Obesity is one of the most burdensome health issues of our time. According to a 2012 World Health Organization report, there are more than 1.4 billion overweight adults worldwide, of whom ∼500 million are obese (1). Strikingly, 40 million children under the age of 5 are currently overweight or obese (1). These statistics are especially alarming because of the long list of obesity-associated conditions. These include insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular disease, and several cancers (2). Among the consequences of the obesity epidemic are an expanding population of chronically ill people, escalating health care expenses, and the prediction that, for the first time in human history, the current generation will have a shorter life span than the previous one (3).
Accumulation of fat results from a prolonged imbalance between energy intake and energy expenditure. One might think that reducing fat mass in obese individuals could be achieved relatively simply by either decreasing food consumption or increasing energy expenditure, thereby attaining a sustained negative energy balance. Unfortunately, this task is not easy to achieve, in large part because of the combination of sedentary lifestyle and the availability of calorie-dense, inexpensive food. With the exception of bariatric surgery (4), most antiobesity interventions that target energy intake result in moderate, and often temporary, weight loss. Sadly, pharmacological strategies aimed at increasing energy expenditure have not delivered on the promise of efficacy or safety when translated from animal models to humans. For instance, supraphysiologic doses of thyroid hormones or sympathomimetic agents, albeit efficacious at increasing energy expenditure, result in systemic adverse events that preclude their use for obesity treatment (5). Notably, physical activity, the most physiological approach to burning energy, is not easy to sustain over the long term.
Very recently, a new weight loss strategy has been proposed that harnesses the energy-dissipating properties of brown adipose tissue (BAT). BAT functions as a thermogenic tissue in small mammals and hibernating species and allows human newborns to cope with the thermal shock of delivery (6). Thermogenesis is achieved through the activity of a large number of mitochondria that express uncoupling protein 1 (UCP1). This uncouples substrate oxidation from ATP production so that heat is produced. BAT is densely innervated by the sympathetic nervous system, the main inducer of BAT thermogenesis, and it is highly vascularized (6). Although BAT deposits have long been assumed to regress shortly after birth, recent imaging studies have revealed that human adults possess BAT in the cervical-supraclavicular (the most common location), perirenal/adrenal, and paravertebral regions, as well as around major arteries (7). BAT activity seems to have a positive relationship with resting energy expenditure (REE) in healthy adult males, although only at low temperatures. It has been shown to decrease with increasing BMI, percent body fat, age, and plasma glucose levels (8).
In this issue of Diabetes, the article by Chondronikola et al. (9) adds important insights into the physiology of human BAT. The authors investigated the effect of prolonged (5–8 h) cold-induced BAT activation on whole-body glucose homeostasis and insulin sensitivity in seven middle-aged overweight men with detectable BAT (BAT+) and five BAT− controls. In the BAT+ group, cold exposure (CE) significantly increased REE, whole-body glucose disposal, plasma glucose oxidation, and insulin sensitivity. These adaptations were absent in the BAT− group. Plasma glucose and free fatty acid accounted for ∼30 and ∼70%, respectively, of the 15% estimated increase in REE. CE-induced BAT activation was associated with higher circulating levels of free triiodothyronine, norepinephrine, and fibroblast growth factor 21 (FGF21). At the tissue level, the BAT thermogenic program was characterized by increased expression of β3-adrenergic receptor, peroxisome proliferator-activated receptor γ coactivator-1α, UCP1, and type 2 deiodinase (Fig. 1).
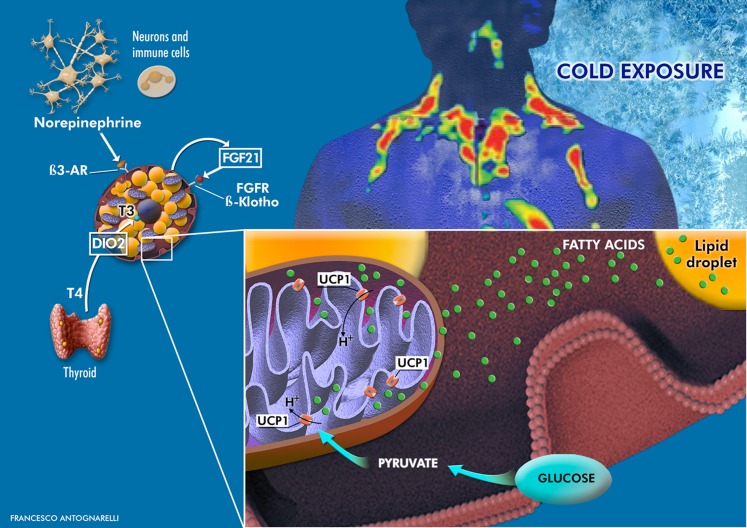
Figure 1.
