Abstract
Rab proteins, small GTPases, are key regulators of mammalian Golgi apparatus organization. Based on the effect of Rab activation state, Rab proteins fall into two functional classes. In Class1, inactivation induces Golgi ribbon fragmentation and/or redistribution of Golgi enzymes to the ER, while overexpression of wild type or activation has little, if any, effect on Golgi ribbon organization. In Class 2, the reverse is true. We give emphasis to Rab6, the most abundant Golgi-associated Rab protein. Rab6 depletion in HeLa cells causes an increase in Golgi cisternal number, longer, more continuous cisternae, and a pronounced accumulation of vesicles; the effect of Rab6 on Golgi ribbon organization is probably through regulation of vesicle transport. In effector studies, motor proteins and their regulators are found to be key Rab6 effectors. A related Rab, Rab41, affects Golgi ribbon organization in a contrasting manner. The balance between minus- and plus-end directed motor recruitment may well be the major Rab-dependent factor in Golgi ribbon organization.
Keywords: Golgi apparatus, Rab6 subfamily, Rab6, Rab41, Golgi ribbon organization
1. Introduction
The cisternal organization of the Golgi apparatus was first identified in the 1950s using electron microscopy (for reviews, see Farquhar and Palade, 1981; Darido and Jane, 2013). Since then, the Golgi apparatus has been extensively studied. The Golgi apparatus occupies a central role in membrane trafficking pathways and, in most mammalian cells, it is organized into a ribbon-like structure composed of multiple Golgi stacks (for review, see Wei and Seemann, 2010). The maintenance of Golgi ribbon organization is essential for cargo proteins to be correctly modified and efficiently sorted. Multiple proteins have been identified to be involved in regulation of Golgi ribbon organization.
Rab proteins, members of the Ras superfamily of small GTPases, have been implicated in Golgi ribbon organization and trafficking. The first example was Ypt1 in yeast and from there Ras-like proteins in mammals were discovered and individually studied (for review, see Bock et al., 2001). Of the ~70 mammalian Rab proteins, approximately 20 are Golgi-associated (for review, see Liu and Storrie, 2012). Multiple approaches ranging from candidate protein studies to genome wide screens have been taken to establish the function of these proteins. Candidate protein studies implicate Rab1 (Nuoffer et al., 1994), Rab6 (Goud et al., 1990; Martinez et al., 1997), Rab30 (Kelly et al., 2012) and Rab41 (Liu et al., 2013) in Golgi ribbon organization. Strikingly, Rab6, the most abundant Golgi-associated Rab protein, has little effect on Golgi ribbon organization in an RNAi experiment or when overexpressed as the GDP-locked mutant (Jiang and Storrie, 2005; Sun et al., 2007; Young et al., 2005). However, overexpression of GTP-locked Rab6 results in the redistribution of Golgi proteins to the endoplasmic reticulum (ER) (Martinez et al., 1997), suggesting that Rab inactivation studies fail to reveal the full importance of these proteins to Golgi ribbon organization.
On the whole, genome wide RNAi screens have revealed hundreds of proteins important to secretion but have exposed little about the role of individual Rab proteins in Golgi ribbon organization. For example, a genome wide RNAi screen of cultured Drosophila cells highlighted only Rab1’s importance to Golgi ribbon organization. The importance of Rab1 and Rab11 were additionally implicated in secretion of soluble cargo proteins, but no other Rab proteins were identified as central to these processes (Bard et al., 2006). Similarly, a genome wide RNAi screen with human HeLa cells revealed a single Rab, Rab18, as being significantly important within the early secretory pathway (Simpson et al., 2012). Rab-centric screens based on reductions in GTPase activity achieved through the expression of mutant GAPs, Rab-specific guanine nucleotide activating proteins (Haas et al., 2005, 2007), or overexpression of wild-type or engineered Rab mutations (Dejgaard et al., 2008) have implicated 3 of ~70 mammalian Rab proteins required for Golgi ribbon organization: Rab1, Rab18, and Rab43 (mis-identified as Rab41 in Haas et al., 2005).
In this review, we focus on the comparative importance of Rab inactivation versus activation on Golgi ribbon organization. Our analysis suggests much of the difference between the two can be explained on the basis of minus- versus plus-end directed motor protein recruitment. In other words, vesicle transport via motors and microtubules must occur for detectable Golgi reorganization by light microscopy.
2. Rab proteins and their subfamilies
With ~70 members in human and 11 in yeast, Rab proteins constitute the largest group within the Ras GTPase superfamily. Most Rab proteins identified so far are ubiquitously expressed. However, in mammals, several Rab proteins are expressed only in specific cell types and tissues. For example, for the Rab6 subfamily, which is important to Golgi ribbon organization (for review, see Liu and Storrie, 2012), Rab6b is selectively expressed in neuronal cells (Opdam et al., 2000), Rab6c is only expressed in brain, testis, prostate and breast (Young et al., 2010), and the other subfamily members, Rab6a, Rab6a′ and Rab41, are ubiquitously expressed. The tissue-specific expression of several Rab proteins in humans may reflect either the complexity of transport pathways in higher eukaryotes or alternate Rab functions. Here, we concentrate on ubiquitously expressed Rab proteins important to Golgi ribbon organization.
Rab proteins switch between inactive GDP-bound and active GTP-bound forms. Conversion between these forms is accelerated by two different kinds of regulatory proteins. Guanine nucleotide exchange factors (GEFs) promote the exchange of GTP for GDP, while GTPase-activating proteins (GAPs) promote GTP hydrolysis to GDP and thus inactivate the Rab proteins (for review, see Barr and Lambright, 2010). GEFs are numerous and belong to several different families. In mammals, GAPs are also numerous, but with one exception all belong to the TBC domain family. GEFs likely play a role in the localization of Rab proteins to individual membrane systems, while GAPs may be less important as some Rab proteins do possess significant endogenous GTPase activity. In the GTP-bound, active form, Rab proteins can recruit specific effectors and mediate distinct stages of membrane trafficking: budding, transport along cytoskeleton, tethering to a target membrane and fusion. Accordingly, Rab effectors fall within many different protein functional classes including sorting adaptors, motors or motor adaptors, tethers and SNARE-interacting proteins (for reviews, see Grosshans et al., 2006; Stenmark, 2009). Most Rab proteins can interact with multiple effectors, and likely, a single protein can be an effector for several different Rab proteins (for review, see Barr, 2009). Using yeast two-hybrid screening and biochemical approaches, more and more Rab effectors are being identified.
