Abstract
We conceptualize brain plasticity as an intrinsic property of the nervous system enabling rapid adaptation in response to changes in an organism's internal and external environment. In prenatal and early postnatal development, plasticity allows for the formation of organized nervous system circuitry and the establishment of functional networks. As the individual is exposed to various sensory stimuli in the environment, brain plasticity allows for functional and structural adaptation and underlies learning and memory. We argue that the mechanisms of plasticity change over the lifespan with different slopes of change in different individuals. These changes play a key role in the clinical phenotype of neurodevelopmental disorders like autism and schizophrenia, as well as neurodegenerative disorders such as Alzheimer's disease. Altered plasticity can trigger maladaptive cascades and be the cause of deficits and disability, but also offers opportunities for novel therapeutic interventions. In this chapter, we discuss the importance of brain plasticity across the lifespan and how neuroplasticity based therapies offer promise for disorders with otherwise limited effective treatment.
Keywords: Plasticity, Aging, Lifespan, Transcranial Magnetic Stimulation, Autism Spectrum Disorders, Schizophrenia, Alzheimer's Disease
Introduction
Brain plasticity is an intrinsic property of the nervous system that allows an individual to adapt to a rapidly changing environment through strengthening, weakening, pruning, or adding of synaptic connections, and by promoting neurogenesis (Pascual-Leone, Amedi et al. 2005; Feldman 2009). Plasticity might be conceptualized as the balanced interplay of mechanisms promoting change and those promoting stability (homeostatic plasticity). At the synaptic level this plays out for example in the balance of long term potentiation (LTP) strengthening connections between presynaptic and postsynaptic neurons (Bliss and Gardner-Medwin 1973)and long term depression (LTD) weakening them(Bear and Abraham 1996). The propensity of a synapse to undergo potentiation or depression relies on the influence of a number of molecular mechanisms (Kandel 2001) as well as the current state of the synapse (whether it has undergone a plastic change in the recent past, so called metaplastic influences (Abraham 2008; Mockett and Hulme 2008).
The molecular mechanisms responsible for plasticity are complex involving multiple cascades eventually culminating in functional and structural changes. Many models of plasticity propose the involvement of the NMDA receptor which, depending on the timing and degree of depolarization of the postsynaptic cell leads to subsequent synaptic LTP or LTD (e.g. (Daw, Stein et al. 1993; Malenka and Nicoll 1993; McBain and Mayer 1994). This process is kept in check by regulatory forms of plasticity to avoid a situation whereby certain cells never fire and others fire constantly. These feedback mechanisms include homeostatic synaptic scaling, whereby uniform increases or decreases in network activity over several hours or days lead to an opposing increase or decrease in excitatory synaptic strength (Turrigiano and Nelson 2004). Metaplasticity is another feedback mechanism, where experience-dependent alterations in inhibitory tone, dendritic excitability, and NMDA receptor function alter the ability of future stimuli to drive LTP and LTD (Abraham and Bear 1996).Plasticity at the synaptic level can be studied using invitro techniques or invivo in animal models. These changes at the synaptic level lead to the development and maintenance of neural circuitry.
Characterization of plasticity in humans is possible. The consequences of brain plasticity can be studied as changes in functional activity and anatomical connectivity using neuroimaging and neurophysiological techniques, and as changes in behavior captured by measures of learning, memory and adaptation. For example, brain imaging studies using structural and functional magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) have provided evidence of circuit modification suggestive of plastic changes(Guye, Bartolomei et al. 2008; Voss and Schiff 2009). These circuit modifications are indirect measures of what is happening at the cellular level.
Cross-sectional anatomical MRI studies have consistently identified age-associated morphometric brain changes encompassing regional cortical thinning, volumetric subcortical reductions, and ventricular enlargement (e.g., (Walhovd, Fjell et al. 2005; Fjell, Westlye et al. 2009; Walhovd, Fjell et al. 2009). Longitudinal studies have demonstrated annual atrophy rates for brain volume, hippocampus and entorhinal cortex (e.g., (Scahill, Frost et al. 2003; Fotenos, Snyder et al. 2005), and atrophy in cortical brain regions over different periods of time (Raz, Lindenberger et al. 2005; Driscoll, Davatzikos et al. 2009). Cortical thickness decreases over the lifespan are estimated at0.5% a year (Thompson, Hayashi et al. 2007). These changes affect different neural systems differently: motor and visual cortices show regional thinning, whereas non-limbic temporal regions and parietal areas are relatively spared in normal aging (Raz, Rodrigue et al. 2004; Salat, Buckner et al. 2004). Furthermore, DTI can reveal structural changes in white matter structure (myelination) and connectivity. For example, DTI has demonstrated that white-matter connections, largely in fronto-striatal areas, have reduced myelination as age increases (Salat, Tuch et al. 2005).
Functional MRI can reveal changes in activation of brain circuits across the age span. One example of this is a reduction in prefrontal hemispheric asymmetry in elderly individuals, referred to as the HAROLD model (hemispheric asymmetry reduction in older adults) (Cabeza, Anderson et al. 2002). According to the HAROLD model, the older brain displays less localizable and more bilateral activation during certain cognitive tasks. A second pattern is a shift in evoked neural activity from posterior to anterior cortex, a model referred to by Davis et al. as PASA (posterior-anterior shift in aging) (Davis, Dennis et al. 2008). The PASA model posits that the aging brain is more likely to recruit prefrontal, rather than occipito-temporal, cortex in the service of task execution. In addition to life-span changes in task-related brain activation patterns, resting-state fMRI is revealing age-related differences in the functional connectivity across large-scale brain networks. One such large-scale brain functional network, the default mode network (DMN) has been shown to undergo notable modifications with advancing age in health and disease (Buckner, Andrews-Hanna et al. 2008). Older individuals reportedly exhibit significantly lower DMN activity in the posterior cingulate as well as a tendency toward lower activity in all other DMN regions as compared to younger subjects (Koch, Teipel et al. 2010). Functional connectivity within the DMN also seems to be reduced in older adults (Grady, Protzner et al. 2010). During performance of a working memory task, the pattern of deactivation of the DMN also seems to be affected by aging, with older individuals not only showing decreased connectivity but also decreased ability to suppress low frequency oscillations of the DMN (Sambataro, Murty et al. 2010). Age-specific changes in activation and connectivity are also seen in the task-positive network (TPN), though the functional significance of this remains uncertain (Grady et al. 2010;Sambataro et al. 2010). During memory encoding and recognition, age-related changes appear to occur mainly in the long-range connections with widespread reductions associated with aging in the fronto-temporal and temporo-parietal regions, and a few age-related increases in the posterior parietal regions (Wang, Li et al. 2010). During developmental years, children and young adults appear to have similar patterns of functionally connected regions, but with differences in the size of functionally connected regions as well as in the strength of functional connectivity between brain regions (Jolles, van Buchem et al. 2011).
Though useful for understanding the consequences of plasticity at the circuit level, brain imaging does not directly probe plasticity but rather reveals its consequences. Direct measures of circuit level plasticity in humans in vivo can be obtained using novel transcranial magnetic stimulation (TMS) paradigms(Ziemann 2004; Huang, Edwards et al. 2005; Thickbroom 2007; Huerta and Volpe 2009). TMS is a noninvasive way to induce, measure, and modify local and network plasticity, and a number of experimental TMS measures of brain plasticity have been introduced. Single-pulse TMS combined with EMG, EEG, fMRI or other brain imaging methods can be used to quantify cortical reactivity before and following a given intervention(Pascual-Leone, Freitas et al. 2011). TMS can provide a controlled and quantifiable input that can be matched across individuals of different ages. Comparison of TMS measures of cortical reactivity before and after an intervention may thus provide an index of brain plasticity in response to said intervention. When the intervention itself involves TMS (as in paired associative stimulation (PAS) or repetitive (r)TMS protocols), it is possible to assess the efficacy of the mechanisms of plasticity in a defined cortical brain region in humans in vivo. PAS builds on the Hebbian principle of spike timing-dependent synaptic plasticity (Classen, Wolters et al. 2004). In its most common form, PAS involves repeated pairing of median nerve electric stimulation with timed TMS over the contralateral primary motor cortex. In this form, PAS has been shown to modulate the excitability of the motor system in either the positive (with an ISI of 25 ms) or negative (with an ISI of 10 ms) direction (Classen, Wolters et al. 2004). Repetitive TMS (rTMS) consists in the application of a train of TMS pulses of the same intensity to a single brain area at a given frequency that can range from 1 to 20 or more stimuli per second (Pascual-Leone, Valls-Sole et al. 1994). Such a train of rTMS can induce a modulation of cortical excitability beyond the duration of the train itself. Depending on the stimulation parameters, particularly frequency and pattern of stimulation, cortical reactivity is potentiated or depressed(Pascual-Leone, Valls-Sole et al. 1994). In general, a continuous train of lower frequencies of rTMS, in the1-Hz range, leads to a transient suppression of excitability in the targeted cortical area, while bursts of high-frequency stimulation (≥ 5-Hz) lead to a temporary increase in cortical reactivity (Kobayashi and Pascual-Leone 2003). Patterned bursting protocols have also been developed that mimic paradigms used to assess synaptic plasticity in animal models (Huang, Wang et al. 2005; Huang, Rothwell et al. 2008).Specifically, Theta burst stimulation (TBS) involves application of 3 bursts of 50-Hz rTMS repeated every 200 milliseconds either continuously for a total of 40 seconds or intermittently (every 8 seconds) for about 3 minutes. When applied to the motor cortex, continuous (cTBS) and intermittent TBS (iTBS) were shown to result in depression and potentiation of cortical reactivity as indexed through suppression and facilitation of motor evoked potentials MEPs, respectively (Huang et al., 2005). Results of animal and human studies are consistent with the notion that the modulatory effects of TMS protocols on cortical reactivity reflect plasticity mechanisms (for review, (Cardenas-Morales, Gron et al. 2011).
