Abstract
Recent insights into the neural circuits controlling energy balance and glucose homeostasis have rekindled the hope for development of novel treatments for obesity and diabetes. However, many therapies contribute relatively modest beneficial gains with accompanying side effects, and the mechanisms of action for other interventions remain undefined. This Review summarizes current knowledge linking the neural circuits regulating energy and glucose balance with current and potential pharmacotherapeutic and surgical interventions for the treatment of obesity and diabetes.
Introduction
Obesity, diabetes, and associated disorders represent a major public health challenge for North America, Europe, and increasingly the rest of the world. Both obesity and diabetes inflict health and economic burdens that require coordinated strategies to both prevent and treat these disorders. Indeed, a major barrier in the management and prevention of obesity is that weight loss due to lifestyle changes alone is inherently difficult. For many, this means that dieting-induced weight loss initially results in tangible beneficial effects but is often followed by a return to previous energy intake and consequently a rebound weight gain.
Numerous neurobiological and physiological mechanisms that regulate energy balance exist. In particular, it has become increasingly evident that the brain plays an important role in sensing energy demands and storage in order to maintain/defend body weight within a rather tight range. Studies ranging from worms, flies, and mice to humans have identified key conserved genes and neural pathways that are critical in regulating energy balance and glucose homeostasis. Moreover, the identification of human mutations in these or analogous pathways has led to hope that it may be possible to develop rational strategies based on animal model studies that may ultimately lead to successful therapeutic intervention in humans. In this Review, we will highlight how advances in understanding the neurophysiology underlying metabolism, including an increased understanding of neural circuits, may hold promise for development of adjunct therapies in the treatment of obesity and associated co-morbidities, including diabetes. Several recent Reviews have provided more detailed information and review of the primary literature regarding the respective circuits and approaches highlighted here (Barsh et al., 2000; Cone, 2005; Deisseroth, 2012; Farooqi and O'Rahilly, 2005; Heisler et al., 2003; Myers and Olson, 2012; Powley et al., 2005; Schwartz and Porte, 2005; Wikberg and Mutulis, 2008).
A Brief Overview of Neural Circuits Regulating Feeding and Energy and Glucose Homeostasis
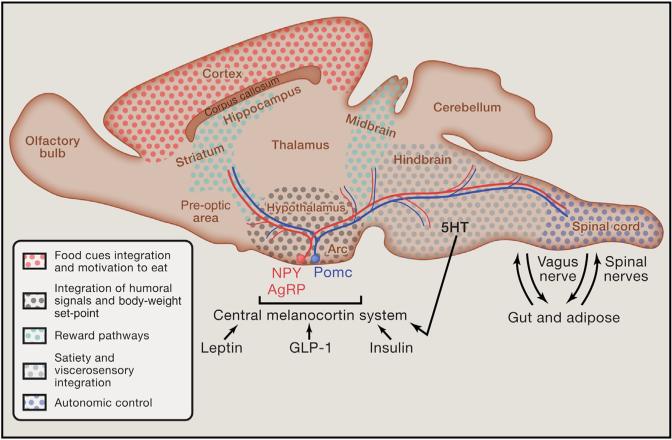
The central melanocortin system is comprised of neurons in the hypothalamic arcuate nucleus and brainstem that produce pro-opiomelanocortin (Pomc), the precursor polypeptide of the biologically active melanocortin receptor peptide agonist, α-melanocyte-stimulating hormone (α-MSH). Additional peptides within the arcuate nucleus that contribute to the melanocortin system include Agouti gene-related peptide (AgRP), an endogenous inverse agonist of the melanocortin 4 receptor (Mc4r), and Neuropeptide Y (NPY), which is co-expressed with AgRP. Elucidating the physiological importance of this system in regulating energy balance and glucose homeostasis brought the hypothalamic arcuate nucleus to the forefront of research aimed at understanding the neural control of energy balance (Cone, 2005; Schwartz and Porte, 2005).
Pomc and NPY/AgRP neurons are prototypical players in the regulation of energy intake and expenditure for several reasons. In particular, exogenous administration of α-MSH potently inhibits food intake via activation of central melanocortin receptor-expressing neurons (Cone, 2005; Rossi et al., 1998; Schwartz and Porte, 2005). Conversely, administration of NPY effectively stimulates food intake via action at NPY-Y receptors in the brain (Clark et al., 1984; Yulyaningsih et al., 2011). Several studies have used opto- and chemogenetic techniques to attempt to manipulate the activity of varying genetically targeted populations of neurons with a role in feeding behavior and metabolism, including but not limited to AgRP neurons (Aponte et al., 2011; Atasoy et al., 2012; Krashes et al., 2011; Krashes et al., 2013) and Pomc neurons (Aponte et al., 2010; Zhan et al., 2013). Stimulation of arcuate Pomc neurons resulted in a reduction in food intake, whereas activation of arcuate AgRP neurons resulted in increased food intake and food-seeking behaviors (Aponte et al., 2010; Krashes et al., 2011; Zhan et al., 2013). The Pomc-induced reduction in food intake was dependent upon melanocortin receptors within the paraventricular hypothalamus (PVH), a hypothalamic nucleus that is a direct target of arcuate melanocortin neurons. Stimulation of arcuate AgRP neurons elicited feeding behavior via projections to the thalamus, the hypothalamus, and the basal forebrain (Atasoy et al., 2012). Interestingly, either the neurotransmitter GABA or NPY is required for the rapid stimulation of feeding, whereas the neuropeptide AgRP, through action on Mc4 receptors, is sufficient to induce feeding over a delayed yet prolonged period (Krashes et al., 2013). GABA from NPY/AgRP neurons of the hypothalamic arcuate nucleus may also play an important role in the regulation of feeding behaviors via direct actions within the hindbrain (Wu et al., 2009). Moreover, neurons of the nucleus of the solitary tract in the caudal medulla might counterbalance this activity (Wu et al., 2012). Neurons of the PVH also reciprocally innervate arcuate NPY/AgRP neurons, providing a regulatory loop in the control of feeding behavior (Krashes et al., 2014). Collectively, these data suggest that in addition to a core circuit, AgRP neuron projections and reciprocal innervations may reveal key modulatory circuit nodes that are gated or otherwise regulated by AgRP neuron projections (Atasoy et al., 2012). Importantly, it's currently unclear which circuits are acutely involved in other complex metabolic processes such as energy expenditure and glucose metabolism. Undoubtedly, similar parallel and redundant mechanisms involved in regulating feeding behavior will be intertwined with various aspects of metabolism.