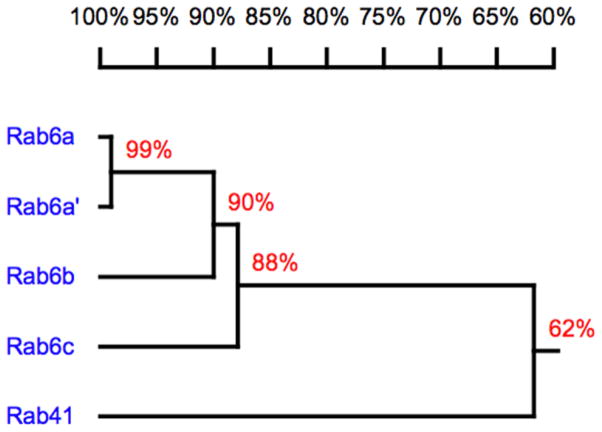
On the basis of homology, mammalian Rab proteins may be grouped into 8 subfamilies (Pereira-Leal and Seabra, 2001) with Golgi organization and trafficking regulators being found in at least 5 of these subfamilies. We give emphasis here to the Rab6 subfamily. Rab6 was the first Rab protein to be associated with the mammalian Golgi apparatus (Goud et al., 1990). The protein is highly enriched in trans Golgi cisternae to the trans-Golgi network. With time, Rab6 was found to consist of two very closely related isoforms, Rab6a and Rab6a′, that differ in only 3 amino acids (Echard et al., 2000). On the basis of sequence homology, Rab6a, Rab6a′, Rab6b, Rab6c and Rab41 co-segregate in the phylogenetic tree, and constitute the Rab6 subfamily (Pereira-Leal and Seabra, 2001). Moreover, based on their electrostatic potential and sequence, the Rab6 isoforms and Rab41 form a subcluster predicted to have similar cellular localization and/or function (Stein et al., 2012). As shown in Figure 1, these proteins are closely related and even the most distant member, Rab41 shares more than 60% identity with the Rab6 isoforms and within central portion, amino acids 9 to 192, has almost 80% identity with other members of Rab6 subfamily (Liu et al., 2013). Despite these similarities, these proteins show distinct functions.
Figure 1.
Homology relationship of human Rab6 subfamily members. The dendrogram was generated using DNAman version 6.2. The numbers on the branches represent amino acid identity.
3. The Golgi apparatus and its organization
It is well known that the Golgi apparatus is composed of flattened cisternal membrane structures. These Golgi cisternae vary in resident enzymes and functions. Thus, they can be classified into four distinct regions: cis, medial, trans and trans-Golgi network (TGN). Different Golgi resident proteins must be localized to the correct cisternae to allow the secretory cargo proteins to be sequentially modified and processed (for review, see Lowe, 2011). Organization of Golgi cisternae depends on the cell types and species. In most mammalian cells, several Golgi cisternae are layered on top of each other to generate compact stacks. Multiple Golgi stacks are then connected into a ribbon-like structure that is usually situated in the perinuclear region of the cell (for review, see Wei and Seemann, 2010). The Golgi apparatus is less organized in lower eukaryotes. In plants and some yeast species including Schizosaccharomyces pombe and Pichia pastoris, the Golgi stacks are unlinked and dispersed throughout the cytoplasm, while in budding yeast Saccharomyces cerevisiae, the stacked structure of Golgi cisternae is absent, i.e., each Golgi cisternae is individually distributed in the cell (for reviews, see Mowbrey and Dacks, 2009; Dupree and Sherrier, 1998; Suda and Nakano, 2012).
Despite the diversity of Golgi organization in eukaryotic cells, its function seems to be highly conserved. The primary role of the Golgi apparatus is processing and sorting of newly synthesized proteins and lipids. Secretory cargo proteins exported from the ER enter the Golgi apparatus at the cis face. Then they undergo post-translational processing and glycosylation within the Golgi apparatus. At the trans face, secretory cargo proteins are sorted and delivered to various destinations in the cell. This forward transport, by which newly synthesized cargo proteins are transferred from cis to trans direction, is actually known as anterograde trafficking pathway (for reviews, see Nickel and Wieland, 1998; Palmer and Stephens, 2004). In addition, delivery of secretory cargo proteins causes ER-resident proteins, Golgi-resident proteins, SNAREs and other cycling machinery proteins to be carried forward. These proteins must be all returned back to their original location through retrograde trafficking pathway, which is mediated by COPI-coated vesicles (for reviews, see Boncompain and Perez, 2013; Cottam and Ungar, 2012). In sum, the Golgi apparatus plays a key role in membrane trafficking pathways. Large amounts of proteins and lipids pass through the Golgi apparatus back and forth. In the face of constant flux, how the Golgi apparatus maintains an organized structure to ensure its normal function in membrane transport remains a question that is at best only partially answered.
Adhesive proteins including Golgi reassembly and stacking proteins (GRASPs) and golgins are known to be key components involved in maintenance of Golgi organization. They mediate the tethering of Golgi cisternae and linking of the Golgi ribbon. They might well be termed “hooks and glue” (for review, see Ramirez and Lowe, 2009). Besides, more and more evidence indicates that the balance of membrane trafficking is essential for the maintenance of Golgi organization. Using electron microscopy followed by quantitative analysis in baby hamster kidney (BHK) cells, Griffiths et al (1989) found that 20°C temperature sensitive inhibition of Golgi exit of newly synthesized G protein of vesicular stomatitis virus results in significant enlargement of TGN, while the size of Golgi stack decreases. Studies by Lee et al (1999) suggest that a block of ER-to-Golgi transport due to osmotic stress leads to tubulation of the Golgi apparatus and redistribution of Golgi resident proteins to the ER in a variety of mammalian cells. In addition, trafficking components are essential. Two examples of this are βCOP and the βCOP interactor Scy1-like 1 protein (Scyl1). Knockdown of the COPI subunit βCOP in HeLa cells leads to the conversion of stacked Golgi structures to a perinculear accumulation of aggregated membranes of about 250 nm in diameter (Guo et al., 2008). Scyl1, a member of the Scy1-like family of protein kinases, was identified as a binding partner of the COPI coat complex. Depletion of Scyl1 in HeLa cells causes disruption of COPI-dependent transport of KDEL receptor from the Golgi apparatus to the ER. In this case, the Golgi apparatus is less organized and the Golgi cisternae are enlarged (Burman et al., 2008, 2010).