Importance of Plasticity for Brain Health Across the Lifespan
Plasticity is a critical component of brain development and maintenance across the lifespan. During development, brain plasticity underlies the formation of functional networks through experience dependent strengthening and weakening of synapses. For example, animal studies have shown that whisker stimulation strengthens the development of excitatory synapses through NMDA mediated LTP in the rat somatosensory barrel cortex(Takahashi, Svoboda et al. 2003). This is not seen in rats with their whiskers trimmed(Takahashi, Svoboda et al. 2003). Visual and auditory cortices also show experience-dependent developmental plasticity. Repeated activation of a specific sensory input (without deprivation) potentiates neural responses to that input and is responsible for the establishment of auditory and visual receptive fields. This can be shown experimentally by exposing young rats to specific auditory stimuli that leads to enhancement of the representation of the presented frequencies and intensities in primary auditory cortex (A1), altering auditory tuning curves and the tonotopic map (Frenkel, Sawtell et al. 2006; Keuroghlian and Knudsen 2007). Similarly, presentation of high-contrast oriented gratings to young mice similarly drives orientation-specific enhancement of visual responses in primary visual cortex (V1)(Frenkel, Sawtell et al. 2006).
The degree and duration of these experience dependent changes to cortical structure are very strictly regulated. During development, there are critical periods where a specific region of cortex has heightened or exclusive capacity for plasticity. The onset of these critical periods is thought to be regulated by the maturation of specific GABAergic neurons (parvalbumin-positive basket cells) (Hensch 2005). How these cells control plasticity is not known but may involve setting a permissive excitatory-inhibitory balance or editing pyramidal cell firing patterns to promote excitatory synaptic plasticity. The regulation of these critical periods during development and the resulting control of plasticity is integral to the healthy establishment of cortical circuits. Consequently, dysfunction of critical period timing, excitatory-inhibitory imbalance and aberrant cortical plasticity have been put forth as a potential pathophysiological mechanisms underlying developmental disorders such as autism and schizophrenia (discussed below) (Rubenstein and Merzenich 2003; LeBlanc and Fagiolini 2011).
During adolescence and adulthood, the brain continues to display capacity to adapt to the ever changing environment, showing both functional and structural changes throughout the lifespan. For example, there is direct evidence that LTP in the hippocampus and amygdala occurs during and is required for adult learning and memory ((Maren 2005; Sossin, Lacaille et al. 2008). Studies have also shown that motor training in adult rats results in LTP-like strengthening of pathways within the primary motor cortex (Rioult-Pedotti, Friedman et al. 2000). Similarly, presentation of temporally precise, flashed visual stimuli to adult rats alters functional synaptic connectivity and visual receptive fields in primary visual cortex and affects visual perception in a manner consistent with induction of spike timing-dependent plasticity. In other brain regions, experience-dependent changes in synaptic strength, or synaptic plasticity, underlie many learning processes. In the reward circuit for example, synaptic plasticity may serve as a cellular substrate for goal-directed behaviors. Addictive drugs, through a surge of dopamine released from neurons of the ventral tegmental area, induce widespread synaptic adaptations within this neuronal circuit (Bonci and Malenka 1999; Liu, Pu et al. 2005; Luu and Malenka 2008). It is thus proposed, that drug-evoked synaptic plasticity may constitute an early cellular mechanism eventually causing compulsive drug-seeking behavior in addiction (Mameli and Luscher 2011)
This ability to change and adapt appears to peak in young adulthood and show a gradual, but consistent decrease into senescence. Animal studies, building on pioneering work from Barnes (Barnes 1979; Rosenzweig and Barnes 2003) in the late 1970s, have demonstrated an age-associated decline in synaptic plasticity in specific brain regions that correlates with neurocognitive impairments. In aged rodents, thresholds for induction of hippocampal LTP and LTD appear to increase and decrease, respectively (Rosenzweig and Barnes 2003). Once induced, LTP decays faster in older rats, and this appears to be associated with a greater degree of forgetfulness (Barnes and McNaughton 1980; Kelly, Nadon et al. 2006). Moreover, deficits in the balance between LTP and LTD result in impaired learning and memory (Larson, Wong et al. 1986; Roman, Staubli et al. 1987; Bliss 2003).
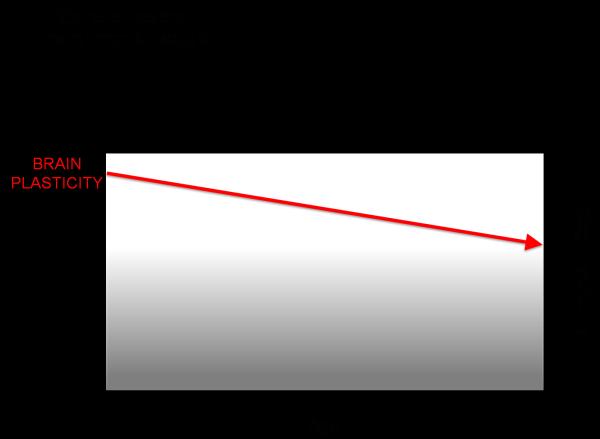
Direct evidence of this age-related decline in plasticity has also been shown in humans through studies using TMS measures of plasticity. For example, in a cross-sectional study of 36 healthy volunteers throughout the adult age-span ranging from 19 to 81 years, Freitas and colleagues (2011) found the duration and magnitude of corticospinal excitability modulation by rTMS was inversely and significantly correlated with age(FIGURE 1). These data provide direct experimental evidence that, in humans, LTD-like plasticity becomes increasingly less efficient with advancing age. Such decreasing plasticity in the motor cortex with advancing age may be associated with the decrement of hand motor function (e.g., longer reaction time) observed during normal aging in both men and women (e.g., (Carmeli, Patish et al. 2003)) and to the age-related deficits in motor learning (e.g., (Brown, Wilson et al. 2009). Such age-related changes in plasticity are also linked to an individual's cognitive ability and age-related cognitive decline may be associated to them. An individual's risk of age-related cognitive decline (and ultimately the manifestation of symptoms of dementia) might then depend on the individual's starting point and slopes of change in plasticity efficiency over the lifespan. Indeed, as will be further discussed below, studies in patients with early Alzheimer's Disease, the most common dementing illness, reveal an abnormally suppressed efficacy of plasticity mechanisms (Freitas, Mondragon-Llorca et al. 2011; Koch, Di Lorenzo et al. 2012).
Figure 1.
Schematic representation of the influence of aging on plasticity and cognitive ability.
