Abstract
The male genital tract plays an important role in protecting sperm by forming a distinct compartment separate from the body which limits exposure to potentially toxic substrates. Transporters along this tract can influence the distribution of xenobiotics into the male genital tract through efflux back into the blood or facilitating the accumulation of toxicants. The aim of this study was to quantitatively determine the constitutive mRNA expression of 30 xenobiotic transporters in caput and cauda regions of the epididymis, vas deferens, prostate, and seminal vesicles from adult Sprague-Dawley rats. The epididymis was found to express at least moderate levels of 18 transporters, vas deferens 15, seminal vesicles 23, and prostate 18. Constitutive expression of these xenobiotic transporters in the male genital tract may provide insight into the xenobiotics that can potentially be transported into these tissues and may provide the molecular mechanism for site specific toxicity of select agents.
1. INTRODUCTION
In order for a xenobiotic compound to influence function of a target tissue, it must be present at sufficient concentrations. Transporters are one of the primary determinants of xenobiotic absorption, distribution, and elimination processes by facilitating uptake into or efflux from cells [1]. Several families of proteins have been demonstrated to transport a wide variety of clinical drugs and endogenous substrates. These transporters include multiple drug resistance (Mdr) proteins, multidrug resistance-associated proteins (Mrp), organic anion transporters (Oat) organic anion transporting polypeptides (Oatp), organic cation transporters (Oct), equilibrative nucleoside transporters (Ent) and concentrative nucleoside transporters (Cnt). These transporters have broad and frequently overlapping substrate specificities that include many clinically used drugs in addition to environmental toxicants [2–4]. Xenobiotic transporters may play an important part in excluding these compounds from tissues, or conversely facilitating the distribution and tissue-specific accumulation of select agents. While drug transport has been well studied in organs such as the kidney, intestine, and liver, there is a paucity of information concerning transporter expression in the male reproductive system [3, 5–6]. This leaves a significant gap in our knowledge concerning the categories of compounds that are excluded from or are able to accumulate within the male reproductive system.
The male genital tract (MGT) begins with the seminiferous tubules located inside the testis and are the site at which spermatogenesis occurs. These tubules converge at the rete testis before connecting to the proximal region of the epididymis (caput). The spermatids mature as they move through the epididymis and become fertile in the distal section of the epididymis (cauda) where they are stored until ejaculation. During ejaculation, the sperm travels through the vas deferens (ductus deferens) while the seminal vesicles and prostate add secretions to the tract which become the primary components of seminal plasma. One of the functions of the MGT is to limit sperm exposure to potentially toxic agents. This function is especially important in the epididymis, where the sperm are stored, and the vas deferens which serves as the route for sperm during ejaculation. However, since sperm become heavily exposed to the secretions from the seminal vesicles and the prostate during ejaculation, any toxicants entering from these tissues could also potentially have an adverse affect sperm, as well as the sexual partners. Surprisingly, little is known concerning the expression of xenobiotic transporters in the male reproductive system. Since transporters play a pivotal role in determining the distribution of xenobiotic compounds, knowledge of tissue-specific expression of xenobiotic transporters along the MGT is essential for understanding the distribution of toxicants within the MGT. The constitutive expression levels of drug transporters in the tissues of the MGT may also provide insight into how well therapeutic agents are able to penetrate the various tissues of the MGT for the purposes of treating diseases, such as cancer or HIV infection [7–8]. While studies have been performed determining the expression of the major xenobiotic transporters in the blood-testis barrier and Mdr1b in the epididymis, a comprehensive analysis of transporter expression for other tissues in the MGT is currently lacking [1, 9]. Therefore, this study was undertaken to determine the constitutive mRNA expression levels of 30 xenobiotic transporters in rat caput and cauda regions of the epididymis, seminal vesicles, vas deferens, and prostate in relation to the liver and kidney. Determining the mRNA expression through branched DNA analysis offers a unique advantage of being able to compare the expression profile of specific transporters with sensitivity comparable to RT-PCR across various tissue samples which is ideal for our interests. Protein analysis of these xenobiotics transporters is unfeasible to perform for a comprehensive study on this scale due to the reliance on specific antibodies which are unavailable for many xenobiotic transporters. Many xenobiotic transporters have overlapping substrate specificities making functional studies unable to discriminate between many of the transporters of interest and therefore impractical for determining expression of xenobiotic transporters in native tissue. For these reasons, studies using mRNA analysis are commonly used and seminal for understanding the transport capabilities in tissues for which little is known [5, 10–11]. It is known that quantitative mRNA levels do not always match protein levels, but mRNA can generally correlate to protein levels. A complete lack of mRNA would indicate no protein present since mRNA is required to translate protein. High mRNA levels would very likely represent at least some protein expression or else the cell would be wasting energy to transcribe unused mRNA. The data presented is foundational regarding the ability of toxicants and drugs to access a variety of tissues in the MGT. An understanding of xenobiotics transport in the male reproductive system is anticipated to serve a variety of interests relevant to male reproductive biology
2. MATERIALS AND METHODS
2.1 Materials
Quantigene HV Signal Amplification Kit and Quantigene Discovery Kit were purchased from Genospectra (Fremont, CA). RNAzol B reagent was purchased from Tel-Test Inc. (Friendswood, TX). All other reagents were purchased from standard scientific suppliers at the highest available purity.
