Abstract
In the past several years, the number of studies investigating enhancement of cognitive functions through non-invasive brain stimulation (NBS) has increased considerably. NBS techniques, such as transcranial magnetic stimulation and transcranial current stimulation, seem capable of enhancing cognitive functions in patients and in healthy humans, particularly when combined with other interventions, including pharmacologic, behavioral and cognitive therapies. The “net zero-sum model”, based on the assumption that brain resources are subjected to the physical principle of conservation of energy, is one of the theoretical frameworks proposed to account for such enhancement of function and its potential cost. We argue that to guide future neuroenhancement studies, the net-zero sum concept is helpful, but only if its limits are tightly defined.
Introduction/background — potential frameworks of enhancement
Cognitive performance can be improved
Learning can be defined as the acquisition or modification of new or already existing skills or knowledge through experience (Terry, 2008). The desire to maximize this effect and improve cognitive functions reaches far back into human history. The Roman orator Cicero, for example, proposed the “Cicero Memory Method” (Method of loci): a simple means to improve memory and rhetorical skills that advocated the use of visualization to structure information. Other approaches that have a long history of use in the general public, such as meditation, are only recently being investigated systematically for their ability to improve cognitive functions (Xiong and Doraiswamy, 2009). Similarly, regular physical exercise has been shown to improve cognitive abilities (Curlik and Shors, 2013). With the technological innovations of the last several decades, attempts at cognitive augmentation now include potentially more direct and specific manipulations of cognitive processes, for example, by computerized training (Kueider et al., 2012). In the wake of advances in the diagnosis and treatment of cognitive dysfunctions in patient populations, researchers have also started to investigate various pharmacological interventions to enhance cognitive performance, for example with methylphenidate (Ritalin®), amphetamine (Adderall®), dopamine agonists (e.g., Mirapex®), acetylcholine esterase inhibitors (e.g., Donepezil®), or modafinil (Provigil®). In fact, the abuse of such interventions by the general public to enhance mental abilities is increasing (Maher, 2008; Müller et al., 2013; Repantis et al., 2010), and ethicists have drawn attention to this worrisome trend and have coined unique terms such as “cosmetic neurology” (Chatterjee, 2004).
Despite the voiced ethical concerns, noninvasive brain stimulation (NBS) techniques, including transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), are also capturing the interest of researchers and clinicians as possible means to enhance human cognition. In fact, the popular press, internet sites, and social networks are broadly advocating its use. TMS and tDCS work through different, not fully understood mechanisms, to modulate the level of cortical excitability and shift or modulate activity in specific neural networks. Beyond their clinical applications, in carefully designed experiments, TMS and tDCS have been shown to enhance cognitive functions in healthy subjects (Fregni et al., 2005; Hilgetag et al., 2001; Zaehle et al., 2011). Other techniques such as EEG-feedback (Gruzelier et al., 2006) and real-time fMRI (deCharms, 2008) may also be counted as NBS methods that can be used to improve brain functions, though they operate via intrinsic mechanisms of plasticity as opposed to externally applied stimulation. As compared with pharmacologic interventions, NBS offers the promise of a deceivingly simple application for a more guided, specific modification of activity in desired brain structures. In the face of growing interest in NBS applications for neuroenhancement, an ethical debate seems warranted (Hamilton et al, 2011; Iuculano and Cohen Kadosh, 2013; Pascual-Leone et al., 2012), and the discussion of possible mechanisms of action and theoretical frameworks seems critical. In the present article we discuss the theoretical framework for the presumed ability of NBS to enhance human cognition, and point to several important implications for our understanding of fundamental aspects of brain function.
Mechanisms to account for enhancement of cognitive performance with NBS
Several, partly overlapping and non-mutually exclusive mechanisms could account for enhancement of cognitive performance using NBS. Ultimately, all are predicated on the notion that activity across specific brain networks is causally linked to behavior. Modulation of such network activity is thus thought to lead to predictable behavioral impact.
The balance effect, first put forward by Kinsbourne (1974), is based on the model of inter-hemispheric rivalry between homologue areas. It has been investigated particularly for complex motor- and space-related functions in healthy subjects and patients. Inter-hemispheric balance effects have been used to account for the paradoxical enhancement of ipsilateral motor function, ipsilateral visuospatial attention, or lateralized verbal memory and language abilities when using NBS to suppress activity in specific cortical regions (Hilgetag et al., 2001; Naeser et al., 2005; Oliveri et al., 2001).
Stochastic resonance refers to the notion that injection of sub-threshold noise into a system can serve to enhance signal detection (Gammaitoni et al., 1998). Stochastic resonance effects may explain recent observations showing that, whereas high levels of TMS impair visual motion detection, low levels of stimulation facilitate the detection of stimuli (Schwarzkopf et al., 2011). Reichenbach et al. (2011) similarly found that TMS delivered within a certain intensity range enhanced neural reactivity to visual stimuli as measured with EEG. Stochastic resonance may also help explain the observation of state-dependent effects of neurostimulation, whereby the basal activity of a target may shape its receptivity to an externally applied stimulation (Silvanto and Pascual-Leone, 2008; Silvanto et al., 2007).
The concept of enhancement through entrainment of oscillatory patterns is predicated on the notion that oscillatory activity in brain networks is associated with specific functions and that NBS can externally simulate these specific oscillations and thus lead to a predictable impact on behavior (Thut et al., 2011). For instance, Marshall et al. (2004,2011) have reported that using NBS to induce the low-frequency oscillations found in slow-wave sleep (SWS) improved declarative memory consolidation. Conversely, tDCS-mediated disruption of SWS can lead to memory consolidation deficits. Others have investigated controlled brain rhythm interventions with the aim of entraining oscillatory patterns linked to perception and attention (Romei et al., 2011; Thut et al., 2011). Ultimately, transcranial alternating current stimulation (tACS) and EEG-gated NBS could be particularly powerful tools to specifically test, and if possible leverage this mechanism of enhancement.
Here we will focus on the notion of net zero-sum. The net zero-sum framework is grounded on the physical principle of conservation of energy in closed systems, and raises fundamental issues regarding the levels and manifold interactions of the nervous system and their evolution. Net-zero sum refers to a mathematical concept put forward by game theorists: if the sum of all payments received or lost by all players is zero at the end of a game, it is called a zero-sum game. This economic concept describes a situation, whereby improvement for one party is always accompanied by a worsening of another party. The degree of interdependence of involved elements can further contribute to variations of the outcome for the parties, though the combined result always remains zero. Applied to the brain, a net zero-sum model of enhancement suggests a situation whereby neural “gains” must be matched by neural “losses”. This notion is appealing, given that the brain operates within the constraints of a finite amount of energy and processing power. Apparent gains (and thus cognitive enhancement) must represent the redirection of shared resources to specific brain circuits. If so, enhancement must be linked to a cost. Thus, identifying and understanding potential cost/benefit relationships may reveal fundamental dynamic interactions across brain and behavior.
Does enhancement come with a cost?
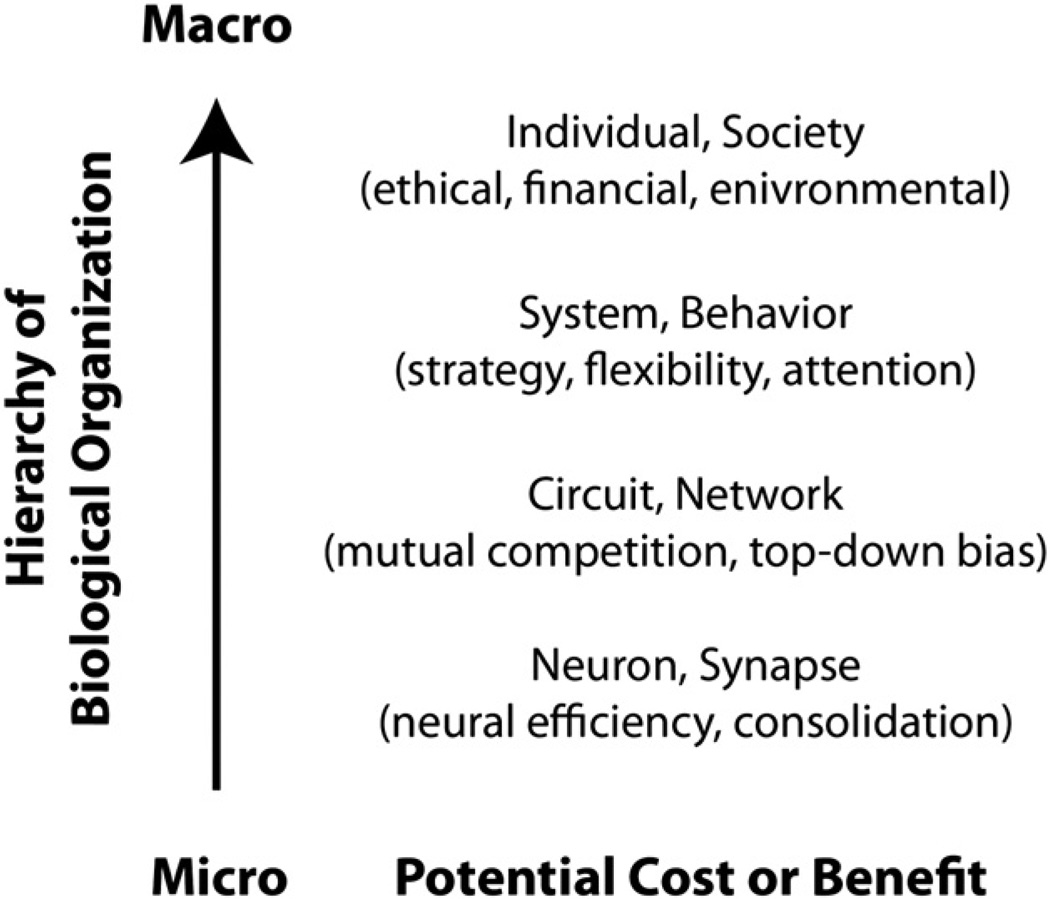
The net zero-sum framework predicts that enhancement of a cognitive function or ability must be associated with a cost. Is there indeed evidence for this? Enhancement of function or ability refers to an improvement in healthy subjects above otherwise normal levels. Thus defined, enhancement is evidently only of interest if we can identify and account for the costs. However, it is a challenging proposition to consider cost in the nervous system, since one has to examine potential cost/ benefit interactions not only within a given domain, but also across multiple domains (Fig. 1.). In general, the following broad parameters need to be taken into account to guide cost-benefit considerations:
-
-
Level of impact (micro-macro cost-benefit): Both enhancement and cost may be observed at various levels ranging from intracellular mechanisms, to synaptic plasticity, gene expression, interactions and connectivity within and between brain networks, interactions between individuals, individuals and environment as well as inter-societal relations.
