Abstract
Background
Understanding the dynamic range for excitatory transmission is a critical component of building a functional circuit diagram for the mammalian brain. Excitatory synaptic transmission is typically studied under optimized conditions, when background activity in the network is low. The range of synaptic function in the presence of inhibitory and excitatory activity within the neocortical circuit is unknown.
Results
Paired-cell recordings from pyramidal neurons in acute brain slices of mouse somatosensory cortex show that excitatory synaptic transmission is markedly suppressed during spontaneous network activity: EPSP amplitudes are two-fold smaller and failure rates are greater than 50%. This suppression is mediated by tonic activation of presynaptic GABAb receptors gated by the spontaneous activity of somatostatin-expressing (Sst) interneurons. Optogenetic suppression of Sst neuron firing was sufficient to enhance EPSP amplitude and reduce failure rates, effects that were fully reversible and occluded by GABAb antagonists.
Conclusions
These data indicate that Sst interneurons can rapidly and reversibly silence excitatory synaptic connections through the regulation of presynaptic release. This is an unanticipated role for Sst interneurons, which have been assigned a role only in fast GABAa-mediated inhibition. Since Sst interneuron activity has been shown to be regulated by sensory and motor input, these results suggest a mechanism by which functional connectivity and synaptic plasticity could be gated in a state-dependent manner.
Introduction
High-resolution anatomical maps will be an essential component for understanding how information flows across neural circuits; however, anatomical analyses will fall short at explaining neural processing without a good understanding of synaptic function across normal variations in brain states, task demands, and experience. Remarkably, the dynamic range for synaptic function in anything but silent network conditions is unknown. For example, how much are synapses changed by excitatory and inhibitory activity across the network? How quickly does this happen, and are modifications reversible? What cell type or circuit regulates synaptic strength? Answering these questions will be critical for predicting circuit output and plasticity.
In the mammalian CNS, synaptic properties have typically been assessed using idealized recording conditions in vitro, where background activity is low and extracellular Ca2+ levels are high to promote neurotransmitter release [1–9]. Although elevated external Ca2+ and network silence have been useful experimental manipulations that facilitate synaptic identification and plasticity, it has been suggested that this approach may inflate estimates of effective synaptic strength between neocortical neurons [1].
Here we show that in the context of network activity and physiological levels of extracellular Ca2+, excitatory synapses between layer 2 (L2) pyramidal neurons are markedly weaker than previous estimates, differences primarily due to the tonic activation of presynaptic GABAb receptors. These receptors have been well-studied at inhibitory synapses, where they act as autoreceptors during high-frequency transmission [10]. GABAb receptors are also present at excitatory terminals, but the conditions under which they are activated during normal network activity have not been determined.
What are the consequences of presynaptic GABAb activation on excitatory synaptic transmission? Depending on the release properties of a given synapse, strong GABAb activation could result in small decrements of synaptic strength [11, 12]. Alternatively, if release probability is very low or the number of anatomical connections is small – such as at neocortical synapses – presynaptic GABAb activation could completely silence synaptic inputs. Because post-synaptic GABAb receptors can change neural excitability and thus the efficacy of extracellular stimulation strength, these questions are best addressed with paired-cell recordings to examine individual connections between neurons. Using this approach, we find that strong GABAb activation is sufficient to completely silence excitatory synapses between L2 pyramidal neurons in barrel cortex, a form of short-term plasticity that is fully reversible.
We show that the spontaneous activity of Sst cells powerfully mediates presynaptic GABAb activation. Although it is well-established that Sst neurons provide fast, GABAa-mediated synaptic input onto the distal dendrites of pyramidal neurons [9, 13, 14] where they are densely wired into the cortical network, with >80% connection probability to nearby pyramidal cells [15]. However, prior studies have not examined their role in mediating slow, GABAb-mediated inhibition. This form of inhibition can persist for 100s of ms – long after fast synaptic transmission has ceased – and is unlikely to be pathway-specific, although its net influence in silencing connections could provide fine-scale control over local subnetworks in the neocortex. Because basal firing rates of Sst neurons are high in awake animals [16–19], these data suggest that neocortical synaptic transmission may exist in a highly suppressed state that can be modulated by the activity of Sst neurons.
Results
Cell-type specific changes in firing during network activity
Levels of network activity in vivo are highly heterogeneous, depending on sleep/wake cycles, attention, movement, and sensory input and thus can be difficult to control and pharmacologically modulate. Instead of measuring synaptic function in vivo, we evaluated excitatory synaptic function between L2 pyramidal cells during spontaneous, recurrent network activity elicited in acute brain slices [20]. The slow oscillation elicited in vitro is similar to that observed in vivo during slow-wave sleep, anesthesia, and quiet wakefulness [21, 22], and consists of short periods of elevated activity – Upstates – separated by longer periods of comparative quiescence, or Downstates. Because the local cellular properties that generate this activity are similar in vivo and in vitro [20, 23, 24], the in vitro preparation has been widely employed to investigate the dynamic interactions between different cell types at a mechanistic level.
The slow oscillation was induced using a modified artificial cerebrospinal fluid (mACSF; Table S1) solution [25]. Although spontaneous spikes in pyramidal cells were still infrequent (Figure 1; <0.02 Hz), overall firing rates of L2 pyramidal neurons in mACSF were on average more than 20x higher than under conventional recording conditions in regular ACSF (rACSF; Table S1) that silence network activity (mACSF/active 0.017±0.007 Hz, n=31 cells vs. rACSF/silent 0.00070±0.0005 Hz n=19 cells; p=0.04).
