Abstract
Background
In rodent and human studies, ethanol (EtOH) exposure is associated with elevated brain levels of the magnetic resonance spectroscopy (MRS) signal representing choline-containing compounds (Cho). One interpretation of elevated brain Cho is that it is a marker of neuroinflammation, and some evidence suggests that EtOH exposure promotes neuroinflammation. This study aimed to determine whether binge EtOH exposure (intragastric 3 g/kg 25% EtOH every 8 hours for 4 days) would induce the expression of certain cytokines in blood, liver, or brain, thereby supporting the neuroinflammation hypothesis of elevated Cho.
Methods
Ten of 18 wild-type male Wistar rats (~322 g at baseline) were exposed to EtOH and attained average blood alcohol levels of ~315 mg/dl across 4 days. Blood for cytokine immunoassays was collected at baseline, after 5 doses of EtOH (binge), and immediately preceding euthanasia either 4 or 24 hours after the last dose of EtOH. Blood was additionally assayed for the levels of thiamine and liver enzymes; liver histopathology was performed postmortem; and tissue from liver and 6 brain regions was assayed for the potential induction of 7 cytokines.
Results
There were no group effects on the levels of thiamine or its phosphate derivatives, thiamine monophosphate or thiamine diphosphate. ANOVAs of liver enzyme levels indicated that only alkaline phosphatase (ALP) levels were higher in the EtOH group than in control group at binge; ALP elevations, however, are difficult to explain in the absence of changes in the levels of additional liver enzymes. Postmortem liver pathology provided evidence for minimal microvesicular lipidosis and portocentric fibrosis in the EtOH group. Group effects on the levels of the measured cytokines in the blood (TNF-α, IFN-γ, IL-1β, IL-4, IL-5, IL-13, and GRO/CXCL1) were not significant. Similarly, postmortem evaluation of liver cytokines did not reveal group effects. Postmortem evaluation of the 7 cytokines in 6 brain regions (anterior cerebellar vermis, cingulate cortex, frontal cortex, hippocampus, hypothalamus, striatum) also failed to identify group effects.
Conclusions
A single 4-day bout of binge EtOH exposure alone was insufficient to induce the expression of 7 cytokines in blood, liver, or 6 brain regions of wild-type Wistar rats. Alternative interpretations for elevations in brain Cho in response to a 4-day binge EtOH treatment are therefore necessary and may include induction of cytokines not measured herein or other non-inflammatory mechanisms.
Keywords: Neuroinflammation, Thiamine, Liver, TNF-α, Withdrawal
IN HUMAN AND rodent studies, recent high-level ethanol (EtOH) exposure is associated with elevated brain levels of the magnetic resonance spectroscopy (MRS) signal representing choline-containing compounds (Cho). Studies using in vivo MRS have reported that Cho levels are higher in the parietal gray matter of alcohol-dependent individuals compared with controls (Meyerhoff et al., 2004), and high Cho levels in the frontal cortex correlate with the amount of alcohol consumed by social drinkers (Ende et al., 2006). Similarly, wild-type rats exposed to EtOH as their sole fluid source for 16 weeks (Lee et al., 2001), to escalating doses of EtOH via vapor inhalation (Zahr et al., 2009), and to 4 days of binge EtOH via oral gavage (Zahr et al., 2010) demonstrate significant elevations in brain Cho. As elevated brain Cho is observed in animal models of acute experimental allergic encephalomyelitis (Brenner et al., 1993) and in the acute inflammatory stage of multiple sclerosis (De Stefano et al., 2005; Tartaglia et al., 2002; Van Au Duong et al., 2007), a posited interpretation for elevated brain Cho in response to EtOH is neuroinflammation.
The hallmark of neuroinflammation is the activation of microglial cells, which may then release pro-inflammatory factors, including cytokines such as tumor necrosis factor (TNF-α) (Kreutzberg, 1996). Cytokines are polypeptides and glycoproteins that form a key part of a complex network that regulate immune and inflammatory responses (Gallin and Snyderman, 1999). Cytokines including TNF-α, interferons (IFN), interleukins (IL), and growth-regulated oncogenes (GRO, also known as chemokine C-X-C motif ligand 1: CXCL1) are mediators that are induced during an immune response.
