Abstract
Cancers associated with immunosuppression and infections have long been recognized as a major complication of HIV/AIDS. More recently, persons living with HIV are increasingly diagnosed with a wider spectrum of HIV-associated malignancies (HIVAM) as they live longer on combination antiretroviral therapy. This has spurred research to characterize the epidemiology and determine the optimal management of HIVAM with a focus on low-and middle-income countries (LMICs). Given background coinfections, environmental exposures, host genetic profiles, antiretroviral therapy usage, and varying capacities for early diagnosis and treatment, one can expect the biology of cancers in HIV-infected persons in LMICs to have a significant impact on chronic HIV care, as is now the case in high-income countries. Thus, new strategies must be developed to effectively prevent, diagnose, and treat HIVAM in LMICs; provide physical/clinical infrastructures; train the cancer and HIV workforce; and expand research capacity—particularly given the challenges posed by the limitations on available transportation and financial resources and the population’s general rural concentration. Opportunities exist to extend resources supported by the President’s Emergency Plan for AIDS Relief and the Global Fund to Fight AIDS, Tuberculosis, and Malaria to improve the health-care infrastructure and train the personnel required to prevent and manage cancers in persons living with HIV. These HIV chronic care infrastructures could also serve cancer patients regardless of their HIV status, facilitating long-term care and treatment for persons who do not live near cancer centers, so that they receive the same degree of care as those receiving chronic HIV care today.
Keywords: HIV/AIDS, low- and middle-income countries, developing countries, Africa, cancer screening, cancer therapy, infrastructure, health workforce, cancer prevention, cancer diagnosis, training, research
INTRODUCTION
Congenital and acquired immunodeficiencies have long been known to modify the incidence and clinical course of a variety of cancers. The etiologies of most, but not all, such cancers have been associated with infectious agents. Cancers associated with immunosuppression and/or infection, notably Kaposi sarcoma (KS), were among the first well-recognized complications of HIV infection and AIDS in 1981. In this review, we examine the association of HIV disease with cancer in low- and middle-income countries (LMICs), concentrating on sub-Saharan Africa (SSA). We explore the historical factors that have shaped the HIV–cancer co-epidemic and the implication of the wider availability of combination antiretroviral therapy (ART) on cancer burden; we examine critical gaps in infrastructure for cancer diagnosis, screening, training, and treatment in SSA and research and training priorities and challenges (Table 1). We conclude that addressing these gaps is an urgent priority that will have a broad impact on the optimal chronic management of cancers in HIV.
TABLE 1.
Perceived Gaps and Recommendations in Research, Training, and Infrastructure Development
| Area | Specific Focus | Perceived Gaps | Recommendations |
|---|---|---|---|
| Surveillance | Knowledge of HIVAM epidemiology |
Systemic population-based information on cancer incidence and survival not widely available |
Support and expand population-based cancer registration |
| Integrate cancer incidence and outcome measurement into large studies and sentinel surveillance of HIV-infected persons |
|||
| Diagnosis | Pathology services | Core cancer diagnostic facilities are underresourced and often limited to basic histopathology. Variable quality and inconsistent turnaround times limit utility of diagnostic pathology in cancer care and research |
Develop a core set of diagnostic pathology services necessary to diagnose most common HIVAM in resource-limited settings |
| Identify models to assure proficiency, quality, and sustainability |
|||
| Early detection | Majority of cancers present at very late stage |
Increase community awareness of cancer through public health campaigns |
|
| Integrate cancer detection into HIV clinical care |
|||
| Improve access to cancer care outside major urban centers |
|||
| Prevention | Cancer screening programs |
Programs to reduce mortality of “preventable” cancers not validated in HIV-positive persons nor widely implemented in resource-limited settings |
Focused research to determine approaches for preventing cancer in HIV |
| Implementation research to identify optimal strategies for using screening for preventable cancers in HIV |
|||
| Comprehensive tobacco programs |
Nonuniversal adoption of comprehensive tobacco control legislation |
Encourage passage and implementation of WHO comprehensive tobacco control legislation |
|
| Few population-based tobacco cessation programs |
Develop community-level interventions for tobacco cessation |
||
| Treatment | Availability of essential medications |
Majority of chemotherapeutic agents are widely unavailable or prohibitively priced |
Expand and update WHO essential medications list to include a wider range of chemotherapeutic agents |
| Adopt strategies for affordable pricing of antiretrovirals to chemotherapeutics |
|||
| Radiotherapy | Limited availability of radiotherapy to majority of population in resource-limited settings |
Increase number of radiotherapy units in LMICs |
|
| Identify sustainable plans for equipment maintenance |
|||
| Train health-care workers in radiation oncology |
|||
| Surgical oncology | Few surgical oncologic specialty units necessitate that cancer care be provided by surgeons with minimal cancer-specific training |
Increase number of surgical oncology units and extend capacity of general surgeons to diagnose and treat cancer |
|
| Supportive care | Availability of transfusion medicine, infectious disease, pain control/palliative care, and nutrition limits ability to deliver effective cancer care |
Develop core set of supportive services for cancer care that can be adopted in LMICs |
|
| Care plans | Few resource-adapted care guidelines for HIVAM exist or have been validated |
Develop specific HIVAM care guidelines for use in LMICs and establish efficacy in clinical trials |
|
| Physical infrastructure | Cancer treatment centers |
Few dedicated and/or comprehensive cancer treatment facilities in LMICs |
Extend HIV care infrastructure to allow treatment of specific HIVAM |
| Invest in centralized specialty cancer treatment facilities in major population centers |
|||
| Linear accelerators | Few LMICs have access to radiotherapy units and those that do often face antiquated equipment |
Model number and distribution of radiotherapy units needed to provide care for HIVAM and establish plan for modernizing and expanding existing facilities |
|
| Diagnostic radiology | Majority of cancer patients in LMICs have regular access only to plain films and ultrasound radiologic technologies |
Identify core set of diagnostic and interventional radiologic services necessitated by HIVAM in LMICs |
|
| Medical informatics | Care for oncology patients in LMICs is commonly documented on paper records which often are isolated from HIV clinics, primary providers, or other members of the care team |
Adapt and adopt electronic medical record systems used for care of patients with HIV in LMICs |
|
| Human capacity | Physicians | Few dedicated physicians trained in oncologic disciplines available in LMICs |
Increase the number of medical, pediatric, surgical, gynecologic, and radiation oncologists |
| Nurses | Few dedicated nurses trained in oncologic disciplines available in LMICs |
Increase the number of nurses with experience in the care of patients with cancer |
|
| Pharmacists | Few dedicated pharmacists trained in oncologic disciplines available in LMICs |
Increase the number of pharmacists with experience in the care of patients with cancer |
|
| Comprehensive cancer planning |
Cost-effective cancer care strategies |
Feasibility of treating cancer in LMICs questioned because of perception of expense |
Conduct cost-effectiveness analyses to prioritize and advocate for cancer treatment |
| Research | Translational and clinical research teams |
Research teams with expertise in cancer-specific research are not widely available in LMICs |
Continue and expand efforts to train research teams in HIVAM |
| Human subjects protection | Limited experience in conducting cancer research in LMICs hampers effective review by Institutional Review Boards in the United States and LMICs |
Conduct capacity-building exercises to increase cancer-specific regulatory functions |
|
| Length and complication of regulatory approvals for cancer studies in LMICs are becoming prohibitive to effective research |
|||
| Laboratory assays | Supportive and correlative laboratory assays for contemporary cancer research not widely available, necessitating costly or prohibited expatriation of research specimens |
Leverage substantial laboratory capacity for HIV research in LMICs to accommodate HIVAM studies |
|
| Investigational drug availability |
Most promising investigational agents for cancer treatment are not widely available in LMICs and importation can be prohibitive |
Develop centralized system for procurement and distribution of investigational cancer therapeutics in LMICs by learning from best practices in HIV research |
|
| Safety monitoring | Infrastructure for rapid identification and mitigation of SAE in cancer clinical trials not widely available |
Leverage HIV research systems to empower cancer SAE reporting |
SAE, serious adverse events; WHO, World Health Organization.
