by mitochondria with fewer genomes, increased ROS, and greater depolarization (Figure 6B, 6D, and 6E, grey), although without evidence for increased biogenesis (Figure 6C, grey). Stunningly, combined cardiac-specific Parkin and MARF suppression normalized heart tube dimension and contractile function (Figure 6A, light grey) and improved each of the indices of mitochondrial health (ie, limited the proportion of ROS-producing and depolarized organelles and increased mitochondrial DNA; Figure 6B, 6D, and 6E, light grey). (It is to be noted that unlike the Parkin-deficient cardiomyopathy, ROS production in the MARF RNAi model is not the major contributory factor; Online Figure V). The beneficial effects of suppressing mitochondrial fusion seemed unrelated to mitochondrial morphology per se, because the mitochondria of combined Parkin/MARF suppressed cardiomyocytes showed characteristics of both parent lines (ie, enlarged mitochondria from Parkin deficiency and fragmented mitochondria from MARF suppression; Figure 6F) rather than normalization of organelle morphometry. Furthermore, the benefits of MARF suppression for Parkin-insufficient hearts did not reflect normalization of some unexpected evoked increase in MARF, because MARF mRNA levels were normal in Parkin−/− and+/− flies (Figure 6G), and we have previously shown that forced mitofusin expression in Drosophila heart tubes is benign.25 Instead, genetic epistasis between Drosophila Parkin and MARF/mitofusin uncovers bidirectional contributory roles for mitochondrial dynamics in the cardiomyopathy evoked by interruption of Parkin-mediated mitophagy, and presumably for mitophagy in the cardiomyopathy produced by suppressing mitochondrial fusion.
Discussion
Here, we show that cardiomyocyte Parkin is essential to mitochondrial and cardiac hemostasis in Drosophila. This warrants re-evaluation of the notion, derived from absence of a basal cardiac phenotype in parkin null mice,16 that Parkin is dispensable to normal heart function. By interrogating genetic epistasis between Drosophila Parkin and mitofusin (MARF), we uncover a completely new mechanism for endorgan dysfunction produced by Parkin insufficiency: ongoing mitochondrial fusion contributes to mitochondrial contagion when Parkin signaling (and presumably Parkin-dependent mitophagic elimination of abnormal organelles) is impaired. These findings suggest that general mitochondrial health and end-organ function could be preserved in mitophagically impaired tissues through chronic suppression of fusion-induced mitochondrial contamination.
The PINK1–Parkin interaction is central to mitophagic quality control in most tissues, but published studies provide an inconsistent picture of the role of PINK1-Mfn2-Parkin– mediated mitophagy in normal hearts. Genetic ablation of mouse PINK1 induces cardiomyopathy,4 and ablation of the critical PINK1–Parkin intermediate, Mfn2, causes a murine cardiomyopathy,5 but genetic ablation of mouse Parkin had no impact on baseline cardiac function.16 Rather than accept that upstream activators of Parkin, but not Parkin itself, are essential to normal heart function, we uncovered compensatory upregulation of multiple functionally related E3 ubiquitin ligases. Functional compensation in Parkin null mice is also observed in neurological tissue24 and can explain minimal cardiac and neurological phenotypes.16,35 As Drosophila have only 1 Parkin-like protein (Parkin),11 fruit flies are excellent models of Parkin dysfunction, perhaps explaining why the major mechanistic insights into Parkin-mediated mitophagy and its role in Parkinson disease have derived from work in Drosophila.6,7,11–14 Our observation that homozygous and heterozygous germline parkin gene ablation and cardiomyocyte-specific Parkin suppression in fruit flies are each detrimental to mitochondrial and cardiac health suggests that a genetic approach in mice that minimizes the opportunity for compensatory regulation of putative alternate mitophagy pathways, such as cardiac-specific Parkin gene ablation, is likely to offer a different view of Parkin’s role in the mammalian heart.