2.2 Sample Collection
Samples were collected from euthanized rats at least 10 weeks old. Sections from the ventral prostate were used for prostate analysis, Protocols for obtaining samples were approved by the University of Arizona Institutional Animal Care and Use Committee (IACUC).
2.3 Development of Specific Oligonucleotide Probe Sets
The probe sets used in this study were described in the following publications: Mdr1a and 1b [10]; Mdr2 [11]; Mrp1, 2, and 3 [12]; Mrp4, 5, and 6 [11]; Mrp7 and 9 [1] Oatp1, 2, 3, 4, and 5 [13]; Oct1, 2, 3, N1, and N2 [14]; Oat1, 2, and 3 [15]; Cnt1 and 2 as well as Ent1 and 2 [11]; and Cnt3 [16].
2.4 Total RNA Isolation
Total RNA was isolated from 4 Sprague Dawley rats using RNAzol B reagent as per manufacturer’s protocol. RNA was pooled and used for all bDNA experiments. Each RNA pellet was resuspended in 0.2 ml of 10 mM Tris-HCl buffer, pH 8.0. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA integrity and quality were analyzed by formaldehyde agarose gel electrophoresis with ethidium bromide staining. The quality of RNA samples was determined by the integrity and relative ratio of 28S and 18S rRNA bands.
2.5 Branched DNA Assay
Specific oligonucleotide probes for each gene were diluted in lysis buffer. Substrate solution, lysis buffer, capture hybridization buffer, amplifier, and label probe buffer used in the analysis were all obtained from the Quantigene Discovery Kit. The assay was performed in 96-well format with 10μg of RNA isolated from various tissues and then added to the capture hybridization buffer and 50 μl of the diluted probe set. The total RNA was then allowed to hybridize to the probe set overnight at 53°C. Hybridization steps were performed per the manufacturer’s protocol the following day. Luminescence of the samples was measured with a Quantiplex 320 bDNA luminometer interfaced with Quantiplex Data Management Software, version 5.02 purchased from Bayer (Walpole, MA). Background for each transporter probe set was determined using negative control wells which had all reagents except for RNA. The background was then subtracted to demonstrate expression above background levels.
3. RESULTS
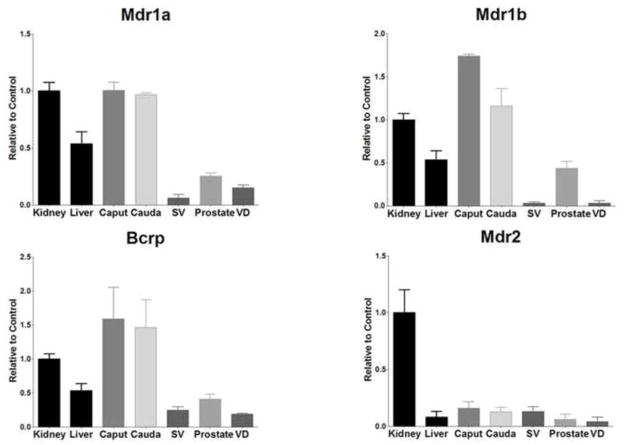
The mRNA expression levels of the major xenobiotic transporters in liver and kidney were compared to that of tissues in the male reproductive system; namely the caput and cauda regions of the epididymis, seminal vesicles, prostate, and vas deferens (ductus deferens). Expression levels for the liver and kidney were used as positive controls since the xenobiotic transporters investigated are expressed by at least one of these tissues [1]. For each transporter, either the kidney or the liver was considered the positive control depending on which tissue exhibited higher expression levels. The mRNA expression levels for the tissues along the MGT were normalized to the control tissue. Any expression that was 90% of control or higher was considered highly expressed, while 20% or higher was considered moderate expression and anything below 20% was considered low expression. Figure 1 shows that Mdr1a, 1b (P-glycoprotein or P-gp) and Bcrp expression was high in both sections of the epididymis (96–174%). The prostate showed moderate expression levels for all three transporters while seminal vesicles only showed modest levels of Bcrp (24%). Expression of Mdr2 mRNA was not found above low levels in any tissue (4–15%).
Figure 1.
Expression level of Mdr and Bcrp mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). Levels for mRNA are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
All MGT tissues highly expressed mRNA for Ent1, except for the caput of the epididymis which had moderate mRNA expression (79%). Ent2 mRNA levels were moderate for all MGT tissues ranging from 24–82% of control (Figure 2). Cnt1 was found to only be slightly expressed in any of the MGT tissues (0.5–10%), but Cnt2 was found at mild levels throughout (28–56%). The epididymis caput had more expression of Cnt3 compared to kidney (228%), while the cauda expression levels were higher than that of the kidney (127%).
Figure 2.
