Abstract
Major therapeutic progress has been accomplished in leukemia and myelodysplastic syndrome (MDS) over the past 40 years, which may not be fully appreciated by the larger medical community. The objective of this review was to briefly highlight the treatment breakthroughs in leukemia and MDS. Therapeutic progress happened through better understanding of disease pathophysiologies and rational development of targeted agents, like imatinib mesylate in chronic myeloid leukemia (CML), and through astute, empirical discoveries in the clinic, like all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia (APL) and chlorodeoxyadenosine in hairy cell leukemia (HCL). Today, the 5- to 10-year survival rates in patients with APL and HCL exceed 80%. In patients with CML, imatinib therapy has been associated with estimated 5- to 7-year survival rates from 85% to 90%. In patients with adult acute lymphocytic leukemia, modern intensive regimens have improved the 5-year survival rates from 20% up to 40%. In patients with chronic lymphocytic leukemia, chemoimmunotherapy recently produced high rates of quality responses and improved long-term outcome. In younger patients with acute myeloid leukemia (AML), the 5-year survival rates range from 40% to 50%, although elderly AML remains a therapeutic challenge. In patients with MDS, it was recently demonstrated that epigenetic therapy with hypomethylating agents improved survival. Much therapeutic progress has been witnessed in leukemia and MDS, and much more is expected to occur soon.
Keywords: acute leukemia, chronic leukemia myelodysplastic syndrome, survival, treatment era, prognosis
Significant therapeutic progress has occurred in leukemia and myelodysplastic syndrome (MDS) over the last 40 years. Therapeutic progress has occasionally occurred in giant leaps (eg, imatinib mesylate therapy in chronic myeloid leukemia [CML]; 2-chlorodeoxyadenosine [CDA] in hairy cell leukemia [HCL]), but has occurred more often in smaller, incremental steps (eg, high-dose cytarabine in acute myeloid leukemia [AML]; modifications of the complex programs in adult acute lymphocytic leukemia [ALL]). At times, discoveries have followed the bench-to-bedside paradigm, evolving rationally from a better understanding of the disease pathophysiology (eg, Bcr-Abl-selective tyrosine kinase inhibitors [TKIs] in CML). At other times, progress was empirical, a result of clinical research for which a biologic rationale was either not elucidated (eg, CDA in HCL) or was clarified post hoc (eg, all-trans retinoic acid [ATRA] and arsenic trioxide in acute promyelocytic leukemia [APL]). This review, on the sixtieth anniversary of the journal Cancer, summarizes the therapeutic milestones in leukemia and MDS. Because of the review scope, the disease biologies are mentioned briefly, and only in the context of their therapeutic implications.
Acute Myeloid Leukemia
Therapeutic perspective
In the 1960s, therapy for AML included the available agents, vincristine, steroids, 6-mercaptopurine, and methotrexate (POMP-like therapy). This produced complete response (CR) rates of 20% to 30%, and cures were rare. The discovery of the activity of cytarabine was the first major therapeutic milestone in the treatment of AML and resulted in CR rates of 40% and long-term survival rates from 10% to 15%. Anthracyclines also proved to be active, leading to studies of cytarabine plus anthracycline combinations and to the testing of different anthracyclines (daunorubicin vs doxorubicin) and dose schedules of daunorubicin plus cytarabine. A series of randomized trials established daunorubicin from 45 mg/m2 to 60 mg/m2 intravenously daily ×3 and cytarabine from 100 mg/m2 to 200 mg/m2 as a continuous infusion daily ×7, known as the `3+7 regimen,' as the gold standard of therapy for the next 30 years.1–3
Understanding the mechanisms of action of cytarabine in AML (phosphorylation and cellular uptake and retention) led to the investigation of high-dose cytarabine schedules. High-dose cytarabine consolidation in AML in first remission was associated with higher disease-free survival rates, particularly in younger patients and those with diploid karyotypes.4–8 High-dose cytarabine during induction also improved disease-free survival.9
Studies that compared a newer anthracycline, idarubicin, to daunorubicin demonstrated that the newer agent was associated with improved rates of CR, survival, and remission duration in various studies, even using equitoxic anthracycline dosages.10–13 The addition of other agents, such as etoposide, fludarabine, or cladribine, to cytarabine plus anthracyclines did not improve the results significantly.14–16 Combinations of fludarabine and cytarabine and combinations of topotecan and cytarabine yielded equivalent results to those achieved with anthracyclines plus cytarabine and were proven as useful therapeutic alternatives for older patients with cardiovascular problems.17 Antibacterial and antifungal prophylaxis, antiemetics, and growth factors have decreased the incidences of infections, morbidities, and hospital stays. Today, the expert use of chemotherapy regimens with supportive care is associated with average CR rates from 60% to 70% and with long-term survival rates from 25% to 35% (Fig. 1).
Figure 1.
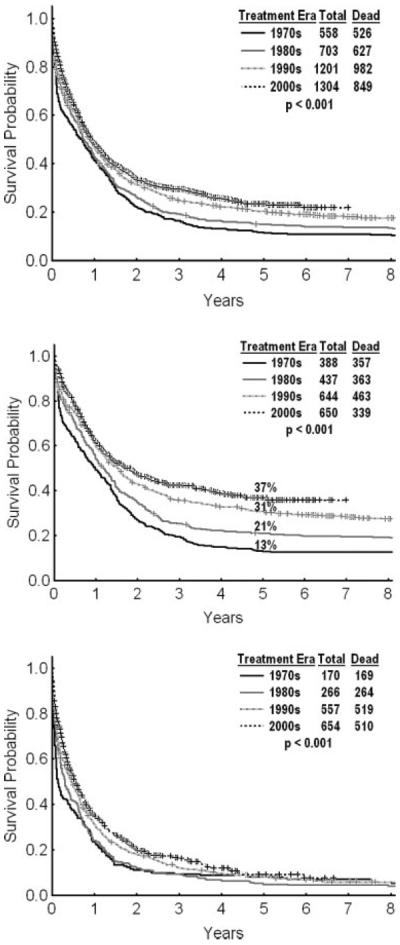
The survival of patients with acute myeloid leukemia (AML) is illustrated by decade overall (Top), among patients aged <60 years (Middle), and among patients aged ≥60 years (Bottom). Data are from 3766 patients with newly diagnosed AML who were referred to the Leukemia Department of the M. D. Anderson Cancer Center from 1973 to the present.
Acute promyelocytic leukemia
APL is an oncologic medical emergency requiring immediate institution of therapy to reduce the risk of bleeding and mortality from disseminated intravascular coagulapathy.18 Intensive chemotherapy in APL resulted in CR rates of 60% and cure rates of 30% to 40%. The discovery of the anti-APL activity of ATRA and arsenic trioxide represented major therapeutic advances in APL, improving the CR rates to 90% and the cure rates to 70% to 85%.19,20 Adverse prognostic factors at presentation are leukocytosis (peripheral blasts >10 × 109/L) and thrombocytopenia (<40 × 109/L).21 Current therapy consists of combinations of ATRA and anthracyclines (with or without cytarabine). The addition of arsenic trioxide as consolidation in first CR improved outcomes.22 The roles of cytarabine and of maintenance with 6 mercaptopurine and methotrexate are being questioned as more effective frontline therapies become available. Arsenic trioxide is probably the most active single agent in the treatment of APL followed by ATRA and gemtuzumab ozogamycin.23 Therapy for APL in the near future likely will consist of combinations of ATRA, arsenic trioxide, and gemtuzumab ozogamycin without the use of anthracyclines, cytarabine, or other chemotherapies.24 The results of such regimens are updated in Figure 2. A `leukocytosis syndrome' is noted in 20% to 30% of patients with APL who receive ATRA and/or arsenic trioxide. This occurs around Days 7 through 14 and may manifest as fever, aches, pulmonary infiltrates and failure, multiorgan failure, or thrombotic events. It often can be prevented or treated with steroids (eg, dexamethasone 20 mg daily ×7–10 days).18
Figure 2.
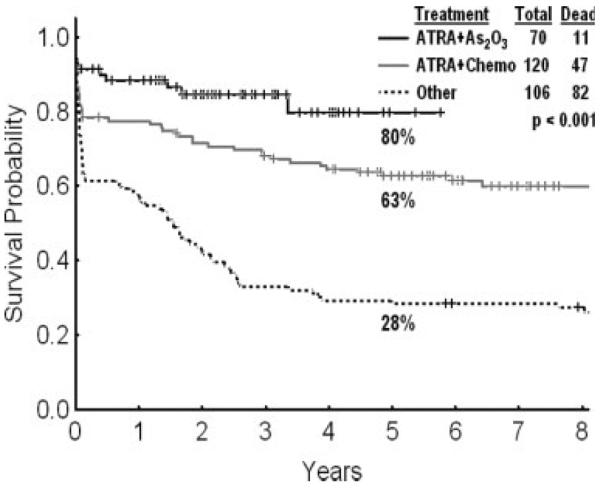
This chart illustrates the survival of patients with acute promyelocytic leukemia at the M. D. Anderson Cancer Center who received chemotherapy (Chemo), all-trans retinoic acid (ATRA) plus chemotherapy, and ATRA plus arsenic trioxide (AS2O3).
Core-binding factor leukemias
Core-binding factor (CBF) leukemias are associated with cytogenetic abnormalities involving translocation 8, 21[t(8;21)], or inversion of chromosome 16. The use of several courses of high-dose cytarabine induction-consolidation in CBF leukemias has improved the CR rates to 90% and the cure rates to 60%.25,26
Acute myeloid leukemia in older patients
AML in older patients presents a common and frustrating problem in leukemia. The median age of patients with AML is 65 to 70 years. Most investigations of AML therapy have focused on younger patients and have often excluded patients aged ≥60 years. The application of the `3+7 regimen' to older patients is associated with unacceptably high early mortality rates (30%–50% in the first 6–8 weeks27–29) (Table 1). Currently, only approximately 33% of older patients with AML are offered any form of chemotherapy because of poor performance status, organ dysfunction, the presence of comorbidities, and the perception that they may be `unfit' for intensive chemotherapy.30 Whereas the survival of younger patients with AML has improved significantly, with an estimated survival rate of 40% (Fig. 1, middle), survival in older patients has not improved markedly (Fig. 1, bottom). Low-intensity `targeted' therapies under investigation in older patients include clofarabine (adenosine nucleoside analogue), hypomethylating agents (decitabine, 5-azacitidine), low-dose cytarabine in combination with gemtuzumab ozogamycin (CD33 monoclonal antibody linked to calicheamycin), arsenic trioxide, and others.31–36
TABLE 1.
