SUMMARY
Low energy states delay aging in multiple species, yet mechanisms coordinating energetics and longevity across tissues remain poorly defined. The conserved energy sensor AMP-activated protein kinase (AMPK) and its corresponding phosphatase calcineurin modulate longevity via the CREB regulated transcriptional coactivator (CRTC)-1 in C. elegans. We show that CRTC-1 specifically uncouples AMPK/calcineurin-mediated effects on lifespan from pleiotropic side effects by reprogramming mitochondrial and metabolic function. This pro-longevity metabolic state is regulated cell-nonautonomously by CRTC-1 in the nervous system. Neuronal CRTC-1/CREB regulates peripheral metabolism antagonistically with the functional PPARα ortholog, NHR-49, drives mitochondrial fragmentation in distal tissues, and suppresses the effects of AMPK on systemic mitochondrial metabolism and longevity via a cell-nonautonomous catecholamine signal. These results demonstrate that while both local and distal mechanisms combine to modulate aging, distal regulation overrides local contribution. Targeting central perception of energetic state is therefore a potential strategy to promote healthy aging.
INTRODUCTION
An organism's energy status is tightly coupled to its rate of aging, as low energy conditions increase longevity and disease resistance across the evolutionary spectrum (Burkewitz et al., 2014). Mechanisms that communicate energetic state between tissues to coordinate organismal health and longevity remain poorly understood, however, and must be defined in order to translate these effects to human therapeutics. AMP-activated protein kinase (AMPK) is a conserved energy sensor activated by increases in the AMP/ADP:ATP ratio, which signals low energy charge (Hardie et al., 2012). AMPK upregulates catabolic processes and shuts down energy-consuming processes to restore cellular energy homeostasis (Hardie et al., 2012). AMPK is also pro-longevity; activating AMPK in C. elegans and Drosophila increases healthy lifespan and mimics a low energy state in well-fed animals (Apfeld et al., 2004; Stenesen et al., 2013). C. elegans lacking AMPK activity fail to respond to low energy conditions, such as dietary restriction, that extend wild type lifespan (Burkewitz et al., 2014).
Both AMPK and its effects on aging are conserved across eukaryotes (Hardie et al., 2012). Metformin, an indirect AMPK agonist, promotes healthy aging in C. elegans (Onken and Driscoll, 2010) and mice (Martin-Montalvo et al., 2013). Deregulation of AMPK results in age-onset human pathologies including cancer and neurodegenerative diseases (Hardie et al., 2012). AMPK signaling therefore plays a critical role linking energetics to pathology, making it an attractive target to treat or prevent multiple age-related diseases.
AMPK has both cell-autonomous effects on energetics through direct phosphorylation of metabolic effectors (Hardie et al., 2012), and cell-nonautonomous effects via integration of hormonal and neuroendocrine signals (Dagon et al., 2012; Lerner et al., 2009; Minokoshi et al., 2004). The extent to which AMPK promotes longevity locally via regulation of metabolism or distally via a secondary signal remains unclear. Additionally, AMPK's pro-longevity effects may not be universal in all tissues, as AMPK activation in certain cell types appears to increase risk for some diseases, and pleiotropic effects of AMPK activation unrelated to aging have detrimental physiological consequences (Burkewitz et al., 2014). Identifying downstream targets and processes regulated by AMPK that specifically mediate its role in longevity would therefore enhance our capacity to harness the link between energetics and aging for treatment of age-related pathologies.
Previously we identified the cyclic AMP-responsive element binding protein (CREB)-regulated transcriptional coactivator (CRTC)-1 as a critical longevity target of AMPK and calcineurin in C. elegans (Mair et al., 2011). AMPK and calcineurin antagonistically regulate CRTC-1 phosphorylation status, thereby modulating its activity and effect on aging. CRTCs are transcriptional coactivators first discovered in mammals for their ability to bind CREB and regulate its transcriptional activity (Altarejos and Montminy, 2011). Mammals possess 3 CRTC family members that act in distinct tissues, including neurons (CRTC1/2), liver (CRTC2) and adipose tissue (CRTC3), and aberrant regulation of CRTCs is implicated in multiple chronic diseases, including obesity, metabolic disease and neurodegeneration (Altarejos and Montminy, 2011). C. elegans possess a single, highly conserved CRTC family member, CRTC-1. AMPK phosphorylates CRTC-1 directly, promoting 14-3-3 binding, cytosolic sequestration and inactivation. Blocking phosphorylation of CRTC-1 at conserved AMPK target sites, serines 76 and 179, renders it refractory to AMPK regulation and constitutively nuclear. CRTC-1S76A,S179A blocks lifespan extension by AMPK activation or inhibition of the corresponding phosphatase calcineurin (Mair et al., 2011).
In this study we demonstrate that CRTC-1 specifically mediates the longevity output of AMPK. We perform transcriptomic analysis to elucidate genes downstream of AMPK/CRTC-1 signaling, which couple specifically to lifespan regulation. Through this approach we identify coordination of mitochondrial metabolism by CRTC-1 and the nuclear hormone receptor NHR-49, a functional PPARα ortholog (Van Gilst et al., 2005). Notably, we demonstrate that these opposing transcriptional effectors act in the nervous system to regulate both longevity and systemic changes in metabolic transcription. NHR-49 is required for AMPK/calcineurin-mediated longevity, and limiting NHR-49 function to neurons is sufficient to mediate both longevity and regulation of metabolic genes in peripheral tissues. In addition, we demonstrate that neuronal CRTC-1 modulates AMPK/calcineurin-mediated longevity cell-nonautonomously via regulation of the neurotransmitter/hormone octopamine. Neuron-specific activation of CRTC-1, like nhr-49 loss, suppresses AMPK/calcineurin-mediated longevity and upregulates expression of key enzymes involved in octopamine synthesis. Correspondingly, neuronal CRTC-1S76A,S179A has no effect on longevity in mutants deficient in octopamine synthesis. Together these data challenge the current paradigm that AMPK, CRTC-1 and NHR-49 act cell-autonomously to regulate metabolism and longevity, and instead highlight their distinct role in communicating perception of energy status in neurons to systemic regulation of metabolism and lifespan.
RESULTS
CRTC-1 is specific to AMPK longevity
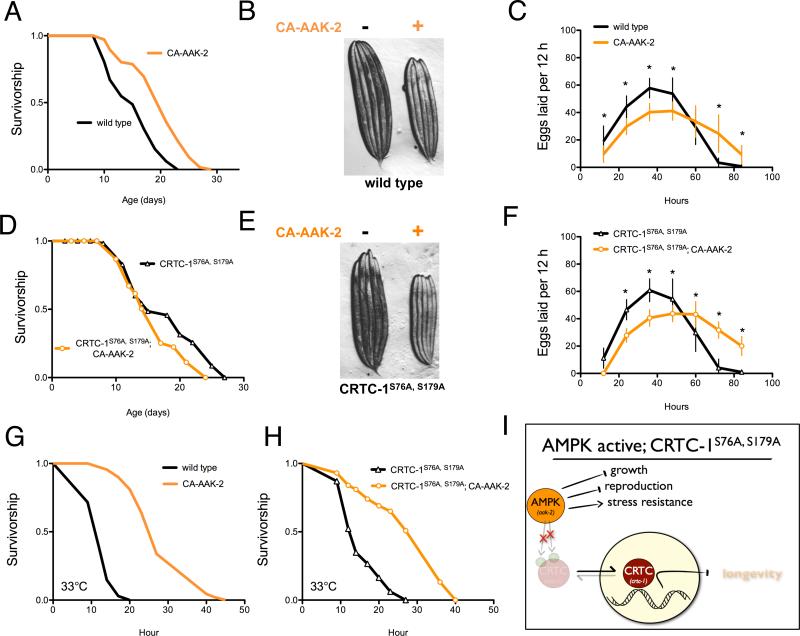
We generated a C. elegans transgenic strain expressing a truncated AMPK α2 catalytic subunit (AAK-2), which results in increased T172 phosphorylation, and constitutively active (CA) AMPK (Mair et al., 2011). CA-AAK-2 increases C. elegans lifespan (Figure 1A), yet induces detrimental pleiotropic side effects including small body size and reduced reproductive capacity (Figure 1B, 1C). As shown previously, CRTC-1S76A,S179A abolishes lifespan extension from AAK-2 activation (Figure 1D) without altering AMPK activity (Figure S1). In contrast to its role in longevity, CRTC-1S76A,S179A does not suppress CA-AAK-2 mediated effects on growth (Figure 1E) or reproductive period (Figure 1F, Figure S1). The physiological effects of CRTC-1S76A,S179A in CA-AAK-2 animals are therefore specific to longevity and do not extend to non-aging related traits.