-
-
Amount of impact and importance: Of what magnitude is the impact of enhancement or cost at the various levels and how important is it?
-
-
Duration (temporal cost-benefit): A momentary cost may be acceptable in exchange for a prolonged improvement, whereas a prolonged cost may not be acceptable.
-
-
Reversibility: Are the costs reversible, without reducing the intended enhancement effects?
Fig. 1.
Factors that may contribute to enhancement and cost effects.
Such multi-parameter considerations raise concerns about the utility of the net-zero sum concept when applied to the nervous system. Therefore, it seems critical to constrain and frame the domains of application if the net-zero sum framework is to be informative. Two key concepts are important in this context: processing power and trade-off.
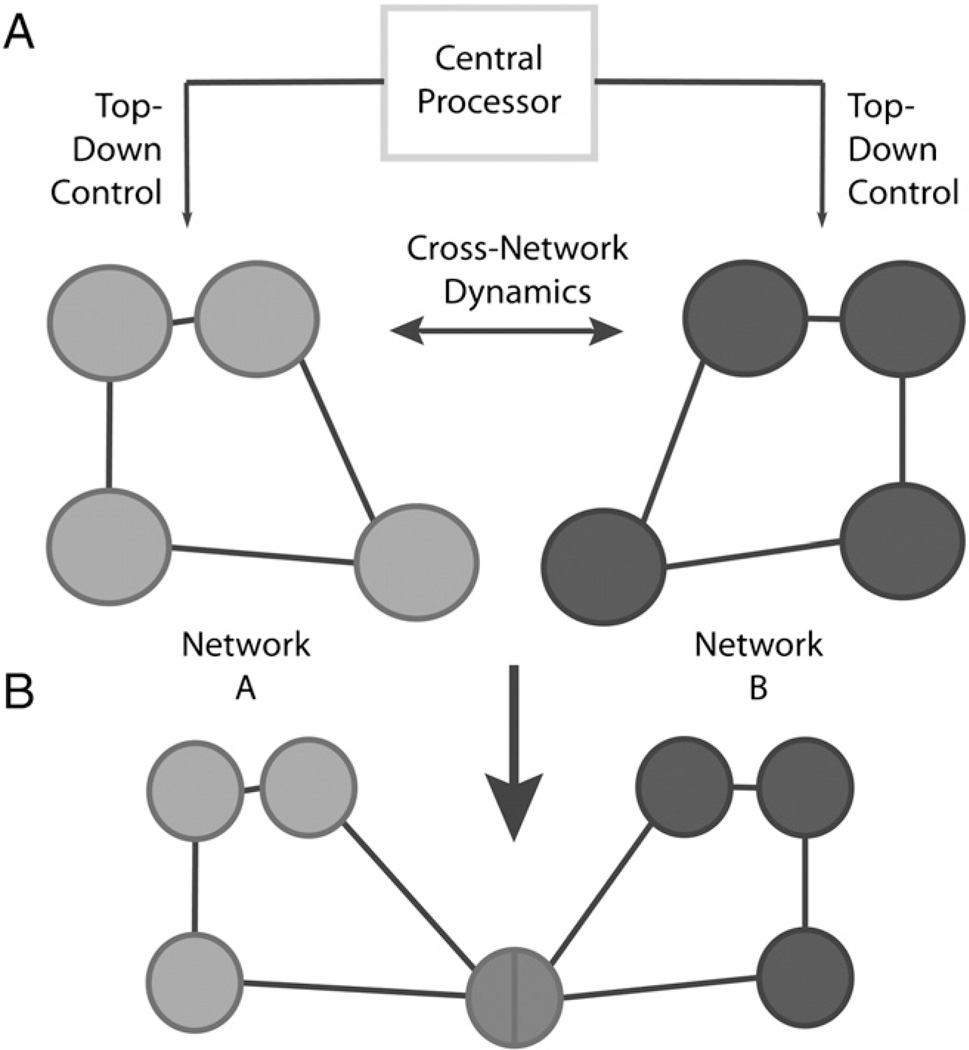
The term “processing power” is borrowed from computer science and indicates the power of the central processing unit of a computer. Processing power in the brain represents the overall power available to all levels of brain function at any given time. During execution of a specific task, processing power is allocated to those networks involved in this task. At the same time however, the brain must reserve sufficient processing power to maintain other ongoing cognitive, sensorimotor, and even basic species-preserving behaviors, the latter of which would always be prioritized. Framed this way, it seems intuitive that the brain must rely on a central processor to distribute processing power appropriately depending on needs and demand (Fig. 2a). One may conceive of top-down modulation as fulfilling such a purpose (Gazzaley and Nobre, 2012). Top-down modulation can be defined as the modulation of activity in neural elements (e.g., single neurons, ensembles or large-scale networks) by hierarchically superior elements. This may involve enhancement of task-relevant representations or suppression of task-irrelevant representations by controlling, timing, and distributing a finite pool of processing power. However, this interpretation fails to fully account for the possibility of temporally independent, dynamic interactions across networks (Smith et al., 2012), which might not be fully dependent on top-down control (Fig. 2b). To this point, the assumption that there is an inherent sharing of resources within functional brain networks has been discussed in various psychological models. These models tap into task-related aspects of attention and executive functions. For example, Kahnemann (1973) described a capacity model of attention based on Moray’s model of a limited capacity processor (1967). Similarly, Posner and Rossman (1965), and Baddeley (1966) investigated cognitive load and suggested that there is a limited central capacity. However, these models do not take into account the impact of resource distribution within a specific function on other ongoing brain activity.
Fig. 2.
A) Processing power is distributed through a central processor across functionally relevant networks (top-down modulation). B) Dynamic interactions between network A and B might not be fully dependent on top-down control. Neural elements and networks can be implied in more than one higher-level network and could thus serve as trade-off switches. Note also that in the figure, networks are meant to possibly represent ensembles of neurons within or across columns, cortico-subcortical networks, or even large-scale bi-hemispheric networks.
Trade-offs, on the other hand, refer to competition between sub-processes, and might capture interactions between neural elements that are independent of top-down control (Fig. 2b). The speed-accuracy trade off (SAT) is particularly well known, and can be described as an adjustment process during decision-making which allows an adaptation to external (Bogacz et al., 2010) and internal demands (Ivanoff et al., 2008), and which can change its function through learning. SAT may arise from threshold shifts (Heitz and Schall, 2012) or baseline shifts (Ivanoff et al., 2008) through various dynamic mechanisms. Trade-off implies a competition between two or more contributing sub-processes, leading to a negative impact on at least one of them. However, this does not necessarily imply measurable behavioral consequences.
