Abstract
Pseudomonas aeruginosa is the primary bacterial pathogen causing contact lens related keratitis. Available ophthalmic agents have reduced efficacy and antimicrobial peptides (AMPs) hold promise as future antibiotics. Here we investigated the in vitro and in vivo anti-Pseudomonal activity of esculentin-1a(1-21)NH2, derived from a frog skin AMP. The data revealed a minimum inhibitory concentration between 2 and 16 μM against reference strains or drug-resistant clinical isolates of P. aeruginosa without showing toxicity to human corneal epithelial cells up to 50 μM. At 1 μM the peptide rapidly killed bacterial cells and this activity was fully retained in 150 mM sodium chloride and 70 % (v/v) human basal tears, particularly against the virulent ATCC 19660 strain. Furthermore, its dropwise administration at 40 μM to the ocular surface in a murine model of P. aeruginosa keratitis (three times daily, for 5 days post-infection) resulted in a significant reduction of infection. The mean clinical score was 2.89 ± 0.26 compared to 3.92 ± 0.08 for the vehicle control. In addition, the corneal level of viable bacteria in the peptide treated animals was significantly lower with a difference of 4 log10 colony counts, compared to 7.7 log10 cells recovered in the control. In parallel, recruitment of inflammatory cells was reduced by half compared to that found in the untreated eyes. Similar results were obtained when esculentin-1a(1-21)NH2 was applied prior to induction of keratitis. Overall, our findings highlight esculentin-1a(1-21)NH2 as an attractive candidate for the development of novel topical pharmaceuticals against Pseudomonas keratitis.
Keywords: Innate immunity, Ocular surface infections, Antibiotic resistance, Amphibian skin antimicrobial peptides
Introduction
Bacterial keratitis is an ocular surface infection following corneal abrasion or contact lens wear with the potential to seriously threaten vision [1–3]. A particular feature is its rapid progression to corneal inflammation, ulceration and tissue destruction which can be completed within 24–48 h with some of the most virulent bacteria if not appropriately treated or if unresponsive to treatment [4]. Amongst the culprits, the gram-negative bacterium Pseudomonas aeruginosa is a leading cause of keratitis associated with contact lens wear [5–7]. This bacterium can easily adhere to contact lenses forming sessile communities, named biofilms, which constitute a protected mode of growth that is difficult to treat [8–10]. Once a colonized lens is placed on the eye, it is easy for the bacterial community to resist the physical removal due to the tear flow combined with the sweeping action of the eyelids [1]. Furthermore, contact lens wear compromises several innate ocular surface defenses [11–13], while adaptation to the ocular surface microenvironment facilitates bacterial survival by enhancing the production of virulence factors that highly concur with the pathogenesis of Pseudomonas keratitis [1, 14]. In this scenario, worsened by the rapid increase of resistant pathogens, including P. aeruginosa, to available antibiotics [15–17], which are not even normally capable of eradicating bacterial biofilms [18], the discovery of new ophthalmic antimicrobial agents with a novel mode of action, has become urgently needed.
Gene-encoded antimicrobial peptides (AMPs) represent valid alternatives for the generation of new feasible anti-infective therapeutics [19–23]. They are produced by almost all living organisms throughout the evolutionary scale as a crucial element of innate defense against infection [24, 25]. Furthermore, especially in higher vertebrates, they have shown relevant immunomodulatory properties so leading to the alternate designation of host-defense peptides [26–28]. In Amphibians, AMPs are produced by dermal glands and stored within granules that are released on the skin surface, by a holocrine mechanism, and act to eliminate invading microorganisms [29–31]. Recently, particular attention has been paid to the peptide esculentin-1a(1-21)NH2 [Esc-1a(1-21)NH2, GIFSKLAGKKIKNLLISGLKG-NH2], consisting of the first 20 amino acids of the frog skin AMP esculentin-1a (isolated from the skin of Pelophylax lessonae/ridibundus, formerly known as Rana esculenta [31, 32]) with a glycinamide residue at its C-terminus [33]. Notably, it has been found that Esc-1a(1-21)NH2 has potent and rapid activity against both free-living and biofilm forms of P. aeruginosa strains, with a concomitant membrane-perturbation process as the major molecular mechanism underlying its bactericidal activity against both motile and sessile phenotypes [34]. This mechanism is based on an initial electrostatic interaction between the cationic peptide and the negatively-charged microbial membrane followed by its disruption through the formation of local-breakages, presumably limiting the induction of microbial resistance [20, 35]. Indeed, bacterial resistance to AMPs would necessitate modification of the composition of the cell membrane, which could not be achieved without causing significant harm to the organism itself [36]. Of note, this is in contrast with commonly-used traditional antibiotics that typically interfere with intracellular processes, e.g. DNA, protein, cell wall synthesis, by recognizing and interacting with single and specific targets which are highly sensitive to mutation [37].
Recent in vivo studies have shown that Esc-1a(1-21)NH2 is able to prolong survival in mouse models of P. aeruginosa sepsis or lung infection, upon intravenous or intra-tracheal administration, respectively [34]. This AMP can also cure inflamed bovine udders in mastitis-affected cows, without inducing irritant effects to the animals, when injected into the teats [33]. Given these promising data, here we investigated both the in vitro anti-Pseudomonal activity of Esc-1a(1-21)NH2 under physiological conditions that better mimic the ocular surface milieu (i.e. at high salt concentration and in the presence of tears) and its in vivo efficacy in a mouse model of bacterial keratitis. Our results suggest that Esc-1a(1-21)NH2 has great potential for development as a novel pharmaceutical for treatment and/or prevention of Pseudomonas keratitis upon topical application to the eye.
Materials and methods
Materials
Synthetic Esc-1a(1-21)NH2 was purchased from Selleck Chemicals (Houston, TX, USA). The purity of the peptide, its sequence and concentration were determined as previously described [33]. Benzalkonium chloride (BAC) and 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (Chemical Co, St. Louis MO). Keratinocyte growth medium-2 supplemented with growth factors was from Lonza (Ltd, Basel, Switzerland) and normocin from Invivogen (Tolouse, France). Mueller-Hinton (MH) broth and Pseudomonas Isolation agar were from Difco, (Detroit, MI). Myeloperoxidase (MPO) was from Calbiochem (San Diego, CA).
Microorganisms
The invasive P. aeruginosa strain ATCC 27853 and the cytotoxic P. aeruginosa strain ATCC 19660 were primarily used in this study [38]. Furthermore, the following clinical isolates from human keratoconjunctivitis and conjunctivitis cases and with a different drug-resistance profile (see Table 1) were included: P. aeruginosa R1; P. aeruginosa 1 Rm; P. aeruginosa n. 2 ME; P. aeruginosa 3; Staphylococcus epidermidis n. 21 (326) ME; Staphylococcus hominis n. 1ME; Staphylococcus aureus n.6 ME. All of these strains were kindly provided by Dr. Anna Rita Blanco, SIFI Pharmaceutical Company, Catania, Italy.
Table 1.