Figure 1. CRTC-1 uncouples AMPK-mediated longevity from other pleiotropic phenotypes.
(A) AMPK activation (CA-AAK-2) extends lifespan in a wild type C. elegans background. See Table S1 for lifespan statistics.
(B) CA-AAK-2 suppresses growth. Image of 5 wild type (left) and 5 CA-AAK-2 (right) adult worms.
(C) Eggs laid per worm over successive 12 h periods. Data are mean ± SD for n=25-30 animals; * denotes p<10−4 via t-test.
(D) CA-AAK-2 fails to promote longevity in a CRTC-1S76A,S179A mutant background.
(E) CRTC-1S76A,S179A does not suppress AMPK-mediated effects on growth.
(F) Eggs laid per worm over successive 12 h periods (data are mean ± SD for n=19–30 animals; * denotes p<10−4 via t-test.
(G and H) Survival curves of AMPK and CRTC-1S76A,S179A transgenic animals exposed to heat stress at 33°C. N=60-100 worms. p<10−5 via Log-rank (Mantel-Cox) analysis.
(I) CRTC-1 is a longevity-specific AMPK target that uncouples growth, reproduction, and stress resistance from lifespan extension.
Increased longevity is often coupled to increased stress resistance. To determine if this was the case for AMPK, we examined the effect of CA-AAK-2 on resistance to heat stress at 33°C. C. elegans lacking aak-2 are sensitive to heat stress compared to wild type (Apfeld et al., 2004). Conversely, activation of AMPK promotes heat resistance, as C. elegans expressing CA-AAK-2 show a 125% increase in median survival at 33°C compared to wild type animals (Figure 1G; 27 and 12 h, respectively). However, unlike its effect on AMPK longevity (Figure 1D), CRTC-1S76A,S179A does not suppress heat resistance conferred by CA-AAK-2 (Figure 1H). Mechanisms that protect CA-AAK-2 animals from heat stress are thus separable from those which promote lifespan extension, and CRTC-1 represents a molecular switch that uncouples the longevity effects of AMPK from pleiotropy unrelated to aging (Figure 1I).
AMPK and CRTC-1 coordinate mitochondrial metabolism to regulate longevity
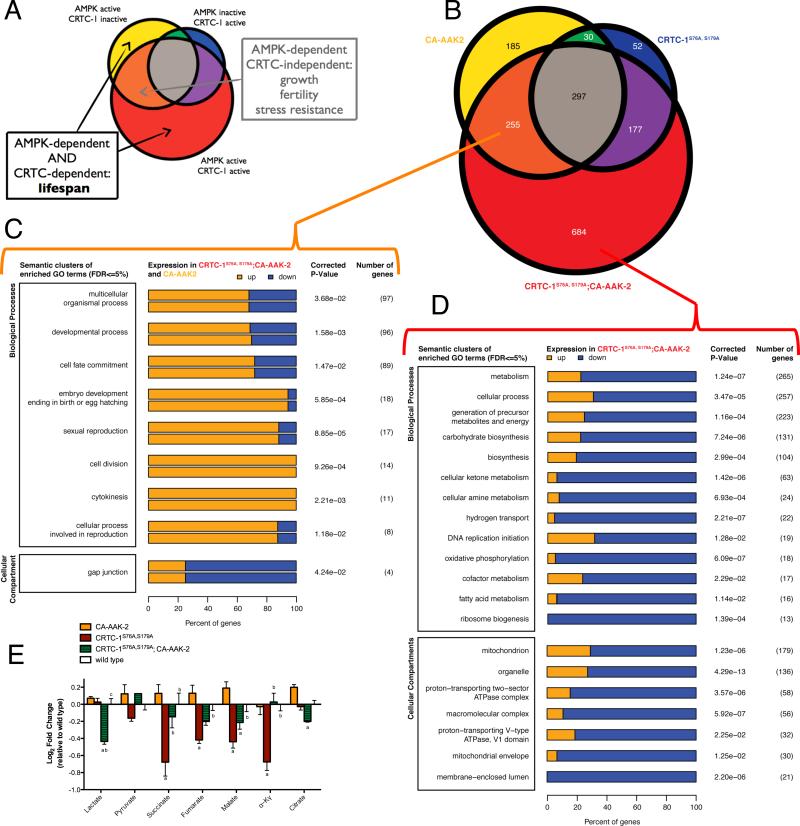
We reasoned that CRTC-1 could be leveraged to identify the mechanisms by which AMPK increases lifespan. As CRTC-1S76A,S179A suppresses only the longevity effects of AMPK activation, genes differentially expressed in CA-AAK-2 animals in a CRTC-1 dependent manner would be enriched for functions specific to AMPK-mediated longevity (Figure 1I). We defined CRTC-1 dependent genes as those differentially regulated in CA-AAK-2 (Figure 2A, Yellow), or in CRTC-1S76A,S179A; CA-AAK-2 double-transgenics (Figure 2A, Red), but not in both (Figure 2A, Orange).
Figure 2. Leveraging CRTC-1 to identify longevity-specific processes downstream of AMPK reveals a critical role for mitochondrial metabolism.
(A) Schematic Venn diagram illustrating how CRTC-1-specific gene expression filters AMPK-induced changes associated with longevity from those involved in other pleiotropic phenotypes.
(B) Venn diagram representing the number of differentially expressed (DE) genes identified through RNA-Seq analysis from each indicated genetic background relative to wild type controls. See Table S2 for complete list of DE genes.
(C and D) Clusters of enriched GO biological processes and cell compartments among the DE genes involved in CRTC-1 independent phenotypes (C, orange) or unique to the CRTC-1S76A,S179A; CA-AAK-2 worms (D, red). Bars represent the percentage of genes within that category that are up- (orange) or downregulated (blue). The number of genes annotated within a cluster is tabulated, along with the smallest multiple-testing corrected p-value for the observed enrichment attributed to a term within the cluster. See also Figure S2 and Table S3.
(E) Metabolomic analyses of transgenic strains to measure levels of organic acids (E), acylcarnitines, and amino acids (Figure S2). Two-way ANOVAs were performed with a Sidak multiple comparisons test after metabolite levels were normalized to total protein. Data are mean ± SEM of n=2-5 replicates per metabolite in each group. ap≤0.05 vs. CA-AAK2, bp≤0.05 vs. CRTC-1S76A,S179A, cp≤0.05 vs. CRTC-1S76A,S179A; CA-AAK-2. See also Table S4.
We performed RNA-Seq analyses and identified 1680 genes differentially expressed in worms with activated AAK-2, activated CRTC-1S76A,S179A, or double-transgenics, relative to wild type animals (Figures 2B, S2A, Table S2). AMPK induces small body size, reduced reproduction and stress resistance independent of CRTC-1 (Figure 1), thus we predicted that AMPK-dependent/CRTC-1-independent genes (Figure 2B, orange region) would be associated with these phenotypes. Supporting this hypothesis, the gene ontology (GO) terms most enriched among those genes include processes involving germline differentiation, growth/development and reproduction (Figure 2C, Table S3). Importantly, this tight association between phenotypes and functional enrichments within the transcriptomic changes validated our hypothesis that CRTC-1 could be used to filter out pleiotropic effects of AMPK activation unrelated to aging.
To identify processes specifically coupled to longevity, we focused on transcriptional changes induced by AMPK that are dependent on CRTC-1 activation status (Figure 2A). Of the 869 genes differentially expressed by AAK-2 activation in a CRTC-1 dependent manner, over 75% are differentially expressed when both AMPK and CRTC-1 are active (Figure 2B, red region). These genes are highly enriched for processes associated with metabolism, and more specifically, processes localized to mitochondria (Figure 2D, Table S3). We examined the directionality of CRTC-1 dependent gene expression changes, and found that suppression of AMPK longevity is associated with a broad downregulation of mitochondrial metabolic processes (Figure 2D).