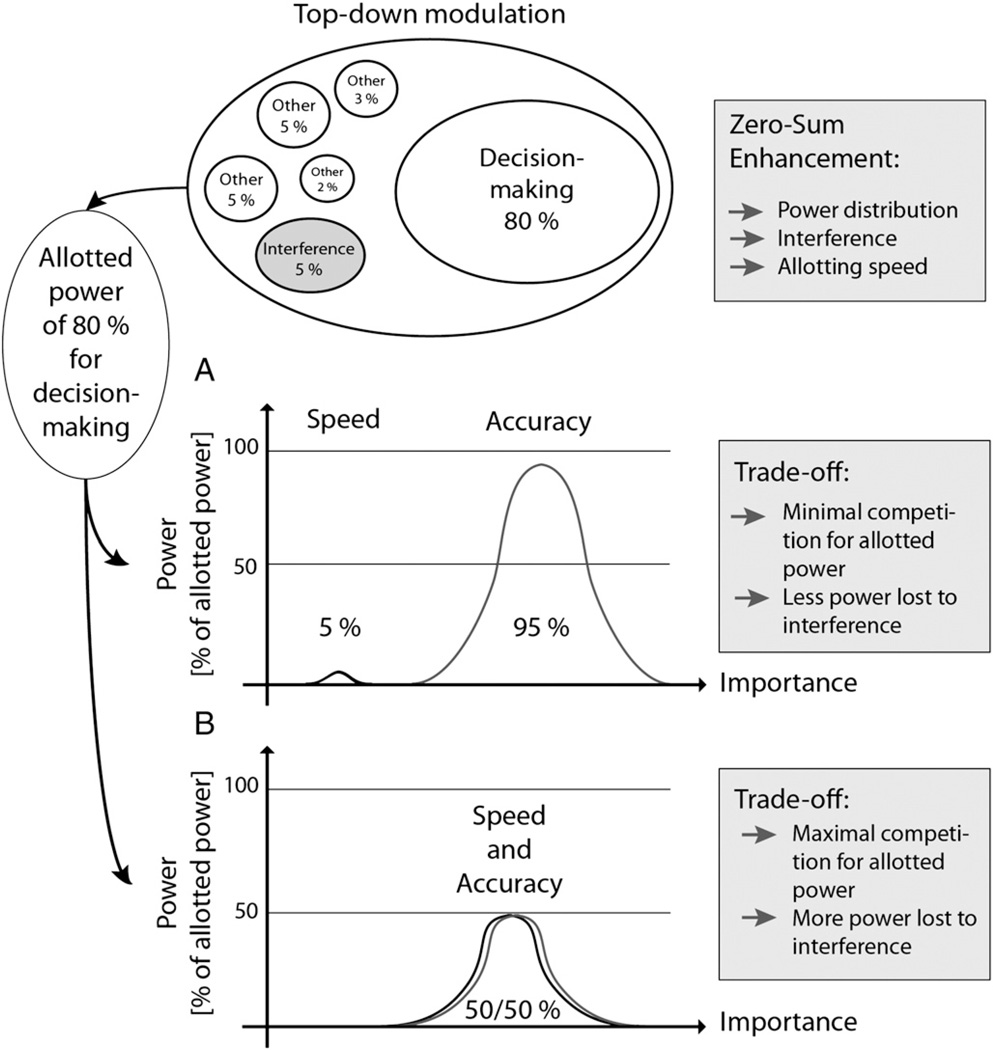
Consider a decision-making task, in which one is required to decide as quickly and accurately as possible. The zero-sum power allocated to solve this task would need to be shared between “speed” and “accuracy”, leading to a competition for limited resources. Competition leads to an additional increase in interference, which further draws on the resources. Ideally, this competition results in an optimal speed-accuracy trade-off. In a situation where either speed or accuracy takes precedent (e.g., accuracy is clearly more important when deactivating a mine), competition decreases and the majority of processing power is invested in either speed or accuracy (Fig. 3).
Fig. 3.
Example of decision-making involving a low (A) or high (B) level speed–accuracy trade-off. Zero-sum refers to 100% of processing power available at any moment. According to internal and external needs, processing power is distributed to fulfill functional demands. A variable part of processing power is lost through interference. Cognitive enhancement within this construct may be achieved through an impact on power distribution, reduction of interference, and impact on the speed by which power distribution is achieved. Respective interference levels are either low or high resulting in a further loss of processing power. Real trade-off processes therefore only account for a small number of situations implying a competing environment.
In accordance with imaging data (see Net zero-sum through the eye of resting-state fMRI section), zero-sum may be driven by the need for processing power. Hence, zero-sum dynamics entails a momentary shift of processing power to high-priority neural elements (e.g. brain networks) by withdrawing processing power from areas that are not behaviorally relevant at a given time or for certain internal and external needs (e.g., when I am reading a book, attention to sensory inputs will be reduced). If so, the consequences of zero-sum dynamics may change in time, and are clearly potentially quite variable across subjects and circumstances.
A perfect orchestration of processing power seems crucial for the brain to function optimally. However, within this orchestra the interaction of “gains” and “losses” of processing power contributes equally to promote salience of currently important concepts and reduce distraction (Lu et al., 2011). “Loss” may not necessarily imply a cost but may, instead, signify a reduction of distraction leading to an enhanced focus of processing power. For example consider the case of attention shifts, which involve three distinct operations: (1) disengagement from a current stimulus, (2) shift of attention to a new target, and (3) engagement of the new target (Posner et al., 1984). In some cases, however, it is desirable to concomitantly direct attention to several targets (divided attention) meaning that the locus of attention continually shifts between different targets. The act of dividing covert attention toward one or across two or several loci necessitates a reduction of resources to any one specific focus, but allows for a quicker shift of attention between loci when needed. Within the activated attention networks we would, therefore, find a trade-off between focused and divided attention.
We hypothesize that such mechanisms do not only account for attention but also explain a more general distribution of processing power within zero-sum processes. In this context any shift in processing power involves the same steps: (1) disengagement from a given neural element (e.g., a brain network), (2) shift of processing power, and (3) engagement of a new neural element (e.g., new brain network).
In summary, it can be hard to find the cost of a given enhancement, which in some casesmay represent an impact at a different time or level of the nervous system. However, enhancement, as here defined, does seem to come with a cost. Nonetheless the operationalization of a metric of cost is challenging and demands careful framing of the zero-cost dynamics.
Can NBS really enhance functions?
A plethora of studies support the basic assumption that NBS can restore cognitive functions in patients, or enhance them in healthy subjects (Guse et al., 2010; Utz et al., 2010; Vallar and Bolognini, 2011). However, the question remains whether NBS is simply shifting processing power more efficiently or reducing interference, leading to behavioral gains with a specific cost? Or to rephrase it: Is there really ever a true enhancement or simply a dynamic trade-off within and across neural levels?
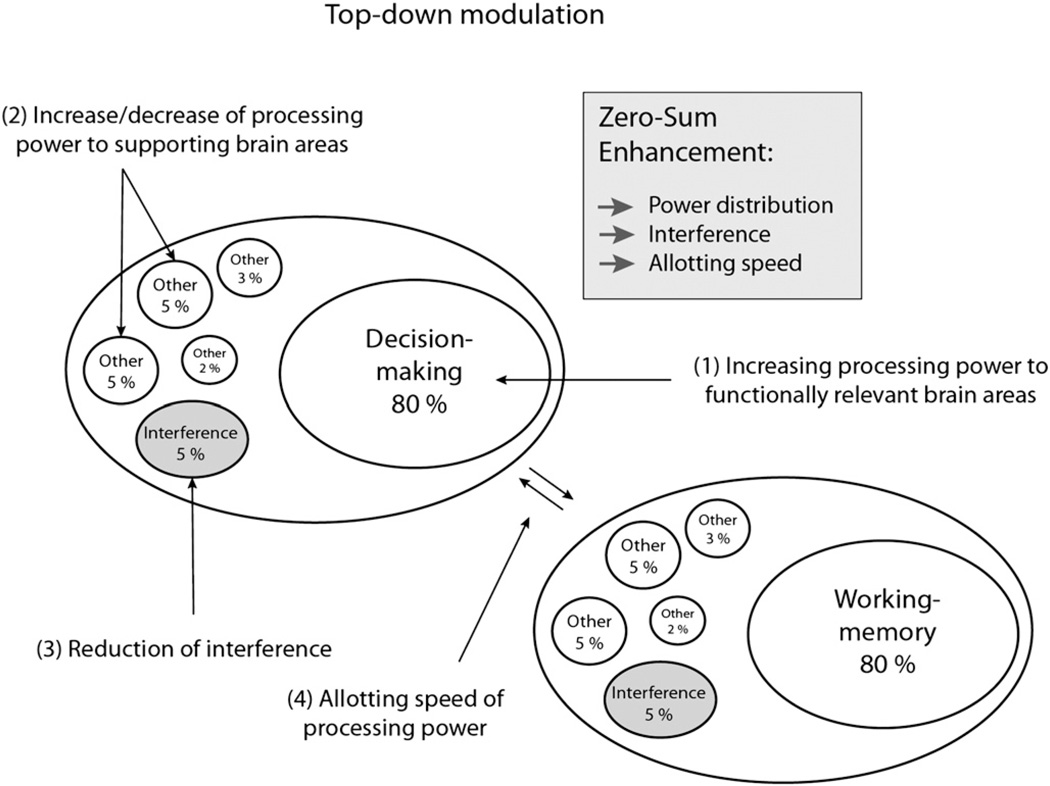
Within the framework of zero-sum, an enhancement that aims to facilitate specific functions could be achieved by a number of different routes: (1) directly, through guiding and increasing allotted processing power to areas that are known to be relevant for the targeted function; (2) indirectly, through guiding and increasing allotted processing power to supportive areas (or conversely, by decreasing power to competitive regions); (3) indirectly, through the reduction of noise/interference effects; and finally (4) through increasing allotment speed (i.e., a change of functional activity patterns) (Fig. 4). Notably, increasing allotted processing power as suggested in (1) and (2) could entail negative effects on other functions if it is accompanied by a commensurate withdrawal of power from other areas.