The present studies are an exemplar of functional insights that can accrue from unanticipated genetic interactions revealed through epistasis. We crossed 2 flies that develop a cardiomyopathy caused by interruption of primary mitochondrial functions (mitochondrial clearance in the cardiac Parkin RNAi and mitochondrial fusion in the cardiac MARF RNAi). The result was flies with normal heart and mitochondrial function, but with mitochondrial structural abnormalities of both parent lines. It may not be intuitively obvious how breeding 2 cardiomyopathies together can result in a rescue, but this result simply reflects functional interconnectivity between mitophagy and fusion. Not only do mitochondrial fusion and mitophagy seem interdependent, but our data also suggest that balance between the 2 processes is essential to overall mitochondrial homeostasis. Thus, a primary disturbance in either the Parkin/mitophagy pathway or the MARF/fusion pathway causes cardiac disease, and restoring balance by concomitant suppression of the other arm is beneficial. Given the unusually slow rate of mitochondrial turnover in hearts compared with other tissues,33,34 it will be interesting to use a similar approach to probe the physiological limits of the mitochondrial dynamics/mitophagy equilibrium in other contexts.
A remarkable feature of the Parkin-deficient fly heart is the correlation between abnormal mitochondrial structure (enlarged or donut mitochondria) and dysfunction as measured by rhodamine 123 staining. Thus, mitochondria exhibit individual organelle abnormalities. This observation is consistent with our paradigm that specific mitochondria that become senescent or are damaged require identification, isolation, and elimination in a manner that preserves members of the normally functioning organelle population. Whereas morphologically and functionally abnormal mitochondria are occasionally observed in normal hearts, Parkin suppression increased the proportion of damaged organelles, presumably because the mitophagic mechanism for their identification and elimination was interrupted. Thus, although it is common to measure mitochondrial respiration, integrity, and toxicity as a population, studies that permit assessments of individual organelles within the overall population can provide insights that might otherwise be overlooked.
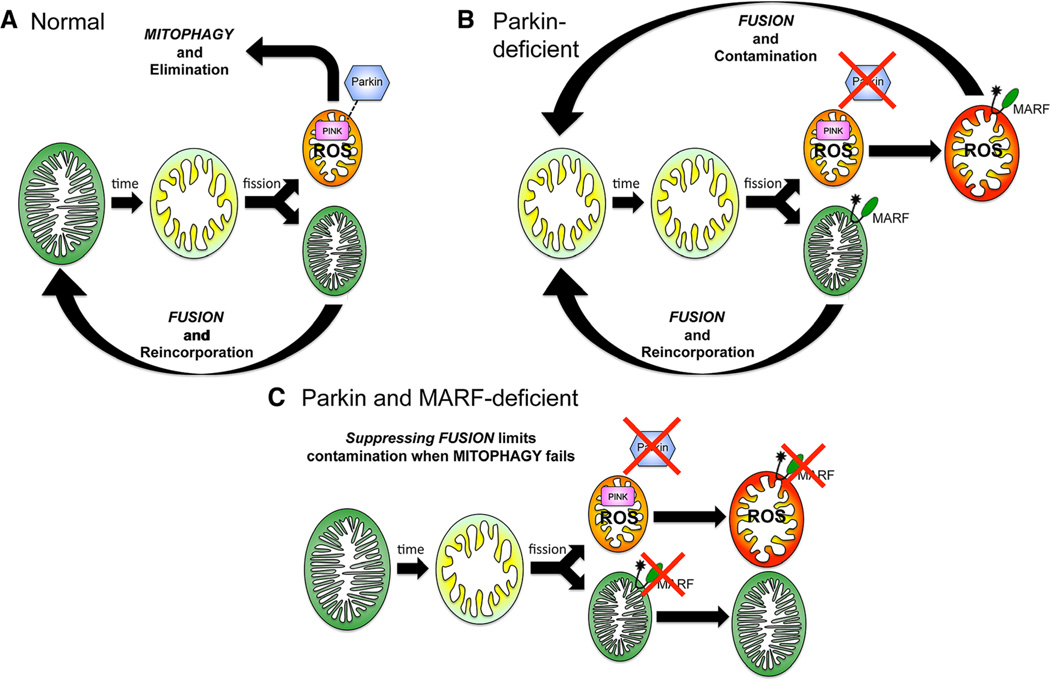
Our work offers a new mechanistic paradigm to explain genetic interactions between Drosophila Parkin and mitofusin/MARF. Deng et al12 described interactions between Parkin and mitochondrial fusion/fission proteins in Drosophila skeletal muscle, which they attributed to a putative pro-fission (or antifusion) activity of Parkin. Although we also found that Parkin-deficient mitochondria are enlarged in Drosophila cardiomyocytes, Parkin overexpression did not induce mitochondrial fragmentation that would be produced by the postulated pro-fission or antifusion effect12 (Online Figure VI). We also did not observe any detrimental effects of Parkin overexpression on normal heart tube function (see Online Figure IIE), and inhibiting mitochondrial fusion by suppressing MARF normalized Parkin-deficient heart tube contraction without normalizing mitochondrial morphology. Thus, opposing effects on mitochondrial dynamics cannot explain the genetic epistasis we detected. Instead, our findings are consistent with the schema depicted in Figure 7. When normal mitophagic