Abstract
OBJECTIVE
To investigate the geographic variation in diabetes prevalence and detection in China.
RESEARCH DESIGN AND METHODS
Self-report and biomedical data were collected from 98,058 adults aged ≥18 years (90.5% response) from 162 areas spanning mainland China. Diabetes status was assessed using American Diabetes Association criteria. Among those with diabetes, detection was defined by prior diagnosis. Choropleth maps were used to visually assess geographical variation in each outcome at the provincial level. The odds of each outcome were assessed using multilevel logistic regression, with adjustment for person- and area-level characteristics.
RESULTS
Geographic visualization at the provincial level indicated widespread variation in diabetes prevalence and detection across China. Regional prevalence adjusted for age, sex, and urban/rural socioeconomic circumstances (SECs) ranged from 8.3% (95% CI 7.2%, 9.7%) in the northeast to 12.7% (11.1%, 14.6%) in the north. A clear negative gradient in diabetes prevalence was observed from 13.1% (12.0%, 14.4%) in the urban high-SEC to 8.7% (7.8%, 9.6%) in rural low-SEC counties/districts. Adjusting for health literacy and other person-level characteristics only partially attenuated these geographic variations. Only one-third of participants living with diabetes had been previously diagnosed, but this also varied substantively by geography. Regional detection adjusted for age, sex, and urban/rural SEC, for example, spanned from 40.4% (34.9%, 46.3%) in the north to 15.6% (11.7%, 20.5%) in the southwest. Compared with detection of 40.8% (37.3%, 44.4%) in urban high-SEC counties, detection was poorest among rural low-SEC counties at just 20.5% (17.7%, 23.7%). Person-level characteristics did not fully account for these geographic variations in diabetes detection.
CONCLUSIONS
Strategies for addressing diabetes risk and improving detection require geographical targeting.
Introduction
Studies have reported that the prevalence of diabetes in China ranges from 9.7% (92.4 million adults) (1) to 11.6% (113.9 million adults) (2), depending on the diagnostic criteria used (3,4). Some researchers have cited China’s rapid urbanization as a key driver (5–7). However, there has been little investigation into the spatial patterning of the epidemic across China’s socioeconomically and topographically diverse landscape. Previous efforts have been limited to comparisons of small groups of provinces (8) or differences between regions crudely defined as north and south (9,10). These approaches are insufficient for identifying drivers of spatial heterogeneity in diabetes risk and detection, which could be explained, for example, by geographic variation in sociodemographics, health literacy, and use of health care. Decision makers require information about geographical variation in diabetes risk and detection efforts at the micro- (e.g., villages, towns) and macroscale (e.g., counties/districts, provinces, regions) level to cost-effectively target local resource allocation and other preventive health policies that address diabetes and related health inequities (11).
The purpose of this study was to investigate and identify potential drivers of geographic variation in diabetes prevalence and detection across China. The questions addressed in this study were as follows:
To what extent do the odds of having diabetes and the odds of being diagnosed with diabetes depend on where a person lives in China?
How much of the geographic variation in diabetes and the diagnosis of diabetes is attributable to differences in urbanization and socioeconomic circumstances (SECs)?
To what degree can geographic variation in diabetes and diagnosed diabetes be attributed to differences in health illiteracy and other person-level risk factors?
Research Design and Methods
Data and Sampling
The data analyzed in this study came from the China Noncommunicable Disease Surveillance 2010, a nationally representative health survey of 109,023 adults aged ≥18 years. It was conducted between August and December 2010 using the National Disease Surveillance Point (DSP) system, which comprises 162 urban districts and rural counties randomly selected from all 7 geographical regions (northeast, north, east, south, southwest, northwest, and central) and 31 provinces, municipalities, and autonomous regions in mainland China. The DSP system covers 7% of the population, and previous work has demonstrated its national representativeness (12). The survey was administered by the National Center for Chronic and Noncommunicable Disease Control and Prevention and Rui-Jin Hospital affiliated with the Shanghai Jiao-Tong University School of Medicine. The ethics committee of the China Center for Disease Control and Prevention approved the study, and written informed consent was obtained from all participants before data collection.
At each data collection site, participants were selected through a complex, multistage probability sampling design. Within each DSP, four subdistricts were selected, with probability proportional to size. Within each subdistrict, three neighborhood communities or administrative villages were selected proportional to size. Within each community or village, all households were listed, and 50 were randomly selected. Only one person from each household was selected at random using a Kish selection table (13). All participants were civilian, noninstitutionalized adults. Only those living at their residence for a period of ≥6 months were eligible to participate. When an individual was ineligible, refused, or unavailable, a replacement household was selected from the initial list minus those households previously selected. These replacements ensured a sufficient sample size and representativeness of the data across the country.
Data collection was performed by trained staff located within examination centers at health stations or community clinics near participants’ homes. A questionnaire including sociodemographics, medical history, lifestyle-related factors, and health service use was administered by trained interviewers. Blood samples were collected from all participants following an overnight fast of at least 10 h. Further information on the survey is available elsewhere (2). Details on data collected for the measures used in this study are provided herein. A total of 98,658 individuals participated in the survey, with an overall response rate of 90.5% and a replacement rate of 9.25%. Six hundred participants with missing values of interest were excluded, leaving a sample size of 98,058.
Outcome Variables
Participants were asked, “Have you ever been told by a doctor or other health-care professional that you had diabetes?” Positive responses identified diagnosed diabetes, although a large proportion of those living with diabetes were expected to be undiagnosed. To identify undiagnosed participants, those responding negatively to the questionnaire item were provided with a standard 75-g glucose solution, and their plasma glucose levels were measured at 0 and 2 h after administration. Each blood specimen was collected using a vacuum collection tube containing anticoagulant sodium fluoride and centrifuged onsite within 2 h of collection. Plasma glucose was measured within 24 h in a local hospital laboratory with a glucose meter using hexokinase or glucose oxidase. Details on the oral glucose tolerance test and glucose measurements are explained elsewhere (2). Diabetes was defined according to American Diabetes Association 2010 criteria: 1) a self-reported previous diagnosis by health professionals, 2) fasting plasma glucose ≥126 mg/dL, 3) 2-h glucose ≥200 mg/dL, or 4) HbA1c concentration of ≥6.5% (4). This information was used to create two outcome variables. The first outcome was whether a person was living with diabetes as identified by a self-reported physician diagnosis or detection through a blood specimen. The second was whether a person living with diabetes had been previously diagnosed by a physician. Because the definition of diabetes through blood sampling is not unequivocal, a second set of these variables was constructed based on alternative criteria (3) used in another study (1) for comparison.