Disease as a Manifestation of Aberrant Plasticity at Different Times in the Lifespan
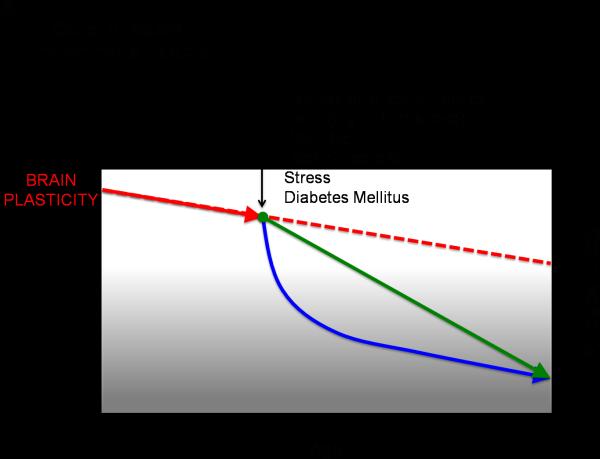
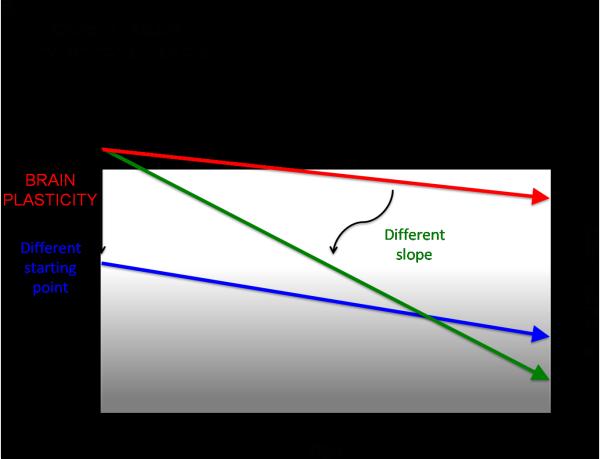
If, as we suggest, plasticity is critical for healthy brain development, it follows that neuropsychological disorders may have a basis in aberrant plasticity mechanisms. Recent theories of the neurological etiology of brain disorders reflect a growing acceptance of this inference (van Spronsen and Hoogenraad 2010; Pascual-Leone, Freitas et al. 2011). A functionally ‘normal’ brain is thus a changing brain, a brain whose capacity and mechanisms of change are shifting appropriately from one time point to another in a given individual's life. Therefore, assessing the mechanisms of brain plasticity across the lifespan is critical to gain insight into an individual's brain health. The timing, site and direction of alterations in plasticity across the lifespan will influence what systems are affected and in turn the behavioral outcome. Important factors to consider that likely contribute to individual differences in changes in the efficacy of mechanisms of plasticity across the lifespan include genetic and epigenetic mechanisms (e.g., polymorphisms, genetic expression), hormonal factors (e.g., gender, menstrual cycle), impact of morbidities (e.g. diabetes, cancer, or infections), and lifetime experiences (e.g., traumatic brain injury, exposure to toxins, stress, sleep deprivation, substance abuse, poor cognitive reserve, poor nutrition, sedentariness, etc.). Therefore, dissimilar ‘starting points’ for different individuals, distinct lifelong ‘slopes of change’, and events that lead to a change in the set point or slope of change in plasticity might be postulated (FIGURE 2A and B). We shall posit that these two factors critically contribute to an individual's predisposition to manifest symptoms of disease. To illustrate this notion, we shall discuss how alterations in plasticity might underlie developmental disorders such as autism spectrum disorders (ASD) and schizophrenia, as well as neurodegenerative disorders such as Alzheimer's disease.
Figure 2A.
Schematic representation of how factors such as expression of certain genes, diseases, brain injury, or behavior can impact the slope of change in plasticity across the lifespan.
Figure 2B.
Schematic representation of how degree of plasticity and cognitive ability at any given time in the lifespan is a consequence of both a given individual's starting point and slopes of change.
Autism Spectrum Disorders (ASD)
Evidence for altered plasticity in ASD comes from multiple lines of research (reviewed in (Oberman in press)). First, genetic linkage studies indicate that genes associated with ASD play critical roles in developmental and experience-dependent plasticity. For example, BDNF (brain-derived neurotrophic factor) plays a critical role in maintenance of synaptic potentiation (Korte, Carroll et al. 1995; Patterson, Abel et al. 1996; Akaneya, Tsumoto et al. 1997; Huber, Sawtell et al. 1998; Jiang, Akaneya et al. 2001) and has been found to be elevated in postmortem tissue of individuals with ASD, specifically, in the basal forebrain(Perry, Lee et al. 2001). Additionally, multiple studies note a reduction in GABAergic receptors (Fatemi, Folsom et al. 2009; Fatemi, Reutiman et al. 2009; Fatemi, Reutiman et al. 2010) as well as a 50% reduction in enzymes that synthesize GABA (glutamic acid decarboxylase (GAD) 65 and 67) (Fatemi, Halt et al. 2002; Yip, Soghomonian et al. 2007). These changes in the GABA system may directly contribute to altered connectivity, especially between the cerebellum and the thalamus, and ultimately the cerebral cortex. This may represent a mechanism by which motor as well as cognitive behaviors may be affected in ASD (Blatt and Fatemi 2011). Other genes coding for molecules such as neuroligin 3 and 4 that are implicated in synaptogenesis (Jamain, Quach et al. 2003), SH3 and multiple ankyrin repeat domains 3 (SHANK3) that encodes a protein involved in dendritic development(Durand, Betancur et al. 2007)and c3orf58, sodium/hydrogen exchanger isoform 9 (NHE), and protocadherin-10 (PCDH10) thought to be critically involved in synaptic development and plasticity(Jamain, Quach et al. 2003; Durand, Betancur et al. 2007; Morrow, Yoo et al. 2008)have all been identified as candidate genes that confer increased risk of ASD (Lamb, Moore et al. 2000; Cook 2001; Persico and Bourgeron 2006).
In addition, single gene disorders associated with autism implicate proteins that play important roles in synaptic plasticity. Among these are mutations in FMRP (fragile X mental retardation protein), thought to contribute to the neurologic deficits of fragile X syndrome by enhancing synaptic potentiation and favoring exaggerated LTD-like plasticity. Other examples include mutations in TSC1 and TSC2 that cause tuberous sclerosis, in NF1 that cause neurofibromatosis, and in phosphatase and tensin homologue (PTEN)that cause PTEN macrocephaly (Dolen and Bear 2009).Although the contributions of these genes and proteins to synaptic plasticity are incompletely described, animal models of these human single gene syndromic causes of autism predictably demonstrate aberrant synaptic plasticity. These genetic findings have inspired others to propose that autism should be thought of as a “synapsopathy” (Dolen and Bear 2009) whereby proteins that are involved in synaptic development and plasticity are affected.
Animal ASD models reveal abnormal plasticity mechanisms (reviewed in(Tordjman, Drapier et al. 2007)). For example, a recent study exploring the parvalbumin (PV)-positive basket cell (a key player for critical period plasticity) in two animal models of autism (valproic acid (VPA)and neuroligin3 knock out models) found a reduction or complete lack of PV-cells in parietal and occipital cortices (Gogolla, Leblanc et al. 2009) suggesting a possible molecular mechanism underlying a proposed hyperpotentiated state. When the microcircuits of these animals were investigated, their reactivity to stimulation, as measured by the number of spikes and the number of postsynaptic potentials following stimulation, was nearly twice that of wild-type animals (Rinaldi, Silberberg et al. 2008). This hyper-reactivity has been found in multiple regions including somatosensory cortex (Rinaldi, Silberberg et al. 2008), prefrontal cortex (Rinaldi, Perrodin et al. 2008)and amygdala (Markram, Rinaldi et al. 2008); thus indicating a widespread enhancement in reactivity of cortical and subcortical neurons. Synaptic responses have also been recorded in pyramidal neurons following a Hebbian pairing stimulation protocol in these animals, and though the presynaptic response was normal, the postsynaptic cell had a more than two-fold increase in response, indicating a state of hyperpotentiation (Rinaldi, Kulangara et al. 2007). Similarly, abnormal synaptic plasticity, specifically exaggerated LTD, has also been shown in mouse models of genetic disorders associated with autism, namely the FMR1-null mouse (fragile X syndrome) and MECP2-null mouse (Rett syndrome) (Huber, Gallagher et al. 2002; Dani, Chang et al. 2005).
In humans with idiopathic autism, the most consistent neuroimaging finding is increased brain volume, with an overall increase in both grey and white matter volume (Courchesne, Karns et al. 2001). Furthermore, there is a distinct developmental trajectory of brain size abnormalities in ASD whereby reduced or normal brain size is present at birth, followed by a rapid rate of brain growth during early childhood. This trajectory suggests that the underlying mechanism is a dynamic process with a timeline consistent with a shift toward increased potentiation of excitatory synapses during early childhood(Courchesne and Pierce 2005). Recent studies reveal that the overall larger brain in individuals with ASD is primarily due to larger white matter volumes, particularly in the outer “radiate” regions including the origins and terminations of projection and sensory fibers(Herbert, Ziegler et al. 2004). Even when accounting for the overall greater brain volume, the proportion of white matter still is greater than normal, suggesting that abnormal axons and neural connections, rather than the neuronal cell bodies themselves, may be responsible for the abnormalities in brain structure. There is also neuropathological data in postmortem tissue supporting brain overgrowth, specifically in the prefrontal cortex (Courchesne, Campbell et al. 2011)and abnormalities at the minicolumn level indicating aberrant minicolumn structure with reduced neuronal size and increased density attributable to reductions in the inhibitory peripheral neuropil space (Casanova, Buxhoeveden et al. 2002). The authors suggest that this lack of inhibition would lead to gross alterations in cortical connectivity.