organelle elimination (Figure 7A) is suppressed by Parkin insufficiency, abnormal undead or zombie mitochondria accumulate and (as zombies will do) contaminate the normal mitochondrial population by fusing with normal organelles (Figure 7B). Mitochondrial fusion that is ordinarily protective, therefore, becomes the mechanism for a general contagion of mitochondrial dysfunction. Interrupting mitochondrial fusion prevents contamination of functionally normal mitochondria by virulent zombie mitochondria (Figure 7C), sequestering abnormal mitochondria that can then be removed by alternate, albeit almost certainly less efficient, elimination pathways.
Figure 7. Interactive mitochondrial dynamics and quality control pathways as revealed by the present results.
Green mitochondria are fully polarized, red mitochondria are fully depolarized, and yellow/orange represents intermediate states.
Inhibiting mitochondrial fusion to contain mitochondrial contagion induced by mitophagic dysfunction may have applications in human diseases caused by defective mitochondrial quality control, most notably hereditary Parkinson disease induced by PINK1 and Parkin mutations. We hypothesize that cytotoxity and end-organ dysfunction can be delayed by interrupting the feed-forward pathway of mitochondrial contamination through suppression of mitochondrial fusion factors. One can envision that the pro-fusion activities of mitofusins-1 or -2 could be suppressed using pharmacological agents that inhibit the critical trans protein–protein interactions mediating mitochondrial tethering and outer membrane fusion. Compared with flies that have only 1 functional mitofusion protein (outside of male germline cells), mammals that have 2 mitofusins may offer greater flexibility for manipulating aspects of the mitochondrial fusion–mitophagy interactome. Thus, because mammalian Mfn1 and Mfn2 both promote mitochondrial fusion, it might be advantageous to individually targeted constitutive organelle fusion mediated by Mfn1 while preserving essential elements of mitophagy, cardiomyocyte differentiation, and SR–mitochondrial calcium crosstalk uniquely mediated by Mfn2.5,36,37 Likewise, the functional redundancy in mammalian mitophagy pathways that initially prompted us to develop the fly as an experimental model for disease caused by cardiac Parkin deficiency will likely enhance the overall efficacy of a therapeutic strategy aimed at reducing the mitochondrial contagion because secondary mammalian mitochondrial quality control mechanisms invoked by Parkin dysfunction will have a greater opportunity to clear damaged organelles.
Supplementary Material
Novelty and Significance.
What Is Known?
Hearts are mitochondria-rich and require mitochondrial ATP to function.
Declining cardiac function with age is associated with deterioration of mitochondrial health.
Damaged cardiac mitochondria must, therefore, constantly be identified and removed to sustain normal heart function.
What New Information Does This Article Contribute?
By studying fruit flies, we found that the Parkinson disease gene, Parkin, mediates targeting and removal of damaged mitochondria from cardiac myocytes.
In the absence of Parkin, damaged mitochondria accumulate, fuse with, and contaminate normal mitochondria, spreading organelle damage and causing heart failure.
Genetically suppressing mitochondrial fusion prevents heart failure in hearts lacking Parkin by interrupting the mitochondrial contagion.