Geographical Variables
The multilevel sampling strategy used in the survey enabled investigation of diabetes prevalence and detection across geographical areas of various scales. Only one participant in each household was surveyed. All participants were nested at the most local level within 1,933 villages, which were clustered within 644 towns, 161 districts/counties (the DSPs), and 31 provinces. Provinces were also nested within seven regions of China: 1) south, Guangdong, Guangxi, and Hainan; 2) north, Beijing, Tianjin, Hebei, Shanxi, and Inner Mongolia; 3) east, Shanghai, Shandong, Jiangsu, Anhui, Jiangxi, Zhejiang, and Fujian; 4) central, Hubei, Hunan, and Henan; 5) southwest, Chongqing, Sichuan, Guizhou, Yunnan, and Tibet; 6) northwest, Shaanxi, Gansu, Ningxia, Xinjiang, and Qinghai; and 7) northeast, Heilongjiang, Jilin, and Liaoning. In addition to classifications of scale, a number of characterizations of areas were also investigated. DSPs were differentiated according to whether they were urban (districts) or rural (counties). Census 2010 data were used to identify the mean number of years of education among residents of each DSP, expressed in tertiles. These variables were cross classified to afford relatively straightforward comparisons of diabetes prevalence and detection between and within urban and rural areas according to their SECs.
Person-Level Explanatory Variables
The survey contained detailed person-level information that was likely to be relevant for explaining geographic variation in diabetes prevalence and detection. Sociodemographic measures included age, sex, employment status, educational qualifications, and marital status. BMI was derived from objectively measured height and weight and dichotomized into normal versus overweight or obese (BMI >24 kg/m2) (14).
Responses to multiple questions in the survey concerned with knowledge and willingness to modify the consumption of salt were used to construct a composite indicator of health literacy and proactive attitude toward health-related behavioral change. Health literacy was derived from cross classifying responses to the questions, “Do you think eating too much salt will affect your health?” and “Which of the following diseases do you think could be caused by eating too much salt?” Participants who answered yes to the first question and correctly identified hypertension for the second were classified as health literate. Participants who believed that too much salt was bad for health but did not identify hypertension for the second question were classified as health semiliterate, and those responding negatively to the first question were automatically classified as health illiterate. This variable was bisected by responses to two further questions: “If you know eating too much salt could harm your health, are you willing to cut down on salt?” and “Are you trying to cut down on salt now?” Participants who were willing to cut down or were attempting to cut down their salt consumption at the time were classified as proactive or willing to change. All others were classified as unwilling to change their health behavior. The final categories in this composite variable were as follows: 1) illiterate, unwilling to change; 2) semiliterate, unwilling to change; 3) literate, unwilling to change; 4) illiterate, proactive or willing to change; 5) semiliterate, proactive or willing to change; and 6) literate, proactive or willing to change.
No comprehensive information on the use of primary health care was available for all participants and, as such, several variables were used to infer this potentially important determinant of diabetes awareness. The survey asked, “How often do you go for a routine health checkup (not including doctor consultations when ill)?” Responses to this question were categorized as never, >2 years, 1–2 years, 6–12 months, and 0–6 months. Minor psychiatric morbidity was taken into account because of the association between psychosocial stress and health (15), which can operate directly through behavioral pathways, such as dietary intake (16). Minor psychiatric morbidity was measured using the General Health Questionnaire (17). A binary variable was constructed using a cut point of 4 (out of 12) on the General Health Questionnaire, which has been shown elsewhere as identifying clinically significant minor psychiatric morbidity in the Chinese population (18,19). A count indicator of the number of unhealthy lifestyles each participant was engaged in was constructed according to published guidelines (20–22), including measures of 1) insufficient participation in physical activity, 2) consumption of too much red meat, 3) insufficient fruit and vegetable intake, 4) alcohol binge drinking, and 5) cigarette smoking.
Statistical Analysis
Choropleth maps were produced within a geographic information system (ArcGIS version 10 software) to visually examine geographical variation in the prevalence and detection of diabetes across China at the provincial level (n = 31). Data illustrated in the choropleth maps were median prevalence and detection estimates with 95% CIs calculated from unadjusted logistic regressions containing the province as a fixed effect. Strata were defined by quintiles of prevalence or detection, with lower prevalence and higher detection considered more favorable.
Cross tabulations and descriptive statistics were used to explore patterns among diabetes prevalence, detection, and each explanatory variable. Multilevel logistic regression (23) was used to investigate geographical and social correlates of diabetes risk and the odds of a person living with diabetes having already been diagnosed. For each outcome variable, an “empty” model was fitted initially to investigate geographic variation across multiple scales. Random intercepts were used to account for the clustering of participants within villages (level 2), towns (level 3), DSPs (level 4), and provinces (level 5). Variances at each level were converted into median odds ratios (MORs). An MOR >1 indicates the extent to which geographical variables play an important role in understanding variation in the odds of reporting a particular outcome (24).
In an attempt to explain geographic variation in each binary outcome, age- and sex-adjusted models were fitted. Geographic region and the cross classification between urban/rural and area-level SECs were then introduced into the models. The prevalence of diabetes at the DSP level was additionally fitted as a potential predictor of detection, given the plausibility that levels of knowledge and detection-related activities by local health services may be higher in areas where the prevalence of diabetes is greater. Person-level health and sociodemographic variables were then fitted sequentially to examine whether they could provide further explanatory value. All fixed-effects parameters were exponentiated to odds ratios with 95% CIs. Median values with 95% CIs were predicted from the multilevel models to report differences in the absolute risk of diabetes and diagnosis across explanatory variables. All statistical analyses were conducted using MLwIN version 2.30 software (25).
