INTRODUCTION AND DEFINITIONS
Acute otitis media (AOM) is a common disorder of early childhood, and among the most common reasons for referral of a young child to the otolaryngologist. Although the majority of children with AOM are managed by primary care providers without the need for specialty consultation, children with recurrent episodes, severe symptoms, or complications of AOM can require prompt otolaryngologic evaluation and surgical treatment. Although AOM affects many children, and tympanostomy tube placement is the most commonly performed operative procedure in young children, consensus is still being reached about the most appropriate use of surgery for children with AOM.
We review the relevant concepts in the management of AOM in children, with an emphasis on changes in microbiology over the last 2 decades. We also discuss management paradigms for AOM advanced by evidence-based clinical practice guidelines published in 2013. Surprisingly, these guidelines are the first to recommend tympanostomy tube placement as an option for children with recurrent AOM, despite decades of tympanostomy tube placement for this indication. New emphasis is placed on accurate diagnosis based on strict criteria, with additional refinement of the selection of children most appropriate for observation without antibiotics at initial diagnosis of AOM. This review focuses on AOM and recurrent AOM, and we do not directly discuss management of middle ear effusion (MEE) that is asymptomatic other than hearing loss (otitis media with effusion [OME]). It is important to distinguish AOM from OME, which are separate entities with unique management considerations (Table 1).
Table 1.
Classification of otitis media
| Term | Definition |
|---|---|
| Acute otitis media (AOM) | The rapid onset of signs and symptoms of inflammation of the middle ear |
| Symptoms include otalgia, irritability, insomnia, anorexia | |
| Signs include fever, otorrhea, full or bulging opaque TM, impaired TM mobility, TM erythema | |
|
| |
| Recurrent acute otitis media (RAOM) | Three or more well-documented and separate AOM episodes in the past 6 mo, or ≥4 well-documented and separate AOM episodes in the past 12 mo with ≥1 in the past 6 mo |
|
| |
| Otitis media with effusion (OME) | The presence of fluid in the middle ear without signs or symptoms of acute ear infection (AOM) |
|
| |
| Chronic otitis media with effusion (COME) | OME persisting for ≥3 mo from the date of onset (if known) or from the date of diagnosis (if onset unknown) |
EPIDEMIOLOGY
AOM is a common disease in children. In the United States, 8.8 million children (11.8%) under the age of 18 were reported to have ear infections in 2006, with an estimated total treatment cost of $2.8 billion.5 Antibiotics are prescribed for AOM more frequently than for any other illness of childhood.6 The epidemiology of AOM has evolved over the past decade, with a decrease in clinician visits for suspected AOM by 33% from 1995–1996 to 2005–2006.6 The reasons for the decrease in clinician visits is unclear, with possible explanations including financial considerations, health care access issues, public educational campaigns about the viral etiology of most upper respiratory tract infections, the introduction of the 7-valent pneumococcal vaccine (PCV7) and influenza vaccines, and publication and implementation of clinical practice guidelines.7
Interestingly, clinician prescribing patterns have not changed significantly for children with AOM, with the rate of antibiotic prescription per visit remaining approximately stable (80% in 1995–1996 to 76% in 2005–2006).6,8 More recent study of prescribing patterns for AOM shows treatment strategy may vary among medical disciplines, with 1 report showing a drop in early antibiotic use for AOM by pediatricians and otolaryngologists between 2002 and 2009, but an increase in antibiotic use by family practitioners over the same period.9 However, with now almost 10 years since the advancement of the concept that prompt antibiotic treatment is not needed for many children with AOM, observation of selected children with a diagnosis of AOM without initial antibiotics has become more accepted by both caregivers and providers. Additionally, physician adherence to the treatment recommendations from clinical practice guidelines may be improved with performance feedback and decision support systems using electronic health records.10
PATHOPHYSIOLOGY AND MICROBIOLOGY
AOM is often, although not always, preceded by a viral upper respiratory tract infection.11 Inflammation leads to edema in the nasal cavities and nasopharynx, causing functional obstruction of the Eustachian tube and the development of negative pressure in the middle ear from a lack of equilibration. Microbe-containing secretions from the upper airway mucosa move into the middle ear owing to the pressure differential, where they become trapped. Bacterial replication and infection may ensue.12-14 Young children are at particularly increased risk for AOM because of increased viral exposure, immunologic naiveté, and impaired Eustachian tube function even at baseline.15
With sensitive assays including culture, polymerase chain reaction and antigen detection, bacteria, viruses, or both, are detected in middle ear fluid in up to 96% of AOM cases. A study of middle ear fluid in 79 children with AOM and indwelling tympanostomy tubes found that 66% had bacteria and viruses, 27% had bacteria alone, and 4% had only viruses.16
The microbiology of AOM has changed over the last 2 decades with increasing penetration of pneumococcal vaccination programs. The most common bacterial species that cause AOM continue to be Streptococcus pneumoniae, nontypeable Haemophilus influenza, and Moraxella catarrhalis.17 The heptavalent S pneumoniae vaccine (PCV7) was introduced in 2000, shortly after which the frequency of S pneumoniae recovery in tympanocentesis studies of AOM decreased relative to that of the other microbes.18 The S pneumoniae serotypes contained in PCV7 continued to decline in AOM patients, and were in fact nearly absent by 2007 through 2009.17 However, they have been replaced by nonvaccine pneumococcal serotypes in both tympanocentesis and nasopharyngeal colonization studies, so that the incidence of S pneumoniae was approximately equal to that of H influenza, with M catarrhalis less frequent.17,19 The new 13-valent S pneumoniae vaccine, PCV13, was licensed in 201020 and will undoubtedly additionally shift the microbiological landscape of AOM.
DIAGNOSIS
Because there is no gold standard for the diagnosis of AOM, short of tympanocentesis and culture of middle ear fluid, there is controversy about the best clinical means to accurately diagnose acute middle ear infection. Diagnostic accuracy is challenging because of the wide spectrum of signs and symptoms that develop throughout the course of the disease, the difficulties in examining the ears of young children who may be uncooperative or have occluding cerumen, and the overlap of symptoms (fever, otalgia, irritability, insomnia) with other entities such as viral illness. In one study of 469 patients ages 6 to 35 months who were suspected by their caregivers to have AOM, only 237 (50%) actually met strict defined criteria for this diagnosis.21 Additionally, the distinction between AOM and chronic OME is unclear to many caregivers and even to many medical professionals.
The diagnostic accuracy for children with AOM is important when we strategize for treatment of children with AOM. Children with upper respiratory infections and chronic OME generally should not be treated with antibiotics.22-24 Many children with AOM do not require antibiotics for cure because the natural history of AOM is in general favorable.25 When we interpret studies of treatment of AOM with antibiotics compared with placebo, those studies that include children with AOM diagnosed less stringently are more likely to include children with respiratory tract infections and OME rather than AOM. Treatment differences may be affected (made smaller) by use of less stringent diagnostic criteria. However, the “real-world” diagnosis of AOM in young children is unfortunately often far from precise, and the conclusions of those studies may be quite applicable to a cohort of children with presumed AOM.
The 2013 American Academy of Pediatrics (AAP) guideline on the management of AOM emphasized diagnostic criteria that focused on otoscopic examination (Table 2). This guideline proposed that AOM should be diagnosed in children with moderate to severe bulging of the tympanic membrane (TM) or new onset of otorrhea, or in children with mild bulging of the TM with recent onset of ear pain or intense TM erythema. In addition, AOM should not be diagnosed in children without a MEE.7 This represents a new emphasis on precise diagnosis compared with the 2004 guidelines, which did not require a bulging TM and made management suggestions when there was an “uncertain diagnosis.”26
Table 2.