Fig. 4.
Zero-sum enhancement could be achieved through different ways (change of power distribution, interference reduction, increase of allotting speed).
Furthermore, several recent reviews have highlighted how and to what extent the internal state of the brain can significantly shape its response to external stimulation (Silvanto and Pascual-Leone, 2008; Sparing and Mottaghy, 2008). Though these so-called state-dependent effects pose a challenge to the goal of a consistent and efficacious response, once identified, they can be exploited as well. To this end, NBS is increasingly combined with regimens of motor or cognitive training (e.g., Ditye et al., 2012; Reis et al., 2009), or other forms of NBS (Grüner et al., 2010; Loo et al., 2009; Takeuchi et al., 2012).
If neuroenhancement by NBS is anet zero-sum proposition, we have to assume that (1) an increase in speed of re-allocation of processing power could play a major role in enhancement effects, and (2) enhancement can take place within a naturally given margin.
Enhancement through improving speed
Optimal functionality may involve keeping a number of possibilities open therefore turning flexibility into a key function, thus allowing one to “keep an eye” on different external and internal inputs, quickly react and adapt to novel inputs, and manage limited resources. Flexibility and the ability to suppress distracting stimuli are not only of importance for cognitive but also for motor functions. Such mechanisms may contribute importantly to survival strategies and may have been promoted through evolutionary processes. Naturally occurring speed improvements arise through automation of processes. Though automatized processes consume less processing power, they nevertheless reduce the amount of available resources for other ongoing (automatized or explicit) processes. Optimal cognitive functioning may, therefore, rely on how fast one is able to switch between brain states (see section Net zero-sum through the eye of resting-state fMRI section). Thus, increasing switching-speed may enhance cognitive functions.
Enhancement through exploiting the margin
The brain might function with a margin of “reserve” that could be exploited under certain circumstances. If so, this margin may define the limit of “normal improvement” and going beyond this margin might require taking resources from other areas. This would result in a conceivable loss elsewhere turning the cost-benefit ratio unfavorable. The notion of a margin of reserve underlays some of the considerations offered for age- or injury-related cognitive adaptations (Stern, 2002, 2009). The concept of “cognitive reserve”, put forward by Stern (2002), originates from the observation that brain damage and cognitive impairment and recovery seem not to be directly related. Cognitive reserves may be “built in” to allow for redundancy and could be tapped into for enhancement purposes. This is similar to the finding of gene redundancy, whereby multiple copies of the same genetic sequence code for the same amino acid. This insures that even if one of these codons were disrupted, it would not have a negative effect on the organism.
Interestingly, some recent experimental data suggest that the margin it self might be variable across individuals, thus allowing for differential amounts of potential enhancement. For example, Berryhill and Jones (2012) found that when older adults perform working memory tasks while receiving tDCS, only subjects with high education profited, while performance of subjects with low education decreased. Whether the same assumptions can be made for young healthy subjects remains to be investigated, but one possible interpretation could be that individuals with higher education have also developed larger cognitive reserves (i.e. larger margins) that can be exploited.
A recent study by Iuculano and Cohen Kadosh (2013) specifically addressed this question further. They investigated positive and negative effects of tDCS on learning and automaticity in the mathematical domain. Subjects underwent a 6-day cognitive training, which was combined with tDCS over the posterior parietal cortices (PPCs) or the dlPFC, or with sham tDCS. They found a double dissociation: while stimulation over PPC facilitated numerical learning, it impaired automaticity. Vice versa, stimulation over dlPFC inhibited learning, while automaticity was improved. This study supports the assumption of zero-sum enhancement and emphasizes the importance of controlling for side effects. However, their finding of inhibitory effects is not surprising as the cathode was placed over areas that are implied in task processing. It would be of importance to investigate similar side effects in protocols that purely aim to enhance functions (e.g., cathode over non-active areas or high-frequency repetitive TMS).
If cognitive reserves represent an intrinsic mechanism built-in to maintain function in the face of an event that strains the system, then extrinsically exploiting such a margin must have a cost, albeit at a rather different level of analysis that may well be highly individually specific, e.g., limiting reserves that might be needed in the case of eventual insult, age-related decline, or other demands. Similarly, enhancement by pharmacological agents may also lead to an unfavorable cost-benefit ratio depending on individual baseline performance level. For example, Farah et al. (2009) found that Adderall had positive effects on creativity for lower-performing subjects, but led to impairments in higher-performing subjects.
With regards to enhancement of cognitive functions in healthy subjects, these results raise the following questions: who could profit from cognitive enhancement interventions; when should we aim to enhance core structures; and when is it preferable to enhance associated structures or inhibit noise-generating structures? Such questions demand thorough consideration and should be addressed in carefully controlled research protocols predicated on efforts to estimate possible effect sizes of enhancement as well as cost.
Estimating NBS-driven enhancement and cost within the net-zero sum framework
Enhancement and cost can be reflected on different levels, yet can be captured with neuropsychological and neurophysiological assessments, as well as brain imaging methods. These measures can inform us on the dynamics that underlay the net zero-sum hypothesis. However, an accurate estimation of enhancement and costs can be extremely challenging and ultimately probably not fully possible, precisely because they can be represented at multiple different levels and time frames. It thus becomes critical to constrain the framework and define the levels under study a priori. In the next two sections we shall focus on lessons from resting-state fMRI and from studies on paradoxical functional facilitation which can help elucidate the challenges in estimating enhancement and cost, as well as the possibilities of NBS-driven neuroenhancement within the net-zero sum framework.
Net zero-sum through the eye of resting-state fMRI
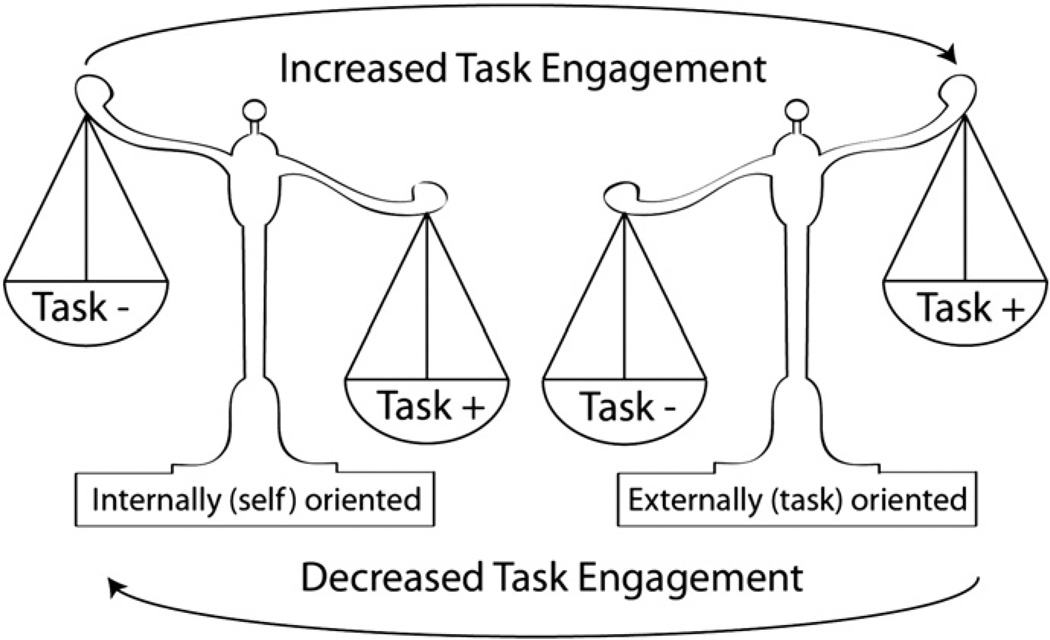
The past decade has seen acceleration in the number of studies investigating the intrinsic activity of the brain at rest. So-called “resting-state functional connectivity” studies have revealed several features of the brain’s innate organization that in turn can inform the question of zero-sum gain. For starters, the brain appears to maintain a dynamic balance between external and internal engagement. Absent a particular task, neural activity is organized into multiple resting-state networks (RSNs). Each RSN is comprised of spatially discrete regions, often termed “nodes,” whose low-frequency oscillatory activity is highly correlated with other nodes within the network (Biswal et al., 1995; Greicius et al., 2003). While nodal activity is positively correlated to other nodes within RSNs, there are strong negative correlations between RSNs associated with extrinsic (i.e., spatial attention, working memory) and intrinsic (i.e., self-reflection, theory of mind) cognition (Damoiseaux et al., 2006; Fox et al., 2005; Greicius et al., 2003; Saxe and Wexler, 2005). These anti-correlated RSNs are often referred to as “task-positive” and “task-negative” networks, respectively (Gao and Lin, 2012; Uddin et al., 2008). Moreover, activity within task-negative nodes declines as engagement in external activities, such as attention-demanding cognitive tasks, increases (Greicius et al., 2003). Given that the brain operates within the restrictions of a finite energy source and that different systems are required for different tasks, it makes sense to think of task-positive and task-negative RSNs as existing in a push-pull or “see-saw” dynamic. In this model, shifting attention between one’s internal state and an externally directed activity is a product of shifting cognitive resources from one RSN to another (Fig. 5).