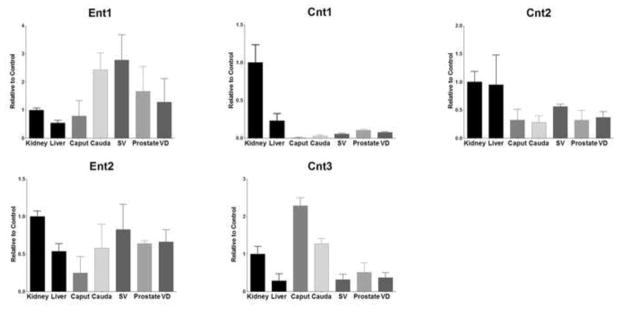
Expression level of nucleoside transporters mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). Levels for mRNA are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
Interestingly, Mrp1 was found to be highly expressed in the epididymis, particularly in the caput (143%), however all tissues expressed Mrp1 at levels ranging from 57–78% (Figure 3). Conversely, the epididymis had almost no detectable expression of either Mrp2 or and Mrp3. The seminal vesicles had the highest expression of all the MGT tissues for both Mrp2 (56%) and Mrp3 (79%) and the vas deferens demonstrated 42% and 74% expression compared to control for Mrp2 and Mrp3 respectively. The caput and cauda showed marked differences in expression of Mrp4 with the cauda possessing 35% expression levels compared to positive control, but the caput exhibited only 5% expression. Mrp5 and Mrp7 expression was strikingly high for both sections of the epididymis and the seminal vesicles (131% and 196%). The caput expressed the highest amounts of Mrp6 (155%), with every other tissue demonstrating expression ranging from 53–85%. Mrp9 expression was only above the low range for the seminal vesicles (47%).
Figure 3.
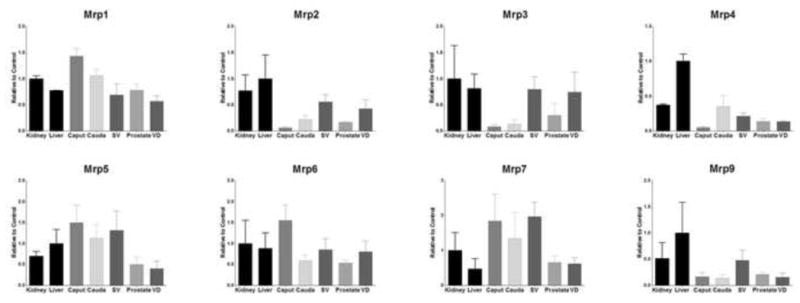
Expression level of Mrp mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). Levels for mRNA are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
Oat2 and Oat3 (Figure 4) were found to be moderately expressed in seminal vesicles (68% and 49%) and the caput (25% and 37%). Other than these exceptions, Oat transporter expression was relatively low in the tissues of the MGT. Oct1 (Figure 5) was primarily expressed in seminal vesicles (64%) and the vas deferens (57%). Oct2 mRNA was not well detected in any tissues of the MGT. Seminal vesicles were found to express Oct3 at levels nearly double that of positive control (174%) and every other MGT tissue expressed Oct3 at intermediate levels (25–55%). OctN1 was expressed at low levels for all the tissues of the MGT except for the caput (22%), seminal vesicles (25%) and the prostate (52%), but OctN2 expression was markedly high in the epididymis, particularly in the proximal region (245%). All tissues exhibited remarkable amounts of OctN3 expression (111–134%).
Figure 4.
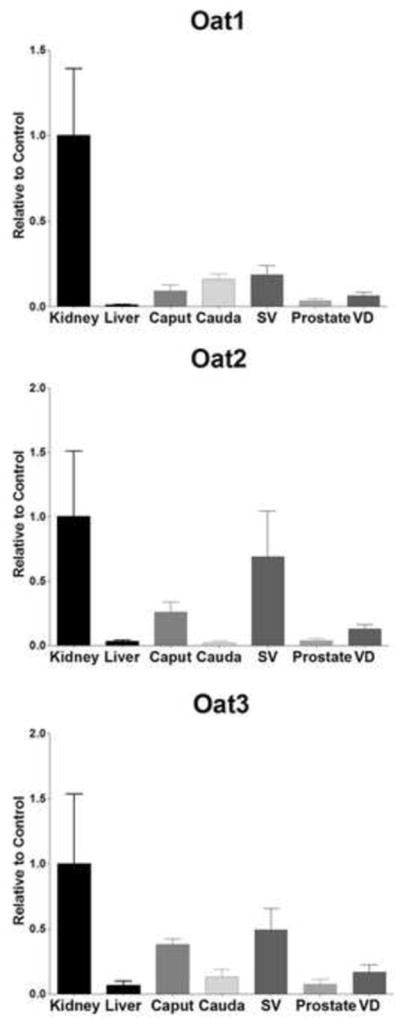
Expression level of Oat mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). Levels for mRNA are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
Figure 5.