Key differences in the 2004 and 2013 American Academy of Pediatrics guidelines for the diagnosis and management of acute otitis media (AOM)
| Subject | 2004 | 2013 | Rationale for 2013 Changes |
|---|---|---|---|
| Children <6 mo | Treat with antibiotic therapy | No recommendations | |
|
| |||
| Diagnosis of AOM | Acute onset of signs and symptoms | Moderate to severe bulging of TM, or new-onset otorrhea not owing to acute otitis externa | 2004 criteria allowed less precise diagnosis, provided treatment recommendation when diagnosis was uncertain. |
| Presence of MEE | Mild bulging of TM and recentb onset ear painc or intense TM erythema | ||
| Signs and symptoms of middle ear inflammationa | Must have MEE | ||
|
| |||
| Uncertain diagnosis | Expected and included in treatment guidelines | Excluded | Emphasized need for diagnosis of AOM for best management. |
|
| |||
| Initial observation option instead of initial antibiotic therapy | Option for observation: | Option for observation:
|
Favorable natural history overall. |
Observation recommended:
|
Observation recommended:
|
Evidence of small benefit of antibiotics in recent trials that used stringent diagnostic criteria. | |
|
| |||
| Initial antibiotic therapy recommended | Antibiotics recommended: | Antibiotics recommended: | More stringent diagnostic guidelines in 2013 should lead to greater antibiotic benefit. |
| Antibiotics an option: | Antibiotics an option:
|
Greater antibiotic benefit for bilateral disease, AOM with otorrhea. | |
| Two recent studies show small benefit of antibiotics for age 6–24 mo. | |||
|
| |||
| Recurrent AOM | No recommendations | Do not prescribe prophylactic antibiotics | Minimal benefit for prophylaxis and antibiotics come with risks (antibiotic resistance and adverse effects). |
| May offer tympanostomy tubes | Modest reduction in AOM with tubes. | ||
Abbreviations: MEE, middle ear effusion; TM, tympanic membrane.
Signs and symptoms of middle ear inflammation include distinct erythema of TM or distinct otalgia (‘discomfort clearly referable to the ear[s] that results in interference with or precludes normal activity or sleep’).
Recent: <48 hours.
Ear pain may be indicated by holding, tugging, or rubbing of the ear in a nonverbal child.
Nonsevere illness defined as mild otalgia and fever <39°C in the past 24 hours in the 2004 guideline; the 2013 guideline modifies this to “mild otalgia for less than 48 hours and temperature less than 39°C.”
Severe signs or symptoms include moderate or severe otalgia or temperature ≥39°C in 2004 guideline; the 2013 guideline also includes otalgia for ≥48 hours.
Adapted from Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics 2013;131(3):e964–99; and American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113(5):1451–65.
Such strict diagnostic criteria were used in 2 randomized, controlled trials of antibiotics for AOM, both of which found benefit of antibiotic compared with placebo. In prior studies, antibiotic therapy resulted in clinical improvement in approximately 6% to 12% of children with AOM. In these 2 recent trials the rate of clinical improvement was 26% to 35%, likely owing to accurate diagnosis of AOM on entry as well as the nature of the measures of clinical improvement.7,27,28
Hoberman and colleagues28 randomized 291 patients 6 to 23 months old with AOM to receive either amoxicillin–clavulanate or placebo for 10 days and recorded symptomatic response with the Acute Otitis Media Severity of Symptoms scale, as well as treatment failure. Diagnostic criteria included (1) onset of symptoms within 48 hours with score of 3+ on the Acute Otitis Media Severity of Symptoms scale, (2) MEE, and (3) moderate or marked bulging of TM, or slight bulging with either otalgia or marked erythema of TM. The treatment group had a lower Acute Otitis Media Severity of Symptoms scale score at 7 days (P =.04), with a lower treatment failure rate at days 4 or 5 and 10 through 12 (4% vs 24% [P<.001] and 16% vs 51% [P<.001], respectively). Tahtinen and colleagues27 randomized 319 patients 6 to 35 months old with AOM to receive either amoxicillin–clavulanate or placebo for 7 days and measured time to treatment failure. Diagnostic criteria were (1) MEE, (2) signs of acute inflammation in TM, and (3) acute symptoms such as fever, ear pain, or respiratory symptoms. Treatment failure occurred in 18.6% of the treatment group, which was significantly lower than the 44.0% treatment failure rate for the placebo group (P<.001).
We should consider that these studies used amoxicillin–clavulanate rather than amoxicillin in the treatment arms. We should also consider that the treatment benefits of antibiotics over placebo were small, with debate about the clinical significance of some outcome measures in these studies.29,30 Of course, small benefits of antibiotics must be assessed in light of side effects, most commonly diarrhea or yeast infections, as well as concerns about antibiotic overuse and bacterial resistance.7,27,28,31,32
Otoscopy
Note that the “red eardrum,” or the ear “with fluid,” is not diagnostic for AOM in the absence of bulging or otorrhea, according to the 2013 AAP guideline. MEE is necessary but alone not sufficient for the diagnosis of AOM, because OME is distinct from AOM (see Table 1). Although OME may precede or follow an episode of AOM, it is not an acute infectious process and in general should not be treated with antibiotics.7,33 The appearance of the TM evolves over the course of the disease, and a change in clinical status warrants repeat examination.
In addition to examination of the color, position, and contour of the TM, pneumatic otoscopy to assess TM mobility is an important component of otoscopy. Absent or reduced TM mobility are diagnostic features of a MEE, as is an air–fluid level behind the TM. Tympanometry, when available, can also provide more quantitative information about TM mobility and middle ear compliance.34
Otoscopy can be challenging in children. Uncooperative patients, cerumen impaction, inadequate instrumentation, and even lack of expertise are common difficulties. Despite these challenges, the 2013 guideline underscores the importance of a thorough physical examination in the management of AOM, and reinforces the need for training pediatric clinicians in otoscopic and pneumatic otoscopic skills.7 Suggestions for cerumen removal can be found in the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) Clinical Practice guidelines on cerumen impaction.35 Diagnostic difficulties may lead to referral to an otolaryngologist for cerumen removal and examination of the ears with the otomicroscope (Box 1).
Box 1. Acute otitis media (AOM): when to refer to the otolaryngologist.
Referral to an otolaryngologist should be considered for the following reasons:
Inability to examine the ear
Unexplained, progressive, or irreversible tympanic membrane abnormality
Poor response to therapy
Recurrent AOM
Associated hearing difficulties that persist or progress
Recurrent AOM in the “at-risk child”
Suspected complication of AOM
MANAGEMENT
Management goals for AOM are to decrease severity and duration of symptoms, principally by controlling pain and fever, to improve hearing outcomes, and to prevent complications.7,36
Analgesia
The assessment and treatment of pain is an important but possibly overlooked component of pediatric care. Poorly controlled pain is associated with suffering and can be emotionally traumatic, causing anxiety for patients and their caregivers.37 Pain control should be actively addressed whether initial treatment included immediate antibiotics or not, because the antibiotics may not begin to provide pain relief for more than 24 hours.7,31,38 Although there are many options for treatment of otalgia, including oral, topical, and homeopathic agents, few have been well studied.7,39
Oral acetaminophen and ibuprofen are commonly used to treat pain in children with AOM. A randomized, blinded, placebo-controlled trial found that both ibuprofen and acetaminophen improved pain control over placebo, but only ibuprofen resulted in a significant increase over placebo, with continued pain in 7%, 10%, and 24% of patients receiving ibuprofen, acetaminophen, and placebo, respectively (P<.01 for ibuprofen).40 Topical drops, both anesthetic and naturopathic, have been found in small trials to improve pain symptoms, but a Cochrane review concluded that there is insufficient evidence to make any statement about their efficacy.39,41,42 The selection of pain medication, as well as the dosing and schedule for administration, should be discussed with the caregivers, who may have valuable experience and preferences.37
Antibiotic Therapy
Antibiotic therapy is intended to target the bacteria present in the MEE of children with AOM.16 Two key decisions regarding antibiotic use occur with every diagnosis of AOM: (1) Should antibiotics be used immediately, and (2) if antibiotics are used, which antibiotic is the best choice for treatment?