Outcome of Older Patients With Acute Myeloid Leukemia Who Received Intensive Chemotherapy*
| Karyotype | CR,% | 8-Week Mortality, % | 2-Year EFS, % |
|---|---|---|---|
| Ages 65–74 y | |||
| Diploid | 60 | 22 | 12 |
| Unfavorable | 39 | 35 | 6 |
| Aged ≥75 y | |||
| Diploid | 43 | 40 | 10 |
| Unfavorable | 35 | 47 | 3 |
CR indicates complete response, EFS, event-free survival.
M. D. Anderson Cancer Center data, 1990–2007.
Biologic perspective
Consistent prognostic factors associated with differences in outcome in AML include host-related factors (age, performance status, organ functions, comorbidities) and leukemia-related factors (cytogenetic and molecular abnormalities). Cytogenetic studies have divided patients with AML into 3 prognostic groups: 1) favorable ([15;17], t[8;21], and inversion 16), 2) intermediate (usually includes diploid karyotype or other categories that are not unfavorable), and 3) unfavorable (particularly chromosome 5 and/or 7 abnormalities and abnormalities that involve ≥3 changes). Patients with favorable karyotypes have CR rates of 90% and cure rates of 60% to 90%. Patients with intermediate karyotypes have CR rates of 60% to 70% and cure rates of 30% to 40%. Patients with unfavorable karyotypes have CR rates of 40% to 50% and cure rates ≤10%.37–39
Abnormalities of the FMS-like tyrosine 3, or FLT3, occur in 30% of patients with AML. An important aberration in FLT3 is the internal tandem duplication (ITD), which results in constitutive activation of the tyrosine kinase (TK) activity of FLT3 and downstream signaling events. Other abnormalities are the TK domain point mutations. FLT3 ITD occurs in 20% to 30% of AML and has been associated with an unfavorable prognosis.40,41 FLT3 inhibitors have demonstrated modest activity as single agents42,43 and are under investigation in combination with chemotherapy in patients with AML and FLT3 mutations.44,45 Mutations of nucleophosmin occur in 50% of patients with diploid karyotype and are associated with a favorable prognosis.46,47 Other relevant molecular abnormalities in AML include partial tandem duplications of the mixed-lineage leukemia or MLL gene (5%–10% of cases; unfavorable), mutations of the CCAAT/enhancer binding protein gene CEBPA (10%; favorable), and mutations of the brain and acute leukemia cytoplasmic gene BAALC (10%; unfavorable).48–51 Gene expression profiling in AML may identify new AML subsets with different prognoses or define new therapeutic targets.52,53 Detection of minimal residual disease in AML and ALL in morphologic remission is associated with relapse and worse prognosis.
The future
A better understanding of the diverse pathophysiology of AML may identify new therapeutically relevant targets. The expression of CD33 on AML cells resulted in several studies of gemtuzumab ozogamycin alone or in combination with chemotherapy. A recent large, randomized Medical Research Council (MRC) trial demonstrated that the addition of a single, small dose of gemtuzumab ozogamycin (3 mg/m2) to standard chemotherapy during induction and 1 consolidation course improved event-free survival and reduced relapse rates.54 Increased site-specific and global methylation in AML have been associated with an unfavorable prognosis,55 and the use of hypomethylating agents has yielded favorable results.31,34 Several new agents with different mechanisms of action also have demonstrated encouraging activity and, when they are incorporated into standard therapy, may improve prognosis in AML.56 APL may be the first acute leukemia to become highly curable with minimal or no chemo-therapy. Allogeneic stem cell transplantation (SCT) and its variations (unrelated donors, nonablative SCT, cord blood) continue to improve the results in AML and are established curative modalities in AML in first CR as well as in refractory-recurrent AML.57–60 The best timing of allogeneic SCT (first CR vs postchemotherapy failure) and within which subsets (favorable vs unfavorable) is under constant reevaluation.
Acute Lymphocytic Leukemia
Therapeutic perspective
Childhood ALL is the poster child for a successful chemotherapy story in cancer.61,62 In contrast to the French proverb, `Plus ça change, plus c'est la méme chose,' therapeutic progress in ALL has followed the line of `ça change en plus, avec les mémes choses.' The use of a multitude of established drugs (vincristine, steroids, anthracyclines, cyclophosphamide, asparaginase, methotrexate, 6 mercaptopurine) and fine tuning schedules and dose-intensity delivery have increased the cure rate in pediatric ALL from 30% to 80%.
The principles of ALL therapy revolve around remission induction, consolidation, maintenance, and central nervous system (CNS) prophylaxis. Using the same principles in adult ALL has increased the CR rates to 80% to 90% and the cure rates to 40% (Fig. 3, top).63–70 Induction chemotherapy relies on the backbone of steroids (with dexamethasone favored over prednisone in recent studies),71,72 anthracyclines (daunorubicin or doxorubicin), and vincristine. The addition of cyclophosphamide, asparaginase, or high-dose cytarabine73–75 to induction therapy did not improve the results significantly. Consolidation therapy used higher dose schedules of cytarabine, methotrexate, or allogeneic or autologous SCT.74–77 Maintenance therapy favored longer durations (usually 2–3 years) of 6 mercaptopurine, vincristine, steroids and methotrexate (POMP) with intermittent consolidations in between. Shorter POMP maintenance produced worse results.78 CNS prophylaxis has gradually shifted from craniospinal irradiation (long-term neurologic sequelae, second cancers, learning disabilities) to the equivalently effective intrathecal prophylaxis.79,80
Figure 3.
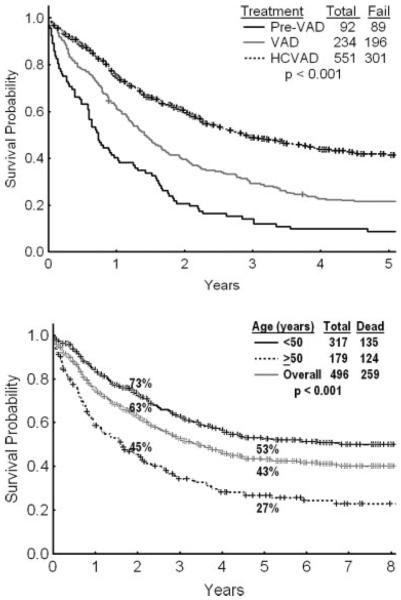
Top: The survival of patients with adult acute lymphoblastic leukemia (ALL) is illustrated in this chart from M. D. Anderson Cancer Center data for 3 successive regimens from 1980 to the present: before vincristine, doxorubicin, and dexamethasone (Pre-VAD) (1980–1985); VAD (1985–1992); and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HCVAD) (1992–2007). Bottom: Survival on the HCVAD regimen is illustrated in patients with adult ALL overall and by age group (<50 years vs ≥50 years).
Several adult ALL regimens closely follow the principles of childhood ALL regimens, yet they do not achieve the high cure rates of childhood ALL. These include the Berlin-Frankfurt-Munster regimen; the Cancer And Leukemia Group B regimen; and the M. D. Anderson Cancer Center regimen of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD).63–68 Programs than exclude older patients (aged ≥60 years) and patients with Philadelphia chromosome (Ph)-positive ALL usually produce better results.69,70 For example, whereas the long-term survival rate with hyper-CVAD is 40% overall (median patient age, 42 years), it increased to 53% if only patients aged ≤50 years were considered. (Fig. 3, bottom).
The difference in prognosis in childhood ALL versus adult ALL is predominantly because of differences in the disease biology. ALL with hyperdiploid karyotype (more than 50 chromosomes) and the cryptic cytogenetic molecular abnormality t(12:21) that produces the TEL-AML1 fusion gene, both favorable ALL subtypes, account for 50% of childhood ALL but only 10% of adult ALL. The Ph-positive ALL subtype, an unfavorable disease until the recent addition of imatinib mesylate to chemotherapy, is observed in only 3% of childhood ALL.81–83
Several studies reported a better outcome among adolescents and young adults (AYAs) who were treated on pediatric regimens versus AYAs who received adult regimens.84–87 These studies did not elaborate on the details of the regimen differences, but the adult regimens often either provided shortened POMP maintenance or omitted it. The abbreviated/omitted POMP maintenance in adult ALL programs may largely explain these differences. Convinced by the accumulating evidence, several investigators have adopted the `augmented' pediatric programs in adult ALL therapy. These regimens often intensify the dose-schedule delivery of the nonmyelosuppressive agents, namely vincristine, steroids, and asparaginase. The early results from these programs are encouraging, and reports have estimated 2-year survival rates of 75%, better than past historic experience88–90 but similar to the results reported with hyper-CVAD in patients aged ≤50 years (Fig. 3).63 A plateau effect of the survival curves in these studies may prove their benefit over hyper-CVAD. It is noteworthy that the `augmented' pediatric regimens had significant toxicities in adult ALL, particularly among older patients (aged ≥50 years), who have been excluded from such trials. Subset analyses have also demonstrated that the pediatric regimens may have benefited patients between ages of 15 and 40 years, but not older patients.90
The role of allogeneic SCT in first CR has been investigated across numerous studies.91,92 A recent MRC-Eastern Cooperative Oncology Group trial that involved >1980 patients (ages 15–e50 years; expanded later to age 55 years) who were randomized genetically either to undergo allogeneic SCT (if a matched sibling donor was available) or otherwise to receive chemotherapy versus autologous SCT demonstrated a better outcome with allogeneic SCT among patients with standard-risk ALL.93 Only 11% of the total study group underwent allogeneic SCT for standard-risk ALL. That trial also demonstrated that autologous SCT was inferior to chemotherapy (Table 2). Some subtypes of ALL benefit from specific therapies.