4. Rab proteins fall into two major functional/phenotypic classes based on their effects on Golgi ribbon organization
Rab proteins are molecular switches that are active in effector recruitment in the GTP-bound state and inactive in the GDP-bound state. One might expect that various Rab proteins important to Golgi ribbon organization would fall into different functional/phenotypic classes based on the effect of Rab inactivation versus activation. The simplest hypothesis is a binary difference in outcomes in which Golgi ribbon organization, the most commonly assayed trait, would appear normal with either Rab inactivation or activation. In reality, the outcomes do fall into 2 functional classes as discussed below and summarized in Table 1. With Class 1 Rab proteins, the Golgi ribbon is disrupted with Rab inactivation but appears normal with Rab overexpression (excess activity). More specifically, in Class 1A, there is over time a redistribution of Golgi enzymes towards the ER with Rab inactivation and little, if any, effect on the Golgi ribbon with Rab overexpression (excess activity). In Class 1B, the Golgi ribbon fragments with Rab inactivation and the ribbon appears normal with Rab overexpression (excess activity). In contrast, for Class 2, Rab inactivation has little, if any, apparent effect on Golgi ribbon organization while Rab overexpression (excess activity) leads to the redistribution of Golgi enzymes to the ER.
Table 1.
Proposed functional grouping of Rab proteins based on Golgi ribbon phenotype
| Golgi ribbon phenotype produced by Rab inactivation or activation | ||
|---|---|---|
|
| ||
| Inactivation (Depletion, GDP-locked/no nucleotide form or GAP overexpression) | Activation (GTP-locked form or wild-type overexpression) | |
| Class 1A | Redistribution of Golgi enzymes to the ER | Normal |
| Class 1B | Fragmented Golgi ribbon | Normal |
| Class 2 | Normal | Redistribution of Golgi enzymes to the ER |
Rab1 and Rab2, members of the Rab1-Sec4 subfamily, are thought to act early and sequentially in ER-to-Golgi trafficking (Tisdale et al., 1992). However, dominant negative overexpression of Rab2 has less effect on Golgi organization than that of Rab1 (Haas et al., 2007). In particular, depletion of Rab1a by RNAi strongly inhibits ER-to-Golgi trafficking and results in dispersal of the Golgi apparatus to the ER. This dispersal is antagonized by Rab1a or Rab1b expression, suggesting that the isoforms are at least partial functionally redundant (Bard et al., 2006). The observed phenotype is similar to that induced by an ER exit block produced by overexpression of mutant Sar1p (Storrie et al., 1998; Jarvela and Linstedt, 2012), the small GTPase that recruits COPII coat protein to the ER. Together these observations raise the question as to whether Golgi organization is dependent on continuous protein input from the ER. As inhibition of protein synthesis has little effect on Golgi ribbon organization even after 10 hours (Storrie et al., 1998), the dependence of Golgi organization on ER input is likely at the level of recycling pre-existing trafficking machinery (see, also Jarvela and Linstedt, 2012). Interestingly, increased Rab1b expression causes increased expression of GalT and other Golgi-associated proteins. These increases are accompanied by elongation of the Golgi cisternae and a pronounced accumulation of round to irregular shaped membrane structures at what is probably the trans side of the Golgi cisternal stack (Romero et al., 2013). The outcome of more recent screening studies supports the conclusion that Rab1 either directly or indirectly is very important to Golgi organization. In screening 38 human Rab GAPs to determine specific Rab proteins that are important for Golgi organization, Haas et al (2007) showed that expression of the Rab1 GAP, TBC1D20, induces loss of Golgi apparatus with punctate structures remaining.
We take Rab1 as being a leading example of Class 1A Rab proteins with respect to phenotype of Golgi ribbon organization (Table 2). In Class 1A, reduced Rab activity, either through overexpression of the GDP-locked form or depletion of the Rab, results in the disruption, in fact, the loss of much of Golgi ribbon organization and the accumulation of Golgi enzymes in the ER (Table 1).
Table 2.
Effects of two major functional classes of Rab proteins on Golgi ribbon organization
| Class | Rab | Isoform | Golgi ribbon phenotype produced by Rab inactivation or activation | References | ||||
|---|---|---|---|---|---|---|---|---|
| Inactivation | Activation | |||||||
| Depletion by RNAi | GDP-locked Rab overexpression | Rab GAP overexpression | Wild-type Rab overexpression | GTP-locked Rab overexpression | ||||
| 1A | Rab1 | Rab1a | Redistribution to the ER | Fragmented (1.5-h expression) | Lost of Golgi apparatus to the ER with punctate structures remaining | Normal | Unknown | (Bard et al., 2006; Haas et al., 2007; Monetta et al., 2007; Nuoffer et al., 1994; Romero et al., 2013; Wilson et al., 1994) |
| Rab1b | Redistribution to the ER with punctate GM130 | Redistribution to the ER with punctate GM130 | Elongation of Golgi cisternae (EM1) | |||||
| 1B | Rab18 | Rab18 | Normal/fragmented2 | Normal | Unknown | Normal/fragmented2 | Fragmented | (Dejgaard et al., 2008) |
| Rab30 | Rab30 | Fragmented | Fragmented | Unknown | Normal | Normal | (Dejgaard et al., 2008; Kelly et al., 2012) | |
| Rab41 | Rab41 | Fragmented | Fragmented | Unknown | Normal | Normal | (Liu et al., 2013) | |
| Rab43 | Rab43 | Unknown | Fragmented | Fragmented | Normal | Unknown | (Dejgaard et al., 2008; Haas et al., 2007) | |
| 2 | Rab63 | Rab6a | Little effect on the Golgi ribbon and by EM1, Golgi cisternae number increases, Golgi ribbon is longer and continuous | Slightly more compact ribbon | Unknown | Redistribution to the ER | Redistribution to the ER | (Ferraro et al., 2014; Jiang and Storrie, 2005; Martinez et al., 1997; Micaroni et al., 2013; Storrie et al., 2012; Sun et al., 2007; Young et al., 2005) |
| Rab6a′ | ||||||||
| Rab33 | Rab33a | Unknown | Unknown | Unknown | Unknown | Unknown | ||
| Rab33b | More continuous Golgi ribbon | Normal | Unknown | Redistribution to the ER | Redistribution to the ER | (Jiang and Storrie, 2005; Starr et al., 2010; Valsdottir et al., 2001) | ||
Golgi ribbon phenotype is obtained by electron microscopy
Results showed by Dejgaard et al (2008) might well be expected to show differences with Rab mutant or careful consideration of expression time. Further studies are needed
The other two isoforms of Rab6, Rab6b and Rab6c, are not shown here. Rab6b is predominantly expressed in neuronal cells and Golgi-associated (Opdam et al., 2000). Rab6c is involved in cell cycle progression (Young et al., 2010)
Rab30 and Rab41, a protein that could be termed Rab6d (Liu et al., 2013) exemplify Class 1B of Golgi ribbon organization regulators (Table 2). Rab30, a ubiquitously expressed Rab protein, is primarily localized to the Golgi apparatus. Loss of Rab30 does not affect anterograde and retrograde trafficking through the Golgi apparatus (Kelly et al., 2012). However, by light microscopy, knockdown of Rab30 using siRNA or overexpression of GDP-locked mutant (Rab30 T23N) fragments the Golgi apparatus into a scattered structure, while overexpression of the GTP-locked mutant (Rab30 Q68L) has no obvious effect on Golgi ribbon organization. By electron microscopy, HeLa cells treated with Rab30 siRNA display fragmented and shorter Golgi cisternae (Kelly et al., 2012). Although Rab41 and Rab6 are in the same subfamily, the role of Rab41 in Golgi ribbon organization is in many ways opposite of Rab6. In contrast to Rab6, Rab41 shows a punctate rather than Golgi concentrated distribution. By light microscopy, both depletion of Rab41 by RNAi and overexpression of GDP-locked Rab41 significantly fragment Golgi apparatus into clustered punctate structures, while the GTP-locked mutant had little, if any, effect on the Golgi ribbon. By electron microscopy, cells lacking Rab41 display comparatively short, isolated Golgi stacks rather than ribbon-like structures. The number of Golgi-associated vesicles also increases in Rab41-depleted cells (Liu et al., 2013). Interestingly, when Rab41 and Rab6 are co-depleted, the Golgi apparatus is fragmented into punctate structures, a phenotype that is similar to Rab41 knockdown alone (Liu et al., 2013). In conclusion, this class of Rab proteins produces disruption of the Golgi ribbon when inactivated, but the activated state has little effect on Golgi ribbon organization. Based on these observations, we term this class, Class 1B (Table 1). Inactivation experiments suggest that Rab18 (Dejgaard et al., 2008) and Rab43 (Dejgaard et al., 2008; Haas et al., 2007) are probably also Class 1B Rab proteins in their effect on Golgi ribbon organization (Table 2).