The decision of whether or not to treat with initial antibiotics is based on age, severity of symptoms, the presence of otorrhea, and laterality (Box 2, see Table 2).7 In the 2013 AAP guideline, antibiotics are recommended for any child with otorrhea, or severe symptoms, or both, and for children younger than 2 years with bilateral AOM. There is an option for initial observation instead of initial antibiotics if children are younger than 2 years old with unilateral disease and no otorrhea, or 2 years or older with either bilateral or unilateral disease and no otorrhea (see Table 2).
Box 2. Initial antibiotic therapy or observation for acute otitis media (AOM).
The decision to treat AOM with antibiotics at time of diagnosis or to observe with close follow-up should include:
Parental/caregiver input and informed shared decision making
Ability to follow patients for improvement or deterioration
Understanding that young children (<6 months of age), children with bilateral AOM, and children with otorrhea should be treated with antibiotics
Understanding that children with severe symptoms (moderate or severe otalgia or otalgia for at least 48 hours, or temperature 39°C [102.2°F] or higher) should be treated with antibiotics
Understanding the controversies about the need to use antibiotics in children between 6 and 24 months who have AOM with mild or moderate symptoms.
The concept of initial observation, without immediate antibiotic treatment, of children diagnosed with AOM was advanced in Europe before the 2004 recommendations of the AAP did so here in the United States. The favorable natural history of AOM, the potential overdiagnosis of AOM, and the potential consequences of antibiotics, including frequent side effects and the emergence of drug resistant microbes, are all considerations here.7,27,28,31,32
A recent Cochrane review found that up to 82% of children with AOM improve spontaneously. Although there is a slight improvement in symptoms associated with antibiotics (relative risk [RR], 0.70; 95% CI, 0.57–0.86 and RR, 0.79; 95% CI 0.66–0.95 for 2–3 days and 4–7 days, respectively), 20 children would need to receive antibiotics to prevent 1 child from experiencing ear pain at 2 to 7 days.31 Antibiotics did significantly reduce TM perforations and contralateral AOM episodes; however, there was no effect on tympanometry findings at 4 weeks to 3 months, or on the number of AOM recurrences. Antibiotics also increased the relative risk of adverse events by 34% (95% CI, 16%–55%), most commonly vomiting, diarrhea, and rash.31
The decision to observe without immediate antibiotics should be made in conjunction with caregivers, with a plan for pain management at the outset and a mechanism for follow-up within 48 to 72 hours so antibiotics can be started for persistent or worsening symptoms.7 There is evidence that in the proper setting this strategy does not increase complication rates.43 Clinicians may also give caregivers an antibiotic prescription with instructions to have it filled only under certain circumstances, the so-called wait-and-see prescription (WASP).44 This approach seems to avoid antibiotic use in up to two thirds of children selected for observation.44,45
Antibiotic Choice
If antibiotic treatment is used for a child with AOM, the choice of antibiotic is based on the most common pathogens and their susceptibility patterns as well as the side effect profile of the drug. High-dose amoxicillin (90 mg/kg per day) is the recommended first-line agent in the 2013 guidelines, with the addition of beta-lactamase coverage for cases in which the patient has recently received amoxicillin, has concurrent purulent conjunctivitis, or has recurrent AOM unresponsive to amoxicillin.7 S pneumoniae is 83 to 87% susceptible to high-dose amoxicillin, and H influenza is 58% to 82% susceptible.7 Interestingly, more than 90% of M catarrhalis is beta-lactamase positive,46 but a high rate of spontaneous clinical improvement and low rate of suppurative complications with AOM from this organism makes amoxicillin treatment an appropriate first choice.7,47,48
For penicillin-sensitive patients, second- or third-generation cephalosporins, including intramuscular ceftriaxone, may be used. Other useful agents, particularly for penicillin-sensitive patients or amoxicillin failures, include second- and third-generation cephalosporins and clindamycin. Patients who do not improve in 48 to 72 hours on their first-line regimen should be reassessed, and alternative therapy considered.7 In this instance, amoxicillin–clavulanate and intramuscular or intravenous ceftriaxone are considered first line, with alternatives including clindamycin or combination treatment with both clindamycin and a third-generation cephalosporin. In difficult cases, tympanocentesis may be considered for drainage and culture-directed therapy.7
COMPLICATIONS
AOM can progress to severe complications, including acute mastoiditis, meningitis, and intracranial abscess. Complications can be thought of as (a) intracranial, extratemporal, (b) intratemporal, extracranial, and (c) extratemporal, extracranial (Table 3). The most common complication seen by the clinician is TM perforation with suppuration and otorrhea, but a busy clinician can expect to see facial nerve paralysis or acute mastoiditis (Fig. 1) in a young child with AOM as well. Such suppurative complications require prompt referral to the appropriate surgical specialists (ie, an otolaryngologist or neurosurgeon) for aggressive antibiotic therapy and for surgical drainage when indicated. Although a full discussion of such complications is beyond the scope of this review, the presentations and diagnostic schemes for such conditions are detailed in Table 3. Importantly, the recommendation of initial observation of AOM without antibiotics in selected children has not caused a substantial increase in suppurative complications such as mastoiditis.43,51
Table 3.
Complications of acute otitis media (AOM)
| Complication | Presentation | Diagnostic Tests | Treatment Options |
|---|---|---|---|
|
Intracranial, extratemporal
| |||
| Meningitis | Headache, altered mental status, nausea, vomiting, lethargy, poor oral intake, seizures, meningismus, focal neurologic deficits | LP after CT or MRI to exclude other intracranial complications | Antibiotics |
| Intracranial abscess | CT first-line and for follow-up | Myringotomy ± tympanostomy tube | |
| Subdural or epidural abscess | MRI more sensitive | Antibiotics | |
| CT first-line and for follow-up | Myringotomy ± tympanostomy tube | ||
| MRI more sensitive | Neurosurgical consultation | ||
|
| |||
| Otitic hydrocephalus (nonobstructing mural thrombus of transverse sinus) | Headache, vomiting, blurred vision, seizures, abducens palsy | LP to measure ICP, after CT to exclude mass effect: high ICP and normal cytology | Antibiotics |
| Measures to decrease ICP | |||
|
| |||
| Thrombosis of dural venous sinuses (lateral or sigmoid sinus thrombophlebitis) | Headache, neck stiffness, fever, otalgia, postauricular pain & erythema | CT-contrast enhanced, MRI/MRA/MRV | Antibiotics ± anticoagulation |
| Myringotomy ± tympanostomy tube ± Mastoidectomy | |||
| Consider clot removal | |||
|
| |||
|
Extracranial, intratemporal
| |||
| Acute mastoiditis (see Fig. 1) | Postauricular erythema, tenderness, edema, protrusion of pinna | CT: bony erosion, destruction of mastoid air cells | Antibiotics |
| Myringotomy ± tympanostomy tube | |||
| Mastoidectomy or aspiration of subperiosteal abscess | |||
|
| |||
| Subperiosteal abscess | Postauricular erythema, tenderness, fluctuance | CT: fluid collection adjacent to eroded mastoid cortex | Antibiotics |
| Myringotomy ± tympanostomy tube | |||
| Mastoidectomy or needle aspiration of abscess | |||
|
| |||
| Petrositis (Gradenigo’s syndrome) | Abducens palsy and retrobulbar pain | CT first-line | Antibiotics |
| MRI for nerve involvement, apical petrositis | Myringotomy ± tympanostomy tube | ||
| Mastoidectomy | |||
|
| |||
| Facial nerve palsy | Often incomplete acute onset facial weakness on affected side | CT to assess extent of disease and rule out cholesteatoma or other lesion | Antibiotics |
| Myringotomy ± tympanostomy tube | |||
|
| |||
| Labyrinthitis | Acute onset SNHL and vertigo | Physical examination | Antibiotics |
| Myringotomy ± tympanostomy tube | |||
| Consider steroids if SNHL persists | |||
|
| |||
| TM perforation | Otorrhea | Physical examination | Antibiotics |
| Close follow-up to determine for surgical repair | |||
|
| |||
|
Extracranial, extratemporal
| |||
| Sepsis | Fever, lethargy, tachycardia, hypotension | Physical examination | Antibiotics |
| Myringotomy ± tympanostomy tube | |||
Fig. 1.