Antimicrobial activity of Esc-1a(1-21)NH2 against reference and clinical isolates from human ocular surface infections, with varying degrees of antibiotic resistance
| Species and strains | Relevant features | MIC (μM) |
|---|---|---|
| Reference strains | ||
| Pseudomonas aeruginosa ATCC 27853a | Reference strain, wild type | 4 |
| Pseudomonas aeruginosa ATCC 19660 | Reference strain, wild type | 16 |
| Clinical ocular isolatesb | ||
| Pseudomonas aeruginosa R1 | CAZ, GEN, IPM, TOB | 2 |
| Pseudomonas aeruginosa 1 Rm | CAZ, CIP, CTX, FEP, GEN, PIP, SXT, TOB | 4 |
| Pseudomonas aeruginosa n. 2 ME | CAZ, IPM | 8 |
| Pseudomonas aeruginosa n. 3 | IPM | 8 |
| Staphylococcus epidermidis n. 21(326) ME | ERY,GEN, OXA,TET, TOB, VAN | 8 |
| Staphylococcus hominis n. 1 ME | AMP, ERY, GEN, RIF, TET,TOB | 1 |
| Staphylococcus aureus n. 6 ME | TET, TOB, | 64 |
a Data were taken from ref [34]
b Relevant resistance traits are indicated as follows: AMP ampicillin, CAZ ceftazidime, CIP ciprofloxacin, CTX cefotaxime, ERY erythromycin, FEP cefepime, GEN gentamicin, IPM imipenem, OXA oxacillin, PIP piperacillin, RIF rifampin, SXT trimethoprim-sulfamethoxazole, TET tetracycline, TOB tobramycin, VAN vancomycin
Antimicrobial activity
Susceptibility testing was performed by adapting the microbroth dilution method outlined by the Clinical and Laboratory Standards Institute using sterile 96-well plates. Aliquots (50 μl) of bacteria in mid-log phase at a concentration of 2 × 106 colony-forming units (CFU)/ml in MH broth were added to 50 μl of MH broth containing the peptide in serial twofold dilutions. The range of peptide concentration used was 0.125–64 μM, each in triplicate. Inhibition of microbial growth was determined by measuring the absorbance at 590 nm, after an incubation of 18 h at 37 °C with a microplate reader (Infinite M200; Tecan, Salzburg, Austria). Antimicrobial activities were expressed as the minimal inhibitory concentration (MIC), the concentration of peptide at which 100 % inhibition of microbial growth is observed.
Bactericidal activity of Esc-1a(1-21)NH2 in the presence of NaCl and human tears
Mid-log phase growth bacteria were centrifuged and resuspended in phosphate buffer (PB, 8.2 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) to an optical density of approximately 1 × 107 CFU/ml. Ten microliters of this suspension were then incubated with vigorous shaking at 37 °C for 20 min with Esc-1a(1-21)NH2 at the indicated concentration, in the presence or absence of different concentrations of NaCl to give a final reaction mixture volume of 50 μl. Controls were bacterial cells in PB supplemented or not with NaCl but without addition of the peptide. At the end of the incubation, aliquots of each reaction mixture were withdrawn, diluted in MH broth and spread onto Luria–Bertani (LB)-agar plates. After overnight incubation at 37 °C, the number of CFU was counted and expressed as % of killed bacteria. To further investigate Esc-1a(1-21)NH2 anti-Pseudomonal activity, another set of experiments was performed using pooled tears from human subjects. Tears were collected from 18 consenting adult volunteers after clinical eye exams performed by an ophthalmologist confirmed normal ocular surface. Tears were collected from the inferior tear meniscus using 5 μl microcapillary tubes (Drummond Scientific, Broomall, PA) without anesthesia and stored at −20 °C until used in experiments. Reaction mixtures containing 50 % (v/v) or 70 % (v/v) human basal tears were incubated with 1 × 105 CFU of P. aeruginosa in the presence or not of the peptide at the indicated concentration for 30, 90 and 120 min at 37 °C (final volume, 50 μl). Controls were bacterial cells in PB not treated with the peptide. At the corresponding time intervals, aliquots of samples were withdrawn and plated on LB-agar plates for counting.
Human corneal epithelial cell culture
Telomerase-immortalized human cornel epithelial (hTCEpi) cells were maintained in serum-free keratinocyte growth medium-2 supplemented with growth factors and normocin (100 μg/ml) as previously described [39]. Cells were subcultured on T25 tissue culture flasks (Falcon NJ, USA) incubated at 37 °C in 5 % CO2 and passaged every 7–10 days.
Cytotoxicity assay
The toxicity of Esc-1a(1-21)NH2 to hTCEpi cells was assessed using an MTT-based colorimetric assay. hTCEpi cells were seeded in triplicate into 96-well plates and exposed to 0.1, 1, 5, 10, 25, 50 and 100 µM of Esc-1a(1-21)NH2 for 24 h. Cells exposed to 0.02 % BAC for 15 min acted as a positive control. MTT solution was then added and the incubation continued at 37 °C for another 3 h until purple formazan crystals were seen by light microscopy. The reaction was stopped by addition of isopropanol with 0.04 N hydrochloric acid followed by trituration to solubilize the formazan crystals. The plate was read using a multi-well spectrophotometer (BMG LABTECH, Ortenberg, Germany) at 590 nm.
In vivo infection with P. aeruginosa
P. aeruginosa ATCC 19660, a strain previously reported to produce severe microbial keratitis in mice [40, 41] was used in the in vivo studies. A colony of P. aeruginosa was grown overnight and expanded. The cultures were centrifuged at 3,100×g for 10 min and the bacterial pellet was resuspended in 2 ml MH broth and the concentration of the bacteria adjusted turbidimetrically to obtain 2 × 108 CFU/ml. A retrospective count of the bacteria was performed by plating the cells.
Infection
All protocols used were approved by the University of Houston Institutional Animal Care and Use Committee and were in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Male 7–10 week old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized by intraperitoneal injection of ketamine, 100 mg/kg; xylazine 10 mg/kg (Vedco, Inc., St. Joseph, MO). One drop topical proparacaine (Bausch + Lomb, Tampa, FL) was applied then the anesthetized animal was placed beneath a stereomicroscope and the cornea of the right eye scratched with a sterile 27-gauge needle. Three parallel 1 mm scratches were made in the central cornea of the right eye so as to abrade the full thickness of the epithelium and penetrate the superficial stroma. A 5 µl aliquot containing 1 × 106 CFU of P. aeruginosa was pipetted directly onto the scarified cornea. The corneas were then topically treated with 40 µM Esc-1a(1-21)NH2 starting 5 h post-infection (PI). Peptide treatment was repeated twice on the day of infection and three times a day (6.30 am, 1.30 pm and 8.30 pm) for the next 4 days. Animals receiving the phosphate buffered saline (PBS) vehicle acted as controls. In some animals (the pre-treatment group), Esc-1a(1-21)NH2 was topically applied, 3 times in 24 h prior to scratching and induction of keratitis.
The clinical progression of infection was monitored and the extent of corneal damage evaluated and documented by digital imaging using a slit-lamp equipped with a camera module CM 01 (Haag Streit USA, Mason, OH) at day 1, 3 and 5 PI. The progression of infection was graded using a previously established scale of 0–4 [42]: grade 0, clear or slight opacity, partially covering the pupil; grade 1, slight opacity, fully covering the cornea; grade 2, dense opacity, partially or fully covering the pupil; grade 3, dense opacity fully covering the cornea; and grade 4, corneal perforation or phthisis.