Rab6 illustrates a second phenotypic class of Golgi regulators. When overexpressed as the GDP-locked form, class 2 Rab proteins have little, if any, effect on Golgi organization as visualized by the juxtanuclear Golgi ribbon as observed by fluorescence microscopy. In striking contrast, when overexpressed as the GTP-locked form, Class 2 members induce the redistribution of Golgi enzymes to the ER (Table 1). Rab6 or more specifically Rab6a was the first reported example of a Class 2 Rab protein. Overall, as first identified, Rab6 is the exemplar protein within the Rab6 subfamily (Goud et al., 1990). Over time, Rab6 itself was found to have two closely related isoforms, Rab6a and Rab6a′ that differ in only 3 amino acids and arise as alternate splice forms (Echard et al., 2000). They are ubiquitously expressed, present in equal amounts, and localize to the trans Golgi and TGN (Antony et al., 1992). Considerable evidence indicates that Rab6a and a′ are functionally redundant. For example, transport of ricin from endosomes to the TGN of Golgi apparatus is regulated by both Rab6a and a′. Depletion of Rab6a causes inhibition of ricin transport to the TGN. However, this inhibition can be abolished by up-regulation of Rab6a′, showing the overlapping roles of Rab6a and a′ in endosome-to-Golgi apparatus transport (Del Nery et al., 2006; Utskarpen et al., 2006). In addition, overexpression of GTP-locked form of either Rab6a or a′ stimulates Golgi-to-ER recycling of Golgi resident glycosylation enzymes (Martinez et al., 1997; Jiang and Storrie, 2005; Young et al., 2005), while knockdown of either of them or overexpression of GDP-locked Rab6a delays the recycling process (Jiang and Storrie, 2005; Young et al., 2005). This suggests that both Rab6a and a′ are associated with Golgi-to-ER transport pathway. The only reported difference in effector binding between Rab6a and Raba′ is in the preferential binding of Kif20A (Rabkinesin-6) to Rab6a′ (Echard et al., 1998). Because of the high degree of biochemical and phenotypic similarity, these two closely related family members are generally collectively referred to as Rab6. Here, for simplicity, we will use Rab6 instead of Rab6a/a′ in the following discussion.
Overexpression of GDP-locked Rab6 or depletion of Rab6 with siRNA that affects the expression of both Rab6a and a′, produces little effect on Golgi ribbon organization with the ribbon being slightly more compact (Jiang and Storrie, 2005; Sun et al., 2007; Young et al., 2005). For Rab33b, a medial Golgi Rab, overexpression of the GDP-locked form also had little, if any, effect on the Golgi ribbon while overexpression of the GTP-locked form induces the redistribution of Golgi cisternal enzymes to the ER (Jiang and Storrie, 2005; Valsdottir et al., 2001). Based on these findings, we suggest that Rab6 and Rab33b are both examples of Class 2 Golgi-associated Rab proteins (Table 2). As observed by electron microscopy, depletion of Rab6 results in increased cisternal continuity of the trans Golgi/TGN in endothelial cells (Ferraro et al., 2014), HeLa cells (Storrie et al., 2012), and macrophages (Micaroni et al., 2013). In addition, in HeLa cells at least, there is increase in cisternal number, a pronounced accumulation of both COPI- and clathrin-coated vesicles and coated membrane fission/fusion figures, and a dilation of the trans cisternae/TGN when Rab6 is depleted (Storrie et al., 2012). In macrophages, cisternal dilatation and vesicle accumulation is less evident (Micaroni et al., 2013). These detailed findings indicate that Rab6 plays a key role in the maintenance of Golgi ribbon organization and vesicle trafficking that is only apparent by observation by electron microscopy. Whether Rab33b depletion would have similar effects remains an untested possibility. In sum, the data indicate that Class 2 Rab proteins regulate Golgi ribbon organization, perhaps through effects on cisternal proximal vesicle transport as indicated by vesicle accumulation in electron micrographs.
5. How do Rab proteins mechanistically affect Golgi ribbon organization?
5.1 General mechanistic predictions
Rab effectors include both motor proteins and their regulatory complexes, various “hooks and glue” proteins such as golgins that are important to holding the Golgi cisternal stack together and machinery proteins for membrane trafficking such as SNAREs (for reviews, see Grosshans et al., 2006; Stenmark, 2009). The Golgi ribbon is located proximal to the microtubule organizing center (MTOC). This is a structure on which the minus-ends of microtubules converge in interphase cells. Golgi organization has been linked to microtubules and motors (for review, see Yadav and Linstedt, 2011). One prediction is that the balance between minus-end and plus-end directed motors might be a major Rab-dependent factor in Golgi ribbon organization. Another possible mechanism is related to the recycling of machinery proteins. Rab1, as suggested earlier, might be an example of this. Rab1 is required for the organization of ER-Golgi intermediate compartment (Jarvela and Linstedt, 2012). In the absence of the intermediate compartment, machinery recycling should be profoundly affected. In the case of an ER exit block, Golgi enzymes accumulate in the ER (Storrie et al., 1998). The relocation of Golgi enzymes to the ER with Rab1 inactivation may be due to its effects on ER exit. Here we concentrate our discussion of Rab6 as the most extensively studied Golgi-associated Rab with respect to effectors and a leading example of Class 2 Rab proteins.