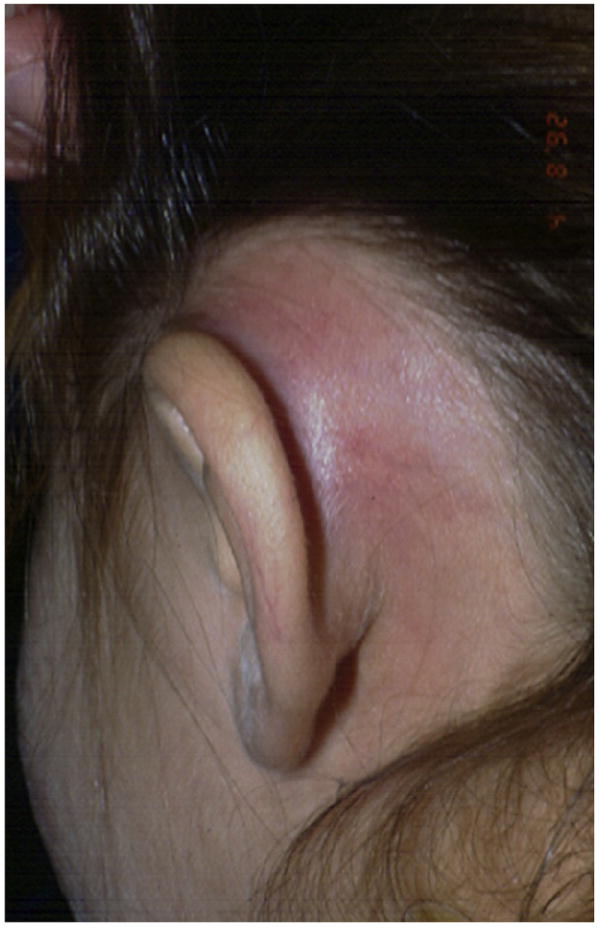
Acute mastoiditis, characterized by postauricular erythema and swelling.
RECURRENT AOM AND TYMPANOSTOMY TUBES
Recurrent AOM is defined as at least 3 episodes of AOM in 6 month or 4 episodes in 1 year, with one of these episodes in the preceding 6 months.7 Risk factors that have been identified for recurrent AOM include group child care attendance, male gender, winter season, passive smoking, pacifier uses, presence of siblings, lack of breast-feeding, and symptoms for longer than 10 days at presentation.52 The 2013 AAP guideline examines the role of both prophylactic antibiotic therapy and tympanostomy tube placement for prevention of recurrent AOM. Prophylactic antibiotics do reduce the incidence of AOM by approximately 0.5 to 1.5 episodes per 12 months of treatment per child, and the treatment of 5 children for 1 year would prevent 1 child from developing AOM during therapy.53 However, given the cost, side effects, potential for antibiotic resistance, and scant clinical benefit with a large number needed to treat to avoid a single episode of AOM, a recommendation against the routine administration of antibiotic prophylaxis for children with recurrent AOM was made in the 2013 AAP AOM guideline.7
Tympanostomy Tubes
Tympanostomy tubes are placed in 667,000 children under the age of 15 every year in the United States.54 One out of 15 children, or 6.8% of the population, had tympanostomy tubes placed before the age of 3 years.55 The health care costs of tympanostomy tubes are enormous, at an estimated cost of $2200 per patient56 for a total of nearly $1.5 billion annually in the United States.
Tympanostomy tubes are most commonly placed for persistent MEE with associated hearing difficulties (OME) or for recurrent AOM. This surgery is less commonly performed for AOM treatment failure, impending complications of AOM, or structural TM changes associated with long-term Eustachian tube dysfunction.57 Indications for tympanostomy tube placement have been subject to recent scrutiny. Possible overuse of tympanostomy tubes has been identified, particularly for children with MEEs of short duration and for children with infrequent or poorly documented episodes of AOM.58 These issues have been addressed by a national “overuse” summit that included tympanostomy tube placement as 1 of the 5 potentially overused treatments,59 as well as by the recent publication of a multidisciplinary clinical practice guideline on the use of tympanostomy tubes in children.1
Most studies of the benefits of tympanostomy tubes focus on utility for MEEs and conductive hearing loss.60,61 The literature studying the use of tympanostomy tubes for recurrent AOM is scant; few studies exclude children with long-term MEE. The reduction of AOM by tube placement is likely modest.
One trial of recurrent AOM treatment that excluded children with MEE randomized children to tympanostomy tube placement, amoxicillin prophylaxis, or placebo and followed them for 2 years. The tympanostomy tube group did not show a decrease in episodes of AOM.2 However, several randomized trials of tympanostomy tubes for recurrent AOM that included children with MEE at entry did show a modest reduction in AOM episodes from 0.55 to 2.5 episodes per child-year.62-64 A recent Cochrane review included 2 of these studies of tympanostomy tubes for recurrent AOM, and concluded that tympanostomy tubes reduced AOM episodes by 1.5 in the 6 months after surgery.65 A systematic review that included 5 studies of recurrent AOM and tympanostomy tubes concluded that 2 to 5 children needed to be treated with tubes to prevent 1 episode of AOM over a 6-month period.66
A recent study of tympanostomy tube insertion with or without adenoidectomy in children 10 months to 2 years with recurrent AOM, and no evidence of chronic OME, showed a modest benefit in preventing recurrent AOM in the 12 months after tube surgery, with a decrease in treatment failure of 13% in the tympanostomy tubes group (95% CI, −25% to −1%) and 18% in the tympanostomy tubes and adenoidectomy group (95% CI, −30% to −6%) compared with controls.62
Children (and caregivers) with AOM may experience improved quality of life after tympanostomy tube insertion. Children with middle ear disease, including OME, recurrent AOM, or both, had improved disease-specific quality of life measures in several studies of tympanostomy tube placement, including improvement in physical suffering, hearing, speech, caregiver concerns, and emotional distress domains.56,67-69
The 2013 AAP AOM guideline states that “clinicians may offer tympanostomy tubes for recurrent AOM.”7 Few if any prior guidelines have included tympanostomy tube placement for this indication, despite widespread accepted use of tubes for recurrent AOM. The use of tympanostomy tubes for recurrent AOM is additionally refined in the recently published AAO-HNS Clinical Practice guideline for Tympanostomy Tubes in Children.1
This AAO-HNS tympanostomy tube guideline contains a key action statement that “clinicians should NOT perform tympanostomy tube insertion in children with recurrent AOM who do not have a MEE in either ear at the time of assessment.” This recommendation is based on evidence from the trials that studied antibiotic prophylaxis for recurrent AOM that excluded children with MEE, where children in the placebo groups improved with fewer subsequent ear infections.25 This may reflect a favorable natural history of recurrent AOM, and also may reflect uncertainties in the diagnosis of AOM in referred children.70 Importantly, this guideline recommendation did not apply to “at-risk” children, or those children who are immunocompromised, had severe or persistent AOM, a prior complication of AOM, or antibiotic allergies/intolerances. Such children may need tympanostomy tubes inserted for prevention of AOM more urgently, even in the absence of obvious ear disease.57
This same guideline contains a key action statement that “clinicians should offer bilateral tympanostomy tube insertion in children with RAOM who have unilateral or bilateral middle ear effusion at the time of assessment.” In these children, the presence of the effusion provides some confidence in the history of AOM, and it suggests ongoing Eustachian tube dysfunction that could lead to more AOM. The tubes provide a small benefit in the reduction of the number of AOM episodes, and perhaps additional benefits with quality of life measures, as well reduction of pain and fever with subsequent infection. Note that this statement is an “offer” of tympanostomy tubes, with the decision to place the tubes based on shared decision making with caregivers.1
Tympanostomy tube insertion is a safe procedure performed on an outpatient basis with risks related mostly to short- and long-term effects on the TM and potential complications of general anesthesia. One quarter of patients experience transient otorrhea while the tympanostomy tube is in place. At least 2% of children may develop persistent TM perforations, and up to one third will develop tympanosclerosis. Other short-term and long-term complications include granulation tissue, premature extrusion or displacement of tympanostomy tubes, focal atrophy, and increased risk of cholesteatoma (RR, 2.6; 95% CI, 1.5–4.4).71
To better understand the potential risks of anesthesia, 1 study of 3198 children undergoing tympanostomy tube placement with general anesthesia at a tertiary care children’s hospital found that 9% had minor complications (upper airway obstruction, agitation, prolonged recovery, and emesis) and 1.9% had major complications (laryngospasm, desaturation, bradycardia, dysrhythmia, and stridor). Rates were significantly increased in patients with acute or chronic illnesses (odds ratio, 2.78; P<.001).72 However another study of 4979 outpatient otolaryngologic procedures at an ambulatory surgical center, including 2045 (41.1%) tympanostomy tube placements, found a complication rate of just 0.2% overall, the majority of which occurred in those who had adenoidectomy and/or tonsillectomy.73 Complications of tympanostomy tube insertion are infrequent but are not negligible, and should be taken into consideration in clinical decision making.
