Abstract
BACKGROUND
Ionic liquids (ILs; salts with melting points below 100 °C) exhibit wide liquid ranges, non-flammability, and thermal stability among other properties. These unique salts are best known as ‘green’ alternatives to traditional volatile organic solvents, which are utilized in both academia and industry. Our current study compares the developmental toxicity potential of three representative ionic liquids, with various chain lengths: 1-ethyl-3-methylimidazolium chloride ([C2mim]Cl), 1-butyl-3-methylimidazolium chloride ([C4mim]Cl), and 1-decyl-3methylimidazolium chloride ([C10mim]Cl).
METHODS
From gestation days (GD) 6-16, mated CD-1 mice were orally dosed with one of the following: 1000, 2000, or 3000 mg/kg/day [C2mim]Cl; 113, 169, or 225 mg/kg/day [C4mim]Cl; 50, 75, or 100 mg/kg/day [C10mim]Cl; or the vehicle only. Dams were sacrificed on GD 17, and their litters were examined for adverse effects.
RESULTS
Fetal weight was significantly decreased in the two highest dosage groups exposed to [C4mim]Cl and [C10mim]Cl in comparison with their controls, but the [C2mim]Cl treated groups were not affected. An apparent teratogenic effect was associated with both [C4mim]Cl and [C10mim]Cl, as the offspring exhibited certain uncommon morphological defects. However, the incidences of malformations were low and no correlation between incidence and dosage could be made. No morphological defects were observed in any of the [C2mim]Cl-treated groups, despite maternal morbidity at the highest dosage level.
CONCLUSIONS
This study indicates that [C4mim]Cl and [C10mim]Cl may have adverse effects on development at high maternal exposures and strongly supports the supposition that the toxicity of imidazolium-based ILs is influenced by alkyl chain length.
Keywords: ionic liquids, imidazolium, developmental toxicity, mouse, mice
INTRODUCTION
Ionic liquids (ILs; salts with melting points below 100 °C) can exhibit wide liquid ranges, non-flammability, and thermal stability among other useful properties (Walden, 1914; Welton, 1999; Olivier-Bourbigou and Magna, 2002; Rogers and Seddon, 2003). The potential properties have attracted attention in the search for ‘green’ alternatives to traditional volatile organic solvents, which are utilized in both academia and industry (Wilkes et al., 1982; Jessop and Heldebrant, 2005). Even though ILs are promoted as ‘green’, few studies have been published regarding the safety of ILs to developing offspring, although general toxicity data has shown that some ILs can be significantly toxic to adult species (Bernot et al., 2005a,b; Pretti et al., 2006; Samori et al., 2007).
One of the most popular cation classes used to form ILs, includes the 1-alkyl-3-methylimidazolium [Cnmim]+ ions. The alkyl chain length at the 1-position varies from short (e.g. methyl) to long (e.g. decyl), however 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) is one of the most widely used and studied ILs in this class. This is a hygroscopic, water soluble salt which, when dissolved in water, yields fully hydrated ions (Pernak et al., 2004; Landry et al., 2005). [Cnmim]+-containing ILs, especially [C4mim]Cl, have numerous applications in solvent replacement, coatings, refractive index, metal plating, fuel cells, catalysis, chromatography columns, biomass processing, and many more (Joglekar et al., 2007; Plechkova and Seddon, 2008).
Because of its widespread use and the corresponding potential for human exposure, the majority of toxicity studies have focused on [C4mim]Cl in various biological models. The models employed have ranged from simpler test systems, such as bacteria, algae, human (HeLa, CaCo-2, HT-29, and MCF7) and rat (ICP-81 and C6 glioma) cell lines, to complex organisms, such as snails, zebrafish, and Fisher 344 rats (Matsumoto et al, 2004; Stepnowski et al., 2004; Bernot et al., 2005a,b; Landry et al., 2005; Latala et al., 2005; Lee et al., 2005; Ganske and Bornscheuer, 2006; Pretti et al., 2006; Chefson and Auclair, 2007; Cho et al., 2007; Frade et al., 2007; Matzke et al., 2007; Salminen et al., 2007; Samori et al.,2007; Stolte et al., 2007; Wang et al., 2007). The results from these studies have indicated that the cation, [C4mim]+, is the primary influence on the toxicity of the IL. This assumption seems reasonable because the cations have typically been paired with simple inorganic anions, such as chloride and bromide (Lee et al., 2005; Salminen et al., 2007; Plechkova and Seddon, 2008). The toxicity of an IL is believed to be partly a consequence of the cation’s ability to intercalate with the cell’s bilayer membrane, thereby increasing membrane permeability and causing cell death (Ranke et al., 2004). This ability increases with increasing alkyl chain length, as the IL becomes more lipophilic and more surfactant-like (Matsumoto et al., 2004; Ranke et al., 2004; Samori et al., 2007).