5.2 Rab6 and the role of individual effectors
Work in this laboratory suggests that retrograde vesicle transport pathways are closely related to Rab6-dependent Golgi ribbon organization. In this work, the emphasis was on vesicle transport in two separate retrograde tether-dependent pathways, ZW10/RINT-1 and COG. Inhibition of either trafficking pathway significantly affects Golgi ribbon organization and can be suppressed by Rab6 inactivation.
Zeste White 10 (ZW10) and RINT-1 were originally discovered as a spindle checkpoint protein and a G2/M cell cycle checkpoint protein, respectively (Williams et al., 1992; Xiao et al., 2001). The multi-subunit conserved oligomeric Golgi (COG) complex is known to be a tethering complex implicated in retrograde intra-Golgi transport (Ungar et al., 2006). ZW10/RINT-1 and COG are associated with maintenance of Golgi ribbon organization. Depletion of ZW10 or its binding partner RINT-1 by RNAi disrupts Golgi apparatus into a cluster of punctate Golgi elements (Hirose et al., 2004; Arasaki et al., 2006; Sun et al., 2007). Likewise, cells treated with COG3 siRNA display a fragmented Golgi ribbon (Zolov and Lupashin, 2005; Shestakova et al., 2006). Importantly, regulation of Golgi ribbon organization by ZW10/RINT-1 and COG is Rab6 dependent. If Rab6 and ZW10/RINT-1 or COG3 are both depleted using siRNAs, the Golgi apparatus displays a Rab6 knockdown phenotype, i.e., a relatively compact Golgi ribbon, rather than a ZW10/RINT-1 or COG3 knockdown phenotype. Additionally, disruption of Golgi apparatus induced by inhibitory antibodies directed against COG3 can also be suppressed by Rab6 depletion. Furthermore, overexpression of GDP-locked Rab6 or a mutant Rab6 effector, BiCD C-fragment, inhibits Golgi fragmentation caused by ZW10/RINT-1 knockdown as well, while expression of BiCD C-fragment alone has little, if any, effect on Golgi ribbon organization (Sun et al., 2007; Suvorova et al., 2002). In conclusion, work in this laboratory indicates the key role of ZW10/RINT-1 and COG in Rab6-dependent Golgi trafficking pathways and Golgi ribbon organization.
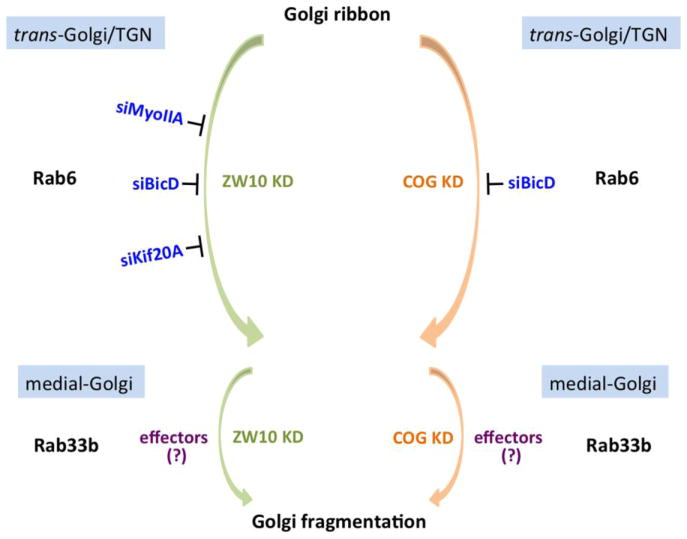
Recent studies by Majeed et al (2014) provide further evidence that ZW10 and COG3 act independently. Taking a candidate protein screening approach, they found that, among Rab6 effectors screened, BicD, MyoIIA and Kif20A are crucial to ZW10 and COG-dependent Golgi ribbon norganization. BicD1 and BicD2, two mammalian homologues of Drosophila Bicaudal-D, act as linkers between motor proteins and Rab6-bound vesicles (Matanis et al., 2002). MyoIIA is a motor protein involved in vesicle fission at the trans-Golgi apparatus and Golgi-to-plasma membrane transport (Valente et al., 2010). Kif20A, originally identified as Rabkinesin-6, functions in retrograde transport from Golgi apparatus to the ER (Echard et al., 1998). Similar to Rab6, treatment of cells with siBicD inhibits fragmentation of Golgi apparatus into clustered punctate Golgi elements induced by either siZW10 or siCOG3. However, double knockdown of either MyoIIA or Kif20A by RNAi can only suppress Golgi dispersal induced by ZW10, but not COG3 depletion (Majeed et al., 2014). Therefore, except BicD, which functions in both pathways, distinct sets of Rab6 effectors are recruited relative to ZW10 and COG (Fig. 2). As observed by electron microscopy, depletion of BicD2 alone mimics much of the Rab6-knockdown phenotype including longer Golgi cisternae and accumulation of coated vesicles, further supporting the role of Rab6 and its effectors in maintenance of Golgi ribbon organization. Other tested Rab6 effectors, including Kif1C, Kif5B, Golgin-97 and OCRL, have little role in ZW10- and COG-associated Golgi ribbon organization (Majeed et al., 2014). It is possible that they act in other pathways involved in Rab6-dependent Golgi ribbon organization. Studies of Arasaki et al (2013) indicate that RINT-1 also functions in endosome-to-TGN trafficking by interacting with a subunit of the COG complex, COG1. This suggests that RINT-1 together with COG1 may be another pathway affecting Rab6-dependent Golgi ribbon organization. Rab1 may be coupled to Rab6 in regulating Golgi ribbon organization and trafficking. Unexpectedly, the protein interaction network of the COG complex includes Rab1 as well as Rab6 and Rab6 effector TMF (Miller et al., 2013). Furthermore, as mentioned previously, increased Rab1b expression causes longer Golgi cisternae, a phenotype that is similar to Rab6 knockdown. Therefore, it is possible that Rab1 and Rab6 work cooperatively in regulating Golgi ribbon organization and trafficking. Further studies are needed to clarify these questions.
Figure 2.