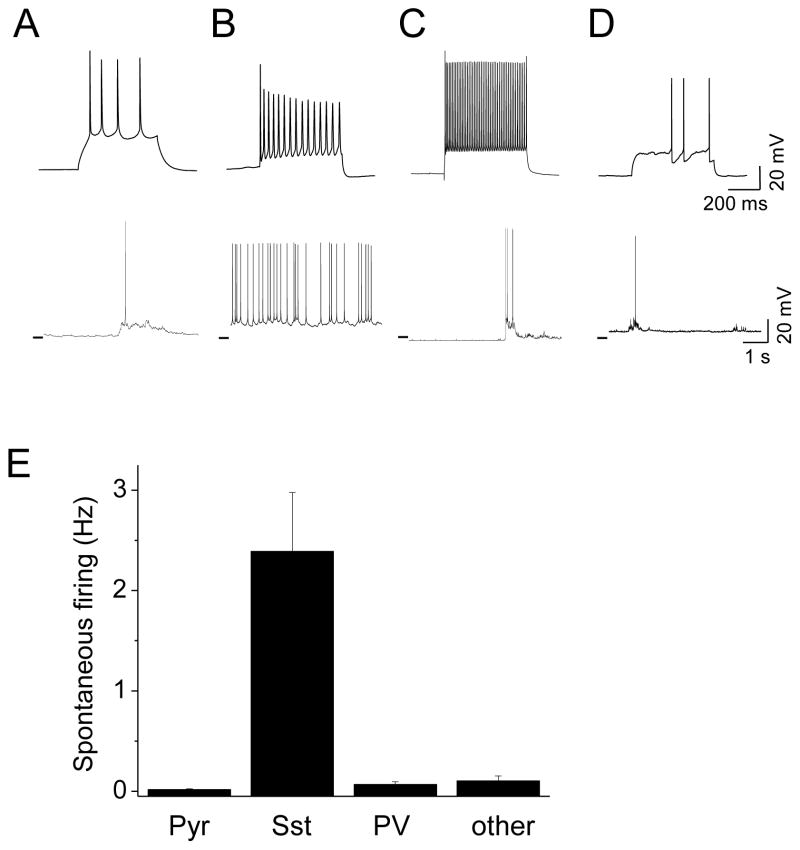
Figure 1. Spontaneous network activity in different types of layer 2/3 neurons of the barrel cortex in vitro.
(A) Top: L2 pyramidal cells firing response to somatic current step injection. Bottom: example trace of pyramidal cell firing in mACSF/active conditions. Horizontal line at left of trace indicates −60 mV for all panels. (B) As in (A) but for LTS/Sst neuron. Bottom trace shows characteristic tonic firing without clear Up- and Downstate transitions. (C) as in (A) but for FS/PV cells. Firing activity is restricted to network Upstates. (D) As in (A) but for non-Pyr, non-LTS/Sst, and non-FS/PV cell in L2/3. Example trace shows spontaneous firing is low and restricted to network Upstates. (E) Overall firing rates calculated across the entire recording period for L2 Pyr, LTS/Sst, FS/PV and other inhibitory cells (n=31, 37, 6, 5 cells respectively).
In contrast to the infrequent spikes observed in excitatory neurons, inhibitory neurons showed significantly higher firing rates during both Up-and also Downstates compared to their activity during conventional recording conditions. L2 inhibitory neurons were divided into 3 groups, identified by differential expression of fluorescent transgenes in Sst-Cre and PV-Cre transgenic mice [26] and by firing response – low-threshold spiking (LTS/Sst), fast-spiking (FS/PV), or non-Sst, non-PV cells characterized by a delayed spike with current injection. Subpopulations of inhibitory neurons exhibited highly divergent firing activity during network activity, similar to what has been observed in vivo and in vitro. Over the recording period (including both Up- and Downstates), we found that Sst neurons exhibited the highest firing rates (Figure 1E; 2.4±0.6 Hz, n=37), similar to those reported for Sst cells in awake, behaving animals. Spontaneous Sst neuron firing frequency exceeded that of other interneuron subtypes by >10-fold (Figure 1E; PV, 0.068±0.02 Hz, n=6; non-Sst and non-PV; 0.1±0.04 Hz, n=5). The firing of non-Sst interneurons was almost entirely restricted to network Upstates (Figure 1C, D), as has also been observed in vivo [17] and in vitro [27, 28].
Although Sst interneurons can be diverse [29] especially across layers, we focused on Sst-Cre L2/3 neurons exhibiting a low-threshold spiking (LTS) phenotype, likely Martinotti cells. Both L2/3 and L5 Sst cells showed high spontaneous firing under our active network conditions (Figure S1A, B). Spontaneous Sst firing was profoundly regulated by the ionic composition of the bath solution, where firing rates fell almost 10-fold when mACSF was replaced with rACSF, likely due to the reduced KCl and increased Mg2+ in this solution (Figure S1C, D; 4.7±1.3 Hz vs. 0.59±0.17 Hz respectively, n=13, p=0.008).
Excitatory synapses are suppressed during network activity
Does high level of inhibition during network activity influence excitatory transmission between pyramidal neurons? To investigate this, pairs of synaptically-coupled L2 pyramidal neurons were identified using whole-cell recording techniques (Figure 2). Initially, basal synaptic function was assessed, comparing EPSP amplitudes and short-term synaptic properties that might be modulated by the presence of spontaneous activity across the network.
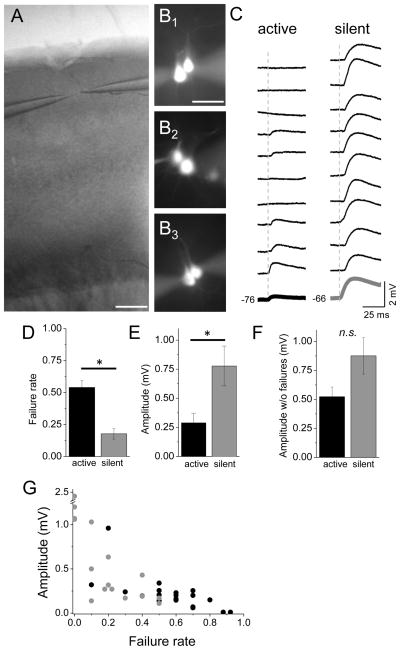
Figure 2. Direct synaptic connections between L2 pyramidal neurons are suppressed by network activity.
(A) A bright field image showing the barrel cortex and the location of patch electrodes in layer 2. Scale bar: 200μm. (B) The location of cell soma for unconnected or connected pyramidal cells was similar. (B1) Two Alexa-fluor- filled, unconnected pyramidal cells. (B2) and (B3) Two pyramidal cells connected unidirectionally and bidirectionally, respectively. (C) Individual response trials for a representative connected pair under active network conditions (left) and for a different connected pair under silent network conditions (right). Bottom trace (bold) is 10-trial average for each connection. (D) Mean failure rates (number of trials without a detectable post-synaptic response) are higher in active networks. (E) Mean EPSP amplitude is smaller in active networks. (F) Mean EPSP amplitude calculated from only successful response trials (i.e., no null responses were included in the average) under the two conditions. All statistical comparisons are with an unpaired, two-tailed t-test. (G) Failure rate and amplitude are highly correlated under both conditions (black, active and gray, silent).