Sipes et al (2008) have determined the toxicokinetics of orally, intravenously, and dermally administered [C4mim]Cl, N-butylpyridinium chloride, and 1-butyl-1-methylpyrrolidinium chloride in mice and rats as these ILs represent the most common cation classes (Cheng et al., 2009;Knudsen et al., 2009). Regardless of the administration route, all of the ILs were almost completely, > 97%, eliminated unchanged from the animal through the urine. No metabolism by the liver or any other mammalian enzymes of the ILs were observed for any of the tested compounds. The authors attribute this efficient clearance to the water solubility and structural features of the IL, as it may be excreted from the body via a sodium-independent cation transporter. These results are interesting as little information is currently available regarding the toxicity, disposition, or metabolism of ILs in mammalian systems.
While the toxicological effects of increasing alkyl chain length in an IL are known, no studies have examined the effects of this length increase of an imidazolium-based IL on development, despite the potential human exposure through groundwater contamination from industrial spills or wastewater. That is the case even though our previous study indicated a dosage-related increase in adverse effects, such as reduced fetal weight and certain morphological defects, as a result of exposure to [C4mim]Cl during development in mice (Bailey et al., 2008). Other studies conducted with zebrafish and frogs have also indicated adverse effects of exposure to ILs during development (Pretti et al., 2006; Lu et al., 2009). To address this issue, we compared the developmental toxicity potential in CD-1 mice of three ILs of various chain lengths: 1-ethyl-3-methylimidazolium chloride ([C2mim]Cl), 1-butyl-3-methylimidazolium chloride ([C4mim]Cl), and 1-decyl-3-methylimidazolium chloride ([C10mim]Cl). The comparison data for the intermediate chain length IL were derived from Bailey et al. (2008).
MATERIALS AND METHODS
Animals and Husbandry
Male and female CD-1 mice, obtained from Charles River Breeding Laboratories International (Wilmington, MA), were maintained at 22±2 °C, with 40-60% humidity and a 12 h photoperiod. The mice were bred naturally, two females with one male. Observation of a copulation plug designated gestation day 0 (GD 0). Mated females were individually housed in shoebox type cages with hardwood bedding and were given Teklad LM-485 rodent diet (Harlan Teklad, Madison, WI) and tap water ad libitum. All procedures performed on these animals were in accordance with established guidelines and were reviewed and approved by The University of Alabama’s or Emporia State University’s Institutional Animal Care and Use Committee.
Test Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated and used as received. Each of the ILs, [C2mim]Cl, [C4mim]Cl, and [C10mim]Cl were prepared via alkylation of methylimidazole with the corresponding chloroalkanes using previously described methods (Huddleston et al., 1998,2001). Before the toxicity studies were carried out, the ILs were rigorously dried by heating to 70 °C under reduced pressure. The water contents were determined using a volumetric Aquastar Karl Fischer titrator (EM Science, Gibbstown, NJ). Triplicate measurements were performed on each sample, and agreed to within 100 ppm. To ensure purity (i.e., complete reaction), the ILs were analyzed using NMR (1H and 13C at 360 MHz), and the analyses indicated no residual reactants. The [C2mim]Cl and [C4mim]Cl were off-white crystalline solids at room temperature, and the [C10mim]Cl was a colorless viscous liquid at room temperature. Stock solutions were prepared by dissolving the ionic liquids in sterile water at the following final concentrations: [C2mim]Cl 500.35 mg mL−1, [C4mim]Cl 103.0 mg mL−1, and [C10mim]Cl 52.5 mg mL−1.
Treatments
Prior to the definitive study, a preliminary range-finding study was conducted at a [C2mim]Cl dosage of 4000 mg/kg/day. Treatments were given by gavage once daily to a total of 9 mated females, commencing on GD 6 and ending on GD 16 (or sooner if morbidity or mortality occurred). A preliminary study was conducted similarly with [C4mim]Cl or [C10mim]Cl gavage dosages of 0, 250, 500, or 1000 mg/kg/day, with five females in each dosage group. Six of the nine female mice in the 4000 mg/kg/day dosage group died after an average of 6 doses and one female died after a non-IL related dosing error. Of the two surviving females, one was not pregnant. Fetuses from the surviving female were not found to have gross malformations.