ZW10 and COG act independently in regulating Rab6-dependent Golgi ribbon organization. Rab6 effectors, MyoIIA, BicD and Kif20A, which are crucial to Golgi ribbon organization were shown. Except Kif20A, which binds Rab6a only, MyoIIA and BicD interact with both Rab6a and a′. BicD functions in both pathways, while MyoIIA and Kif20A are pathway selective and act in ZW10-dependent pathway only. Rab33b and Rab6 regulate Golgi ribbon organization sequentially within the same pathway.
In sum, motors appear to be key Rab6 effectors contributing to Golgi ribbon organization. Moreover, the long-standing observation that overexpression of wild-type or GTP-locked Rab6 puts Golgi enzymes into the ER leading to the loss of the ribbon may also be explained on the basis of increased recruitment of motor effectors. This is a hypothesis that remains to be tested in detail.
5.3 Role of Rab33b in Rab6-dependent Golgi ribbon organization
Rab33b, together with Rab33a, are two members of Rab33 subfamily. In contrast with Rab33a, which is restricted to brain and the immune system, Rab33b is universally expressed in mammalian tissues. Analysis by light microscopy and electron microscopy both indicate that Rab33b is localized to the Golgi apparatus, especially in the medial Golgi cisternae (Zheng et al., 1998). Rab33b has been implicated in Golgi-to-ER retrograde trafficking, as well as Golgi ribbon organization. Like Rab6, overexpression of wild-type Rab33b or its GTP-locked isoform induces the redistribution of Golgi enzymes into the ER (Valsdottir et al., 2001). Depletion of Rab33b activity by RNAi or the competitive overexpression of GDP-locked Rab33b suppresses Golgi fragmentation induced by siRNA knockdown of ZW10 or COG3, showing that Rab33b and Rab6 both contribute to regulating ZW10- and COG-dependent Golgi ribbon organization (Fig. 2). Furthermore, Rab33b depletion partially inhibits GTP-locked Rab6 induced retrograde trafficking of Golgi enzymes to the ER. However, knockdown of Rab6 has no apparent effect on GTP-locked Rab33b induced relocation of Golgi enzymes (Valsdottir et al., 2001; Starr et al., 2010), suggesting that Rab33b acts downstream of Rab6 in retrograde Golgi trafficking pathway. Biochemical data from the Pfeffer laboratory (Pusapati et al., 2012) indicate that Rab33b and Rab6 are functionally linked in a Rab cascade, in which they regulate Golgi ribbon organization and trafficking sequentially within the same pathway (Fig. 2). Whether knockdown of Rab33b has other phenotypic effects similar to Rab6 such as vesicle accumulation and increased cisternal number requires electron microscopy.
5.4 Rab41 and Rab6 affect Golgi ribbon organization in an opposing manner
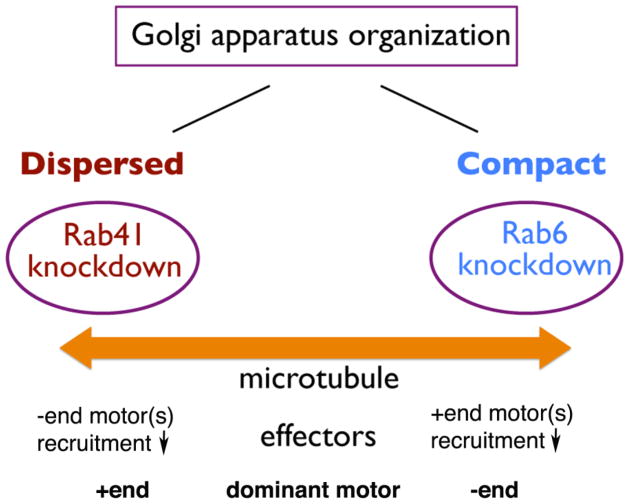
As a member of Rab6 subfamily, Rab41 was supposed to have similar effects on Golgi ribbon organization. However, as described previously, in contrast to Rab6, Rab41 depletion produces shorter and less connected Golgi stacks. Although Rab41 and Rab6 are in the same Rab subfamily, they affect Golgi ribbon organization in a contrasting manner. Rab41 and Rab6 may recruit opposing motors in regulating Golgi ribbon organization (Fig. 3).
Figure 3.
Schematic model of the distinct role of Rab41 and Rab6 in regulating Golgi ribbon organization. By light microscopy, depletion of Rab6 produces a slightly more compact Golgi ribbon, while depletion of Rab41 causes dispersal of Golgi apparatus. It is possible that Rab41 and Rab6 recruit opposing motors in regulating Golgi ribbon organization.
6. Conclusions and perspectives
Of the ~70 human Rab proteins, roughly 20 have been associated with the Golgi apparatus (for review, see Liu and Storrie, 2012). Of these, only one, Rab1, as pointed out by Haas et al. (2007), is essential to ER-to-plasma trafficking in the typical screen. Only a handful has been implicated in the organization of the juxtanuclear Golgi apparatus. From this, we can conclude that few Golgi-associated Rab proteins are functionally important to Golgi organization. However, that may well be a false conclusion. Knockdown or inactivation of Rab6 has little, if any, effect on Golgi ribbon organization, an easy fluorescence assay (Jiang and Storrie, 2005; Sun et al., 2007; Young et al., 2005). However, at the higher resolution of electron microscopy, Rab6 depletion induces a significant increase in Golgi cisternal number and continuity and the Golgi proximal accumulation of coated vesicles (Storrie et al., 2012). Conceivably, other Rab proteins may produce similar effects. Moreover, overexpression of Rab6, as either the wild-type or GTP-locked protein, induces the relocation of Golgi enzymes to the ER with a concomitant loss of juxtanuclear Golgi cisternae (Jiang and Storrie, 2005; Young et al., 2005; Martinez et al., 1997). Only some Golgi-associated Rab proteins have been tested for the phenotypic outcome of the competitive overexpression of the GDP- and GTP-locked form of the protein. Such experiments should be done as they lead to functionally important distinctions. We propose that Golgi-associated Rab proteins may be divided into two major functional classes, Class 1 and 2, based on the effect of inactivation versus activation on Golgi ribbon organization. For Class 1 Rab proteins, Rab inactivation leads to disruption of the Golgi ribbon. For Class 2 Rab proteins, Rab inactivation has little to no obvious effect on Golgi ribbon organization while activation results in ribbon disruption and often the relocation over time of Golgi enzymes to the ER (Table 1).