Although alcohol exposure can modulate the human immune system (e.g., McClain and Cohen, 1989; Szabo et al., 1996), it is not obvious that cytokine induction occurs in the absence of alcoholic liver disease (e.g., Jarvelainen et al., 1999; McClain et al., 2004; Tilg et al., 2003) or thiamine deficiency (Hazell and Butterworth, 2009), known concomitants of severe and chronic alcoholism. Brain tissue recovered from chronic alcoholics compared with tissue from healthy donors was found to express higher levels of the cytokine monocyte chemoattractant protein-1 (MCP-1, also known as chemokine C-C motif ligand 2: CCL2) in the ventral tegmental area (VTA), substantia nigra, hippocampus, and amygdala and to have higher microglial activation in the cingulate cortex, VTA, and midbrain (He and Crews, 2008); in 4 of the 8 alcoholic brains analyzed, however, cause of death involved complications related to liver disease (He and Crews, 2008). The potential relevance of liver pathology to human alcohol studies requires acknowledgment because nearly every stage in the transition from steatosis to cirrhosis is associated with an inflammatory component and cytokine response (e.g., Bode and Bode, 2005; McMullen et al., 2005; Valles et al., 2003). Confirming this relation, knock-out mice lacking the TNF-α receptor are resistant to the effects of chronic EtOH in that they fail to develop steatosis, inflammation, or necrosis of the liver (Yin et al., 1999).
Thiamine deficiency is another potential attendant of chronic alcoholism (Harper, 2006) and may also contribute to neuroinflammation (Hazell and Butterworth, 2009). The levels of thiamine and its phosphate derivatives, thiamine monophosphate (TMP) and thiamine diphosphate (TDP), can be low in human alcoholics, even without a history of Wernicke’s Encephalopathy (Mancinelli et al., 2003; Tallaksen et al., 1992a,b). Animal models of thiamine deficiency have revealed reactive gliosis (Karuppagounder et al., 2007; Todd and Butterworth, 1999) and increases in the levels of pro-inflammatory markers such as TNF-α (Karuppagounder et al., 2007; Vemuganti et al., 2006). Thus, experimental EtOH models seeking evidence of inflammatory processes should also assess thiamine status.
While in vitro studies indicate that EtOH can modify microglial function (e.g., Choi et al., 2005; Fernandez-Lizarbe et al., 2009; Lee et al., 2004), there is a scarcity of data regarding the in vivo effects of EtOH, especially on the brain’s immune response. In in vivo animal models of EtOH intoxication, challenge with the bacterial toll-like receptor ligand endotoxin lipopolysaccharide (LPS) is often necessary to achieve a robust immune response (e.g., Jarvelainen et al., 1999; Qin et al., 2008). Alternatively, intermittent EtOH exposure may be necessary to observe neuroinflammation. For example, intermittent compared with continuous EtOH exposure increased the number of microglia in the cerebellar vermis of wild-type male Wistar rats (Riikonen et al., 2002), and intermittent compared with no EtOH exposure increased microglial activation as indicated by elevated OX-6 staining in the dentate gyrus of the wild-type female adolescent Wistar rat hippocampus (Ward et al., 2009). Cyclooxygenase-2 (COX-2), another inflammatory mediator, is upregulated in the wild-type male adolescent Wistar rat neocortex, hippocampus, and cerebellum following exposure to 3 cycles of 2 days with EtOH (3 g/kg) followed by 2 days without EtOH administration (Pascual et al., 2007). This pattern of findings suggests a significant role of repeated EtOH withdrawals in promoting inflammation. Exceptions to such examples include the reporting of elevations in COX-2 in various brain regions of the adult male Sprague–Dawley rat following a 4-day binge EtOH exposure as used herein (but see Knapp and Crews, 1999; Valles et al., 2004).
To provide evidence that elevations in brain Cho are associated with EtOH-induced brain cytokine induction independent of repeated bouts of withdrawal, biochemical signs of liver pathology, or thiamine deficiency, the levels of TNF-α, IFN-γ, IL-1β, IL-4, IL-5, IL-13, and GRO/CXCL1 were determined in serum, liver, and brain samples from adult wild-type Wistar rats exposed to the same 4 days of binge EtOH treatment via oral gavage used for the MRS studies. Of these 7 cytokines, 4 (i.e., TNF-α, IFN-γ, IL-1β, IL-4) have previously been explored in the alcohol literature. Liver pathology and thiamine deficiency were investigated contemporaneously for potential discounting of these factors as contributors to any alcohol-exposure effect detected.
METHODS
Animals
We examined 18 wild-type male Wistar rats (Charles River Laboratories, Hollister, CA) that were singly housed with free access to food and water until the experiment began. Rats weighed 322.78 ±2.14 g (range = 312.0 to 339.5 g) at the beginning of the experiment. The Institutional Animal Care and Use Committees at SRI International and Stanford University approved all procedures.