To determine whether the transcriptional changes in metabolic genes ultimately alter metabolic function we performed metabolomic analyses. We measured organic acids, amino acids and acylcarnitines, which represent metabolites of the major energy producing pathways, in CAAAK-2 animals with and without CRTC-1S76A,S179A. While there are few significant differences between CA-AAK-2 and CRTC-1S76A,S179A; CA-AAK-2 in amino acids or acylcarnitines (Figure S2C-D, Table S4), the data show widespread differential regulation of TCA cycle intermediates (Figure 2E). Congruent with the changes observed at the transcriptional level, the TCA cycle intermediates exhibit a pattern consistent with altered mitochondrial metabolism being causal to AMPK/CRTC-1 regulation of longevity: while TCA intermediates and associated organic acid levels are maintained or increased by AMPK activation, CRTC-1S76A,S179A opposes these effects for several organic acids, including malate, citrate and lactate (Figure 2E). These metabolomic data support a role for AAK-2 and CRTC-1 in coordinating central metabolic processes, and highlight a new role for CRTCs in mediating transcriptional links between AMPK status and mitochondrial metabolism. Although AMPK is a known sensor and regulator of mitochondrial function and biogenesis (Hardie et al., 2012), these data now specifically couple these processes to the role of AMPK in longevity assurance.
Transcriptional regulation of metabolism is required for AMPK and calcineurin longevity
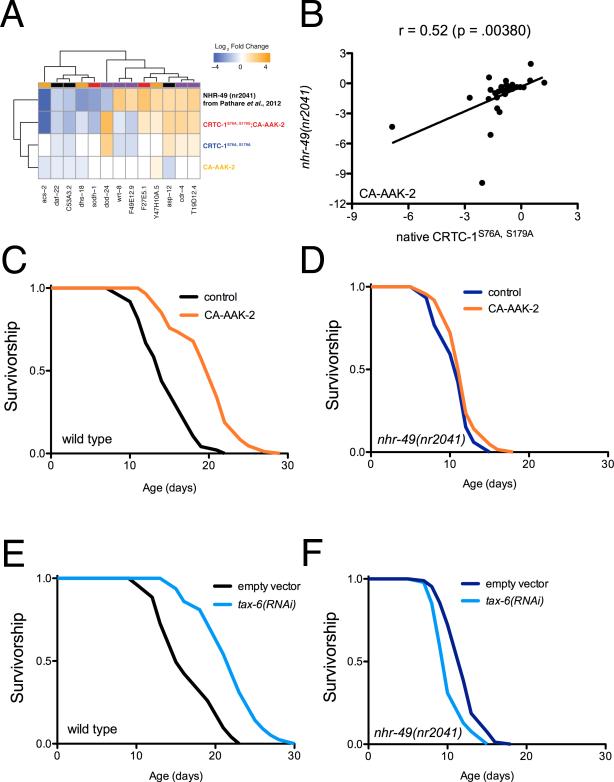
To determine if the metabolic effects of CRTC-1 are causal to AMPK longevity we searched for known alternative interventions that broadly regulate cellular metabolic processes to examine their effects on lifespan. Like AMPK, the nuclear hormone receptor and functional PPARαortholog, NHR-49, activates during low energy status such as fasting, and transcriptionally promotes genes required for mitochondrial function (Pathare et al., 2012). We compared genes differentially expressed in CRTC-1S76A,S179A; CA-AAK-2 transgenic animals with genes previously reported to be differentially expressed in nematodes lacking functional nhr-49 (Pathare et al., 2012). Of the 47 NHR-49 dependent genes identified by Pathare et al., we observe 30% overlap (Χ2=13.45, p=10−5), suggesting that NHR-49 and CRTC-1 coordinately regulate shared metabolic targets (Figure 3A; Table S5). We selected a candidate list of 29 metabolic genes, including genes regulated by CRTC-1 from our RNA-Seq dataset and by NHR-49 from previously published data (Table S6), and validated the high degree of correlation between CRTC-1 activation and NHR-49 loss of function in a CA-AAK-2 background relative to wild type (Figure 3B; p<0.01). When AMPK is activated, loss of nhr-49 mirrors activation of CRTC-1 to promote a transcriptional reprogramming of metabolic genes.
Figure 3. NHR-49 is required for AMPK- and calcineurin-mediated longevity.
(A) Heat map of genes differentially expressed in CRTC-1S76A,S179A; CA-AAK-2 and nhr-49(nr2041) worms, demonstrating significant overlap in gene expression patterns. See also Table S5.
(B) Mean mRNA expression levels (average log2 of fold change relative to wild type worms) of 29 metabolic genes. In a CA-AAK-2 background, native CRTC-1S76A,S179A (crtc-1 promoter) correlates with whole-organism loss of NHR-49 (Pearson correlation coefficient, r), validating the comparison made in (A). Note r=0.66; p≤0.0001 after 10% winsorization of strong outliers. See Table S6.
(C and D) CA-AAK-2 extends lifespan in a wild type background (C), but not in the absence of NHR-49 function (D). See Table S1 for lifespan statistics.
(E and F) tax-6 RNAi extends lifespan in a wild type background (E), but not in the absence of NHR-49 function (F). The genetic background in B – F is noted next to the origin.
If transcriptional regulation of metabolism by CRTC-1 is causal to AMPK lifespan extension, we hypothesized that AMPK should also fail to promote longevity in nhr-49 mutants, since they recapitulate similar transcriptional changes in metabolic processes. In support of this hypothesis, an nhr-49(nr2041) deletion allele suppresses lifespan extension via CA-AAK-2 (Figure 3C-D). Our previous work established that AMPK and calcineurin mediate longevity through a shared signaling pathway that converges on CRTC-1. Calcineurin, a protein phosphatase, directly opposes AMPK by dephosphorylating and activating CRTC-1. RNAi-mediated knockdown of tax-6, the catalytic subunit of calcineurin, mimics AMPK activation by increasing C. elegans lifespan in a CRTC-1 dependent manner (Mair et al., 2011) and additionally activates expression of the NHR-49 dependent target, acs-2 (Figure S3). Strikingly, tax-6 RNAi also requires intact NHR-49 function to promote longevity (Figure 3 E-F). These results suggest that AMPK/calcineurin signaling promotes a shift in metabolic programs by orchestrating the activity of opposing transcriptional effectors, CRTC-1 and NHR-49, and that this metabolic switch is required for longevity.
CRTC-1 acts through CREB in neurons to mediate longevity
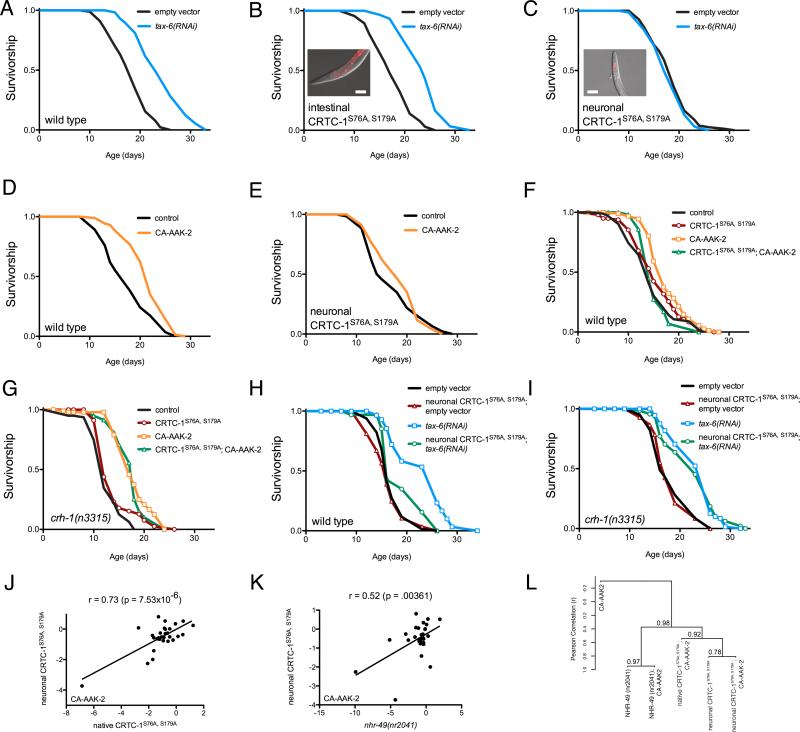
Both aak-2 and nhr-49 are expressed ubiquitously (Mair et al., 2011; Van Gilst et al., 2005), and are believed to function as cell-autonomous regulators of metabolism. In contrast, crtc-1 expression is limited to the intestine and neurons in C. elegans (Mair et al., 2011). We reasoned that CRTC-1 may directly regulate transcription of genes involved in metabolism in the intestine, one of the major organs of cellular metabolic activity and fat storage in C. elegans, and that this effect may be sufficient to systemically modulate longevity. To test this hypothesis we expressed CRTC-1S76A,S179A from the ges-1 promoter, limiting its expression to intestinal cells. Surprisingly, intestinal expression of CRTC-1S76A,S179A has no effect on tax-6 RNAi-mediated longevity (Figure 4A-B). Ruling out the intestine as the site of action, we examined the role of neuronal CRTC-1 in AMPK/calcineurin-mediated longevity. Expressing CRTC-1S76A,S179A from the pan-neuronal rab-3 promoter (Figure 4C, inset) fully suppresses lifespan extension by both tax-6 RNAi (Figure 4C) and AAK-2 activation (Figure 4D-E). Upon finding that selective CRTC-1 activation in neurons is sufficient for its effects on lifespan, we asked whether activating AMPK in select tissues is also sufficient to promote longevity. We expressed CA-AAK-2 from pan-neuronal, muscle and intestinal-specific promoters. AMPK activation is not sufficient for longevity in any of the individual tissues tested (Figure S4A), suggesting this longevity mechanism in C. elegans requires local AMPK-mediated programming of mitochondrial function in multiple tissues. Taken together these results indicate that CRTC-1 activity in neurons cell-nonautonomously modulates AMPK/calcineurin-mediated longevity. Moreover, signals downstream of neuronal CRTC-1 dominantly override the effects AMPK exerts locally in peripheral tissues.