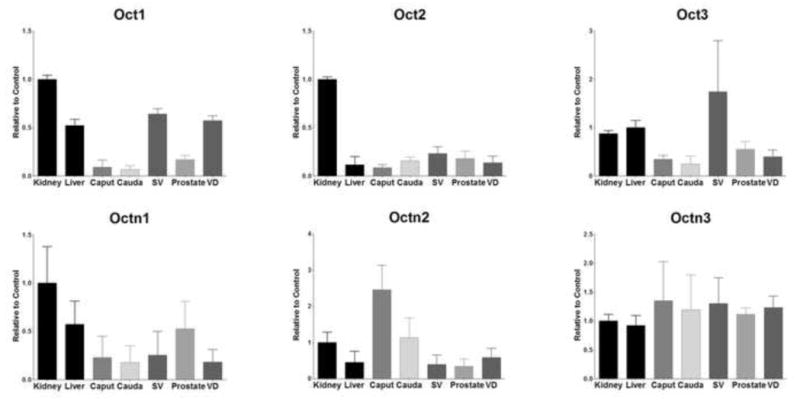
Expression level of Oct mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). Levels for mRNA are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
Oatp1a1 and Oatp1a6 were not well expressed in the MGT with expression levels ranging from 2–6% compared to control (Figure 6). However, Oatp1a4 was found with moderate expression in the prostate and vas deferens (31% and 65%). Particularly high expression was detected in the seminal vesicles and epididymis with 139% for seminal vesicles, 146% for the caput and 105% for the cauda. Oatp1a5 was found to be modestly expressed by seminal vesicles (33%), but all other tissues demonstrated low expression (4–11%).
Figure 6.

Expression level of Oatp mRNA levels in kidney, liver, caput, cauda, seminal vesicles (SV), prostate, and vas deferens (VD). mRNA levels are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n=3).
4. DISCUSSION
An important spermatoprotective function of the blood-testis barrier is to prevent the entry of potential toxicants and thus limit their exposure to the developing germ cells. Once immature sperm leave the testis, this vital function is maintained in the epididymis where the blood-epididymal barrier separates the adluminal compartment from the rest of the body [17–18]. The epithelia of the seminal vesicles and the prostate may also serve to keep potentially toxic compounds out of the secretions to which the sperm will be exposed [17].
The purpose of this study was to quantitatively determine the constitutive expression of the major xenobiotic transporters in caput and cauda regions of the epididymis, vas deferens, prostate, or seminal vesicles from adult Sprague-Dawley rats to assess the potential impact of xenobiotics distribution. The transporters investigated in this study play an important role in determining the ADME processes of xenobiotics in the body. The goal of determining xenobiotic expression is to give mechanistic insight into xenobiotic distribution within the male genital tract. During xenobiotic exposure, knowledge of transporter expression can predict which tissues of the MGT will be exposed the most. If an antagonist of a transporter is introduced, it would either increase or decrease exposure of the xenobiotic in the tissue depending on the characteristics of the transporter. For example, if a basolateral uptake transporter was inhibited, exposure to a substrate for that transporter would decrease. If a basolateral efflux transporter was inhibited, then exposure would increase since the substrate isn’t being pumped back into the blood. The results demonstrated a complex expression pattern across these various tissues which helps define the role of transport in drug distribution within the MGT. While xenobiotic transporter expression in rat tissue should generally represent that of humans, it should be noted that there may be some species differences. One example is multidrug resistance protein 1 (MDR1). Humans only have 1 isoform of the transporter MDR1, but rodents express 2 isoforms (Mdr1a and Mdr1b). Both the rodent and human isoforms of MDR1 have similar function and are typically expressed in the same tissues [20].
The epididymis can be divided into 4 regions, the initial segment, caput, corpus, and the cauda, each with distinct transcriptional profiles [21]. Due to the small size of the corpus, we were not able to harvest sufficient mRNA to investigate the expression profile of this region. There has been recent interest in the influence of the epididymis in male fertility, especially with the secretions’ impact on sperm development [22]. A potential transepithelial pathway using xenobiotic uptake transporters, such as Ent1, Ent2, Oat3, Oatp1a4, OctN2 and OctN3, which were determined to be expressed in the epididymis, could represent a mechanism for toxicants, such as alpha-chlorohydrin, to accumulate in epididymal secretions. The toxicant alpha-chlorohydrin (also known as 3-MCPD) is an organic molecule found in soy products and has been reported to cause edema of the caput and epididymal sperm immotility [23]. Since alpha-chlorohydrin is a polar compound, transporters are likely to play a key role in the disposition of alpha-chlorohydrin into the epididymal cells and thereby into the epididymal secretions to which the sperm are exposed. Carbendazim, a fungicide, is another classic epididymal toxicant that is reported to cause duct necrosis and have deleterious effects on epididymal sperm [21, 24]. Since it is known to affect epididymal sperm, carbendazim requires penetration of the MGT, likely due to a transport-mediated mechanism, in order to have a toxicological effect. A transport-dependent mechanism seems relevant since carbendazim has been reported to be transported by fungal transporters [25].