All mice in the 500 and 1000 mg/kg/day [C4mim]Cl or [C10mim]Cl treatment groups died immediately or became moribund and were subsequently euthanized by CO2 overdose approximately 8 h after the initial dose. The moribund mice displayed ataxia, hypoactivity, and labored breathing. Three of the five mice in the 250 mg/kg/day [C10mim]Cl treatment group died or became moribund and were subsequently euthanized within 8 hours of the initial dose. Two of the five mice in the 250 mg/kg/day [C4mim]Cl treatment group appeared sluggish after the initial dose, but they recovered within 24 h and survived multiple doses.
For the definitive study, mated female CD-1 mice were randomly assigned to one of the following treatment groups: (1) DI H2O (vehicle control), (2) 1000, 2000, or 3000 mg/kg/day [C2mim]Cl, (3) 113, 169, or 225 mg/kg/day [C4mim]Cl, or (4) 50, 75, or 100 mg/kg/day [C10mim]Cl. Initially, dosages of 113, 169, or 225 mg/kg/day [C10mim]Cl were employed; however these dosages were lowered to the definitive dosages because of significant maternal morbidity and mortality. Eleven out of 11 dams died after an average of 3 doses of 225 mg/kg/d (3 were moribund and subsequently sacrificed), 5/11 dams in the 169 mg/kg/d died after an average of 4 doses, and 1 dam (out of 11) was moribund and subsequently sacrificed in the 113 mg/kg/d dosage group after 6 doses. Animals were dosed once daily from GD 6 – 16, with an average of 25 mated females per treatment group. The dosage volume was 0.01 mL/g body weight. Clinical signs were recorded daily and females were weighed on GD 0, as well as prior to each dosing.
Data Collection
On GD 17, mated females were euthanized by CO2 overdose, their uteri were exposed, and the numbers of resorptions and dead or live fetuses were recorded. Live fetuses were removed from the uterus, weighed individually, and examined for gross malformations. Maternal body weight, minus the gravid uterine weight, was then obtained. Fetuses were initially fixed in 70% ethanol and then cleared and stained by the double-staining technique described by Webb and Byrd (1994). All fetuses were subsequently examined for skeletal abnormalities.
Data Analysis
The litter was used as the experimental unit for statistical analysis. This study was performed in multiple replicates. The data from each study replicate were calculated independently, tested for homogeneity of variance by means of the Levene statistic, using SPSS (SPSS, Inc., Chicago, IL), and then pooled and analyzed to give the results reported. All tabular data are presented as the mean ± standard error (SEM). Data were analyzed by one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) post-hoc test to determine specific significant differences (P ≤ 0.05) or by a Pearson chi-square test (P ≤ 0.05).
RESULTS
Maternal Data
Maternal weight gain was not significantly affected by exposure to any of the ILs (Tables 1-3). There was an apparent treatment effect on maternal weight gain in the highest [C2mim]Cl and [C4mim]Cl dosage groups; however, the differences from their respective control values were not statistically significant. There was evidence of dosage-dependent maternal morbidity/mortality in the [C2mim]Cl and [C4mim]Cl exposed mice (Tables 1 and 2). The percentage of animals dying or becoming moribund and necessitating euthanization prior to the completion of dosing were significantly greater in the 3000 mg/kg/day [C2mim]Cl and 225 mg/kg/day [C4mim]Cl treatment groups as compared to both the controls and the lower IL doses . There was an apparent treatment effect on maternal morbidity/mortality in the [C10mim]Cl exposed animals; however this effect was not statistically significant at the revised dosage levels (Table 3).
Table 1.
Maternal weight gain and litter parameters of CD-1 mice exposed to [C2mim]Cl
| Treatment and dose (mg/kg/day) | ||||
|---|---|---|---|---|
| Vehicle Control |
[C2mim]Cl (1000) |
[C2mim]Cl (2000) |
[C2mim]Cl (3000) |
|
| Maternal weight gain (g ± SEM) |
12.46 ± 0.68 | 13.51 ± 0.80 | 13.72 ± 0.74 | 11.31 ± 0.90 |
| Maternal | ||||
| Morbidity/Mortality (No./% affected dams) | 0 | 0 | 0 | 8/33.3a |
| Fetuses/litters examined | 243/20 | 192/17 | 206/17 | 72/6 |
| Implantations (mean ± SEM) |
12.30 ± 0.53 | 12.12 ± 0.54 | 11.70 ± 0.68 | 12.33 ± 0.84 |
| Resorbed or dead fetuses (No. ± SEM) |
0.90 ± 0.64 | 0.47 ± 0.47 | 2.51 ± 1.22 | 2.67 ± 1.69 |
| Litters with resorbed or dead fetuses (No./%) |
2/10.0 | 1/5.9 | 4/22.2 | 2/33.3 |
| Malformed fetuses (% ± SEM)b,c |
0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 |
| Malformed fetuses (% affected litters)b |
0.0 | 0.0 | 0.0 | 0.0 |
| Supernumerary Ribs (% ± SEM) |
13.64 ± 3.47 | 15.41 ± 3.66 | 19.36 ± 5.82 | 23.00 ± 3.47 |
| Rudimentary Ribs (% ± SEM) |
11.12 ± 3.84 | 15.91 ± 3.42 | 10.43 ± 2.59 | 10.33 ± 2.72 |
Significantly different from all other mean values (P ≤ 0.01)
Fetuses displaying any gross malformation.