TABLE 2.
Outcome of Patients With Adult Acute Lymphoblastic Leukemia After Postremission Consolidation With Allogeneic Stem Cell Transplantation (SCT), Autologous SCT, or Chemotherapy
| Parameter | No. of Patients | 5-Year Survival, % | P | EFS, % | P |
|---|---|---|---|---|---|
| Donor available | <.05 | <.05 | |||
| Yes | 384 | 54 | 50 | ||
| No | 418 | 44 | 40 | ||
| Allogeneic SCT | |||||
| High risk | NS | NS | |||
| Yes | 168 | 41 | 38 | ||
| No | 190 | 35 | 31 | ||
| Standard risk | <.05 | <.05 | |||
| Yes | 216 | 64 | 59 | ||
| No | 223 | 51 | 45 | ||
| No allogeneic SCT | <.05 | <.05 | |||
| Autologous SCT | 220 | 37 | 33 | ||
| Chemotherapy | 215 | 46 | 42 |
EFS indicates event-free survival; NS, not significant.
Burkitt or mature B-cell acute lymphocytic leukemia
Burkitt ALL or mature B-cell ALL is uncommon and constitutes 5% to 10% of adult ALL. It is often associated with L3 morphology (according to the French-American-British classification) and/or with translocations involving chromosome 8 and 14, 2, or 22 (t[8;14], t[2;8]; t[8;22]). It is highly proliferative (its doubling time often is <12 hours), it may present with bulky adenopathy and hepatosplenomegaly, and it is associated with a high incidence of CNS disease at presentation (30%) and with tumor lysis and renal failure. Together with APL and severe leukocytosis, it represents one the few true chemotherapy emergencies in leukemia and cancer in which immediate implementation of therapy is necessary. Historically, mature B-cell ALL was associated with a hopeless prognosis and a cure rate of <5% with standard regimens. Recent short-term, dose-intensive regimens incorporating high doses of fractionated cyclophosphamide and high doses of cytarabine and methotrexate with intensive CNS prophylaxis and therapy have improved the results dramatically, producing CR rates of 90% and cure rates of 70%.94 The addition of rituximab to these regimens has improved the results further.95 Maintenance therapy in mature B-cell ALL is not needed.
Philadelphia chromosome-positive acute lymphoblastic leukemia
Ph-positive acute ALL used to be associated with a very poor prognosis. The addition of imatinib to chemotherapy has improved the results in most, but not all, studies. Combinations of chemotherapy plus imatinib have now improved the 3- to 4-year survival rates to >50%.96–98 The dose intensity of imatinib may be important. In the 1 negative study to date, imatinib was added to chemotherapy only after induction and for limited and interrupted periods.99 Like in CML, extended continuous therapy with imatinib may be critical for improved survival.
T-cell acute lymphocytic leukemia
The outcomes of patients with T-cell ALL, which includes approximately 15% of ALL, have also improved with the addition of multiple courses of cytarabine, methotrexate, and asparaginase. The reported cure rate in adult ALL with such regimens is 40% to 50%.100 Nelarabine, a guanosine nucleoside analogue, is highly active in T-cell ALL, produces CR rates of 40% to 50% in refractory-recurrent ALL, and has been approved for this condition. Its incorporation into frontline T-cell-specific ALL regimens may further improve prognosis.101,102
Biologic perspective
Like in patients with AML, the prognosis in patients with ALL is determined by host-associated factors (age, performance status, comorbidities) and disease-associated factors. The immunophenotypic and cytogenetic subsets described above are associated with differences in prognosis.103,104 The persistence of minimal residual disease (MRD) in morphologic CR, detected by molecular or multiparameter flow-cytometric studies, has been associated now with a consistently worse prognosis. Although such studies have not yet been incorporated into routine monitoring procedures, they should be adopted. The detection of MRD in CR should lead to therapeutic modifications in investigational programs. Genomicproteomic profiling of ALL may identify new prognostic-pathophysiologic subsets or new targetable gene clusters.105–107
The future
Although the cure rate in adult ALL has improved to approximately 40%, the current status quo is unsatisfactory. Several strategies are promising: 1) the addition of rituximab to chemotherapy in mature B-cell ALL and perhaps in CD20-positive pre-B ALL,95,108 2) optimizing the delivery of Bcr-Abl TKIs (particularly the more potent dual Src-Abl inhibitors like dasatinib) in chemotherapy-targeted therapy combinations,109 3) the introduction of T-cell-targeted agents (nelarabine) to T-cell ALL,101,102 and 4) addressing the problem of persistent MRD with intensive or modified regimens (allogeneic SCT, others). Several anti-ALL agents also are emerging. These include clofarabine (which has been approved for the treatment of refractory-recurrent pediatric ALL),110 modified formulations of liposomal vincristine (perhaps more potent and less toxic),111 polyethylene glycolconjugated formulations of asparaginase (longer acting and perhaps more potent),112,113 liposomal formulations of cytarabine for CNS prophylaxis-therapy (longer acting, less frequent delivery),114 and new monoclonal antibodies.115,116 An intense, ongoing search for anti-ALL-specific agents (eg, Notch inhibitors) may yield positive therapeutic discoveries in ALL.
Chronic Myeloid Leukemia
Therapeutic perspective
The discovery of the activity of imatinib mesylate, a selective Bcr-Abl TKI, represented a true therapeutic paradigm shift in CML.117,118 Before the broad availability of imatinib, CML was considered a poor-prognosis leukemia with a median survival of 3 to 4 years. Frontline therapies included 1) allogeneic SCT (curative in 40%–70% of patients; 1-year mortality rate, 10%–40%,) which was available potentially to 20% to 40% of patients (limitations of patient age and donor availability); and 2) interferon-α therapy, which produced cytogenetic CR rates of 10% to 35% and improved the median survival to 6 to 7 years and the 10-year survival rate to 30% to 40%.119–121 Patients with CML after the failure of such strategies had no subsequent options.122,123
Since 2000, the year imatinib became widely available, the course of CML has changed to an `indolent' form. The estimated annual mortality was reduced from the historic rate of 10% to 20% to ≤2% in the first 6 years of follow-up (Fig. 4). The CML-specific annual mortality is approximately 1%. If the current experience continues to be as favorable, then the estimated median survival in CML would exceed 25 years.124–128 With an incidence of 5000 cases per year in the United States and an annual mortality of 2%, the prevalence of CML will continue to rise until it plateaus at approximately 250,000 cases around the Year 2040.
Figure 4.
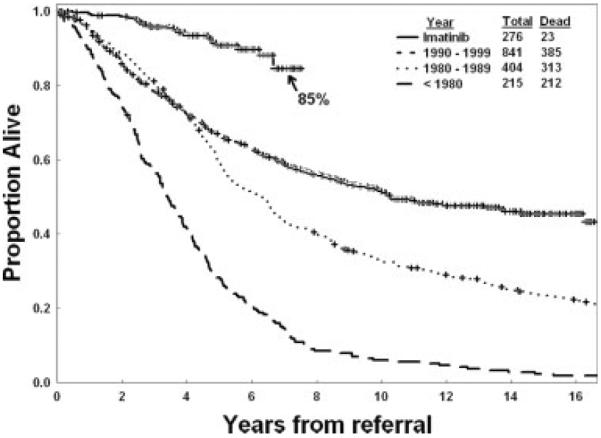
This chart illustrates the survival of patients with chronic myeloid leukemia (CML) before and after imatinib (based on M. D. Anderson Cancer Center data from 1736 patients with newly diagnosed CML who were referred from 1970 to the present). Excluding 11 patients who died from causes other than CML, the estimated 7-year survival rate is 89%.
The updated results of the IRIS trial (International Randomized Study of Interferon vs STI571) at 6 years continue to demonstrate the efficacy and safety of imatinib therapy in CML. The estimated 6-year survival rate was 88%, the progression-free survival rate (no progression to accelerated or blastic phase) was 93%, and the event-free survival rate was 83%.129 The cumulative incidence of cytogenetic CR (as the single best response) was 87%, and 71% of patients were in cytogenetic CR on imatinib therapy at 6 years. The annual rate of progression to accelerated or blastic phase was 2% to 3% in the first 2 years and decreased to ≤1% in Years 4 and 5. Among the patients who achieved a cytogenetic CR, the rate of transformation has decreased to <0.5%, suggesting the stability and predictability of the course of CML in patients who respond to imatinib.125,129 Treatment-related side effects are mild to moderate and are manageable. These include gastrointestinal disturbances, fluid retention (peripheral edema, periorbital edema), skin rashes, muscle cramps, and rare organ dysfunctions (renal, hepatic, cardiac).130
The standard dose of imatinib is 400 mg daily given orally. Dose escalation to from 600 to 800 mg daily may overcome relative resistance to imatinib and produce durable cytogenetic CRs, particularly in patients with cytogenetic resistance-recurrence, but not in patients with hematologic resistance-recurrence.131,132 The incidence of major molecular response (3-log reduction or greater in BCR-ABL transcripts; a BCR-ABL:ABL transcript ratio ≤0.05–0.1%) has increased to 70% as the data have matured, and the incidence of undetectable BCR-ABL transcripts (detection sensitivity, of 4.5 log) has increased to 25% to 40%.133,134 Some studies in newly diagnosed patients have demonstrated that high-dose imatinib (400 mg orally twice daily) is associated with higher and faster rates of cytogenetic CR and complete and major molecular responses.135–137 High-dose imatinib is more costly and is associated with a higher incidence of side effects and myelosuppression.
There is a poor correlation between imatinib plasma levels and body weight or body surface area.138 In other words, the flat dose of 400 mg daily of imatinib, on average, is effective in most patients regardless of their size or weight. However, there is a correlation between the average daily imatinib dose and the incidence of cytogenetic CR: Daily doses <300 mg were associated with significantly lower cytogenetic CR rates.139 There also is a correlation between higher 4-week trough plasma levels of imatinib and rates of cytogenetic CR, molecular response, and event-free survival.138 Among patients who develop `resistance' to imatinib, measuring imatinib plasma levels may identify patients with pseudoresistance (low levels) because of poor compliance, poor absorption, or other factors compared with patients who have true resistance (reasonable plasma levels) and who will benefit from changing therapy.