Tympanostomy Tube Otorrhea
An advantage of tympanostomy tube insertion is the ability to deliver topical antibiotic therapy in the place of systemic medication should AOM occur after tube placement. In children with tympanostomy tubes, AOM is often manifested by acute tympanostomy tube otorrhea (TTO), discharge from the middle ear through the tympanostomy tube into the external auditory canal. TTO is also a common postoperative complication of tympanostomy tube insertion,71 defined as such if it occurs within 4 weeks postoperatively. Beyond 4 weeks after surgery, TTO is referred to as delayed otorrhea. It may also be chronic (≥3 months) or recurrent (≥3 episodes).57
Acute TTO is generally caused by the same pathogens as AOM in children without tympanostomy tubes, such as, S pneumonia, H influenza, or M catarrhalis.16 In older children and those with a history of water exposure, pathogens may include Pseudomonas aeruginosa and Staphylococcus aureus.1,74 The AAO-HNS tympanostomy tube guideline contains a strong recommendation that clinicians “should prescribe topical antibiotic eardrops only, without oral antibiotics, for children with uncomplicated acute tympanostomy tube otorrhea.”1 This is based on randomized, controlled trials comparing topical with systemic oral antibiotics that demonstrated superiority for topical therapy in treatment, eradication of bacterial pathogens, and patient satisfaction.75-77 Approved topical drops include ofloxacin or ciprofloxacin–dexamethasone. In general, aminoglycoside-containing drops should be avoided owing to the potential for ototoxicity. Systemic antibiotics should be considered if TTO persists despite adequate ototopical therapy, if delivery of the ear drops is impeded by an obstructed external auditory canal or uncooperative child, or if there is concern for more severe disease (ie, cellulitis, fever, severe otalgia, concurrent sinusitis or pharyngitis, immune compromise). Oral antibiotics may be given concurrently with topical antibiotics.1
PREVENTION
Several anticipatory health interventions and environmental factors can reduce the incidence of AOM and are endorsed in the 2013 AAP guideline. Vaccination with the conjugate pneumococcal vaccine (PCV7) decreased physician visits for AOM, although there was a subsequent trend toward serotype replacement where AOM (and complications) were caused by nonvaccine serotypes.17,19 Vaccination with the more recent PCV13, which covers 6 additional S pneumoniae serotypes, should be encouraged.17,19,20,78 The influenza vaccine is recommended for all children over 6 months of age.79 AOM frequently complicates influenza owing to predisposing inflammation of the upper respiratory mucosa, and this vaccination can reduce the frequency of AOM with up to 55% efficacy.7,80-83
Exclusive breastfeeding for 4 to 6 months after birth reduces frequency of AOM and recurrent AOM.84,85 Passive tobacco smoke exposure significantly increases the risk of middle ear disease,86,87 and parental smoking cessation is a preventative measure that should be addressed by clinicians. Group childcare attendance and pacifier use are other modifiable risk factors that have also been associated with OM.87,88
The literature does contain low-level evidence that nutritional supplementation may have a role in preventing OM.89,90 A large, randomized, placebo-controlled trial in Bangladesh of weekly zinc supplementation found a significant reduction in suppurative otitis media in the group receiving zinc (RR, 0.58; 95% CI, 0.41–0.82; P = .002), although the significance of these results in developed countries is unclear. A recent randomized, placebo-controlled trial of vitamin D supplementation taking place in Italy enrolled 116 children with a history of recurrent AOM and randomized to 4 months of either vitamin D or placebo. Fewer children in the treatment group experienced 1 or more episode of AOM (P = .03). It was also noted that the likelihood of AOM was significantly lower among patients with higher serum concentrations of vitamin D (≥30 ng/mL).89 Additional well-designed, randomized trials are necessary to further define the role of nutritional supplementation in the prevention of AOM.
COMPLEMENTARY AND ALTERNATIVE MEDICINE
Although many dietary modifications and complementary and alternative medicine options have been proposed and are available for prevention and treatment of AOM, high-quality evidence does not exist to support their use. Naturopathic herbal extracts have been used as topical analgesics and have been found to provide similar rates of pain relief to conventional anesthetic ear drops,41 although good evidence of benefit is lacking.39
A randomized, double-blind, placebo-controlled study involving 328 Israeli children examined the impact on upper respiratory infections of a mixture of Echinacea with several other substances including propolis and vitamin C in an elixir called “Chizukit” administered twice daily during the winter months. This study found a 68% reduction in AOM in the treatment group (19.4% vs 43.5% incidence AOM; P<.001).91 Additional studies of individual components of such mixtures using more rigorous study design are necessary before routine recommendation of such for prevention of AOM.
The general health benefits of probiotics have been widely endorsed but sparsely studied. Studies of probiotics given to children in milk, capsule form, nasal sprays, and formula have conflicting results, but some have found a modest benefit in the prevention of AOM.92 One placebo-controlled study of probiotic formula supplementation for infants from younger than 2 months through 12 months of age found a significantly decreased frequency of AOM, with fewer antibiotic prescriptions, in the first 7 months of life in the treatment group.93 Other randomized, controlled trials, however, have found no difference in the frequency of AOM.94,95
Xylitol is a natural sugar found in fruit and administered as a gum, syrup, or lozenge that has been extensively studied as a preventative compound for AOM. It decreases microbial adherence to nasopharyngeal cells, and alters Streptococcus pneumoniae gene expression.92 A recent Cochrane review found a 25% reduced risk of AOM (RR, 0.75; 95% CI, 0.65–0.88) in otherwise healthy children receiving xylitol compared with controls.96 Despite these findings, the clinical relevance of xylitol is limited by the need for frequent dosing (5 times daily in most studies), frequent gastrointestinal side effects, and poor compliance.