Figure 4. Neuronal CRTC-1/CRH-1 activation suppresses longevity downstream of AMPK and calcineurin signaling and cell-nonautonomously regulates metabolic transcription.
(A) tax-6 RNAi increases longevity. See Table S1 for lifespan statistics. The genetic background in A – I is noted next to the origin.
(B) Intestine-specific CRTC-1S76A,S179A (ges-1 promoter) fails to suppress tax-6 RNAi longevity. Inset: image of intestine-specific tdTOMATO-tagged CRTC-1S76A,S179A; scale bar = 100 μm.
(C) Neuronal-specific (rab-3 promoter) CRTC-1S76A,S179A fully suppresses tax-6 RNAi-mediated longevity. Inset: image of neuron-specific tdTOMATO-tagged CRTC-1S76A,S179A; scale bar = 100 μm.
(D and E) CA-AAK-2 extends lifespan in a wild type background (D), but neuronal CRTC-1S76A,S179A suppresses AMPK-mediated longevity (E).
(F and G) CA-AAK-2 extends lifespan and this effect is blocked by expressing CRTC-1S76A,S179A from its native promoter (F). In crh-1 null mutants, CRTC-1 activation fails to suppress AMPK-mediated longevity (G).
(H and I) Neuron-specific activation of CRTC-1 S76A,S179A suppresses longevity mediated by tax-6 RNAi (H), but neuronal CRTC-1S76A,S179A requires intact crh-1 function to mediate its effects on aging (I).
(J and K) Expression levels of 29 metabolic genes in CA-AAK-2 animals reveal that the effects of neuron-specific CRTC-1S76A,S179A correlate strongly with expression of CRTC-1S76A,S179A from its native promoter (J); and neuron-specific CRTC-1S76A,S179A activation correlates with the transcriptional effects of loss of NHR-49 function (K). r and p values were derived by Pearson correlation. See also Table S6.
(L) Dendrogram summarizing similarities in metabolic gene expression between AMPK, CRTC-1 and NHR-49 mutants. Strains were clustered by their pair-wise Pearson correlations using Ward's minimum variance method. Vertical heights of branches indicate the degree of correlation (r; y-axis). Multiscale bootstrap resampling p values on each branch were calculated via pvclust R package [http://www.sigmath.es.osaka-u.ac.jp/shimo-lab/prog/pvclust/].
CRTCs lack DNA-binding activity and depend on partner transcription factors for recruitment to DNA in order to stimulate gene transcription (Altarejos and Montminy, 2011). Though first identified as CREB modulators, CRTCs also bind and regulate other bZIP transcription factor family members. To determine whether the effects of neuronal CRTC-1 on aging occur via CREB, we tested whether CREB was necessary for longevity suppression by CRTC-1S76A,S179A. In animals lacking CRH-1, the C. elegans CREB ortholog, CRTC-1S76A,S179A expressed under its endogenous promoter no longer suppressed CA-AAK-2 mediated longevity (Figure 4F-G). Additionally, neuronal expression of CRTC-1S76A,S179A in crh-1 null animals had no effect on lifespan extension by tax-6 RNAi (Figure 4H-I). Lifespan extension via AMPK/calcineurin therefore requires inhibition of the CRTC-1/CRH-1 transcriptional complex in neurons.
Neuronal CRTC-1 cell-nonautonomously regulates metabolic genes
Since CRTC-1 regulates longevity through a systemic metabolic program (Figure 2) and neuron-limited CRTC-1 activation is sufficient to suppress longevity, we sought to determine if neuronal CRTC-1 is sufficient to produce the lifespan-related effects on metabolic transcription. Analyzing the same panel of 29 metabolic genes used previously (Figure 3B), we found that limiting CRTC-1S76A,S179A expression to neurons recapitulates the effects on peripheral metabolic genes seen in animals expressing CRTC-1S76A,S179A from its native promoter (Figure 4J; p<0.0001).
Therefore, similarly to lifespan, CRTC-1 regulates metabolism cell-nonautonomously from neurons. Finally, we determined that neuron-specific activation of CRTC-1 mimics genetic deletion of nhr-49 regarding peripheral expression of genes involved in cellular metabolism (Figure 4K; p<0.01). Despite the presence of CA-AAK-2 in every background, AAK-2 activated animals alone exhibit no correlation in gene expression with any of the double transgenics, indicating these transcriptional effects are all attributable to NHR-49 and CRTC-1 (Figure S4BD; p>0.05). Taken together, the striking overlap between gene expression profiles (Figure 4L; Table S6) reveals an antagonistic relationship between metabolic programs regulated by NHR-49 and neuronal CRTC-1.
NHR-49 regulates metabolism and lifespan cell-nonautonomously
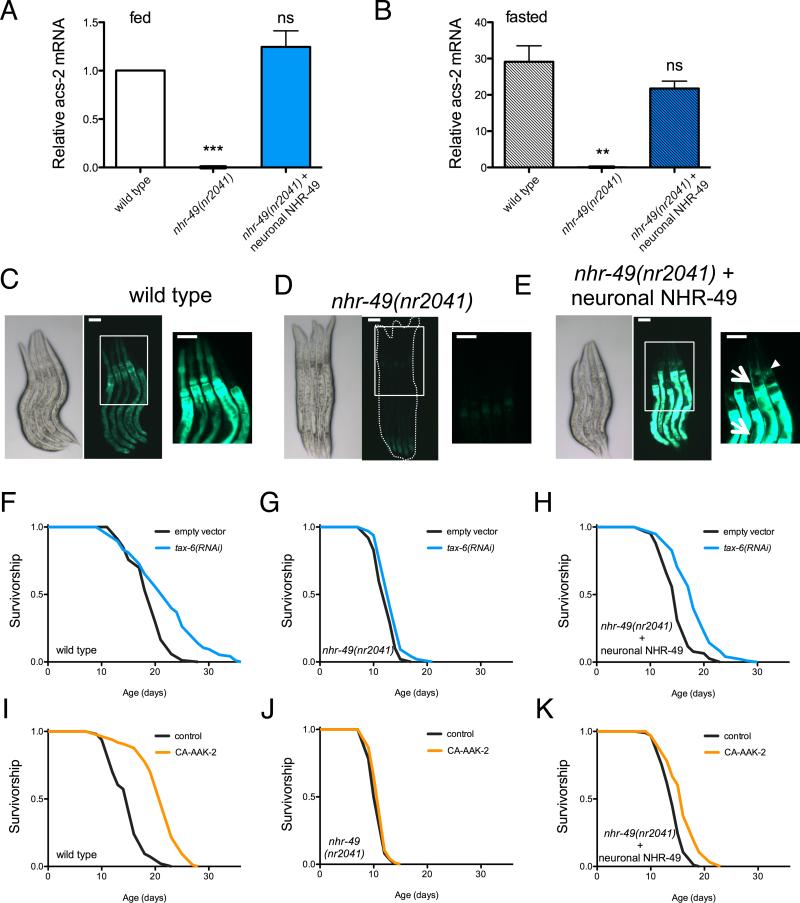
NHR-49 has previously been characterized as a functional ortholog of mammalian PPARα, as nhr-49 mutants fail to activate mitochondrial fatty acid oxidation (FAO) genes during starvation (Van Gilst et al., 2005). However, whether this effect is cell autonomous is unknown, as the NHR-49 DNA-binding motif has not been determined and direct targets of NHR-49 remain elusive. To determine whether NHR-49 can mediate lifespan and metabolism cell-nonautonomously we selectively restored NHR-49 function to neurons in an nhr-49 null background by driving expression with the rab-3 promoter. As previously described, C. elegans subjected to 24 hours of fasting show strong upregulation of a key gene involved in beta-oxidation, acyl-CoA synthetase (ACS)-2, which is dependent on nhr-49 (Figure 5A). Supporting a role for NHR-49 beyond cell-autonomous regulation of metabolic gene expression, neuronal rescue of NHR-49 is sufficient to restore both basal acs-2 expression (Figure 5A) and the induction of acs-2 by fasting (Figure 5B). Since mRNA measurements were obtained using whole-animal preparations, we generated animals expressing GFP under control of the acs-2 promoter to examine tissue specific induction. Fasting for 24 h induces expression of GFP in multiple tissues, including pharynx, muscle and intestine (Figure 5C). This induction is abrogated by loss of nhr-49 (Figure 5D). Remarkably, neuronal expression of nhr-49 is sufficient to restore induction of acs-2 in both neurons and distal tissues, including muscle and intestine, but not pharynx (Figure 5E).