Another common neuropathological finding in ASD is a reduction in the number of cerebellar Purkinje cells (Bauman and Kemper 1996). Such a reduction is thought to release the deep cerebellar nuclei from inhibition, producing abnormally strong physical connectivity and potentially abnormally weak computational connectivity along the cerebello-thalamo-cortical circuit and furthermore, possibly aberrant activity-dependent plasticity along this pathway(Belmonte, Allen et al. 2004). Abnormally high and indiscriminate physical connectivity may lead to abnormally low and ineffective functional connectivity due to excessive noise and poor temporal precision secondary to activity of superfluous connections. Consistent with this assertion, structural and functional MRI studies have confirmed anatomical and functional connectivity abnormalities in individuals with ASD (for a review see (Geschwind and Levitt 2007).
Two recent studies have been published exploring plasticity using TMS in individuals with ASD. The first (Oberman, Eldaief et al. 2012)explored modulation in cortical excitability in response to a train of rTMS in 20 adults with Asperger's syndrome and found them to have greater and longer-lasting modulation of cortical reactivity following rTMS as compared to age-, gender-, and IQ-matched controls. The latency to return to baseline following rTMS was on average between 80 and 90 minutes in the ASD group compared to 25-30 minutes in the controls. This finding was confirmed in a separate cohort of 15 individuals (Oberman, Eldaief et al. 2012). Interestingly, consistent with other studies, there was no significant group difference in measures of basic excitability as measured by resting and active motor threshold (Theoret, Halligan et al. 2005; Enticott, Kennedy et al. 2012; Oberman, Eldaief et al. 2012) or response to single pulse TMS (Enticott, Kennedy et al. 2011; Oberman, Eldaief et al. 2012). Thus, this excessive modulation of excitability in response to stimulation (a putative measure of LTD-like and LTP-like plasticity) is not primarily attributable to differences in baseline excitability. A second study was subsequently published exploring response to the PAS in nine patients with high functioning ASD (HFA)/Asperger's Syndrome (AS) and typically developing age-matched controls. In contrast to the findings by Oberman and colleagues (2012), this study found that individuals with ASD showed a marked absence of the expected modulation of excitability following PAS (Jung, Janzarik et al. 2013). The authors contend that their results indicate an impairment in LTP-like plasticity induced by PAS in individuals with HFA/AS compared with typically developing participants. The conflicting findings could reflect paradigmatic differences (i.e. Hebbian vs. non-Hebbian plasticity) or the heterogeneity of ASD, but in any case the effects are opposite (i.e. absent vs. long-lasting) and emphasize the importance of large studies, with detailed clinical and genetic information, to examine functional neurobiology in ASD. Regardless, these TMS studies support the notion of alterations in plasticity mechanisms being central to the pathophysiology of ASD.
Schizophrenia
Schizophrenia is another neurodevelopmental disorder where researchers are beginning to implicate neuroplasticity mechanisms in its pathophysiology. Several lines of evidence suggest that the neurotransmitter mechanisms mediating plasticity in the cortex are altered in schizophrenia. For example, both NMDA and GABA receptor–mediated neurotransmission have been implicated in the pathophysiology of schizophrenia. Blockade of NMDA receptor– mediated neurotransmission is associated with worsening of psychosis in patients with schizophrenia (Krystal, Anand et al. 2002) and produces behaviors in healthy subjects that are similar to the positive and negative symptoms experienced by patients with schizophrenia (Krystal, Karper et al. 1994). Moreover, neuroanatomical (Benes and Berretta 2001) and neurophysiological evidence (Freedman, Adams et al. 2000; Daskalakis, Christensen et al. 2002; Fitzgerald, Brown et al. 2002) suggests that both a decrease and a disruption of cortical GABAergic inhibitory neurotransmission is associated with the pathophysiological findings of schizophrenia. In addition, genetic and postmortem studies have implicated abnormalities in dysbindin, neuregulin, and reelin proteins involved in synaptic plasticity, as possible contributors to pathological findings in schizophrenia (Fatemi, Earle et al. 2000; Straub, Jiang et al. 2002; Weeber, Beffert et al. 2002; Stefansson, Sarginson et al. 2003).
Behaviorally, patients with schizophrenia demonstrate an inability to learn complex motor skills. For example, studies suggest that patients with schizophrenia show impaired motor learning as indexed through the rotary pursuit task and a lack of increase in blood oxygen level-dependent premotor activity following one week of training as compared to healthy subjects (Schwartz, Rosse et al. 1996; Kodama, Fukuzako et al. 2001). A recent TMS study confirms these findings showing that following motor training, both medicated and unmedicated patients with schizophrenia demonstrated significantly reduced motor reorganization as indexed by TMS-induced motor evoked potentials compared with healthy subjects (Daskalakis, Christensen et al. 2008).
Several other TMS studies have been conducted which also support plasticity abnormalities in schizophrenia. Fitzgerald and colleagues (Fitzgerald, Brown et al. 2004) showed reduced plastic brain responses in medicated and unmedicated patients with schizophrenia. Specifically, LTD-like suppression of cortical excitability was reduced in patients in response to a single 15-min train of 1-Hz rTMS applied to the motor cortex, compared with a healthy control group. Frantseva and colleagues (2008) conducted a study using PAS and demonstrated that schizophrenia patients, compared with healthy subjects, showed deficits in MEP facilitation indicating disrupted LTP-like plasticity, which appeared to be associated with impaired motor skill learning. Finally, McClintock et al.(McClintock, Freitas et al. 2011)report the findings of an rTMS study in a group of six first episode patients with schizophrenia who had 42% reduced duration of rTMS-induced aftereffects compared with age and gender-matched healthy control subjects, suggesting that corticomotor plasticity mechanisms are already abnormally reduced in early stages of schizophrenia.
Alzheimer's Disease (AD)
Large strides have been conducted in investigating the pathophysiology of AD (Jack Jr 2010). The leading hypothesis about the cause of AD argues that toxic forms of the amyloid ß (Aβ) protein initiate a cascade of events ending in synaptic dysfunction and cell death, and where “plaques” and “tangles” are conceived as residues of this pathological process (Mattson 2004; Walsh and Selkoe 2004). Aβ is critical as when it is isolated directly from human AD brains it can cause impaired synaptic plasticity and memory in rodents (Shankar, Li et al. 2008).Furthermore, when Aβ is released into the extracellular fluid it triggers signaling cascades on the postsynaptic membrane, sharing remarkable similarities with LTD, including increased synaptic AMPA receptor endocytosis and dendritic spine loss(Hsieh, Boehm et al. 2006).
Consistent with the clinical observation that initial symptoms of AD include memory impairment, the medial temporal lobe and other cortical structures linked to memory are affected early in AD. The reason why memory structures are particularly vulnerable to AD and critically involved in disease progression, remains unclear, though proposed theories include concepts based on anatomy (Hyman, Van Hoesen et al. 1990) and on mechanisms of plasticity (Mesulam 2000). On the other hand, early pathological studies and more recent morphometric brain studies also reveal distributed cortical regions as vulnerable to AD, prompting further exploration of systems-level causes (Saper, Wainer et al. 1987). In any case, our understanding remains insufficient to guide novel interventions and current therapeutic options remain disappointing. Therefore, novel conceptualizations of AD pathogenesis seem worth entertaining.
We often consider how aberrant molecular and cellular processes can affect brain circuits and cognitive processes. However, the opposite causal direction is also possible: dysfunctional brain activity patterns may directly modulate molecular cascades that are relevant to disease. We propose that AD is an illustrative example of this pathophysiologic instance, where alterations in plasticity ultimately trigger a cascade of maladaptive responses leading to pathology.
Direct evidence of a dysfunction in plasticity in AD is provided by recent TMS studies. The first, conducted by Inghilleri and colleagues (Inghilleri, Conte et al. 2006) tested the effects of cortical motor modulation induced by suprathreshold high-frequency (5 Hz) rTMS and found the amplitude of MEPs progressively decreased in patients while increasing in controls. This suggests impaired LTP-like plasticity. Another study, conducted by Battaglia and colleagues (Battaglia, Wang et al. 2007), studied neocortical (motor) LTP-like plasticity in AD and healthy individuals using a paired associative stimulation (PAS) protocol and found it to be significantly reduced in AD patients.