Both NPY/AgRP and Pomc neurons are also sensitive to metabolic status (Cone, 2005; Yulyaningsih et al., 2011). In particular, NPY/AgRP neurons are activated during fasting, whereas Pomc neurons are activated following feeding and inhibited during fasting. NPY/AgRP and Pomc neurons are also well positioned to sense and integrate numerous nutrient and humoral signals (Schwartz and Porte, 2005). Indeed, many of these signals in the periphery and/or the central nervous system (CNS) have been attributed to regulating feeding behavior and energy expenditure via activity at these neurons (Figure 1). For example, increased serum levels of the adipocyte-derived anorexigenic peptide leptin activate Pomc neurons and stimulate the production as well as the release of α-MSH (Cone, 2005; Schwartz et al., 1997). Leptin inhibits NPY/AgRP neurons concomitantly suppressing the production and release of NPY and AgRP. These data and others support a primary role of arcuate Pomc and NPY/AgRP neurons as first-order neurons in the neural control of energy balance. However, it must be noted that leptin-sensitive neurons distributed across the brain may contribute to the control of feeding. For instance, loss of leptin receptors in either arcuate Pomc neurons or adjacent neurons of the ventromedial hypothalamus (VMH) results in modest increases in adiposity largely dependent upon deficits in energy expenditure (Myers and Olson, 2012). Moreover, deletion of leptin receptors in both Pomc and VMH neurons resulted in an additive effect on body weight. Among other examples, leptin receptors on GABAergic neurons of the lateral hypothalamic area (LHA) modulate the mesolimbic dopaminergic system and decrease feeding (Leinninger et al., 2009). Also, deficiency of leptin receptors in a brainstem site called the dorsal vagal complex causes hyperphagia, resulting in modest weight gain (Scott et al., 2011; Skibicka and Grill, 2009). Collectively, these data support a model of a distributed network of melanocortin- and leptin-responsive neurons contributing to the central regulation of energy homeostasis. Not surprisingly, numerous nutrient and humoral receptors are also widely distributed within the CNS, and researchers are identifying multiple divergent and redundant roles for these receptors in the regulation of metabolism.
Figure 1. Integrated Model of the Central Melanocortin System and Connected Regions within the Nervous System Involved in Obesity and Diabetes.
Recent discoveries highlight a circuitry within the brain that includes the hypothalamus as well as midbrain/hindbrain areas in the acute regulation of energy expenditure. This circuitry has also been demonstrated to have an overlapping role in the management of glucose homeostasis. Importantly, several neurotransmitters and peptides contribute to a distributed network of receptor systems within this circuit and provide potential substrates for non-surgical therapeutic intervention. Similarly, advancing technologies have raised potential surgical and non-surgical manipulation of cellular and nerve activity as a viable strategy in this circuit to combat obesity and diabetes. Abbreviations: NPY = neuropeptide Y; Pomc = pro-opiomelanocortin; AgRP = Agouti gene-related peptide; Arc = arcuate nucleus; GLP-1 = glucagon-like peptide 1.
The aforementioned evidence supports a well-defined action of the central melanocortin system in regulating food intake and body weight. However, it is less appreciated that the central melanocortin system plays an important role in regulating glucose metabolism, including insulin release and action (Farooqi and O'Rahilly, 2005; Schwartz and Porte, 2005). In fact, deficits in melanocortin signaling result in hyperinsulinemia, fasting hyperglycemia, and frank diabetes (Berglund et al., 2014; Cone, 2005; Myers and Olson, 2012). However, restoring Mc4r expression selectively in the PVH reduces hyperphagia and largely restores deficits in body weight independent of improved glucose or insulin intolerance (Myers and Olson, 2012). In parallel, loss of Mc4rs in parasympathetic and sympathetic nuclei of the brainstem and spinal cord results in glucose and insulin intolerance largely independent of changes in food intake or body-weight gain (Berglund et al., 2014; Myers and Olson, 2012). Thus, the wide distribution of melanocortin receptors concomitant with projection patterns of Pomc and NPY/AgRP neurons contributes to their divergent effects on energy and glucose homeostasis.
Several mutations in Mc4r have been identified and may represent the most common form of monogenic obesity in humans (Barsh et al., 2000; Farooqi and O'Rahilly, 2005). Importantly, the melanocortin system is remarkably conserved across species from zebrafish and rodents to humans (Farooqi and O'Rahilly, 2005; Sebag et al., 2013). Thus, neural circuits regulating metabolic homeostasis in the mammalian hypothalamus have an ancient origin, dating back to teleost fish. This long evolution may explain the complexity as well as some of the main anatomical and functional principles of these circuits governing energy homeostasis in the mammalian hypothalamus.
In Search of Effective Appetite Suppressants
Although dieting and balanced nutrition are key to the management of most, if not all, metabolic disorders, most dieters struggle to maintain a rigid dietary regimen. Why is dieting so difficult? There is certainly no single straightforward answer to this question; however, brain-imaging studies in humans have offered clues to neural correlates of obesity and responses to dieting. In particular, dieting in humans changes the activity of interconnected brain regions involved in the neural representations of hunger and satiety and the anticipation of reward (Rosenbaum et al., 2008). Neural activity induced by food cues also differs between obese and lean individuals in the prefrontal cortex (Zheng and Berthoud, 2008). Paradoxically, these regions are involved in the motivation to eat and are normally stimulated by dieting and starvation. Typically, the brains of obese individuals appear to react to food cues as if these individuals were in a state of negative energy balance (instead of energy repletion). Put another way, defective brain processing in response to food cues in obesity may result in inadequate reward and/or motivational processes and, ultimately, hyperphagia.
Driven by various biological and environmental factors, hyper-phagia is largely responsible for the current epidemic of obesity and type 2 diabetes (T2DM). Thus, any successful weight-loss strategy must address the issue of appetite. Accordingly, many drugs approved by the US Food and Drug Administration (FDA) for the treatment of obesity directly act on the nervous system to suppress appetite (Table 1 and Figure 2) (Vetter et al., 2010). The only peripherally acting drug currently approved to treat obesity is Orlistat (trade name: Xenical or Alli), an inhibitor of lipase activity that prevents fatty-acid absorption from the diet (Bray, 2014). However, this drug commonly promotes loose stools and, due to its lack of effect on the brain, minimally reduces appetite. As the field continues to build an understanding of neural circuitry regulating appetite, it has become evident that a very large number of neural pathways and molecules are implicated in modulating appetite levels. Consequently, the neural circuits involved in metabolism offer numerous avenues for therapeutic intervention in the treatment of hyperphagia (Figure 2). Below we include a brief discussion on current appetite suppressants and promising pharmacotherapeutics, with a special emphasis on their potential mechanisms of action (Table 1 and Figure 2). Information on the clinical features and medical recommendations for the drugs presented below can be found elsewhere (Bray, 2014).
Table 1.
Select List of Current and Potential Future Therapeutics for the Treatment of Obesity and Diabetes
| Pharmacology | |||||
|---|---|---|---|---|---|
| Drug/Compound | Trade/Brand Name | Receptors/Molecular Pathways |
FDA Approval | Applications(s) | Brain Area and/or Tissues Involved |
| Lorcaserin | Belviq | 5ht2cr agonist | yes–2012 | obesity | hypothalamus, cortex, midbrain, and brainstem |
| Liraglutide | Victoza | GLP-1r agonist | yes–2012 | obesity and diabetes | hypothalamus |
| Topiramate/Phentermine | Qysemia | inhibit several ionic conductances as well as carbonic anhydrase isozymes/stimulate the release of serotonin-norepinephrine-dopamine | yes–2012 | obesity | hypothalamus and brainstem |
| Buproprion/Naltrexone | Contrave | dopamine and norepinephrine reuptake inhibitor/opioid receptor antagonist | yes–2014 | obesity | hypothalamus |
| D-fenfluramine | Pondimin, Ponderax, Adifax | 5ht2cr agonist | withdrawn | obesity | hypothalamus and brainstem |
| Orlistat | Xenical and Alli | inhibit gastric and pancreatic lipases | yes–1999 | obesity | gut |
| Sibutramine | Meridia | serotonin-norepinephrine-dopamine reuptake inhibitor | withdrawn | obesity | hypothalamus and brainstem |
| Ribonamant | Zimulti | CB1R antagonist | withdrawn | obesity | hypothalamus and brainstem |
| Mc4R agonists | N/A | Mc4R | no | obesity | hypothalamus and brainstem |
| Exenatide | Byetta, Bydureon | GLP-1r agonist | yes –2005 | T2DM | hypothalamus |
| Metformin | Glucophage, Glumetza, Glucophage XR, Fortamet | suppress gluconeogenesis pathways | yes–1995 | T2DM | liver |
| Pramlintide | Symlin | amylin receptor agonist | yes–2005 | T2DM | hypothalamus and brainstem |
| Leptin | Metreleptin | LepRB agonist | nob | type 1 and 2 diabetes | hypothalamus |
| Device-Assisted | ||||
|---|---|---|---|---|
| Device | Receptors/Molecular Pathways |
FDA Approval | Application(s) | Brain Area and/or Tissues Involved |
| hfDBS | neuronal excitability | noa | obesity | cortex |
| tDCs | neuronal excitability | noa | obesity | cortex |
| VNS | neuronal excitability | noa | obesity | cervical vagus nerve |
| VBLOC | neuronal excitability | yes–2015 | obesity | gastric vagus nerve |
| Lap-band – laproscopic adjustable gastric band | unknown | yes–2001 | obesity | gut-brain communication |
| Roux-en-Y gastric bypass | unknown | N/A | obesity and T2DM | gut-brain communication |
| Vertical sleeve gastrectomy | unknown | N/A | obesity and T2DM | gut-brain communication |
Approved for indications not related to metabolic outcomes.