Grand mean of litter mean percentages.
Table 3.
Maternal weight gain and litter parameters of CD-1 mice exposed to [C10mim]Cl
| Treatment and dose (mg/kg/day) |
||||
|---|---|---|---|---|
| Vehicle Control |
[C10mim]Cl (50) |
[C10mim]Cl (75) |
[C10mim]Cl (100) |
|
| Maternal weight gain (g ± SEM) | 11.42 ± 0.74 | 12.11 ± 0.70 | 11.42 ± 0.77 | 12.01 ± 0.85 |
| Maternal Morbidity/Mortality (No./% affected dams) |
0 | 3/12.5 | 2/9.5 | 3/12.5 |
| Fetuses/litters examined | 271/23 | 241/21 | 233/19 | 234/21 |
| Implantations (mean ± SEM) | 12.28 ± 0.62 | 12.22 ± 0.60 | 12.80 ± 0.49 | 11.39 ± 0.67 |
| Resorbed or dead fetuses (No. ± SEM) |
2.17 ± 0.93 | 1.49 ± 0.90 | 2.41 ± 1.44 | 4.16 ± 2.01 |
| Litters with resorbed or dead fetuses (No./%) |
5/21.7 | 3/14.3 | 3/15.8 | 5/23.8 |
| Malformed fetuses (% ± SEM)a,b | 0 ± 0.0 | 0.62 ± 0.62 | 0.74 ± 0.51 | 0.95 ± 0.66 |
| Malformed fetuses (% affected litters)a |
0.0 | 5.0 | 10.5 | 10.0 |
| Supernumerary Ribs (% ± SEM) |
13.57 ± 3.91 | 14.70 ± 5.04 | 10.48 ± 3.14 | 9.40 ± 2.91 |
| Rudimentary Ribs (% ± SEM) |
10.38 ± 2.78 | 11.71 ± 3.56 | 11.61 ± 2.82 | 4.43 ± 2.23 |
Fetuses displaying any gross malformation.
Grand mean of litter mean percentages.
Table 2.
Maternal weight gain and litter parameters of CD-1 mice exposed to [C4mim]Cl
| Treatment and dose (mg/kg/day) |
||||
|---|---|---|---|---|
| Vehicle Control |
[C4mim]Cl (113) |
[C4mim]Cl (169) |
[C4mim]Cl (225) |
|
| Maternal weight gain (g ± SEM) |
10.67 ± 0.39 | 10.29 ± 0.44 | 10.38 ± 0.42 | 9.28 ± 0.64 |
| Maternal | ||||
| Morbidity/Mortality (No./% affected dams) |
0 | 1/3.3 | 2/6.7 | 10/28.3a |
| Fetuses/litters examined | 489/36 | 288/23 | 319/26 | 314/23 |
| Implantations (mean ± SEM) |
13.97 ± 0.36 | 12.78 ± 0.55 | 13.40 ± 0.36 | 13.91 ± 0.44 |
| Resorbed or dead fetuses (No. ± SEM) |
2.77 ± 0.84 | 2.24 ± 0.88 | 6.20 ± 2.82 | 3.24 ± 0.98 |
| Litters with resorbed or dead fetuses (No./%) |
10/27.8 | 6/26.1 | 8/30.8 | 9/39.1 |
| Malformed fetuses (% ± SEM)b,c |
0.91 ± 0.44 | 1.09 ± 0.60 | 1.56 ± 0.76 | 2.47 ± 0.93 |
| Malformed fetuses (% affected litters)b |
11.1 | 13.0 | 15.4 | 26.1 |
| Supernumerary Ribs (% ± SEM) |
24.53 ± 4.16 | 16.49 ± 4.42 | 16.97 ± 4.17 | 18.10 ± 5.28 |
| Rudimentary Ribs (% ± SEM) |
18.85 ± 2.70 | 21.47 ± 4.50 | 12.72 ± 2.16a | 7.92 ± 2.35a |
Significantly different from all other mean values (P ≤ 0.01)
Fetuses displaying any gross malformation.
Grand mean of litter mean percentages.