An understanding of constitutive drug transporter expression in the epididymis may also be useful in the treatment of HIV infection. It has been speculated that the MGT acts as a sanctuary site for HIV by shielding the virus from therapy [16]. Epididymal efflux transporters such as Mdr1 Mrp1, Mrp5, Mrp6, and Mrp7 may be contributing to the MGT acting as a sanctuary site. One class of drugs used to treat HIV infection, nucleoside reverse transcriptase inhibitors (NRTI) have been shown to accumulate in seminal plasma at concentrations higher than that of the blood [26–27]. One potential mechanism for NRTI accumulation within seminal plasma involves transport via ENT1/2 into seminiferous tubules to concentrations equal to that of the blood, and subsequent concentration within MGT as the epididymis removes 99% of the water in the ducts [16, 28]. Knowledge of which transporters are constitutively expressed in the epididymis can help assess the impact of drug transport at the blood-testis barrier (BTB) by determining if drugs that are able to get into the seminiferous tubules will be reabsorbed distally in the epididymis. This mechanism of testicular transport followed by epididymal concentration may also be applicable to epididymal toxicants.
The vas deferens is a muscular tube that allows for sperm to travel from the epididymis to the urethra during ejaculation. Since contractility of the vas deferens is important for proper ejaculation, drugs that interfere with muscular contraction (notably, α-adrenergic blockers) may cause side effects that interfere with patient compliance [29]. Drug transporters expressed in the vas deferens such as Oct1, Oct3, OctN2, and Oatp1a4 can affect the distribution of xenobiotics into the vas deferens and thus potentiate undesirable side effects for several drugs. Secretions of the vas deferens are also suspected to play an important role in male fertility [29]. As with the epididymis, xenobiotic transporters of the vas deferens could secrete potential toxicants to the MGT via transepithelial transport which may adversely affect fertility [30].
Here we report several xenobiotic transporters expressed in the prostate that may play roles in several clinical observations and can potentially impact a variety of drug therapies. It has been reported that a blood-prostate barrier exists with properties similar to the blood-testis barrier and may limit the distribution of therapeutic compounds [19, 31]. It is reasonable to expect that efflux transporters such as Mdr1, Mrp1, Mrp3, Mrp5, Mrp6, Mrp7, and Mrp9 are important for the blood-prostate barrier through the efflux of xenobiotics compounds out of the prostate back into the blood. However, these transporters may be acting as an obstacle for drug therapy requiring access to the prostate to achieve full therapeutic effect. It is also possible that uptake transporters such as Ent1, Ent2, and Oatp1a4 represent a route of bypassing the epithelial barrier and achieve therapeutic concentrations in the prostate. Success of these therapies may depend on interactions with these transporters.
Both uptake and efflux transporters in the prostate may be involved in the treatment or the development of cancer. The most well studied role for transporters in the context of cancer is on the distribution of chemotherapeutics as many ABC transporters are known to interact with anticancer drugs [32]. Cancerous cells have also been observed to upregulate many ABC transporters such as Mdr1 and BCRP which may contribute to therapeutic resistance [33–34]. Knowledge of the constitutive expression of transporters may provide a background to which prostate cancer cells can be compared in future studies. In addition to determining the disposition of chemotherapeutics, xenobiotic transporters may also affect the exposure of carcinogens, chemopreventative agents (such as sulforaphane or catechins), and endocrine disrupting chemicals to the prostate, all of which are suspected of being involved in carcinogenesis [35–39].
An oddity of the seminal vesicles is despite being in close proximity, and thus exposed to a similar environment as the prostate, the seminal vesicles rarely develop cancer [40–41]. The tremendously reduced incidence of cancer may be at least partially related to efflux transporters, such as Mrp2, Mrp5, Mrp7, and Mrp9, that we report to be expressed at higher levels in seminal vesicle tissue than prostatic tissue. In addition to low carcinogenesis rates, there is also a paucity of information concerning select toxic agents that are specific for seminal vesicles. The main toxicant reported in the literature is flutamide, an antiandrogen drug used for the treatment of prostate cancer, which causes a decrease in weight for both seminal vesicles and ventral prostate and interestingly, flutamide is a known substrate for human MRP1 [22, 42]. The fact that flutamide is transported by MRP1 is especially intriguing since our results demonstrated that mRNA levels for Mrp1 was roughly equal for seminal vesicles and prostate. Drug transport into seminal vesicles could also be important for the treatment of HIV infection. Macrophages infected with HIV have been detected in seminal vesicles and may contribute to the MGT acting as a sanctuary site for the virus [7]. Increasing the bioavailability of antiretroviral drugs will be essential in an effort to eliminate the virus from a patient. Additionally, an understanding of constitutive expression of transporters in the seminal vesicles is valuable information for predicting xenobiotics appearance in seminal vesicle secretions which represent the primary component of ejaculate [43–44]. It is conceivable that xenobiotic transporters could form a transepithelial pathway though epithelial cells of the seminal vesicles which would lead to xenobiotic presence in the seminal vesicle secretions. This indicates that the seminal vesicles may be a major contributor to the secretion of drugs or toxicants into the ejaculate.