Koch and colleagues (Koch, Esposito et al. 2011) studied the effects of low frequency (1 Hz) rTMS over the primary motor cortex in a group of patients with a diagnosis of probable AD, compared to healthy age-matched controls (HS) and tested the effects of a single dose of orally administered L-dopa, one of the key neurotransmitters in modulating synaptic plasticity mechanisms, on rTMS induced plasticity. They found that in AD patients the 1 Hz rTMS protocol did not induce the expected inhibitory effect, while a long lasting inhibition of MEP was observed in control participants. In addition, L-dopa induced a clear form of reversal of the direction of plasticity in healthy controls that was not evident in AD. In a follow up study, Koch and colleagues (Koch, Di Lorenzo et al. 2012) applied repetitive transcranial magnetic stimulation over the primary motor cortex (M1) in AD patients and in age-matched healthy controls, using protocols of TBSAD patients showed consistent LTD-like effects that were comparable to those obtained in healthy controls when submitted to 40 seconds of continuous TBS. Conversely, AD patients did not show any LTP-like after effect when submitted to two different TBS protocols that induced an LTP-like effect in healthy controls such as intermittent TBS and 20 seconds of continuous TBS followed by one minute of muscular contraction. These results demonstrate the impairment of LTP-like together with normal LTD-like cortical plasticity in AD patients. Finally, a study conducted by Frietas and colleagues (2011) indicates that the duration and magnitude of the modulation of corticospinal excitability by cTBS, an index of LTD-like plasticity is significantly shorter in individuals with early AD than in controls (Frietas et al., 2011). Thus, it is unclear to what extent LTD-like plasticity is affected in this population, but studies consistently reveal early alteration of mechanisms in plasticity that may antedate, and contribute to trigger a molecular maladaptive cascade culminating in the manifestation of symptoms of dementia.
The use of Transcranial Magnetic Stimulation as a Novel Treatment Strategy for Neuropsychiatric Disorders of Plasticity
If, as we propose, brain plasticity is critically tied to brain health across the lifespan and a dysfunction in plasticity underlies the symptoms of many neuropsychiatric disorders, then normalizing plasticity mechanisms may represent novel and effective therapeutic interventions. In the future, interventions aimed at modulating plasticity mechanisms could potentially prevent the structural and functional pathology underlying these disorders and in doing so prevent the behavioral symptoms from developing (Cramer, Sur et al. 2011).
The potential of rTMS to induce a long-lasting modulation of cortical excitability and plasticity offers the possibility of its use for therapeutic purposes in neurological and psychological conditions thought to be a result of altered excitability or plasticity of specific neural circuits. Studies examining behavioral performance prior to and following rTMS have shown rTMS-induced changes in sensory (Kosslyn, Pascual-Leone et al. 1999), cognitive (Hilgetag, Theoret et al. 2001; Mottaghy, Doring et al. 2002), and affective processing (see (Lee, Blumberger et al.) for a review). Low frequency rTMS protocols and a specific type of theta burst stimulation (continuous, cTBS) generally induce lasting suppression of the excitability, while high-frequency rTMS and a different type of theta burst stimulation (intermittent, iTBS) generally induce lasting facilitation (Maeda, Keenan et al. 2000). However, it should be noted that these effects are state-dependent and there is significant inter-subject and intra-subject variability (Silvanto and Pascual-Leone 2008). Thus, in order to induce the desired effect, one must consider (1) the brain region, as even a small shift in the targeted region may greatly affect the behavioral impact; (2) the current state of the stimulated cortex as state-dependent changes have been observed, and (3) the exact stimulation protocol being applied as opposite effects can be induced by even slight modifications of the parameters. rTMS-based treatments are already being proposed and tested in the aforementioned disorders.
Autism Spectrum Disorders
Recent studies from two sites in the United States (Harvard Medical School, Boston, MA and University of Louisville School of Medicine, Louisville, KY) and one site in Australia (Monash University, Melbourne, Australia) have reported preliminary data suggesting an improvement in both physiological indices and specific behavioral symptoms in individuals with ASD following rTMS.
The first of these studies, was based on the finding that individuals with ASD showed abnormal structure of minicolumns with reduced neuronal size and increased density attributable to reductions in the inhibitory peripheral neuropil space (Casanova, Buxhoeveden et al. 2002). This finding was most prominent in the prefrontal cortex (Casanova 2006). Thus, using a rTMS protocol aimed at increasing inhibitory tone, Sokhadze and colleagues (Sokhadze, El-Baz et al. 2009) applied low-frequency (0.5 Hz, 150 pulses) stimulation to left DLPFC two times per week for three weeks in a small sample of eight individuals with ASD. The results of this first study showed an abnormally increased amplitude and latency of the P300 event related potential (ERP) and abnormally high induced gamma frequency electroencephalographic (EEG) activity over frontal and parietal sites at baseline in the ASD group that were normalized (not significantly different from healthy controls) in amplitude and latency following the series of rTMS sessions. There was also a reduction in repetitive-ritualistic behavior in ASD subjects as reported by their caregivers. This result is quite promising, though the study should be considered extremely preliminary given its small sample size and lack of sham control condition. Following this initial study, the same group conducted several follow-up studies with slightly larger samples. In the first of these follow-up studies the group replicated their previous finding of normalized ERPs and a reduction in repetitive-ritualistic behaviors following the same protocol (Sokhadze, Baruth et al. 2010) in 13 individuals with ASD. In the second follow-up study the same investigators applied bilateral low-frequency TMS (1Hz) once a week for 12 weeks, with the first six treatments to the left DLPFC and the next six to the right DLPFC in 16 patients with ASD. EEG and behavioral evaluations pre- and post-rTMS revealed normalization of induced gamma activity and a reduction in both repetitive behaviors and irritability(Baruth, Casanova et al. 2010). Using this same protocol, this group explored error monitoring pre- and post rTMS and found improvements in both ERP indices and behavioral measures of error monitoring following 1 Hz stimulation once a week first to left then to right DLPFC in 20 individuals with ASD (Sokhadze, Baruth et al. 2012). Lastly, using a similar design the same group also recently published a paper describing improvements in ERP indices of visual processing, accuracy on a selective attention task, and behavioral measures of repetitive behavior and irritability of 25 individuals with ASD following the 12-week protocol described above (Casanova 2012). Again, these studies provide promising preliminary data for the use of low-frequency rTMS to DLPFC for the alleviation of aberrant behavior and physiological indices in ASD, but are limited by small sample size and unblinded designs. It is also unclear in the paradigms where both left and right hemisphere were stimulated whether the effect was driven by one or the other hemisphere, or whether the effect was a result of the combination of both. Finally, the behavioral improvements appear to be limited to repetitive behaviors, irritability, and specific measures of attention.
We have also published reports showing improved performance on a behavioral task in patients with ASD following a TMS protocol. Fecteau and colleagues (Fecteau, Agosta et al. 2011) conducted a study where they applied a single session of low-frequency (1 Hz) rTMS to left and right pars triangularis and pars opercularis (the two regions that comprise Broca's area) in 10 individuals with ASD and 10 matched neurotypical control participants in a double-blind, pseudorandomized, sham-controlled study. Compared to the sham condition all 10 individuals with ASD showed reduced latency to name objects on the Boston Naming Test following stimulation to the left pars triangularis (BA 45) while 9/10 showed an increased latency following stimulation to the adjacent left pars opercularis (BA44). The findings suggest that in individuals with ASD left BA45 exerts an abnormally excessive amount of inhibition on left BA44, thus inhibiting left BA45 results in a suppression of the excessive inhibitory control and a behavioral improvement. However this interpretation has yet to be empirically tested. Findings from this study though short-lived, given the single session design, suggest that rTMS to BA45 may lead to improvements in language processing in ASD and warrant further studies aimed at long-term improvements in this domain (Fecteau, Agosta et al. 2011). This study also demonstrated the importance of strict anatomical targeting as the opposite result was found when the target region was in the adjacent BA44 region.
Fitzgerald's group based in Melbourne Australia is also exploring the potential of rTMS to improve specific symptoms of ASD. In a recent paper they describe a study in which a single session of 1 Hz rTMS was applied to one of two motor cortical regions (Left M1 and Supplementary Motor Area (SMA)) in 11 individuals with ASD. Though not often considered a core impairment in ASD, motor dysfunction is often noted as an associated feature. Following stimulation of M1, there was a significant improvement in a late movement-related cortical potential (MRCP) thought to be associated with the execution of movement while stimulation of SMA resulted in an improvement of the early MRCP suggesting enhanced motor preparation. Though post-stimulation improvements were seen, their MRCPs still remained outside of what would be considered neurotypical levels, and despite improvements in the electrophysiological response, there was not a significant improvement in behavioral measures of motor functioning (Enticott, Rinehart et al. 2012).