Synaptic connections were identified by generating ten presynaptic spikes with a 50 ms interspike interval at 0.1 Hz for each cell in the pair. Post-synaptic EPSPs were monitored over at least 100 spike trials. For connected pairs, this stimulation frequency did not alter EPSP properties, as responses were stable over the analysis period (5–60+ minutes; data not shown).
For connections recorded under active network conditions, EPSP failures to the first presynaptic spike were high: more than half of presynaptic stimuli failed to elicit a postsynaptic EPSP in connected pairs (Figure 2C, D; failure rate 0.54±0.05, n=20), and for some connections the failure rate was >85%. This was notable, since previous studies have reported near-zero failure rates for neocortical synapses [2, 5], and failure rates were low in silent networks (0.18±0.04, n=18; active vs. silent p<0.00001). Were weak connections missed entirely? This is unlikely, since overall connection probability in active networks was 10.9%, similar to previous reports under silent network conditions [2, 5]. Comparison of connection probabilities observed in active and silent conditions showed that the frequency of identifying connected cells was identical (active 10.7% vs. silent 11.1%, n=149 and 99 connections tested).
EPSP amplitudes were also reduced in active compared to silent conditions (Figure 2E–F; active 0.29±0.08 mV, n=20 vs. silent 0.78±0.17 mV, n=18, p=0.02; n’s different from above connection frequency because of exclusion of the same connection recorded under two different bath conditions). This was due in large part because of the high failure rates, although EPSP amplitude calculated only from successes also showed a reduction in mean amplitude (Figure 2F; active 0.52±0.08 mV, n=20 vs. silent 0.88±0.16 mV, n=18, p=0.06).
Individual synaptic connections were highly variable for amplitude and failure rates (Figure 2G; amplitude could vary 10-fold and failure rates ranged from 0 to >90%), making statistical comparisons across groups difficult. Thus, we compared the same EPSP for a single connection while varying the ACSF composition of the bath solution (Figure S2). Network activity was associated with a significant decrease in EPSP amplitude and increase in failure rates (Figure S2). For all connections under both conditions, EPSP amplitude and failure rate were inversely related (Figure 2G).
Network activity is associated with reduced release probability at excitatory synapses
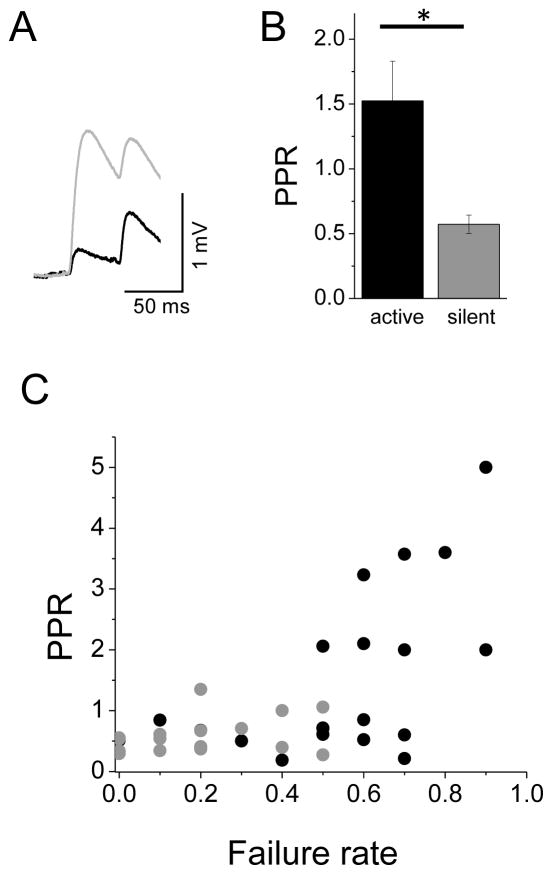
The increase in failure rates suggested that the presence of spontaneous activity in the network might influence presynaptic release probability (Pr), although not via short-term synaptic depression at excitatory synapses, since pyramidal cell firing was <0.01 Hz and spikes in pyramidal neurons were typically isolated (i.e., cells did not burst). The paired-pulse ratio (PPR; amplitude of the 2nd/1st EPSP) is typically used to assess release probability, where synapses with a low Pr will exhibit facilitation. Presynaptic stimuli delivered at 20 Hz showed that synapses between connected L2 pairs in active networks were strongly facilitating, with a PPR of 1.5 (Figure 3A, B). This was significantly different from the PPR in silent networks (PPR=0.6; active n=20 vs. silent n=18; p=0.006), consistent with previous studies showing excitatory synapses in L2 are, on average, depressing [2]. PPR was directly related to failure rate for connections recorded under active conditions (Figure 3C).
Figure 3. Paired-pulse ratio of excitatory synapses changes from depression to facilitation during network activity.
(A) Example traces showing the PPR for the same cell under silent (grey) and active (black) network conditions. (B) PPR shows that excitatory synapses are facilitating under active conditions and depressing under silent conditions. (C) Failure rate and PPR are positively correlated under active conditions (black, active; and gray, silent).
It is well-established that high external Ca2+ levels will enhance Pr. In vivo, free Ca2+ has been estimated to be ~1 mM [1, 30], significantly lower than what has typically been used for most in vitro studies (range 2–3 mM). Although the lower Ca2+ levels used in mACSF are similar to levels found in vivo (Table S1), the difference in Ca2+ levels in active and silent network conditions might explain the difference in PPR observed. Within-cell comparisons of synaptically-connected pairs in rACSF with either 1 or 2.5 mM Ca2+ showed that lowering extracellular Ca2+ did reduce EPSP amplitude (but did not influence Vrest or input resistance (Ri); Table S2). However, simply reducing Ca2+ concentration did not by itself significantly change failure rates, probably because Pr was already quite high (Figure S2 B–D). Recording temperature did not significantly alter synaptic properties in active networks (Figure S2 E–G). Thus, reduced extracellular Ca2+ concentration is not a sufficient explanation for the increased failure rates observed in active networks.