Ethanol Treatment
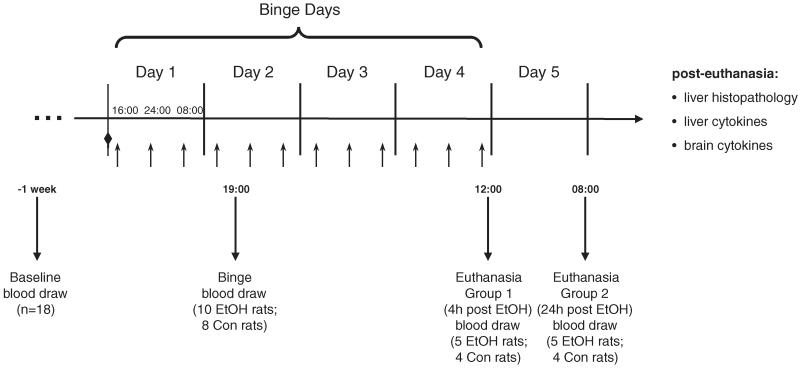
Figure 1 presents the timeline of procedures. The EtOH group comprised 10 animals, randomly selected from the full group of 18 rats. The EtOH rats received an initial dose of 5 g/kg 25% EtOH w/v (in deionized water) via oral gavage (at 14:00 on day 1), then a maximum of 3 g/kg every 8 hour for 4 days (average dose: 3.07 ± 0.10 g/kg; Fig. 1). Control (Con) animals (N = 8 rats) received a similar volume (2cc’s) of 5% dextrose, and treatments occurred at comparable times to the EtOH rats: ~16:00, 24:00, and 8:00 hours. On each of the 4 EtOH dosing days, animals were weighed and blood samples (50 μl) were taken from a tail vein (3 times per day preceding EtOH or dextrose dosing) to determine blood alcohol levels (BALs) in plasma assayed for alcohol content based on direct reaction with the enzyme alcohol oxidase (Analox Instruments Ltd., London, UK). The control animals were treated the same as the EtOH animals even though BALs were understood to be 0. EtOH was administered according to body weight, BALs, and behavioral intoxication state assessed using the modified Majchrowicz 6-point scale (range 0 to 5, Majchrowicz, 1975; Zahr et al., 2010). To maintain similar weights between animals in the EtOH and Con groups, food was restricted to a maximum of 2 pellets (Certified Rodent Diet; LabDiet, Richmond, IN) per animal per day. Wild-type Wistar rats eat ~5% of their body weight each day (Technical Bulletin, Charles River Laboratories, 1999). For a 325 -g rat, that equals 16.25 g per day. Certified Rodent Diet rat chow pellets weigh ~4.4 g each, meaning the average Wistar rat eats approximately 3.7 pellets a day. Experience from earlier experiments (e.g., Zahr et al., 2010) indicated that binge EtOH-treated animals typically consume no more than 2 pellets per day and therefore lose weight. In an effort to weight-yoke the 2 groups of animals, both the EtOH and Con animals were limited to 2 food pellets per day. At the end of 4 days of treatment, the EtOH animals had received a total average cumulative EtOH dose of 39.9 ± 1.3 g/kg per animal, had average BALs of 314.93 ± 16.42 mg/dl, and reached peak BALs of 421.33 ± 15.04 mg/dl.
Fig. 1.
Time course of experiment: downward arrows indicate time-points at which blood was drawn for thiamine, liver enzyme, and cytokine analysis. Diamond indicates loading dose (at 14:00 on day 1). Upward arrows indicate 3 time-points per day (exact times for first day included; times consistent across 4 days) during which animals received treatment (i.e., 3 g/kg 25% EtOH or 5% dextrose).
Blood Collection
A total of ~2 ml of whole blood for the various assays was collected via retro-orbital bleed at 3 time-points (Fig. 1): baseline (left eye, 1 week prior to beginning EtOH dosing), after 5 doses of EtOH (binge, right eye, at 19:00 on day 2), and at euthanasia (left eye). Half of the animals from the EtOH group (n = 5) and half from the Con group (n = 4) were euthanized 4 hours after the last dose of EtOH (early euthanasia, at 12:00 on day 4), and the remaining half of each group was euthanized 24 hours after the last dose of EtOH (late euthanasia, at 08:00 on day 5) to determine whether 24 hours of abstinence would result in changes different from those achieved by continuous EtOH exposure.
For the thiamine assay, 1.5 of the 2 ml of whole blood was collected in EDTA (ethylenediaminetetraacetic acid) tubes and transferred to 15-ml conical centrifuge tubes, to which 10 ml normal saline was added. The suspension was mixed gently and then centrifuged at 164×g at 2 to 8°C for 10 minutes. The supernatant was then discarded with care to prevent disturbing red blood cells (RBCs). This saline wash was repeated 2 more times. After the third wash, 3 to 5 ml of saline was left with the RBCs in the tubes. The tubes were wrapped in aluminum foil to protect from light and shipped to Ani-Lyitics Inc. (Gaithersburg, MD) cold but not frozen for the measurement of thiamine and its phosphate derivatives. Baseline bloods were shipped immediately, binge bloods remained at 4°C for 2 days prior to shipment, and the early euthanasia bloods were stored at 4°C for 24 hour and shipped with the late euthanasia bloods.