This same group is currently conducting a sham-controlled, double blind clinical trial of a specific type of high frequency rTMS (deep rTMS) to the medial prefrontal cortex (mPFC) a region thought to play a key role in theory of mind abilities (understanding the mental state of others) (Frith and Frith 1999; Amodio and Frith 2006; Mitchell, Cloutier et al. 2006; Saxe and Powell 2006). The goal of this study is to develop a therapeutic intervention aimed at improving the individual's capacity for understanding other's mental states. Though this study is still ongoing, the group has reported that several participants have responded to the treatment resulting in a reduction of self-reported clinical symptoms (Enticott, personal communication). An individual who had a very pronounced response (Ms. D) was featured in a case report (Enticott, Kennedy et al. 2011). This patient showed improvements on the Interpersonal Reactivity Index (IRI), the Autism Spectrum Quotient (AQ) and the Ritvo Autism-Asperger Diagnostic Scale. She also reported that she found eye contact “less uncomfortable” and found social situations “more natural” even joining a social club and making new friends. She noted that she “did not have to think so much of what to say” and was more aware of instances when she might be making someone uncomfortable. She also reported an increased capacity for empathy and perspective taking, even for incidents that occurred many years before. She also experienced greater consideration for and affection toward family members following the stimulation protocol. These changes were also noted by her family. Her mother described her as more considerate of others following the stimulation. These improvements seemed to remain at the one month and six month follow-up (Enticott, Kennedy et al. 2011). Still other groups including one in Israel (NCT 01388179) and one in France (NCT 01648868) also have ongoing clinical trials applying rTMS for the treatment of specific ASD symptoms, the results of which have yet to be published.
Schizophrenia
Studies using TMS and rTMS in schizophrenia have been more extensively reviewed in Freitas et al. (Freitas, Fregni et al. 2009). Initial rTMS studies focused on the clinical efficacy of rTMS on the positive and negative symptoms of the disease but overall the results were inconsistent and effect sizes rather small. For positive symptoms (specifically auditory hallucinations), the goal was to inhibit the left temporoparietal cortex via 1 Hz rTMS, based on the rationale that increased temporal activity correlates with positive symptoms (for a review see(Freitas, Fregni et al. 2009)). In regards to negative symptoms, numerous studies attempted to increase the activity in the left prefrontal region via high-frequency rTMS as this might regulate the dopamine release and ameliorate the negative symptoms.
Among numerous studies that targeted the negative symptoms, only five randomized controlled trials assessed the cognitive effects ((Novak, Horacek et al. 2006; Mogg, Purvis et al. 2007; Fitzgerald, Herring et al. 2008; Schneider, Schneider et al. 2008; Mittrach, Thunker et al. 2010).Mogg et al. applied 10 consecutive daily sessions of 10 Hz rTMS to the left DLPFC and reported a significant improvement in verbal learning in a series of patients with prominent negative symptoms. In addition, two intra-individual crossover studies applied 10sessions of 20 Hz rTMS to the left DLPFC (Rollnik, Huber et al. 2000; Huber, Schneider et al. 2003) and though initially failed to detect a significant effect of rTMS on cognition (Rollnik et al., 2000), when analyzed stratifying for gender, an improvement of visuomotor tracking was observed in females (Huber et al., 2003).
Further studies seem warranted, specifically considering the encouraging findings of open, proof-of-principle trials. For example, Cohen et al.(Cohen, Bernardo et al. 1999) stimulated the PFC bilaterally with 20 Hz using a double-cone coil, a special coil considered to stimulate deeper brain regions compared to standard figure-of-eight coil. Following 10sessions of rTMS, the authors reported an improvement in visual memory. In a recent study, Levkovitz et al. (Levkovitz, Rabany et al. 2011)performed bilateral deeper stimulation of the prefrontal cortex (L>R) using an H-coil and reported improvement in executive functions, spatial working memory, attention, and rapid visual information processing. It seems that indeed the use of special TMS coils that enable direct stimulation of deeper brain structures may be important in this setting. Studies using more conventional TMS coils with limited depth penetrance have yielded less encouraging results. For example, Sachdev et al. applied 20 sessions of20 Hz rTMS to the left DLPFC and found no improvement in cognitive functions (Sachdev, Loo et al. 2005).
Another promising approach in schizophrenia appears to be the targeting of nodes of identified neural networks. Specifically, targeting cerebellar vermis to have an impact of distributed bihemispheric neural networks is an intriguing notion(Demirtas-Tatlidede, Freitas et al. 2011). Schutter et al (Schutter, van Honk et al. 2003) reported early promising results targeting the cerebellar vermis. In a carefully designed open-safety study, we embraced this novel approach and targeted the cerebellar vermis using an intermittent TBS paradigm (Demirtas-Tatlidede, Freitas et al. 2010). Following 10sessions of stimulation in5 days (twice per day with a minimum gap of 4 h), we observed an improvement in working memory and visual learning domains while no significant decline was found. The direction of improvement in 70% of the neuropsychological variables suggests a trend toward improvement in cognition. A double-blind, sham-controlled Phase-II study is currently underway.
Another important consideration in this setting is the possibility of employing stimulation paradigms tuned to specific brain oscillations and targeting bihemispheric structures. The combination of TMS with EEG, and specifically EEG-gated TMS protocols enable such approaches (Shafi, Westover et al. 2012).For example, rTMS can lower the excessive gamma oscillatory activity found in patients with schizophrenia when applied at appropriate stimulation frequency bilaterally over the DLPFC (Barr, Farzan et al. 2011). This was associated with significant improvements in working memory.
Alzheimer's Disease
We hypothesize that a therapy that targets specific brain circuits that are impaired in AD in order to promote their functional integrity and restore their plasticity might preserve cognitive function and effectively reduce the burden of the disease. Data from several small, single-site, randomized controlled trials reveal extremely encouraging results. If confirmed in appropriately powered and controlled clinical efficacy trials such an approach would represent a major advance in the treatment of AD that could be truly transformative for the care of patients, reducing the impact on their families, and potentially producing a substantial financial saving for society. Furthermore, such an approach might serve as a proof-of-concept for the notion of harnessing and modulating plasticity as a corner-stone of neurologic therapeutics.
The hypothesis underlying the proposed novel therapeutic approach is that repetitive TMS targeting specific nodes of brain networks affected in AD, can enhance plasticity and modulate connectivity in the targeted brain circuit thus making it more responsive to circuit specific CR tasks and altering the pathologic metabolic cascade.
Two randomized controlled trials have been published using TMS for AD and both reported positive changes following consecutive sessions of rTMS application. Cotelli et al. (2011)applied 20 sessions of 20 Hz rTMS over the left DLPFC and performed a series of language tests in patients with moderate AD. The authors reported a significant effect of rTMS on auditory comprehension. Secondly, Ahmed et al.(2012)tested the effects high and low frequency rTMS applied over the bilateral DLPFCs. A significant improvement in global cognitive functioning was reported following 5 consecutive sessions of bilateral high-frequency stimulation and this effect was maintained for 3 months.
In an open trial, Bentwich et al. (2011) tested the effects of 10 Hz rTMS together with cognitive training in patients with AD. This combined therapy was applied for 6 weeks while the authors stimulated 6 different locations (Broca, Wernicke, right and left DLPFC, right and left parietal somatosensory association cortices) with an aim to cover the cognitive domains affected by the disease .A significant improvement in the primary outcome measure, Alzheimer Disease Assessment Scale-Cognitive (ADAS-cog), was detected at 6 weeks and 4.5 months. MMSE revealed a significant change at 6 weeks only.
Subsequently, Bentwich and colleagues (Bentwich, Dobronevsky et al. 2011) completed a small, randomized double blind controlled study of TMS-CR in 15 patients with mild-moderate AD on cholinesterase inhibitor therapy (stable dose for ≥ 2 months). Seven patients were randomized to TMS-CR while eight were double sham controls. They followed exactly the same protocol as our pilot study and found a mean improvement on the ADAS-cog of 3.8 points in the active TMS-CR group, as compared with a mean improvement of 0.5 in the control group (p =0.04, mean difference in ADAS-Cog between groups at endpoint of 4.3 points). There was also a significant improvement in the average CGIC score in the real versus sham groups (p<0.05): The CGIC is a 7-point “global change” rating in which 4=no change, and 3,2,1 or 5,6,7 is “minimal”, “moderate” and “marked” improvement or worsening, respectively. The real TMSCR group showed an average change rating of 3.6 and the sham group an average change rating of 4.3, representing slight average improvement and worsening, respectively. The difference between means, in this case 0.7, is what represents the degree of difference between treatments in global change. This mean difference compares favorably to the one in encountered in trials of marketed treatments, which has been in the 0.3 to 0.4 range. There were no reported side effects of treatment. A double-blind, multiple site European study is under way to confirm these promising findings.