The BAT is mainly found in deposits located in the cervical-supraclavicular, perirenal/adrenal, and paravertebral regions. As described by Chondronikola et al. (9), a prolonged CE triggers a thermogenic program through the elevation of norepinephrine (secreted by neurons and alternatively activated macrophages) and thyroid hormone levels. BAT activation is marked by the expression of 1) UCP1, which causes proton leakage from the inner mitochondrial membrane, thereby dissipating energy and generating heat; 2) type 2 deiodinase (DIO2), which converts thyroxine (T4) in triiodothyronine (T3), which in turn induces UCP1 transcription; and 3) FGF21, which ameliorates whole-body glucose homeostasis and insulin sensitivity by acting through systemic, autocrine, and paracrine mechanisms. Cold-induced BAT activation also stimulates lipolysis. Free fatty acids released from lipid droplets bind to and activate UCP1, fueling mitochondrial thermogenesis. β3-AR, β3-adrenergic receptor.
The value of this study lies in its use of an individualized and prolonged CE protocol that allowed the characterization of the changes induced by maximal nonshivering BAT thermogenesis. The use of gold standard direct measures of whole-body glucose metabolism provides robust information on the involvement of BAT in systemic energy metabolism and further advances our understanding on the topic (10–15).
On the other hand, some limitations in the study design suggest that future investigations are needed. First, it remains to be established whether BAT-driven thermogenesis under CE is consistent across ethnic groups and climatic areas. Indeed, the prevalence, mass, and activity of BAT differ depending on age, sex, BMI, plasma glucose levels, outdoor temperature, and day length (8,15). Second, although 18F-fluordeoxyglucose positron emission tomography/computed tomography (18FDG-PET/CT) is highly sensitive for detecting metabolically active BAT, it provides an incomplete picture on BAT physiology and morphology (16,17). Further, 18FDG-PET/CT is expensive, technically difficult, and involves exposing subjects to relatively high doses of ionizing radiation, all factors that impede the large-scale use of this imaging technique. In addition, although Chondronikola et al. (9) provided an extensive characterization of study participants, more in-depth mechanistic investigations are warranted to fully uncover the signaling pathways governing the BAT thermogenic program. This information is necessary to develop safe and effective pharmaceutical interventions to harness BAT thermogenesis. To this end, a promising strategy may involve transforming the more abundant white fat cells into tissue that behaves like BAT, the so-called “beige” or “brite” (brown-in-white) fat (18,19). Although a number of factors and pathways have been discovered that enhance brown and beige fat recruitment and function in rodents, the mechanisms that activate these tissues in humans are still poorly understood (19,20). Finally, since energy balance is tightly controlled by homeostatic mechanisms, the body may compensate for a sustained cold-induced activation of BAT thermogenesis by inducing hunger or increasing the metabolic efficiency of other tissues such as the muscle. In other words, we may end up with chilled, yet obese people hunting for food. Not so cool after all!
In conclusion, findings by Chondronikola et al. (9) add valuable information to the field of human BAT physiology by showing that activation of this tissue can be exploited to improve systemic glucose homeostasis and increase energy expenditure. An unexpected ally in the BATtle against obesity has entered the arena.
Article Information
Acknowledgments. The authors thank Francesco Antognarelli for his invaluable assistance with illustrations.
Funding. This work was partly supported by the “Centro Studi Achille e Linda Lorenzon” (E.M. and R.C.), Innovative Medicines Initiative Joint Undertaking (IMI–JU 115621; E.M. and R.C.), and the University of Florida's Institute on Aging and Claude D. Pepper Older Americans Independence Center (NIA 1P30AG028740; C.L.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 4089.
References
- 1.World Health Organization. Obesity and overweight. Fact sheet no. 311. WHO Media Centre. Updated August 2014. Available from http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 4 September 2014
- 2.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 2006;29:109–117 [DOI] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005;352:1138–1145 [DOI] [PubMed] [Google Scholar]
- 4.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;8:CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen M, Ewald MB. Toxicity of weight loss agents. J Med Toxicol 2012;8:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature 2014;510:76–83 [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96:192–199 [DOI] [PubMed] [Google Scholar]
- 9.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 11.Yoneshiro T, Aita S, Matsushita M, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–16 [DOI] [PubMed] [Google Scholar]
- 12.Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012;122:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 2013;54:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orava J, Nuutila P, Noponen T, et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 2013;21:2279–2287 [DOI] [PubMed] [Google Scholar]
- 15.Persichetti A, Sciuto R, Rea S, et al. Prevalence, mass, and glucose-uptake activity of ¹⁸F-FDG-detected brown adipose tissue in humans living in a temperate zone of Italy. PLoS ONE 2013;8:e63391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab 2014;20:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lans AA, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in human adults - methodological issues. Am J Physiol Regul Integr Comp Physiol. 28 May 2014 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19:1252–1263 [DOI] [PubMed] [Google Scholar]
- 19.Yoneshiro T, Saito M. Activation and recruitment of brown adipose tissue as anti-obesity regimens in humans. Ann Med. 5 June 2014 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 2014;20:396–407 [DOI] [PubMed] [Google Scholar]