Fig. 5.
Schematic showing purported interactions between externally-focused task-positive network (+) and internally-focused task-negative (−) network.
Recent evidence suggests that the strength and direction of correlations between regions, both within and between RSNs, is not a trivial coincidence or a by product of data processing techniques such as global mean signal regression (Chai et al., 2012). Rather, functional connectivity may serve as an important biomarker of abnormal brain function in diseases such as schizophrenia and bipolar disorder (Chai et al., 2011), and attention deficit/hyperactivity disorder (Cocchi et al., 2012), and chart the progression of age-related changes in cognition (Schlee et al., 2012). These studies suggest that a breakdown in certain cognitive abilities may arise from maladaptive coupling of brain regions responsible for different, potentially competing functions. Thus, problems with attention or working memory, for example, could result from a failure to properly disengage task-negative networks during extrinsic activities, or to put it another way, an inability to shift attention from one’s internal state to the external world.
Relevant to the question of zero-sum game, several recent studies have investigated changes in RSNs in response to interventions associated with neuroenhancement, including cognitive training (Evers et al., 2012; Waites et al., 2005), meditation (Jang et al., 2011), and NBS. In healthy participants and under normal circumstances, RSNs show robust consistency when assessed days, weeks or even months apart (Damoiseaux et al., 2006; Shehzad et al., 2009). This relative stability makes resting-state functional connectivity a suitable and potentially powerful assessment of experimentally induced neuroplasticity.
In one experiment (Eldaief et al., 2011), a group of healthy individuals underwent resting-state fMRI immediately before and after receiving repetitive TMS (rTMS) to a node of the task-negative “default mode network” (DMN). The target, within the left posterior inferior parietal lobule (lpIPL), was derived individually for each subject using a seed-based analysis of his or her baseline resting-state fMRI. The authors then stimulated the same region with both high (20 Hz) and low (1 Hz) frequency rTMS in separate sessions, at least one week apart. The effects of rTMS were assessed in terms of changes in the strength of correlations between a seed region placed in the rTMS target node and seeds located in other nodes within the DMN. The authors found different outcomes for high and low frequency both in terms of the direction of change and the spatial distribution of the correlated pairs: following 1 Hz rTMS, there was an increase in functional connectivity between the lpIPL and the hippocampal formation bilaterally, while 20 Hz rTMS led to a decrease between the lpIPL and both the posterior cingulate cortex and the medial prefrontal cortex. These results indicate that the intrinsic functional connectivity of different regions within the same RSN is not fixed, but can be causally manipulated through external stimulation. Moreover, the opposing and spatially distinct response of 20 Hz and 1 Hz rTMS suggests that high and low frequency stimulation may operate differently on sub-populations of neurons within the same region.
Using a different approach, Keeser et al. (2011) obtained resting-state fMRI on healthy adults before and after the subjects received tDCS to the prefrontal cortex (PFC). The authors compared the effects of real and sham stimulation in a double blind, randomized-crossover design. The anode, often referred to as the stimulating electrode, was placed on EEG coordinate F3, which overlies the left dorsolateral pre-frontal cortex (dlPFC), with the cathode located on the contralateral supraorbital region (approximately over right frontal pole). Unlike direct depolarization of rTMS, tDCS modulates spontaneous excitability by polarizing the extracellular ionic concentration in the general vicinities of the anode and cathode. The consequence of spreading the stimulation over a wider area, as compared to rTMS in the previous experiment, is the potential to stimulate the nodes of multiple RSNs simultaneously. Using a dual-regression group-level independent components analysis (ICA), the authors assessed the effects of tDCS in terms of a change in coactivation for a number of task-positive and task-negative RSNs with nodes in the prefrontal cortex. Following real tDCS, significant clusters of increased coactivation were observed within the DMN and frontoparietal “control” network (FPN). While the majority of these clusters were located in superior and middle frontal gyri, bilaterally, increases were also observed in posterior nodes, including the posterior cingulate and inferior parietal lobule. The results of this study demonstrate that tDCS of the prefrontal cortex can modulate neural excitability within task-positive and task-negative RSNs simultaneously.
While investigating the effect of NBS on functional connectivity can provide insight into the question of whether neuroenhancement is a zero-sum game, there are several limitations in the these two particular studies. Neither the Eldaief nor the Keeser study examined the effect of modulation on inter-network relationships. Since several neuropsychiatric disorders show abnormal anti-correlations between task-positive and task-negative networks, it would be valuable to known if this relationship could be manipulated in normal individuals by neuromodulation. In the case of the Eldaief study, the authors stimulated just a single node of the DMN and only assessed changes to intra-network correlations within that RSN. While Keeser and colleagues assessed changes within multiple RSNs, their choice of an ICA-based approach may have precluded an investigation of relationships between networks. Furthermore, the broad field of tDCS meant that stimulation likely impacted the nodes of multiple networks simultaneously. Hence, it is not possible to separate the effects of modulating the DMN from the FPN. In the discussion, the authors acknowledge that interpretation of their results showing increased coactivation within multiple RSNs may be limited to the particular parameters and configuration of tDCS.
A second major limitation is that neither Eldaief et al. nor Keeser et al. included a behavioral task, which could be used to probe the functional significance of manipulating the intrinsic activity of the brain. As such, it is not known whether these changes in functional connectivity or coactivation are also accompanied by changes in cognitive functions (although this is the focus of ongoing research). Addressing this question will shed light on whether neuroenhancement is a zero-sum game. At the same time, relating changes in intrinsic activity to changes in behavior could tie together previous studies showing that NBS can alter cognitive performance (e.g., Fregni et al., 2005; Mottaghy et al., 2000) with recent evidence that individual differences in resting-state functional connectivity are predictive of cognitive performance (Meier et al., 2012; Sala-Llonch et al., 2011). We know that the strength and directions of correlations within and between networks have functional implications and that NBS can modulate this connectivity. However, we do not yet know the relationship between NBS-driven changes in behavior and changes in functional connectivity. If augmentation of cognitive functions by NBS reflects the strengthening of connectivity within a relevant network while weakening a competing one, this would support the zero-sum game argument. Alternatively, if NBS altered RSNs completely independently of each other, or could be applied in such a way as to make the ability to switch between networks more efficient, this might represent a net gain in function without a corresponding loss.
Net zero-sum through the eye of paradoxical functional facilitation
Kapur (1996) first introduced the term “paradoxical functional facilitation” and described two major types: (1) Restorative effects: damage to an intact area of the brain normalizes a previously reduced level of functioning, such as for example in the Sprague effect; (2) Enhancing effects: a patient performs certain tasks better than at baseline or compared to a healthy control subject.
The Sprague effect is well known: Unilateral damage to the network of cortical, thalamic, and tectal regions responsible for orienting to visual stimuli can cause neglect of the contralateral hemifield (Brain, 1941). This decline in visuospatial attention results not simply from the loss of uniquely critical neurons but rather from the disruption in the dynamic balance that normally exists between the left and right hemispheres, which biases attention to the ipsilateral field (Bartolomeo, 2007; Danckert and Ferber, 2006; Gabrieli and Whitfield-Gabrieli, 2007). Correct the balance, the model predicts, and you will regain function (Corbetta et al., 2005). As evidence, Sprague (1966) was able to restore orienting abilities in cats with unilateral damage to striate and extrastriate cortex by making a lesion of the contralateral superior colliculus. Sprague concluded that the initial loss of function was not due to the loss of reticulothalamic input to the cortex (as had been previously assumed), but rather a disruption in the commissural and corticotectal projections that normally keep the left and right systems in balance. A subsequent lesion of the now unconstrained and hyperactive contralesional colliculus thus removed its suppression of the ipsilesional colliculus leading to a restoration in bilateral orienting behavior. Further lesion and reversible deactivation studies have confirmed (Sprague, 1996) and extended this effect to other regions (Lomber and Payne, 2001; Payne and Rushmore, 2004).