Although this study focuses on xenobiotic transporters, it is worth noting that there are several transporters with endogenous substrates that have been investigated. For example, in the epididymis water reabsorption is important for proper sperm maturation and as such, salt transporters and aquaporins present in the epididymis have been characterized [45–48]. Additionally, transporters that contribute to the microenvironment of the MGT, such as those controlling pH or calcium concentrations, have also been studied [49–50]. OCTN2 has also been characterized for its ability to transport carnitine, an important nutrient for male fertility, in MGT tissues [51]. Several ABC transporters have also been implicated in cholesterol transport within the testis, spermatozoa, and epididymis [52–55].
4.1 Conclusion
This study presents a comprehensive analysis of the mRNA expression of the 30 major xenobiotic transporters located in tissues along the MGT. The findings from this study can serve as a valuable reference to a variety of interests in medical research including male fertility, toxicology of the MGT, HIV infection and cancer chemotherapy. Some future studies may include localizing these xenobiotic transoprters through immunohistochemical analysis, functional transport analysis using tissues from MGT, and in vivo studies determining drug-drug interactions and the distribution of xenobiotics in knockout animal models. The data presented here would also be foundational for studies interested in how transporter expression may be induced/repressed following exposure to a xenobiotic. As such, the constitutive expression of these xenobiotic transporters may provide insight into the molecular mechanisms associated with the transport of drugs into and out of the MGT.
Organs of the MGT express a complex pattern of xenobiotic transporter expression.
Seminal vesicles express the most xenobiotic transporters, vas deferens the least.
Many of these transporters are known to affect the disposition of toxicants.
These data lays the foundation for future work to determine induction effects.
Footnotes
This work was supported by the National Institutes of Health [Grants NIAID AI083927, ES006694, and HD062489] and the National Institute of Environmental Health Science Toxicology Training Grant [ES007091].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Augustine LM, Markelewicz RJ, Jr, Boekelheide K, Cherrington NJ. Xenobiotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab Dispos. 2005;33:182–189. doi: 10.1124/dmd.104.001024. [DOI] [PubMed] [Google Scholar]
- 2.Bart J, Groen HJ, van der Graaf WT, Hollema H, Hendrikse NH, Vaalburg W, Sleifer DT, de Vries EG. An oncological view on the blood-testis barrier. Lancet Oncol. 2002;3(6):357–363. doi: 10.1016/s1470-2045(02)00776-3. [DOI] [PubMed] [Google Scholar]
- 3.Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65(3):944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 4.Lozano E, Herraez E, Briz O, Robledo VS, Hernandez-Iglesias J, Gonzalez-Hernandez A, Marin JJ. Role of the Plasma Membrane Transporter of Organic Cations OCT1 and Its Genetic Variants in Modern Liver Pharmacology. Biomed Res Int. 2013;2013:692071. doi: 10.1155/2013/692071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768(3):637–647. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Cangoz S, Chang YY, Chempakaseril SJ, Guduru RC, Huynh LM, John JS, John ST, Joseph ME, Judge R, Kimmey R, Kudratov K, Lee PJ, et al. The kidney as a new target for antidiabetic drugs: SGLT2 inhibitors. J Clin Pharm Ther. 2013;38(5):350–359. doi: 10.1111/jcpt.12077. [DOI] [PubMed] [Google Scholar]
- 7.Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jégou B, Dejucq-Rainsford N. Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo. Am J Pathol. 2011;179(5):2397–2408. doi: 10.1016/j.ajpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dueregger A, Guggenberger F, Barthelmes J, Stecher G, Schuh M, Intelmann D, Abel G, Haunschild J, Klocker H, Ramoner R, Sampson N. Attenuation of nucleoside and anti-cancer nucleoside analog drug uptake in prostate cancer cells by Cimicifuga racemosa extract BNO-1055. Phytomedicine. 2013;20(14):1306–1314. doi: 10.1016/j.phymed.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Jones SR, Cyr DG. Regulation and characterization of the ATP-binding cassette transporter-B1 in the epididymis and epididymal spermatozoa of the rat. Toxicol-Sci. 2011;119(2):369–379. doi: 10.1093/toxsci/kfq318. [DOI] [PubMed] [Google Scholar]
- 10.Brady JM, Cherrington NJ, Hartley DP, Buist SC, Li N, Klaassen CD. Tissue distribution and chemical induction of multiple drug resistance genes in rats. Drug Metab Dispos. 2002;30(7):838–844. doi: 10.1124/dmd.30.7.838. [DOI] [PubMed] [Google Scholar]
- 11.Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31(2):153–167. doi: 10.1124/dmd.31.2.153. [DOI] [PubMed] [Google Scholar]