Figure 5. NHR-49 regulates mitochondrial metabolism and longevity cell-nonautonomously from neurons.
(A and B) Analysis of acs-2 transcript levels by RT-PCR in L4/young adult worms fed (A) and fasted 16 h (B). Data are mean ± SEM of 3-4 independent experiments. By 1-sample (A) or 2-sample (B) t-test relative to fed wild type animals, *** denotes p<0.001; ** = p<0.01; ns = p> 0.05).
(C – E) Brightfield (left) and fluorescence (middle and right panels) imaging of L4-stage 16 h fasted worms expressing GFP driven by the acs-2 promoter. (C) Fasting activates the acs-2 promoter ubiquitously in C. elegans (middle panel), and higher magnification reveals strongest expression in the intestine and pharynx (right panel). (D) nhr-49 mutants fail to activate acs-2. (E) Neuron-limited rescue of NHR-49 restores acs-2 levels in neurons (arrowhead) and peripheral tissues (arrows). Boxes outline areas magnified in the right panels. Scale bars represent 50 μm.
(F – I) Survival analysis demonstrating that tax-6 RNAi (F) and CA-AAK-2 (I) extend lifespan in wild type worms, but not worms lacking nhr-49 (G, J). Restoring NHR-49 function selectively to neurons via the rab-3 promoter rescues tax-6 RNAi- (H) and CA-AAK-2-mediated longevity (K). Common genetic backgrounds are indicated next to the origin. See also Figure S5 and Table S1 for lifespan statistics.
Induction of acs-2 expression in multiple tissues lacking NHR-49 suggests alternative transcription factors might respond to signals downstream of neuronal CRTC-1. To identify transcriptional regulators in this pathway we revisited our RNA-Seq dataset and performed Hypergeometric Optimization of Motif EnRichment (HOMER) analysis of the ‘CRTC-1 dependent genes’ (Figure 2B, Red section). We identified a motif with consensus TGATAACG or CGTTATCA enriched in the putative promoter regions of genes downstream of AMPK/CRTC-1 (p=1e-25, found in 38% of targets vs. 21% of background) (Figure S5A). The motif strongly resembles the GATA-like DAE (DAF-16 Associated Element), recently identified as the binding site for PQM-1 (Tepper et al., 2013). modENCODE ChIP-Seq data also suggests that both DAF-16 and PQM-1 bind the acs-2 promoter directly. To determine whether DAF-16 can regulate expression of acs-2 we examined the effect of fasting on our acs-2P::GFP reporter under control conditions, and in animals subjected to RNAi for daf-16, along with two other transcription factors known to mediate DR longevity, skn-1 and pha-4. While inhibition of skn-1 and pha-4 does not alter induction of acs-2 by fasting, daf-16 RNAi significantly suppresses acs-2 induction (Figure S5B-C). These data therefore suggest that DAF-16 and/or PQM-1 might be acting downstream of the neuronal CRTC-1 signal to modulate metabolic gene expression.
Given the ability of NHR-49 to cell-nonautonomously regulate metabolic genes, we next asked whether, like CRTC-1, NHR-49 mediates longevity through its effects in neurons. Restoring NHR-49 function in neurons exclusively was sufficient to restore lifespan extension via both tax-6 RNAi (Figure 5F-H) and AMPK activation (Figure 5I-K) in nhr-49 deletion mutants. However, unlike the effects of CRTC-1 on AMPK/calcineurin-mediated longevity, which is specific to neurons, the role of NHR-49 is more complex, as intestinal rescue can also partially reverse suppression of tax-6 RNAi lifespan in nhr-49 deletion mutants and is sufficient to restore acs-2 induction exclusively in intestinal cells (Figure S6A-B). Additionally, while nhr-49 overexpression in intestine does not affect C. elegans longevity, overexpression of nhr-49 in neurons is sufficient to extend lifespan, further highlighting tissue-specific functions (Figure S6C-D).
Neuronal CRTC-1 cell-nonautonomously regulates mitochondrial dynamics
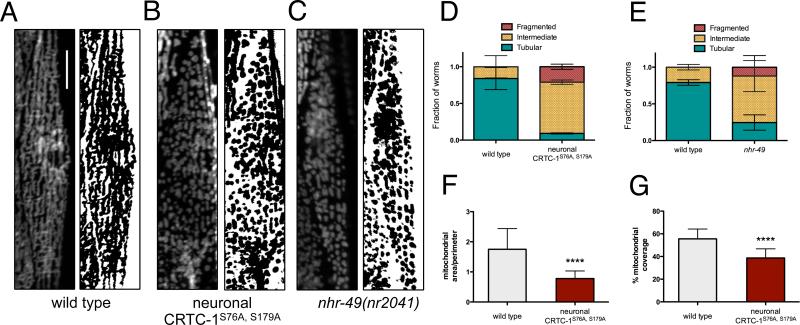
Recent studies of mitochondrial dynamics suggest that remodeling of the mitochondrial network itself may impact metabolic function (Liesa and Shirihai, 2013), and loss of NHR-49 has been shown to disrupt mitochondrial morphology and function (Pathare et al., 2012). Given neuronal CRTC-1 and NHR-49 antagonistically regulate AMPK/calcineurin-mediated longevity and metabolic processes, we explored whether changes in mitochondrial architecture were involved in the metabolic reprogramming and longevity by AMPK/CRTC-1. To observe the mitochondrial network directly in distinct tissues, we employed nematodes expressing mRFP targeted to the outer mitochondrial membrane via fusion to TOM20. Typically, the mitochondria of muscle cells of young (day 1) adult worms are fused and tubular, running parallel among the myofilaments (Figure 6A). Activation of CRTC-1 exclusively in neurons of young adult worms, however, results in significant fragmentation of the mitochondrial network in muscle cells, demonstrating a cell-nonautonomous role for CRTC-1 in regulating mitochondrial dynamics (Figure 6B). This effect is consistent with the opposing transcriptional effects of NHR-49 and neuronal CRTC-1, as loss of nhr-49 also causes mitochondrial fragmentation and altered morphology (Figure 6C-E). To quantify the degree of fragmentation in these animals, we determined the ratio of mitochondrial area to perimeter in muscle cells of neuronal CRTC-1S76A,S179A mutants and found a 56% reduction relative to control animals (Figure 6F; p<0.0001). The area of muscle cells occupied by mitochondria was also decreased 30% upon neuronal CRTC-1 activation (Figure 6G; p<0.0001), supporting CRTC-1 mediated suppression of mitochondrial function observed at the transcriptomic and metabolomic levels (Figure 2).
Figure 6. Neuronal CRTC-1 and NHR-49 regulation of mitochondrial dynamics mirrors their respective roles in longevity.
(A – C) Fluorescence imaging (left) and binary representations (right) of mitochondrial networks in body wall muscle cells of day 1 adult worms. Neuronal CRTC-1S76A,S179A (B) and nhr-49 loss-of-function (C) induce fragmentation of the mitochondrial network in muscle cells relative to wild type (A). Scale bars represent 20 μm.
(D – E) Quantification of neuronal CRTC-1S76A,S179A (D) and nhr-49 loss-of-function (E) dependent mitochondrial fragmentation in a population of worms demonstrates loss of tubular morphology (n = 3 groups of 10-17 worms; p = 0.001 by t-test).