The remainder of the whole blood (~0.5 ml) was centrifuged at 2,620×g for 10 minutes at room temperature, and the supernatant (i.e., serum) was removed and reserved in separate Eppendorf tubes for the liver enzyme assay (~250 μl) and for the cytokine assay (~50 μl).
Tissue Collection
Liver and brain were extracted from each animal at euthanasia. Livers were dissected, and the left lateral lobe was placed in a 50 -ml falcon tube containing formalin for histopathology. Tissue from the right lateral lobe was cut into small pieces with 2 scalpel blades, and ~0.2 ml tissue was shock-frozen in liquid nitrogen and stored at −80°C until further processing for cytokine analysis. Whole brains were likewise quickly frozen in liquid nitrogen and stored at −80°C until regional dissection for cytokine analysis.
Thiamine Assay
The procedure followed was based on published methods (Mancinelli et al., 2003). All reagents were purchased from Sigma–Aldrich (St. Louis, MO). Upon arrival at AniLytics, Inc. (Gaithersburg, MD), samples were pelleted at 73×g for 10 minutes at 10°C. The buffy coat was removed to eliminate TDP-rich leukocytes. The cell suspension was then centrifuged at 143×g for 20 minutes at 10°C to obtain well-packed RBCs, and then the saline was partially removed leaving a 1:1 cell suspension, thus obtaining analytical samples with a final hematocrit of ~48 to 52%. The 1:1 suspension was well mixed to form a uniform suspension and immediately transferred to a glass tube for extraction, yielding a packed-erythrocyte sample. For each 1 ml of cell suspension (equivalent to 0.5 ml packed cells), 0.125 ml of trichloroacetic acid (TCA, 40%) was added, mixed by vortex, and left for 1 hour in the dark at room temperature to complete protein precipitation. The tube was then centrifuged at 655×g for 15 to 20 minutes. The clear supernatant was carefully transferred into a polypropylene microcentrifuge tube and centrifuged at 7,280×g for 10 minutes. The supernatant (100 to 200 μl) was transferred to another tube, and for each 100 μl of extract, 0.57 ml of water-saturated diethyl ether (anhydrous) was added, mixed by vortex, then centrifuged at 164×g for 5 minutes. Tubes were placed at −50°C or below for at least 1 hour, during which the aqueous layer froze. The ether layer was then poured off, and samples were thawed to room temperature. The sample was derivatized as follows: for each 100 μl of sample, 5 μl of potassium hexacyanoferrate (30.4 mM) was added, mixed by vortex, and allowed to sit for 5 minutes, and then 5 μl of NaOH (0.8 M) was added. The derivatized sample was then ready for high performance liquid chromatography (HPLC) fluorimetric determination of thiamine, TMP, and TDP.
Liver Enzyme Assay and Liver Histopathology
The Stanford Department of Comparative Medicine processed serum collected from each animal to determine the levels of liver enzymes including aspartate aminotransferase (AST), alanine amino-transferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and total bilirubin. Left lateral lobe liver specimens from all rats were prepared with a standard hematoxylin and eosin (H&E) stain and a Masson’s Trichrome stain and evaluated by a trained liver pathologist (RL). Scores given were as follows: 0 = absence of pathology, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe pathology.
Cytokine Assay
Liver tissue was thawed on ice, and approximately 0.2 ml glass beads (Sigma–Aldrich) and 1 ml phosphate-buffered saline (PBS) containing protease inhibitors were added to the frozen tissue to avoid degradation on thawing. Brain regions were quickly dissected using a rat coronal (1 mm) brain matrix on dry ice. The dissection of 6 different brain regions took ~20 minutes per brain, during which time the brain remained frozen. As each dissected region was collected, the tissue was placed in microcentrifuge tubes containing 0.2 ml glass beads and 1 ml PBS containing protease inhibitors. Brain regions collected included the anterior vermis of the cerebellum, cingulate cortex, frontal cortex, hippocampus, hypothalamus, and striatum, regions chosen because of their sensitivity to EtOH exposure in rodents (e.g., Vilpoux et al., 2009) and humans (e.g., Sullivan et al., 2003; Vilpoux et al., 2009). Subsequently, both liver and brain samples were homogenized 3 times for 5 minutes at 4°C using a Bullet-Blender (Next Advance, Averill Park, NY). Homogenates were then centrifuged using 2 sequential centrifugation steps (164×g, 4°C, 10 minutes). Supernatants stored at −80°C were analyzed for cytokine levels by electrochemiluminescence within 1 week of tissue processing.