GABAb receptors at excitatory synapses
High levels of inhibition during spontaneous network activity might be involved in reducing EPSP efficacy. Because spontaneous IPSP frequency was ~3 Hz [31], it was unlikely that precisely-timed GABAa-mediated synaptic input had a prominent role in the suppression of the excitatory responses observed. Instead, we hypothesized that presynaptic GABAb receptors might be involved in regulating high failure rates. Presynaptic GABAb receptors are found in many brain areas at both inhibitory and excitatory synapses where they can suppress neurotransmitter release [10] via modulation of Ca2+ channels at the axon terminal [32]. Typically, presynaptic GABAb activation in vitro has required block of GABA reuptake mechanisms or induction of high-frequency bursting of nearby inhibitory neurons to enable sufficient GABA accumulation at the synapse [8, 11]. Despite their well-documented presence at excitatory neocortical synapses, the conditions and cell types that gate GABAb activation during normal network activity remain unclear.
We next determined whether GABAb receptors might be responsible for reduced synaptic efficacy observed in active networks. In the presence of spontaneous network activity, excitatory connections could be almost completely silenced by application of a GABAb agonist (Figure 4 and S3; EPSP amplitude baseline 0.23±0.06 mV to 0.02±0.01 mV in baclofen, p=0.003 for paired comparisons in Figure S3; failure rate baseline 0.56+0.06 to 0.92+0.05 in baclofen, p=0.01 for paired comparisons in Figure S3). For silenced connections, the PPR could not be calculated; however, action potential trains would often show EPSP responses at the end of the stimulus series (Figure 4B, E), indicating that those connections had not been lost during the recording.
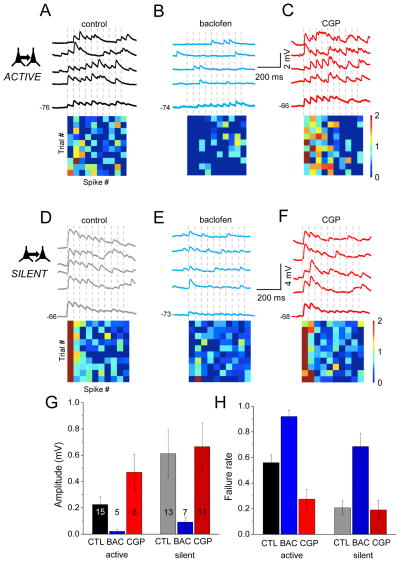
Figure 4. Network activity is associated with tonic GABAb activity.
(A) Individual response trials for a connected pair of L2 pyramidal neurons under active network conditions. Ten presynaptic spikes (dashed vertical lines) at 20 Hz were delivered on each trial. Bold trace shows average of 10 trials. Heatmap at bottom shows response amplitudes for 10 individual trials each with 10 spikes, using a linear scale where red is maximum amplitude. (B) The same cell as in (A) but in baclofen. (C) The same cell as in (A) but in CGP. (D) As in A but for a connected pair isolated in silent network conditions. (E) The same cell as in (D) but in baclofen. (F) The same cell as in (D) but in CGP. (G) High GABAb activity in active states suppresses EPSP amplitude. Mean EPSP amplitude can be increased from baseline under active conditions, but cannot be increased under silent conditions (grey and stippled red bars). Amplitudes were calculated for the first spike in the train, for all conditions. Numbers in bars represent number of cells for each measurement, and are the same for (H). (H) High GABAb activity in active states increases failure rates. Mean failure rates can be up- or downregulated from baseline using GABAb agonists and antagonists under active conditions, but cannot be reduced further under silent conditions (grey and stippled red bars). Failure rates were calculated for the first spike in the train, for all conditions.
With spontaneous network activity, GABAb receptor blockade using CGP-55845 (CGP) significantly enhanced synaptic efficacy: EPSP amplitude was increased more than two-fold to 0.47+0.14 mV (p=0.04 for paired comparisons in Figure S3) and failure rates dropped to 0.28+0.08 (p=0.002 for paired comparisons in Figure S3). CGP reduced the PPR, consistent with a presynaptic effect.
Abundant electron microscopy (EM) and electrophysiological studies show that postsynaptic GABAb receptors are present in neocortical neurons. Immuno-EM indicates that they are localized to the dendritic spine [33], and they can activate a slow hyperpolarizing current of several mV in response to stimulation of GABAergic afferents [6, 34–36]. Postsynaptic GABAb receptors have also been shown to regulate pyramidal cell dendritic Ca2+ spikes in vivo [37, 38], where they act through activation of K+ channels and inhibition of Ca2+ channels [10].
Indeed, CGP application depolarized Vrest of the post-synaptic cell by 3.4±1.03 mV (Figure S4; p=0.005, n=15 cells). Could the effects of CGP in enhancing synaptic transmission be attributed to inhibition of postsynaptic GABAb receptors that depolarize the cell and reduce apparent EPSP amplitude due to changes in the driving force? This was the opposite of what we observed, which was an increase in EPSP amplitude. Thus, we conclude that presynaptic GABAb effects predominate in regulating synaptic transmission between coupled excitatory L2 neurons. These data show that GABAb receptors are tonically activated during network activity, and that GABAb signaling can exert powerful effects on synaptic strength at physiological Ca2+ levels.
Presynaptic GABAb receptors are not activated in silent networks
Are presynaptic GABAb receptors tonically active in silent networks, i.e., conventional recording conditions? We thus examined the effect of CGP on synaptic efficacy in silent slices, predicting that CGP would have no effect without tonic GABAb activation. This was the case; CGP did not change EPSP amplitudes or failure rates compared to baseline, pre-drug conditions (Figure 4D, F, G; EPSP amplitude control 0.61+0.18 to CGP 0.66+0.18 mV; failure rate control 0.21±0.05 to CGP 0.19±0.07). In silent networks, baclofen suppressed EPSP amplitude and increased failure rates (Figure 4E, G; EPSP amplitude baclofen 0.09±0.03 mV; p=0.02 for paired comparisons in Figure S3 and failure rate baclofen 0.68±0.1 p=0.002 for paired comparisons in Figure S3). Once GABAb receptors were blocked, EPSP amplitude and failure rates were indistinguishable from active networks (Figure 4G, H). This occurred despite the fact that external Ca2+ levels were higher in silent network recording conditions, consistent with the conclusion that Ca2+ levels are not the critical factor in differentiating synaptic efficacy between the two conditions.