The 2013 AAP guideline calls for additional research to compare complementary and alternative medicine therapies with the observation option for AOM. Clinicians should take appropriate caution when discussing complementary and alternative medicine therapies, given the lack of supporting evidence for many approaches, potential side effects, and costs.92 Of course, any study of such interventions should be interpreted in light of our knowledge of the inconsistencies in diagnosis of AOM as well as the general favorable natural history of children identified as having recurrent AOM.
“AT-RISK” CHILDREN AND OTITIS MEDIA
Children with medical comorbidities, neurocognitive and/or communication impairment, and craniofacial anomalies that affect Eustachian tube function may be at increased risk for frequent AOM or consequences of middle ear disease and associated conductive hearing loss. These children are usually excluded from clinical trials that evaluate the management and outcomes of otitis media, and in fact are excluded from the 2013 AAP AOM guideline recommendations. The AAO-HNS Clinical Practice guideline on Tympanostomy Tubes in Children specifically recommended identification of such “at-risk” children, with acknowledgment that these children may need more urgent and chronic management of otitis media, often with tympanostomy tubes.
At-risk children include those with underlying hearing loss, autism spectrum and other developmental disorders, Down syndrome, craniofacial disorders with cognitive delays, vision impairment, cleft palate with or without an associated syndrome, and developmental delay.22 Children with craniofacial disorders, Down syndrome, cleft palate, and many other syndromes often have anatomic and functional impairment of the Eustachian tubes that increase the risk of AOM.97,98 Underlying immune dysfunction and unique group care settings may further exacerbate middle ear disease in these patients. Physical examination and hearing assessment may be difficult in these children because of behavioral and communication issues. Some have stenotic external auditory canals, particularly children with Down syndrome, prone to cerumen impaction and difficult otoscopy.99
The AAO-HNS 2004 guideline for Otitis Media with Effusion and the 2013 guideline for Tympanostomy Tubes in Children acknowledge the special needs of this population and encourage the optimization of conditions for hearing, speech and language.1,22 Some of these “at-risk” children have medical issues that increase the risk of general anesthesia, a consideration when tympanostomy tubes are recommended. General anesthesia for tympanostomy tube placement carries an higher rate of complications in children with chronic illnesses.72 Children with developmental abnormalities have high rates of sleep apnea, which increases the risks of general anesthesia, and skeletal deformities may present airway management challenges during anesthesia.98,100 These risks should be taken into account when planning for tympanostomy tube insertion.
SUMMARY
Management of AOM requires keen diagnostic skills to recognize the signs, symptoms, and severity of the illness and to determine the otoscopic hallmarks of acute middle ear infection. Recent clinical practice guidelines emphasize the need for precise diagnosis, whereas understanding the generally favorable natural history of AOM may allow many children to be observed without initial antibiotic treatment. Prevention of AOM can include vaccinations, environmental modifications, maintenance of breastfeeding, as well as other interventions. Tympanostomy tubes may modestly lower the number of infections in children with recurrent AOM, and improve diseaserelated quality of life for children as well.
KEY POINTS.
Acute otitis media (AOM) should be distinguished from chronic otitis media with effusion.
Clinical practice guidelines have been updated to refine the “observation” option for treatment of AOM, with an emphasis on precise diagnosis.
The bacteriology of AOM has been changed by the use of pneumococcal vaccines, but high dose amoxicillin or amoxicillin–clavulanate are good choices when initial antibiotic therapy is prescribed for AOM.
Tympanostomy tubes are an option for children with recurrent AOM, particularly when there is evidence of ongoing Eustachian tube dysfunction.
Complications of AOM are rare, but must be detected early to avoid serious morbidity.
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013;149(Suppl 1):S1–35. doi: 10.1177/0194599813487302. [DOI] [PubMed] [Google Scholar]
- 2.Casselbrant ML, Kaleida PH, Rockette HE, et al. Efficacy of antimicrobial prophylaxis and of tympanostomy tube insertion for prevention of recurrent acute otitis media: results of a randomized clinical trial. Pediatr Infect Dis J. 1992;11(4):278–86. doi: 10.1097/00006454-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone CD, Klein JO. Otitis media in infants and children. 4. Hamilton (Ontario): BC Decker; 2007. [Google Scholar]
- 4.Rosenfeld RM, Bluestone CD. Evidence-based otitis media. 2. Hamilton (Ontario); Lewiston (NY): B.C. Decker; 2003. [Google Scholar]
- 5.Soni A. Statistical Brief No. 228. Vol. 2008. Rockville (MD): Agency for Healthcare Research and Quality; Ear infections (otitis media) in children (0-17): use and expenditures, 2006. [Google Scholar]
- 6.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 8.Coco A, Vernacchio L, Horst M, et al. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125(2):214–20. doi: 10.1542/peds.2009-1115. [DOI] [PubMed] [Google Scholar]
- 9.Grossman Z, Silverman BG, Miron D. Physician specialty is associated with adherence to treatment guidelines for acute otitis media in children. Acta Paediatr. 2013;102(1):e29–33. doi: 10.1111/apa.12051. [DOI] [PubMed] [Google Scholar]
- 10.Forrest CB, Fiks AG, Bailey LC, et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics. 2013;131(4):e1071–81. doi: 10.1542/peds.2012-1988. [DOI] [PubMed] [Google Scholar]
- 11.Winther B, Alper CM, Mandel EM, et al. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119(6):1069–75. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 12.Rovers MM, Schilder AG, Zielhuis GA, et al. Otitis media. Lancet. 2004;363(9407):465–73. doi: 10.1016/S0140-6736(04)15495-0. [DOI] [PubMed] [Google Scholar]
- 13.Chonmaitree T, Heikkinen T. Role of viruses in middle-ear disease. Ann N Y Acad Sci. 1997;830:143–57. doi: 10.1111/j.1749-6632.1997.tb51886.x. [DOI] [PubMed] [Google Scholar]
- 14.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16(2):230–41. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluestone CD, Swarts JD. Human evolutionary history: consequences for the pathogenesis of otitis media. Otolaryngol Head Neck Surg. 2010;143(6):739–44. doi: 10.1016/j.otohns.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruohola A, Meurman O, Nikkari S, et al. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin Infect Dis. 2006;43(11):1417–22. doi: 10.1086/509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grubb MS, Spaugh DC. Microbiology of acute otitis media, Puget sound region, 2005-2009. Clin Pediatr. 2010;49(8):727–30. doi: 10.1177/0009922810361365. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196(8):1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morbid Mortal Wkly Rep. 2010;59(9):258–61. [PubMed] [Google Scholar]
- 21.Laine MK, Tahtinen PA, Ruuskanen O, et al. Symptoms or symptom-based scores cannot predict acute otitis media at otitis-prone age. Pediatrics. 2010;125(5):e1154–61. doi: 10.1542/peds.2009-2689. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130(Suppl 5):S95–118. doi: 10.1016/j.otohns.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. [September 26, 2013];Get smart: know when antibiotics work. Available at: http://www.cdc.gov/getsmart/campaign-materials/about-campaign.html.