Mitochondrial quality control is critical to normal heart function, but the responsible pathways are unclear. The PTEN-inducible kinase 1 (PINK1)–Parkin pathway is the primary mechanism by which dysfunctional mitochondria are identified and selectively eliminated in many tissues, and mutations in PINK1 and Parkin cause Parkinson disease in humans. Whereas PINK1 knockout mice develop neurological and cardiac diseases, Parkin knockout mice are inexplicably normal. Here, we uncovered compensatory upregulation of Parkin-like factors in mouse hearts lacking Parkin, explaining this apparent discrepancy. In fruit flies, which do not have multiple compensatory Parkin-like factors, systemic deletion or cardiac-specific suppression of Parkin caused mitochondrial dysmorphology and dysfunction and evoked heart failure. Remarkably, preventing fusion between abnormal and normal mitochondria by suppressing the fusion protein mitochondrial assembly regulatory factor in fly hearts prevented the cardiomyopathy induced by Parkin insufficiency. These results uncover a new mechanism that explains disproportionate cell and end-organ damage when the PINK1–Parkin mitophagy pathway is interrupted: mitochondrial contagion spreads through organelle fusion. Our findings demonstrate the feasibility of moderating organ damage in mitophagically impaired tissues by targeting mitochondrial fusion.
Acknowledgments
Sources of Funding
Supported by National Institutes of Health grants HL59888 and HL107276.
Nonstandard Abbreviations and Acronyms
- MARF
mitochondrial assembly regulatory factor
- Mfn2
mitofusin-2
- PINK1
PTEN-inducible kinase 1
- Romo1
ROS modulator 1
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.114.302734/-/DC1.
Disclosures
None.
References
- 1.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 2.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 3.Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Dorn GW., II PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 7.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 8.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, Pon LA. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8:CR241–CR246. [PubMed] [Google Scholar]
- 20.Zesiewicz TA, Strom JA, Borenstein AR, Hauser RA, Cimino CR, Fontanet HL, Cintron GB, Staffetti JF, Dunne PB, Sullivan KL. Heart failure in Parkinson’s disease: analysis of the United States medicare current beneficiary survey. Parkinsonism Relat Disord. 2004;10:417–420. doi: 10.1016/j.parkreldis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Guo M. Drosophila as a model to study mitochondrial dysfunction in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009944. doi: 10.1101/cshperspect.a009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., II Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res. 2010;106:1459–1467. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn GW, II, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, Matkovich SJ, Jowdy CC. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res. 2011;108:12–17. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eschenbacher WH, Song M, Chen Y, Bhandari P, Zhao P, Jowdy CC, Engelhard JT, Dorn GW., II Two rare human mitofusin 2 mutations alter mitochondrial dynamics and induce retinal and cardiac pathology in Drosophila. PLoS One. 2012;7:e44296. doi: 10.1371/journal.pone.0044296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandebring A, Dehvari N, Perez-Manso M, Thomas KJ, Karpilovski E, Cookson MR, Cowburn RF, Cedazo-Mínguez A. Parkin deficiency disrupts calcium homeostasis by modulating phospholipase C signalling. FEBS J. 2009;276:5041–5052. doi: 10.1111/j.1742-4658.2009.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calì T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta. 2013;1832:495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka A. Parkin-mediated selective mitochondrial autophagy, mitophagy: Parkin purges damaged organelles from the vital mitochondrial network. FEBS Lett. 2010;584:1386–1392. doi: 10.1016/j.febslet.2010.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ, Chung JS, Yoo YD. Replicative senescence induced by Romo1-derived reactive oxygen species. J Biol Chem. 2008;283:33763–33771. doi: 10.1074/jbc.M805334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Y, Dorn GW., II Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim TY, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–1594. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey BK, Wang Y, Hoffer BJ. Transgenic rodent models of Parkinson’s disease. Acta Neurochir Suppl. 2008;101:89–92. doi: 10.1007/978-3-211-78205-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Csordás G, Jowdy C, Schneider TG, Csordás N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, II, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasahara A, Cipolat, Chen Y, Dorn GW, II, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin/notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.