Patients who respond to imatinib should continue on treatment indefinitely. Interruption of therapy, even in patients in molecular CR for ≥2 years, was associated with molecular recurrence in 50% of patients.140 The development of chromosomal abnormalities in the Ph-negative cells in patients in cytogenetic response was noted in 5% to 8%. These may include chromosome 5 or 7 abnormalities, trisomy 8, 20q–, and other aberrations observed in MDS and AML. However, these abnormalities are transient in most patients and disappear with continued therapy. Rare cases of AML or MDS have been reported that evolved in the Ph-negative cells.141,142 This may indicate the instability of the stem cell, which led to the initial development of Ph-positive disease and which may transform again as part of the natural course of the disease once the Ph-positive clones have been suppressed.
Resistance to imatinib occurs at an annual rate of 3% to 4%. Approximately 50% of patients with CML resistance exhibit mutations in the BCR-ABL kinase domain. Mutations may occur in the ATP-binding site, in the P-loop, the activation site, catalytic sites, or other areas in the BCR-ABL structure. Such mutations may confer absolute or relative resistance to imatinib or may be clinically irrelevant.143–145 The causes of imatinib resistance in the other 50% of patients are being elucidated. A particular mutation involving amino acid 315 with a threonine→isoleucine mutation, T315I, is a `gatekeeper' mutation that is associated with absolute resistance to currently developed second-generation TKIs.
Patients who develop CML resistance or recurrence on imatinib have several treatment options. If they progress in chronic phase, then they have an excellent response to the new generation TKIs with an estimated cytogenetic CR rate of 50% to 60% and an estimated 1.5- to 2-year survival of 90%.146 In such patients, an initial trial of a second-generation TKI may be appropriate. The choice of referral for an allogeneic SCT may depend on the response to the TKI and the risk of allogeneic SCT (related vs unrelated donor, degree of matching, patient age). Patients who progress on imatinib to accelerated or blastic phase must consider allogeneic SCT immediately, regardless of the risk of the procedure. In such patients, a second-generation TKI may be offered to reduce disease burden in an attempt to improve the results of allogeneic SCT.146 Patients who undergo allogeneic SCT in chronic phase after imatinib exposure have a significantly better outcome than patients who have not been exposed to imatinib.147
Second-generation TKIs include more potent selective Bcr-Abl inhibitors, such as nilotinib, or dual Src-Abl kinase inhibitors like dasatinib, bosutinib, or INNO406. Dasatinib and nilotinib are approved in the United States and in several other countries for the treatment of CML after imatinib failure in all phases and in Ph-positive ALL (dasatinib) and for CML in chronic and accelerated phases (nilotinib).148–154 Bosutinib and INNO406 are investigational.155,156 In chronic phase CML, dasatinib and nilotinib are associated with major cytogenetic response rates of 50% to 60% (CR in 40%–50%) and with 1- to 2-year survival rates from 85% to 95%. Some mutations may be more responsive to 1 TKI or another. T315I mutations are resistant to all 4 TKIs. In a randomized study of oral dasatinib 70 mg twice daily versus high-dose oral imatinib 400 mg twice daily in patients with resistance to imatinib 400 to 600 mg daily, dasatinib was associated with higher rate of major and complete cytogenetic responses, molecular responses, and progression-free survival.157 Dasatinib was associated with severe myelosuppression in 50% of patients and with severe pleural effusions in 5% to 10% of patients.158 Randomized trials have demonstrated that dasatinib 100 mg as a single daily dose in chronic phase CML resulted in similar response rates and better progression-free survival compared with 70 mg orally twice daily and was associated with significantly lower rates of myelosuppression and pleural effusion.159 Promising investigational studies include selective T315I inhibitors, homoharringtonine, decitabine, farnesyl transferase inhibitors, and others.160–163
Biologic perspective
Animals models have established a causal association between BCR-ABL molecular events and the development of CML.164,165 This encouraged the development of the selective Bcr-Abl TKIs, and imatinib was the first of these to enter clinical trials and to be approved (and become established frontline therapy) for treating CML. The development of imatinib resistance and its common association with mutations involving the BCR-ABL kinase domain, led to the development of more potent (20- to 300-fold increased potency) selective Bcr-Abl and dual Src-Abl inhibitors.
Because >70% of patients now achieve a cytogenetic CR with imatinib, more precise and more convenient methods to monitor treatment response and resistance have been developed. These include fluorescence in situ hybridization (FISH) studies, quantitative polymerase chain reaction (QPCR) studies, and mutational analyses. The uses/misuses of these methods are discussed in other reviews.166,167 In simple terms: 1) FISH studies can substitute for bone marrow studies until Ph-positive levels are <5%, at which time a cytogenetic CR needs to be confirmed by standard cytogenetics; 2) in patients who are in stable cytogenetic CR, bone marrow studies for cytogenetics may be performed every 1 to 2 years, and QPCR studies should be obtained every 6 months; 3) variations of QPCR for patients who are in cytogenetic CR should lead to more cautious monitoring, eg, QPCR studies every 3 to 6 months, but not to a change in therapy167; and 4) mutational studies are useful only in patients with suspected resistance or recurrence on imatinib or other TKIs. This helps to identify patients who have T315I mutations that require specific approaches, including referral for allogeneic SCT, as well as selective mutations that may benefit from specific TKI therapies.
The future
The prognosis in CML is excellent. Most patients who receive treatment with TKIs will exhibit a `functional cure' and will live close to a normal life span with continued therapy, without the need for disease eradication. The development of safe, nontoxic, and more potent agents that encompass in their activity particular resistant mutations (eg, T315I, F317V/L) may improve outcomes further. Combinations of TKIs with downstream Bcr-Abl-mediated or Bcr-Abl-independent signal inhibitors (eg, farnesyl transferase inhibitors, Janus kinase 2 inhibitors) may eradicate dormant CML cells. Immunotherapy may also result in the eradication of minimal persistent disease and, thus, may lead to `molecular cures' that persist after the discontinuation of therapy.
Chronic Lymphocytic Leukemia
Therapeutic perspective
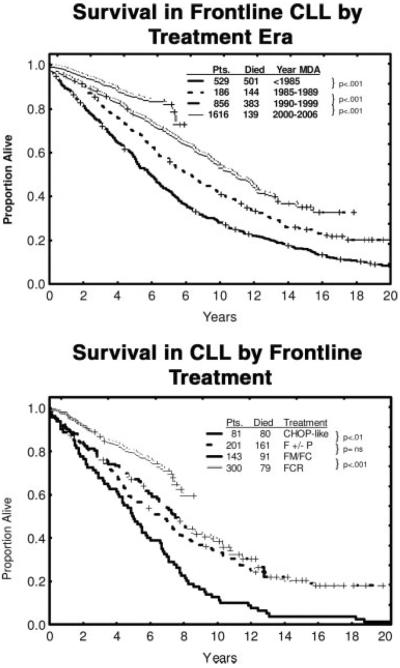
The discovery of the activity of fludarabine, an aden-osine nucleoside analog, in CLL has ushered in a new therapeutic era and a change of emphasis from palliation to the pursuit of durable CR as a step toward potential cure of CLL.168 Before fludarabine, patients were treated with chlorambucil, vincristine, steroids, cyclophosphamide, and anthracyclines. These strategies produced low CR rates of 10% to 20% and had minimal survival benefits. Several randomized trials have confirmed the superiority of fludarabine over alkylating agents as initial treatment for CLL.169–172 Combinations of fludarabine and cyclophosphamide were also superior to fludarabine alone.173–175 The CR rates with single-agent fludarabine in frontline CLL therapy were 15% to 30%. The combination of fludarabine and cyclophosphamide increased the CR rate to 30% to 40%. The introduction of rituximab, the chimeric anti-CD20 monoclonal antibody, to chemotherapy in CLL further improved the results. In preclinical models, rituximab sensitized chemotherapy-resistant cells to fludarabine.176 Fludarabine also down-regulated expression of CD55 and CD59, thus increasing the susceptibility of tumor cells to rituximab-mediated complement effect.177 Chemoimmunotherapy combinations of fludarabine and rituximab (FR) and fludarabine, cyclophosphamide, and rituximab (FCR) improved the results over those observed with fludarabine-based therapy (Fig. 5).178,179 The M. D. Anderson Cancer Center experience in over 300 patients who received frontline FCR therapy demonstrated an overall response rate of 95%, including a CR rate of 72%. Molecular CRs, determined by PCR-based ligase assay, were observed in 42% of patients in morphologic CR. The estimated 5-year survival and freedom-from-progression survival rates were 79% and 60%, respectively. Among patients with CR or partial response, the median time to progression was 80 months, and the estimated 5-year progression-free survival rate was 70%.
Figure 5.
These charts illustrate the survival of patients (Pts) with chronic lymphocytic leukemia (CLL) by treatment era (Top) and specific regimens (Bottom) from M. D. Anderson Cancer Center (MDA) data. CHOP indicates combined cyclophosphamide, doxorubicin, vincristine, and prednisone; F±P, fludarabine with or without prednisone; FMFC, fludarabine and mitoxan trone/fludarabine and cyclophosphamide; FCR, fludarabine, cyclophosphamide, and rituximab.
Among patients who progress on fludarabine-based therapy, treatment options include allogeneic SCT,180 alemtuzumab (a CD52 monoclonal antibody),181 lenalidomide (an immunomodulatory inhibitor derivative of thalidomide),182 and new monoclonal antibodies (humanized CD20 monoclonal antibody; CD23 monoclonal antibody [lumilixumab]). Allogeneic SCT in patients after failure on fludarabine is associated with 3- to 5-year survival rates of 50% to 70%.180 Alemtuzumab in fludarabine failure produced response rates of 30% to 40% and a median survival of 9 to 18 months.181 Similarly, lena-lidomide resulted in response rates of 30% to 50% in patients with recurrent CLL.182 Combinations of chemoimmunotherapy (FCR) with alemtuzumab and lenalidomide, in different schedules and sequences, are under investigation.