Ultimately, understanding the importance of Rab proteins to Golgi organization will require the mechanistic characterization of how Rab effectors “seed” protein machines. A candidate protein approach has been taken for Rab6, the most abundant Golgi Rab (Gilchrist et al., 2006). The net conclusion from these studies has been that motor proteins and their regulators are key Rab effectors with respect to Golgi ribbon organization and cisternal continuity and number (Majeed et al., 2014). This is consistent with the effect of Rab6 overexpression in stimulating dynamic tubular membrane extensions from the Golgi apparatus (White et al., 1999) and promoting the relocation of Golgi enzymes to the ER (Martinez et al., 1997). These results may well indicate that the regulation of motors is more important than that of golgins and other potential “hooks and glue” to Rab-dependent Golgi organization at least as observed by light microscopy. Only the extension of such studies to other Rab proteins will provide evidence as to whether this is a general answer. In conclusion, this is a hopeful time as new patterns of Rab action begin to emerge to be important organizers.
Acknowledgments
We appreciate the generosity of XXXX in taking their time to read and comment on this review. Work in the Storrie laboratory has been supported by grants from the NIH and NSF. Current support is from NIGMS grant R01 GM09620.
References
- Antony C, Cibert C, Géraud G, Santa Maria A, Maro B, Mayau V, Goud B. The small GTP-binding protein rab6p is distributed from medial Golgi to the trans-Golgi network as determined by a confocal microscopic approach. J Cell Sci. 1992;103( Pt 3):785–796. doi: 10.1242/jcs.103.3.785. [DOI] [PubMed] [Google Scholar]
- Arasaki K, Takagi D, Furuno A, Sohda M, Misumi Y, Wakana Y, Inoue H, Tagaya M. A new role for RINT-1 in SNARE complex assembly at the trans-Golgi network in coordination with the COG complex. Mol Biol Cell. 2013;24(18):2907–2917. doi: 10.1091/mbc.E13-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasaki K, Taniguchi M, Tani K, Tagaya M. RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex. Mol Biol Cell. 2006;17(6):2780–2788. doi: 10.1091/mbc.E05-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439(7076):604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22(4):461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. Rab GTPase function in Golgi trafficking. Semin Cell Dev Biol. 2009;20(7):780–783. doi: 10.1016/j.semcdb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409(6822):839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Boncompain G, Perez F. The many routes of Golgi-dependent trafficking. Histochem Cell Biol. 2013;140(3):251–260. doi: 10.1007/s00418-013-1124-7. [DOI] [PubMed] [Google Scholar]
- Burman JL, Bourbonniere L, Philie J, Stroh T, Dejgaard SY, Presley JF, McPherson PS. Scyl1, mutated in a recessive form of spinocerebellar neurodegeneration, regulates COPI-mediated retrograde traffic. J Biol Chem. 2008;283(33):22774–22786. doi: 10.1074/jbc.M801869200. [DOI] [PubMed] [Google Scholar]
- Burman JL, Hamlin JN, McPherson PS. Scyl1 regulates Golgi morphology. PLoS One. 2010;5(3):e9537. doi: 10.1371/journal.pone.0009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam NP, Ungar D. Retrograde vesicle transport in the Golgi. Protoplasma. 2012;249(4):943–955. doi: 10.1007/s00709-011-0361-7. [DOI] [PubMed] [Google Scholar]
- Darido C, Jane SM. Golgi Feels Its Own Wound. Adv Wound Care (New Rochelle) 2013;2(3):87–92. doi: 10.1089/wound.2011.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R, Simpson JC, Presley JF. Rab18 and Rab43 have key roles in ER-Golgi trafficking. J Cell Sci. 2008;121(Pt 16):2768–2781. doi: 10.1242/jcs.021808. [DOI] [PubMed] [Google Scholar]
- Del Nery E, Miserey-Lenkei S, Falguières T, Nizak C, Johannes L, Perez F, Goud B. Rab6A and Rab6A′ GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7(4):394–407. doi: 10.1111/j.1600-0854.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- Dupree P, Sherrier DJ. The plant Golgi apparatus. Biochim Biophys Acta. 1998;1404(1–2):259–270. doi: 10.1016/s0167-4889(98)00061-5. [DOI] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279(5350):580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Echard A, Opdam FJ, de Leeuw HJ, Jollivet F, Savelkoul P, Hendriks W, Voorberg J, Goud B, Fransen JA. Alternative splicing of the human Rab6A gene generates two close but functionally different isoforms. Mol Biol Cell. 2000;11(11):3819–3833. doi: 10.1091/mbc.11.11.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J Cell Biol. 1981;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Kriston-Vizi J, Metcalf DJ, Martin-Martin B, Freeman J, Burden JJ, Westmoreland D, Dyer CE, Knight AE, Ketteler R, Cutler DF. A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev Cell. 2014;29(3):292–304. doi: 10.1016/j.devcel.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127(6):1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Goud B, Zahraoui A, Tavitian A, Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Fuller SD, Back R, Hollinshead M, Pfeiffer S, Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989;108(2):277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Punj V, Sengupta D, Linstedt AD. Coat-tether interaction in Golgi organization. Mol Biol Cell. 2008;19(7):2830–2843. doi: 10.1091/mbc.E07-12-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AK, Fuchs E, Kopajtich R, Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7(9):887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci. 2007;120(Pt 17):2997–3010. doi: 10.1242/jcs.014225. [DOI] [PubMed] [Google Scholar]
- Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23(6):1267–1278. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvela T, Linstedt AD. Irradiation-induced protein inactivation reveals Golgi enzyme cycling to cell periphery. J Cell Sci. 2012;125(Pt 4):973–980. doi: 10.1242/jcs.094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Storrie B. Cisternal rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol Biol Cell. 2005;16(5):2586–2596. doi: 10.1091/mbc.E04-10-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EE, Giordano F, Horgan CP, Jollivet F, Raposo G, McCaffrey MW. Rab30 is required for the morphological integrity of the Golgi apparatus. Biol Cell. 2012;104(2):84–101. doi: 10.1111/boc.201100080. [DOI] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol Biol Cell. 1999;10(5):1445–1462. doi: 10.1091/mbc.10.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hunt L, Storrie B. Rab41 is a novel regulator of Golgi apparatus organization that is needed for ER-to-Golgi trafficking and cell growth. PLoS One. 2013;8(8):e71886. doi: 10.1371/journal.pone.0071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Storrie B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol Life Sci. 2012;69(24):4093–4106. doi: 10.1007/s00018-012-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23(1):85–93. doi: 10.1016/j.ceb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Majeed W, Liu S, Storrie B. Distinct sets of Rab6 effectors contribute to ZW10--and COG-dependent Golgi homeostasis. Traffic. 2014;15(6):630–647. doi: 10.1111/tra.