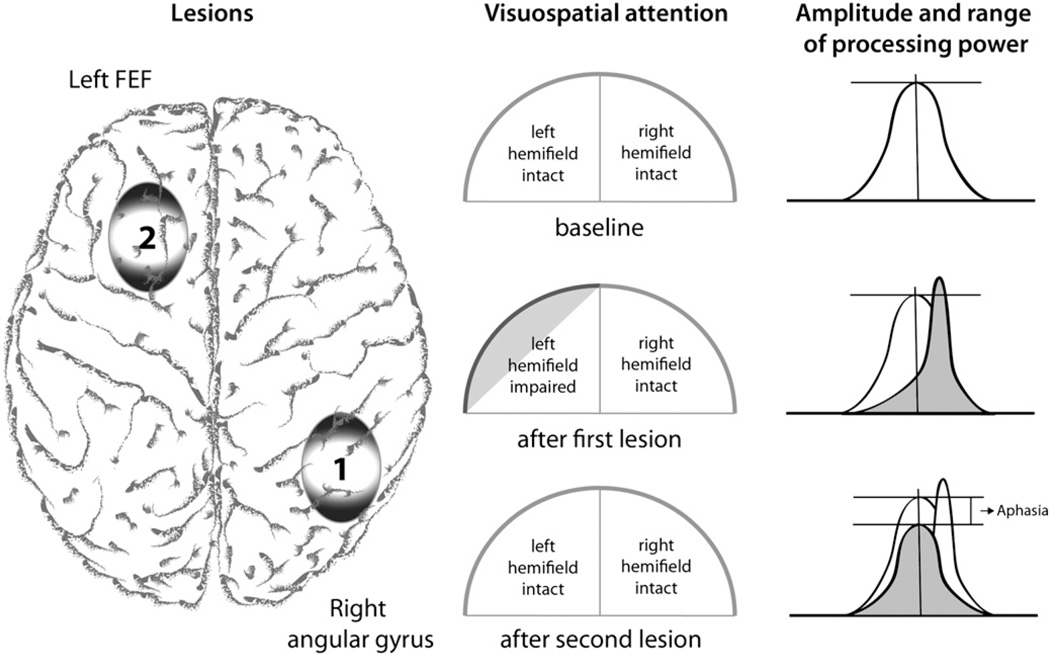
Similar phenomena have been observed in humans in at least two cases in which both the initial lesion that induced neglect and the second lesion that restored function were of natural causes. In the case described by Weddell (2004), a 34-year-old right-handed man developed a left hemi-spatial neglect after incurring right frontal damage in the management of hydrocephalus. The neglect was subsequently resolved following invasion of the left superior colliculus by a cyst. Vuilleumier et al. (1996) presented a case of “natural” paradoxical facilitation (Fig. 6). They described a patient who suffered from visuospatial neglect and hemianopia after a stroke of the right posterior parietal area, which however disappeared after a second, well circumscribed stroke of the left middle frontal gyrus (including the frontal eye field). This means that although the second stroke entailed an inhibition in the area of the lesion, it had a paradoxical facilitatory effect leading to a reduction of neglect symptoms, while the patient newly developed aphasic symptoms. The authors argued that this abrupt recovery was related to the second stroke and inferred from this that the visuospatial neglect must have been caused by an imbalance between two opposing attention systems in the first place (Kinsbourne, 1970). The second stroke therefore must have re-established balance within the visuospatial attention network, which led to a recovery of neglect symptoms. Both stroke locations are critical within this network and one could argue that injuries within the same brain network would hence interact to a certain degree. In this case an injury led to a paradoxical functional improvement.
Fig. 6.
Example of “natural” paradoxical facilitation (Vuilleumier et al., 1996). After a first stroke (lesion 1) the patient suffered from a left visuospatial neglect and hemianopia which disappeared after a second stroke that affected the frontal eye fields (lesion 2). The second stroke had a paradoxical facilitatory effect on visuospatial attention, while the patient newly developed aphasic symptoms. While the first lesions shifted and increased processing power availability towards the left hemisphere (over-excitability) hereby increasing attention towards the right visual hemifield, the second lesion re-shifted and therefore normalized attention allocation while losing overall processing power.
Even in the face of an injury, activity within the brain and its networks would result in zero-sum. Accordingly, activity across a perfectly balanced healthy attention network, as well as in an imbalanced attention network resulting from injury, would in both cases summate to zero. In this case, the magnitude of activity may either be smaller than before the injury, but activity foci could also be more dispersed including increased activity of associated brain areas that were previously less or not at all involved. With regard to the above-described special scenario, the first stroke led to a reduction of excitability of the left attention network entailing an over-excitability of the right attention network resulting in a visuospatial neglect. Though these symptoms improved markedly after the second stroke, by reducing the over-excitability of the left hemisphere, and therefore re-establishing the balance, it also led to the onset of aphasic symptoms (Fig. 6).
Several researchers have achieved improvements in visuospatial neglect through paradoxical facilitation. Sparing et al. (2009) applied tDCS to 10 stroke patients and found an improvement in line-bisection with either the anode over the injured or the cathode over the intact parietal cortex (opposing electrode over Cz). Several other studies have examined the potential of NBS to restore balance to a disrupted network and ameliorate neglect symptoms (Brighina et al., 2003; Kim et al., 2013; Koch et al., 2008; Lim et al., 2010; Nyffeler et al., 2009; Oliveri et al., 2001; Shindo et al., 2006; Song et al., 2009); either by enhancing the activity of the injured hemisphere or by reducing the excitability of (and thus the inhibitory competition by) intact structures in the contralateral hemisphere. The assumption that visuospatial attention relies on a widespread network is supported by neuroimaging studies showing that changed patterns of corticocortical connectivity are related to neglect symptoms and neglect recovery is based on the re-weighting of activity within implicated networks (Bartolomeo et al., 2007; Corbetta et al., 2005).
This type of paradoxical facilitation of behavior seen after neuronal damage can occur in the healthy brain as well (Najib and Pascual-Leone, 2011). In relation to NBS, paradoxical facilitation can occur when a regimen of stimulation associated with suppressing activity (i.e., low-frequency rTMS or cathodal tDCS) is applied to intact regions (e.g., the contralesional homologue area) to restore the balance of activity in patients, or is applied to areas that exert suppressive control over a primary (enhanced) area of interest in healthy subjects. Hence, the net effects of stimulation are always a product of the stimulation applied (suppressive or facilitative), the temporary role of the target area within local and extended functional networks, and the state of the target.
Interactions between homologue brain areas take a special place in net zero-sum enhancement. Paradoxical enhancement involving the interaction of homologue brain areas has been mainly studied with regards to language functions and visuospatial attention. In healthy humans, modulation of the posterior parietal cortex (PPC) with rTMS (Bjoertomt et al., 2002; Brighina et al., 2002; Dambeck et al., 2006; Fierro et al., 2000) and tDCS (Giglia et al., 2011) has been shown to induce a temporary neglect-like bias in spatial attention. For example, Fierro et al. (2006) applied single pulse TMS over the right PPC 150 milliseconds (ms) after stimulus presentation and were able to induce a significant rightward bias in a line-length judgment task. At an inter-stimulus interval (ISI) of 5 ms (but not at 1 or 3 ms ISI), paired-pulse TMS restored baseline levels. Lastly, the most direct support for the notion of the net zero-sum model comes from two studies (Hilgetag et al., 2001; Jin and Hilgetag, 2008) showing that a similar rTMS-induced bias, while diminishing the ability to detect targets in one field, facilitates detection in the other. At the very least, the study of visuospatial neglect, and its cancelation, reinforces the idea that there exist certain systems within the brain whose proper function depends on a delicate balance between competitive circuits.
A growing body of literature reports neuroenhancement in healthy subjects with NBS predicated upon such paradoxical facilitation concepts. Galea et al. (2010) investigated interfering processes between declarative and procedural consolidation. Disrupting the left or right dlPFC immediately after training a serial reaction time task (SRTT) resulted in improvements in the SRTT several hours later. The authors argued that disruption of the dlPFC, which is important for declarative memory formation and is believed to have a negative effect on procedural memory formation, led to a reduction of the said negative effect. Disrupting the dlPFC therefore had a paradoxical facilitatory effect on procedural memory formation. A later study specifically explored interference effects between declarative and motor memory (Cohen and Robertson, 2011). Applying TMS immediately after encoding motor and declarative stimuli in quick succession interrupted interference effects and hence left both memories unimpaired. These findings indicate that the brain may actively produce interference resulting in impairment. An explanation for such an apparently detrimental process could be that naturally occurring events following in quick succession would usually draw on similar processes, which makes such an interaction valuable. The quick succession of two very different memory processes, however, may turn this valuable interaction into a disruptive interference.