Results
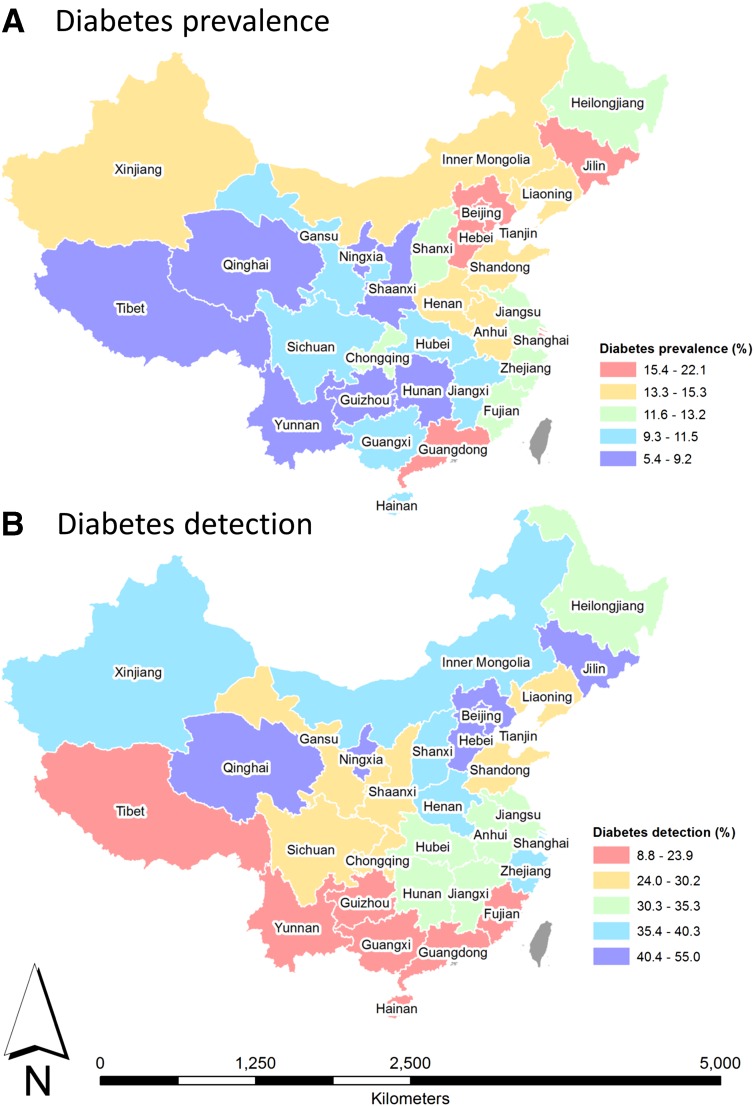
Figure 1 illustrates the geographical variation in prevalence and detection of diabetes at the provincial level, stratified into quintiles. In the lowest quintile for diabetes prevalence were Tibet, Qinghai, Yunnan, Guizhou, Ningxia, Shaanxi, and Hunan, whereas Jilin, Beijing, Hebei, and Guangdong were in the highest prevalence quintile. In contrast, provinces in the highest detection quintile were Qinghai, Ningxia, Hebei, Beijing, and Jilin, but those in the lowest were Tibet, Yunnan, Guizhou, Guangxi, Hainan, Guangdong, and Fujian. Visual comparisons between Fig. 1A and B suggest that aside from Beijing, Hebei, and Jilin, provinces with more successful detection of diabetes were not necessarily those with a greater disease burden. It is notable that Qinghai and Ningxia were in the lowest quintile for prevalence and highest quintile for detection. In contrast, Tibet, Yunnan, and Guizhou were in the lowest prevalence quintile and lowest detection quintile. Guangdong was in the highest prevalence quintile but lowest detection quintile. Although providing no explanatory information, these choropleth maps illustrate the marked geographic variation in diabetes prevalence and detection across China at the provincial level.
Figure 1.
Choropleth maps of diabetes prevalence (A) and detection (B) across Chinese provinces by DSPs. Quintiles were used to define map strata. Map coverage corresponds to DSPs only. Red indicates less favorable SECs.
Substantive regional variation in the prevalence and detection of diabetes is reported in Table 1, with the highest prevalence in the north and the lowest in the northeast and northwest. Supplementary Table 1 shows the small difference in estimates across regions made by the choice of diabetes definition. Urban high-SEC DSPs were most prevalent in the south, north, and central counties/districts. Participants in the southwest, northwest, and east reported the least health literacy and willingness or proactive attitude toward behavioral change, whereas those in the northwest and northeast were most likely to report never having a health check. Cross tabulations also showed that health literacy tended to be more evident in socioeconomically advantaged participants. Minor psychiatric morbidity was higher in the northeast and east than in other regions, whereas the north had the highest prevalence of overweight and obesity. Only 12.4% of participants in the northwest met all health behavioral guidelines, but 61.6% reported no educational qualifications. Both employment and the proportion living as a couple were lowest in the north.
Table 1.
Descriptive statistics by geographic region
| Geographic region |
|||||||
|---|---|---|---|---|---|---|---|
| South | North | Northeast | East | Central | Southwest | Northwest | |
| Participants (n) | 13,128 | 13,938 | 11,989 | 13,823 | 20,991 | 8,388 | 15,801 |
| Diabetes prevalence | |||||||
| n (%) | 1,461 (11.1) | 2,185 (15.7) | 1,181 (9.9) | 1,972 (14.3) | 2,852 (13.6) | 1,155 (13.8) | 1,431 (9.1) |
| 95% CI | 10.6, 11.7 | 15.1, 16.3 | 9.3, 10.4 | 13.7, 14.9 | 13.1, 14.1 | 13.0, 14.5 | 8.6, 9.5 |
| Diabetes detection† | |||||||
| n (%) | 495 (33.9) | 920 (42.1) | 409 (34.6) | 689 (34.9) | 905 (31.7) | 189 (16.4) | 296 (20.7) |
| 95% CI | 31.5, 36.3 | 40.1, 44.2 | 32.0, 37.4 | 32.9, 37.1 | 30.1, 33.5 | 14.3, 18.6 | 18.7, 22.9 |
| Urban DSPs, highest SEC tertile‡ | |||||||
| n (%) | 4,577 (34.9) | 4,231 (30.4) | 2,600 (21.7) | 3,689 (26.7) | 7,642 (36.4) | 2,000 (23.8) | 1,100 (7.0) |
| 95% CI | 34.1, 35.7 | 29.6, 31.1 | 21.0, 22.4 | 26.0, 27.4 | 35.8, 37.1 | 22.9, 24.8 | 6.6, 7.4 |
| Female sex | |||||||
| n (%) | 7,195 (54.8) | 7,669 (55.0) | 6,566 (54.8) | 7,416 (53.6) | 11,407 (54.3) | 4,478 (53.4) | 8,476 (53.6) |
| 95% CI | 54.0, 55.7 | 54.2, 55.8 | 53.9, 55.7 | 52.8, 54.5 | 53.7, 55.0 | 52.3, 54.5 | 52.9, 54.4 |
| Aged ≥60 years | |||||||
| n (%) | 2,591 (19.7) | 2,857 (20.5) | 2,390 (19.9) | 2,622 (19.0) | 5,023 (23.9) | 1,711 (20.4) | 2,641 (16.7) |
| 95% CI | 19.1, 20.4 | 19.8, 21.2 | 19.2, 20.7 | 18.3, 19.6 | 23.4, 24.5 | 19.5, 21.3 | 16.1, 17.3 |
| Health literate and proactive or willing to change | |||||||
| n (%) | 3,545 (27.0) | 5,345 (38.3) | 3,798 (31.7) | 4,694 (34.0) | 7,387 (35.2) | 1,818 (21.7) | 2,847 (18.0) |
| 95% CI | 26.3, 27.8 | 37.5, 39.2 | 30.9, 32.5 | 33.2, 34.8 | 34.5, 35.8 | 20.8, 22.6 | 17.4, 18.6 |
| Never had a health check | |||||||
| n (%) | 8,966 (68.3) | 9,206 (66.0) | 8,919 (74.4) | 10,227 (74.0) | 11,229 (53.5) | 5,724 (68.2) | 12,218 (77.3) |