Postsynaptic GABAb receptors hyperpolarize neurons during network activity
Is network activity associated with postsynaptic GABAb receptor activation? To test this, we examined whether pharmacological manipulation of GABAb receptors could change resting membrane potential of L2 pyramidal neurons. In active networks, there was evidence for tonic activation of postsynaptic GABAb receptors in pyramidal cells, since blocking GABAb receptors by bath application of CGP significantly depolarized Vrest (Figure S4; +3.44±1.03 mV; n=15 cells, p=0.005). In contrast, GABAb receptor blockade had no effect on Vrest in silent networks (−0.24mV±1.38; n=20 cells, p=0.9), suggesting there was no tonic GABAb activation postsynaptically.
Under active network conditions, GABAb activation with baclofen did not alter Vrest in pyramidal neurons (Figure S4; −0.68±0.87 mV; n=10 cells), suggesting that these receptors might be fully activated. In contrast, under silent network conditions, baclofen significantly hyperpolarized Vrest (Figure S4; −5.4±0.8 mV; n=15 cells; p<0.0001). These data indicate that postsynaptic GABAb receptor activity may be fully saturated during some network states, and that under conditions where network activity is negligible there is little tonic activity.
Heterosynaptic GABAb activation from Sst interneurons
What neuron subtype regulates presynaptic GABAb signaling in active networks? High firing rates in Sst cells suggested they might provide a source of GABA that activates this receptor. To test whether acute silencing of Sst neurons could enhance EPSP strength and reliability, the hyperpolarizing proton pump Archaerhodopsin (Arch) [39] was introduced into this cell population, using virus-mediated transduction or transgenic introduction by crossing Sst-Cre with floxed Arch animals (Figure S5). Illuminating tissue with yellow-green light (535 nm, LED) was sufficient to hyperpolarize Sst neurons by 2.1–21.5 mV (Figure S5) and virtually eliminate spontaneous firing during a 1 s light pulse (>100-fold reduction; Figure S5).
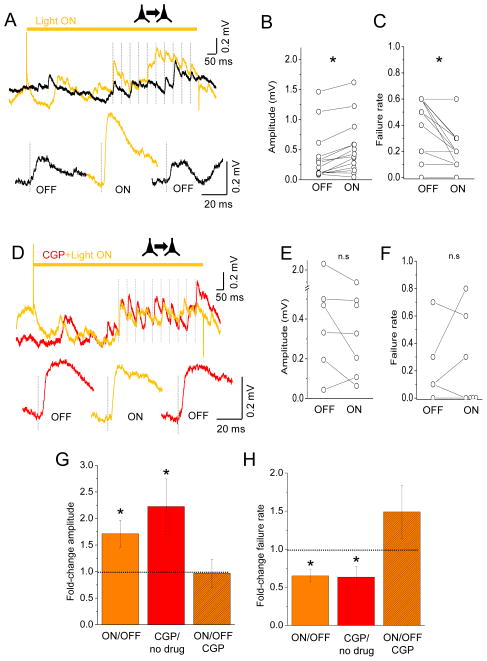
Connected pairs of L2 pyramidal neurons were identified in slices from Sst-Arch transgenic mice, and EPSPs were collected for a short baseline period (~5 min) to calculate amplitude and failure rates. A 1 s light pulse was initiated 500 ms prior to the 20 Hz spike train, in order to allow sufficient time for signaling pathways to extinguish. During light ON trials, EPSP amplitude was significantly increased (Figure 5A, B; mean 1.6-fold increase over baseline, n=14 connections, p=0.0003). EPSP amplitude returned to baseline levels after illumination trials, indicating that this effect was fully reversible. EPSP failure rates were reduced nearly 2-fold during light ON trials, from a baseline failure rate of 0.42±0.05 to 0.25±0.05 during Sst cell silencing (Figure 5C; n=14 connections; p=0.001). Thus, Sst interneuron silencing is sufficient to enhance synaptic transmission between L2 pyramidal neurons, increasing EPSP amplitude and decreasing failure rates.
Figure 5. Somatostatin cell silencing enhances EPSP efficacy by reducing GABAb activation.
(A) Top line (amber) indicates duration of light-activated Sst silencing initiated 500 ms prior to presynaptic spike train. Black trace is EPSP response during baseline/light OFF stimulus trials (10 sweep average); amber trace, the same but during Sst silencing. Dashed vertical lines indicate spikes. Bottom left, EPSP after the first spike (10-sweep average; black) for baseline/light OFF trials; middle, EPSP (10-sweep average; amber) for light ON trials; right, EPSP recovery (10-sweep average; black) for directly following, light OFF trials. (B) Within-cell comparisons for EPSP amplitude for light OFF and light ON trials. (C) As in (B) but for failure rate. (D) Top line (amber) indicates duration of light-activated Sst silencing initiated 500 ms prior to presynaptic spike train. Red trace is EPSP response after CGP application during baseline/light OFF stimulus trials (10 sweep average); amber trace, the same but during Sst silencing, right, EPSP recovery (10-sweep average; red) for directly following, light OFF trials. (E) Within-cell comparisons for EPSP amplitude in CGP for light OFF and light ON trials. (F) As in (B) but for failure rate. (G) Change in amplitude during light ON versus OFF trials, with CGP versus baseline/no drug trials, and for light ON versus light OFF trials in CGP. (H) As in (G) but for failure rate. All statistical comparisons by two-tailed paired t-test.