- 12.Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther. 2002;300(1):97–104. doi: 10.1124/jpet.300.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Hartley DP, Cherrington NJ, Klaassen CD. Tissue expression, ontogeny, and inducibility of rat organic anion transporting polypeptide 4. J Pharmacol Exp Ther. 2002;301(2):551–560. doi: 10.1124/jpet.301.2.551. [DOI] [PubMed] [Google Scholar]
- 14.Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD. Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab Dispos. 2002;30(2):212–219. doi: 10.1124/dmd.30.2.212. [DOI] [PubMed] [Google Scholar]
- 15.Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther. 2002;301(1):145–151. doi: 10.1124/jpet.301.1.145. [DOI] [PubMed] [Google Scholar]
- 16.Klein DM, Evans KK, Hardwick RN, Dantzler WH, Wright SH, Cherrington NJ. Basolateral uptake of nucleosides by Sertoli cells is mediated primarily by equilibrative nucleoside transporter 1. J Pharmacol Exp Ther. 2013;346(1):121–129. doi: 10.1124/jpet.113.203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubé E, Cyr DG. The blood-epididymis barrier and human male fertility. Adv Exp Med Biol. 2012;763:218–236. doi: 10.1007/978-1-4614-4711-5_11. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Yi S, Zhang J, Fang Z, Zhou F, Jia W, Liu Z, Ye G. Effect of microbubble-enhanced ultrasound on prostate permeability: a potential therapeutic method for prostatedisease. Urology. 2013;81(4):921.e1–7. doi: 10.1016/j.urology.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston DS, Turner TT, Finger JN, Owtscharuk TL, Kopf GS, Jelinsky SA. Identification of epididymis-specific transcripts in the mouse and rat by transcriptional profiling. Asian J Androl. 2007;9(4):522–527. doi: 10.1111/j.1745-7262.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;14;146(1):R21–35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 23.Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol. 2001;29(1):64–76. doi: 10.1080/019262301301418865. [DOI] [PubMed] [Google Scholar]
- 24.Akbarsha MA, Kadalmani B, Girija R, Faridha A, Hamid KS. Spermatotoxic effect of carbendazim. Indian J Exp Biol. 2001;39(9):921–924. [PubMed] [Google Scholar]
- 25.Song TT, Ying SH, Feng MG. High resistance of Isaria fumosorosea to carbendazim arises from the overexpression of an ATP-binding cassette transporter (ifT1) rather than tubulin mutation. J Appl Microbiol. 2012;112(1):175–184. doi: 10.1111/j.1365-2672.2011.05188.x. [DOI] [PubMed] [Google Scholar]
- 26.Prins JM, Lowe SH, van Leeuwen E, Droste JA, van der Veen F, Reiss P, Lange JM, Burger DM, Repping S. Semen quality and drug concentrations in seminal plasma of patients using a didanosine or didanosine plus tenofovir containing antiretroviral regimen. Ther Drug Monit. 2007;29(5):566–570. doi: 10.1097/FTD.0b013e31811fef29. [DOI] [PubMed] [Google Scholar]
- 27.Dumond JB, Reddy YS, Troiani L, Rodriguez JF, Bridges AS, Fiscus SA, Yuen GJ, Cohen MS, Kashuba AD. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. J Acquir Immune Defic Syndr. 2008;48:156–162. doi: 10.1097/QAI.0b013e31816de21e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornwall GA. New Insights into Epididymal Biology and Function. Hum Reprod. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koslov DS, Andersson KE. Physiological and pharmacological aspects of the vas deferens-an update. Front Pharmacol. 2013;4:101. doi: 10.3389/fphar.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi S, Pierucci-Alves F, Schultz BD. Transforming growth factor beta1 impairs CFTR-mediated anion secretion across cultured porcine vas deferensepithelial monolayer via the p38 MAPK pathway. Am J Physiol Cell Physiol. 2013;305(8):C867–876. doi: 10.1152/ajpcell.00121.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulmer BR, Turner TT. A blood-prostate barrier restricts cell and molecular movement across the rat ventral prostate epithelium. J Urol. 2000;163(5):1591–1594. [PubMed] [Google Scholar]
- 32.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63(5):1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 33.Rothweiler F, Michaelis M, Brauer P, Otte J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, Nicoletti F, Cinatl J., Jr Anticancer effects of the nitric oxide-modified saquinavir derivative saquinavir-NO against multidrug-resistant cancer cells. Neoplasia. 2010;12(12):1023–1030. doi: 10.1593/neo.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin ML. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights. 2013;7:27–34. doi: 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, Ho E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28(12):3171–3179. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubelska K, Milczarek M, Modzelewska K, Krzyszto-Russjan J, Fronczyk K, Wiktorska K. Interactions between drugs and sulforaphane modulate the drug metabolism enzymatic system. Pharmacol Rep. 2012;64(5):1243–1252. doi: 10.1016/s1734-1140(12)70920-9. [DOI] [PubMed] [Google Scholar]