Biologic perspective
Several prognostic factors may help determine the outcome of patients with CLL. Older age is an adverse prognostic factor that may be related to a different disease biology or poor tolerance to current therapies. Elevated β2-microglobulin levels have been associated consistently with an adverse prognosis.183,184 Other adverse prognostic factors include CD38 expression, the absence of a mutation of the immunoglobulin-variable region, and overexpression of ζ-associated protein 70 (ZAP 70).185–188 Cytogenetic abnormalities involving chromosome 17 (deletion of the p53 locus) also have been associated with an adverse outcome in CLL.189,190
The future
Discovering more active anti-CLL agents, improving the safety and efficacy of allogeneic SCT, and gaining a better understanding of the disease pathophysiology and targetable signals may improve prognosis further in patients with CLL.
The particular subset of hairy cell leukemia
HCL is an uncommon lymphoproliferative disease that often occurs in older men who present with cytopenias and splenomegaly. The diagnosis is suspected by the morphology of the hairy cells and is confirmed by particular stains (tartrate-resistant acid phosphatase) and cell surface markers (CD11c, CD22, CD25, CD103). The median survival in HCL before the discovery of effective therapies was 5 years.191 Interferon-α therapy and the later discovery of the activities of pentostastin and CDA improved 5- to 10-year survival rates to 90%.191–194 Approximately 50% of the deaths are because of secondary cancers or are unrelated to HCL progression. HCL is a leukemia associated with excellent long-term outcome after 1 or 2 5-day courses of a relatively nontoxic chemotherapy, CDA. Commonly used regimens include CDA 4 mg/m2 as a continuous infusion daily for 7 days (standard), or CDA 5.6 mg/m2 intravenously over 2 hours daily for 5 days (common practice). The addition of rituximab to CDA may improve progression-free survival195
Myelodysplastic Syndrome
Treatment perspective
MDS includes heterogeneous hematopoietic disorders characterized by cytopenias, hypercellular bone marrows with dysplastic changes in 1 one or more of the hematopoietic series, a variable percentage of blasts, chromosomal abnormalities in 50% of patients, and a predilection for transformation to AML in 50% of patients.196,197 Several prognostic models have been designed to account for the disease heterogeneity.198–200 The International Prognostic Scoring System (IPSS) divides patients into low, intermediate 1, intermediate 2, and high-risk groups with corresponding median survivals of 5.6 years, 3.1 years, 1.2 years, and 0.4 years, respectively, based on the percentage of bone marrow blasts, the karyotype, and the number of cytopenias.200 In a simple practice, patients can be divided into higher risk MDS (blasts ≥10%) and lower risk MDS based on the percentage of bone marrow blasts. The percentage of patients with higher risk MDS ranges from 30% (newly diagnosed in community practice) to 60% (referral after treatment failure to tertiary cancer centers). Although MDS is considered `indolent' by many specialists, the median survival range in newly diagnosed MDS is 2 to 3 years. Survival is worse in patients with secondary MDS, patients who have received prior transfusions,201 and patients who are referred to tertiary centers after failing some form of therapy; in such patients, the median survival is 1 to 2 years.202
A decade ago, no treatment options were available for patients with MDS. Patients were observed or received supportive care or transfusions as indicated by their symptoms. Today, numerous treatment options are available, and 4 drugs have received approval from the US Food and Drug Administration in the past 2 years for the treatment of MDS and chronic myelomonocytic leukemia (CMML) or 1 of the subsets. The 2 hypomethylating agents azacitidine and decitabine are approved for the broad treatment of MDS and CMML.203–207 Lenalidomide is approved for the treatment of lower risk MDS with transfusion dependence and abnormalities involving deletions of chromosome 5 (5q–).208,209 Imatinib is approved for the treatment of MDS or CMML with translocations involving chromosome 5q33.
Patients with lower risk MDS usually are observed until cytopenias or symptoms require interventions. These could include growth factors (erythropoietins, granulocyte-colony-stimulating factor) and transfusions. Response rates to growth factors range from 40% to 60%, and responses generally are durable (median response duration, 2.5 years).210 Recent studies have suggested a survival prolongation with growth factors in patients with low-risk MDS and low transfusion requirement (<2 erythrocyte transfusions per month).211 Patients who progress on these measures with worsening cytopenias but with a low percentage of bone marrow blasts (eg, ≥5%) may benefit from immunotherapy in the form of antithymocyte globulin, steroids, or cyclosporine A. The response rate to immunotherapy ranges from 20% to 50% with a median response duration of 2 to 3 years.212
Cytogenetic studies allow the identification of patients with deletions of chromosome 5 who would benefit from lenalidomide therapy and patients with translocations involving chromosome 5q33 who respond well to imatinib therapy. Among patients with low-risk MDS and deletion of chromosome 5, lenalidomide has been associated with a transfusion-independence rate of 66%, a median response duration of 116 weeks, and a cytogenetic CR rate of 40% to 50%.208 Among patients with low-risk MDS and transfusion dependence but without the presence of chromosome 5 deletions, lenalidomide also resulted in a transfusion-independence rate of 26%, a median response duration of 41 weeks, and a cytogenetic CR rate of 8%.209
Recent studies have demonstrated an adverse effect of transfusion dependence and iron overload on prognosis, presumably as a result of organ damage (cardiac, pulmonary, hepatic, endocrine). Iron chelation therapy may benefit some patients with MDS and transfusion dependence, although the issue is controversial and requires further rigorous investigations.201,213–215 Patients with MDS should be monitored for the number of transfusions and iron overload (rising serum ferritin levels). Patients who have received ≥20 transfusions or who show rising serum ferritin levels (>1000 ng/mL) may be offered iron chelation therapy. Iron chelation has been associated with survival prolongation in some retrospective studies.214,215 Patients who progress on the above measures should be considered for treatment with hypomethylating agents or other strategies used in higher risk MDS.
Patients with higher risk MDS may be offered initial therapy with hypomethylating agents, particularly if they are older (ages ≥50 to 60 years). Azacitidine is usually given as 75 mg/m2 subcutaneously daily for 7 days every 4 weeks for at least 1 or 2 years or until progression.203 Alternative schedules include subcutaneous azacitidine 75 mg/m2 per day for 5 days every 4 weeks.216 Azacitidine is associated with CR rates of 7% to 20% and overall response rates of 50% to 70%. A recent randomized trial of azacitidine versus best standard of care demonstrated a survival advantage with azacitidine therapy.217 The median survival was 24.4 months with azacitidine versus 15 months with best standard of care, and the estimated 2-year survival rates were 51% and 26%, respectively (P<.0001) (Table 3). The addition of growth factors or antitumor necrosis factor (anti-TNF) agents (eg, etanercept) to azacitidine therapy may improve the response rate.218,219 Decitabine may be given as 15 mg/m2 over 4 hours every 8 hours for 3 days (135 mg/m2 per course) every 4 weeks or as 20 mg/m2 intravenously over 1 hour daily for 5 days every 4 weeks. It also can be given subcutaneously. The optimal schedules of hypomethylating agents are under evaluation. It is believed generally that courses given every 4 weeks are optimal for induction of the hypomethylating effect. Patients should be treated for at least 3 courses before considering response or failure. Treatment delays should not be implemented unless patients have cytopenias and hypoplastic bone marrow without evidence of MDS or if they have serious, unresolved complications (pneumonia, organ failure). Decitabine has demonstrated a survival benefit compared with historical experience in intensive chemotherapy using either matched controls or multivariate analysis. In a poor prognosis population (cytopenias in 96%, cytogenetic abnormalities in 62%, prior therapy in 56%, bone marrow blasts >10% in 47%), decitabine was associated with a median survival of 22 months versus 12 months with intensive chemotherapy.206,207
TABLE 3.
Azacitidine Versus Conventional Care in Patients with Myelodysplastic Syndrome (n=358)*
| Response, % |
|||||
|---|---|---|---|---|---|
| Therapy | No. Treated | CR | PR | Overall | Median Survival, mo |
| Azacitidine | 179 | 18 | 12 | 29 | 24.4 |
| Conventional care | 179 | 8 | 4 | 12 | 15.5 |
| Best support | 1 | 4 | 5 | 11.5 | |
| Low-dose cytarabine | 8 | 4 | 12 | 15.3 | |
| Chemotherapy (3+7) | 36 | 4 | 40 | 15.7 | |
CR indicates complete response; PR, partial response; 3+7, intravenous daunorubicin daily X3 plus continuous-infusion cytarabine daily X7.
See Fenaux 2007.217
Intensive chemotherapy is usually offered either after failure on hypomethylating agents or in younger patients with diploid karyotype who are treated with curative intent.220 Allogeneic SCT is curative in 30% to 50% of patients with MDS.221,222 The best results are achieved with transplantation in patients who have bone marrow blasts ≥5%. Therefore, achieving MRD in patients before SCT by using either hypomethylating agents or chemotherapy may improve the results of SCT. An analysis of the timing of transplantation (at diagnosis, 2 years into MDS, at the time of transformation) in lower risk MDS versus higher risk MDS indicated that the best timing for SCT is later in patients with lower risk MDS and earlier in patients with higher risk MDS.222 A sequence of treatment strategies in MDS is summarized in Table 4.
TABLE 4.
Strategies in Myelodysplastic Syndrome
| Lower Risk (Blasts<10%) | Higher Risk (Blasts ≥10%; cytogenetics with ≥ 3 abnormalities or with chromosome 7 abnormality) |
|---|---|
| Growth factors: Erythropoietin, G-CSF | Decitabine, 5-azacitidine |
| Immune therapy: Steroids, cyclosporin, antithymocyte globulin | Investigational |
| Lenalidomide: 5q31 | Intensive chemotherapy (younger, karyotype diploid) |
| Decitabine, 5-azacitidine | Allogeneic stem cell transplantation |
| Investigational: clofarabine, homoharringtonine | |
| Iron chelation | Iron chelation |
| Translocation (5; 12) or 5q33 variant (PDGFR-B): Imatinib | Translocation (5; 12) or 5q33 variant (PDGFR-B): Imatinib |
G-CSF indicates granulocyte–colony-stimulating factor; PDGFR-B, platelet-derived growth factor receptor B.