Approved for the metabolic disorders of lipodystrophies.
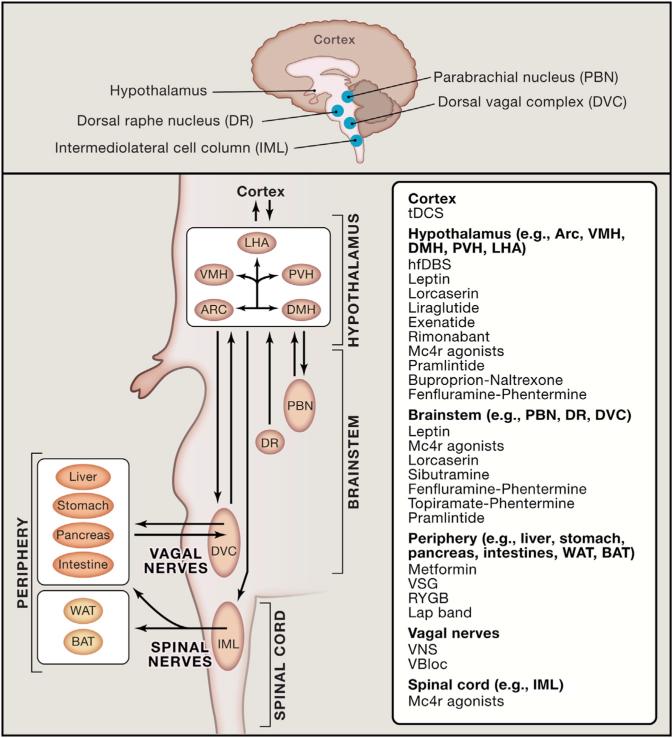
Figure 2. Selected Therapeutic Options for Treating Obesity and Diabetes by Targeting the Brain.
Current and promising therapeutics in the treatment of both obesity and diabetes have targeted neural connections including the melanocortin system, via pharmacological and/or device-assisted methods. Here we summarize a select number of targets (i.e., receptors and brain regions) that have been demonstrated to regulate at least in part effects of energy balance and glucose homeostasis.
Abbreviations: hfDBS = high-frequency deep brain stimulation; VBLOC = vagal blocking; VNS = vagal nerve stimulation; Mc4r = melanocortin 4 receptor; DVC = dorsal vagal complex; PBN = parabrachial nucleus; tDCS = transcranial direct current stimulation; Arc = arcuate nucleus; VMH = ventrome-dial hypothalamic nucleus; LHA = lateral hypothalamic area; DMH = dorsal medial hypothalamic nucleus; PVH = paraventricular hypothalamus; DVC = dorsal vagal complex; IML = intermedio-lateral cell column; BAT = brown adipose tissue; WAT = white adipose tissue; DR = dorsal raphe nucleus.
Overview of Current Anti-Obesity Drugs
Serotoninergic neurons play critical roles in the suppression of feeding (Heisler et al., 2003). Accordingly, treatments that suppress central serotoninergic signaling result in hyperphagia and weight gain in humans and rodents. Lorcaserin (trade name: Belviq), an agonist of the serotonin receptor 5-HT2CR, reduces body weight largely dependent upon lowered food intake in both humans and rodents (Martin et al., 2011; Smith et al., 2010; Thomsen et al., 2008). Although 5-HT2CRs are widely expressed across the neuraxis (Julius et al., 1988), accumulating evidence in rodents indicates that 5-HT2CR reduces appetite and body weight by acting on Pomc neurons (Xu et al., 2008, 2010b). Notably, Lorcaserin is currently the only FDA-approved drug monotherapy for obesity that specifically targets the brain. However, clinical trials indicate that Lorcaserin yields weight losses of 5%–10% of initial body weight after 1 year (Smith et al., 2010) and is not marketed in Europe.
The naturally occurring peptide glucagon-like peptide 1 (GLP-1) induces multiple desirable anti-diabetic and anti-obesity actions, and protease-resistant long-acting GLP-1 analogs are currently available for the treatment of T2DM (Drucker, 2006). Initial interest in the beneficial effects of GLP-1 on body weight stemmed from the observation that GLP-1 inhibits food intake (Turton et al., 1996). Both Exenatide (trade name: Byetta or Bydureon—a synthetically derived exendin-4) and Liraglutide (trade name: Victoza—a fatty acid-modified GLP-1) are long-acting GLP-1 analogs that effectively lower blood glucose in diabetic patients and are approved by the FDA (see Table 1) to treat T2DM (Juhl et al., 2002; Madsbad et al., 2004). Recent evidence further suggests that both Exenatide and Liraglutide crosse the blood-brain barrier (Drucker, 2006; Hunter and Hölscher, 2012) and specifically act at the level of the hypothalamus to reduce appetite (Drucker, 2006; Secher et al., 2014; Sisley et al., 2014). Importantly, the FDA recently approved Liraglutide for the treatment of chronic weight management. In particular, Liraglutide results in sustained weigh loss of 5%–10% of initial body weight in obese patients (Astrup et al., 2009), which can be attributed to both reduced appetite (concomitant with inhibition of gastric emptying) and elevated energy expenditure in T2DM individuals (Horowitz et al., 2012). While Liraglutide is a well-tolerated drug, its long-term side effects are not well known, and some concern has been raised due to a possible risk of pancreatic cancer associated with GLP-1 agonist treatment (Cohen, 2013). Moreover, GLP-1 receptor agonists increase heart rate and blood pressure in rats by stimulating the sympathetic outflow to the cardiovascular system (Yamamoto et al., 2002).