The cytokine assay of blood, liver, and brain samples was carried out in duplicate according to the instructions provided with Mesoscale Discovery’s Rat 7-PlexUltra-Sensitive Kit (Gaithersburg, MD). The kit allowed for the assessment of 7 cytokines involved in various immune responses (i.e., TNF-α, IFN-γ, IL-1β, IL-4, IL-5, IL-13, GRO/CXCL1). Protein content of liver and brain samples was measured using a BCA kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Liver and brain cytokine levels were computed relative to total protein content of the sample, i.e., pg/mg protein. For blood cytokine measurements, robust duplicate measures were sometimes not possible because of insufficient blood volume; consequently, statistics were conducted on the first measurement only. For liver and brain tissue, statistics were carried out on the mean values of the duplicated assays.
Statistical Analysis
Group differences were analyzed using 2-group (Con vs. EtOH)-by-3 time-point (baseline, binge, and euthanasia) repeated-measures analysis of variance (ANOVA). Only group effects and interactions were of interest to this analysis. Follow-up between-group differences were determined by two-tailed t-tests and confirmed by the nonparametric Mann–Whitney U-test. The nonparametric Mann–Whitney U-test was used to evaluate group differences in scores on the behavioral assessments and postmortem liver histopathology.
RESULTS
Binge Ethanol Effects on Weight and Behavior
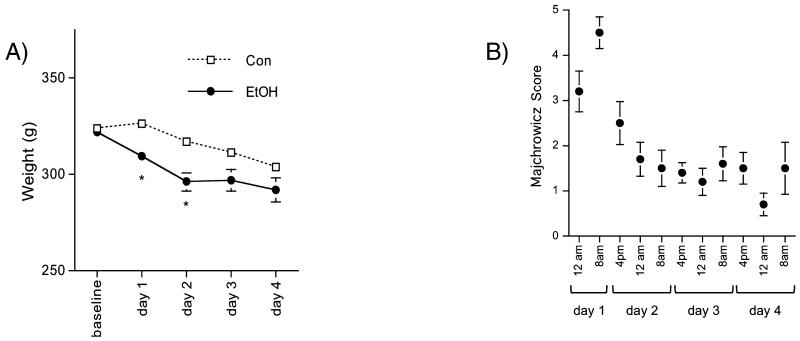
Figure 2A presents weights per group at baseline and on each day of EtOH treatment. Group differences in body weight were evident on day 1 (t16 = 3.51, p = 0.0029) and day 2 (t16 = 3.37, p = 0.0039) of EtOH treatment when EtOH animals were losing weight more rapidly than the Con group. On day 1, EtOH rats had lost 4% of their body weight, while Con animals gained 1%; on day 2, EtOH rats had lost 8% of their body weight, while Con animals had lost 2%.
Fig. 2.
(A) Animals’ weights (mean ± SE) on each of the 4 days of binge EtOH exposure; (B) Scores animals in the EtOH group demonstrated on the Majchrowicz 6-point scale, first recorded at 24:00 on day 1 of EtOH exposure. 0 = normal animal; 1 = hypoactive/mildly ataxic; 2 = ataxic; 3 = ataxic, dragging abdomen, delayed righting reflex; 4 = loss of righting reflex; 5 = loss of righting reflex and loss of eye-blink reflex.
EtOH rats scored a mean of 3.2 on the behavioral scale after the second EtOH dose (evaluated at 24:00 on day 1) and 4.5 after their third EtOH dose, indicating that most EtOH animals had lost their righting reflex and that some had also lost their eye-blink reflex (Fig. 2B). EtOH animals scored an average of 2.5 after the fourth dose (ataxic and delayed righting reflex), but then nothing higher than an average of 1.7 (moderately ataxic) for the remainder of the experiment, indicating that the animals exposed to EtOH were in overall good health during the course of the experiment and showed remarkable behavioral tolerance to the high doses of EtOH. Furthermore, although half of the EtOH-treated animals endured 24 hour of abstinence between the last dose of EtOH and euthanasia, they remained healthy: furled coat, tremors, spasticity, aggressive running, “wet-dog” shakes, and teeth chattering, potential indicators of EtOH withdrawal-induced seizures (Majchrowicz, 1975) were not observed in any EtOH or Con animal.