Sst interneurons influences EPSP efficacy through GABAb receptors
To test whether the effects of Sst interneurons silencing were mediated in part or entirely by the activation of GABAb receptors, we investigated whether EPSP efficacy could be changed when GABAb receptors were pharmacologically blocked by CGP. An increase in EPSP amplitude under these conditions might suggest that GABAa currents, mediated through direct synaptic input from Sst to pyramidal neurons, might be shunting excitation and reducing measured EPSP amplitude during somatic recordings. Importantly, Sst-silencing did not increase EPSP amplitude or decrease failure rates in CGP, indicating that this effect was fully mediated by GABAb receptors (Figure 5D–F). Overall, Sst-silencing and CGP application resulted in similar changes in EPSP amplitude (Figure 5G).
Both Sst-silencing alone and CGP application also reduced failure rates (Figure 5C, H), further supporting the conclusion that Sst silencing affects EPSP efficacy primarily through presynaptic GABAb receptors. We note that the assessment of failure rates in CGP was difficult to calculate; because these values frequently fell to near zero when GABAb receptors were blocked, there was no room for further reduction during Sst silencing.
Sst silencing might change tonic GABAa currents, which provide a significant Cl− current that can shunt excitatory input or hyperpolarize neocortical neurons. Thus, eliminating GABAa currents could increase EPSP amplitude by decreasing shunting inhibition. This is unlikely to explain the observed effects for several reasons. First, post-synaptic resting membrane potential was typically at or around ECl− (−82 mV) reducing the overall effect of Cl− currents in our recordings (although local shunting remains possible). Second, to isolate the effect of Sst silencing on GABAb receptors, in a subset of experiments GABAa currents were blocked by including DNDS in the intracellular recording solution, a compound that blocks >90% of GABAa mediated currents from the intracellular face of the channel [40]. Consistent with the blockade of GABAa receptor channels by DNDS, Ri did not change during Sst silencing in these experiments, a phenomenon that might occur if both synaptic and tonic GABAa inhibition were suddenly eliminated (Ri light OFF 133 MΩ vs. light ON 129 MΩ; n=15 cells, p=0.15). In summary, silencing of Sst neurons can enhance EPSP efficacy within 500 ms, and these effects were fully attributable to the activation of GABAb receptors.
Discussion
Here we show that spontaneous Sst firing activates presynaptic GABAb receptors at excitatory pyramidal cell synapses in the neocortex, profoundly suppressing transmission and, in many cases, effectively silencing synapses (Figure 6). Optogenetic suppression of Sst neuron activity was sufficient to reduce synaptic failure rates and enhance mean EPSP amplitude, an effect that was fully reversible and occluded by GABAb antagonists. Although previous studies have indicated that presynaptic GABAb receptors are ubiquitous at cortical synapses, the endogenous conditions under which these receptors are activated have been obscure. These data not only show that network activity is sufficient to activate presynaptic GABAb receptors, but identify an unanticipated role for Sst neurons in regulating presynaptic release through these receptors, where they act as local neuromodulators that can reversibly silence synaptic connections.
Figure 6. Excitatory synaptic transmission is regulated by Sst neurons via presynaptic GABAb receptors.
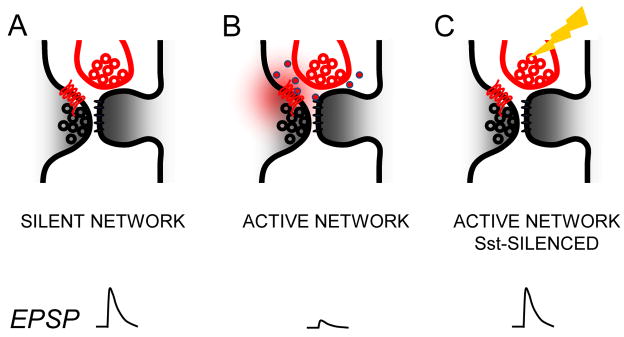
(A) GABAb receptor (red 7-transmembrane line) activation is negligible in silent networks and EPSPs between layer 2 pyramidal neurons are large. Presynaptic pyramidal neuron (left; black) and Sst interneuron (top; red) are depicted as synapsing onto nearby regions of a dendritic spine, although effects may not be spatially restricted to a single spine. (B) Spontaneous activity of Sst neurons in active networks leads to GABA release (red dots; GABA released into extracellular space) driving tonic GABAb presynaptic receptor activation (red cloud) to reduce EPSP efficacy. (C) Optogenetic silencing of Sst neurons by Arch (yellow lightning) under active network conditions is sufficient to increase EPSP efficacy by reducing GABAb receptor activity.
Other interneurons that activate GABAb signaling
Our data do not exclude the possibility that other inhibitory neuron subtypes could also regulate excitatory synaptic transmission through GABAb receptors. We cannot rule out the possibility that PV cell firing, could under some conditions, activate presynaptic GABAb receptors, although prior studies have been unsuccessful at inducing this effect [6, 12], and the axons from these cells are typically localized to the soma, far from the distal synapses where pyramidal-pyramidal cell synapses are found. In addition, this class of interneurons was relatively inactive under our recording conditions suggesting that they do not regulate the phenomenon described here. Neurogliaform (NGF) cells have been implicated in both pre- and post-synaptic GABAb activation at excitatory synapses [6, 12, 36, 41], although their activation yielded only modest reductions in synaptic strength. Additional studies to identify the specific network states associated with NGF-driven GABAb activation will be required.
Do network states and sensory input regulate the activity of Sst cells in vivo? Suppression of Sst firing has been described in a number of behavioral states or during learning [17, 42, 43], providing an immediate context for interpreting these results. For example, Sst neuron activity can be suppressed by sensory stimulation [17] or motor input through the inhibitory action of synaptically-coupled VIP-expressing interneurons [43, 44]. Our findings provoke specific hypotheses about the role of Sst firing in regulating information flow across the network. For example, we predict that Sst cells can regulate the sparseness of layer 2 firing during tasks that engage attention or invoke reward, and may gate plasticity induction by revealing silent connections.