- 37.Dankers AC, Roelofs MJ, Piersma AH, Sweep FC, Russel FG, van den Berg M, van Duursen MB, Masereeuw R. Endocrine Disruptors Differentially Target ATP-binding Cassette Transporters in the Blood-Testis Barrier and Affect Leydig Cell Testosterone Secretion in vitro. Toxicol Sci. 2013;136(2):382–391. doi: 10.1093/toxsci/kft198. [DOI] [PubMed] [Google Scholar]
- 38.González-Sarrías A, Miguel V, Merino G, Lucas R, Morales JC, Tomás-Barberán F, Alvarez AI, Espín JC. The gut microbiota ellagic acid-derived metabolite urolithin A and its sulfate conjugate are substrates for the drug efflux transporter breast cancer resistance protein (ABCG2/BCRP) J Agric Food Chem. 2013;61(18):4352–4359. doi: 10.1021/jf4007505. [DOI] [PubMed] [Google Scholar]
- 39.Mulware SJ. The mammary gland carcinogens: the role of metal compounds and organic solvents. Int J Breast Cancer. 2013;2013:640851. doi: 10.1155/2013/640851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Yue X, Zhao R, Cheng B, Wazir R, Wang K. Primary squamous cell carcinoma of seminal vesicle: an extremely rare case report with literature review. Int Urol Nephrol. 2013;45(1):135–138. doi: 10.1007/s11255-012-0373-z. [DOI] [PubMed] [Google Scholar]
- 41.Ormsby AH, Haskell R, Jones D, Goldblum JR. Primary seminal vesicle carcinoma: an immunohistochemical analysis of four cases. Mod Pathol. 2000;13(1):46–51. doi: 10.1038/modpathol.3880008. [DOI] [PubMed] [Google Scholar]
- 42.Grzywacz MJ, Yang JM, Hait WN. Effect of the multidrug resistance protein on the transport of the antiandrogen flutamide. Cancer Res. 2003;63(10):2492–2498. [PubMed] [Google Scholar]
- 43.Schramm P, Schopf RE, Wildfeuer A. Josamycin concentration in human ejaculate and its influence on sperm motility--a contribution to antibiotic therapy in andrological patients. Andrologia. 1988;20(6):521–525. [PubMed] [Google Scholar]
- 44.Simbini T, Umapathy E, Jacobus E, Tendaupenyu G, Mbizvo MT. Study on the origin of seminal leucocytes using split ejaculate technique and the effect of leucocytospermia on sperm characteristics. Urol Int. 1998;61(2):95–100. doi: 10.1159/000030296. [DOI] [PubMed] [Google Scholar]
- 45.Pholpramool C, Borwornpinyo S, Dinudom A. Role of Na+/H+ exchanger 3 in the acidification of the male reproductive tract and male fertility. Clin Exp Pharmacol Physiol. 2011;38(7):403–409. doi: 10.1111/j.1440-1681.2011.05525.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang HF, He RH, Sun CC, Zhang Y, Meng QX, Ma YY. Function of aquaporins in female and male reproductive systems. Hum Reprod Update. 2006;12(6):785–95. doi: 10.1093/humupd/dml035. [DOI] [PubMed] [Google Scholar]
- 47.Da Silva N, Piétrement C, Brown D, Breton S. Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim Biophys Acta. 2006;1758(8):1025–33. doi: 10.1016/j.bbamem.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Danyu L, Ying L, Zhenwu B, Heming Y, Xuejun L. Aquaporin 1 expression in the testis, epididymis and vas deferens of postnatal ICR mice. Cell Biol Int. 2008 May;32(5):532–41. doi: 10.1016/j.cellbi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Kujala M, Hihnala S, Tienari J, Kaunisto K, Hästbacka J, Holmberg C, Kere J, Höglund P. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction. 2007;133(4):775–784. doi: 10.1530/rep.1.00964. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira AG, Aquino DJ, Mahecha GA, Oliveira CA. Involvement of the transepithelial calcium transport disruption and the formation of epididymal stones in roosters. Reproduction. 2012;143(6):835–844. doi: 10.1530/REP-12-0034. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi D, Irokawa M, Maeda T, Tsuji A, Tamai I. Carnitine/organic cation transporter OCTN2-mediated transport of carnitine in primary-cultured epididymal epithelial cells. Reproduction. 2005;130(6):931–937. doi: 10.1530/rep.1.00737. [DOI] [PubMed] [Google Scholar]
- 52.Palme N, Becher A, Merkl M, Glösmann M, Aurich C, Schäfer-Somi S. Immunolocalization of the Cholesterol Transporters ABCA1 and ABCG1 in Canine Reproductive Tract Tissues and Spermatozoa. Reprod Domest Anim. 2014 doi: 10.1111/rda.12294. [DOI] [PubMed] [Google Scholar]
- 53.Ouvrier A, Cadet R, Vernet P, Laillet B, Chardigny JM, Lobaccaro JM, Drevet JR, Saez F. LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner. J Lipid Res. 2009;50(9):1766–1775. doi: 10.1194/jlr.M800657-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales CR, Ni X, Smith CE, Inagaki N, Hermo L. ABCA17 mediates sterol efflux from mouse spermatozoa plasma membranes. Histol Histopathol. 2012;27(3):317–328. doi: 10.14670/HH-27.317. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz A, Wennemuth G, Post H, Brandenburger T, Aumüller G, Wilhelm B. Vesicular transfer of membrane components to bovine epididymal spermatozoa. Cell Tissue Res. 2013;353(3):549–561. doi: 10.1007/s00441-013-1633-7. [DOI] [PubMed] [Google Scholar]