Current monotherapeutic approaches in the treatment of obesity and diabetes have not been optimally effective. Emerging strategies include combination drug therapy targeting multiple signaling mechanisms. Combination therapies have been more efficacious than the aforementioned monotherapies, presumably due to the additive and synergic effects previously observed by the drugs separately. There are two combination drug therapies currently marketed in the treatment of obesity, Phentermine/Topiramate (trade name: Qysemia) and Bupro-pion/Naltrexone (trade name: Contrave). All four compounds have been previously approved on an individual basis by the FDA in the treatment of various neurological disorders. Phenter-mine and Bupropion are well-known stimulators of serotonin and norepinephrine release from nerve endings. The enhanced release of norepinephrine in sympathetically innervated tissues is likely to increase metabolism (Nedergaard and Cannon, 2014). Moreover, at high doses, these two drugs effectively reduce food intake and body weight in laboratory rodents (Roth et al., 2008; Wright and Rodgers, 2013). Topiramate is an anti-convulsant anecdotally reported to induce weight loss (Astrup et al., 2004). Naltrexone is an antagonist of opioid receptors with little effect on food intake when administered alone (Greenway et al., 2009). Although Naltrexone may act by increasing the firing rates of Pomc neurons (Greenway et al., 2009), the exact mechanisms of action of Naltrexone and Topiramate on energy balance are unclear. Importantly, the FDA has recently approved the drug combinations Contrave and Qysemia as an adjunct for chronic weight management (Table 1) with both medications promoting weight losses of 8% to 10% of initial body weight (Allison et al., 2012; Garvey et al., 2012; Greenway et al., 2009). Interestingly, another combination medication called Empatic (trade name) that contains both Bupropion and Zonisamide (another anti-convulsant) is currently under phase 2 clinical trial (Jackson et al., 2014). Thus, with the recent FDA approval and/or promising clinical trial results, combination therapies show promise for therapeutic efficacy in the treatment of obesity.
Novel Paradigms in Anti-Obesity Pharmacotherapy
Although many compounds acting on neural pathways controlling appetite, glucose metabolism, and energy expenditure were found to induce marked body-weight loss in animal models and human clinical trials, leading to FDA approval, many of these pharmacotherapies were subsequently abandoned due to serious safety concerns (Bray, 2009) (Table 1). This was the case for D-fenfluramine (D-fen; trade name: Pondimin, Ponderax, or Adifax), a pharmacological agent that increases serotonin content by stimulating synaptic release of serotonin and blocking its reuptake into presynaptic terminals (Connolly et al., 1997). Although D-fen showed potent anorexigenic activity in humans (McGuirk et al., 1991), it was removed from the market in 1997 due to its link to the development of cardiovascular complications (Connolly et al., 1997). Other centrally acting drugs have succumbed to a similar fate, including Rimonabant (trade name: Zimulti), an antagonist of the type I cannabinoid receptor Cb1R. Rimonabant's anti-obesity actions have been attributed to altered excitability of Cb1Rs located in key hypothalamic, striatal, and brainstem neurons (Cota et al., 2006). Although Rimonabant was highly effective in reducing food intake and body weight, its development was halted in 2009 due to its serious gastrointestinal and adverse psychiatric effects. Although combinatorial approaches have already raised hope for safer ways of treating obese patients, even currently FDA-approved drugs are not without safety concerns. Another caveat of currently available drugs is their modest efficacy when compared to bariatric surgery (Colquitt et al., 2014). The issues discussed above have stimulated research aimed at identifying new classes of centrally acting appetite suppressants, some of which are presented below.
Mc4 receptors have been considered prime targets in the search for effective therapeutics managing obesity (Van der Ploeg et al., 2006; Wikberg and Mutulis, 2008). In fact, several Mc4 receptor agonists were reported to produce significant reduction in adiposity in humans and monkeys (Fehm et al., 2001; Kievit et al., 2013; Wellhöner et al., 2012). However, Mc4 receptor agonists have not progressed to the clinic due to potential side effects, in particular those affecting the autonomic nervous system. For example, Mc4 receptor agonists such as melanotan II may result in hypertension and priapism (Greenfield et al., 2009; Van der Ploeg et al., 2002). These undesirable effects are likely caused by the stimulation of preganglionic autonomic neurons expressing Mc4 receptors (Sohn et al., 2013). However, all Mc4 receptor agonists may not similarly alter autonomic function. A small peptide, RM-493, has been demonstrated to effectively induce weight loss in non-human primates and humans with no major impact on cardiovascular function (Kievit et al., 2013). This agent is currently in clinical trials for genetic obesity. Interestingly, Mc4 receptors may also be modulated by the melanocortin receptor accessory proteins (Mrap1 and Mrap2) (Asai et al., 2013; Sebag et al., 2013). These Mraps may provide a substrate to differentially target the beneficial effects of Mc4 receptor activity on energy expenditure, glucose utilization, and other metabolic parameters while minimizing the adverse side effects. Recent work has also highlighted a potential role for the melanocortin system to regulate brown adipose tissue thermogenesis as well as the browning/beiging of white adipose tissues (Berglund et al., 2014; Dodd et al., 2015; Nedergaard and Cannon, 2014; Ruan et al., 2014; Williams et al., 2014). It's currently unclear what contribution this thermogenesis may play in the regulation of body composition and glucose homeostasis; however, these data may aid in the development of Mc4 receptor agonists to combat obesity and diabetes.
Recent efforts have also focused on exploring new ways of potentiating the efficacy of currently available appetite suppressants, including, most notably, leptin. Shortly after its discovery (Zhang et al., 1994), leptin was shown to completely reverse the hyperphagic and obese phenotype of leptin-deficient animals and humans (Farooqi and O'Rahilly, 2005; Licinio et al., 2004; Pelleymounter et al., 1995). Unfortunately, obesity is characterized by hyperleptinemia (Considine and Caro, 1996), rather than leptin deficiency. Thus, to the vast majority of morbidly obese individuals, leptin monotherapy is ineffective in lowering food intake or body weight. However, since the normal fall of leptin that accompanies weight loss is detected by the brain as a starvation signal (Ahima and Flier, 2000), it has been proposed that leptin administration during the course of dieting may amplify weight loss and reinforce compliance (Rosenbaum et al., 2008).
Several gut peptides have been demonstrated to potently enhance or restore leptin sensitivity in diet-induced obesity. Most notably, amylin, a pancreatic-derived small peptide, has been shown to successfully enhance the effects of leptin administration on energy balance in obese rodents as well as in humans (Ravussin et al., 2009; Trevaskis et al., 2008). In addition, recent advances in the synthesis of peptides with co-agonistic properties have permitted the development of a new generation of anti-obesity molecules. This includes a GLP-1::glucagon unimolecular co-agonist (Day et al., 2009). This molecule was shown to effectively reduce body weight in obese animals to almost 30% of initial body weight in only 1 month. Leptin-induced body-weight loss has also been shown to be considerably potentiated by the GLP-1::glucagon co-agonist (Clemmensen et al., 2014). The chronic co-administration of leptin and GLP-1::glucagon yielded an impressive 50% weight loss in obese mice over 1 month and normalized glucose intolerance.
Another promising co-agonistic (dual peptide) strategy consists of delivering peptide agonists with an attached (linker) small molecule. This strategy allows for the selective delivery of a complex molecule to particular target cells (Finan et al., 2012). In the first usage of this strategy, researchers capitalized on the monoagonistic properties of GLP-1 and the sex steroid hormone, estrogen, to improve metabolic parameters of obesity and T2DM. A fully active GLP-1 agonist stably linked to estrogen consistently proved to be more efficacious in lowering body weight than either molecule alone. Additionally, these effects of the GLP-1::estrogen conjugates were independent of adverse gyneco-logical and oncogenic outcomes. This strategy appears to uniquely combine potency with specificity; however, the molecular mechanism of these beneficial effects remains to be elucidated. Importantly, these co-agonist data suggest that the maximally achievable and sustainable body-weight loss of 10%, observed with currently available pharmaceutics, may not reflect an insurmountable physiological barrier (Day et al., 2009). Hence, co-agonistic pharmacotherapy as well as varied leptin sensitizers and new classes of chemical compounds hold promise as an efficacious approach in the management of obesity.