| 95% CI | 67.5, 69.1 | 65.3, 66.8 | 73.6, 75.2 | 73.2, 74.7 | 52.8, 54.2 | 67.2, 69.2 | 76.7, 78.0 |
| Minor psychiatric morbidity | |||||||
| n (%) | 1,572 (11.9) | 1,400 (10.0) | 1,884 (15.7) | 2,145 (15.5) | 1,828 (8.7) | 846 (10.1) | 1,710 (10.8) |
| 95% CI | 11.4, 12.5 | 9.6, 10.6 | 15.1, 16.4 | 14.9, 16.1 | 8.3, 9.1 | 9.5, 10.7 | 10.3, 11.3 |
| Overweight or obese (BMI ≥24 kg/m2) | |||||||
| n (%) | 6,001 (45.7) | 8,149 (58.5) | 5,332 (44.5) | 6,823 (49.4) | 9,720 (46.3) | 2,676 (31.9) | 5,510 (34.9) |
| 95% CI | 44.9, 46.6 | 57.6, 59.3 | 43.6, 45.5 | 48.5, 50.2 | 45.6, 47.0 | 30.9, 32.9 | 34.1, 35.6 |
| Met all health guidelines | |||||||
| n (%) | 2,914 (22.2) | 2,551 (18.3) | 2,469 (20.6) | 2,775 (20.1) | 4,504 (21.5) | 1,338 (16.0) | 1,952 (12.4) |
| 95% CI | 21.5, 22.9 | 17.7, 19.0 | 19.9, 21.3 | 19.4, 20.8 | 20.9, 22.0 | 15.2, 16.8 | 11.8, 12.9 |
| No education | |||||||
| n (%) | 5,168 (39.4) | 4,902 (35.2) | 5,593 (46.7) | 4,944 (35.8) | 9,007 (42.9) | 3,491 (41.6) | 9,734 (61.6) |
| 95% CI | 38.5, 40.2 | 34.4, 36.0 | 45.8, 47.5 | 35.0, 36.6 | 42.2, 43.6 | 40.6, 42.7 | 60.8, 62.4 |
| Employed | |||||||
| n (%) | 8,811 (67.1) | 8,591 (61.6) | 8,947 (74.6) | 9,298 (67.3) | 13,384 (63.8) | 5,375 (64.1) | 12,367 (78.3) |
| 95% CI | 66.3, 67.9 | 60.8, 62.4 | 73.8, 75.4 | 66.5, 68.0 | 63.1, 64.4 | 63.0, 65.1 | 77.6, 78.9 |
| Living as a couple | |||||||
| n (%) | 1,483 (11.3) | 1,084 (7.78) | 1,322 (11.0) | 1,579 (11.4) | 2,028 (9.7) | 928 (11.1) | 1,354 (8.6) |
| 95% CI | 10.8, 11.8 | 7.3, 8.2 | 10.5, 11.6 | 10.9, 12.0 | 9.3, 10.1 | 10.4, 11.8 | 8.1, 9.0 |
†Numerator = number of participants diagnosed with diabetes within a particular region; denominator = number of participants living with diabetes within the same region.
‡Numerator = number of participants living in an urban DSP classified as high SEC within a particular region; denominator = total population within the same region.
MORs from empty multilevel logistic regressions showed a twofold geographic variation in diabetes prevalence and detection across China. This variation partitioned across micro- and macroscale geographies (Table 2). Diabetes prevalence varied more at the village and DSP level, although variation at the provincial and town level were also not trivial. Variation in the detection of diabetes was greatest at the DSP, provincial, and village levels but very small between towns. In additional analyses, MORs ≥2 were calculated at the DSP level for binary expressions of each candidate risk factor, revealing important subregional geographical variation that could potentially explain the spatial patterning of diabetes prevalence and detection (Supplementary Table 2). Geographic variation was especially notable for the odds of a participant having no educational qualifications (MOR 2.64), low health literacy (MOR 2.69), and at least one health check within 6 months before the survey (MOR 2.79).
Table 2.
Random effects from empty multilevel logistic regression models
| Geographic scale | Province | DSP | Town | Village |
|---|---|---|---|---|
| Diabetes prevalence | ||||
| n | 31 | 161 | 644 | 1,933 |
| Variance (SE) | 0.056 (0.023) | 0.121 (0.020) | 0.065 (0.012) | 0.131 (0.012) |
| MOR (95% CI) | 1.25 (1.10, 1.35) | 1.39 (1.31, 1.46) | 1.28 (1.21, 1.33) | 1.41 (1.37, 1.45) |
| Diabetes detection | ||||
| n | 31 | 161 | 644 | 1,887 |
| Variance (SE) | 0.144 (0.053) | 0.197 (0.036) | 0.044 (0.024) | 0.100 (0.031) |
| MOR (95% CI) | 1.44 (1.21, 1.61) | 1.53 (1.40, 1.64) | 1.00 (1.00, 1.00) | 1.35 (1.21, 1.47) |
Adjusting for age, sex, region, and urban SEC cross classification explained 94% of the provincial variation in diabetes prevalence and between 22.3% and 37.4% of the variation at other geographical levels (Table 3, model 1). Compared with the south, diabetes was more common in the north, east, and southwest. A clear negative gradient in diabetes prevalence was observed from urban high SEC to rural low SEC. Adjusting for health literacy and other person-level characteristics attenuated this gradient, but regional variation remained substantial, as did variation at the DSP, town, and village levels (model 2). Increased health literacy was positively associated with diabetes prevalence regardless of willingness or proactive attitude toward behavioral change. The odds of having diabetes was also more common among those who had a more recent health check or minor psychiatric morbidity, were overweight or obese, had three or more unhealthy lifestyle factors, and were unemployed.
Table 3.