Fetal Data
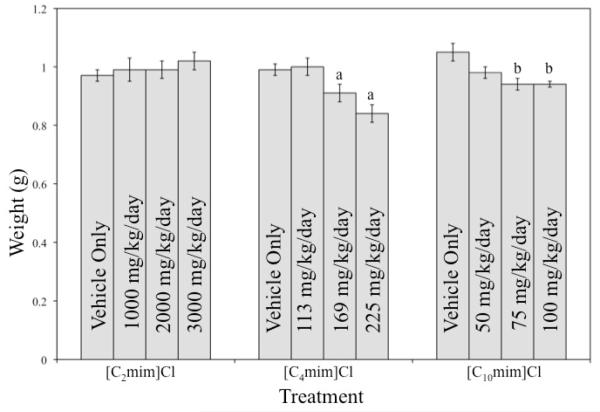
The numbers of implantations and the percentages of resorbed or dead fetuses did not differ significantly among treatment groups (Tables 1-3). In the [C4mim]Cl and [C10mim]Cl treatment groups, fetal weight was significantly affected (Fig. 1). Fetuses exposed to either 169 or 225 mg/kg/day [C4mim]Cl weighed significantly less than either the control or 113 mg/kg/day [C4mim]Cl exposed fetuses. Fetuses in the 75 and 100 mg/kg/day [C10mim]Cl treatment group weighed significantly less than fetuses in the control group. There was no effect on fetal weight among the [C2mim]Cl exposed fetuses.
Figure 1.
Fetal Weights of Offspring Exposed to Imidazolium-Based Ionic Liquids
a Significantly differs from control and 113 mg/kg/day groups (P ≤ 0.05).
b Significantly differs from control group (P ≤ 0.05).
Several of the gross malformations observed in the [C4mim]Cl exposed fetuses were reported in a previous publication and are included in Table 4 for comparison purposes (Bailey et al., 2008). Malformations were also observed in fetuses exposed to the higher dosages of [C10mim]Cl, including ectopia cordis, exencephaly, and ablepharia. Two fetuses in the 100 mg/kg/day [C10mim]Cl dosage group displayed malformations, with one fetus exhibiting gastroschisis and another exhibiting exencephaly (Table 4). No gross malformations were observed in fetuses exposed to [C2mim]Cl.
Table 4.
| Treatment and Dose (mg/kg/day) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malformation (No./%) |
[C4mim]Cla | [C10mim]Cl | [C10mim]Cl | |||||||||
|
|
||||||||||||
| 0 | 113 | 169 | 225 | 0 | 113 | 169 | 225 | 0 | 50 | 75 | 100 | |
|
|
||||||||||||
| Fetuses | 489 | 288 | 319 | 314 | 126 | 170 | 117 | 35 | 273 | 267 | 251 | 239 |
| Examined (No.) | ||||||||||||
| Bent Tail | 2 | 3 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Talipes | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemimelia | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ablepharia | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Exencephaly | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Craniorachischis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ectopia Cordis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cleft Palate | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastroschisis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
|
| ||||||||||||
| Total (No./%) | 4 | 3 | 9 | 8 | 0 | 2 | 2 | 1 | 0 | 0 | 2 | 2 |
[C4mim]Cl data previously reported in Bailey et al., 2008.
No malformations seen in any [C2mim]Cl dosage group.
In both the [C4mim]Cl and [C10mim]Cl treatment groups, the mean litter percentages of fetuses and the per litter incidences of any specific malformation were not statistically significantly greater than the incidences in the fetuses of control dams. Both the fetal and litter incidences of total malformations increased in an apparently dosage-dependent manner in the [C4mim]Cl and [C10mim]Cl treatment groups, although the increase was not statistically significant (Tables 2 and 3).
When fetal skeletons were examined for malformations and variations, it appeared that exposure to [C2mim]Cl increased the litter incidence of supernumerary ribs. However, this increase was not statistically significant (Table 1). A statistically significant decrease in the incidence of fetuses with rudimentary ribs, as well as affected litters, was found in fetuses exposed to [C4mim]Cl, which we reported previously (Bailey et al., 2008)(Table 2), and a similar effect was also observed at the highest dosage of [C10mim]Cl (Table 3).
The present study did not find significant differences in the numbers of implantations, viable fetuses, or resorbed/dead fetuses among any of the treatment groups. Fetal weight was significantly reduced in the two highest dosage groups for both [C4mim]Cl and [C10mim]Cl, while no such reduction was observed among [C2mim]Cl exposed fetuses (Figure 1). An apparent teratogenic effect was also seen in the [C4mim]Cl and [C10mim]Cl treatment groups, with fetuses exhibiting a variety of uncommon morphological defects, such as ectopia cordis, craniorachischisis, and hemimelia. There were no morphological aberrations in fetuses exposed to [C2mim]Cl. Historical control data from more than 180 litters/2300 fetuses contained 2 incidences of bent tail and 2 incidences of ablepharia, but in the current study there were no incidences of any of the other malformations observed in fetuses from IL treated dams .