LANDSCAPE: HISTORY
Cancer has been recognized as an important comorbidity of HIV infection since the start of the global pandemic and was heralded by an outbreak of KS among previously healthy young men in the United States.1 A diagnosis of KS in a person with HIV was subsequently considered an indication of progression to AIDS, and KS became one of the first AIDS-defining conditions and the first AIDS-defining cancer (ADC). Two other cancers, invasive cervical cancer and a subset of aggressive non-Hodgkin lymphoma (NHL), were later included in the category of ADCs by the Centers for Disease Control and Prevention.2,3 The aggressive NHLs include diffuse large B-cell lymphomas, Burkitt lymphoma (BL), and primary central nervous system lymphomas.4 KS, cervical cancer, and central nervous system lymphomas are all attributable to infection with oncogenic viruses [human herpes virus 8 (HHV-8), human papillomavirus (HPV), and Epstein–Barr virus (EBV)]. A proportion of diffuse large B-cell lymphomas and BL are also EBV associated.
Before the HIV epidemic, 2 of the ADCs (KS and BL) were endemic in some countries in equatorial Africa5 and the high prevalence of the etiological oncogenic viruses in these populations posed an increased risk. Unsurprisingly, after the emergence of HIV, KS has become one of the most frequently reported cancers among persons living with HIV (PLHIV) in SSA.6 As ART has become widely available in resource-rich regions, the prevalence of virus-associated cancers, such as KS and, to a lesser extent, aggressive NHLs, has declined dramatically, whereas the prevalence of cervical cancer has risen modestly,7 suggesting a dynamic clinical milieu.
Other cancers have been reported in excess among people with HIV. These cancers, referred to collectively as non-ADCs, are increasingly recognized as a threat to the health of PLHIV and include some with viral associations, such as anal cancer (associated with HPV), liver cancer [associated with hepatitis B virus (HBV) and hepatitis C virus (HCV)], and Hodgkin disease (associated with EBV).
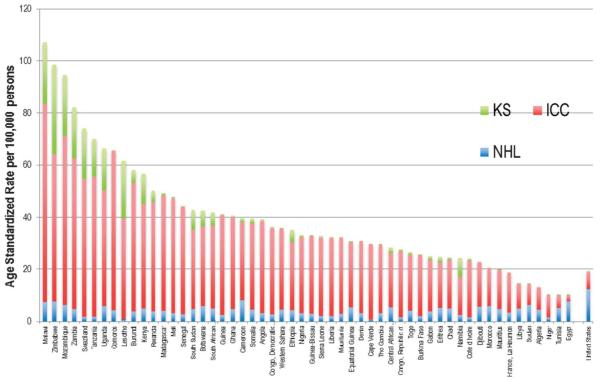
The bulk of our knowledge on the epidemiology of HIV-associated cancers comes from high-income countries (HICs), that is, the United States, Australia, and those in Western Europe, where about 10% of PLHIV live. Beyond a few limited studies, our knowledge of the epidemiology of HIV-associated cancers in LMICs remains incomplete. The reasons for sparse data on the burden of HIV-associated malignancies (HIVAM) in LMICs include weaknesses in health-care infrastructure for diagnosing cancers and limited epidemiological expertise in LMICs. Thus, many cancers go undiagnosed or untreated, and accurate comprehensive information from cancer registries cannot be obtained. These weaknesses make it difficult to carry out record linkage studies of HIV and cancer databases as has been done in HICs.8 Despite the limitations in available data, the incidence patterns of HIVAM in SSA are consistent with a substantial burden relative to HICs (Fig. 1). There is an urgent need to obtain systematic population-based information about HIV-associated cancers from countries where the preponderance of the global HIV resides.
FIGURE 1.
Incidence of ADCs in the general population of African countries compared with the United States (data from IARC, Globocan 2012). ICC, invasive cervical carcinoma. Note: Nations/territories from left to right: Malawi, Zimbabwe, Mozambique, Zambia, Swaziland, Tanzania, Uganda, Comoros, Lesotho, Burundi, Kenya, Rwanda, Madagascar, Mali, Senegal, South Sudan, Botswana, South Africa, Guinea, Ghana, Cameroon, Somalia, Angola, Democratic Republic of Congo, Western Sahara, Ethiopia, Nigeria, Guinea-Bissau, Sierra Leone, Liberia, Mauritania, equatorial Guinea, Benin, Cape Verde, Gambia, Central African Republic, Republic of Congo, Togo, Burkina Faso, Gabon, Eritrea, Chad, Namibia, Côte d’Ivoire, Djibouti, Morocco, La Réunion, Libya, Sudan, Algeria, Niger, Tunisia, Egypt, United States.