- 24.Weissman J, Besser RE. Promoting appropriate antibiotic use for pediatric patients: a social ecological framework. Semin Pediatr Infect Dis. 2004;15(1):41–51. doi: 10.1053/j.spid.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope. 2003;113(10):1645–57. doi: 10.1097/00005537-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 27.Tahtinen PA, Laine MK, Huovinen P, et al. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364(2):116–26. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 28.Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364(2):105–15. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman DH, Shreves AE. Treatment of acute otitis media in children. N Engl J Med. 2011;364(18):1775. doi: 10.1056/NEJMc1102207. [DOI] [PubMed] [Google Scholar]
- 30.Darby-Stewart A, Graber MA, Dachs R. Antibiotics for acute otitis media in young children. Am Fam Physician. 2011;84(10):1095–7. [PubMed] [Google Scholar]
- 31.Venekamp RP, Sanders S, Glasziou PP, et al. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2013;(1) doi: 10.1002/14651858.CD000219.pub3. CD000219. [DOI] [PubMed] [Google Scholar]
- 32.Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2007;26(Suppl 10):S12–6. doi: 10.1097/INF.0b013e318154b25d. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld RM, Culpepper L, Yawn B, et al. Otitis media with effusion clinical practice guideline. Am Fam Physician. 2004;69(12):2776, 2778–9. [PubMed] [Google Scholar]
- 34.Onusko E. Tympanometry. Am Fam Physician. 2004;70(9):1713–20. [PubMed] [Google Scholar]
- 35.Roland PS, Smith TL, Schwartz SR, et al. Clinical practice guideline: cerumen impaction. Otolaryngol Head Neck Surg. 2008;139(3 Suppl 2):S1–21. doi: 10.1016/j.otohns.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Shaikh N, Hoberman A, Paradise JL, et al. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatr Infect Dis J. 2009;28(1):5–8. doi: 10.1097/INF.0b013e318185a387. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Pediatrics, Committee on Psychosocial Aspects of Child and Family Health, Task Force on Pain in Infants, Children, and Adolescents. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108(3):793–7. doi: 10.1542/peds.108.3.793. [DOI] [PubMed] [Google Scholar]
- 38.Burke P, Bain J, Robinson D, et al. Acute red ear in children: controlled trial of non-antibiotic treatment in general practice. BMJ. 1991;303(6802):558–62. doi: 10.1136/bmj.303.6802.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foxlee R, Johansson A, Wejfalk J, et al. Topical analgesia for acute otitis media. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD005657.pub2. CD005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10(4):387–92. doi: 10.1111/j.1472-8206.1996.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarrell EM, Mandelberg A, Cohen HA. Efficacy of naturopathic extracts in the management of ear pain associated with acute otitis media. Arch Pediatr Adolesc Med. 2001;155(7):796–9. doi: 10.1001/archpedi.155.7.796. [DOI] [PubMed] [Google Scholar]
- 42.Bolt P, Barnett P, Babl FE, et al. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93(1):40–4. doi: 10.1136/adc.2006.110429. [DOI] [PubMed] [Google Scholar]
- 43.Marcy M, Takata G, Chan LS, et al. Management of acute otitis media. Evid Rep Technol Assess (Summ) 2000;(15):1–4. [PMC free article] [PubMed] [Google Scholar]
- 44.Spiro DM, Tay KY, Arnold DH, et al. Wait-and-see prescription for the treatment of acute otitis media: a randomized controlled trial. JAMA. 2006;296(10):1235–41. doi: 10.1001/jama.296.10.1235. [DOI] [PubMed] [Google Scholar]
- 45.McCormick DP, Chonmaitree T, Pittman C, et al. Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics. 2005;115(6):1455–65. doi: 10.1542/peds.2004-1665. [DOI] [PubMed] [Google Scholar]
- 46.Doern GV, Jones RN, Pfaller MA, et al. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY antimicrobial Surveillance Program (United States and Canada, 1997) Antimicrobial Agents Chemother. 1999;43(2):385–9. doi: 10.1128/aac.43.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmu AA, Herva E, Savolainen H, et al. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin Infect Dis. 2004;38(2):234–42. doi: 10.1086/380642. [DOI] [PubMed] [Google Scholar]
- 48.Petersen CG, Ovesen T, Pedersen CB. Acute mastoidectomy in a Danish county from 1977 to 1996 with focus on the bacteriology. Int J Pediatr Otorhinolaryngol. 1998;45(1):21–9. doi: 10.1016/s0165-5876(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 49.Naseri I, Sobol SE. Regional and intracranial complications of acute otitis media. In: Bell LM, editor. Pediatric otolaryngology: the requisites in pediatrics. 1. Vol. 2007. Philadelphia: Mosby, Inc; pp. 105–17. [Google Scholar]
- 50.Ropposch T, Nemetz U, Braun EM, et al. Management of otogenic sigmoid sinus thrombosis. Otol Neurotol. 2011;32(7):1120–3. doi: 10.1097/MAO.0b013e31822a1ec0. [DOI] [PubMed] [Google Scholar]
- 51.Thompson PL, Gilbert RE, Long PF, et al. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the United kingdom general practice research database. Pediatrics. 2009;123(2):424–30. doi: 10.1542/peds.2007-3349. [DOI] [PubMed] [Google Scholar]
- 52.Damoiseaux RA, Rovers MM, Van Balen FA, et al. Long-term prognosis of acute otitis media in infancy: determinants of recurrent acute otitis media and persistent middle ear effusion. Fam Pract. 2006;23(1):40–5. doi: 10.1093/fampra/cmi083. [DOI] [PubMed] [Google Scholar]
- 53.Leach AJ, Morris PS. Antibiotics for the prevention of acute and chronic suppurative otitis media in children. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD004401.pub2. CD004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. [PubMed] [Google Scholar]
- 55.Kogan MD, Overpeck MD, Hoffman HJ, et al. Factors associated with tympanostomy tube insertion among preschool-aged children in the United States. Am J Public Health. 2000;90(2):245–50. doi: 10.2105/ajph.90.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mui S, Rasgon BM, Hilsinger RL, Jr, et al. Tympanostomy tubes for otitis media: quality-of-life improvement for children and parents. Ear Nose Throat J. 2005;84(7):418, 420–2, 424. [PubMed] [Google Scholar]
- 57.Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: tympanostomy tubes in children–executive summary. Otolaryngol Head Neck Surg. 2013;149(1):8–16. doi: 10.1177/0194599813490141. [DOI] [PubMed] [Google Scholar]
- 58.Keyhani S, Kleinman LC, Rothschild M, et al. Overuse of tympanostomy tubes in New York metropolitan area: evidence from five hospital cohort. BMJ. 2008;337:a1607. doi: 10.1136/bmj.a1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Advisory Panel Work Group for Tympanostomy Tubes. Tympanostomy tubes for middle ear effusion of brief duration; Paper presented at: The National Summit on Overuse; Oakbrook Terrace. September 24, 2012. [Google Scholar]
- 60.Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10) doi: 10.1002/14651858.CD001801.pub3. CD001801. [DOI] [PubMed] [Google Scholar]
- 61.Agency for Healthcare Research and Quality (AHRQ) Number 101, otitis media with effusion: comparative effectiveness of treatments, executive summary. Vol. 2013. Rockville (MD): Agency for Healthcare Research and Quality; [PubMed] [Google Scholar]
- 62.Kujala T, Alho OP, Luotonen J, et al. Tympanostomy with and without adenoidectomy for the prevention of recurrences of acute otitis media: a randomized controlled trial. Pediatr Infect Dis J. 2012;31(6):565–9. doi: 10.1097/INF.0b013e318255ddde. [DOI] [PubMed] [Google Scholar]
- 63.Gebhart DE. Tympanostomy tubes in the otitis media prone child. Laryngoscope. 1981;91(6):849–66. doi: 10.1288/00005537-198106000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez C, Arnold JE, Woody EA, et al. Prevention of recurrent acute otitis media: chemoprophylaxis versus tympanostomy tubes. Laryngoscope. 1986;96(12):1330–4. [PubMed] [Google Scholar]