Bacterial load and myeloperoxidase assay
Control and infected corneas from four to six mice were harvested at day 1, 3 and 5 PI and homogenized (8–10 strokes for 10 s, repeated 3 times until all tissue was uniformly homogenized) on ice in 1 ml of sterile PBS, pH 7.4. A 100 µl aliquot of the homogenate was serially diluted in sterile PBS and duplicate aliquots were plated onto Pseudomonas Isolation agar plates which were incubated overnight at 37 °C then the number of CFU counted. The remaining homogenate was processed to quantitate the number of infiltrating inflammatory cells by MPO activity, a standard and well-established method in the study of infectious keratitis [43]. To determine MPO activity, hexadecyltrimethylammonium bromide at a final concentration of 0.5 % (wt/v) in 50 mM phosphate buffer (pH 6.0) was added to 90 µl corneal homogenate. Samples were then freeze-thawed three times and centrifuged at 13,000g for 20 min at 4 °C. Ten microliters of supernatant were pipetted in triplicate into a microtiter plate and the reaction initiated by the addition of 90 µl of 0.0167 % (wt/v) o-dianisidinedihydrochloride and 0.002 % (v/v) H2O2 in PBS. The absorbance was measured for 90 min at 450 nm and plotted in comparison with a standard curve generated using purified MPO on the same plate [43]. Results are expressed as relative units of MPO activity per cornea (1 MPO unit is proportional to 2 × 105 infiltrating neutrophils).
Statistical analysis
Statistical analysis was performed using Student’s t test or analysis of variance (ANOVA), with PRISM software (GraphPad, San Diego, CA). A Tukey’s HSD test was used for the analysis of the data from in vivo experiments. Differences were considered to be statistically significant for p < 0.05. All experiments were repeated at least three times to ensure reproducibility.
Results
MIC of Esc-1a(1-21)NH2 against P. aeruginosa and Staphylococcus genus
The susceptibility of P. aeruginosa ATCC 19660 to increasing concentrations of Esc-1a(1-21)NH2 was determined using the MIC assay as described. Data obtained by visual and spectrophotometric observation were comparable and showed clear/non-turbid wells indicating no bacterial growth with 64, 32 and 16 µM peptide. However, wells containing Esc-1a(1-21)NH2 at a concentration of 8, 4, 2, 1, 0.5 and 0 µM exhibited considerable turbidity indicating significant bacterial growth and therefore the MIC was 16 μM. As reported in Table 1, a lower MIC (4 μM) was displayed by this peptide against P. aeruginosa ATCC 27853. Furthermore, four P. aeruginosa clinical isolates from human ocular surface infections (keratitis and conjunctivitis) and with varying degrees of resistance to commonly used antibiotics were included for comparison, as well as other three bacterial strains belonging to the Staphylococcus genus (i.e. S. aureus, S. epidermidis, S. hominis) which are relevant not only for cornea, but also for conjunctival infections [44, 45]. Importantly, Esc-1a(1-21)NH2 was found to be active against the selected clinical isolates with MIC values measured in the range of 2–8 μM for P. aeruginosa strains compared with a MIC of 1 or 8 μM for S. hominis or S. epidermidis, respectively. An exception was given by S. aureus toward which a higher MIC (64 μM), was detected (Table 1).
Esc-1a(1-21)NH2 anti-Pseudomonal activity in the presence of NaCl
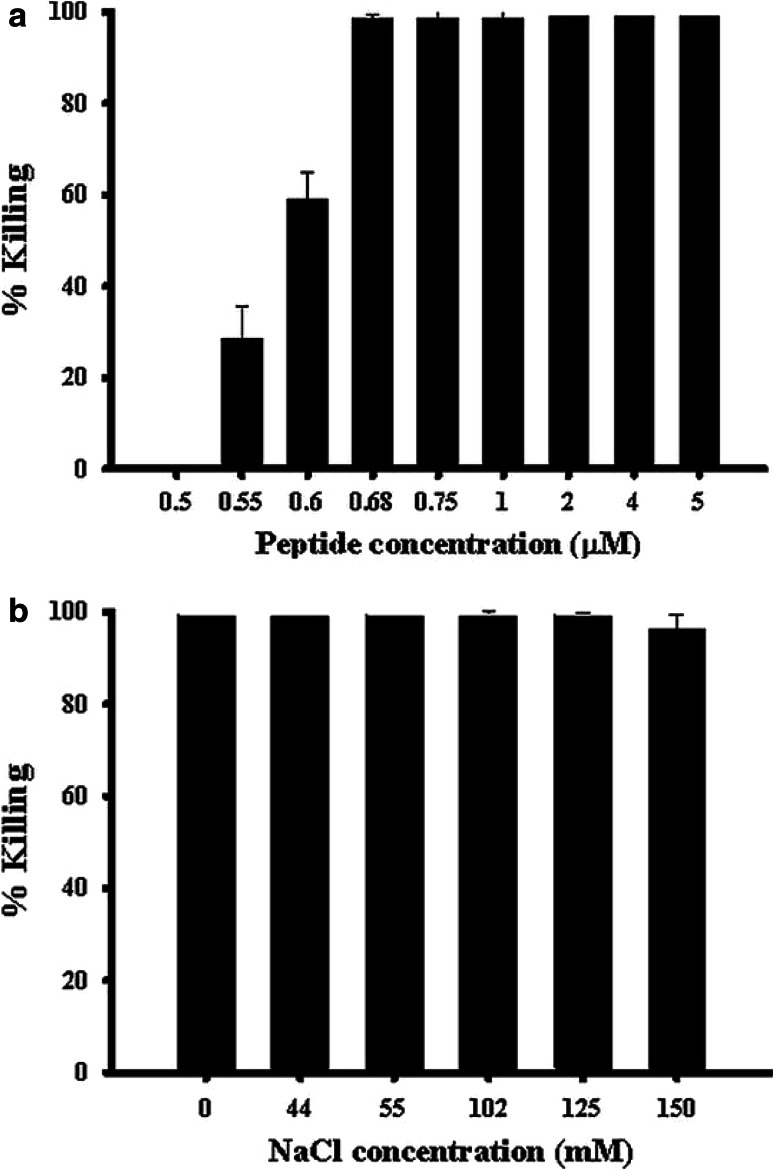
The bactericidal activity of Esc-1a(1-21)NH2 against P. aeruginosa ATCC 27853 was initially analyzed in the presence of 150 mM NaCl, the physiological salt concentration of the tear film [11]. As reported in Fig. 1a, concentrations of peptide at 0.68 μM and above caused approximately 100 % killing of the bacterial population within 20 min. These data are in keeping with recent studies showing that Esc-1a(1-21)NH2 provokes a 3-log reduction in the number of viable P. aeruginosa ATCC 27853 cells when tested at high ionic strength, i.e. PBS, at 1 μM [34].
Fig. 1.
Effects of NaCl on the bactericidal activity of Esc-1a(1-21)NH2 against P. aeruginosa ATCC 27853. a Bacterial cells were incubated with different concentrations of Esc-1a(1-21)NH2 in the presence of 150 mM NaCl for 20 min at 37 °C. b Bacterial cells were incubated with the peptide (1 μM) in the absence or in the presence of varying concentrations of NaCl at 37 °C for 20 min. The data are the mean ± standard deviation (SD) of three independent experiments and are reported as percentage of bacteria killed by the peptide compared with the corresponding control (see “Materials and methods”)
In order to determine whether NaCl affected the anti-Pseudomonal activity of this peptide by hampering its electrostatic interactions with the anionic bacterial membrane, we then evaluated the bactericidal activity of Esc-1a(1-21)NH2 in the presence of different concentrations of salt. The lethal activity of 1 μM peptide against P. aeruginosa ATCC 27853 was well-retained after 20 min treatment in the presence of increasing concentrations of NaCl from 44 to 150 mM (Fig. 1b).