EVOLUTION
The evolution of the incidence and spectrum of HIV-associated cancers in LMICs will be influenced by many external factors. Primary among these is the successful rollout of ART by initiatives, such as the President’s Emergency Plan for AIDS Relief (PEPFAR) and The Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM). By significantly increasing the access of LMIC populations to life-extending ART,9-12 these initiatives will lead to reduced mortality from infectious complications of HIV13 and an increase in the mean age of PLHIV, which will likely be accompanied by a corresponding increase in the incidence of selected malignancies, particularly those more common among older persons.14-18 As many as 11% of individuals on ART in Africa are older than 50 years,19 a time when there is a marked increase in cancer risk as a function of age.20
These changes present opportunities for prevention through well-implemented cancer screening programs, strengthening of immunizations against oncogenic viruses, and other initiatives for early cancer detection. Cancer prevention initiatives that harness HIV treatment and prevention programs are likely to be the most cost effective and also to have the broadest impacts.21-23 The increased professional and public awareness of cancer in LMICs as a treatable and preventable comorbidity in people with controlled HIV infection is likely to have spin-off benefits to the general population by strengthening general oncologic practice and cancer registration, increasing our understanding of the spectrum of cancers, and characterizing trends in cancer incidence.24
Excellent examples of such programs can be found in cervical cancer screening initiatives, including those that are now supported by PEPFAR. Programs for early detection of other ADCs, such as KS, particularly in SSA, are likely to allow better treatment outcomes by increasing the proportion of patients detected at a stage early enough to limit the need for chemotherapy. The incidence of anal cancer is rapidly increasing among PLHIV, particularly among gay, bisexual, and other men who have sex with men (collectively referred to as MSM). Screening strategies to detect and treat anal lesions could thus positively affect outcome of anal cancer in PLHIV.
Although it is difficult to predict the trajectory of cancers in HIV-infected populations in LMICs, it is likely that the trends will be shaped by interactions between long-term immunosuppression, because ART does not restore immune competence fully; the prevalence of other risk factors, such as tobacco use; chronic infections (eg, HBV, HPV, and HCV); other environmental exposures (eg, ultraviolet light) and aging; and host genetic predisposition to cancers. These factors are likely to affect the risk of cancers at disparate body sites, including the oral cavity, salivary glands, nasopharynx, esophagus, stomach, colon, anus, lung, bronchus, penis, and eyes.8,25-28 Dramatically increased rates of primary hepatocellular carcinoma have been reported in multiple studies in several LMICs, further highlighting the possible interaction between HIV-related immunosuppression and known hepato-carcinogenic exposures, including HBV and HCV.29,30
Elevated risk of ocular surface squamous cancer (OSSC) in PLHIV has been reported in many countries in SSA outside of South Africa and in India.31 The existence of an infectious risk factor for OSSC is unknown, but some studies have suggested an association with cutaneous types of HPV.32,33 Although data are limited, there is some evidence that among PLHIV in LMICs lung cancer risk is increased.34 The reasons for this increase are not clear but may include smoking (which is concerning because LMICs, particularly in Africa, have been targets for aggressive marketing by the tobacco industry), inflammation from chronic infection, or environmental pollution.35-37
Cancers are difficult to treat in low-resource settings, even in a non-HIV context. Expanded screening programs are likely to increase the number of diagnosed cases of cancer in regions where resources are not sufficient to handle the existing cancer burden. Thus, screening and prevention programs will need to be accompanied by expansion of treatment capacity. Access to chemotherapy depends on a consistent drug supply and the ability of patients to pay. Radiation therapy is available at only a few centers in a few LMICs. Beyond relatively uncomplicated procedures suitable for certain cancers, access to cancer surgery is also limited in LMICs. The increase in the burden of cancers arising from a surge in the population of PLHIV who are living longer because of ART is likely to be substantial. Therefore, given the resource limitations outlined above, this increase is likely to overwhelm current health-care infrastructures, even in those LMICs that have operational oncology services, unless urgent steps are taken to buttress capacity.15
CANCER INFRASTRUCTURE IN LMICs
Diagnosis and treatment of cancer requires a health system with functional, interactive, and responsive physical and human resources to identify, diagnose, and treat cases. The infrastructure necessary for the provision of cancer services is underdeveloped in LMICs, especially those in SSA.38 Although few studies have directly addressed this issue, we suggest that the infrastructure for cancer should comprise 4 elements: physical infrastructure, qualified staff, supportive policies, and supportive laws.
The main components of the physical infrastructure include buildings, such as purpose-built or adapted oncology hospitals, training institutions, laboratories, imaging units, radiation therapy centers, and medical stores; communication infrastructure, such as roads, telephone systems, and Internet availability; and utility support, including water, sewerage, and electricity. Intuitively, countries with better established physical infrastructure have better cancer services, including stable routine facilities for cancer surveillance, diagnosis, treatment, follow-up, and palliation.
The components of qualified and experienced staff include effective team interactions and a goal-driven staff working in multidisciplinary departments. To be effective, research must be integral to practice and should serve to consolidate local knowledge for cancer care. Cancer centers also play an important role in the education of health-care professionals and the public about cancer.
Supportive policies articulated through various documents, including National Cancer Control Plans, acts of parliament, and government white papers, are useful for bringing stability to cancer work, including supporting training plans, conducting monitoring and evaluation, and guiding utilization of scarce resources.