GABAb activation at excitatory synapses via spillover
Because axo-axonic synapses in the neocortex are rare (and are typically confined to parvalbumin-expressing chandelier cells that innervate the axon initial segment but see [45]), we hypothesize that Sst-mediated GABAb activation occurs via GABA spillover to nearby excitatory synapses. Indeed, inputs from Sst neurons are anatomically close to excitatory synapses at the distal dendrites [14, 46–48] where L2 neurons synapse with each other [2]. Previous studies have shown that under some conditions, GABA spillover from nearby inhibitory interneurons can activate postsynaptic GABAb receptors [8], and that it can heterosynaptically activate presynaptic GABAb receptors at hippocampal mossy fiber synapses [49]. Thus, we propose a mechanism by which Sst-mediated GABA release can activate presynaptic GABAb receptors at nearby excitatory synapses (Figure 6). Because at least one class of Sst neurons in layer 2/3, Martinotti cells, elaborate axons in layer 1 of the neocortex [29] where L5 dendrites branch extensively, these data suggest that Sst-mediated presynaptic GABAb activation may be poised to regulate excitatory transmission at other synapse types within the neocortex.
Sst neuron firing rates varied between 2–10 Hz when network activity was enabled in vitro, a frequency that is close to observed in vivo firing rates [17]. Interestingly, although these neurons can sometimes be coupled via gap junctions, we did not observe correlated firing across coupled neurons under active network conditions (data not shown). Thus, we predict that Sst spikes are tiled across time, a mechanism that could increase ambient GABA to trigger GABAb receptor activation. Because neuromodulators such as acetylcholine can increase Sst firing [50, 51], we predict that some brain states may further suppress excitatory synaptic transmission between neocortical neurons. Although Sst interneurons can fire independently of synaptic activity [51], they can also be driven by input from as few as 4 pyramidal neurons and then powerfully suppress activity in the column via GABAa mechanisms [52]. These two regimes of Sst activity will inhibit network activity at two different timescales: a few 10s of ms for fast GABAa feedback inhibition that may be synapse-specific [47], and 100s of ms for slow GABAb, synapse non-specific inhibition.
Sst interneurons and postsynaptic GABAb receptors
Although our pharmacological experiments confirm the presence of postsynaptic GABAb receptors in L2 pyramidal neurons, we noted that Sst silencing had no clear effect on postsynaptic resting membrane potential. This might be explained by a longer duration of downstream target modulation in the dendrite compared to presynaptic GABAb targets, which are predominantly Ca2+ channels, or a lower affinity of post-synaptic receptors for ambient GABA. These data may further uncouple the roles of pre- and postsynaptic GABAb receptors across different network states [53, 54]. Future experiments will be required to determine how Sst activity can regulate postsynaptic GABAb receptors and dendritic excitability [37].
Presynaptic GABAb receptor activation can silence synapses
Our data suggest a specific signaling pathway by which excitatory synaptic transmission can be controlled by Sst activity via the activation of presynaptic GABAb receptors. These effects were profound: strong GABAb activation was sufficient to completely silence excitatory neurotransmission (>95% failure rates), especially for EPSPs elicited by a single presynaptic spike.
Although a train of presynaptic spikes would frequently reveal an EPSP response for later spikes – confirming that the connection had not been lost – the low firing rates of pyramidal neurons especially in superficial layers [55] suggests that this facilitation is unlikely to occur under normal conditions. The high failure rates observed under these conditions of elevated network activity may be substantially different than previous reports, in part because the high levels of Ca2+ employed previously have occluded the profound effect of presynaptic GABAb activation.
Silent synapses have typically been defined as NMDAR-only synapses, and may provide a substrate for synaptic potentiation to generate functional synaptic connections based on coordinated pre- and post-synaptic activity. GABAb-mediated synaptic silencing is likely to have a very different purpose: it can be rapidly activated or reversed based on the population activity of Sst neurons, and will effectively rewire networks at much faster timescales than are typically associated with postsynaptically-generated synaptic potentiation. The current data may be of interest in interpreting recent findings that input from superficial layers does not modulate the firing of layer 5 neurons in quiet awake animals [56], despite estimates that approximately 10% of L2 neurons are connected to layer 5 [5]. In addition, because almost all models of long-lasting synaptic strengthening require coincident pre- and post-synaptic activity, we predict that GABAb-mediated synaptic silencing will play a critical role in gating synaptic plasticity. Previous studies have shown that transgenic mice deficient in presynaptic GABAb receptors have impaired synaptic plasticity and memory deficits [33], and it will be interesting to examine whether selective modulation of presynaptic GABAb receptors by Sst activity can regulate synaptic plasticity in vitro and in vivo.
Anatomical vs. functional connectivity
These data help establish the dynamic range for synaptic function for excitatory synapses in the neocortex, with a focus on L2. Previous studies have characterized the anatomical and electrophysiological properties of synapses between these neurons under silent network conditions [2, 5, 31], providing an excellent context to interpret the current data. For example, connected L2 neurons have been shown to be connected by 2–4 anatomical synapses, as assessed by biocytin-post-hoc reconstructions [2]. Regardless of extracellular Ca2+ levels, we found that mean failures rates in the absence of spontaneous network activity were low (~20%), mean EPSP amplitudes were similar (0.2–2.5 mV), and connectivity was ~10%, all in accordance with previous reports.
Because the number of anatomically verified synapses between connected cells is small, the effect of GABAb release suppression is poised to have profound consequences, completely silencing connections in many cases. Although prior studies have provided evidence for GABAb-modulation of release, the effects were typically small, incrementally reducing EPSP amplitude [6, 12]. The use of paired-cell recordings in the current study has enabled us to precisely evaluate the consequences of GABAb activation for functional connectivity across layer 2 neurons. If neurons are connected by a large number of synapses with high Pr, presynaptic GABAb activation may have a smaller contribution in changing the wiring diagram of neocortical circuits.
We predict that the effect of GABAb-mediated synaptic silencing on information flow through the cortical network will be pronounced, effectively rewiring excitatory neural circuits so that only the strongest connections will be maintained. These data are relevant for understanding how Sst cells can regulate the output of neocortical circuits under different network states, as well as in elucidating the requirements for plasticity in vivo. Indeed, it remains unknown under what conditions somatostatin is released from Sst interneurons, and how this peptide can influence synaptic transmission.
The analysis of synaptic function in active networks, under different brain states, is likely to elucidate the role of many different neuromodulators in the control of synaptic efficacy in vivo. We anticipate that these studies will lead the way for an evaluation of many more factors in regulating information flow across synapses under dynamic activity conditions.