We have recently completed an investigator-initiated randomized, double-blind clinical trial in 12 patients with mild-moderate AD (MMSE 18-26). Patients were randomized to active (n=6) or sham (n=6) intervention. Patients underwent six weeks of daily one-hour sessions of active or sham TMS-CR as adjunct to their stable pharmacologic therapy (five sessions per week, Monday to Friday, total of 30 sessions). A short train of repetitive TMS was applied to a given brain region immediately before cognitive training tailored to engage the targeted brain circuit. Six different brain regions engaged in major cognitive functions affected by AD were targeted, as identified using the patient's own brain MRI scan. The cognitive tasks were developed to fit these regions and engaged the modulated brain circuits. The primary outcome measure was to assess improvement relative to sham on the ADAS-cog score at the end of the 6 weeks of intervention, and at a 3 month follow-up. The active treatment group improved by 2.9 points relative to baseline, whereas the sham treatment group worsened by 2.7 points (p< 0.01). Therefore, a primary analysis for the difference between groups at endpoint, controlling for baseline (effectively a covariance analysis or a test of difference scores) revealed a mean difference in ADAS-Cog between groups at endpoint of 5.6 points, markedly greater than the reported effect of pharmacologic or non-pharmacologic interventions. It is further compelling that relative to baseline, all patients in the active TMS-CR group showed an improvement (either immediately after the intervention or within one month), while none of the patients in the sham group showed improvement.
The few trials conducted to date reveal positive effects and provide initial evidence on the potential of noninvasive brain stimulation for cognitive enhancement in AD. However, these studies have not been replicated and the evidence remains preliminary. While the initial target in patients with mild cognitive impairment and mild AD should be to halt the progression of the disease, cognitive enhancement strategies in moderate to severe AD should target multiple cognitive domains in conjunction with cognitive training in order to achieve a clinically meaningful effect. Further systematically designed, sham-controlled trials will establish whether noninvasive brain stimulation might prove an effective cognitive enhancing strategy for this implacable disease.
Conclusion
The brain changes across the lifespan. First, growing evidence demonstrates that the brain undergoes a complex array of neuroanatomical and neurophysiologic modifications from birth till death, so that concepts such as ‘development’ and ‘senescence’ have become increasingly arbitrary in their definition. Instead, the lifespan and the aging process itself might be best viewed as a ‘life-long developmental process’, which is thought to constitute the underpinnings of shifts in cognition and behavior throughout each individual's life. Second, along with changes in brain structure and function, the mechanisms by which structure and function can be modified (the mechanisms of brain plasticity themselves) appear to also change over the lifespan. This developmental process is very well-controlled by the processes described above including LTP, LTD, as well as homeostatic and metaplastic control of these processes. Over the course of development the brain goes through critical periods where a specific region of cortex has heightened or exclusive capacity for plasticity.
This chapter highlights the importance of brain plasticity throughout the lifespan for optimal brain health. In health, local cortical and network plasticity might keep a fine-tuned balance, which optimizes functionality (Pascual-Leone et al., 2011). Such a ‘life-long dynamic, plastically changing brain’ poses several challenges, including the definition of a functionally ‘normal’ brain at a given point in time in a given individual. A functionally ‘normal’ brain is a changing brain, a brain whose capacity and mechanisms of change are shifting appropriately from one time-point in life to another.
We've also highlighted how pathology of brain plasticity may underlie a number of neuropsychological disorders across the lifespan. ASD and schizophrenia may represent two sides of the same coin with the symptoms of ASD potentially stemming from uncontrolled excitatory plasticity and an overall potentiated cortex, and symptoms of schizophrenia stemming from a lack of excitatory plasticity. At the other end of the lifespan, in late adulthood, maintaining the capacity for plastic change may be critical for avoiding age-related cognitive decline with dementia and Alzheimer's disease representing an inability for plastic change.
If, as we propose, these diseases and disorders stem from aberrant plasticity mechanisms, then modulating such systems using TMS may represent a novel alternative to drug treatments. Pilot studies suggest promise for the treatment of ASD, Schizophrenia, and Alzheimer's Disease using specific rTMS protocols. As of now, these treatments should be considered highly experimental and in need for further replication in properly powered and controlled trials. However, they offer valuable proof-of-principle support for the concept of harnessing and guiding brain plasticity for neurotherapeutics.
The future of translational neuroscience, with the ultimate goal of understanding the mechanisms driving brain health and disease and developing therapeutic interventions that optimally treat brain diseases depends on our ability to: 1. Understand the mechanisms of plasticity across the lifespan and how they are optimized in neurologically healthy individuals, 2. Identify how dysfunction in these mechanisms can account for the clinical phenotype of neuropsychological diseases across the lifespan, and finally 3. Further develop approaches and tools to (ideally noninvasively) treat disorders of brain plasticity. If one assumes that abnormalities in plasticity predate any structural or functional brain alterations or any behavioral symptom, then therapeutic approaches to normalize brain plasticity may reduce or prevent the anatomical and functional brain pathology underlying these disorders and in doing so prevent the clinical manifestation of the disease.
Acknowledgement statement (including conflict of interest and funding sources)
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Work on this study was supported by a grant from the National Institutes of Health - Harvard Clinical and Translational Science Center/Harvard Catalyst (UL1 RR025758), and the Sidney Baer Foundation.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Tsumoto T, et al. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17(17):6707–16. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–85. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, et al. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One. 2011;6(7):e22627. doi: 10.1371/journal.pone.0022627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruth JM, Casanova MF, et al. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Evoked-Gamma Frequency Oscillations in Autism Spectrum Disorder (ASD). J Neurother. 2010;14(3):179–194. doi: 10.1080/10874208.2010.501500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, et al. Cortical plasticity in Alzheimer's disease in humans and rodents. Biol Psychiatry. 2007;62(12):1405–12. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper T. Observations of the Purkinje cells in the cerebellar vermis in autism. . Neuropath Exp Neurol. 1996;55:613. [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–62. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, et al. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bentwich J, Dobronevsky E, et al. Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer's disease: a proof of concept study. J Neural Transm. 2011;118(3):463–71. doi: 10.1007/s00702-010-0578-1. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec (Hoboken) 2011;294(10):1646–52. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV. A journey from neocortex to hippocampus. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):621–3. doi: 10.1098/rstb.2002.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):357–74. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19(10):3723–30. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, et al. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci. 2009;21(5):1013–22. doi: 10.1162/jocn.2009.21079. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, et al. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, et al. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cardenas-Morales L, Gron G, et al. Exploring the after-effects of theta burst magnetic stimulation on the human motor cortex: a functional imaging study. Hum Brain Mapp. 2011;32(11):1948–60. doi: 10.1002/hbm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli E, Patish H, et al. The aging hand. J Gerontol A Biol Sci Med Sci. 2003;58(2):146–52. doi: 10.1093/gerona/58.2.m146. [DOI] [PubMed] [Google Scholar]
- Casanova M, Buxhoeveden DP, et al. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF. Neuropathological and genetic findings in autism: the significance of a putative minicolumnopathy. Neuroscientist. 2006;12(5):435–41. doi: 10.1177/1073858406290375. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Baruth JM, El-Baz A, Tasman A, Sears L, Sokhadze E. Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. . Translational Neuroscience. 2012;3(2):170–180. doi: 10.2478/s13380-012-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Wolters A, et al. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–9. [PubMed] [Google Scholar]
- Cohen E, Bernardo M, et al. Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67(1):129–30. doi: 10.1136/jnnp.67.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH., Jr. Genetics of autism. Child Adolesc Psychiatr Clin N Am. 2001;10(2):333–50. [PubMed] [Google Scholar]
- Courchesne E, Campbell K, et al. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–45. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23(2-3):153–70. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(Pt 6):1591–609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102(35):12560–5. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, et al. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59(4):347–54. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, et al. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65(4):378–85. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, et al. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Stein PS, et al. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–22. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, et al. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124(1-3):91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, et al. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum. 2011;10(3):495–503. doi: 10.1007/s12311-010-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Fragile x syndrome and autism: from disease model to therapeutic targets. J Neurodev Disord. 2009;1(2):133–40. doi: 10.1007/s11689-009-9015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–13. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, et al. GABAergic activity in autism spectrum disorders: An investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. 2011;71(5):427–33. doi: 10.1016/j.biopsych.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, et al. Deep repetitive transcranial magnetic stimulation associated with improved social functioning in a young woman with an autism spectrum disorder. J ECT. 2011;27(1):41–3. doi: 10.1097/YCT.0b013e3181f07948. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, et al. Repetitive transcranial magnetic stimulation (rTMS) improves movement-related cortical potentials in autism spectrum disorders. Brain Stimul. 2012;5(1):30–7. doi: 10.1016/j.brs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, et al. Hippocampal CA4 Reelin-positive neurons. Mol Psychiatry. 2000;5(6):571. doi: 10.1038/sj.mp.4000794. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, et al. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8(1):64–9. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, et al. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40(6):743–50. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, et al. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39(2):223–30. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, et al. Brain stimulation over Broca's area differentially modulates naming skills in neurotypical adults and individuals with Asperger's syndrome. Eur J Neurosci. 2011;34(1):158–64. doi: 10.1111/j.1460-9568.2011.07726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, et al. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res. 2002;114(1):11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71(1):17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Herring S, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. 2008;1(1):27–32. doi: 10.1016/j.brs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19(9):2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, et al. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–9. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams CE, et al. Inhibitory neurophysiological deficit as a phenotype for genetic investigation of schizophrenia. Am J Med Genet. 2000;97(1):58–64. doi: 10.1002/(sici)1096-8628(200021)97:1<58::aid-ajmg8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Freitas C, Fregni F, et al. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Mondragon-Llorca H, et al. Noninvasive brain stimulation in Alzheimer's disease: systematic review and perspectives for the future. Exp Gerontol. 2011;46(8):611–27. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, et al. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51(3):339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, et al. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1(2):172–81. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20(6):1432–47. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bartolomei F, et al. Imaging structural and functional connectivity: towards a unified definition of human brain organization? Curr Opin Neurol. 2008;21(4):393–403. doi: 10.1097/WCO.0b013e3283065cfb. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, et al. Enhanced visual spatial attention ipsilateral to rTMS-induced 'virtual lesions' of human parietal cortex. Nat Neurosci. 2001;4(9):953–7. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JC, Wang WK, et al. Phylogeography and conservation genetics of Hygrophila pogonocalyx (Acanthaceae) based on atpB-rbcL noncoding spacer cpDNA. J Plant Res. 2005;118(1):1–11. doi: 10.1007/s10265-004-0185-z. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, et al. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18(3):563–70. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, et al. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37(4-5):571–9. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Schneider U, et al. Gender differences in the effect of repetitive transcranial magnetic stimulation in schizophrenia. Psychiatry Res. 2003;120(1):103–5. doi: 10.1016/s0165-1781(03)00170-7. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009;6:7. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, et al. Memory-related neural systems in Alzheimer's disease: an anatomic study. Neurology. 1990;40(11):1721–30. doi: 10.1212/wnl.40.11.1721. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, et al. Altered response to rTMS in patients with Alzheimer's disease. Clin Neurophysiol. 2006;117(1):103–9. doi: 10.1016/j.clinph.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Jack CK,DS, Jr, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–9. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Akaneya Y, et al. Brain-derived neurotrophic factor induces long-lasting potentiation of synaptic transmission in visual cortex in vivo in young rats, but not in the adult. Eur J Neurosci. 2001;14(8):1219–28. doi: 10.1046/j.0953-816x.2001.01751.x. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, et al. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21(2):385–91. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- Jung NH, Janzarik WG, et al. Impaired induction of long-term potentiation-like plasticity in patients with high-functioning autism and Asperger syndrome. Dev Med Child Neurol. 2013;55(1):83–9. doi: 10.1111/dmcn.12012. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Nadon NL, et al. The neurobiology of aging. Epilepsy Res 68 Suppl. 2006;1:S5–20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82(3):109–21. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, et al. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer's disease patients. J Alzheimers Dis. 2012;31(3):593–9. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- Koch G, Esposito Z, et al. Altered dopamine modulation of LTD-like plasticity in Alzheimer's disease patients. Clin Neurophysiol. 2011;122(4):703–7. doi: 10.1016/j.clinph.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, et al. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage. 2010;51(1):280–7. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kodama S, Fukuzako H, et al. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychol Med. 2001;31(6):1079–88. doi: 10.1017/s0033291701004196. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(19):8856–60. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Pascual-Leone A, et al. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284(5411):167–70. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, et al. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59(7):663–4. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Moore J, et al. Autism: recent molecular genetic advances. Hum Mol Genet. 2000;9(6):861–8. doi: 10.1093/hmg/9.6.861. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, et al. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–50. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- LeBlanc JJ, Fagiolini M. Autism: a “critical period” disorder? Neural Plast. 2011;2011:921680. doi: 10.1155/2011/921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Blumberger DM, et al. The Role of Transcranial Magnetic Stimulation in Treatment-Resistant Depression: A Review. Curr Pharm Des. doi: 10.2174/138161212803523644. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Rabany L, et al. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. Int J Neuropsychopharmacol. 2011;14(7):991–6. doi: 10.1017/S1461145711000642. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, et al. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437(7061):1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Malenka RC. Spike timing-dependent long-term potentiation in ventral tegmental area dopamine cells requires PKC. J Neurophysiol. 2008;100(1):533–8. doi: 10.1152/jn.01384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–5. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16(12):521–7. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Mameli M, Luscher C. Synaptic plasticity and addiction: learning mechanisms gone awry. Neuropharmacology. 2011;61(7):1052–9. doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47(6):783–6. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Markram K, Rinaldi T, et al. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33(4):901–12. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74(3):723–60. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, et al. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70(1):19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. A plasticity-based theory of the pathogenesis of Alzheimer's disease. Ann N Y Acad Sci. 2000;924:42–52. doi: 10.1111/j.1749-6632.2000.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Cloutier J, et al. Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Soc Cogn Affect Neurosci. 2006;1(1):49–55. doi: 10.1093/scan/nsl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrach M, Thunker J, et al. The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry. 2010;43(3):110–7. doi: 10.1055/s-0029-1242824. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR. Metaplasticity: new insights through electrophysiological investigations. J Integr Neurosci. 2008;7(2):315–36. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- Mogg A, Purvis R, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1-3):221–8. doi: 10.1016/j.schres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Doring T, et al. Bilateral parieto-frontal network for verbal working memory: an interference approach using repetitive transcranial magnetic stimulation (rTMS). Eur J Neurosci. 2002;16(8):1627–32. doi: 10.1046/j.1460-9568.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- Novak T, Horacek J, et al. The double-blind sham-controlled study of high-frequency rTMS (20 Hz) for negative symptoms in schizophrenia: negative results. Neuro Endocrinol Lett. 2006;27(1-2):209–13. [PubMed] [Google Scholar]
- Oberman LM, Eldaief M, et al. Abnormal modulation of corticospinal excitability in adults with Asperger's syndrome. Eur J Neurosci. 2012;36(6):2782–8. doi: 10.1111/j.1460-9568.2012.08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LMP-L,A. Altered Brain Plasticity as the Proximal Cause of Autism Spectrum Disorders. In: Tracy J, Hampstead B, Sathian K, editors. Plasticity of Cognition in Neurologic Disorders. Oxford University Press; New York: in press. [Google Scholar]
- Pascual-Leone A, Amedi A, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24(3-4):302–15. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, et al. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–45. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Perry EK, Lee ML, et al. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158(7):1058–66. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29(7):349–58. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, et al. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, et al. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci U S A. 2007;104(33):13501–6. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Perrodin C, et al. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, et al. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18(4):763–70. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, et al. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rollnik JD, Huber TJ, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport. 2000;11(18):4013–5. doi: 10.1097/00001756-200012180-00022. [DOI] [PubMed] [Google Scholar]
- Roman F, Staubli U, et al. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418(2):221–6. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–79. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Loo C, et al. Transcranial magnetic stimulation for the deficit syndrome of schizophrenia: a pilot investigation. Psychiatry Clin Neurosci. 2005;59(3):354–7. doi: 10.1111/j.1440-1819.2005.01382.x. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–52. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Wainer BH, et al. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer's disease. Neuroscience. 1987;23(2):389–98. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17(8):692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60(7):989–94. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schneider AL, Schneider TL, et al. Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul. 2008;1(2):106–11. doi: 10.1016/j.brs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, et al. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett. 2003;336(2):73–6. doi: 10.1016/s0304-3940(02)01077-7. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, et al. Impaired motor skill learning in schizophrenia: implications for corticostriatal dysfunction. Biol Psychiatry. 1996;39(4):241–8. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Shafi MM, Westover MB, et al. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur J Neurosci. 2012;35(6):805–25. doi: 10.1111/j.1460-9568.2012.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21(1):1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback. 2010;35(2):147–61. doi: 10.1007/s10484-009-9121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, Baruth JM, et al. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback. 2012;37(2):91–102. doi: 10.1007/s10484-012-9182-5. [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz A, et al. Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. J Autism Dev Disord. 2009;39(4):619–34. doi: 10.1007/s10803-008-0662-7. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Lacaille JC, et al. The Essence of Memory. Preface. Prog Brain Res. 2008;169:ix–x. doi: 10.1016/S0079-6123(07)00028-3. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72(1):83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–48. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, et al. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299(5612):1585–8. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Theoret H, Halligan E, et al. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol. 2005;15(3):R84–5. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180(4):583–93. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, et al. Tracking Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet. 2007;37(1):61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10(3):207–14. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss HU, Schiff ND. MRI of neuronal network structure, function, and plasticity. Prog Brain Res. 2009;175:483–96. doi: 10.1016/S0079-6123(09)17532-5. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, et al. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage. 2009;45(1):215–23. doi: 10.1016/j.neuroimage.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-8. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11(3):213–28. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, et al. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 2010;50(3):862–72. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277(42):39944–52. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, et al. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113(5):559–68. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15(4):253–66. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]