DISCUSSION
Although the number of malformed fetuses observed was relatively modest, it must be noted that the multiple-dose regimen employed is relatively inefficient at producing malformed offspring, even if the test compound is capable of doing so. It should also be mentioned that the positive fetal findings all occurred at maternal dosages associated with at least some maternal morbidity or mortality. In the dose ranges tested, the apparent No Observed Adverse Effect Level (NOAEL) for fetal effects in mice exposed to [C2mim]Cl was greater than 3000 mg/kg/day, as no adverse effects were seen. However, the [C4mim]Cl NOAEL for fetal effects was 113 mg/kg/day, while the Lowest Observed Adverse Effect Level (LOAEL) was 169 mg/kg/day, based on effects on fetal weight. The NOAEL for [C10mim]Cl was 50 mg/kg/day, while the LOAEL was 75 mg/kg/day. Nonetheless, these values may have been lower if visceral defects had been evaluated, and although the low dose fetal weights for [C10mim]Cl did not differ significantly from the control value, they appeared to have been affected.
Solvents such as benzene, toluene, acetonitrile, xylene, and commercial polychlorinated biphenyl mixtures tend to produce effects similar to those observed with the tested ILs, including reduced fetal weight and skeletal anomalies, particularly at high maternal exposures (Stepnowski et al., 2004; Bernot et al., 2005b). However, comparison of the tested IL’s toxicities to those of traditional solvents is difficult, primarily because of the different routes of administration i.e. inhalation for volatile traditional solvents or animal species utilized in other studies.
The results of the present study strongly indicate that the toxicity of ILs increases with increasing alkyl chain length. The dosages employed for the [C2mim]Cl treatment groups were some 10-30 times greater than the highest dosage tolerated by the [C10mim]Cl treated dams. In the preliminary study, a [C10mim]Cl dosage of 1000 mg/kg/day was immediately lethal, while multiple administrations of the same dosage of [C2mim]Cl were well tolerated and caused no obvious maternally toxic or embryo/fetotoxic effects. Indications of developmental toxicity, such as reductions in fetal weight and the occurrence of morphological defects, were observed primarily in the [C4mim]Cl and [C10mim]Cl treatment groups, despite using dosages of [C2mim]Cl that were an order of magnitude higher.
The mechanism for the developmental toxicity of ILs remains unknown. It is known that imidazolium-based ILs can act as cationic surfactants, which have the potential to be incorporated into biological membranes, disrupting the lipid bilayer and increasing membrane permeability (Jastorff et al., 2003; Kumar et al., 2008). The hydrophobicity and surfactant quality of ILs increase with alkyl chain length, therefore, increasing the toxicity (Zhao et al., 2007). Enzyme inhibition is another possible means of toxicity, as several imidazolium- and pyridinium-based ILs, including [C4mim]Cl, have been shown to inhibit enzymes, such as acetylcholinesterase, in vitro (Stock et al., 2006). ILs have also been shown to inhibit the P450 enzyme CYP3A4 to a degree comparable to the effects of organic co-solvents such as acetonitrile (Chefson and Auclair, 2007). Any of these mechanisms might negatively affect embryonic development and viability as well as contribute to maternal toxicity; however, further investigation to elucidate the mechanisms of developmental toxicity of ILs would be needed to address such questions, as the current data are only observational.
CONCLUSIONS
The present comparison study conducted with pregnant mice gavaged with imidazolium-based ionic liquids supports the contention that toxic effects of the ILs increase with increasing alkyl chain length. That relationship was manifested by maternal morbidity and mortality, and by effects on fetal weight. The same may be true of malformations, as they were seen only in fetuses from dams exposed to the two longer chain ILs, although differences in dosage levels among the three test compounds make direct comparison more difficult. Although the term ‘green solvent’ is typically misused as a synonym for ‘safe’, it is important that all users of ILs understand the fate, transport, and toxicity of these chemicals and take appropriate measures to avoid unnecessary exposure.
Acknowledgments
This work was made possible by NIH grant number P20 RR016475 from the INBRE Program of the National Center for Research Resources to Emporia State University. It was also supported in part by a Howard Hughes Medical Institute Undergraduate Biological Sciences Program grant to The University of Alabama, by The Alabama Institute for Manufacturing Excellence, and by The University of West Alabama.