Finally, it must be noted that novel centrally acting molecules regulating metabolism are regularly being discovered. Among other examples, FGF21 was recently described as a potent anti-obesity factor predominately affecting energy expenditure (Bookout et al., 2013; Owen et al., 2014; Sarruf et al., 2010) and, in humans, exhibiting a marked lipid-lowering effect (Gaich et al., 2013). Based on genetic studies in rodents, the anti-obesity actions of FGF21 have been attributed to its direct action on the nervous system (Bookout et al., 2013; Owen et al., 2014; Sarruf et al., 2010). However, concurrent data indicate that the adipose tissue, rather than the nervous system, is required for FGF21 anti-obesity actions (Adams and Kharitonenkov, 2012). The regulation of glucose metabolism by FGF21 has also been attributed to direct actions on the liver (Adams and Kharitonenkov, 2012). Further work should paint a fuller picture of FGF21's activities, and overall, the field is hopeful that existing and/or novel ligands currently under development will be efficacious in the treatment of obesity.
Device-Assisted Neuromodulatory Techniques
Device-assisted neuromodulation refers to delivery of an electrical current to either a specific nerve or a particular brain region in order to influence brain activity and autonomic outflow. Devices include but are not limited to stimulators of the spinal cord, vagus nerve, and sacral nerve; deep brain stimulators; and gastric electric stimulators. Although many of these devices have not been approved by the FDA for the treatment of metabolic disorders, accumulating evidence suggests that modulation of targeted brain sites or peripheral nerve activity by device-assisted means is a valuable tool in the treatment of many chronic diseases (Famm et al., 2013).
The idea of being able to target a particular brain site is attractive, considering the unwanted effects of many pharmacological compounds. Furthermore, peripheral nerves are increasingly recognized to play a critical role in metabolic functions (Bartness et al., 2014; Powley et al., 2005). The vagus nerve is a mixed (sensory and motor) nerve that innervates most of the thoracic and the abdominal viscera, including the entire gastrointestinal tract, pancreas, and liver (Berthoud and Neuhuber, 2000). It is well established that vagal sensory neurons convey a wide variety of signals originating from the gastrointestinal tract, including mechanical stretch, changing levels of nutrients, lipids, immune signals, and gut peptides (de Lartigue et al., 2011). Experimental observations also indicate the reduced ability of vagal afferents to respond to dietary and endogenous metabolic signals in animals fed on high-fat diets (Kentish et al., 2012). These observations strongly support the idea that the vagus nerve serves as a critical link between the gut and the brain and that this link is impaired in obesity. Based on the aforementioned literature linking vagal afferents to post-prandial functions and the regulation of feeding, stimulation of vagal afferents has been hypothesized to be a promising anti-obesity approach and a potential alternative to bariatric surgery (Powley et al., 2005). Pre-clinical studies in large animals and humans suggest that vagus nerve stimulation (VNS) might show efficacy in reducing food craving and weight gain (Pardo et al., 2007; Val-Laillet et al., 2010). A technique of vagal blocking has also been tested in pre-clinical studies (VBLOC) and recently approved by the FDA as a weight-loss treatment device in obese individuals. Briefly, this technique targets the nerve pathway between the brain and the stomach by stimulating the vagal trunks at high frequency, thus interfering with normal gastric functions and leading to early satiation (Camilleri et al., 2008). However, contradictory results have been obtained as to the benefits of this novel weight-loss approach in obese subjects, with a couple of studies suggesting a significant weight loss and reduced food craving (Camilleri et al., 2008; Shikora et al., 2013), and others finding no significant benefits (Ikramuddin et al., 2014; Sarr et al., 2012).
Central neurostimulatory techniques may also be of interest in the treatment of obesity. In particular, high-frequency deep brain stimulation (hfDBS, Table 1) has been effective at treating the symptoms associated with Parkinson's disease and other disabling neurological disorders by normalizing pathological patterns of neuronal activity (Wichmann and Delong, 2006). This technique involves the chronic implantation by stereotaxic surgery of stimulation electrodes in a targeted brain site. Electric current is delivered to electrodes connected to a pulse generator similar to a pace maker. Stimulation of the hypothalamus in humans is feasible and has been recently used in morbidly obese humans to target the LHA (Whiting et al., 2013). Stimulation of the LHA succeeded in increasing resting metabolic rate, leading to reduced binge-eating scores and/or body weight in all three test subjects. Similar results were obtained in a rat model, supporting the idea that this technique could be considered as an option for the treatment of obesity (Soto-Montenegro et al., 2014).
It should be noted that brain surgery is associated with inherent risks of hemorrhage, infection, and post-surgical complication. Less invasive strategies that target the activity of sub-populations of neurons may pose fewer concerns. In particular, transcranial direct current stimulation (tDCS) is emerging as a promising technique for non-invasive neuromodulation in a variety of clinical conditions (Dayan et al., 2013). This approach allows for the modification of neuronal excitability in regions involved in specific behaviors by delivering a weak current through the scalp (Dayan et al., 2013). Thus, tDCS is suited for cortical targets, specifically lateral and dorsomedial sectors of the prefrontal cortex that contribute to cognitive control. Importantly, this technique has shown promise for acutely reducing food craving (Fregni et al., 2008; Montenegro et al., 2012) and may be suitable for obese individuals (Boggio et al., 2009; Truong et al., 2013). Although the field embraces these novel methodologies, others are still needed in order to facilitate therapeutic opportunities in the treatment of various neurological pathologies, including those contributing to obesity.
Treating Diabetes by Targeting the Brain
Many of the currently available drugs to treat T2DM are peripherally acting compounds (Table 1). This includes molecules acting on glucose co-transporters, incretin (gastrointestinal hormone that stimulates a decrease in blood glucose levels) degradation enzymes, nuclear receptor signaling, and bile acid metabolism. The most widely prescribed anti-diabetic drug in the world is Metformin (Glucophage, Glumetza, Glucophage XR, or Fortamet), a suppressant of hepatic glucose production with few severe side effects (Garber et al., 1997). Likely due to their minimal effect on the brain, these peripherally acting agents marginally affect appetite. Overall, the popularity of each of these drugs in the medical community is largely determined by their benefit-risk profile.
Importantly, Claude Bernard's idea that mechanisms behind diabetes may have an origin in the CNS has been largely validated by modern physiological approaches (Obici and Rossetti, 2003; Pocai et al., 2005; Seeley and Tschöp, 2006). Over the past two decades, it has become apparent that many of the currently available anti-diabetic drugs also act directly in the CNS (Table 1 and Figure 2). For instance, Liraglutide and Exenatide are known to act both in the periphery and at the level of the brain (Mul et al., 2013; Sisley et al., 2014). However, one recent study demonstrates that the glucose-lowering effects of Liraglutide are independent of GLP-1 receptor signaling in the brain (Sisley et al., 2014). Instead, the GLP-1 anti-diabetic actions in the periphery have been well studied, as the naturally occurring pancreatic-derived peptide GLP-1 was initially described as an incretin-like peptide that controls blood glucose (Drucker, 2001).