Anti-Pseudomonal activity in the presence of human tears
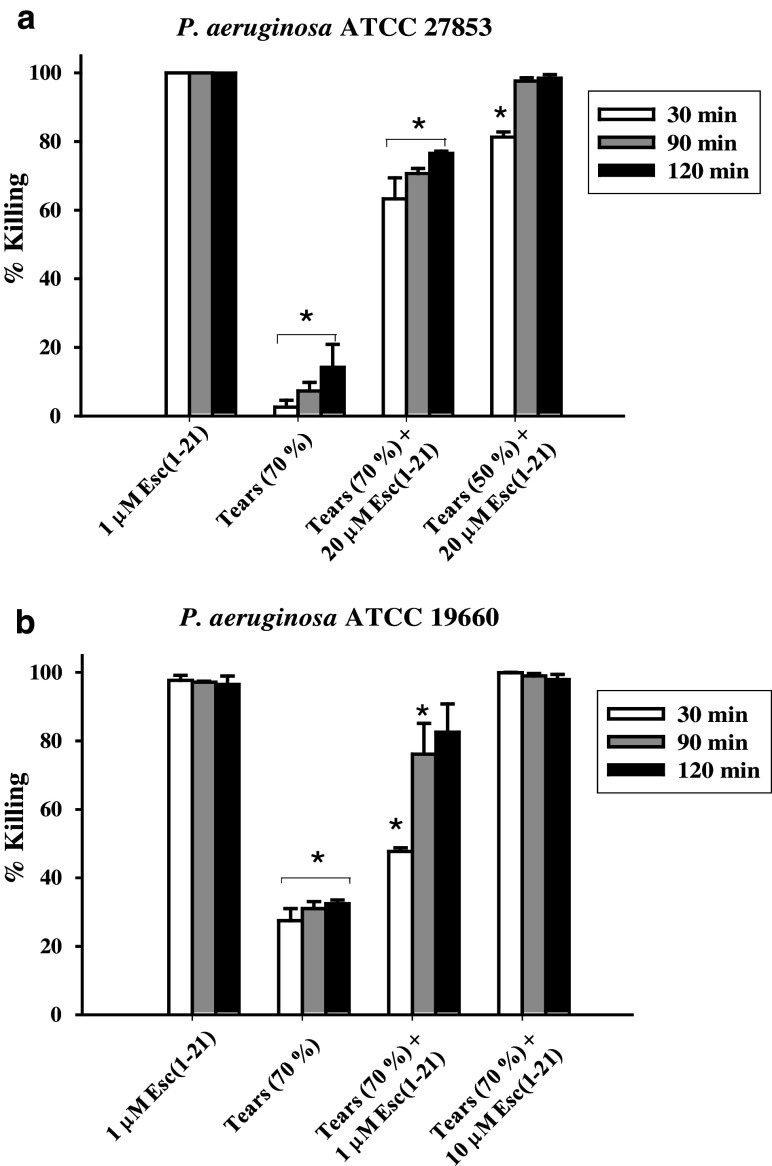
In addition to salt, mucins in tears have also been shown to hinder the antimicrobial activity of some AMPs, such as hBD2 [38]. Surprisingly, in the present experiments, 70 % or 100 % killing of bacterial cells (strain ATCC 27853) was obtained after 90 min incubation with 20 μM Esc-1a(1-21)NH2 in the presence of 70 % (v/v) or 50 % (v/v) human basal tears, respectively (Fig. 2a). For comparison, only approximately 10 % killing of Pseudomonas was previously demonstrated by 100 μg/ml hBD2 (~20 μM), 2 h after treatment in 70 % (v/v) human basal tears [38].
Fig. 2.
Effects of human basal tears on the bactericidal activity of Esc-1a(1-21)NH2 against P. aeruginosa ATCC 27853 (a) and ATCC 19660 (b). Bacterial cells were incubated with the peptide in the presence of 70 % (v/v) or 50 % (v/v) basal tears, as indicated, for 30, 90 and 120 min at 37 °C. Cells treated with 1 μM peptide [indicated as Esc(1-21) in the figure] in PB or with 70 % (v/v) tears were included for comparison. After incubation, aliquots were withdrawn for cell counting. The data are the mean ± standard deviation (SD) of three independent experiments and are reported as percentage of bacteria killed compared with the control (see “Materials and methods”). Significance was assessed using values of all groups compared with those of peptide treated samples in the absence of tears by t test. *Indicates significant difference, p < 0.05
Most importantly, when Esc-1a(1-21)NH2 was studied against the cytotoxic strain of P. aeruginosa ATCC 19660, which is capable of inducing severe ocular infection in experimentally infected mouse models of bacterial keratitis [41, 46], we found even more promising results. Indeed, despite Esc-1a(1-21)NH2 displaying a higher MIC against this strain with respect to the invasive ATCC 27853 (Table 1), 10 μM peptide was sufficient to induce the complete killing of P. aeruginosa ATCC 19660 within 30 min in the presence of 70 % (v/v) basal tears (Fig. 2b). Note also that, compared with the results obtained with 1 μM of peptide alone, only ~20 % reduction in killing efficacy was observed 90 min after addition of Esc-1a(1-21)NH2 in basal tears at the same concentration of 1 μM (Fig. 2b). However, in comparison with the invasive ATCC 27853, the cytotoxic strain was more susceptible to the toxic activity inherent to basal tears themselves, which gave rise to approximately 30 % microbial death (Fig. 2b), whilst ~10 % was recorded in the case of P. aeruginosa ATCC 27853 (Fig. 2a).
Cytotoxic effects of Esc-1a(1-21)NH2 on hTCEpi cells
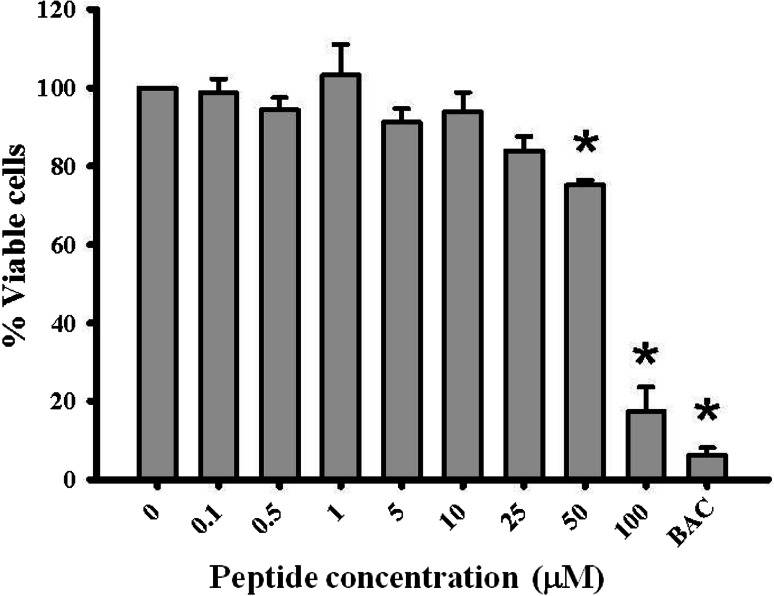
Cytotoxicity of Esc-1a(1-21)NH2 to hTCEpi cells was examined by MTT assay. As shown in Fig. 3, cell viability after 24 h exposure at concentrations of peptide 0.1–10 µM, was 93.77–100 %, and at 25 µM, viability was 83.93 %. At 50 µM peptide, viability was statistically significantly reduced to 75.73 % and at 100 µM Esc-1a(1-21)NH2 this was reduced even further to 17.35 %. Exposure to the positive control BAC resulted in a viability of only 6.18 %. These results revealed that Esc-1a(1-21)NH2 became cytotoxic to hTCEpi cells at concentrations of 50 µM and above and guided the choice of peptide concentration for the in vivo studies.