Finally, progressive policies must be backed by the establishment of a regulatory environment to guide expenditures and improve accountability. We suggest that the establishment of additional cancer centers, which are still a rarity in LMICs, is a necessary starting point to guide and shape the development of an effective cancer infrastructure in LMICs.39
Because there are only a few established cancer centers in most LMICs, cancer services are usually obtained from general hospitals in general surgical or medical departments. Some of these hospitals are at regional or district levels, but there is generally little or no information about quantity or quality of the services provided. Most often, the cancer centers that do exist are located in large urban centers, whereas the majority of the population lives in rural areas. Despite their urban location, the hospitals often lack reliable routine pathology, imaging, oncology nursing, and specialized pharmacy departments or have units that offer a low quality of service. Because these hospitals lack multidisciplinary staff, cases are not evaluated by a multi-disciplinary team (like tumor boards) that would optimize treatment and significantly improve patients’ quality of care.40 Rural patients who make it to these hospitals find themselves in an unfamiliar environment, which is difficult to navigate, and have to confront numerous hurdles, including unaffordable co-payments for services, transportation difficulties, and stigmatization.38,41-53
The lack or limited scope of pathology services, which are central to cancer diagnosis and care, is a major gap in most countries in SSA, where there is typically only one pathologist for every 500,000 to 1 million people. The corresponding number in HICs is 1 pathologist for every 20,638 people. By necessity, the few pathologists available in SSA have to provide a broad range of services, may lack specialized training, and may not work in well-functioning laboratories with standard operating procedures for handling and processing of pathology specimens.54,55 The low quality of pathology services in LMICs has been noted in numerous studies.54,56 This constraint not only results in poor clinical care because of low diagnostic accuracy but also limits the ability of researchers working in those settings to contribute to high-impact research and knowledge generation.57 Other specialties crucial to oncologic practice are similarly underdeveloped and critically understaffed. This state of affairs is the result of inadequate investment in cancer care, perpetuated in part by the lack of data, which creates the wrong impression that cancer is comparatively rare in these countries.58-60 Efforts to improve cancer registration coverage of SSA populations, such as the African Cancer Registry Network (http://afcrn.org/), will contribute to redressing this situation.58-60
Despite the challenges noted above, this is an opportune moment to introduce changes to prepare LMICs to confront the looming HIV-related cancer epidemic. We suggest that the basic solution lies in establishing efficient and effective cancer centers that can collect initial information about cancer and conduct training activities to develop qualified and well-trained staff, who will then become leading advocates for policy and regulatory change. Cancer centers can foster harmony and create stable working environments that will lead to long-term staff commitment.38 They can also serve as the hub, with the spokes representing the outlying hospitals (and perhaps HIV clinics) that can provide simpler chronic care, including ongoing chemotherapy for persons who cannot travel to the cancer center often. This approach can encourage the development and integration of complementary services, guide training, assist with the retention of qualified staff through the provision of incentive schemes, and support the systematic collection of data that will inform policy and resource allocation.
The unprecedented investments in malaria and HIV in LMICs through programs, such as PEPFAR and GFATM, have led to improvements in health-care infrastructure and personnel. These include upgrades to laboratory infrastructure, disease surveillance, infection control, monitoring adherence to treatments, and training and retention of key staff.61 These investments and the unprecedented success they have garnered in LMICs increase our confidence that now is the time for action against cancer.62,63 They also present opportunities to galvanize and leverage efforts to introduce cancer surveillance, diagnosis, treatment, and prevention services.22 Examples of groups building on a foundation nested within PEPFAR- or GFATM-supported program include the Uganda Cancer Institute/Fred Hutchinson Cancer Center Alliance in Kampala, Academic Model Providing Access to Healthcare Program (AMPATH)-Oncology in Kenya, and clinical trials groups, such as the AIDS Malignancy Consortium and the AIDS Clinical Trials Group, funded by the National Institutes of Health.64,65 These programs are working to develop and enhance international clinical and research capacity in AIDS-associated cancers through training, protocol development, and enhancements to physical infrastructure. Local, regional, and international nongovernmental organizations, including patient advocacy groups, are being formed with a mission to promote cancer awareness and improve cancer diagnosis and treatment and can be tapped to foster the development of an enabling policy and regulatory framework.
Strategic investments, such as the creation of histopathology core laboratories, could simultaneously support cancer care, research, and registration.54,55 The creation of histopathology core laboratories could provide stable structured training and mentoring programs and introduce best practices in pathology. The laboratories would meet the need for high-quality histopathology clinical care and research, including discovery and biomarker validation. Following the same principle, model cancer centers could be established to improve clinical cancer care, treatment, and surveillance; train multidisciplinary health-care professionals; and educate the public.38 With such strategic investment, nascent cancer centers would be able to establish physical footprints that would strengthen the cancer research infrastructure and foster the training and mentoring of local champions for long-term sustainability.
RESEARCH PRIORITIES AND CHALLENGES
Epidemiological Research: Trends in Cancers Among PLHIV in LMICs
The expansion of access to ART, particularly for PLHIV who still have relatively high CD4+ T-cell counts and associated longevity, creates opportunities for new research initiatives to study the frequency and types of cancers that may emerge in PLHIV in LMICs.6,7,15,52 These studies could be conducted using the well-established HIV/AIDS–cancer record linkage methodology or via cohort or cross-sectional studies.66-69 Etiological studies should also shed light on the interactions between different viral agents and other environmental factors on cancer development in this setting. This may be particularly relevant to childhood cancers.
Pathogenesis: Investigate the Pathogenesis of Unusual HIV-Associated Cancers More Common in LMICs
Much is known about the etiology and pathogenesis of ADCs. The significant proportion of ADCs attributable to oncogenic viruses, including EBV, HPV, and HHV-8, highlight the enhanced risk of infection-related cancers in people with HIV. Several mechanisms may explain the increased incidence of cancer in PLHIV. First, reactivation of oncogenic viruses because of their escape from immune surveillance has been implicated as a primary mode of carcinogenesis. Second, HIV integration at genomic loci near oncogenes and tumor suppressor genes could affect cell proliferation.70 Third, some HIV-encoded proteins (eg, Tat) have been implicated in interactions with known cancer suppressor genes, such as p53.71 Fourth, HIV replication may contribute to carcinogenesis by directly or indirectly producing proteins that promote inflammation or angiogenesis. One such example is the interaction between HIV tat and HHV-8 viral interleukin-6 to promote angiogenesis.72 Finally, HIV replication creates a pro-inflammatory state through its expression of cytokines, inhibition of T-regulatory cells, T-cell activation, B-cell activation, or immune exhaustion. The potentiation of this inflammatory milieu may also contribute to cancer pathogenesis.73
Similar data are needed for cancers that are less frequently diagnosed among PLHIV, such as OSSC.74 There is currently no consensus on an association between HPV infection and OSSC or whether invasive tumors are preceded by dysplastic precursors. If such lesions were found, detection and prevention strategies analogous to those for cervical and anal cancers might prove effective. An association between HIV infection and cancers like hepatocellular carcinoma that are well known in LMICs has been described,26 but none of the studies has been adequately powered statistically to investigate this association and its interaction with potentially modifiable risk factors, such as HBV and HCV infections, alcohol intake, and aflatoxin.