Central 5-HT2C receptor agonism is involved in glycemic control (Berglund et al., 2013; Nonogaki et al., 1998; Xu et al., 2010a), actions that are independent of its effects on food intake and body weight. In particular, deletion of the 5-HT2CR in in mice deficient for the peptide leptin leads to synergistic impairment of glucose balance, independent of additional obesity compared to ob/ob mice (Wade et al., 2008). Furthermore, 5-HT2CR signaling in the hypothalamus has also been linked to glucose metabolism (Xu et al., 2010a). It is therefore conceivable that the reported beneficial effect of Lorcaserin on T2DM (O'Neil et al., 2012) may be linked to its central actions in neurons regulating glycemia.
It is now clear that the anti-diabetic and anti-obesity actions of Mc4 receptors are independent and mediated by distinct neural substrates. For example, humans deficient for Mc4 receptors are hyperinsulinemic, more so than would be expected from their degree of obesity alone (Farooqi and O'Rahilly, 2005). Recent genetic studies in the mouse have elegantly demonstrated that restoration of Mc4 receptors in brainstem cholinergic neurons normalizes hyperinsulinemia in an Mc4 receptor-deficient background (Myers and Olson, 2012). Conversely, the selective deletion of Mc4 receptors in the cholinergic neurons alone is sufficient to raise insulin levels (Sohn et al., 2013). However, the deletion of Mc4 receptors in both sympathetic and parasympathetic neurons is required in order to lead to hyperglycemia and insulin resistance (Berglund et al., 2014). Hence, Mc4 receptor agonism in the nervous system would appear as a promising anti-diabetic approach if these beneficial effects can be isolated from the aforementioned adverse side effects.
New experimental data indicate that targeting the brain is highly relevant to the treatment of type I diabetes (T1D). Leptin has recently been shown to normalize glucose levels in rodents with T1D, an effect that might be dependent upon leptin receptor signaling in Pomc neurons and the regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Fujikawa et al., 2013; Perry et al., 2014). Surprisingly, leptin appears sufficient to prevent death and restore normoglycemia, even in the absence of insulin therapy (Fujikawa et al., 2013). Leptin has also been shown to be useful for the treatment of other metabolic disorders characterized by hypoleptinemia, which can be the result of congenital or acquired lypodystrophies involving selective loss of fat and, in some cases, severe insulin resistance, dyslipidaemia, hepatic steatosis, and diabetes (Javor et al., 2005; Oral et al., 2002; Petersen et al., 2002). The FDA has approved MYALEPT Metreleptin (trade name: MYALEPT), a synthetically derived analog of leptin, for the treatment of lipodystrophy-related metabolic disorders. Leptin monotherapy in mice (Gavrilova et al., 2000; Shimomura et al., 1999) and in patients suffering from generalized lipodystrophy results in improvements of several metabolic parameters, including lowering of triglyceride and glucose levels as well as hepatic steatosis (Javor et al., 2005; Oral et al., 2002; Petersen et al., 2002). Moreover, after just a few months of leptin therapy, many lipodystrophic patients no longer require pharmacotherapy (e.g., insulin) to regulate glucose levels.
As previously outlined, amylin is an endogenous peptide directly acting on the nervous system and able to potentiate leptin sensitivity (Ravussin et al., 2009). An amylin analog (pramlintide) is approved for T1D and T2DM in combination with insulin under the trade name Symilin (Herrmann et al., 2014). Whereas the mechanism of action of pramlintide on glucose regulation is not well documented, the involvement of the brain in its anti-diabetic actions cannot be ruled out.
At first glance, given the efficacy and large choice of currently available anti-diabetic drugs acting in the periphery, the necessity for developing centrally acting anti-diabetic compounds is not immediately clear. However, it is also evident that centrally acting agents can effectively alleviate diabetes. This may become useful when peripherally acting agents are not well tolerated in certain T2DM patients and also in subjects with poorly controlled T1D. Furthermore, centrally acting agents commonly show a broader spectrum of actions on varied metabolic functions. In particular, the ability of many central agents to simultaneously affect energy expenditure, appetite, cardiovascular function, and glucose metabolism makes them particularly relevant for the treatment of the metabolic syndrome. In summary, the brain should be (re)-considered as a prime target in designing therapeutics for diabetes, especially in individuals with multiple comorbidities.
Is the Brain Involved in the Metabolic Outcomes of Bariatric Surgery?
A number of different approaches to bariatric surgery, also known as gastric bypass, have been described (for review, see Lutz and Bueter, 2014; Stefater et al., 2012). Roux-en-Y gastric bypass is the most efficacious and frequently performed weight-loss surgery (Buchwald, 2014; Lo Menzo et al., 2014; Schauer et al., 2003). However, the use of sleeve gastrectomy has been gaining in popularity in recent years since the approval of laparoscopic sleeve gastrectomy in 2005. Patients eligible for either sleeve gastrectomy or Roux-en-Y gastric bypass can reasonably expect a near normalization of their body mass index and reversal of most co-morbidities within 2 years. Despite their remarkable efficacy, these surgeries remain costly procedures associated with many complications. Perhaps weight-loss surgeries could be simplified and their complications avoided if the biological mechanisms underlying their effects were understood. Thus, many investigators acknowledge that identifying the mechanisms underlying the beneficial effects of gastric bypass is an important challenge. There is now ample evidence that neither the mere physical restriction of the stomach nor nutrient malabsorption is sufficient to explain weight loss and glucose-metabolism improvement after bariatric surgery (Lutz and Bueter, 2014; Stefater et al., 2012). At the physiological level, a combination of modified parameters, including reduced appetite, modified food preference, and augmented energy expenditure, may collectively mediate weight loss after bariatric surgery. At the cellular level, numerous non-exclusive factors have been implicated in the benefits of bariatric surgery, including, but not limited to, elevated levels of gut peptides and bile acids secretion, enhanced intestinal growth, and changes in the microbiome composition (Lutz and Bueter, 2014). At the molecular level, farsenoid-X receptor (FXR), a nuclear receptor for bile acids, has been recently demonstrated to play a key role in mediating weigh loss after Roux-en-Y gastric bypass (Ryan et al., 2014). Interestingly, a recent study further demonstrated that intestinal FXR agonism is sufficient to attenuate diet-induced obesity and insulin resistance in the mouse (Fang et al., 2015).
The role played by the brain in mediating the benefits of bariatric surgery remains open to discussion. At the phenomenological level, the absence of compensatory hunger after bariatric surgery in the face of considerable weight loss suggests major adaptations in hunger pathways and the neural circuits involved in the regulation of body-weight set-point. Whereas the nature of these changes remains unknown, numerous experimental and clinical observations indicate that profound adaptations occur in the gut-to-brain axis after bariatric surgery: (1) Gastric bypass surgery significantly improves post-prandial functions (Borg et al., 2006; Thirlby et al., 2006), suggesting a modified functioning of the gut-brain axis. (2) Augmented c-Fos expression in the brainstem in response to gavage administration of lipid emulsion (trade name: Intralipid) further supports the view that vagal sensory neurons may behave differently after sleeve gastrectomy (Chambers et al., 2012). (3) Following Roux-en-Y gastric bypass and sleeve gastrectomy, animals eat more frequent meals of smaller size (Stefater et al., 2010; Zheng et al., 2009); a similar phenomenon has been described in humans after Roux-en-Y gastric bypass (Laurenius et al., 2012). (4) Roux-en-Y gastric bypass in rats reduces the excitability of vagal efferents (Browning et al., 2013). Furthermore, Mc4 receptor signaling in mouse vagal efferents is required for diabetes reversal after Roux-en-Y gastric bypass (Zechner et al., 2013). (5) Destroying sensory afferents using capsaicin largely prevents the anti-diabetic actions of entero-gastro anastomosis in rats (Troy et al., 2008). (6) Indirect evidence also suggests that the activity of high-order brain sites may be modified after bariatric surgery. For instance, altered food preference (Shin et al., 2013; Wilson-Pérez et al., 2013) and increase in the risk of alcoholism after gastric bypass in humans (Ostlund et al., 2013; Suzuki et al., 2012) strongly suggest modified reward functions. Brain-imaging studies have also revealed altered dopamine receptor 2 availability after Roux-en-Y gastric bypass (Steele et al., 2010). (7) One study reported a reduced hypothalamic activity in response to food cues after gastric banding (Bruce et al., 2012). According to another study (Stefater et al., 2010), sleeve gastrectomy in the rat does not impact the expression levels of hypothalamic neuropeptides. Further research is needed to determine to what extent the vagal and brain changes discussed above may, at least in part, be causally implicated in the benefits of bariatric surgery.