Fig. 3.
Esc-1a(1-21)NH2 toxicity to human corneal epithelial cells. hTCEpi cells exposed to Esc-1a(1-21)NH2 for 24 h were tested for cytotoxicity using an MTT assay. Data shown are the average from 3 independent experiments (n = 3–4 wells/experiment). BAC benzalkonium chloride, the positive control. *Indicates p < 0.0002 using ANOVA with Tukey’s HSD for post hoc analysis
Effect of Esc-1a(1-21)NH2 on P. aeruginosa keratitis in C57BL/6 mice
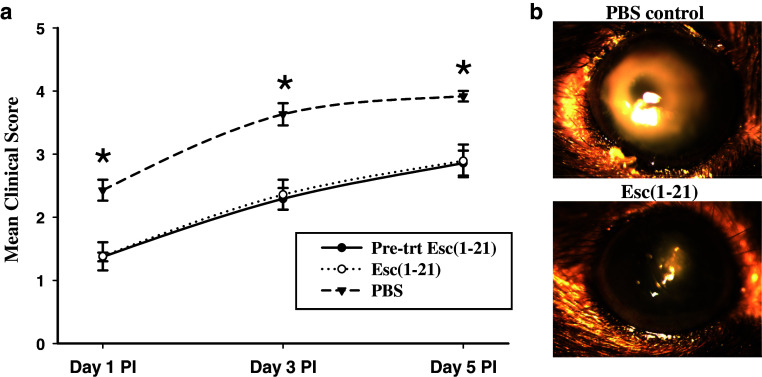
Clinical progression of corneal infection, inflammatory cell recruitment and bacterial load with and without treatment with 40 μM Esc-1a(1-21)NH2 were examined in C57BL/6 mice infected with P. aeruginosa ATCC 19660. Figure 4a shows the mean clinical scores from mice examined by slit-lamp biomicroscopy. Corneas of the PBS treated control animals demonstrated severe disease with rapid progression beginning with a dense opacity fully covering the pupil at day 1 PI to corneal perforation or phthisis in some animals at day 5 PI. The mean clinical scores for this group at days 1, 3 and 5 PI were 2.43 ± 0.17, 3.63 ± 0.17 and 3.92 ± 0.08. Infection was significantly less severe at all time points (p < 0.009, 0.005 and 0.009 at day 1,3 and 5 PI) for mice treated (or pre-treated) with Esc-1a(1-21)NH2, although as with controls, the severity of the infection was progressive. Figure 4b shows the appearance of P. aeruginosa keratitis infection at day 3 PI in the peptide and PBS control treated groups. In the latter, there was a very dense opacity fully covering the cornea with significant edema whereas the peptide treated animals had a much smaller opacity partially covering the cornea. Data from the Esc-1a(1-21)NH2 pre-treated and treated animals were not significantly different at day 1, 3 or 5 PI (p < 0.95, 0.83 and 0.92 respectively). At day 1 PI, the corneas of mice in the peptide pre-treatment and treatment groups appeared cloudy with a slight opacity and a mean clinical score of 1.37 ± 0.07 and 1.38 ± 0.22 respectively. At day 3 PI, corneas of the peptide treated and pre-treated animals exhibited a dense opacity covering up to 40 % of the cornea with a mean clinical score of 2.29 ± 0.17 and 2.36 ± 0.24. At day 5 PI, a dense opacity covering about 50–60 % of the cornea was observed in the Esc-1a(1-21)NH2 treated animals with a mean clinical score of 2.89 ± 0.26 (2.86 ± 0.20 in the pre-treated animals). The severity of ocular disease was significantly greater at day 3 PI and day 5 PI compared to day 1 in all groups (p < 0.05), but not at day 3 compared to day 5.
Fig. 4.
Effect of Esc-1a(1-21)NH2 on P. aeruginosa keratitis in C57BL/6 mice. a Clinical scores indicated that severity of infection was significantly less at all time points in mice treated (or pre-treated) with the peptide [indicated as Esc(1-21) in the figure] compared to controls. Data are mean clinical scores from 4 independent experiments with 4–6 mice per experiment. *Indicates significant difference, p < 0.05, among PBS control and peptide treated groups. b Representative photographs of infected animals in the control and peptide treatment group at day 3 PI
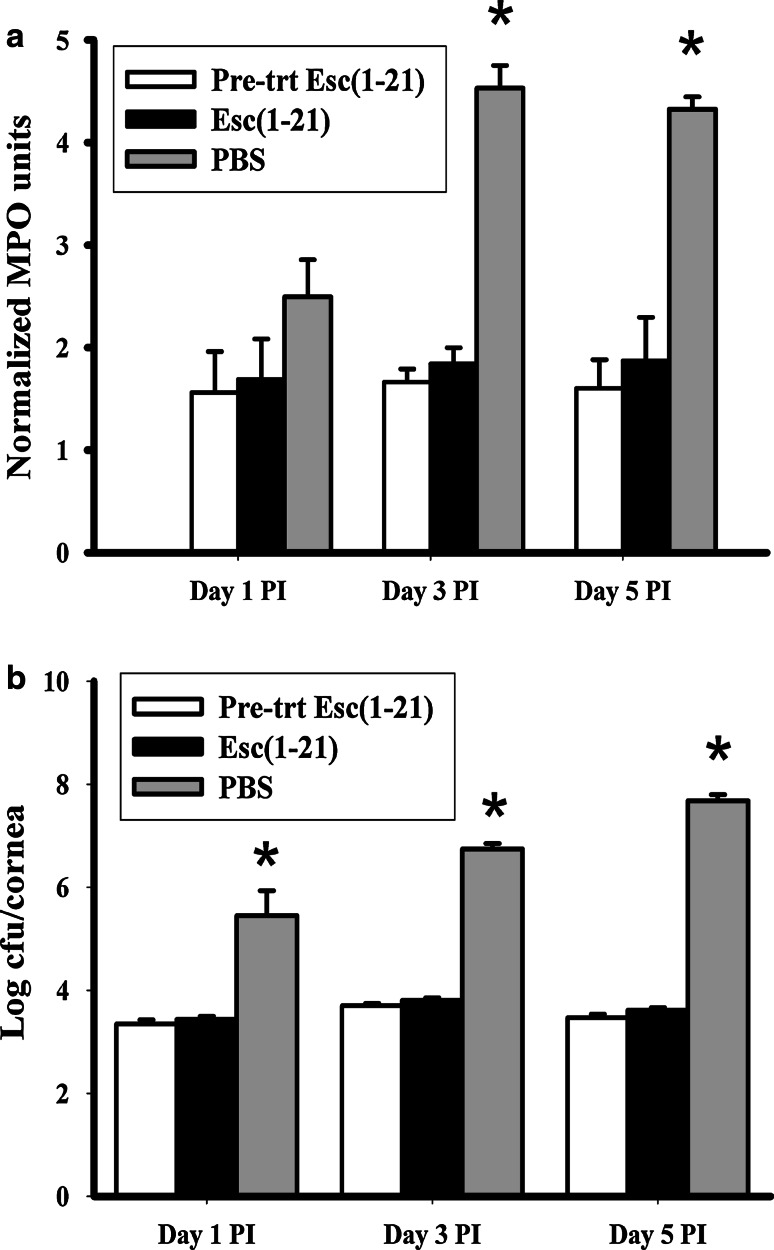
Inflammatory cell recruitment to the site of infection was determined by measuring the relative MPO activity in corneas harvested at day 1, 3 and 5 PI (Fig. 5a). MPO activity for the control PBS treated animals at days 1, 3 and 5 PI was 2.5 ± 0.36, 4.53 ± 0.22 and 4.2 ± 0.12 respectively. These values were significantly higher than for both groups of peptide treated animals at days 3 and 5 PI (p < 0.0004 and 0.0012 for the pre-treated group and 0.0006 and 0.005 for the Esc-1a(1-21)NH2 treated group), indicating a substantially lower influx of inflammatory cells into peptide treated corneas than corneas receiving the vehicle PBS. The latter demonstrated a significantly higher recruitment of inflammatory cells and hence higher MPO activity at day 3 and 5 PI compared to day 1 (p < 0.008 and 0.008), although there was no significant difference between days 3 and 5 PI. The pattern was different for peptide treated animals in that the level of inflammatory cell recruitment was similar at all time points with mean MPO values for the pre-treatment and Esc-1a(1-21)NH2 treated group of 1.55 ± 0.4 and 1.69 ± 0.40 at day 1; 1.66 ± 0.13 and 1.84 ± 0.16 at day 3 and 1.55 ± 0.28 and 1.82 ± 0.42 at day 5 PI. There was no significant difference in MPO values at day 1, 3 and 5 PI among peptide pre-treated and peptide treated animals (p < 0.83, 0.42 and 0.62 respectively).