Clinical Research: Conduct Cancer Screening, Diagnosis, and Prevention
In LMICs, many cancers are diagnosed at late stages when palliative care is the only option. Although late diagnosis is not unique to HIVAM, patients and care providers may fail to recognize cancer signs and symptoms (eg, fevers, lymph node enlargement, lung infiltrates), attributing them instead to opportunistic infections, such as tuberculosis.75,76 Research into early diagnosis of cancer, cancer precursors, or modifiable risk factors for cancer development has the potential to significantly reduce HIV-associated cancer morbidity and mortality. Examples of key research priorities include the following: (1) identification of optimal methods to detect and ablate cervical cancer precursor lesions in women with HIV; (2) evaluation of methods to enhance clinical recognition of early KS lesions, the implementation of pharmacologic methods to inhibit HHV-8 viremia in individuals at high risk for KS development, and the implementation of novel, field-implementable low-cost methods to definitively diagnose KS in areas lacking access to pathology laboratories and trained pathologists; and (3) investigation of methods to rapidly and definitively diagnose and discriminate among the multiple potential causes of lymphadenopathy in PLHIV.77
Clinical Research: Develop Resource-Appropriate Strategies for Treatment of HIV-Associated Cancer
In HICs, effective therapeutic strategies have been identified for HIVAM in adults. Whether the same approaches are optimal for cancer management in LMICs has not been systematically determined and should be a major focus of research. Although there is no reason, a priori, to expect that established treatments for invasive cancer in HICs will be less likely to induce tumor regression in LMICs, their overall efficacy in these settings will likely depend on the availability of resources to administer complex therapy, the ability to provide appropriate supportive care and manage complex drug interactions, and the economics of providing costly cancer therapeutics and managing their side effects. Thus, research is urgently needed to evaluate and compare existing therapeutic approaches to treatment of ADC/HIVAM, including reduced-dose regimens that may be associated with lower objective response rates but also a reduced incidence of adverse effects, which may have a significant impact on both quality of life and overall survival.78 Among HIV-infected children with cancer, there have been no systematic investigations to identify optimal therapeutic strategies in either HICs, where such tumors are rare, or LMICs, particularly in SSA, where pediatric HIV infection, KS, and BL are relatively common.79-81 Prospective evaluations of optimal management strategies for pediatric patients with HIV in LMICs should be a high priority.
Operational Research: Integrate Cancer Care With HIV Treatment and Evaluate Cost-Effectiveness and Comparative Efficacy of Cancer Prevention and Treatment Strategies
Given the increasing number of people living with HIV, their growing cancer burden, and the increasingly constrained resources of the international community for care, strategies for more efficient and effective delivery of care of HIVAM are needed. Operational research could help define how cancers could be detected at earlier stages by engaging HIV clinics, how the laboratory infrastructure created by HIV care can be applied to cancer diagnosis, and whether the initial or subsequent care of HIVAM can be accomplished by nononcologists practicing in HIV clinics. These studies should be coupled with economic analyses to inform the use of limited resources and with formal evaluations of the interactions of cancer therapy with ART and drugs commonly used in LMICs to treat HIV comorbidities.
RESEARCH TRAINING PRIORITIES AND CHALLENGES
Comprehensive and sustained training is needed to develop the health-care workforce and scientific leaders who will meet the challenges of cancer care in LMICs. The critical shortage of skilled personnel is one of the greatest difficulties facing health-care delivery in SSA today, and cancer specialists are particularly rare in most LMICs.20,82 For example, Kenya has had only 5 public cancer physicians in the entire country and Uganda has had fewer than 8 oncologists in the sole national cancer center serving a population of 32 million.65,83 Targeted training in the diagnosis and management of HIVAM is needed to meet the growing patient demand for these services. This training should be offered to a range of health-care professionals, including physicians, surgeons, nurses, radiotherapists, pathologists, pharmacists, palliative care providers, and other ancillary staff. To complement the expansion in local specialist care and research capacity, there is also a need to train local health managers, logistical experts, and policy experts to build strong multidisciplinary and well-supported local institutions in LMICs.
To improve cancer training of clinicians and researchers in LMICs, cancer centers designated as “centers of excellence” should be established and linked to regional-level institutions to facilitate sharing of training resources and specialized expertise. The network approach lends itself to creation of educational hubs for cancer training, reduction of duplication in closely located regions, and increased regional responsiveness to the development and conduct of training programs ranging from subspecialist fellowships to train-the-trainer activities.84,85 The well-established HIV care infrastructure in SSA would be a catalyst to support the training of cancer care professionals generally.8 In particular, PEPFAR’s mandate to train care providers in the management of HIV and its complications represents an opportunity to expand the cadre of health-care workers who are knowledgeable about the diagnosis and management of cancer. Other important areas that need addressing include the development of standardized clinical guidelines for the management of HIVAM, a harmonized approach for disseminating knowledge, and a general strategy for supporting the underserved regions with less-experienced providers. To be successful and sustainable, these guidelines and harmonization activities should be developed in collaboration with trained experts and be based on evidence generated by research conducted in LMICs. They should include resource-appropriate diagnostic and treatment recommendations. Although we acknowledge that the crafting of such recommendations will be complex and that critics may ask why patients in LMICs should receive anything less than optimal cancer treatment, the success of these activities will be measured by the substantial and steady progress that they have the potential to achieve, even with the use of lower cost options. The development of more practical, affordable, and sustainable approaches to cancer prevention and therapies holds the promise of saving many lives now lost because of the unattainability of HIC standards of cancer care.
The development of research capacity in LMICs will require the extended training and support of aspiring investigators. International partnerships between institutions with established research infrastructures and those without have proven successful in building research capacity, as exemplified by several collaborations between universities in HICs and cancer centers in SSA.86,87 Essential elements of these investigator training programs include the following: (1) didactic training in cancer epidemiology, biostatistics, and research methodology, including research integrity and human subjects protection; (2) exposure to research through participation in collaborative research projects at the home institution; and (3) career paths at the home institution that support the development and retention of HIVAM specialists. Establishing a system of mentorship and career development is also critical to the professional growth and retention of clinical scientists.88-90 However, most academic medical centers in LMICs report an inadequate number of potential mentors for their junior faculty and postdoctoral trainees.91 Facilitated peer-mentoring programs, in which peer mentees develop their own mentoring groups, may offer an approach to leverage the limited number of senior faculty available for mentoring while building a robust culture and practical experience in mentoring for the next generation of researchers in resource-limited settings. Although these strategies provide a map to begin essential training for providers and researchers in HIVAM, building and maintaining a dedicated oncology workforce in LMICs will ultimately require years of sustained commitment and funding for cancer training by local governments and international agencies.