LITERATURE CITED
- Bailey MM, Townsend MB, Jernigan PL, Sturdivant J, Hough-Troutman WL, Rasco JF, Swatloski RP, Rogers RD, Hood RD. Developmental toxicity assessment of the ionic liquid 1-butyl-3-methylimidazolium chloride in CD-1 mice. Green Chem. 2008;10:1213–1217. [Google Scholar]
- Bernot R, Brueseke M, Evans-White M, Lamberth G. Acute and chronic toxicity of imidazolium-based ionic liquids on Daphnia magna. Environ Toxicol Chem. 2005a;24(1):87–92. doi: 10.1897/03-635.1. [DOI] [PubMed] [Google Scholar]
- Bernot R, Kennedy E, Lamberti G. Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail Physa acuta. Environ Toxicol Chem. 2005b;24:1759–1765. doi: 10.1897/04-614r.1. [DOI] [PubMed] [Google Scholar]
- Chefson A, Auclair K. CYP3A4 activity in the presence of organic cosolvents, ionic liquids, or water-immiscible organic solvents. ChemBioChem. 2007;8:1189–1197. doi: 10.1002/cbic.200700128. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Wright SH, Hooth MJ, Sipes IG. Characterization of the Disposition and Toxicokinetics of N-Butylpyridinium Chloride in Male F-344 Rats and Female B6C3F1 Mice and Its Transport by Organic Cation Transporter 2. Drug Metab Disp. 2009;37:909–916. doi: 10.1124/dmd.108.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CW, Pham T, Jeon YC, Vijayaraghavan K, Choe WS, Yun YS. Toxicity of imidazolium salt with anion bromide to a phytoplankton Selenastrum capricornutum: Effect of alkyl-chain length. Chemosphere. 2007;69:1003–1007. doi: 10.1016/j.chemosphere.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Frade R, Matias A, Branco L, Afonso C, Duarte C. Effect of ionic liquids on human colon carcinoma HT-29 and CaCo-2 cell lines. 2007;9:873–877. [Google Scholar]
- Ganske F, Bornscheuer U. Growth of Escherichia coli, Pichia pastoris and Bacillus cereus in the presence of the ionic liquids [BMIM][BF4] and [BMIM][PF6] and organic solvents. Biotechnol Lett. 2006;28:465–469. doi: 10.1007/s10529-006-0006-7. [DOI] [PubMed] [Google Scholar]
- Huddleston JG, Rogers RD. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem Commun. 1998;16:1765–1766. [Google Scholar]
- Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3:156–164. [Google Scholar]
- Jastorff B, Stormann R, Ranke J, Molter K, Stock F, Oberheitmann B, Hoffmann W, Hoffmann J, Nuchter M, Ondruschka B, Filser J. How hazardous are ionic liquids? Structure–activity relationships and biological testing as important elements for sustainability evaluation. Green Chem. 2003;5:136–142. [Google Scholar]
- Jessop PG, Heldebrant DJ. Green Biphasic Homogeneous Catalysis. In: Grassian V, editor. Environmental Catalysis. Marcel Dekker; NY: 2005. 2005. pp. 627–648. [Google Scholar]
- Joglekar HG, Rahman I, Kulkarni BD. The path ahead for ionic liquids. Chem Eng Technol. 2007;30:819–828. [Google Scholar]
- Knudsen GA, Cheng Y, Kuester RK, Hooth MJ, Sipes IG. Effects of Dose and Route on the Disposition and Kinetics of 1-Butyl-1-methylpyrrolidinium Chloride in Male F-344 Rats. Drug Metab Disp. 2009;37:2171–2177. doi: 10.1124/dmd.109.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, Papiconomou N, Lee JM, Salminen J, Clark DS, Prausnitz JM. In vitro cytotoxicities of ionic liquids: Effect of cation rings, functional groups, and anions. Environ Toxicol. 2008;24:388–395. doi: 10.1002/tox.20443. [DOI] [PubMed] [Google Scholar]
- Landry T, Brooks K, Poche D, Woolhiser M. Acute toxicity profile of 1-butyl-3-methylimidazolium chloride. Bull of Environ Contam Toxicol. 2005;74:559–565. doi: 10.1007/s00128-005-0620-4. [DOI] [PubMed] [Google Scholar]
- Latala A, Stepnowski P, Nedzi M, Mrozik W. Marine toxicity assessment of imidazolium ionic liquids: Acute effects on the Baltic algae Oocystis submarina and Cyclotella meneghiniana. Aquat Toxicol. 2005;73:91–98. doi: 10.1016/j.aquatox.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Lee SM, Chang WJ, Choi AR, Koo YM. Influence of ionic liquids on the growth of Escherichia coli. Kor J Chem Eng. 2005;22:687–690. [Google Scholar]