Fig. 5.
Effect of Esc-1a(1-21)NH2 on corneal neutrophil infiltration and bacterial load in P. aeruginosa keratitis. a MPO activity revealed a significantly greater inflammatory cell recruitment in infected corneas of the PBS treated control animals compared to the pre-treatment and peptide [indicated as Esc(1-21) in the figure] treated groups. b Viable bacterial count in the infected cornea was significantly greater in the PBS control animals compared to peptide treated or pre-treated animals. Data represent averages from 3 independent experiments with 2–4 mice/group. *Indicates significant difference, p < 0.05, among PBS control and peptide treated groups
The viable bacterial load that was determined by quantifying P. aeruginosa colonies at day 1, 3 and 5 PI is shown in Fig. 5b. The levels of recoverable viable bacteria obtained in the PBS control group were significantly greater than for the pre-treated or Esc-1a(1-21)NH2 treated groups at day 1, 3 and 5 PI (p < 0.01, 0.0001, 0.0001, respectively) with a difference in the number of CFU of ~2–3 log10 at days 1 and 3, and 4 log10 at day 5 PI. There was no significant difference between the pre-treated and Esc-1a(1-21)NH2 treated groups (p < 0.41, 0.15 and 0.15 at days 1, 3 and 5 PI respectively). The pre-treated corneas demonstrated a significantly higher CFU at day 3 PI (3.7 log10 CFU) compared to day 1 (3.35 log10 CFU) and day 5 PI (3.47 log10 CFU) (p < 0.01, 0.04 respectively). The peptide treated infected corneas demonstrated a similar pattern, with statistically significant differences (p < 0.008, 0.04 at day 1 and 5 PI respectively). However, in the PBS control animals there was a much greater increase in the viable bacteria recovered at day 3 PI (6.76 log10 CFU) compared to day 1 PI (5.8 log10 CFU) and for day 5 PI (7.7 log10 CFU) as compared to day 3 PI (p < 0.02).
Discussion
P. aeruginosa, a common cause of contact-lens associated bacterial keratitis [47–49], induces rapid destruction of the cornea, initially owing to bacterial proteases and toxins [40, 50]. Although host reactions to Pseudomonas keratitis rapidly occur after disease is initiated, they are not adequate to defeat this pathogen and contribute significantly to corneal damage. Further, the antimicrobials currently used, e.g. aminoglycosides and fluoroquinolones, are problematic, due to their side effects and the growing emergence of resistant bacterial strains [51, 52]. Of note, AMPs hold promise as future therapeutics. Thus, our current investigation aimed at studying the potential of the amphibian AMP-derived Esc-1a(1-21)NH2 as a novel pharmaceutical agent for bacterial keratitis.
Prior studies have characterized AMPs for their activity against ocular pathogens in vitro [38, 53–55], a preservative for eye bank corneal storage media [56] and as contact lens disinfectants [57]. Efficacy of two AMPs has also been investigated in in vivo studies in a rabbit model of P. aeruginosa keratitis. In one study, a synthetic AMP COL-1, administered topically at 10 or 50 μg/ml, resulted in greater inflammation than that observed in controls, and despite its potent antimicrobial activity in vitro showed no efficacy in vivo [49]. In another study, infected rabbit eyes topically treated with a hybrid cecropin A-melittin peptide showed higher clinical scores after 6 h than those from control animals, likely owing to a toxic effect of the peptide [58]. After 24 h however, more promising results were seen as clinical scores for the peptide treated animals were lower than the control group, although not as low as the gentamicin treated positive control. Possibly contributing to the lack of robust AMP efficacy in these two studies is the fact that the antimicrobial activity of some AMPs is significantly compromised by physiological salt concentrations and other tear components such as mucins [38, 54, 59]. Thus, to determine if an exogenously applied AMP is potentially active at the ocular surface it is important to establish if its activity is hampered by salt and tears. Here, we found that the bactericidal activity of Esc-1a(1-21)NH2 against P. aeruginosa is fully preserved at increasing salt concentrations. Notably, this response clearly differs from that previously reported for some endogenously expressed mammalian AMPs, e.g. hBD-1 and hBD-2 produced by corneal and conjunctival epithelial cells [54, 60, 61]. Indeed, their bacterial killing activity is reduced by at least 30 % in reaction mixtures containing NaCl solutions at an osmolality equivalent to that of tears [38, 62, 63]. In addition, in contrast with what has been observed for mammalian AMPs, e.g. rabbit NP-3a [59] and human hBD-1 and -2 [38, 54], whose ability to kill Pseudomonas in the presence of 70 % (v/v) tears is completely abolished or very markedly diminished, Esc-1a(1-21)NH2 significantly retains bactericidal activity in basal human tears, especially against the cytotoxic strain P. aeruginosa ATCC 19660. Furthermore, our previous studies demonstrated the ability of Esc-1a(1-21)NH2 to be similarly active against both reference and clinical strains of P. aeruginosa, with a multi-drug resistance or mucoid phenotype (isolated from cystic fibrosis sufferers), with a MIC value measured in the range of 4–8 μM [34]. This is now strengthened by the findings that the antimicrobial activity of this peptide is preserved also against a range of P. aeruginosa isolates from keratitis and conjunctivitis cases and with varying degrees of antibiotic resistance (MIC value from 2 to 8 μM).
All of these promising in vitro data prompted us to test the efficacy of this peptide in a mouse model of P. aeruginosa keratitis.