Geographical and social correlates of the odds of having diabetes: fixed- and random-effects parameters estimated from multilevel logistic regression
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Median % (95% CI) | Odds ratio (95% CI) | Median % (95% CI) | Odds ratio (95% CI) | |
| Fixed effects | ||||
| Region (reference: south) | 9.1 (7.8, 10.7) | 1 | 8.9 (7.8, 10.2) | 1 |
| North | 12.7 (11.1, 14.6)* | 1.45 (1.15, 1.84)* | 11.2 (9.9, 12.8)* | 1.29 (1.05, 1.59)* |
| Northeast | 8.3 (7.2, 9.7) | 0.90 (0.71, 1.15) | 7.9 (6.8, 9.1) | 0.88 (0.70, 1.09) |
| East | 12.0 (10.4, 14.0)* | 1.36 (1.07, 1.72)* | 11.3 (9.9, 12.7)* | 1.29 (1.05, 1.59)* |
| Central | 10.9 (9.6, 12.2) | 1.21 (0.97, 1.51) | 10.1 (9.0, 11.3) | 1.14 (0.94, 1.38) |
| Southwest | 11.6 (9.7, 13.9)* | 1.30 (1.00, 1.70)* | 12.4 (10.5, 14.5)* | 1.44 (1.14, 1.83)* |
| Northwest | 9.0 (7.7, 10.4) | 0.98 (0.77, 1.25) | 8.9 (7.8, 10.2) | 0.99 (0.80, 1.23) |
| Urbanization × SEC (reference: urban, high SEC) | 13.1 (12.0, 14.4) | 1 | 10.6 (9.7, 11.7) | 1 |
| Urban, moderate SEC | 11.7 (10.5, 13.1) | 0.88 (0.76, 1.03) | 11.0 (9.9, 12.1) | 1.03 (0.88, 1.20) |
| Urban, low SEC | 10.5 (9.2, 11.9)* | 0.78 (0.65, 0.92)* | 10.4 (9.2, 11.7) | 0.97 (0.82, 1.16) |
| Rural, high SEC | 9.7 (8.3, 11.1)* | 0.71 (0.61, 0.82)* | 9.0 (7.8, 10.3)* | 0.82 (0.71, 0.96)* |
| Rural, moderate SEC | 9.3 (8.4, 10.2)* | 0.68 (0.59, 0.78)* | 9.3 (8.5, 10.3)* | 0.86 (0.74, 1.00) |
| Rural, low SEC | 8.7 (7.8, 9.6)* | 0.63 (0.54, 0.74)* | 9.4 (8.5, 10.4) | 0.87 (0.75, 1.02) |
| Sex × age-group (reference: male × 18–29 years) | 4.6 (4.1, 5.3) | 1 | 4.9 (4.3, 5.5) | 1 |
| Male × 30–39 years | 7.7 (7.1, 8.5)* | 1.71 (1.49, 1.96)* | 7.4 (6.7, 8.1)* | 1.54 (1.34, 1.77)* |
| Male × 40–49 years | 12.9 (12.0, 13.8)* | 3.03 (2.68, 3.43)* | 12.0 (11.2, 12.9)* | 2.65 (2.34, 3.01)* |
| Male × 50–59 years | 16.7 (15.7, 17.9)* | 4.12 (3.64, 4.66)* | 15.9 (14.9, 16.9)* | 3.67 (3.24, 4.16)* |
| Male × 60+ years | 20.1 (18.9, 21.3)* | 5.13 (4.55, 5.80)* | 18.6 (17.4, 19.7)* | 4.44 (3.90, 5.05)* |
| Female × 18–29 years | 3.5 (3.0, 4.0)* | 0.74 (0.63, 0.87)* | 3.9 (3.4, 4.5)* | 0.80 (0.67, 0.94)* |
| Female × 30–39 years | 4.6 (4.1, 5.0) | 0.98 (0.85, 1.13) | 4.7 (4.2, 5.2) | 0.96 (0.82, 1.11) |
| Female × 40–49 years | 8.1 (7.6, 8.8)* | 1.82 (1.60, 2.06)* | 7.6 (7.0, 8.2)* | 1.60 (1.41, 1.83)* |
| Female × 50–59 years | 15.5 (14.6, 16.5)* | 3.77 (3.34, 4.26)* | 13.9 (13.0, 14.8)* | 3.13 (2.75, 3.56)* |
| Female × 60+ years | 23.0 (21.7, 24.2)* | 6.13 (5.44, 6.91)* | 20.6 (19.3, 21.8)* | 5.04 (4.42, 5.74)* |
| Health literacy (reference: illiterate, unwilling to change) | — | — | 9.1 (8.5, 9.7) | 1 |
| Semiliterate, unwilling to change | — | — | 8.6 (7.9, 9.3) | 0.94 (0.86, 1.03) |
| Literate, unwilling to change | — | — | 10.4 (9.7, 11.1) | 1.16 (1.07, 1.25)* |
| Illiterate, proactive or willing to change | — | — | 9.3 (8.7, 10.0) | 1.03 (0.96, 1.10) |
| Semiliterate, proactive or willing to change | — | — | 9.8 (9.1, 10.5) | 1.09 (1.00, 1.18)* |
| Literate, proactive or willing to change | — | — | 11.2 (10.6, 11.8) | 1.26 (1.18, 1.35)* |
| Time since health check (reference: never) | — | — | 9.6 (9.1, 10.1) | 1 |
| >2 years | — | — | 9.4 (8.6, 10.2) | 0.97 (0.89, 1.05) |
| 1–2 years | — | — | 10.4 (9.6, 11.2) | 1.09 (1.01, 1.17)* |
| 6–12 months | — | — | 11.3 (10.4, 12.3) | 1.20 (1.10, 1.30)* |
| 0–6 months | — | — | 11.2 (10.4, 12.1) | 1.19 (1.10, 1.28)* |
| Minor psychiatric morbidity (reference: no) | — | — | 9.8 (9.4, 10.3) | 1 |
| Yes | — | — | 10.8 (10.1, 11.6) | 1.11 (1.05, 1.18)* |
| Weight status (reference: normal) | — | — | 7.4 (7.0, 7.8) | 1 |
| Overweight or obese | — | — | 14.0 (13.3, 14.7) | 2.03 (1.95, 2.12)* |
| Number of unhealthy lifestyles (reference: 0) | — | — | 9.5 (8.9, 10.1) | 1 |
| 1 | — | — | 9.8 (9.3, 10.3) | 1.04 (0.98, 1.10) |
| 2 | — | — | 10.0 (9.4, 10.6) | 1.06 (0.99, 1.13) |
| ≥3 | — | — | 10.8 (10.1, 11.6) | 1.16 (1.07, 1.26)* |
| Highest educational qualification (reference: none) | — | — | 10.1 (9.5, 10.7) | 1 |
| Primary | — | — | 10.1 (9.5, 10.7) | 1.00 (0.95, 1.05) |
| Secondary | — | — | 9.5 (8.8, 10.1) | 0.93 (0.87, 0.99)* |
| University | — | — | 9.3 (8.5, 10.2) | 0.92 (0.83, 1.01) |
| Economic status (reference: employed) | — | — | 9.4 (8.9, 9.9) | 1 |
| Housewife/husband | — | — | 10.9 (10.0, 11.7) | 1.17 (1.08, 1.28)* |
| Retired | — | — | 12.2 (11.3, 13.2) | 1.34 (1.24, 1.45)* |
| Unemployed/student/other | — | — | 10.8 (10.1, 11.6) | 1.17 (1.10, 1.24)* |
| Couple status (reference: living with another person) | — | — | 10.3 (9.6, 11.0) | 1 |
| Living alone | — | — | 9.9 (9.4, 10.4) | 0.95 (0.89, 1.01) |
| Random effects | ||||
| Variance among provinces (SE) | 0.003 (0.007) | <0.001 (<0.001) | ||
| MOR (provinces) | 1.05 | ∼1 | ||
| PCV (provinces) (%) | 94.6 | ∼100 | ||
| Variance among DSPs (SE) | 0.094 (0.016) | 0.088 (0.014) | ||
| MOR (DSPs) | 1.34 | 1.33 | ||
| PCV (DSPs) (%) | 22.3 | 27.3 | ||
| Variance among towns (SE) | 0.045 (0.01) | 0.039 (0.009) | ||
| MOR (towns) | 1.22 | 1.21 | ||
| PCV (towns) (%) | 30.8 | 40.0 | ||
| Variance among villages (SE) | 0.082 (0.011) | 0.078 (0.011) | ||
| MOR (villages) | 1.31 | 1.31 | ||
| PCV (villages) (%) | 37.4 | 40.5 | ||
PCV, proportional change in variance in model compared with the empty model.