Looking ahead to Unconventional Brain Therapies in Metabolism
In 1974, the stereotaxic destruction of the lateral hypothalamus was attempted in obese humans. It had been firmly anticipated that this procedure would lead to reduced appetite and weight loss, based on the previously described anorectic phenotype after lateral hypothalamus lesions in the rat. Unfortunately, the obese individuals enrolled in this trial did not respond to the procedure. Important ethical and technical constraints have limited progress in this area, and to the best of our knowledge, similar invasive neurosurgical interventions have not been attempted since. Nonetheless, the concept of altering the integrity of select brain circuits to cure obesity still garners support in the scientific community, and at least two major technical avenues for therapeutic intervention are currently being explored.
One involves cell transplantation technologies. For instance, the transplant of leptin-sensitive neurons into the hypothalamus of leptin receptor-deficient mice resulted in a diminished obesity phenotype (Czupryn et al., 2011). The other involves chemoand optogenetics in which experimenters selectively silence or activate subtypes of neurons and their connections (Lichtman et al., 2008; Deisseroth, 2012; Urban and Roth, 2015). Unfortunately, there is no currently available experimental evidence to suggest that sustained weight loss and/or T2DM reversal could be achieved using this kind of approach, and it remains unclear whether opto- and chemogenetic techniques in their current iterations will be a viable therapeutic in the treatment of various diseases in humans. However, the following observations offer clues that the application of these technologies to the human brain may be feasible in the near future: (1) Virally mediated gene delivery has been attempted on many occasions in the non-human primate brain and at least once in the human brain. (2) Clozapine-N-oxide, an exogenous ligand commonly used in chemogenetic experiments, has been safely administered to humans. (3) The applicability of optogenetics to the non-human primate brain has been validated. Certainly, for both transplantation and acute manipulation of selectively labeled neurons, ethical and safety concerns will be an important consideration for clinical application.
Conclusions
In order for currently available drug therapies and weight-loss surgeries to be maximally effective in severely obese subjects, a careful nutritional management and monitoring of dietary habits is required. A major goal of current obesity research is to identify pharmacological compounds that would mimic the effect of bariatric surgery without the inherent complexity and potential risks of the surgery. New drugs are continuously in development, but to date, none have reached the efficacy and spectrum of action of bariatric surgery. It is admittedly disappointing that the tremendous progress in our understanding of the neural pathways regulating metabolism has not yet resulted in more clinically relevant progress and clearly not a cure for obesity or T2DM. Nonetheless, the burgeoning field of combinatorial pharmacology, device-assisted neuromodulation medicine, and chemo- and optogenetics holds promise for more effective and safer anti-obesity and anti-diabetic therapies. In parallel, it is necessary to treat the symptoms and complications of obesity with appropriate pharmacological and surgical means. The field should also address the biology underlying human preferences for hypercaloric food, especially given the constant or growing availability of these foods throughout the world. Undoubtedly, an increased understanding of the brain pathways regulating energy balance and glucose homeostasis will provide insights that will facilitate the development of multi-faceted approaches to combat both obesity and diabetes.
ACKNOWLEDGMENTS
This work was supported by grants to K.W.W. (NIH R01 DK100699), L.G. (ANMS Research Grant 2014), and J.K.E. (R01DK53301, R01DK088423, and RL1DK081185).
REFERENCES
- Adams AC, Kharitonenkov A. FGF21: The center of a transcriptional nexus in metabolic regulation. Curr. Diabetes Rev. 2012;8:285–293. doi: 10.2174/157339912800840505. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Leptin. Annu. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Caterson I, Zelissen P, Guy-Grand B, Carruba M, Levy B, Sun X, Fitchet M. Topiramate: long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes. Res. 2004;12:1658–1669. doi: 10.1038/oby.2004.206. [DOI] [PubMed] [Google Scholar]
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME, Group, N.N.S. NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu T, Kong X, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Boggio PS, de Macedo EC, Schwartzman JS, Brunoni D, Teixeira MC, Fregni F. Transcranial direct current stimulation: a novel approach to control hyperphagia in Prader-Willi syndrome. J. Child Neurol. 2009;24:642–643. doi: 10.1177/0883073808322339. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br. J. Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medications for obesity: mechanisms and applications. Clin. Chest Med. 2009;30:525–538. ix. doi: 10.1016/j.ccm.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medical treatment of obesity: the past, the present and the future. Best Pract. Res. Clin. Gastroenterol. 2014;28:665–684. doi: 10.1016/j.bpg.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J. Physiol. 2013;591:2357–2372. doi: 10.1113/jphysiol.2012.249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM, Savage CR. Changes in brain activation to food pictures after adjustable gastric banding. Surg. Obes. Relat. Dis. 2012;8:602–608. doi: 10.1016/j.soard.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Buchwald H. Revisional metabolic/bariatric surgery: a moral obligation. Obes. Surg. 2014;25:547–549. doi: 10.1007/s11695-014-1458-9. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Toouli J, Herrera MF, Kulseng B, Kow L, Pantoja JP, Marvik R, Johnsen G, Billington CJ, Moody FG, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D'Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am. J. Physiol. Endocrinol. Metab. 2012;303:E1076–E1084. doi: 10.1152/ajpendo.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Chabenne J, Finan B, Sullivan L, Fischer K, Küchler D, Sehrer L, Ograjsek T, Hofmann SM, Schriever SC, et al. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422–1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- Cohen D. European drugs agency clashes with scientists over safety of GLP-1 drugs. BMJ. 2013;347:f4838. doi: 10.1136/bmj.f4838. [DOI] [PubMed] [Google Scholar]
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Considine RV, Caro JF. Leptin in humans: current progress and future directions. Clin. Chem. 1996;42:843–844. [PubMed] [Google Scholar]
- Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res. Brain Res. Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Crick FHC. Thinking about the brain. Scientific American. 1979;241:3. doi: 10.1038/scientificamerican0979-219. [DOI] [PubMed] [Google Scholar]
- Czupryn A, Zhou YD, Chen X, McNay D, Anderson MP, Flier JS, Macklis JD. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. 2011;334:1133–1137. doi: 10.1126/science.1209870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009;5:749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat. Neurosci. 2013;16:838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol. Psychiatry. 2012;71:1030–1032. doi: 10.1016/j.biopsych.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Münzberg H, Zhang ZY, Kahn BB, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Development of glucagon-like peptide-1-based pharmaceuticals as therapeutic agents for the treatment of diabetes. Curr. Pharm. Des. 2001;7:1399–1412. doi: 10.2174/1381612013397401. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu. Rev. Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Smolnik R, Kern W, McGregor GP, Bickel U, Born J. The melanocortin melanocyte-stimulating hormone/adrenocorticotropin(4-10) decreases body fat in humans. J. Clin. Endocrinol. Metab. 2001;86:1144–1148. doi: 10.1210/jcem.86.3.7298. [DOI] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Müller TD, Yi CX, Habegger K, Schriever SC, García-Cáceres C, Kabra DG, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51:34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, et al. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am. J. Med. 1997;103:491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J. Clin. Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA, Group, N.B.S. NB-201 Study Group Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J. Clin. Endocrinol. Metab. 2009;94:4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro J, Zigman JM, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann. N Y Acad. Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Shan K, Brunell SC, Chen S. Effects of pramlin-tide in patients with type 2 diabetes mellitus: an analysis using daily insulin dose tertiles. Endocr. Pract. 2014;20:1070–1075. doi: 10.4158/EP13477.OR. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, Doran S, Jax T, Zdravkovic M, Chapman IM. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res. Clin. Pract. 2012;97:258–266. doi: 10.1016/j.diabres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikramuddin S, Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915–922. doi: 10.1001/jama.2014.10540. [DOI] [PubMed] [Google Scholar]