Results from our in vivo study established that Esc-1a(1-21)NH2 treated C57BL/6 mice demonstrated significantly less severe keratitis compared to their PBS treated counter parts at all time points. This improved clinical appearance is accompanied by reduced inflammatory cell infiltration and reduced bacterial counts compared to PBS treated mice. The peptide does not appear to lead to an inflammatory process, suggesting that the clinical benefit is presumably due to a direct effect of Esc-1a(1-21)NH2 on the bacterial load rather than being mediated by additional events e.g. recruitment of neutrophils to the site of infection which may result in severe damage to the corneal tissue. To better explore whether this peptide had immunomodulatory activity, as discovered for other mammalian AMPs [25–28], we also investigated if initiating treatment with Esc-1a(1-21)NH2 before infection may affect the clinical outcome. However, we found that this was not the case, with results from mice where treatment of the peptide was started before infection and those where Esc-1a(1-21)NH2 administration was started post-infection being virtually identical. This suggests that pre-treatment with Esc-1a(1-21)NH2 does not induce innate defenses at the ocular surface, supporting the notion that the amelioration of keratitis by Esc-1a(1-21)NH2 is mainly due to direct antibacterial activity of the peptide once applied to the eye. Note, however, that in our study, although Esc-1a(1-21)NH2 treatment, at the concentration tested, significantly reduced severity of the infection compared to PBS treated controls, the disease was still progressive. This may be related to our use of the ATCC 19660 cytotoxic strain. Thus, while peptide treatment was associated with reduced inflammatory cell infiltration and viable bacteria within the cornea, there may have been sufficient bacterial toxin produced early on to cause persistent damage.
In the experiments, a balance needed to be achieved among antimicrobial efficacy and lack of toxicity. Thus, we selected 40 μM for the topical treatment as this was below the concentration at which the Esc-1a(1-21)NH2 began to show cytotoxicity in vitro and was higher than the MIC for ATCC 19660. It should also be noted that the peptide was only applied topically three times per day. This is in stark contrast to typical treatment regimens for bacterial keratitis in human patients in which antibiotic drops every 15 min to 1 h are recommended for the first 2 days of treatment. Thus, more frequent application likely would result in greater efficacy. Further, other studies from our lab have shown that tethering of AMPs to surfaces and nanoparticles greatly enhances their antimicrobial efficacy compared to an AMP in solution [64, 65]. Formulating Esc-1a(1-21)NH2 for delivery in such a fashion would therefore also be expected to increase its effectiveness while not causing significant toxicity, as much lower amounts can be utilized.
In summary, our findings highlight that simple topical application of Esc-1a(1-21)NH2 in solution or likely its attachment to a “surface” (e.g. contact lens or nanoparticles) may represent a very promising mode of drug delivery for the prevention/treatment of P. aeruginosa keratitis. In regards to development of an AMP based-antimicrobial contact lens, Willcox and colleagues have already shown the feasibility of this with a hybrid melittin-protamine peptide, named melimine, which when conjugated to contact lenses, reduced bacterial colonization [66–68], as well as the degree of ocular inflammation and corneal infiltrates in animal models of contact lens related adverse events [68]. Despite some frog-skin AMPs having already been shown to have anti-Pseudomonal activity at high salt concentration [69], our study is the first to report such an activity by a frog skin AMP-derived peptide in the presence of either high ionic strength or human tears (in contrast with what found for some mammalian AMPs) as well as when administered, in solution, in an animal model of bacterial keratitis, with clear resulting signs of beneficial effect. Overall, our data, coupled with the wide spectrum of activity of Esc-1a(1-21)NH2, either against reference microbial strains [33, 34] or clinical isolates from human ocular surface infections (Table 1), and with the low risk of development of bacterial resistance, due to the mechanism of action of this peptide [34], suggest Esc-1a(1-21)NH2 as an excellent candidate for development as a novel drug against microbial keratitis.
Acknowledgments
We thank Prof. Santi Maria Recupero, Head of DESMOS Department, Faculty of Medicine and Psychology, Sapienza University of Rome, for allowing access to the ambulatory centre of S. Andrea Hospital to collect tears from consenting volunteers. We thank Dr. Anna Rita Blanco, at SIFI, Catania, Italy, for providing the clinical isolates from human ocular surface infections. This work was supported by grants NIH EY13175 (AMM), NIH EY07551 (UHCO Core grant), Sapienza University of Rome (prot. C26A12NPXZ).
Abbreviations
- AMP
Antimicrobial peptide
- BAC
Benzalkonium chloride
- CFU
Colony-forming units
- Esc-1a(1-21)NH2
Esculentin-1a(1-21)NH2
- hTCEpi
Telomerase-immortalized human cornel epithelial cells
- LB
Luria-Bertani
- MH
Mueller–Hinton
- MIC
Minimal inhibitory concentration
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MPO
Myeloperoxidase
- PB
Phosphate buffer
- PBS
Phosphate buffered saline
References
- 1.Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013;155:961–970. doi: 10.1016/j.ajo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, Stapleton F. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129–141. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J. 2009;9:184–195. [PMC free article] [PubMed] [Google Scholar]
- 5.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 6.Mela EK, Giannelou IP, Koliopoulos JX, Gartaganis SP. Ulcerative keratitis in contact lens wearers. Eye Contact Lens. 2003;29:207–209. doi: 10.1097/01.icl.0000078102.30635.A7. [DOI] [PubMed] [Google Scholar]
- 7.Schein OD, McNally JJ, Katz J, Chalmers RL, Tielsch JM, Alfonso E, Bullimore M, O’Day D, Shovlin J. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112:2172–2179. doi: 10.1016/j.ophtha.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, Molin S, Givskov M, Tolker-Nielsen T, Bjarnsholt T. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3:55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilm: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 10.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 11.McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013;117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson DM. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013;39:67–72. doi: 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans DJ, Fleiszig SM. Microbial keratitis: could contact lens material affect disease pathogenesis? Eye Contact Lens. 2013;39:73–78. doi: 10.1097/ICL.0b013e318275b473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa . Microbiol Mol Biol Rev. 2010;74:621–641. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willcox MD. Review of resistance of ocular isolates of Pseudomonas aeruginosa and staphylococci from keratitis to ciprofloxacin, gentamicin and cephalosporins. Clin Exp Optom. 2011;94:161–168. doi: 10.1111/j.1444-0938.2010.00536.x. [DOI] [PubMed] [Google Scholar]
- 16.Szczotka-Flynn LB, Imamura Y, Chandra J, Yu C, Mukherjee PK, Pearlman E, Ghannoum MA. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28:918–926. doi: 10.1097/ICO.0b013e3181a81835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maviglia R, Nestorini R, Pennisi M. Role of old antibiotics in multidrug resistant bacterial infections. Curr Drug Targets. 2009;10:895–905. doi: 10.2174/138945009789108846. [DOI] [PubMed] [Google Scholar]