*P < 0.05.
Contrasting findings were reported for those living with diabetes and their odds of being diagnosed (Table 4). Detection was lower in the southwest and northwest than in the south but were higher in the north (model 3). The odds of being diagnosed were substantively lower in rural low-SEC DSPs but were not associated with the local prevalence of diabetes. Adjustment for these geographical characteristics explained 79.2% of the provincial and 39.6% of the DSP-level variation in diagnoses. Adjusting for person-level characteristics (model 4) had little impact on the regional variation but did attenuate the SEC gradient in urban DSPs. The odds of being diagnosed in a rural moderate- or low-SEC DSP, however, remained very low. Diagnosis was positively associated with health literacy combined with a proactive attitude or willingness to change; among those who were unwilling to change, health literacy was not associated with diagnosis. Participants with a recent health check, experiencing minor psychological morbidity, or not employed were more likely to have been diagnosed. Lifestyles, educational qualifications, and weight status were not associated with a diagnosis of diabetes. These person-level characteristics accounted for a further 8% of the provincial variation in diagnoses. Supplementary Tables 3 and 4 show that these associations were largely similar, regardless of the definition of diabetes used.
Table 4.
Geographical and social correlates of the odds of being diagnosed with diabetes (i.e., detection): fixed- and random-effects parameters estimated from multilevel logistic regression
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Median % (95% CI) | Odds ratio (95% CI) | Median % (95% CI) | Odds ratio (95% CI) | |
| Fixed effects | ||||
| Region (reference: south) | 30.8 (25.4, 36.9) | 1 | 31.6 (26.1, 37.8) | 1 |
| North | 40.4 (34.9, 46.3)* | 1.53 (1.04, 2.24)* | 40.1 (34.6, 45.5)* | 1.44 (1.02, 2.05)* |
| Northeast | 34.9 (29.6, 41.2) | 1.21 (0.82, 1.78) | 33.8 (28.0, 39.8) | 1.11 (0.78, 1.58) |
| East | 34.5 (29.2, 40.3) | 1.18 (0.81, 1.72) | 34.3 (29.4, 40.3) | 1.14 (0.81, 1.60) |
| Central | 30.0 (25.9, 34.5) | 0.97 (0.68, 1.38) | 28.6 (24.8, 32.8) | 0.86 (0.62, 1.19) |
| Southwest | 15.6 (11.7, 20.5)* | 0.42 (0.27, 0.64)* | 16.0 (12.3, 20.5)* | 0.41 (0.28, 0.62)* |
| Northwest | 21.6 (17.3, 26.8)* | 0.62 (0.42, 0.92)* | 21.5 (17.4, 26.1)* | 0.59 (0.41, 0.85)* |
| Urbanization × SEC (reference: urban, high SEC) | 40.8 (37.3, 44.4) | 1 | 35.5 (32.4, 38.8) | 1 |
| Urban, moderate SEC | 32.4 (28.6, 36.5)* | 0.70 (0.56, 0.87)* | 32.7 (28.9, 36.6) | 0.88 (0.71, 1.10) |
| Urban, low SEC | 28.3 (24.1, 32.9)* | 0.58 (0.44, 0.75)* | 29.8 (25.5, 34.9) | 0.77 (0.59, 1.01) |
| Rural, high SEC | 30.6 (25.7, 35.9)* | 0.64 (0.51, 0.81)* | 30.1 (25.4, 35.4) | 0.78 (0.61, 1.00) |
| Rural, moderate SEC | 24.5 (21.5, 27.8)* | 0.47 (0.38, 0.58)* | 26.2 (23.1, 29.6)* | 0.64 (0.52, 0.80)* |
| Rural, low SEC | 20.5 (17.7, 23.7)* | 0.38 (0.30, 0.47)* | 22.9 (20.0, 26.3)* | 0.54 (0.43, 0.68)* |
| County diabetes prevalence (reference: low) | 32.6 (29.6, 35.5) | 1 | 32.3 (29.4, 35.1) | 1 |
| Moderate | 30.1 (27.2, 33.3) | 0.89 (0.74, 1.07) | 29.4 (26.6, 32.3) | 0.88 (0.73, 1.06) |
| High | 28.2 (24.9, 32.0) | 0.82 (0.65, 1.02) | 27.8 (24.5, 31.6) | 0.81 (0.65, 1.01) |
| Sex (reference: male) | 29.9 (27.7, 32.4) | 1 | 30.4 (28.2, 32.7) | 1 |
| Female | 30.5 (28.3, 32.9) | 1.03 (0.95, 1.12) | 29.3 (27.1, 31.4) | 0.95 (0.86, 1.04) |
| Age-group (reference: 18–29 years) | 16.3 (13.5, 19.9) | 1 | 16.7 (13.7, 20.4) | 1 |
| 30–39 years | 21.7 (18.9, 25.1)* | 1.43 (1.09, 1.87)* | 22.7 (19.7, 26.0)* | 1.47 (1.12, 1.93)* |
| 40–49 years | 26.6 (24.3, 29.1)* | 1.86 (1.46, 2.37)* | 26.8 (24.5, 29.4)* | 1.83 (1.43, 2.34)* |
| 50–59 years | 32.6 (30.3, 35.2)* | 2.49 (1.97, 3.16)* | 32.4 (30.2, 34.8)* | 2.39 (1.87, 3.06)* |
| 60+ years | 35.1 (32.7, 37.7)* | 2.78 (2.20, 3.51)* | 33.5 (31.1, 35.9)* | 2.51 (1.95, 3.22)* |
| Health literacy (reference: illiterate, unwilling to change) | — | — | 25.8 (23.4, 28.4) | 1 |
| Semiliterate, unwilling to change | — | — | 27.2 (23.4, 31.1) | 1.07 (0.88, 1.32) |
| Literate, unwilling to change | — | — | 28.2 (25.3, 31.2) | 1.13 (0.96, 1.34) |
| Illiterate, proactive or willing to change | — | — | 26.3 (23.7, 29.0) | 1.03 (0.89, 1.20) |
| Semiliterate, proactive or willing to change | — | — | 31.2 (28.2, 34.6)* | 1.31 (1.11, 1.54)* |
| Literate, proactive or willing to change | — | — | 34.4 (32.1, 36.9)* | 1.51 (1.32, 1.73)* |
| Time since health check (reference: never) | — | — | 26.7 (24.8, 28.6) | 1 |
| >2 years | — | — | 29.4 (26.1, 33.3) | 1.15 (0.97, 1.36) |
| 1–2 years | — | — | 34.6 (31.3, 37.9)* | 1.46 (1.26, 1.68)* |
| 6–12 months | — | — | 37.7 (34.0, 41.7)* | 1.67 (1.43, 1.95)* |
| 0–6 months | — | — | 40.8 (37.2, 44.3)* | 1.89 (1.64, 2.19)* |
| Minor psychiatric morbidity (reference: no) | — | — | 30.6 (28.5, 32.9) | 1 |
| Yes | — | — | 29.3 (27.4, 31.4)* | 1.58 (1.40, 1.78)* |
| Weight status (reference: normal) | — | — | 28.6 (26.7, 30.5) | 1 |
| Overweight or obese | — | — | 38.7 (35.5, 42.0) | 0.94 (0.86, 1.03) |
| Number of unhealthy lifestyles (reference: 0) | — | — | 30.5 (27.9, 33.2) | 1 |
| 1 | — | — | 30.2 (28.1, 32.5) | 0.99 (0.88, 1.11) |
| 2 | — | — | 30.1 (27.9, 32.7) | 0.98 (0.86, 1.12) |
| ≥3 | — | — | 27.1 (24.5, 30.2) | 0.85 (0.72, 1.00) |
| Highest educational qualification (reference: none) | — | — | 30.1 (28.0, 32.4) | 1 |
| Primary | — | — | 29.4 (27.1, 31.8) | 0.96 (0.86, 1.08) |
| Secondary | — | — | 30.3 (27.2, 33.1) | 1.00 (0.87, 1.15) |
| University | — | — | 28.1 (24.5, 32.1) | 0.91 (0.75, 1.10) |
| Economic status (reference: employed) | — | — | 28.5 (26.5, 30.5) | 1 |
| Housewife/husband | — | — | 29.7 (26.1, 33.5) | 1.06 (0.89, 1.26) |
| Retired | — | — | 33.0 (30.0, 36.3)* | 1.24 (1.07, 1.43)* |