- Jackson VM, Price DA, Carpino PA. Investigational drugs in Phase II clinical trials for the treatment of obesity: implications for future development of novel therapies. Expert Opin. Investig. Drugs. 2014;23:1055–1066. doi: 10.1517/13543784.2014.918952. [DOI] [PubMed] [Google Scholar]
- Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, Pørksen N, Schmitz O. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–429. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- Julius D, MacDermott AB, Jessel TM, Huang K, Molineaux S, Schieren I, Axel R. Functional expression of the 5-HT1c receptor in neuronal and nonneuronal cells. Cold Spring Harb. Symp. Quant. Biol. 1988;53:385–393. doi: 10.1101/sqb.1988.053.01.046. [DOI] [PubMed] [Google Scholar]
- Kentish S, Li H, Philp LK, O'Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, Page AJ. Diet-induced adaptation of vagal afferent function. J. Physiol. 2012;590:209–221. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lönroth H, Fändriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 2012;36:348–355. doi: 10.1038/ijo.2011.217. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat. Rev. Neurosci. 2008;9:417–422. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl. Acad. Sci. USA. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Menzo E, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand. J. Surg. 2014;104:18–23. doi: 10.1177/1457496914552344. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Bueter M. Physiological mechanisms behind Roux-en-Y gastric bypass surgery. Dig. Surg. 2014;31:13–24. doi: 10.1159/000354319. [DOI] [PubMed] [Google Scholar]
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, Group, N.N.I.S. NN2211-1310 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, Ravussin E. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J. Clin. Endocrinol. Metab. 2011;96:837–845. doi: 10.1210/jc.2010-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk J, Goodall E, Silverstone T, Willner P. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behav. Pharmacol. 1991;2:113–119. [PubMed] [Google Scholar]
- Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EB, Farinatti PT. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite. 2012;58:333–338. doi: 10.1016/j.appet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R68–R77. doi: 10.1152/ajpregu.00588.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr., Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology. 2003;144:5172–5178. doi: 10.1210/en.2003-0999. [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, Näslund E. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148:374–377. doi: 10.1001/jamasurg.2013.700. [DOI] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 2007;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Powley TL, Chi MM, Schier LA, Phillips RJ. Obesity: should treatments target visceral afferents? Physiol. Behav. 2005;86:698–708. doi: 10.1016/j.physbeh.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J. Clin. Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Roth JD, Trevaskis JL, Wilson J, Lei C, Athanacio J, Mack C, Kesty NC, Coffey T, Weyer C, Parkes DG. Antiobesity effects of the beta-cell hormone amylin in combination with phentermine or sibutramine in diet-induced obese rats. Int. J. Obes. (Lond.) 2008;32:1201–1210. doi: 10.1038/ijo.2008.91. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, et al. EMPOWER Study Group The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes. Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Tschöp M. How diabetes went to our heads. Nat. Med. 2006;12:47–49. doi: 10.1038/nm0106-47. discussion 49. [DOI] [PubMed] [Google Scholar]
- Shikora S, Toouli J, Herrera MF, Kulseng B, Zulewski H, Brancatisano R, Kow L, Pantoja JP, Johnsen G, Brancatisano A, et al. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J. Obes. 2013;2013:245683. doi: 10.1155/2013/245683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Townsend RL, Patterson LM, Holmes GM, Berthoud HR. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obes. Surg. 2013;23:531–540. doi: 10.1007/s11695-012-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J. Clin. Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral M, Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Montenegro ML, Pascau J, Desco M. Response to deep brain stimulation in the lateral hypothalamic area in a rat model of obesity: in vivo assessment of brain glucose metabolism. Mol. Imaging Biol. 2014;16:830–837. doi: 10.1007/s11307-014-0753-0. [DOI] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. e1–e3. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr. Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes. Surg. 2012;22:201–207. doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- Thirlby RC, Bahiraei F, Randall J, Drewnoski A. Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J. Gastrointest. Surg. 2006;10:270–277. doi: 10.1016/j.gassur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, Weyer C, Koda J, Baron AD, Parkes DG, Roth JD. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–5687. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: Impact of head fat and dose guidelines. Neuroimage Clin. 2013;2:759–766. doi: 10.1016/j.nicl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, Biraben A, Randuineau G, Malbert CH. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55:245–252. doi: 10.1016/j.appet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg LH, Kanatani A, MacNeil D, Ming Fong T, Strack A, Nargund R, Guan XM. Design and synthesis of (ant)-agonists that alter appetite and adiposity. Prog. Brain Res. 2006;153:107–118. doi: 10.1016/S0079-6123(06)53005-5. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, et al. A role for the melanocortin 4 receptor in sexual function. Proc. Natl. Acad. Sci. USA. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat. Rev. Endocrinol. 2010;6:578–588. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JM, Juneja P, MacKay AW, Graham J, Havel PJ, Tecott LH, Goulding EH. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;149:955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhöner P, Hörster R, Jacobs F, Sayk F, Lehnert H, Dodt C. Intranasal application of the melanocortin 4 receptor agonist MSH/ACTH(4-10) in humans causes lipolysis in white adipose tissue. Int. J. Obes. (Lond.) 2012;36:703–708. doi: 10.1038/ijo.2011.105. [DOI] [PubMed] [Google Scholar]
- Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, Alcindor D, Prostko ER, Cheng BC, Angle C, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J. Neurosurg. 2013;119:56–63. doi: 10.3171/2013.2.JNS12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat. Rev. Drug Discov. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–482. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J Obes. (Lond.) 2013;37:288–295. doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright FL, Rodgers RJ. Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash. Psychopharmacology (Berl.) 2013;228:291–307. doi: 10.1007/s00213-013-3036-6. [DOI] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, Rossi J, Williams KW, Jones JE, Zigman JM, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat. Neurosci. 2010a;13:1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J. Neurosci. 2010b;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulyaningsih E, Zhang L, Herzog H, Sainsbury A. NPY receptors as potential targets for anti-obesity drug development. Br. J. Pharmacol. 2011;163:1170–1202. doi: 10.1111/j.1476-5381.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM, Still CD, Gerhard GS, Burgess SC, Mirshahi T, Aguirre V. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144:580–590. e7. doi: 10.1053/j.gastro.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 2008;23:75–83. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]