- 18.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 19.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 20.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 21.Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 22.Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey CE, Hawrani A, Howe RA, Walsh TR. Amphipathic antimicrobial peptides—from biophysics to therapeutics? Protein Pept Lett. 2010;17:1334–1344. doi: 10.2174/0929866511009011334. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 25.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haney EF, Hancock RB. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. 2013;100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- 28.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Mangoni ML, Miele R, Renda TG, Barra D, Simmaco M. The synthesis of antimicrobial peptides in the skin of Rana esculenta is stimulated by microorganisms. FASEB J. 2001;15:1431–1432. doi: 10.1096/fj.00-0695fje. [DOI] [PubMed] [Google Scholar]
- 30.Mangoni ML. Temporins, anti-infective peptides with expanding properties. Cell Mol Life Sci. 2006;63:1060–1069. doi: 10.1007/s00018-005-5536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conlon JM, Iwamuro S, King JD. Dermal cytolytic peptides and the system of innate immunity in anurans. Ann N Y Acad Sci. 2009;1163:75–82. doi: 10.1111/j.1749-6632.2008.03618.x. [DOI] [PubMed] [Google Scholar]
- 32.Simmaco M, Mignogna G, Barra D, Bossa F. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J Biol Chem. 1994;269:11956–11961. [PubMed] [Google Scholar]
- 33.Islas-Rodriguez AE, Marcellini L, Orioni B, Barra D, Stella L, Mangoni ML. Esculentin 1-21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J Pept Sci. 2009;15:607–614. doi: 10.1002/psc.1148. [DOI] [PubMed] [Google Scholar]
- 34.Luca V, Stringaro A, Colone M, Pini A, Mangoni ML. Esculentin(1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa . Cell Mol Life Sci. 2013;70:2773–2786. doi: 10.1007/s00018-013-1291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock RE, Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett. 2002;206:143–149. doi: 10.1111/j.1574-6968.2002.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 36.Mangoni ML, Shai Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: similarities and differences in cell specificities and modes of action. Cell Mol Life Sci. 2011;68:2267–2280. doi: 10.1007/s00018-011-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy SB. The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother. 2002;49:25–30. doi: 10.1093/jac/49.1.25. [DOI] [PubMed] [Google Scholar]
- 38.Huang LC, Redfern RL, Narayanan S, Reins RY, McDermott AM. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrob Agents Chemother. 2007;51:3853–3860. doi: 10.1128/AAC.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 40.O’Callaghan RJ, Engel LS, Hobden JA, Callegan MC, Green LC, Hill JM. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest Ophthalmol Vis Sci. 1996;37:534–543. [PubMed] [Google Scholar]
- 41.Hobden JA, Masinick SA, Barrett RP, Hazlett LD. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci. 1995;36:1107–1114. [PubMed] [Google Scholar]
- 42.Hazlett LD, Moon MM, Strejc M, Berk RS. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987;28:1978–1985. [PubMed] [Google Scholar]
- 43.Cole N, Hume E, Khan S, Krockenberger M, Thakur A, Husband AJ, Willcox MD. Interleukin-4 is not critical to pathogenesis in a mouse model of Pseudomonas aeruginosa corneal infection. Curr Eye Res. 2005;30:535–542. doi: 10.1080/02713680590968583. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Satani D, Patel A, Vhankade R. Colored cosmetic contact lenses: an unsafe trend in the younger generation. Cornea. 2012;31:777–779. doi: 10.1097/ICO.0b013e31823cbe9c. [DOI] [PubMed] [Google Scholar]
- 45.Knauf HP, Silvany R, Southern PM, Jr, Risser RC, Wilson SE. Susceptibility of corneal and conjunctival pathogens to ciprofloxacin. Cornea. 1996;15:66–71. doi: 10.1097/00003226-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Estrellas PS, Jr, Alionte LG, Hobden JA. A Pseudomonas aeruginosa strain isolated from a contact lens-induced acute red eye (CLARE) is protease-deficient. Curr Eye Res. 2000;20:157–165. doi: 10.1076/0271-3683(200003)2031-9FT157. [DOI] [PubMed] [Google Scholar]
- 47.Pinna A, Usai D, Sechi LA, Molicotti P, Zanetti S, Carta A. Detection of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens-associated corneal ulcers. Cornea. 2008;27:320–326. doi: 10.1097/ICO.0b013e31815c5a3f. [DOI] [PubMed] [Google Scholar]
- 48.Ormerod LD, Smith RE. Contact lens-associated microbial keratitis. Arch Ophthalmol. 1986;104:79–83. doi: 10.1001/archopht.1986.01050130089027. [DOI] [PubMed] [Google Scholar]
- 49.Mannis MJ. The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc. 2002;100:243–271. [PMC free article] [PubMed] [Google Scholar]
- 50.Twining SS, Kirschner SE, Mahnke LA, Frank DW. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Invest Ophthalmol Vis Sci. 1993;34:2699–2712. [PubMed] [Google Scholar]
- 51.Chin GJ, Marx J. Resistance to antibiotics. Science. 1994;264:359. doi: 10.1126/science.264.5157.359. [DOI] [PubMed] [Google Scholar]
- 52.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106:1319–1323. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 53.Cullor JS, Mannis MJ, Murphy CJ, Smith WL, Selsted ME, Reid TW. In vitro antimicrobial activity of defensins against ocular pathogens. Arch Ophthalmol. 1990;108:861–864. doi: 10.1001/archopht.1990.01070080105044. [DOI] [PubMed] [Google Scholar]
- 54.Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang FD, Woodruff E, Mohrmann R, Broadie K. Rolling blackout is required for synaptic vesicle exocytosis. J Neurosci. 2006;26:2369–2379. doi: 10.1523/JNEUROSCI.3770-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwab IR, Dries D, Cullor J, Smith W, Mannis M, Reid T, Murphy CJ. Corneal storage medium preservation with defensins. Cornea. 1992;11:370–375. doi: 10.1097/00003226-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Sousa LB, Mannis MJ, Schwab IR, Cullor J, Hosotani H, Smith W, Jaynes J. The use of synthetic Cecropin (D5C) in disinfecting contact lens solutions. CLAO J. 1996;22:114–117. [PubMed] [Google Scholar]
- 58.Nos-Barbera S, Portoles M, Morilla A, Ubach J, Andreu D, Paterson CA. Effect of hybrid peptides of cecropin A and melittin in an experimental model of bacterial keratitis. Cornea. 1997;16:101–106. doi: 10.1097/00003226-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 59.McDermott AM, Rich D, Cullor J, Mannis MJ, Smith W, Reid T, Murphy CJ. The in vitro activity of selected defensins against an isolate of Pseudomonas in the presence of human tears. Br J Ophthalmol. 2006;90:609–611. doi: 10.1136/bjo.2005.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDermott AM, Redfern RL, Zhang B. Human beta-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 62.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/S0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 64.Santos CM, Kumar A, Kolar SS, Contreras-Caceres R, McDermott A, Cai C. Immobilization of antimicrobial peptide IG-25 onto fluoropolymers via fluorous interactions and click chemistry. ACS Appl Mater Interfaces. 2013;5:12789–12793. doi: 10.1021/am404591n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar A, Kolar SS, Zao M, McDermott AM, Cai C. Localization of antimicrobial peptides on polymerized liposomes leading to their enhanced efficacy against Pseudomonas aeruginosa . Mol Bio Syst. 2011;7:711–713. doi: 10.1039/c0mb00207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willcox MD, Hume EB, Aliwarga Y, Kumar N, Cole N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J Appl Microbiol. 2008;105:1817–1825. doi: 10.1111/j.1365-2672.2008.03942.x. [DOI] [PubMed] [Google Scholar]
- 67.Dutta D, Cole N, Kumar N, Willcox MD. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Invest Ophthalmol Vis Sci. 2013;54:175–182. doi: 10.1167/iovs.12-10989. [DOI] [PubMed] [Google Scholar]
- 68.Cole N, Hume EB, Vijay AK, Sankaridurg P, Kumar N, Willcox MD. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci. 2010;51:390–395. doi: 10.1167/iovs.09-4068. [DOI] [PubMed] [Google Scholar]
- 69.Conlon JM, Sonnevend A, Patel M, Al-Dhaheri K, Nielsen PF, Kolodziejek J, Nowotny N, Iwamuro S, Pal T. A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica . Regul Pept. 2004;118:135–141. doi: 10.1016/j.regpep.2003.12.003. [DOI] [PubMed] [Google Scholar]