If we take into account top-down modulation, which is said to “underlie our ability to focus attention on task-relevant stimuli and ignore irrelevant distractions” (Gazzaley and Nobre, 2012) we may be able to explain seemingly contradictory results from the literature. For example, Gallate et al. (2009) found decreased “false memories” after inhibiting excitability of the left anterior temporal lobe (ATL) with low-frequency rTMS, while Boggio et al. (2009) found similar results after increasing excitability of the same area with unilateral tDCS (anode over the left ATL, enlarged cathode over right ATL) and bilateral tDCS (anode over left ATL, cathode over right ATL). At first sight these results indeed seem contradictory; however, we could interpret them as follows: while the inhibiting protocol may have led to a suppression of the influence of irrelevant distractors, the facilitating protocol may have led to improved focused attention on relevant stimuli. This could explain the success of both protocols. We therefore need to consider contradictory effects arising within one stimulated brain area, though this may be highly dependent on the assessed function. In this specific case, identifying a false memory, which is defined as the false recollection of an event, indicates that one or several relevant stimuli (correct memory) are surrounded by several distracting stimuli (false memory) that need to be distinguished. Different mechanisms can lead to similar behavioral improvement.
Moreover, when applying NBS to alter brain functions one needs to take into consideration that behavioral effects are not solely due to direct effects of the stimulated area, but may also be influenced by interactive network effects. Kahn et al. (2005) investigated the contribution of the ventrolateral prefrontal cortices (vlPFCs) in verbal encoding with single-pulse TMS. While stimulation of the left vlPFC resulted in an inhibitory effect, stimulation of the right vlPFC led to a paradoxical facilitatory effect on verbal memory. The authors argued that this facilitation might be due to a functional shift in mechanisms involved in learning.
Implications for future studies and ethical considerations
Current research aiming to improve brain functions shows that the brain’s capability to enhance functions may not be limited from a merely neurophysiological perspective, but is likely accompanied by a cost. Until now, most research protocols have focused measures on functions that were to be improved, and control measures were sparsely used. However, paradoxical functional facilitation inspired experimental designs and neuroimaging studies investigating large-scale correlation/ anti-correlation networks help illustrate the importance of a more holistic assessment. Thus, it is imperative to emphasize both the assessment of cost and the estimation of enhancement versus cost balance in future studies.
Net zero-sum implies a limitation of brain enhancement and can guide us in defining hypothesis-driven constraints in order to estimate cost-benefit ratios for enhancement protocols. Within this context, enhancement through NBS could result from changes in the distribution and/or amplitude of processing power, reduction of neuronal interference processes, and/or changes in how fast processing power can be re-distributed.
The promise of brain enhancement in an otherwise healthy individual inevitably raises important bioethical concerns (Chatterjee, 2004; Farah et al., 2004; Hamilton et al., 2011). Is it acceptable to improve certain brain functions at the cost of others and can we take the responsibility for its impact on the individual and on society? Assessment of cost seems particularly central to answer this question. Current neuroenhancement studies emphasize positive outcomes of specific functions and concentrate on individual improvements, while related topics such as risk and safety, as well as social and moral factors are neglected or restricted to specific inquiries (Rossi et al., 2009). An ongoing discussion of underlying theoretical frameworks like the net-zero sum construct are important to increase awareness for ethical concerns and help researchers define control parameters.
Acknowledgments
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroenceph-alography (EEG) and magnetic resonance imaging (MRI). Work on this project was supported in part by grant number 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Science or the National Institutes of Health. Dr. Brem was supported by the Stiefel-Zangger Foundation and the Swiss National Foundation (PBZHP1_147196). Dr. Fried was supported by the Department of Anatomy and Neurobiology at Boston University School of Medicine.
Footnotes
Conflict of interest statement
The authors declare that this article was written in the absence of any commercial, financial, or personal relationships that could be construed as a potential conflict of interest.
Contributor Information
Anna-Katharine Brem, Email: abrem@bidmc.harvard.edu.
Peter J. Fried, Email: pfried@bidmc.harvard.edu.
Jared C. Horvath, Email: jch155@mail.harvard.edu.
Edwin M. Robertson, Email: emrobert@bidmc.harvard.edu.
Alvaro Pascual-Leone, Email: apleone@bidmc.harvard.edu.
References
- Baddeley AD. The capacity for generating information by randomization. Q. J. Exp. Psychol. 1966;18:119–129. doi: 10.1080/14640746608400019. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. Visual neglect. Curr. Opin. Neurol. 2007;20:381–386. doi: 10.1097/WCO.0b013e32816aa3a3. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb. Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci. Lett. 2012;521:148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar. MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bjoertomt O, Cowey A, Walsh V. Spatial neglect in near and far space investigated by repetitive transcranial magnetic stimulation. Brain. 2002;125:2012–2022. doi: 10.1093/brain/awf211. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers E-J, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Fregni F, Valasek C, Ellwood S, Chi R, Gallate J, Pascual-Leone A, Snyder A. Temporal lobe cortical electrical stimulation during the encoding and retrieval phase reduces false memories. PLoS One. 2009;4:e4959. doi: 10.1371/journal.pone.0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain WR. Visual disorientation with special reference to lesions of the right cerebral hemisphere. Brain. 1941;64:244–272. [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Piazza A, Oliveri M, La Bua V, Daniele O, Fierro B. Perceptual and response bias in visuospatial neglect due to frontal and parietal repetitive transcranial magnetic stimulation in normal subjects. Neuroreport. 2002;13:2571–2575. doi: 10.1097/00001756-200212200-00038. [DOI] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci. Lett. 2003;336:131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JDE, Nieto Castañón A, McCarthy JM, Cohen BM, Ongür D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. Cosmetic neurology: the controversy over enhancing movement, mentation, and mood. Neurology. 2004;63:968–974. doi: 10.1212/01.wnl.0000138438.88589.7c. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, Tripp G, Mattos P. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J. Neurosci. 2012;32:17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Robertson EM. Preventing interference between different memory tasks. Nat. Neurosci. 2011;14:953–955. doi: 10.1038/nn.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Curlik DM, II, Shors TJ. Training your brain: do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambeck N, Sparing R, Meister IG, Wienemann M, Weidemann J, Topper R, Boroojerdi B. Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Res. 2006;1072:194–199. doi: 10.1016/j.brainres.2005.05.075. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckert J, Ferber S. Revisiting unilateral neglect. Neuropsychologia. 2006;44:987–1006. doi: 10.1016/j.neuropsychologia.2005.09.004. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nat. Rev. Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp. Brain Res. 2012;219:363–368. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EA, Klaassen EB, Rombouts SA, Backes WH, Jolles J. The effects of sustained cognitive task performance on subsequent resting state functional connectivity in healthy young and middle-aged male schoolteachers. Brain Connect. 2012;2:102–112. doi: 10.1089/brain.2011.0060. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Illes J, Cook-Deegan R, Gardner H, Kandel E, King P, Parens E, Sahakian B, Wolpe PR. Neurocognitive enhancement: what can we do and what should we do? Nat. Rev. Neurosci. 2004;5:421–425. doi: 10.1038/nrn1390. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Haimm C, Sankoorikal G, Smith ME, Chatterjee A. When we enhance cognition with Adderall, do we sacrifice creativity? A preliminary study. Psychopharmacol. (Berl.) 2009;202:541–547. doi: 10.1007/s00213-008-1369-3. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, Bisiach E. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport. 2000;11:1519–1521. [PubMed] [Google Scholar]
- Fierro B, Brighina F, Giglia G, Palermo A, Francolini M, Scalia S. Paired pulse TMS over the right posterior parietal cortex modulates visuospatial perception. J. Neurol. Sci. 2006;247:144–148. doi: 10.1016/j.jns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MTA, Paulus W, Pascual-Leone A. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli J, Whitfield-Gabrieli S. Attention to neglect. Neuron. 2007;53:776–777. doi: 10.1016/j.neuron.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J. Cogn. Neurosci. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallate J, Chi R, Ellwood S, Snyder A. Reducing false memories by magnetic pulse stimulation. Neurosci. Lett. 2009;449:151–154. doi: 10.1016/j.neulet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Gammaitoni L, Haenggi P, Jung P, Marchesoni F. Stochastic resonance. Rev. Mod. Phys. 1998:223–288. [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. (Regul. Ed.) 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglia G, Mattaliano P, Puma A, Rizzo S, Fierro B, Brighina F. Neglect-like effects induced by tDCS modulation of posterior parietal cortices in healthy subjects. Brain Stimul. 2011;4:294–299. doi: 10.1016/j.brs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüner U, Eggers C, Ameli M, Sarfeld A-S, Fink GR, Nowak DA. 1 Hz rTMS preconditioned by tDCS over the primary motor cortex in Parkinson’s disease: effects on bradykinesia of arm and hand. J. Neural Transm. 2010;117:207–216. doi: 10.1007/s00702-009-0356-0. [DOI] [PubMed] [Google Scholar]