| Unemployed/student/other | — | — | 30.9 (28.4, 33.8)* | 1.13 (1.00, 1.27)* |
| Couple status (reference: living with another person) | — | — | 31.0 (28.2, 34.0) | 1 |
| Living alone | — | — | 29.6 (27.7, 31.5) | 0.94 (0.83, 1.06) |
| Random effects | ||||
| Variance among provinces (SE) | 0.030 (0.019) | 0.018 (0.016) | ||
| MOR (provinces) | 1.18 | 1.14 | ||
| PCV (provinces) (%) | 79.2 | 87.5 | ||
| Variance among counties/districts (SE) | 0.119 (0.026) | 0.122 (0.026) | ||
| MOR (counties/districts) | 1.39 | 1.4 | ||
| PCV (counties/districts) (%) | 39.6 | 38.1 | ||
| Variance among towns (SE) | — | — | ||
| MOR (towns) | — | — | ||
| PCV (towns) (%) | — | — | ||
| Variance among villages (SE) | 0.144 (0.029) | 0.144 (0.029) | ||
| MOR (villages) | 1.44 | 1.44 | ||
| PCV (villages) (%) | 0.0 | 0.0 | ||
Town-level variance not significant in empty model and omitted thereafter. PCV, proportional change in variance in model compared with the empty model.
*P < 0.05.
Conclusions
Consistent with many other countries around the world, the prevalence of diabetes in China is high (1,2), and the potential consequences of ineffective prevention and management are daunting (26). With a country of China’s enormously varied geography, demographics, and SECs, data on how the epidemic is spatially distributed are required to allocate resources equitably and efficiently. Previous spatially orientated work in this regard has been limited by comparisons across singular and coarse geographic scales, for example, higher prevalence in northern (6.41%) than southern regions (4.83%) (9,10). The current study is the first in our knowledge to use nationally representative surveillance data to demonstrate a more nuanced, multilevel geography of the diabetes epidemic in China. Within the northern region, for example, diabetes prevalence ranged from 9.1% in the northwest and 9.9% in the northeast to 15.7% in the north. Systematic variation in the prevalence of diabetes was observed across multiple geographical levels below the regional level, including provinces, counties/districts (DSPs), towns, and villages. This highlights a key strength of the study: the multilevel analysis of a large source of nationally representative data using objective and biomedical outcome measures as well as a linkage of study participant information to place-based data from the 2010 census. The results imply that strategies for allocating health-care resources exclusively by large-scale regional classifications risk being inefficient. Investing in a multilevel geographical perspective provides the necessary information for eliminating spatial inequities in diabetes.
Some studies have reported a lower prevalence of diabetes in rural compared with urban areas (2,9,10). Few, however, investigated heterogeneity within urban and rural areas according to SECs. One study approximated economic development using data on the provincial gross domestic product per capita, reporting higher diabetes prevalence in developed urban (12%) and rural (12%) areas and in underdeveloped urban areas (10.4%) but lower prevalence in underdeveloped rural areas (5.8%) (1). By contrast, diabetes prevalence in the current study was slightly lower in rural than in urban areas, regardless of the mean years of education. An individual’s level of education was not associated with the odds of having diabetes in this study. Although it is unclear what mechanisms link provincial gross domestic product per capita with diabetes, previous research has argued strongly for a causal relationship between education and health outcomes (27). The lack of a pattern in this study does not refute that relationship but does suggest that the socioeconomic patterning of diabetes, considered among many as a so-called “disease of affluence” in developing countries, is as much in transition as China’s urban environment (7,11). Spatiotemporal modeling of finer-scale time-series surveillance data are needed to monitor the shifting epidemiology of diabetes incidence, and health more generally, toward the socioeconomic gradient commonly seen in North American and western European countries (28,29).
Monitoring the prevalence of diabetes is, however, of little value if people living with the condition are not being diagnosed. People living with undiagnosed diabetes will not receive the medical attention that can reduce the risk of coronary heart disease, stroke, renal failure, lower limb amputations, and blindness (30). Unfortunately, like in many countries around the world, a nontrivial proportion of people living with diabetes in China has not been diagnosed (31,32). Previous work has suggested a north-south gradient, with detection of 29.0% and 18.5%, respectively (9). Results from the current study suggest greater spatial heterogeneity in diabetes detection. Although only one-third of participants living with diabetes had been diagnosed, detection varied from 42.1% in the north to only 16.4% in the southwest. Detection was poorest among rural communities with moderate to low SECs. Crucially, detection was not proportional to the underlying prevalence; interventions to increase the diagnosis of diabetes should not be exclusive to areas of high prevalence. Much of the difference between urban/rural and area-level SECs appeared to be explained by adjustment for participant interactions with health services and health literacy combined with proactive attitudes. Health-literate participants with no motivation for change were less likely to be diagnosed. Although some bidirectionality of the association is expected, this cannot be explored fully with cross-sectional data, but it is unlikely to be evident in those with undiagnosed diabetes, as biomedical assessments were provided to participants within the 3-month period after the completion of face-to-face interviews.