Experimental Procedures
Animals
Mice were wild-type C57Bl6 mice (Harlan), Sst-Cre or Pvalb-2A-Cre mice crossed to either Ai14 (floxed-Tdt) reporter mice or Ai35D (floxed-Arch).
Brain slice preparation
Experiments were performed in mice aged P12–P21, where P0 indicates the day of birth. Brain slices (350 μm thick) were prepared by an “across-row” protocol in which the anterior end of the brain was cut along a 45° plane toward the midline [3].
Whole-cell recording
Recordings were carried out as previously described [31]. Recordings were performed in 3 ACSF solutions that differed only by concentrations of Mg2+, Ca2+, and K+. Ionic concentrations were as follows (in mM): mACSF – 0.5 MgSO4, 1 CaCl2, 3.5 KCl; rACSF – 1.3 MgSO4, 2.5 CaCl2, 2.5 KCl; and low-Ca rACSF – 1.3 MgSO4, 1 CaCl2, 2.5 KCl.
Neuron classification
Neurons were classified as pyramidal neurons according to pyramidal-like soma shape, the presence of an apical dendrite and spines visible after Alexa filling reconstruction and regular-spiking phenotype. Inhibitory neurons were identified either by fluorescent gene expression or firing phenotype. Apparent Sst cells identified by reporter expression exhibiting FS firing patterns were excluded [57].
Connectivity analysis
EPSP properties were evaluated for cells only with normal Vrest of the post-synaptic cell <−55 mV and Ri was >200 MΩ. Because recurrent activity in network Upstates made EPSP identification difficult, only responses collected during Downstates were evaluated.
Pharmacology
The GABAb receptor agonist baclofen (10 μM, Sigma) or antagonist CGP 55845 (1μM, Tocris) were bath applied for at least 10 minutes before data acquisition. Typically, either baclofen or CGP was applied, although in a subset of experiments both drugs were applied in sequence, where baclofen was followed by CGP, since the effects of CGP did not wash out.
Virus injection and optical stimulation
In all but a small number of cases (where Arch was virally transduced; see expanded Methods), Sst-IRES-Cre homozygous mice were crossed with homozygous Ai35D mice carrying a floxed Arch-GFP transgene for Sst-cell silencing. Photo stimulation was produced by a light-emitting diode (white LED with 535 nm 41002 HQ filter, set to maximum range, Prizmatix, Israel) and delivered through a 40x water-immersion objective. Sst silencing was initiated 500 ms prior to the 10 pulse presynaptic train. Trials were delivered at 0.1 Hz, and at least 20 baseline (light-OFF) trials were collected before initiating light-ON trials. CGP was applied for at least 10 minutes before assessing EPSP properties. Stimuli were not delivered during drug wash-on.
Supplementary Material
Highlights.
Excitatory transmission is suppressed in active neocortical networks
Tonic GABAb receptor activation with spontaneous somatostatin cell firing
Silencing somatostatin neuron firing enhances EPSP efficacy
Acknowledgments
Special thanks to Joanne Steinmiller for expert animal care, and members of the Barth lab for helpful comments on the manuscript. Work was supported by the McKnight Foundation and NIH DA0171-88 to ALB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borst JG. The low synaptic release probability in vivo. Trends in neurosciences. 2010;33:259–266. doi: 10.1016/j.tins.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- 4.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan BX, Dong Y, Ito W, Yanagawa Y, Shigemoto R, Morozov A. Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron. 2009;61:917–929. doi: 10.1016/j.neuron.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 9.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Chalifoux JR, Carter AG. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacson JS, Hille B. GABA(B)-mediated presynaptic inhibition of excitatory transmission and synaptic vesicle dynamics in cultured hippocampal neurons. Neuron. 1997;18:143–152. doi: 10.1016/s0896-6273(01)80053-2. [DOI] [PubMed] [Google Scholar]
- 12.Logie C, Bagetta V, Bracci E. Presynaptic control of corticostriatal synapses by endogenous GABA. J Neurosci. 2013;33:15425–15431. doi: 10.1523/JNEUROSCI.2304-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. The Journal of physiology. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2013;19:228–237. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 18.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 21.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 23.Beltramo R, D’Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat Neurosci. 2013;16:227–234. doi: 10.1038/nn.3306. [DOI] [PubMed] [Google Scholar]
- 24.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. Journal of neurophysiology. 2010;104:596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahvildari B, Wolfel M, Duque A, McCormick DA. Selective functional interactions between excitatory and inhibitory cortical neurons and differential contribution to persistent activity of the slow oscillation. J Neurosci. 2012;32:12165–12179. doi: 10.1523/JNEUROSCI.1181-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somjen GG. Ions in the brain: Normal function, seizures, and stroke. Oxford University Press; 2004. [Google Scholar]
- 31.Yassin L, Benedetti BL, Jouhanneau JS, Wen JA, Poulet JFA, Barth AL. An embedded subnetwork of highly active neurons in the neocortex. Neuron. 2010;68:1043–1050. doi: 10.1016/j.neuron.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 33.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. The Journal of physiology. 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald AM, Doiron B, Rinzel J, Reyes AD. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J Neurosci. 2009;29:10321–10334. doi: 10.1523/JNEUROSCI.1703-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. J Neurophysiol. 1996;75:2167–2173. doi: 10.1152/jn.1996.75.5.2167. [DOI] [PubMed] [Google Scholar]
- 41.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 42.Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonchar Y, Turney S, Price JL, Burkhalter A. Axo-axonic synapses formed by somatostatin-expressing GABAergic neurons in rat and monkey visual cortex. J Comp Neurol. 2002;443:1–14. doi: 10.1002/cne.1425. [DOI] [PubMed] [Google Scholar]
- 46.Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 49.Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, Brauner-Osborne H, Shigemoto R, Kretz O, Frotscher M, et al. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 51.Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger TK, Silberberg G, Perin R, Markram H. Brief bursts self-inhibit and correlate the pyramidal network. PLoS biology. 2010:8. doi: 10.1371/journal.pbio.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity. The Journal of physiology. 2013;591:835–843. doi: 10.1113/jphysiol.2012.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Neubauer FB, Luscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. European Journal of Neuroscience. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- 55.Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu H, Cavendish JZ, Agmon A. Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits. 7:195. doi: 10.3389/fncir.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.