CONCLUSIONS
The evolving global response to the HIV epidemic has resulted in millions of persons in LMICs gaining access to ART. Although KS declines with ART, living longer in an immunosuppressed state may increase the risk for many other cancers, such as cervical cancer, primary hepatocellular carcinoma, and lung cancer. In this dynamic environment, the evidence base for the causes, optimal screening strategies, and feasible therapies of HIVAM in LMICs remains limited. Diagnosis and therapy for HIVAM have remained focused on HIC needs, with a host of unresolved dilemmas facing LMICs. We urge a focus on the challenges of cancer and HIV/AIDS in LMICs to illuminate modifiable cofactors, elucidate feasible screening strategies, and develop therapies that can be implemented effectively. The lack of a basic cancer diagnostic and treatment infrastructure is a daunting challenge, especially for patients who live far from cancer services,92 and the mission of the core infrastructures that have been developed for HIV care in LMICs through PEPFAR and GFATM can and should be expanded to include HIVAM and malignancies in general.
Acknowledgments
Supported in part by the National Institutes of Health grants: D43 CA153792 (IHV-UM Capacity Development for Research into AIDS-Associated Malignancies) and 1U54HG006947 (African Collaborative Center for Microbiome and Genomics Research) (C.A.A.); D43 CA153720 (C.C. and W.P.), R24 TW007988 [Fogarty International Clinical Research Scholars Support Center at Vanderbilt (S.H.V.)], and U01 CA121947 [AIDS Malignancy Consortium (S.E.K. and C.C.)].
The views expressed in this article are those of the authors and do not reflect the official policy of the National Cancer Institute, the National Institutes of Health, or the US Government.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hymes KB, Cheung T, Greene JB, et al. Kaposi’s sarcoma in homosexual men-a report of eight cases. Lancet. 1981;2:598–600. doi: 10.1016/s0140-6736(81)92740-9. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control Opportunistic non-Hodgkin’s lymphomas among severely immunocompromised HIV-infected patients surviving for prolonged periods on antiretroviral therapy—United States. MMWR Morb Mortal Wkly Rep. 1991;40:591. 597–600. [PubMed] [Google Scholar]
- 3.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 4.Classification system for human T-lymphotropic virus type III/lymphadenopathy-associated virus infections. MMWR Morb Mortal Wkly Rep. 1986;35:334–339. [PubMed] [Google Scholar]
- 5.Parkin DM, Ferlay J, Hamdi-Cherif M, et al. Cancer in Africa: Epidemiology and Prevention. IARC Press; Lyon, France: 2003. [Google Scholar]
- 6.Parkin DM, Sitas F, Chirenje M, et al. Part I: cancer in indigenous Africans–burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 7.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annu Rev Med. 2011;62:157–170. doi: 10.1146/annurev-med-050409-103711. [DOI] [PubMed] [Google Scholar]
- 9.Goosby E, Dybul M, Fauci AS, et al. The United States President’s Emergency Plan for AIDS Relief: a story of partnerships and smart investments to turn the tide of the global AIDS pandemic. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S51–S56. doi: 10.1097/QAI.0b013e31825ca721. [DOI] [PubMed] [Google Scholar]
- 10.El-Sadr WM, Holmes CB, Mugyenyi P, et al. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S96–S104. doi: 10.1097/QAI.0b013e31825eb27b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.April MD, Wood R, Berkowitz BK, et al. The survival benefits of anti-retroviral therapy in South Africa. J Infect Dis. 2014;209:491–499. doi: 10.1093/infdis/jit584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermund SH. Massive benefits of antiretroviral therapy in Africa. J Infect Dis. 2014;209:483–485. doi: 10.1093/infdis/jit586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 14.Chokunonga E, Levy LM, Bassett MT, et al. Aids and cancer in Africa: the evolving epidemic in Zimbabwe. AIDS. 1999;13:2583–2588. doi: 10.1097/00002030-199912240-00012. [DOI] [PubMed] [Google Scholar]
- 15.Adebamowo CA, Akarolo-Anthony S. Cancer in Africa: opportunities for collaborative research and training. Afr J Med Med Sci. 2009;38(suppl 2):5–13. [PubMed] [Google Scholar]
- 16.Bender E. Putting the brakes on cancer in Africa. Cancer Discov. 2013;3:8. doi: 10.1158/2159-8290.CD-ND2012-042. [DOI] [PubMed] [Google Scholar]
- 17.Hontelez JAC, Lurie MN, Newell M-L, et al. Ageing with HIV in South Africa. AIDS. 2011;25:1665–1667. doi: 10.1097/QAD.0b013e32834982ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomon JA, Wang H, Freeman MK, et al. Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet. 2012;380:2144–2162. doi: 10.1016/S0140-6736(12)61690-0. [DOI] [PubMed] [Google Scholar]
- 19.Greig J, Casas EC, O’Brien DP, et al. Association between older age and adverse outcomes on antiretroviral therapy: a cohort analysis of programme data from nine countries. AIDS. 2012;26(suppl 1):S31–S37. doi: 10.1097/QAD.0b013e3283558446. [DOI] [PubMed] [Google Scholar]
- 20.Boyle P, Levin B. World Cancer Report. International Agency for Research on Cancer; Geneva, Switzerland: 2008. [Google Scholar]
- 21.Ononogbu U, Almujtaba M, Modibbo F, et al. Cervical cancer risk factors among HIV-infected Nigerian women. BMC Public Health. 2013;13:582. doi: 10.1186/1471-2458-13-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a “screen-and-treat” program integrated with HIV/AIDS care in Zambia. PLoS One. 2013;8:e74607. doi: 10.1371/journal.pone.0074607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parham GP, Mwanahamuntu MH, Sahasrabuddhe VV, et al. Implementation of cervical cancer prevention services for HIV-infected women in Zambia: measuring program effectiveness. HIV Ther. 2010;4:713–722. doi: 10.2217/hiv.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jedy-Agba E, Adebamowo C. Knowledge, attitudes and practices of AIDS associated malignancies among people living with HIV in Nigeria. Infect Agent Cancer. 2012;7:28. doi: 10.1186/1750-9378-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV/AIDS related cancers in India. Cancer Causes Control. 2008;19:147–153. doi: 10.1007/s10552-007-9080-y. [DOI] [PubMed] [Google Scholar]