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O, Antony C, Pehau-Arnaudet G, Berger EG, Salamero J, Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1997;94(5):1828–1833. doi: 10.1073/pnas.94.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4(12):986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- Micaroni M, Stanley AC, Khromykh T, Venturato J, Wong CX, Lim JP, Marsh BJ, Storrie B, Gleeson PA, Stow JL. Rab6a/a′ are important Golgi regulators of pro-inflammatory TNF secretion in macrophages. PLoS One. 2013;8(2):e57034. doi: 10.1371/journal.pone.0057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VJ, Sharma P, Kudlyk TA, Frost L, Rofe AP, Watson IJ, Duden R, Lowe M, Lupashin VV, Ungar D. Molecular insights into vesicle tethering at the Golgi by the conserved oligomeric Golgi (COG) complex and the golgin TATA element modulatory factor (TMF) J Biol Chem. 2013;288(6):4229–4240. doi: 10.1074/jbc.M112.426767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18(7):2400–2410. doi: 10.1091/mbc.E06-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583(23):3738–3745. doi: 10.1016/j.febslet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Nickel W, Wieland FT. Biosynthetic protein transport through the early secretory pathway. Histochem Cell Biol. 1998;109(5–6):477–486. doi: 10.1007/s004180050249. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Davidson HW, Matteson J, Meinkoth J, Balch WE. A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125(2):225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdam FJ, Echard A, Croes HJ, van den Hurk JA, van de Vorstenbosch RA, Ginsel LA, Goud B, Fransen JA. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J Cell Sci. 2000;113( Pt 15):2725–2735. doi: 10.1242/jcs.113.15.2725. [DOI] [PubMed] [Google Scholar]
- Palmer KJ, Stephens DJ. Biogenesis of ER-to-Golgi transport carriers: complex roles of COPII in ER export. Trends Cell Biol. 2004;14(2):57–61. doi: 10.1016/j.tcb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313(4):889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Pusapati GV, Luchetti G, Pfeffer SR. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J Biol Chem. 2012;287(50):42129–42137. doi: 10.1074/jbc.M112.414565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20(7):770–779. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Romero N, Dumur CI, Martinez H, García IA, Monetta P, Slavin I, Sampieri L, Koritschoner N, Mironov AA, De Matteis MA, Alvarez C. Rab1b overexpression modifies Golgi size and gene expression in HeLa cells and modulates the thyrotrophin response in thyroid cells in culture. Mol Biol Cell. 2013;24(5):617–632. doi: 10.1091/mbc.E12-07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakova A, Zolov S, Lupashin V. COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic. 2006;7(2):191–204. doi: 10.1111/j.1600-0854.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Simpson JC, Joggerst B, Laketa V, Verissimo F, Cetin C, Erfle H, Bexiga MG, Singan VR, Heriche JK, Neumann B, Mateos A, Blake J, Bechtel S, Benes V, Wiemann S, Ellenberg J, Pepperkok R. Genome-wide RNAi screening identifies human proteins with a regulatory function in the early secretory pathway. Nat Cell Biol. 2012;14(7):764–774. doi: 10.1038/ncb2510. [DOI] [PubMed] [Google Scholar]
- Starr T, Sun Y, Wilkins N, Storrie B. Rab33b and Rab6 are functionally overlapping regulators of Golgi homeostasis and trafficking. Traffic. 2010;11(5):626–636. doi: 10.1111/j.1600-0854.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- Stein M, Pilli M, Bernauer S, Habermann BH, Zerial M, Wade RC. The interaction properties of the human Rab GTPase family--comparative analysis reveals determinants of molecular binding selectivity. PLoS One. 2012;7(4):e34870. doi: 10.1371/journal.pone.0034870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Storrie B, White J, Röttger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143(6):1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Micaroni M, Morgan GP, Jones N, Kamykowski JA, Wilkins N, Pan TH, Marsh BJ. Electron tomography reveals Rab6 is essential to the trafficking of trans-Golgi clathrin and COPI-coated vesicles and the maintenance of Golgi cisternal number. Traffic. 2012;13(5):727–744. doi: 10.1111/j.1600-0854.2012.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Nakano A. The yeast Golgi apparatus. Traffic. 2012;13(4):505–510. doi: 10.1111/j.1600-0854.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Shestakova A, Hunt L, Sehgal S, Lupashin V, Storrie B. Rab6 regulates both ZW10/RINT-1 and conserved oligomeric Golgi complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18(10):4129–4142. doi: 10.1091/mbc.E07-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157(4):631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119(4):749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16(2):113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Utskarpen A, Slagsvold HH, Iversen TG, Wälchli S, Sandvig K. Transport of ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A′. Traffic. 2006;7(6):663–672. doi: 10.1111/j.1600-0854.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Valente C, Polishchuk R, De Matteis MA. Rab6 and myosin II at the cutting edge of membrane fission. Nat Cell Biol. 2010;12(7):635–638. doi: 10.1038/ncb0710-635. [DOI] [PubMed] [Google Scholar]
- Valsdottir R, Hashimoto H, Ashman K, Koda T, Storrie B, Nilsson T. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 2001;508(2):201–209. doi: 10.1016/s0014-5793(01)02993-3. [DOI] [PubMed] [Google Scholar]
- Wei JH, Seemann J. Unraveling the Golgi ribbon. Traffic. 2010;11(11):1391–1400. doi: 10.1111/j.1600-0854.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147(4):743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BC, Karr TL, Montgomery JM, Goldberg ML. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol. 1992;118(4):759–773. doi: 10.1083/jcb.118.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Nuoffer C, Meinkoth JL, McCaffery M, Feramisco JR, Balch WE, Farquhar MG. A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J Cell Biol. 1994;125(3):557–571. doi: 10.1083/jcb.125.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Liu CC, Chen PL, Lee WH. RINT-1, a novel Rad50-interacting protein, participates in radiation-induced G(2)/M checkpoint control. J Biol Chem. 2001;276(9):6105–6111. doi: 10.1074/jbc.M008893200. [DOI] [PubMed] [Google Scholar]
- Yadav S1, Linstedt AD. Golgi positioning. Cold Spring Harb Perspect Biol. 2011;3(5) doi: 10.1101/cshperspect.a005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Ménétrey J, Goud B. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J Mol Biol. 2010;397(1):69–88. doi: 10.1016/j.jmb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Young J, Stauber T, del Nery E, Vernos I, Pepperkok R, Nilsson T. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A′. Mol Biol Cell. 2005;16(1):162–177. doi: 10.1091/mbc.E04-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JY, Koda T, Fujiwara T, Kishi M, Ikehara Y, Kakinuma M. A novel Rab GTPase, Rab33B, is ubiquitously expressed and localized to the medial Golgi cisternae. J Cell Sci. 1998;111( Pt 8):1061–1069. doi: 10.1242/jcs.111.8.1061. [DOI] [PubMed] [Google Scholar]
- Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168(5):747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]