Biologic perspective
The cytogenetic changes in MDS are important for determining prognosis and therapy. Patients with aberrations that involve chromosome 7 or ≥3 abnormalities have a very poor prognosis (as poor as the presence of ≥20% blasts) that is not accounted for readily by the IPSS.223 The presence of chromosome 5 deletion in low-risk MDS favors lenalidomide therapy, whereas a translocation that involves 5q33 (the site of the platelet-derived growth factor receptor β) favors imatinib therapy. In early MDS, a subset of patients may have clonal T cells that suppress hematopoiesis. In such patients, immunotherapy may be beneficial. Increased global and site-specific methylation is adverse in MDS and increases with disease progression, which may be one reason for the efficacy of epigenetic therapy, including hypomethylating agents and histone deacetylase inhibitors. Recently, the ribosomal protein S14 gene RPS14 was suggested as the gene involved in the 5q syndrome.224 This finding may produce targeted therapies relevant to this MDS subset. High TNF-α levels in early MDS suggests a possible role for TNF-α inhibitors (eg, etanacerpt) in combinations.219
The future
The gradual unraveling of the heterogeneous pathophysiology of MDS may result in new beneficial treatments. Combinations of epigenetic agents (hypomethylating agents and histone deacetylase inhibitors) may improve results over those obtained with single agents. New anti-MDS agents with promise include clofarabine,225 homoharringtonine, sapacitabine (oral cytosine analogue), arsenic trioxide,226 CD33 monoclonal antibodies, thrombopoiesis-stimulating agents,227 and others.
Summary
For leukemia and MDS, as the poet Bob Dylan declared, `the times they are a-changin'. HCL is potentially curable with 1 or 2 courses of CDA, a relatively nontoxic chemotherapy. APL is highly curable with ATRA plus anthracycline regimens and is possibly curable without chemotherapy (ATRA plus arsenic trioxide) or with minimal chemotherapy (ATRA, arsenic trioxide, and gemtuzumab ozogamycin). In CML, oral nontoxic therapy with imatinib or other TKIs produces `functional cures' and results in an estimated 6-year survival rate of 90%. In CBF acute leukemias, high-dose cytarabine regimens are associated with cure rates of 50% to 70%. The 5- to 10-year survival rates continue to improve in ALL, AML, and CLL. In MDS, new treatments are showing significant promise. Allogeneic SCT is an established curative modality in many of these disorders and may have broader applications as the safety and efficacy of such procedures improve.
REFERENCES
- 1.Freireich EJ, Bodey G, Harris J, Hart J. First continuous infusion (12) hour Ara-C 14 patients, 6 CRs and 2 PRs. Therapy for acute granulocytic leukemia, Cancer Res. 1967;27:2573–2577. [PubMed] [Google Scholar]
- 2.Boiron M, Weil M, Levey D, et al. Daunorubicin in the treatment of acute myeloblastic leukemia. Lancet. 1969;1:330–333. doi: 10.1016/s0140-6736(69)91296-3. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, Burnett AK, Agura ED, Kantarjian H. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110:1900–1910. doi: 10.1002/cncr.23000. [DOI] [PubMed] [Google Scholar]
- 4.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive post remission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 5.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996;88:2841–2851. [PubMed] [Google Scholar]
- 6.Buchner T, Hiddemann W, Wormann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93:4116–4124. [PubMed] [Google Scholar]
- 7.Bishop JF, Matthews JP, Young GA, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- 8.Bloomfield CD, Lawrence D, Byrd J, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- 9.Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: review of 3 randomized trials. Cancer. 2006;107:116–124. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- 10.Berman E, Heller G, Santorsa J, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–1674. [PubMed] [Google Scholar]
- 11.Wiernik PH, Banks PL, Case D, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–319. [PubMed] [Google Scholar]
- 12.Vignetti M, De Witte TM, Suciu S, et al. Daunorubicin (DNR) vs mitoxantrone (MTZ) vs idarubicin (IDA) administered during induction and consolidation in acute myelogenous leukemia (AML) followed by autologous or allogeneic stem transplantation (SCT): results of the EORTC-GIMEMA [ASH Annual Meeting Abstracts] Blood. 2003;102 Abstract 175. [Google Scholar]
- 13.Pautas C, Thomas X, Merabet F, et al. Randomized comparison of standard induction with daunorubicin (DNR) for 3 days vs idarubicin (IDA) for 3 or 4 days in AML pts aged 50 to 70 and of maintenance with interleukin 2. Final analysis of the Alfa 9801 Study [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 162. [Google Scholar]
- 14.Estey EH, Thall PF, Pierce S, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin ± all-trans retinoic acid ± granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–2484. [PubMed] [Google Scholar]
- 15.Juliusson G, Hoglund M, Karlsson K, et al. Increased remissions from 1 course for intermediate-dose cytosine arabinoside and idarubicin in elderly acute myeloid leukaemia when combined with cladribine. A randomized population-based phase II study. Br J Haematol. 2003;123:810–818. doi: 10.1046/j.1365-2141.2003.04702.x. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JF, Lowenthal RM, Joshua D, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group. Blood. 1990;75:27–32. [PubMed] [Google Scholar]
- 17.Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer. 2006;106:1099–1109. doi: 10.1002/cncr.21699. [DOI] [PubMed] [Google Scholar]
- 18.Tallman M, Nabhan C, Feusner J, Rowe J. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 19.Tallman M, Anderson J, Schiffer C, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100:4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 20.Mathews V, George B, Lakshmi K, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 21.Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARα-positive acute promyelocytic leukemia. Blood. 1999;94:3015–3021. [PubMed] [Google Scholar]
- 22.Powell BL, Moser B, Stock W, et al. Effect of consolidation with arsenic trioxide (As2O3) on event-free survival (EFS) and overall survival (OS) among patients with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Protocol C9710. 2007 ASCO Annual Meeting Proceedings [abstract] J Clin Oncol. 2007;25(18S) Abstract 2. [Google Scholar]
- 23.Lo-Coco F, Cimino G, Breccia M, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104:1995–1999. doi: 10.1182/blood-2004-04-1550. [DOI] [PubMed] [Google Scholar]
- 24.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 25.Marcucci G, Mrozek K, Ruppert A, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–5717. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 26.Byrd J, Ruppert A, Mrozek K, et al. Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv (16) (p13q22) or t (16;16) (p13;q22): results from CALGB 8461. J Clin Oncol. 2004;22:1087–1094. doi: 10.1200/JCO.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Grimwade D, Walker G, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 29.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 30.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162:1597–603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 31.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 32.Faderl S, Ravandi F, Ferrajoli A, et al. Clofarabine and clofarabine plus low dose cytarabine (ara-C) as induction acute myeloid leukemia (AML) [ASH Annual Meeting Abstracts] Blood. 2005;106 Abstract 786. [Google Scholar]
- 33.Giles F, Verstovsek S, Faderl S, et al. A phase II study of cloretazine (VNP40101M), a novel sulfonylhydrazine alkylating agent, in patients with very high risk relapsed acute myeloid leukemia. Leuk Res. 2006;30:1591–1595. doi: 10.1016/j.leukres.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Lubbert M, Rüter B, Claus R, et al. Continued low-dose decitabine (DAC) is an active first-line treatment in all cytogenetic subgroups of older AML patients: results of the FR00331 multicenter phase II study [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 300. [Google Scholar]
- 35.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 36.Kantarjian H. Therapy for elderly patients with acute myeloid leukemia: a problem in search of solutions. Cancer. 2007;109:1007–1010. doi: 10.1002/cncr.22502. [DOI] [PubMed] [Google Scholar]
- 37.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 38.Byrd J, Mrozek K, Dodge R, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 39.Slovak M, Kopecky K, Cassileth P, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 40.Gilliland D, Griffin J. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 41.Kottaridis P, Gale R, Frew M, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 42.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 43.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 44.Levis M, Smith BD, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: clinical response correlates with successful FLT3 inhibition [abstract] Blood. 2005;106:121a. [Google Scholar]
- 45.Stone RM, Fischer T, Paquette R, et al. Phase IB study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed patients with AML [abstract] Blood. 2006;108:50a. [Google Scholar]
- 46.Dohner K, Schlenk R, Habdank M, et al. AML Study Group (AMLSG) Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 47.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 48.Dohner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–3261. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 49.Preudhomme C, Sagot C, Boissel N, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 50.Frohling S, Schlenk RF, Stolze I, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–633. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 51.Baldus CD, Thiede C, Soucek S, Bloomfield CD, Thiel E, Ehninger G. BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol. 2006;24:790–797. doi: 10.1200/JCO.2005.01.6253. [DOI] [PubMed] [Google Scholar]
- 52.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 53.Valk PJ, Verhaak RG, Beijen MA, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 54.Burnett AK, Kell WJ, Goldstone AH, et al. The addition of gemtuzumab ozogamicin to induction chemotherapy for AML improves disease free survival without extra toxicity: preliminary analysis of 1115 patients in the MRC AML15 trial [abstract] Blood. 2006;108:8a. [Google Scholar]
- 55.Grovdal M, Khan R, Aggerholm A, et al. Negative effect of DNA hypermethylation on the outcome of intensive chemotherapy in older patients with high-risk myelodys-plastic syndromes and acute myeloid leukemia following myelodysplastic syndrome. Clin Cancer Res. 2007;13:7107–7112. doi: 10.1158/1078-0432.CCR-07-1193. [DOI] [PubMed] [Google Scholar]
- 56.Ravandi F, Kantarjian H, Giles F, Cortes J. New agents in acute myeloid leukemia and other myeloid disorders. Cancer. 2004;100:441–454. doi: 10.1002/cncr.11935. [DOI] [PubMed] [Google Scholar]
- 57.Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 58.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 59.Archimbaud E, Thomas X, Michallet M, et al. Prospective genetically randomized comparison between intensive postinduction chemotherapy and bone marrow transplantation in adults with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2994;12:262–267. doi: 10.1200/JCO.1994.12.2.262. [DOI] [PubMed] [Google Scholar]
- 60.Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–3059. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 61.Pui CH, Evans W. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 62.Pui C-H, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 63.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 64.Faderl S, Jeha S, Kantarjian H. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98:1337–1354. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 65.Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1327–1352. doi: 10.1016/s0889-8588(05)70189-1. [DOI] [PubMed] [Google Scholar]