(F) Quantification of the ratio between mitochondrial area and perimeter (Mean ± SD from muscle cells of 32 worms; p<0.0001 by t-test).
(G) Neuronal CRTC-1S76A,S179A activation decreases the area of muscle cells occupied by mitochondria (Mean ± SD from muscle cells of 32 worms; p<0.0001 by t-test).
CRTC-1 mediates lifespan via a catecholamine signal
Having determined neuronal CRTC-1 mediates longevity and mitochondrial function cell-nonautonomously, we reasoned it might regulate a signal that relays energy status from neurons to coordinate aging and metabolism in peripheral tissues. Monoamine signals, e.g., dopamine and serotonin, act in nutrient-sensing pathways to regulate behavioral and peripheral metabolic changes conserved from nematodes to humans (Ashrafi, 2007). We therefore examined whether monoamine signaling might provide a potential mechanism by which neuronal CRTC-1 could regulate longevity and mitochondrial dynamics. Monoamines are secreted via exocytosis in dense core vesicles (DCVs), which in C. elegans requires the Ca2+-dependent activator protein for secretion (CAPS), UNC-31 (Grishanin et al., 2004). Null mutation of unc-31 suppresses acs-2 expression, as determined by our acs-2P::GFP reporter strain and qRT-PCR (Figure S7A-C), suggesting secreted signals within DCVs may mediate distal metabolic gene regulation.
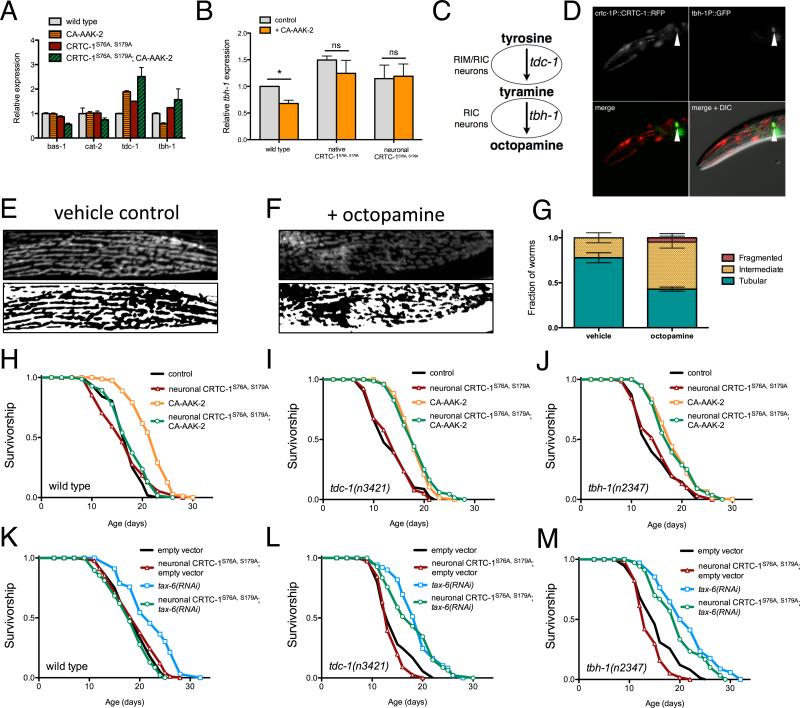
In our RNA-Seq analysis we identified multiple enzymes involved in the synthesis of biogenic amines that were differentially mediated by AMPK and CRTC-1 (Figure 7A). We further examined the genes involved in monoamine synthesis by qRT-PCR and identified a catecholamine biosynthetic enzyme, tyramine beta-hydroxylase (TBH)-1, among the genes regulated by CRTC-1 that couples to AMPK longevity (Table S2). Downregulation of tbh-1 expression by AMPK activation is attenuated by CRTC-1S76A,S179A (Figure 7B). Acting in a pathway with tyrosine decarboxylase (TDC)-1, TBH-1 catalyzes the synthesis of octopamine, which functions as the invertebrate noradrenaline equivalent (Figure 7C). Analysis of the tdc-1 and tbh-1 promoters revealed putative cAMP response elements (not shown), suggesting that CRTC-1/CRH-1 may directly regulate transcription of these genes. As previously reported, expressing GFP downstream of the tbh-1 promoter results in expression exclusively in two octopaminergic RIC neurons (Alkema et al., 2005), notably CRTC-1 also localizes to RIC neurons (Figure 7D) and thus may be capable of directly regulating octopamine signaling. We therefore hypothesized that octopamine may play a role in intercellular AMPK/CRTC-1 longevity signaling.
Figure 7. Neuronal CRTC-1 regulates longevity and mitochondrial function cell-nonautonomously through catecholamine signaling.
(A) Normalized read counts for enzymes involved in synthesis of biogenic amines from the RNA-Seq analysis (see also Table S2).
(B) qRT-PCR validating AMPK/CRTC-1 regulation of tbh-1 transcript levels (Mean ± SEM of mRNA levels extracted from 2-4 samples of 50-100 animals; * denotes p<0.05 by t-test).
(C) The biosynthetic pathway of octopamine.
(D) Fluorescence imaging of a worm co-expressing CRTC-1::tdTOMATO from the native crtc-1 promoter (top left) and GFP driven by the tbh-1 promoter reveals CRTC-1 expression in octopaminergic RIC neurons.
(E – F) Fluorescence imaging (top) and binary representations (bottom) of the mitochondrial network in muscle cells of animals vehicle-treated (water) or grown on media with 5 mM octopamine (F).
(G) Classifying worms by their mitochondrial morphology reveals a 45% decline in the fraction of worms with tubular mitochondria treated with octopamine versus control (p<0.001 by t-test; n = 3 samples of 11-19 worms).
(H – M) Survival curves demonstrating that neuron-specific activation of CRTC-1S76A,S179A suppresses both AMPK- (H) and calcineurin-mediated (K) lifespan extension. However, neuronal CRTC-1S76A,S179A has no effect on AMPK or calcineurin-mediated longevity in animals lacking functional tdc-1 (I, L) or tbh-1 (J, M). Genetic backgrounds are noted next to the origin. See Table S1 for lifespan statistics.
If octopamine mediates neuronal CRTC-1 signaling to other tissues, we reasoned it might be sufficient to generate the mitochondrial and metabolic phenotypes observed upon neuronal CRTC-1 activation. To test this hypothesis, we cultured nematodes expressing the mitoRFP reporter in the presence of exogenous octopamine. Strikingly, octopamine treatment elicits a similar degree of mitochondrial fragmentation in C. elegans muscle to that observed when CRTC-1 is activated neuronally, further suggesting that octopamine signaling may mediate CRTC-1 regulation of metabolism to peripheral tissues (Figure 7E-G). In direct support of octopamine as a relay signal, tax-6 RNAi increases expression of acs-2 but this effect is blunted in animals lacking TDC-1 or TBH-1 (Figure S7D).
To define the functional requirement of octopamine signaling in the modulation of aging by the AMPK/CRTC-1/NHR-49 pathway, we tested whether the suppression of lifespan by neuronal CRTC-1S76A,S179A or nhr-49 deletion required either TDC-1 or TBH-1. As shown previously, AMPK activation robustly increases lifespan of wild type animals and this effect is suppressed in worms expressing neuronal CRTC-1S76A,S179A (Figure 7H) or nhr-49(nr2041) (Table S1). While suppression of lifespan by nhr-49 deletion is independent of octopamine signaling (Figure S7E-F), the ability of neuronal CRTC-1S76A,S179A to suppress longevity is completely abolished in animals harboring null mutations in either tdc-1 (Figure 7I) or tbh-1 (Figure 7J), both of which lack octopamine (Alkema et al., 2005). Confirming these findings, neuronal CRTC-1S76A,S179A suppresses longevity mediated by tax-6 RNAi (Figure 7K), and this suppression requires TDC-1 (Figure 7L) and TBH-1 (Figure 7M) function. The ability of CRTC-1 to regulate aging in C. elegans therefore requires functional octopamine signaling. Together these results suggest that neuronal CRTC-1 modulates AMPK/calcineurin-mediated longevity and metabolism via an octopamine signal. Moreover, this cell-nonautonomous signal is dominant over the cell-autonomous effects of AMPK in peripheral tissues (Figure S7G).