It is possible that some residual confounding is also present, with detection likely to be influenced by unmeasured area-level characteristics, such as geographic access to health services. Such phenomena may correlate with the area-level measure of SECs used, but to what extent remains uncertain. As such, the development and exploration of these types of area-level measures across DSPs will be an important next step for enhancing understanding of geographic variation in diabetes prevalence and detection in China. The current results suggest that integrated responses incorporating both population-level and individual-level approaches (33) toward increasing public knowledge of diabetes, enhancing access to routine health checks, and encouraging proactive self-risk assessment through noninvasive screening tools (34,35) should be a key priority for policymakers tasked with reducing geographic inequality in diabetes risk and detection in China.
Supplementary Material
Article Information
Acknowledgments. The authors thank all individuals who have contributed to the data collection in all DSPs.
Funding. This study was supported by the China National Science and Technology Pillar Program 2013 (2013BAI04B02) from the Ministry of Science and Technology, Science and Technology Outstanding Program for Scholars Coming Back from Overseas from the Ministry of Human Resources and Social Security, the Key Laboratory for Endocrine and Metabolic Diseases of the Ministry of Health (1994DP131044), the National Key New Drug Creation and Manufacturing Program of the Ministry of Science and Technology (2012ZX09303006-001), and the National Clinical Research Center for Metabolic Diseases of the Ministry of Health (2013BAI09B13). T.A.-B., X.F., and A.P. were funded through the Australia-China Science and Research Fund (ACSRF17120). T.A.-B.’s time was also supported by the National Heart Foundation of Australia.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.Z., T.A.-B., and Y.B. contributed equally to the study design, data analysis and interpretation, and drafting of the manuscript. X.F. contributed to the study design, data interpretation, and writing of the manuscript. Y.J., Y.L., Lim.W., and Y.X. contributed to the data analysis and interpretation and writing of the manuscript. A.P. contributed to the data interpretation and editing of the manuscript. Lin.W., W.Z., and G.N. obtained the raw data and contributed to the study design, data analysis and interpretation, and writing of the manuscript. M.Z. and G.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-1100/-/DC1.
References
- 1.Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group . Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 3.Department of Noncommunicable Disease Surveillance Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999 [Google Scholar]
- 4.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2013;381:1987–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Oldenburg B, Chamberlain C, et al. Diabetes prevalence and determinants in adults in China mainland from 2000 to 2010: a systematic review. Diabetes Res Clin Pract 2012;98:226–235 [DOI] [PubMed] [Google Scholar]
- 7.Gong P, Liang S, Carlton EJ, et al. Urbanisation and health in China. Lancet 2012;379:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, Ming J, Xing Y, et al. Regional differences in diabetes prevalence and awareness between coastal and interior provinces in China: a population-based cross-sectional study. BMC Public Health 2013;13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu D, Fu P, Xie J, et al. MS for the InterASIA Collaborative Group . Increasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA study. Diabetes Res Clin Pract 2008;81:250–257 [DOI] [PubMed] [Google Scholar]
- 10.Hu D, Sun L, Fu P, et al. Prevalence and risk factors for type 2 diabetes mellitus in the Chinese adult population: the InterASIA Study. Diabetes Res Clin Pract 2009;84:288–295 [DOI] [PubMed] [Google Scholar]
- 11.Tang S, Meng Q, Chen L, Bekedam H, Evans T, Whitehead M. Tackling the challenges to health equity in China. Lancet 2008;372:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou MG, Jiang Y, Huang ZJ, Wu F. Adjustment and representativeness evaluation of national disease surveillance points system. Ji Bing Jian Ce 2010;25:239–244 [Google Scholar]
- 13.Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc 1949;44:380–387 [Google Scholar]
- 14.Bei-Fan Z, Cooperative Meta-Analysis Group of Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr 2002;11(Suppl. 8):S685–S693 [PubMed] [Google Scholar]
- 15.Marmot MG. Status syndrome: a challenge to medicine. JAMA 2006;295:1304–1307 [DOI] [PubMed] [Google Scholar]
- 16.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet 2007;370:859–877 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg DP, Gater R, Sartorius N, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 1997;27:191–197 [DOI] [PubMed] [Google Scholar]
- 18.Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet 2009;373:2041–2053 [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Huang L, Wu Z. Study on the appropriateness of the Chinese version of the General Health Questionnaire as a screening instrument for psychological disorders in mainland China. Chin J Epid 2003;24:769–773 [Google Scholar]
- 20.World Health Organization Global Recommendations on Physical Activity for Health. Geneva, World Health Organization, 2010 [PubMed] [Google Scholar]
- 21.World Cancer Research Fund International Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC, American Institute for Cancer Research, 2009 [Google Scholar]
- 22.World Health Organization WHO STEPS Surveillance Manual: The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance. Geneva, WHO, 2005 [Google Scholar]
- 23.Leyland AH, Goldstein H. Multilevel Modelling of Health Statistics. Chichester, U.K., Wiley, 2001 [Google Scholar]
- 24.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006;60:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasbash J, Browne W, Goldstein H, et al. A User’s Guide to MLwiN. London, Institute of Education, 2000, p. 286 [Google Scholar]
- 26.Zhao W, Zhai Y, Hu J, et al. Economic burden of obesity-related chronic diseases in Mainland China. Obes Rev 2008;9(Suppl. 1):62–67 [DOI] [PubMed] [Google Scholar]
- 27.Richards M, Sacker A. Is education causal? Yes. Int J Epidemiol 2011;40:516–518 [DOI] [PubMed] [Google Scholar]
- 28.Williams ED, Magliano DJ, Zimmet PZ, et al. Area-level socioeconomic status and incidence of abnormal glucose metabolism: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care 2012;35:1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Commission on Social Determinants of Health Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of health: Final Report of the Commission on Social Determinants of Health. Geneva, World Health Organization, 2008 [Google Scholar]
- 30.Colagiuri S. Optimal management of type 2 diabetes: the evidence. Diabetes Obes Metab 2012;14(Suppl. 1):3–8 [DOI] [PubMed] [Google Scholar]
- 31.Li MZ, Su L, Liang BY, et al. Trends in prevalence, awareness, treatment, and control of diabetes mellitus in mainland China from 1979 to 2012. Int J Endocrinol 2013;2013:753150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning G, Bloomgarden Z. Diabetes in China: prevalence, diagnosis, and control. J Diabetes 2013;5:372. [DOI] [PubMed] [Google Scholar]
- 33.Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32–38 [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Qiao Q, Ji L, et al. Nonlaboratory-based risk assessment algorithm for undiagnosed type 2 diabetes developed on a nation-wide diabetes survey. Diabetes Care 2013;36:3944–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YL, Gao WG, Pang ZC, et al. Diabetes self-risk assessment questionnaires coupled with a multimedia health promotion campaign are cheap and effective tools to increase public awareness of diabetes in a large Chinese population. Diabet Med 2012;29:e425–e429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.