- 66.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and auto-logous and allogeneic transplantation: a follow-up report of the French Protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1365. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 67.Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter study group for adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 68.Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. doi: 10.1016/s0889-8588(05)70191-x. [DOI] [PubMed] [Google Scholar]
- 69.Linker CA, Levitt LJ, O'Donnell M, et al. Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report. Blood. 1991;78:2814–2822. [PubMed] [Google Scholar]
- 70.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 71.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL 97 randomized trial. Br J Haematol. 2005;129:734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 73.Hallbook H, Simonsson B, Ahlgren T, et al. High-dose cytarabine in upfront therapy for adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2002;118:748–754. doi: 10.1046/j.1365-2141.2002.03685.x. [DOI] [PubMed] [Google Scholar]
- 74.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–0. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 75.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 76.Pession A, Valsecchi MG, Masera G, et al. Long-term results of a randomized trial on extended use of high dose of L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:7161–7167. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 77.Schrappe M, Reiter A, Ludwig WD, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 78.Toyoda Y, Manabe A, Tsuchida M, et al. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. doi: 10.1200/JCO.2000.18.7.1508. [DOI] [PubMed] [Google Scholar]
- 79.Pui C-H. Toward optimal central nervous system-directed treatment in childhood acute lymphoblastic leukemia. J Clin Oncol. 2003;21:179–181. doi: 10.1200/JCO.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 80.Cortes J, O'Brien SM, Pierce S, et al. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86:2091–2097. [PubMed] [Google Scholar]
- 81.Mancini M, Scappaticci D, Cimino G, et al. A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood. 2005;105:3434–34415. doi: 10.1182/blood-2004-07-2922. [DOI] [PubMed] [Google Scholar]
- 82.Armstrong SA, Look AT. Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol. 2005;23:6306–6315. doi: 10.1200/JCO.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 83.Gleissner B, Gokbuget N, Bartram CR, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99:1536–1543. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 84.Stock W, Sather H, Dodge RK, et al. Outcome of adolescents and young adults with ALL: a comparison of Children's Cancer Group (CCG) and Cancer and Leukemia Group B (CALGB) regimens [abstract] Blood. 2000;96:467a. [Google Scholar]
- 85.Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 86.de Bont JM, Holt B, Dekker AW, et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 87.Hallook H, Gustafsson G, Smedmyr B, Soderhall S, Heyman M, Swedish Adult Lymphocytic Leukemia Group. Swedish Childhood Leukemia Group Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107:1551–1561. doi: 10.1002/cncr.22189. [DOI] [PubMed] [Google Scholar]
- 88.DeAngelo D, Dahlberg S, Silverman L, et al. A multicenter phase II study using a dose intensified pediatric regimen in adults with untreated acute lymphoblastic leukemia [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 587. [Google Scholar]
- 89.Haiat S, Vekhoff A, Marzac C, et al. Improved outcome of adult acute lymphoblastic leukemia treated with a pediatric protocol: results of a pilot study [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 2822. [Google Scholar]
- 90.Huguet F, Raffoux E, Thomas X, et al. Towards a pediatric approach in adults with acute lymphoblastic leukemia (ALL): the GRAALL-2003 Study [ASH Annual Meeting Abstracts] Blood. 2006;108 Abstract 147. [Google Scholar]
- 91.Zhang M, Hoelzer D, Horowitz M, et al. Long-term follow-up of adults with acute lymphoblastic leukemia in first remission treated with chemotherapy or bone marrow transplantation. Ann Intern Med. 1995;123:428–431. doi: 10.7326/0003-4819-123-6-199509150-00006. [DOI] [PubMed] [Google Scholar]
- 92.Attal M, Blaise D, Marit G, et al. Consolidation treatment of adult acute lymphoblastic leukemia: a prospective, randomized trial comparing allogeneic versus autologous bone marrow transplantation and testing the impact of recombinant interleukin-2 after autologous bone marrow transplantation. BGMT Group. Blood. 1995;86:1619–1628. [PubMed] [Google Scholar]
- 93.Goldstone AH, Richards SM, Lazarus HM, et al. adults with standard-risk acute lyphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogenic transplantation in first complete remission, and an auto-logous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 94.Patte C, Auperin A, Michon J, et al. The Societe Francaise d'Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 95.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 96.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 97.de Labarthe A, Rousselot P, Huguet-Rigal F, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2006;109:1408–1413. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 98.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24:460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 99.Fielding A, Richards S, Lazarus H, et al. Does imatinib change the outcome in Philadelphia chromosome positive acute lymphoblastic leukaemia in adults? Data from the UK ALL XII/ECOG2993 study [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 8. [Google Scholar]
- 100.Thomas D, O'Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 101.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 102.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 103.Wetzler M, Dodge RK, Mrozek K, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the Cancer and Leukemia Group B experience. Blood. 1999;93:3983–3993. [PubMed] [Google Scholar]
- 104.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UK ALL XII/Eastern Cooperative Oncology Group (ECOG) 2993 Trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 105.Holleman A, Cheok MH, den Boer ML, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 106.Lugthart S, Cheok MH, den Boer ML, et al. Identification of genes associated with chemotherapy cross resistance and treatment response in childhood acute lymphoblastic leukemia. Cancer Cell. 2005;7:375–386. doi: 10.1016/j.ccr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Yeoh EJ, Ross ME, Shurtleff SA, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 108.Thomas DA, Cortes J, O' Brien S, et al. Update of the modified hyper-CVAD regimen with or without rituximab in newly diagnosed adult acute lymphocytic leukemia (ALL) [abstract] Blood. 2005;106:521a. Abstract 1831. [Google Scholar]
- 109.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 110.Faderl S, Gandhi V, Keating MJ, Jeha S. Plunkett W, Kantarjian H. The role of clofarabine in hematologic and solid malignancies—development of a next-generation nucleoside analog. Cancer. 2005;103:1985–1995. doi: 10.1002/cncr.21005. [DOI] [PubMed] [Google Scholar]
- 111.Thomas DA, Sarris AH, Cortes J, et al. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer. 2006;106:120–127. doi: 10.1002/cncr.21595. [DOI] [PubMed] [Google Scholar]
- 112.Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 113.Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegasparginase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109:2744–2750. doi: 10.1182/blood-2006-07-035006. [DOI] [PubMed] [Google Scholar]
- 114.Jabbour E, O'Brien S, Kantarjian H, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109:3214–3218. doi: 10.1182/blood-2006-08-043646. [DOI] [PubMed] [Google Scholar]
- 115.Tibes R, Keating MJ, Ferrajoli A, et al. Activity of alemtuzumab in patients with CD52-positive acute leukemia. Cancer. 2006;106:2645–2651. doi: 10.1002/cncr.21901. [DOI] [PubMed] [Google Scholar]
- 116.Strauss SJ, Morschhauser F, Rech J, et al. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:3880–3886. doi: 10.1200/JCO.2006.05.6291. [DOI] [PubMed] [Google Scholar]
- 117.Jabbour E, Cortes J, Giles F, O'Brien S, Kantarjian H. Current and emerging treatment options in chronic myeloid leukemia. Cancer. 2007;109:2171–2181. doi: 10.1002/cncr.22661. [DOI] [PubMed] [Google Scholar]
- 118.Goldman J, Melo J. Chronic myeloid leukemia—advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 119.Kantarjian H, Smith TL, O'Brien S, Beran M, Pierce S, Talpaz M, Leukemia Service Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-α. Ann Intern Med. 1995;122:254–261. doi: 10.7326/0003-4819-122-4-199502150-00003. [DOI] [PubMed] [Google Scholar]
- 120.Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid Leukemia Trialists' Collaborative Group. J Natl Cancer Inst. 1997;89:1616–1620. [No authors listed] [PubMed] [Google Scholar]
- 121.Kantarjian H, O'Brien S, Cortes J, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 122.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 123.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia diagnosis and treatment. Mayo Clin Proc. 2006;81:973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 124.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 125.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;12:908–916. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 126.Kantarjian H, Talpaz M, O'Brien S, et al. Imatinib mesylate for Philadelphia-chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-α: follow-up results. Clin Can Res. 2002;8:2177–2187. [PubMed] [Google Scholar]
- 127.Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-α-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 128.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-α plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006;108:1478–1484. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 129.Hochhaus A, Druker BJ, Larson R, O'Brien SG, Gathmann I, Guilhot F. IRIS 6-year follow-up: sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 25. [Google Scholar]
- 130.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 131.Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 132.Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- 133.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 134.Branford S, Seymour J, Grigg A, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–7085. doi: 10.1158/1078-0432.CCR-07-0844. [DOI] [PubMed] [Google Scholar]
- 135.Kantarjian H, Talpaz M, O'Brien S, et al. High dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 136.Hughes TP, Brandford S, Matthews J, et al. Trial of higher dose imatinib with selective intensification in newly diagnosed CML patients in chronic phase [abstract] Blood. 2003;102:31a. [Google Scholar]
- 137.Aoki E, Kantarjian H, O' Brien S, et al. High-dose imatinib mesylate treatment in patients (pts) with untreated early chronic phase (CP) chronic myeloid leukemia (CML): 2.5-year follow-up [abstract] Proc Am Soc Clin Oncol. 2006;24 Abstract 6535. [Google Scholar]
- 138.Larson R, Druker B, Guilhot F, et al. on behalf of the IRIS Study Group Correlation of pharmacokinetic data with cytogenetic and molecular response in newly diagnosed patients with chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib; an analysis of IRIS study data [ASH Annual Meeting Abstracts] Blood. 2006;108 Abstract 429. [Google Scholar]
- 139.Ohnishi K, Nishimura M, Takeuchi J, et al. on behalf of JALSG. Lower dose of imatinib provides outcomes similar to the standard dose imatinib in Japanese patients with early chronic-phase CML: the interim analyses of JALSG CML202 study [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 1044. [Google Scholar]
- 140.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 1007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 141.Jabbour E, Kantarjian H, Abruzzo L, et al. Chromosomal abnormalities in Philadelphia chromosome-negative metaphases appearing during imatinib mesylate therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2007;110:2991–2995. doi: 10.1182/blood-2007-01-070045. [DOI] [PubMed] [Google Scholar]