- Gruzelier J, Egner T, Vernon D. Validating the efficacy of neurofeedback for optimising performance. Prog. Brain Res. 2006;159:421–431. doi: 10.1016/S0079-6123(06)59027-2. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J. Neural Transm. 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R, Messing S, Chatterjee A. Rethinking the thinking cap: ethics of neural enhancement using noninvasive brain stimulation. Neurology. 2011;76:187–193. doi: 10.1212/WNL.0b013e318205d50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron. 2012;76:616–628. doi: 10.1016/j.neuron.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Iuculano T, Cohen Kadosh R. The mental cost of cognitive enhancement. J. Neurosci. 2013;33:4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff J, Branning P, Marois R. fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS One. 2008;3:e2635. doi: 10.1371/journal.pone.0002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang D-H, Byun MS, Kwon SJ, Choi C-H, Kwon JS. Increased default mode network connectivity associated with meditation. Neurosci. Lett. 2011;487:358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Jin Y, Hilgetag CC. Perturbation of visuospatial attention by high-frequency offline rTMS. Exp. Brain Res. 2008;189:121–128. doi: 10.1007/s00221-008-1449-y. [DOI] [PubMed] [Google Scholar]
- Kahn I, Pascual-Leone A, Theoret H, Fregni F, Clark D, Wagner AD. Transient disruption of ventrolateral prefrontal cortex during verbal encoding affects subsequent memory performance. J. Neurophysiol. 2005;94:688–698. doi: 10.1152/jn.01335.2004. [DOI] [PubMed] [Google Scholar]
- Kahnemann D. Attention and Effort. Prentice Hall: Englewood Cliffs, NJ; 1973. [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Möller H-J, Reiser M, Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BR, Chun MH, Kim D-Y, Lee SJ. The effect of high and low frequency repetitive transcranial magnetic stimulation on visuospatial neglect in acute stroke patients: a double-blind, sham-controlled trial. Ach. Phys. Med. Rehabil. 2013;94:803–807. doi: 10.1016/j.apmr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. A model for the mechanism of unilateral neglect of space. Trans. Am. Neurol. Assoc. 1970;95:143–146. [PubMed] [Google Scholar]
- Kinsbourne M. Direction of gaze and distribution of cerebral thought processes. Neuropsychologia. 1974;12:279–281. doi: 10.1016/0028-3932(74)90013-x. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One. 2012;7:e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Kang EK, Paik N-J. Repetitive transcranial magnetic stimulation to hemispatial neglect in patients after stroke: an open-label pilot study. J. Rehabil. Med. 2010;42:447–452. doi: 10.2340/16501977-0553. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. Task-specific reversal of visual hemineglect following bilateral reversible deactivation of posterior parietal cortex: a comparison with deactivation of the superior colliculus. Vis. Neurosci. 2001;18:487–499. doi: 10.1017/s0952523801183148. [DOI] [PubMed] [Google Scholar]
- Loo C, Martin D, Pigot M, Arul-Anandam P, Mitchell P, Sachdev P. Transcranial direct current stimulation priming of therapeutic repetitive transcranial magnetic stimulation: a pilot study. J. ECT. 2009;25:256–260. doi: 10.1097/YCT.0b013e3181a2f87e. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Li X, Tjan BS, Dosher BA, Chu W. Attention extracts signal in external noise: a BOLD fMRI study. J. Cogn. Neurosci. 2011;23:1148–1159. doi: 10.1162/jocn.2010.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B. Poll results: look who’s doping. Nature. 2008;452:674–675. doi: 10.1038/452674a. [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J. Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Mölle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One. 2011;6:e16905. doi: 10.1371/journal.pone.0016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Wildenberg JC, Liu J, Chen J, Calhoun VD, Biswal BB, Meyerand ME, Birn RM, Prabhakaran V. Parallel ICA identifies sub-components of resting state networks that covary with behavioral indices. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moray N. Where is capacity limited? A survey and a model. Acta Psychol. (Amst.) 1967;27:84–92. doi: 10.1016/0001-6918(67)90048-0. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Krause BJ, Kemna LJ, Töpper R, Tellmann L, Beu M, Pascual-Leone A, Müller-Gärtner HW. Modulation of the neuronal circuitry subserving working memory in healthy human subjects by repetitive transcranial magnetic stimulation. Neurosci. Lett. 2000;280:167–170. doi: 10.1016/s0304-3940(00)00798-9. [DOI] [PubMed] [Google Scholar]
- Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490–495. doi: 10.1016/j.neuropharm.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Najib U, Pascual-Leone A. Paradoxical functional facilitation with noninvasive brain stimulation. In: Kapur N, editor. The Paradoxical Brain. Cambridge: University Press; 2011. pp. 234–260. [Google Scholar]
- Nyffeler T, Cazzoli D, Hess CW, Muri RM. One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke. 2009;40:2791–2796. doi: 10.1161/STROKEAHA.109.552323. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, Fierro B. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology. 2001;57:1338–1340. doi: 10.1212/wnl.57.7.1338. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Horvath JC, Robertson EM. Enhancement of normal cognitive abilities through noninvasive brain stimulation. In: Chen R, Rothwell JC, editors. Cortical Connectivity. Springer Berlin Heidelberg: Berlin, Heidelberg; 2012. pp. 207–249. [Google Scholar]
- Payne BR, Rushmore RJ. Functional circuitry underlying natural and interventional cancellation of visual neglect. Exp. Brain Res. 2004;154:127–153. doi: 10.1007/s00221-003-1660-9. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rossman E. Effect of size and location of informational transforms upon short-term retention. J. Exp. Psychol. 1965;70:496–505. doi: 10.1037/h0022545. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J. Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Whittingstall K, Thielscher A. Effects of transcranial magnetic stimulation on visual evoked potentials in a visual suppression task. Neuro Image. 2011;54:1375–1384. doi: 10.1016/j.neuroimage.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repantis D, Schlattmann P, Laisney O, Heuser I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: a systematic review. Pharmacol. Res. 2010;62:187–206. doi: 10.1016/j.phrs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Romei V, Driver J, Schyns PG, Thut G. Rhythmic TMS over parietal cortex links distinct brain frequencies to global versus local visual processing. Curr. Biol. 2011;21:334–337. doi: 10.1016/j.cub.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2011;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schlee W, Leirer V, Kolassa I-T, Weisz N, Elbert T. Age-related changes in neural functional connectivity and its behavioral relevance. BMC Neurosci. 2012;13 doi: 10.1186/1471-2202-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Silvanto J, Rees G. Stochastic resonance effects reveal the neural mechanisms of transcranial magnetic stimulation. J. Neurosci. 2011;31:3143–3147. doi: 10.1523/JNEUROSCI.4863-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb. Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo K, Sugiyama K, Huabao L, Nishijima K, Kondo T, Izumi S-I. Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J. Rehabil. Med. 2006;38:65–67. doi: 10.1080/16501970500441807. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur. J. Neurosci. 2007;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen DC, Feinberg DA, Yacoub ES, Ugurbil K. Temporally-independent functional modes of spontaneous brain activity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Du B, Xu Q, Hu J, Wang M, Luo Y. Low-frequency transcranial magnetic stimulation for visual spatial neglect: a pilot study. J. Rehabil. Med. 2009;41:162–165. doi: 10.2340/16501977-0302. [DOI] [PubMed] [Google Scholar]
- Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction. Methods. 2008;44:329–337. doi: 10.1016/j.ymeth.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of inter hemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Neural mechanisms of visual orienting responses. Prog. Brain Res. 1996;112:1–15. doi: 10.1016/s0079-6123(08)63317-8. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Matsuo Y, Ikoma K. Low-frequency repetitive TMS plus anodal transcranial DCS prevents transient decline in bimanual movement induced by contralesional inhibitory rTMS after stroke. Neurorehabil. Neural Repair. 2012;26:988–998. doi: 10.1177/1545968311433295. [DOI] [PubMed] [Google Scholar]
- Terry S. Learning and Memory. 4th ed. Pearson; 2008. [Google Scholar]
- Thut G, Schyns PG, Gross J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol. 2011;2:170. doi: 10.3389/fpsyg.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anti correlation, and causality. Hum. Brain Mapp. 2008;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—a review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bolognini N. Behavioural facilitation following brain stimulation: implications for neurorehabilitation. Neuropsychol. Rehabil. 2011;21:618–649. doi: 10.1080/09602011.2011.574050. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Hester D, Assal G, Regli F. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46:184–189. doi: 10.1212/wnl.46.1.184. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum. Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddell AR. Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain Cogn. 2004;55:497–506. doi: 10.1016/j.bandc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Xiong GL, Doraiswamy PM. Does meditation enhance cognition and brain plasticity? Ann. N. Y. Acad. Sci. 2009;1172:63–69. doi: 10.1196/annals.1393.002. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jäncke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12 doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]