- Lu XY, Zhou J, Yu M, Wang JJ, Pei YC. Toxic effects of 1-methyl-3-octylimidazolium bromide on the early embryonic development of the frog Rana nigromaculata. Ecotoxicology and Environmental Safety. 2009;72:552–556. doi: 10.1016/j.ecoenv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Mochiduki K, Kondo K. Toxicity of ionic liquids and organic solvents to lactic acid-producing bacteria. J Biosci Bioeng. 2004;98:334–347. doi: 10.1016/S1389-1723(04)00293-2. [DOI] [PubMed] [Google Scholar]
- Matzke M, Stolte S, Thiele K, Juffernholz T, Arning J, Ranke J, Welz-Biermann U, Jastorff B. The influence of anion species on the toxicity of 1-alkyl-3-methylimidazoliumionic liquids observed in an (eco)toxicological test battery. Green Chem. 2007;9:1198–1207. [Google Scholar]
- Olivier-Bourbigou H, Magna L. Ionic liquids: perspectives for organic and catalytic reactions. J Mol Catal A: Chem. 2002;182-183:419–437. [Google Scholar]
- Pernak J, Sobaszkiewicz K, Foksowicz-Flaczyk J. Ionic liquids with symmetrical dialkoxymethyl-substituted imidazolium cations. Chem Eur J. 2004b;10:3479–3485. doi: 10.1002/chem.200400075. [DOI] [PubMed] [Google Scholar]
- Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008;37:123–150. doi: 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- Pretti C, Chiappe C, Pieraccini D, Gregori M, Abramo F, Monni G, Intorre L. Acute toxicity of ionic liquids to the zebrafish (Danio rerio) Green Chem. 2006;8:238–240. [Google Scholar]
- Ranke J, Molter K, Stock F, Bottin-Weber U, Poczobutt J, Hoffmann J, Ondruschka B, Filser J, Jastorff B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol Environ Saf. 2004;58:396–404. doi: 10.1016/S0147-6513(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Seddon K. Ionic liquids – solvents of the future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Salminen J, Papaiconomou N, Kumar RA, Lee JM, Kerr J, Newman J, Prausnitz JM. Physicochemical properties and toxicities of hydrophobic piperidinium and pyrrolidinium ionic liquids. Fluid Phase Equil. 2007:421–426. [Google Scholar]
- Samori C, Pasteris A, Galletti P, Tagliavini E. Acute toxicity of oxygenated and nonoxygenated imidazolium-based ionic liquids to Daphnia magna and Vibrio fischeri. Environ Toxicol Chem. 2007;26:2379–2382. doi: 10.1897/07-066R2.1. [DOI] [PubMed] [Google Scholar]
- Sipes IG, Knudsen GA, Kuester RK. The Effects of Dose and Route on the Toxicokinetics and Disposition of 1-Butyl-3-methylimidazolium Chloride in Male F-344 Rats and Female B6C3F1 Mice. Drug Metab Disp. 2008;36:284–293. doi: 10.1124/dmd.107.018515. [DOI] [PubMed] [Google Scholar]
- Stepnowski P, Skladanowski A, Ludwiczak A, Laczynska E. Evaluating the cytotoxicity of ionic liquids using human cell line HeLa. Hum Exp Toxicol. 2004;23:513–517. doi: 10.1191/0960327104ht480oa. [DOI] [PubMed] [Google Scholar]
- Stock F, Hoffman J, Ranke J, Stormann R, Ondruschka B, Jastorff B. Effects of ionic liquids on the acetylcholinesterase – a structure–activity relationship consideration. Green Chem. 2004;6:286–290. [Google Scholar]
- Stolte S, Matzke M, Arning J, Boeschen A, Pitner WR, Welz-Biermann U, Jastorff B, Ranke J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007;9:1170–1179. [Google Scholar]
- Walden P. Molecular weights and electrical conductivity of several fused salts. Bull Acad Sci St Petersburg. 1914:405. [Google Scholar]
- Wang X, Ohlin CA, Lu Q, Fei Z, Hu J, Dyson PJ. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007;9:1191–1197. [Google Scholar]
- Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. 1999;99:2071–2084. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Wilkes JS, Levisky JA, Wilson RA, Hussey CL. Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg Chem. 1982;21:1263–1264. [Google Scholar]
- Webb G, Byrd R. Simultaneous differential staining of cartilage and bone without glacial acetic acid. Biotech Histochem. 1994;69:181–185. doi: 10.3109/10520299409106284. [DOI] [PubMed] [Google Scholar]
- Zhao D, Liao Y, Zhang Z. Toxicity of ionic liquids. Clean. 2007;35(1):42–48. [Google Scholar]