Binge Ethanol Effects on the Levels of Thiamine and Its Phosphate Derivates
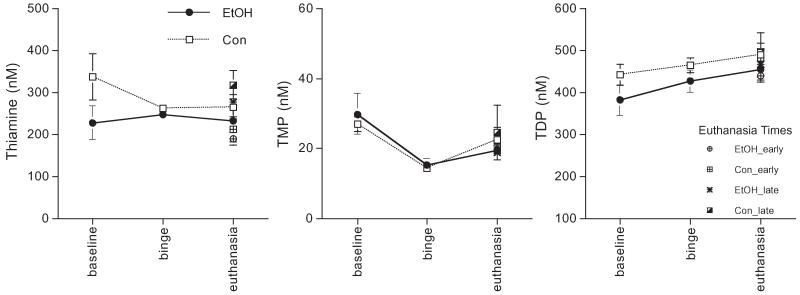
Figure 3 presents the levels of thiamine, TMP, and TDP per group at baseline, binge, and euthanasia; also shown are levels for the early and late euthanasia groups separately. Three separate 2-group repeated-measures (3 time-point: baseline, binge, euthanasia) ANOVAs for thiamine, TMP, and TDP revealed no significant group effects.
Fig. 3.
Thiamine, thiamine monophosphate (TMP), and thiamine diphosphate (TDP) levels (mean ± SE) at baseline (EtOH n = 10, Con n = 8), binge (EtOH n = 10, Con n = 8), and euthanasia time-points including separate points demonstrating levels for the early (EtOH n = 5, Con n = 4) and late (EtOH n = 5, Con n = 4) euthanasia groups.
Binge Ethanol Effects on Liver Enzyme Levels
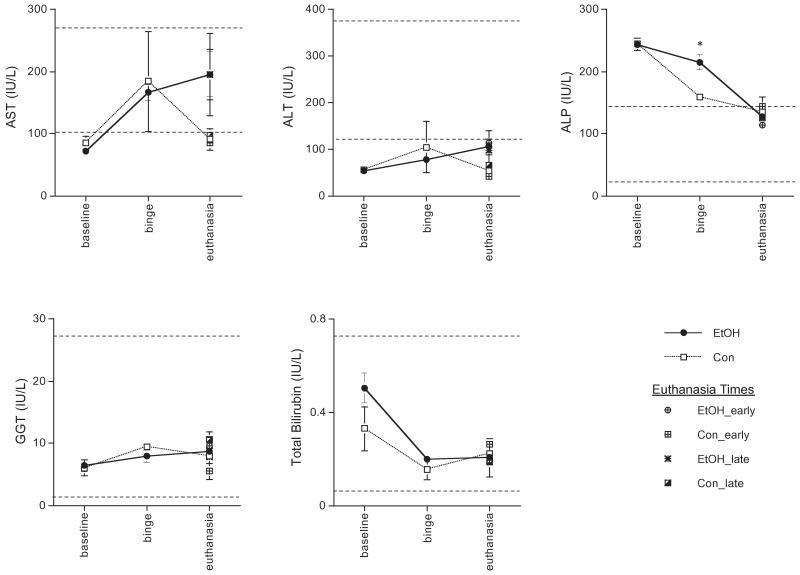
For each of the 5 liver enzymes monitored, a 2-group repeated-measures ANOVA was conducted. Only the ANOVA for ALP revealed a group effect (F2,2 = 10.66, p = 0.0003). Follow-up analysis determined that ALP was higher in the EtOH than in the Con group at binge (t16 =4.02, p = 0.001) and was confirmed with a Mann–Whitney nonparametric test (U = 2.45, p = 0.014) (Fig. 4).
Fig. 4.
Levels (mean ± SE) of liver enzymes at baseline (EtOH n = 10, Con n = 8), binge (EtOH n = 10, Con n = 8), and euthanasia including separate points demonstrating levels for early (EtOH n = 5, Con n = 4) and late (EtOH n = 5, Con n = 4) euthanasia groups separately. IU/l, institutional units per liter; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase. Dashed lines indicate typical levels.
Binge Ethanol Effects on Liver Histopathology
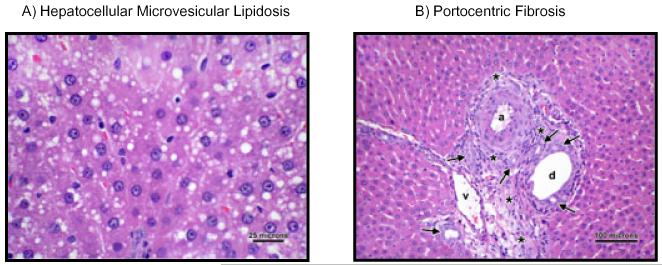
Postmortem analysis of the left liver lobe revealed significant group differences for the presence of hepatocellular microvesicular lipidosis (U = 2.02, p = 0.044, Fig. 5A) and portocentric fibrosis (U = 2.27, p = 0.023, Fig. 5B). Six of 10 EtOH livers, but only 1 of 8 Con livers revealed evidence for minimal hepatocellular microvesicular lipidosis. Because average scores were EtOH = 0.75 ± 0.2 and Con =0.125 ± 0.125, where a score of 1 indicates only minimal pathology, these results suggest that despite the statistically higher lipidosis scores in EtOH than in Con animals, differences were not clinically meaningful. Similarly, although none of the Con livers had portocentric fibrosis, 5 of 10 EtOH livers revealed the presence of minimal portocentric fibrosis (average score: EtOH = 0.6 ± 0.2), again indicating that pathology was minimal. Evidence for hepatic steatosis, alcoholic hepatitis, or alcoholic cirrhosis was absent.