Discussion
These data challenge current thinking regarding strategies to translate the link between energetics and longevity for therapeutics; perception and cell-nonautonomous communication of energy status in neurons can override direct activation of pro-longevity factors in distal tissues and might therefore be targeted for healthy aging. Although AMPK and PPARs are both targeted peripherally to promote metabolic homeostasis in humans (Hardie et al., 2012; Wahli and Michalik, 2012), both can affect metabolism cell-nonautonomously from the central nervous system (Bantubungi et al., 2012; Kocalis et al., 2012; Minokoshi et al., 2004). To date the relative contributions of these local and distal effects to healthy aging have not been explored. Here we demonstrate a cell-nonautonomous role these metabolic regulators play in coordinating energetics and longevity via effects on neuronal catecholamine signaling. AMPK locally and cell autonomously promotes remodeling of mitochondrial metabolic networks to increase longevity; however, AMPK must also inactivate CRTC-1 dependent transcription in neurons to systemically and cell-nonautonomously generate a permissive transcriptional landscape for its local metabolic programming. Critically, these cell-nonautonomous signals dominantly impact longevity, irrespective of AMPK activation and energetic state in receiving cells. Intriguingly, both neuronal activation of CRTC-1 and octopamine supplementation promote mitochondrial network fragmentation, suggesting dynamics of the mitochondrial network can be shaped from a distance and are critical for the ability of AMPK to promote longevity. However, this study also raises key questions going forward, including the sufficiency of the neuronal signal for longevity assurance, and how these mechanisms might translate to mammalian systems and therapeutics designed to promote metabolic homeostasis. Specifically, perhaps treatments targeting peripheral metabolic effectors to promote healthy aging will have reduced efficacy if cell-nonautonomous CNS signals remain discordant.
Although AMPK, PPARs and CRTCs are key peripheral metabolic regulators, all have emerging roles in neuroendocrine control of organismal metabolism that may become dysfunctional with age or obesity. Early studies of CRTCs focused on regulation of glucose metabolism in the mammalian liver, but there are three CRTC family members in mammals, two of which are expressed in the nervous system. Though less studied, recently elucidated roles of neuronal CRTCs include regulating expression of peptide signals and metabolic homeostasis in the periphery. Deletion of CRTC1, which is expressed primarily in the brain in mammals, results in hyperphagia and obesity in mice (Altarejos et al., 2008; Breuillaud et al., 2009). More recently, knockout of TRPV1 pain receptors was shown to both promote metabolic fitness and extend lifespan through effects in mouse sensory neurons (Riera et al., 2014). Interestingly, TRPV1 mutant mice exhibit nuclear exclusion of CRTC1 and reduced expression of a neuropeptide important for regulating glucose homeostasis. Although the requirement of neuronal CRTC1 inhibition for the longevity effects associated with TRPV1 loss of function in mice remains untested, our results point to a potentially conserved, neuronally-mediated mechanism by which CRTCs regulate systemic metabolic homeostasis and impact the aging process in worms and mice.
AMPK cell-autonomously regulates numerous physiological processes known to play roles in aging, including autophagy, protein synthesis, mitochondrial biogenesis, and both lipid and glucose metabolism (Burkewitz et al., 2014). Our data indicate mitochondrial metabolism is causally associated with AMPK longevity. Moreover, they suggest that AMPK regulation of both longevity and metabolism can be divided into two components: acute remodeling of metabolic pathways through direct regulation of enzymatic activity, and long-term remodeling of cell function via transcriptional reprogramming. Surprisingly, the transcriptional effects of AMPK are induced via cell-nonautonomous signals that override local enzymatic effects; CRTC-1 transcription in neurons suppresses lifespan despite AMPK being constitutively active in all tissues. Cunningham et al. (2014) recently identified a cell-nonautonomous role for neuronal AMPK in modulating peripheral lipid storage in nematodes, which supports cell-nonautonomous effects of AMPK/CRTC on metabolism. The role of AMPK in the central regulation of peripheral metabolism is conserved in mammals; AMPK integrates hormonal signals in the hypothalamus to control energy homeostasis, satiety and metabolism (Minokoshi et al., 2004). Further, in response to changes in glucose levels, AMPK regulates CRTC2 activity in the murine hypothalamus to modulate insulin signaling via IRS2 (Lerner et al., 2009). Communication between central and peripheral AMPK activity and its effect on metabolic homeostasis and aging in other organisms will be an exciting area for future research. While expressing truncated CA-AAK-2 in individual tissues of C. elegans in our study failed to promote longevity, work in Drosophila has shown that overexpressing wild type AMPK in muscle or fat body (Stenesen et al., 2013) or activated AMPK mutants in brain or gut (Ulgherait et al., 2014) is sufficient to extend lifespan. Differential effects seen in C. elegans and Drosophila may be due to methods employed to generate tissue specific strains. Moving forward, more work is needed to better elucidate how tissue-specific roles of AMPK coordinate to control longevity across different model systems.
Like AMPK and CRTCs, PPAR family transcription factors are best known for their primarily cell-autonomous roles in regulating metabolism, including lipid uptake, storage and oxidation. Here we demonstrate that the proposed worm PPARα, NHR-49, acts antagonistically to CRTC-1/CREB, regulating the shift in metabolic and mitochondrial programming, and that neuronal nhr-49 is sufficient for AMPK-mediated longevity (Figure 5). Notably, novel PPAR functions in the mammalian brain have also begun to emerge. The thiazolidinedione (TZD) class of anti-diabetic drugs is associated with weight gain, and two complementary studies identified brain PPARγ as the critical mediator of TZD-induced effects on food intake, thermogenic energy expenditure and peripheral glucose metabolism (Lu et al., 2011; Ryan et al., 2011). In addition, PPARα null mice show increases in glucose turnover, body weight and adipogenesis that are not rescued by restoring hepatic PPARα function. Pharmacologically activating PPARα in the brain of these mice, however, decreases glucose usage in peripheral tissues (Knauf et al., 2006). How PPARs and NHR-49 function in neurons to systemically regulate metabolic homeostasis with age remains unknown and is an important scope for future work.
An exciting key finding of this study is the novel role of octopamine, the invertebrate equivalent to the catecholamine (nor)adrenaline, as a signal communicating energetic state between neuronal AMPK/CRTC-1 and the periphery to modulate longevity (Figure 7). Interestingly, there is precedent for both AMPK and CRTCs in the regulation of analogous bioamine pathways in mammals. In mice, AMPKα2 suppresses sympathetic catecholamine release (Viollet et al., 2003), while CRTC1 enhances monoamine signaling in the prefrontal cortex (Viollet et al., 2003). However, it remains unclear whether octopamine is acting as a neurotransmitter or a neuroendocrine signal to mediate longevity in C. elegans. Octopamine and 5-HT were recently shown to act through a positive regulatory loop in neurons to promote release of an unidentified endocrine factor capable of activating the nuclear hormone NHR-76 to regulate lipid oxidation in the C. elegans intestine (Noble et al., 2013). That at least 2 nuclear hormone receptors (NHR-49 and NHR-76) act downstream of octopamine suggests that perhaps octopamine regulates the release of a lipophilic hormone. Alternatively, given our finding that DAF-16/FOXO may be involved in metabolic transcription downstream of neuronal CRTC-1, octopamine may regulate the secretion of specific insulin-like peptides. Beyond serving as a signaling molecule between neurons, octopamine could also act as an endocrine molecule itself. C. elegans possesses three putative octopamine receptors, ser-3, ser-6 and octr-1, whose expression outside the nervous system has not been extensively examined. Interestingly, a small-molecule screen for drugs capable of extending C. elegans lifespan identified a molecule that was shown to be an antagonist of the SER-3 receptor (Petrascheck et al., 2007). Future studies characterizing the respective roles of each octopamine receptor will be enlightening in understanding how octopamine elicits metabolic and longevity-related responses in the periphery.
Although our studies point towards remodeling of mitochondrial metabolism as being required for AMPK longevity, they do not preclude a role for other cellular processes. AMPK and CRTCs are known regulators of autophagy (Egan et al., 2011; Seok et al., 2014), and autophagy is required for lifespan extension by AMPK activation in Drosophila (Ulgherait et al., 2014) and calcineurin inhibition in C. elegans (Dwivedi et al., 2009). Highlighting the role of inter-tissue communication in AMPK longevity, tissue-specific activation of AMPK in the fly promotes systemic tissue homeostasis via the autophagic effector, Atg1, which subsequently and cell-nonautonomously promotes activation of autophagy in other tissues (Ulgherait et al., 2014). DAF-16/FOXO is a known regulator of autophagy and is directly regulated by both AMPK and calcineurin and required for their effects on longevity (Greer et al., 2007; Tao et al., 2013). We saw enrichment of the DAE element in genes downregulated when both AMPK and CRTC-1 were active, suggesting CRTC-1 might remotely regulate DAF-16/FOXO activity and/or activate its transacting antagonist PQM-1 (Tepper et al., 2013). Understanding how neuronal CRTC-1 interacts with DAF-16 and/or PQM-1 and if they function intra- or intercellularly to modulate AMPK/calcineurin-mediated longevity will be informative.