- 26.Tanon A, Jaquet A, Ekouevi DK, et al. The spectrum of cancers in West Africa: associations with human immunodeficiency virus. PLoS One. 2012;7:e48108. doi: 10.1371/journal.pone.0048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biggar RJ, Chaturvedi AK, Bhatia K, et al. Cancer risk in persons with HIV/AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4. doi: 10.1186/1750-9378-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YX, Gui XE, Zhong YH, et al. Cancer in cohort of HIV-infected population: prevalence and clinical characteristics. J Cancer Res Clin Oncol. 2011;137:609–614. doi: 10.1007/s00432-010-0911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petoumenos K, Hui E, Kumarasamy N, et al. Cancers in the TREAT Asia HIV Observational Database (TAHOD): a retrospective analysis of risk factors. J Int AIDS Soc. 2010;13:51. doi: 10.1186/1758-2652-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wild CP. Aflatoxin exposure in developing countries: the critical interface of agriculture and health. Food Nutr Bull. 2007;28(suppl 2):S372–S380. doi: 10.1177/15648265070282S217. [DOI] [PubMed] [Google Scholar]
- 31.Nagaiah G, Stotler C, Orem J, et al. Ocular surface squamous neoplasia in patients with HIV infection in sub-Saharan Africa. Curr Opin Oncol. 2010;22:437–442. doi: 10.1097/CCO.0b013e32833cfcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiong T, Borooah S, Msosa J, et al. Clinicopathological review of ocular surface squamous neoplasia in Malawi. Br J Ophthalmol. 2013;97:961–964. doi: 10.1136/bjophthalmol-2012-302533. [DOI] [PubMed] [Google Scholar]
- 33.Pradeep TG, Gangasagara SB, Subbaramaiah GB, et al. Prevalence of undiagnosed HIV infection in patients with ocular surface squamous neoplasia in a tertiary center in Karnataka, South India. Cornea. 2012;31:1282–1284. doi: 10.1097/ICO.0b013e3182479aed. [DOI] [PubMed] [Google Scholar]
- 34.Winstone TA, Man SF, Hull M, et al. Epidemic of lung cancer in patients with HIV infection. Chest. 2013;143:305–314. doi: 10.1378/chest.12-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glynn T, Seffrin JR, Brawley OW, et al. The globalization of tobacco use: 21 challenges for the 21st century. CA Cancer J Clin. 2010;60:50–61. doi: 10.3322/caac.20052. [DOI] [PubMed] [Google Scholar]
- 36.Ipp H, Zemlin AE, Erasmus RT, et al. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab Sci. 2014;51:98–111. doi: 10.3109/10408363.2013.865702. [DOI] [PubMed] [Google Scholar]
- 37.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10:455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orem J, Wabinga H. The roles of national cancer research institutions in evolving a comprehensive cancer control program in a developing country: experience from Uganda. Oncology. 2009;77:272–280. doi: 10.1159/000259258. [DOI] [PubMed] [Google Scholar]
- 39.Garfield DH, Brenner H, Lu L. Practicing western oncology in Shanghai, China: one group’s experience. J Oncol Pract. 2013;9:e141–e144. doi: 10.1200/JOP.2012.000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann K, Guller U, Bugnon S, et al. Interdisciplinary tumour boards in Switzerland: quo vadis? Swiss Med Wkly. 2008;138:123–127. doi: 10.4414/smw.2008.12078. [DOI] [PubMed] [Google Scholar]
- 41.Maree JE, Langley G, Nqubezelo L. “Not a nice experience, not at all”: underprivileged women’s experiences of being confronted with cervical cancer. Palliat Support Care. 2014:1–9. doi: 10.1017/S1478951513001247. [epub ahead of print, February 13, 2014] [DOI] [PubMed] [Google Scholar]
- 42.Maranga IO, Hampson L, Oliver AW, et al. Analysis of factors contributing to the low survival of cervical cancer patients undergoing radiotherapy in Kenya. PLoS One. 2013;8:e78411. doi: 10.1371/journal.pone.0078411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suneja G, Ramogola-Masire D, Medhin HG, et al. Cancer in Botswana: resources and opportunities. Lancet Oncol. 2013;14:e290–291. doi: 10.1016/S1470-2045(13)70283-3. [DOI] [PubMed] [Google Scholar]
- 44.Stefan DC, Elzawawy AM, Khaled HM, et al. Developing cancer control plans in Africa: examples from five countries. Lancet Oncol. 2013;14:e189–195. doi: 10.1016/S1470-2045(13)70100-1. [DOI] [PubMed] [Google Scholar]
- 45.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14:e158–167. doi: 10.1016/S1470-2045(12)70472-2. [DOI] [PubMed] [Google Scholar]
- 46.Gouws L, Eedes D, Marais E, et al. Revolutionising cancer care in South Africa. Lancet Oncol. 2012;13:447–448. doi: 10.1016/S1470-2045(12)70159-6. [DOI] [PubMed] [Google Scholar]
- 47.Hadley LG, Rouma BS, Saad-Eldin Y. Challenge of pediatric oncology in Africa. Semin Pediatr Surg. 2012;21:136–141. doi: 10.1053/j.sempedsurg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Burki TK. Recent developments for cancer care in Africa. Lancet Oncol. 2012;13:e6. doi: 10.1016/s1470-2045(11)70341-2. [DOI] [PubMed] [Google Scholar]
- 49.Strother RM, Rao KV, Gregory KM, et al. The oncology pharmacy in cancer care delivery in a resource-constrained setting in western Kenya. J Oncol Pharm Pract. 2012;18:406–416. doi: 10.1177/1078155211434852. [DOI] [PubMed] [Google Scholar]
- 50.Ugwumba FO, Aghaji AE. Testicular cancer: management challenges in an African developing country. S Afr Med J. 2010;100:452–455. doi: 10.7196/samj.3871. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf AJ, Adamu A, Nuhu FT. Caregiver burden among poor caregivers of patients with cancer in an urban African setting. Psychooncology. 2011;20:902–905. doi: 10.1002/pon.1814. [DOI] [PubMed] [Google Scholar]
- 52.Sitas F, Parkin DM, Chirenje M, et al. Part II: cancer in indigenous Africans–causes and control. Lancet Oncol. 2008;9:786–795. doi: 10.1016/S1470-2045(08)70198-0. [DOI] [PubMed] [Google Scholar]
- 53.Dye TD, Bogale S, Hobden C, et al. Complex care systems in developing countries: breast cancer patient navigation in Ethiopia. Cancer. 2010;116:577–585. doi: 10.1002/cncr.24776. [DOI] [PubMed] [Google Scholar]
- 54.Ogwang MD, Zhao W, Ayers LW, et al. Accuracy of Burkitt lymphoma diagnosis in constrained pathology settings: importance to epidemiology. Arch Pathol Lab Med. 2011;135:445–450. doi: 10.1043/2009-0443-EP.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152–157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 56.Orem J, Sandin S, Weibull CE, et al. Agreement between diagnoses of childhood lymphoma assigned in Uganda and by an international reference laboratory. Clin Epidemiol. 2012;4:339–347. doi: 10.2147/CLEP.S35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adebamowo CA, Famooto A, Ogundiran TO, et al. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–188. doi: 10.1007/s10549-007-9694-5. [DOI] [PubMed] [Google Scholar]