Fig. 5.
(A) Demonstrates an H&E-stained liver sample from an EtOH animal showing mild microvesicular lipidosis. (B) Demonstrates an H&E-stained liver sample from an EtOH animal presenting the portal triad, consisting of a hepatic arteriole (a), hepatic venule (v), and bile ductule (d). Note the prominence of the portal triad because of moderate expansion of the portal interstitium (asterisks) by a combination of fibroplasia; infiltration by moderate numbers of lymphocytes and plasma cells; and mild bile ductular proliferation/hyperplasia (arrows). 200× magnification.
Binge Ethanol Effects on Blood and Liver Cytokines
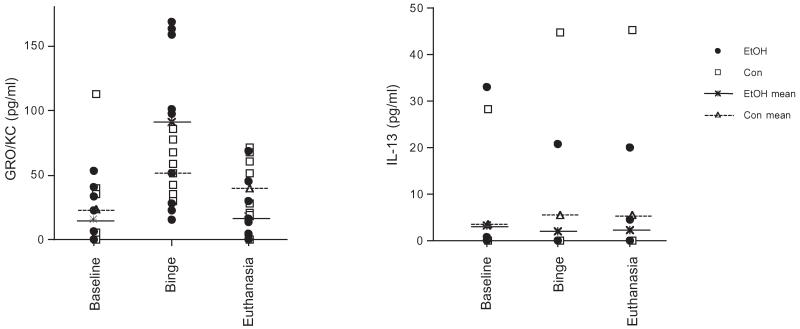
For all measurements in the serum and liver besides GRO/KC and IL-13 in the serum, cytokines did not reach detectable levels (Table 1). Two-group-by-3 time-point (baseline, binge, euthanasia) ANOVAs carried out on the non-duplicated sample (i.e., measurement 1) of serum GRO/KC and IL-13 did not reveal group effects (Fig. 6). For confirmation, t-tests comparing serum GRO/KC and IL-13 between EtOH and Con groups were conducted and revealed no group differences. Similarly, there were no between-group differences in the levels of the 7 cytokines assessed in the liver (average of the duplicated samples assessed, Table 1, Fig. 7).
Table 1.
Cytokine Levels (Mean ± SE)
| GRO/KC |
GRO/KC |
IFN-γ |
IFN-γ |
TNF-α |
TNF-α |
IL-1β |
IL-1β |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EtOH | Con | LOD | EtOH | Con | LOD | EtOH | Con | LOD | EtOH | Con | LOD | |
| Serum baseline | 15.74 ± 6.40 | 24.26 ± 13.98 | 22.00 | 0.48 ± 0.30 | 0.09 ± 0.07 | 2.96 | 0.45 ± 0.30 | 0.15 ± 0.15 | 37.90 | 1.28 ± 0.84 | 0.22 ± 0.22 | 35.20 |
| Serum binge | 90.89 ± 18.85 | 56.26 ± 7.06 | 22.00 | 0.28 ± 0.20 | 0.65 ± 0.25 | 2.96 | 0.17 ± 0.17 | 0 | 37.90 | 16.08 ± 5.95 | 7.20 ± 4.32 | 35.20 |
| Serum euth 4 hour | 13.35 ± 8.56 | 30.30 ± 14.94 | 30.20 | 1.02 ± 0.89 | 0 | 5.18 | 0 | 0 | 57.90 | 2.03 ± 2.03 | 0 | 68.10 |
| Serum euth 24 hour | 23.13 ± 12.45 | 50.03 ± 10.87 | 30.20 | 0.41 ± 0.35 | 0.57 ± 0.50 | 5.18 | 0 | 0 | 57.90 | 0.57 ± 0.57 | 2.72 ± 2.72 | 68.10 |
| Liver | 6.00 ± 1.06 | 4.48 ± 1.36 | 30.20 | 0.136 ± 0.038 | 0.23 ± 0.05 | 5.18 | 0.16 ± 0.10 | 0.02 ± 0.02 | 57.90 | 4.51 ± 0.77 | 6.42 ± 1.09 | 68.10 |