In summary this study highlights ‘mito-centric’ metabolism as the critical target of AMPK/CRTC-mediated effects on aging, and establishes that neurons are the causal and crucial site for CRTC-1-dependent regulation of longevity. Though both sensory perception of nutrient availability in neurons (Petrascheck et al., 2007) and organismal energy status (Burkewitz et al., 2014) are known to modulate aging, our data suggest an emerging paradigm: the optimal pro-longevity intervention requires coordination of energy perception in the neurons with accurately executed metabolic programs in peripheral tissues. Indeed, we show here that the pro-longevity, AMPK-mediated metabolic program in peripheral tissues is overridden when the regulatory link between AMPK and CRTC-1 is broken exclusively in neurons, completely suppressing all gains in longevity for the organism. If neuronal energy-sensing mechanisms are dominant, as our data indicate, selectively targeting central sensors and regulators of energy homeostasis may be sufficient to generate a peripheral metabolic program that promotes healthier aging.
Experimental Procedures
Additional details are provided in the Extended Experimental Procedures.
Lifespans
Lifespan experiments were performed on standard nematode growth media (NGM) at 20 °C. Worms were synchronized by timed egg lays using gravid adults. When the progeny reached adulthood (~72 h), 100 worms were transferred to fresh plates at 10-25 worms per plate and this was considered time = 0. Worms were transferred to fresh bacterial lawns every other day until the first deaths (10-14 d). Survival was scored every 1-2 days and a worm was deemed dead when unresponsive to 3 taps on the head and tail. Worms were censored due to contamination on the plate, leaving the NGM, eggs hatching inside the adult or loss of vulval integrity during reproduction. Only in the lifespans noted (TABLE S1), 5-Fluoro-2’-deoxyuridine (FUDR) was added to media to prevent excessive censoring. FUDR (100 μl; 1 mg ml−1) was added 24 h before picking worms to the plate on the first day of adulthood, and worms were transferred off FUDR-containing plates once reproduction had ceased (7 d), after which the assays continued normally.
RNA sequencing
Experiment was performed with three biological replicates. Eggs were synchronized to L1 larvae overnight in M9 and 1000 larvae were grown to L4 on NGM seeded with OP50-1 E. coli. Animals were collected and washed with M9 media to remove bacteria. Worms were then snap frozen in liquid nitrogen. RNA was extracted by five freeze/thaw cycles in Qiazol then purified by RNeasy mini kit (Qiagen). RNA quality was checked using an Agilent Technologies 2100 Bioanalyzer. All samples had an RNA integrity number of 10. cDNA libraries were prepared from 4 μgs of total RNA using the TruSeq RNA Sample Preparation v2 kit (Illumina). See Extended Experimental Procedures for more details of the data analysis.
Metabolomics
Synchronized L1 larvae were grown to L4 on NGM/OP50-1 before being washed off plates with M9, resuspended in 0.6% formic acid, snap-frozen, and thawed immediately before lysis by sonication. Aliquots were taken for total protein quantification, then an equal volume of acetonitrile was added to reach a final concentration of 0.3% formic acid and 50% acetonitrile. Samples were then subject to metabolomic analysis as detailed in Extended Experimental Procedures.
Mitochondrial analysis
Mitochondria were analyzed in muscle cells from ≥10 d1 adult worms per genotype. Qualitative assessment of mitochondrial morphology was made by scoring worms based on three categories: tubular (interconnected mitochondrial network), intermediate (combination of interconnected network and isolated smaller mitochondria) or fragmented (mostly fragmented mitochondria). Quantitative assessments of percent mitochondrial coverage of the cell and mitochondrial area/perimeter ratio were made by measuring >30 muscle cells per genotype using a macro for ImageJ, as previously described (Dagda et al., 2009).
Supplementary Material
Acknowledgements
Funding support was provided by the Ellison Medical Foundation (WBM and MDH) the NIH [1R01AG044346 (WBM), U54CA155626 (WBM), 1R01AG045351 (MDH), 1F32AG044944 (KB)], and the American Diabetes Association/Canadian Diabetes Association PF-3-13-4342 (FH). We thank the Caenorhabditis Genetic Center for providing several strains, Mark Alkema for the crh-1(n3315) mutant, and Sean Curran/Paul Sternberg for the mitochondrial marker strain. We also thank Tinatini Tavhelidse and Ana Paula Morales Allende for preliminary contributions to the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. 260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Altarejos JY, Goebel N, Conkright MD, Inoue H, Xie J, Arias CM, Sawchenko PE, Montminy M. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nature medicine. 2008;14:1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature reviews Molecular cell biology. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes & development. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K. Obesity and the regulation of fat metabolism. WormBook : the online review of C elegans biology. 2007:1–20. doi: 10.1895/wormbook.1.130.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantubungi K, Prawitt J, Staels B. Control of metabolism by nutrient-regulated nuclear receptors acting in the brain. The Journal of steroid biochemistry and molecular biology. 2012;130:126–137. doi: 10.1016/j.jsbmb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Breuillaud L, Halfon O, Magistretti PJ, Pralong FP, Cardinaux JR. Mouse fertility is not dependent on the CREB coactivator Crtc1. Nature medicine. 2009;15:989–990. doi: 10.1038/nm0909-989. author reply 991. [DOI] [PubMed] [Google Scholar]
- Burkewitz K, Zhang Y, Mair WB. AMPK at the Nexus of Energetics and Aging. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Bouagnon AD, Barros AG, Lin L, Malard L, Romano-Silva MA, Ashrafi K. Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions. PLoS Genet. 2014;10:e1004394. doi: 10.1371/journal.pgen.1004394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. The Journal of biological chemistry. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell metabolism. 2012;16:104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi M, Song HO, Ahnn J. Autophagy genes mediate the effect of calcineurin on life span in C. elegans. Autophagy. 2009;5:604–607. doi: 10.4161/auto.5.5.8157. [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Current biology : CB. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, Martinez E, Bringart M, Waget A, Kersten S, et al. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: Role in liver and brain. Endocrinology. 2006;147:4067–4078. doi: 10.1210/en.2005-1536. [DOI] [PubMed] [Google Scholar]
- Kocalis HE, Turney MK, Printz RL, Laryea GN, Muglia LJ, Davies SS, Stanwood GD, McGuinness OP, Niswender KD. Neuron-specific deletion of peroxisome proliferator-activated receptor delta (PPARdelta) in mice leads to increased susceptibility to diet-induced obesity. PloS one. 2012;7:e42981. doi: 10.1371/journal.pone.0042981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner RG, Depatie C, Rutter GA, Screaton RA, Balthasar N. A role for the CREB co-activator CRTC2 in the hypothalamic mechanisms linking glucose sensing with gene regulation. EMBO reports. 2009;10:1175–1181. doi: 10.1038/embor.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell metabolism. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature medicine. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Noble T, Stieglitz J, Srinivasan S. An integrated serotonin and octopamine neuronal circuit directs the release of an endocrine signal to control C. elegans body fat. Cell metabolism. 2013;18:672–684. doi: 10.1016/j.cmet.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS one. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare PP, Lin A, Bornfeldt KE, Taubert S, Van Gilst MR. Coordinate regulation of lipid metabolism by novel nuclear receptor partnerships. PLoS genetics. 2012;8:e1002645. doi: 10.1371/journal.pgen.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–556. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. TRPV1 Pain Receptors Regulate Longevity and Metabolism by Neuropeptide Signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nature medicine. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014 doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, Graff JM. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell metabolism. 2013;17:101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Xie Q, Ding YH, Li ST, Peng S, Zhang YP, Tan D, Yuan Z, Dong MQ. CAMKII and Calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. eLife. 2013;2:e00518. doi: 10.7554/eLife.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell. 2013;154:676–690. doi: 10.1016/j.cell.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell reports. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS biology. 2005;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. The Journal of clinical investigation. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends in endocrinology and metabolism: TEM. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.