- 58.Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6:603–612. doi: 10.1038/nrc1948. [DOI] [PubMed] [Google Scholar]
- 59.Adebamowo AC. The realities of providing cancer care in Nigeria. Paper presented at: American Society of Clinical Oncology Annual Meeting; Chicago, IL. Jun 3, 2013. [Google Scholar]
- 60.Jedy-Agba E, Curado MP, Ogunbiyi O, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. 2012;36:e271–e278. doi: 10.1016/j.canep.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mbulaiteye SM, Talisuna AO, Ogwang MD, et al. African Burkitt’s lymphoma: could collaboration with HIV-1 and malaria programmes reduce the high mortality rate? Lancet. 2010;375:1661–1663. doi: 10.1016/S0140-6736(10)60134-1. [DOI] [PubMed] [Google Scholar]
- 62.Mbulaiteye SM, Bhatia K, Adebamowo C, et al. HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. Infect Agent Cancer. 2011;6:16. doi: 10.1186/1750-9378-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lingwood RJ, Boyle P, Milburn A, et al. The challenge of cancer control in Africa. Nat Rev Cancer. 2008;8:398–403. doi: 10.1038/nrc2372. [DOI] [PubMed] [Google Scholar]
- 64.Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–259. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strother RM, Asirwa FC, Busakhala NB, et al. AMPATH-Oncology: a model for comprehensive cancer care in sub-Saharan Africa. J Cancer Policy. 2013;1:e42–e48. [Google Scholar]
- 66.Akarolo-Anthony SN, Maso LD, Igbinoba F, et al. Cancer burden among HIV-positive persons in Nigeria: preliminary findings from the Nigerian AIDS-cancer match study. Infect Agent Cancer. 2014;9:1. doi: 10.1186/1750-9378-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalal S, Beunza JJ, Volmink J, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 68.Holmes MD, Dalal S, Volmink J, et al. Non-communicable diseases in sub-Saharan Africa. Part II—the case for cohort studies. PLoS Med. 2010;7:e1000244. doi: 10.1371/journal.pmed.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mbulaiteye SM, Katabira ET, Wabinga H, et al. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 70.Cohen J. HIV/AIDS. Cancer genes help HIV persist, complicating cure efforts. Science. 2014;343:1188. doi: 10.1126/science.343.6176.1188. [DOI] [PubMed] [Google Scholar]
- 71.Li CJ, Wang C, Friedman DJ, et al. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995;92:5461–5464. doi: 10.1073/pnas.92.12.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou F, Xue M, Qin D, et al. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpes virus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3beta signaling pathway. PLoS One. 2013;8:e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24:501–506. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Chokunonga E, Borok MZ, Chirenje ZM, et al. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991-2010. Int J Cancer. 2013;133:721–729. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 75.Puvaneswaran B, Shoba B. Misdiagnosis of tuberculosis in patients with lymphoma. S Afr Med J. 2013;103:32–33. doi: 10.7196/samj.6093. [DOI] [PubMed] [Google Scholar]
- 76.Mosam A, Carrara H, Shaik F, et al. Increasing incidence of Kaposi’s sarcoma in black South Africans in KwaZulu-Natal, South Africa (1983-2006) Int J STD AIDS. 2009;20:553–556. doi: 10.1258/ijsa.2008.008372. [DOI] [PubMed] [Google Scholar]
- 77.Jiang L, Mancuso M, Lu Z, et al. Solar thermal polymerase chain reaction for smartphone-assisted molecular diagnostics. Sci Rep. 2014;4:4137. doi: 10.1038/srep04137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krown SE. Cancer in resource-limited settings. J Acquir Immune Defic Syndr. 2011;56:297–299. doi: 10.1097/QAI.0b013e31820c0b0f. [DOI] [PubMed] [Google Scholar]
- 79.Gantt S, Kakuru A, Wald A, et al. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatr Blood Cancer. 2010;54:670–674. doi: 10.1002/pbc.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orem J, Maganda A, Mbidde EK, et al. Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infection. Pediatr Blood Cancer. 2009;52:455–458. doi: 10.1002/pbc.21769. [DOI] [PubMed] [Google Scholar]
- 81.Stefan DC, Wessels G, Poole J, et al. Infection with human immunodeficiency virus-1 (HIV) among children with cancer in South Africa. Pediatr Blood Cancer. 2011;56:77–79. doi: 10.1002/pbc.22672. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization . World Health Statistics 2010. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 83.Anonymous Parliament of the Republic of Kenya. 2011 Available at: http://www.parliament.go.ke. Accessed March 14, 2014.
- 84.Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e142–151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 85.Sloan FA, Gelband H. Cancer Control Opportunities in Low- and Middle-Income Countries. Institute of Medicine of the National Academies, National Academic Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- 86.Ribeiro RC, Pui CH. Saving the children–improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352:2158–2160. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 87.Hopkins J, Burns E, Eden T. International twinning partnerships: an effective method of improving diagnosis, treatment and care for children with cancer in low-middle income countries. J Cancer Policy. 2013;1:e8–e19. [Google Scholar]
- 88.Feldman MD, Arean PA, Marshall SJ, et al. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:5063. doi: 10.3402/meo.v15i0.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pololi LH, Krupat E, Civian JT, et al. Why are a quarter of faculty considering leaving academic medicine? A study of their perceptions of institutional culture and intentions to leave at 26 representative U.S. medical schools. Acad Med. 2012;87:859–869. doi: 10.1097/ACM.0b013e3182582b18. [DOI] [PubMed] [Google Scholar]
- 90.Sambunjak D, Straus SE, Marusic A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103–1115. doi: 10.1001/jama.296.9.1103. [DOI] [PubMed] [Google Scholar]
- 91.Nakanjako D, Byakika-Kibwika P, Kintu K, et al. Mentorship needs at academic institutions in resource-limited settings: a survey at Makerere University College of Health Sciences. BMC Med Educ. 2011;11:53. doi: 10.1186/1472-6920-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slone JS, Chunda-Liyoka C, Perez M, et al. Pediatric malignancies, treatment outcomes and abandonment of pediatric cancer treatment in Zambia. PLoS One. 2014;9:e89102. doi: 10.1371/journal.pone.0089102. [DOI] [PMC free article] [PubMed] [Google Scholar]