- 142.Deininger M, Cortes J, Paquette R, et al. The prognosis for patients with chronic myeloid leukemia who have clonal cytogenetic abnormalities in Philadelphia chromosome-negative cells. Cancer. 2007;110:1509–1518. doi: 10.1002/cncr.22936. [DOI] [PubMed] [Google Scholar]
- 143.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 144.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 145.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 146.Kantarjian H, O'Brien S, Talpaz M, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer. 2007;109:1556–1560. doi: 10.1002/cncr.22569. [DOI] [PubMed] [Google Scholar]
- 147.Lee S, Maziarz R, Kukreja M, et al. Impact of prior therapy with imatinib mesylate on the outcome of hematopoietic cell transplantation (HCT) for chronic myeloid leukemia (CML) [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 738. [Google Scholar]
- 148.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 149.Hochhaus A, Kantarjian H, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 150.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–50. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 151.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 152.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 153.Kantarjian H, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 154.le Coutre P, Ottmann OG, Giles F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 155.Cortes J, Bruemmendorf T, Kantarjian H, et al. Efficacy and safety of bosutinib (SKI-606) among patients with chronic phase Ph+ chronic myelogenous leukemia (CML) [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 733. [Google Scholar]
- 156.Kantarjian H, Cortes J, Coutre P, et al. A phase I study of INNO-406 in patients with advanced Philadelphia (Ph+) chromosome-positive leukemias who are resistant or intolerant to imatinib and second generation tyrosine kinase inhibitors [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 469. [Google Scholar]
- 157.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase II trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 158.Quintas-Cardama A, Kantarjian H, O'Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 159.Shah NP, Kantarjian HM, Kim D-W, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 160.Giles F, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman S. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 161.Cortes J, Albitar M, Thomas D, et al. Efficacy of the farnesyl transferase inhibitor R115777 in chronic myeloid leukemia and other hematologic malignancies. Blood. 2003;101:1692–1697. doi: 10.1182/blood-2002-07-1973. [DOI] [PubMed] [Google Scholar]
- 162.Issa JP, Gharibyan V, Cortes J, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 163.Marin D, Kaeda JS, Andreasson C, et al. Phase I/II trial of adding semisynthetic homoharringtonine in chronic myeloid leukemia patients who have achieved partial or complete cytogenetic response on imatinib. Cancer. 2005;103:1850–1855. doi: 10.1002/cncr.20975. [DOI] [PubMed] [Google Scholar]
- 164.Daley GQ, Van Etten RA, Baltimore D, et al. Induction of chronic myelogenous leukemia in mice by the P210BCR/ABL gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 165.Kelliher MA, McLaughlin J, Witte ON, Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with V-ABL and BCR/ABL. Proc Natl Acad Sci USA. 1990;87:6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kantarjian H, Schiffer C, Jones D, Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 168.Keating MJ, O'Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 169.Leporrier M, Chevret S, Cazin B, et al. French Cooperative Group on Chronic Lymphocytic Leukemia. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–2325. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- 170.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 171.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomized controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 172.Johnson S, Smith AG, Loffler H, et al. Multicentre prospective randomized trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996;347:1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- 173.O'Brien S, Kantarjian H, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:1414–1420. doi: 10.1200/JCO.2001.19.5.1414. [DOI] [PubMed] [Google Scholar]
- 174.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 175.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 176.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of BCL-2 and sensitization of B-cell non-Hodgkin's lymphoma to apoptosis. Clin Cancer Res. 2001;7:709–723. [PubMed] [Google Scholar]
- 177.Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 178.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 179.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 180.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 181.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 182.Chanan-Khan A, Miller KC, Musial L, Lawrence D, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 183.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 184.Wierda WG, O'Brien S, Wang X, et al. Prognostic nomo-gram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 185.Damie RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD 38 expression as novel progmostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 186.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 187.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 188.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 189.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 190.Dohner H, Fischer K, Bentz M, et al. p53 Gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85:1580–1589. [PubMed] [Google Scholar]
- 191.Saven A, Piro LD. Treatment of hairy cell leukemia. Blood. 1992;79:1111–1120. [PubMed] [Google Scholar]
- 192.Flinn IW, Kopecky KJ, Foucar MK, et al. Long-term follow-up of remission duration, mortality, and second malignancies in hairy cell leukemia patients treated with pentostastin. Blood. 2000;96:2981–2986. [PubMed] [Google Scholar]
- 193.Goodman GR, Burian C, Koziol JA, Saven A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21:891–896. doi: 10.1200/JCO.2003.05.093. [DOI] [PubMed] [Google Scholar]
- 194.Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol. 1995;13:974–982. doi: 10.1200/JCO.1995.13.4.974. [DOI] [PubMed] [Google Scholar]
- 195.Ravandi F, Jorgensen JL, O'Brien SM, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006;107:4658–4662. doi: 10.1182/blood-2005-11-4590. [DOI] [PubMed] [Google Scholar]
- 196.Faderl S, Kantarjian H. Myelodysplastic syndromes. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 7th ed Lippincott Williams & Wilkins; Philadelphia, Pa: 2005. pp. 2144–2154. [Google Scholar]
- 197.Faderl S, Kantarjian H. Novel therapies for myelodysplastic syndromes. Cancer. 2004;101:226–241. doi: 10.1002/cncr.20381. [DOI] [PubMed] [Google Scholar]
- 198.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Heamatol. 1982;51:189–199. [PubMed] [Google Scholar]
- 199.Harris NJ, Jaffe ES, Diebold J, et al. World Health Organization lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1977. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 200.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 201.Malcovati L, Della Porta M, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision-making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 202.Estey E, Keating M, Pierce S, et al. Application of the international scoring system for myelodysplasia to M. D. Anderson patients. Blood. 1997;90:2843–2844. [PubMed] [Google Scholar]
- 203.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 204.Kantarjian H, Issa J-P, Rosenfeld CS, et al. Decitabine improves patient outcome in myelodysplastic syndrome. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 205.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 206.Kantarjian H, O'Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome. Cancer. 2007;109:1133–1137. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kantarjian H, O'Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 208.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 209.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1-risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 210.Jadersten M, Montgomery SM, Dybedal I, et al. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106:803–811. doi: 10.1182/blood-2004-10-3872. [DOI] [PubMed] [Google Scholar]
- 211.Jaädersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26:3607–3613. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 212.Lim Z, Killick S, Germing U, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21:1436–1441. doi: 10.1038/sj.leu.2404747. [DOI] [PubMed] [Google Scholar]
- 213.Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective survey of Japanese patients with transfusion-dependent myelodysplastic syndromes and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality [abstract] Blood. 2006;108:781a. doi: 10.1111/j.1600-0609.2007.00842.x. Abstract 2656. [DOI] [PubMed] [Google Scholar]
- 214.Leitch HA, Goodman TA, Wong KK, et al. Improved survival in patients with myelodysplastic syndrome (MDS) receiving iron chelation therapy [abstract] Blood. 2006;108:78a. Abstract 249. [Google Scholar]
- 215.Rose C, Brechignac S, Vassilief D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly trans-fused MDS patients. a prospective analysis by the GFM [abstract] Blood. 2007;110:78a. Abstract 249. [Google Scholar]
- 216.Lyons R, Cosgriff T, Modi S, et al. Results of the initial treatment phase of a study of three alternative dosing schedules of azacitidine (Vidaza®) in patients with myelodysplastic syndromes (MDS) [abstract] Blood. 2007;110:246a. Abstract 819. [Google Scholar]
- 217.Fenaux P, Mufti G, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional cure regimens (CCR); results of the AZA-001 phase III study [abstract] Blood. 2007;110:245a. Abstract 817. [Google Scholar]
- 218.Rossetti J, Falke E, Shadduck R, et al. G-CSF increases hematological response among patients with myelodysplasia treated with azacitidine [abstract] Blood. 2006;108:292b. Abstract 4868. [Google Scholar]
- 219.Holsinger A, Ramakrishnan A, Storer B, et al. Therapy of myelodysplastic syndrome (MDS) with azacitidine given in combination with etanercept: a phase II study [abstract] Blood. 2007;110:420a. Abstract 1452. [Google Scholar]
- 220.Beran M, Shen Y, Kantarjian H, et al. Chemotherapy in high-risk myelodysplastic syndrome—Covariate-adjusted comparison of five regimens. Cancer. 2001;92:1999–2015. doi: 10.1002/1097-0142(20011015)92:8<1999::aid-cncr1538>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 221.Sierra J, Perez WS, Rozman C, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100:1997–2004. [PubMed] [Google Scholar]
- 222.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 223.Haase D, Estey E, Steidl C, et al. Multivariate evaluation of the prognostic and therapeutic relevance of cytogenetics in a merged European-American cohort of 3860 patients with MDS [abstract] Blood. 2007;110:77a. Abstract 247. [Google Scholar]
- 224.Ebert BL, Pretz J, Bosco J, et al. Identification of RPS14 as a 5q2 syndrome gene by RNA interference screen. Nature. 2008;451:335. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Faderl S, Garcia-Manero G, Gandhi V, et al. Results of an exploratory study of oral (po) and intravenous (iv) clofarabine in patients with myelodysplastic syndrome [abstract] Blood. 2007;110:421a. Abstract 1455. [Google Scholar]
- 226.Vey N, Bosly A, Guerci A, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006;24:2465–2472. doi: 10.1200/JCO.2005.03.9503. [DOI] [PubMed] [Google Scholar]
- 227.Kantarjian H, Fenaux P, Sekeres MA, et al. Phase 1/2 study of AMG 531 in thrombocytopenic patients (pts) with low-risk myelodysplastic syndrome (MDS): update including extended treatment [ASH Annual Meeting Abstracts] Blood. 2007;110 Abstract 250. [Google Scholar]