- 65.McDonald S, Langton Hewer CD, Nunez DA. Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD004741.pub2. CD004741. [DOI] [PubMed] [Google Scholar]
- 66.Lous J, Ryborg CT, Thomsen JL. A systematic review of the effect of tympanostomy tubes in children with recurrent acute otitis media. Int J Pediatr Otorhinolaryngol. 2011;75(9):1058–61. doi: 10.1016/j.ijporl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeld RM, Bhaya MH, Bower CM, et al. Impact of tympanostomy tubes on child quality of life. Arch Otolaryngol Head Neck Surg. 2000;126(5):585–92. doi: 10.1001/archotol.126.5.585. [DOI] [PubMed] [Google Scholar]
- 68.Witsell DL, Stewart MG, Monsell EM, et al. The Cooperative Outcomes Group for ENT: a multicenter prospective cohort study on the effectiveness of medical and surgical treatment for patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2005;132(2):171–9. doi: 10.1016/j.otohns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Witsell DL, Stewart MG, Monsell EM, et al. The Cooperative Outcomes Group for ENT: a multicenter prospective cohort study on the outcomes of tympanostomy tubes for children with otitis media. Otolaryngol Head Neck Surg. 2005;132(2):180–8. doi: 10.1016/j.otohns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Pichichero ME. Acute otitis media: part I. Improving diagnostic accuracy. Am Fam Physician. 2000;61(7):2051–6. [PubMed] [Google Scholar]
- 71.Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg. 2001;124(4):374–80. doi: 10.1067/mhn.2001.113941. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann KK, Thompson GK, Burke BL, et al. Anesthetic complications of tympanostomy tube placement in children. Arch Otolaryngol Head Neck Surg. 2002;128(9):1040–3. doi: 10.1001/archotol.128.9.1040. [DOI] [PubMed] [Google Scholar]
- 73.Shah RK, Welborn L, Ashktorab S, et al. Safety and outcomes of outpatient pediatric otolaryngology procedures at an ambulatory surgery center. Laryngoscope. 2008;118(11):1937–40. doi: 10.1097/MLG.0b013e3181817b9a. [DOI] [PubMed] [Google Scholar]
- 74.Dohar J. Microbiology of otorrhea in children with tympanostomy tubes: implications for therapy. Int J Pediatr Otorhinolaryngol. 2003;67(12):1317–23. doi: 10.1016/j.ijporl.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 75.Dohar J, Giles W, Roland P, et al. Topical ciprofloxacin/dexamethasone superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrhea through tympanostomy tubes. Pediatrics. 2006;118(3):e561–9. doi: 10.1542/peds.2005-2033. [DOI] [PubMed] [Google Scholar]
- 76.Goldblatt EL, Dohar J, Nozza RJ, et al. Topical ofloxacin versus systemic amoxicillin/clavulanate in purulent otorrhea in children with tympanostomy tubes. Int J Pediatr Otorhinolaryngol. 1998;46(1–2):91–101. doi: 10.1016/s0165-5876(98)00150-5. [DOI] [PubMed] [Google Scholar]
- 77.Heslop A, Lildholdt T, Gammelgaard N, et al. Topical ciprofloxacin is superior to topical saline and systemic antibiotics in the treatment of tympanostomy tube otorrhea in children: the results of a randomized clinical trial. Laryngoscope. 2010;120(12):2516–20. doi: 10.1002/lary.21015. [DOI] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59(9):253–7. [PubMed] [Google Scholar]
- 79.Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2013-2014. Pediatrics. 2013;132:e1089–104. doi: 10.1542/peds.2013-2377. [DOI] [PubMed] [Google Scholar]
- 80.Block SL, Heikkinen T, Toback SL, et al. The efficacy of live attenuated influenza vaccine against influenza-associated acute otitis media in children. Pediatr Infect Dis J. 2011;30(3):203–7. doi: 10.1097/INF.0b013e3181faac7c. [DOI] [PubMed] [Google Scholar]
- 81.Heikkinen T, Ruuskanen O, Waris M, et al. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145(4):445–8. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 82.Clements DA, Langdon L, Bland C, et al. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149(10):1113–7. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- 83.Marchisio P, Cavagna R, Maspes B, et al. Efficacy of intranasal virosomal influenza vaccine in the prevention of recurrent acute otitis media in children. Clin Infect Dis. 2002;35(2):168–74. doi: 10.1086/341028. [DOI] [PubMed] [Google Scholar]
- 84.Duncan B, Ey J, Holberg CJ, et al. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics. 1993;91(5):867–72. [PubMed] [Google Scholar]
- 85.Scariati PD, Grummer-Strawn LM, Fein SB. A longitudinal analysis of infant morbidity and the extent of breastfeeding in the United States. Pediatrics. 1997;99(6):E5. doi: 10.1542/peds.99.6.e5. [DOI] [PubMed] [Google Scholar]
- 86.Jones LL, Hassanien A, Cook DG, et al. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(1):18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 87.Lubianca Neto JF, Hemb L, Silva DB. Systematic literature review of modifiable risk factors for recurrent acute otitis media in childhood. J Pediatr (Rio J) 2006;82(2):87–96. doi: 10.2223/JPED.1453. [DOI] [PubMed] [Google Scholar]
- 88.Jackson JM, Mourino AP. Pacifier use and otitis media in infants twelve months of age or younger. Pediatr Dent. 1999;21(4):255–60. [PubMed] [Google Scholar]
- 89.Marchisio P, Consonni D, Baggi E, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J. 2013;32:1055–60. doi: 10.1097/INF.0b013e31829be0b0. [DOI] [PubMed] [Google Scholar]
- 90.Elemraid MA, Mackenzie IJ, Fraser WD, et al. Nutritional factors in the pathogenesis of ear disease in children: a systematic review. Ann Trop Paediatr. 2009;29(2):85–99. doi: 10.1179/146532809X440707. [DOI] [PubMed] [Google Scholar]
- 91.Cohen HA, Varsano I, Kahan E, et al. Effectiveness of an herbal preparation containing Echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004;158(3):217–21. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 92.Levi JR, Brody RM, McKee-Cole K, et al. Complementary and alternative medicine for pediatric otitis media. Int J Pediatr Otorhinolaryngol. 2013;77(6):926–31. doi: 10.1016/j.ijporl.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 93.Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101(11):1722–6. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 94.Hatakka K, Savilahti E, Ponka A, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322(7298):1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hatakka K, Blomgren K, Pohjavuori S, et al. Treatment of acute otitis media with probiotics in otitis-prone children-a double-blind, placebo-controlled randomised study. Clin Nutr. 2007;26(3):314–21. doi: 10.1016/j.clnu.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Azarpazhooh A, Limeback H, Lawrence HP, et al. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst Rev. 2011;(11) doi: 10.1002/14651858.CD007095.pub2. CD007095. [DOI] [PubMed] [Google Scholar]
- 97.Sando I, Takahashi H. Otitis media in association with various congenital diseases. Preliminary study. Ann Otol Rhinol Laryngol Suppl. 1990;148:13–6. doi: 10.1177/00034894900990s605. [DOI] [PubMed] [Google Scholar]
- 98.Lyford-Pike S, Hoover-Fong J, Tunkel DE. Otolaryngologic manifestations of skeletal dysplasias in children. Otolaryngol Clin North Am. 2012;45(3):579–98. vii. doi: 10.1016/j.otc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Shott SR, Joseph A, Heithaus D. Hearing loss in children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2001;61(3):199–205. doi: 10.1016/s0165-5876(01)00572-9. [DOI] [PubMed] [Google Scholar]
- 100.Rosen D. Management of obstructive sleep apnea associated with Down syndrome and other craniofacial dysmorphologies. Curr Opin Pulm Med. 2011;17(6):431–6. doi: 10.1097/MCP.0b013e32834ba9c0. [DOI] [PubMed] [Google Scholar]