| Brain_AV | 4.98 ± 1.41 | 2.84 ± 0.68 | 27.60 | 1.76 ± 0.41 | 2.29 ± 0.47 | 24.00 | 0.70 ± 0.33 | 0.11 ± 0.09 | 51.40 | 7.00 ± 3.67 | 6.88 ± 4.09 | 496.00 |
| Brain_CC | 80.79 ± 55.32 | 11.33 ± 6.76 | 27.60 | 2.60 ± 1.10 | 7.031 ± 1.71 | 24.00 | 2.42 ± 1.41 | 5.76 ± 2.60 | 51.40 | 53.43 ± 23.50 | 19.24 ± 8.75 | 496.00 |
| Brain_FC | 34.59 ± 24.90 | 7.19 ± 1.76 | 48.60 | 1.90 ± 0.46 | 2.70 ± 1.25 | 39.70 | 3.96 ± 1.58 | 1.84 ± 1.24 | 61.60 | 21.31 ± 5.28 | 23.18 ± 10.66 | 165.00 |
| Brain_Hip | 27.53 ± 8.60 | 9.09 ± 1.37 | 48.60 | 1.28 ± 0.87 | 0 | 39.70 | 6.24 ± 4.13 | 2.29 ± 1.58 | 61.60 | 43.51 ± 16.44 | 26.36 ± 7.48 | 165.00 |
| Brain_Hyp | 104.60 ± 74.66 | 22.71 ± 4.39 | 23.30 | 0.45 ± 0.33 | 0.93 ± 0.62 | 10.30 | 15.64 ± 7.00 | 6.74 ± 2.91 | 7.40 | 89.19 ± 30.20 | 73.10 ± 25.82 | 339.00 |
| Brain_Str | 14.00 ± 4.14 | 6.93 ± 0.59 | 23.30 | 1.22 ± 0.81 | 1.95 ± 0.47 | 10.30 | 3.93 ± 0.35 | 2.31 ± 0.86 | 7.40 | 38.28 ± 22.75 | 27.79 ± 6.78 | 339.00 |
| IL-4 |
IL-4 |
IL-5 |
IL-5 |
IL-13 |
IL-13 |
||||
|---|---|---|---|---|---|---|---|---|---|
| EtOH | Con | LOD | EtOH | Con | LOD | EtOH | Con | LOD | |
| Serum baseline | 0 | 0 | 1.71 | 0.38 ± 0.38 | 0 | 46.80 | 3.41 ± 3.31 | 3.56 ± 3.56 | 1.35 |
| Serum binge | 0 | 0 | 1.71 | 7.07 ± 3.85 | 0 | 46.80 | 2.08 ± 2.08 | 5.62 ± 5.62 | 1.35 |
| Serum euth 4 hour | 0.06 ± 0.06 | 0 | 2.42 | 0 | 0 | 60.90 | 4.97 ± 3.90 | 11.35 ± 11.35 | 1.53 |
| Serum euth 24 hour | 0.45 ± 0.40 | 0.44 ± 0.26 | 2.42 | 0 | 4.5 ± 3.3 | 60.90 | 0 | 0 | 1.53 |
| Liver | 0.03 ± 0.01 | 0.03 ± 0.01 | 2.42 | 0.47 ± 0.12 | 0.63 ± 0.23 | 60.90 | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.53 |
| Brain_AV | 0.14 ± 0.08 | 0.06 ± 0.03 | 4.14 | 2.12 ± 0.79 | 0.79 ± 0.60 | 78.30 | 0.56 ± 0.23 | 0.47 ± 0.19 | 10.20 |
| Brain_CC | 0.75 ± 0.53 | 0.45 ± 0.25 | 4.14 | 10.65 ± 7.96 | 3.22 ± 1.86 | 78.30 | 6.26 ± 1.69 | 6.17 ± 1.73 | 10.20 |
| Brain_FC | 0.16 ± 0.13 | 0.13 ± 0.06 | 4.38 | 3.59 ± 1.51 | 3.38 ± 2.18 | 78.90 | 0.61 ± 0.35 | 1.63 ± 0.90 | 9.82 |
| Brain_Hip | 0.05 ± 0.03 | 0 | 4.38 | 3.50 ± 3.04 | 0.55 ± 0.36 | 78.90 | 1.38 ± 0.95 | 0.24 ± 0.24 | 9.82 |
| Brain_Hyp | 0.40 ± 0.28 | 0 | 10.10 | 8.70 ± 7.59 | 1.24 ± 0.92 | 156.00 | 0.91 ± 0.58 | 0.23 ± 0.23 | 10.50 |
| Brain_Str | 0 | 0.06 ± 0.04 | 10.10 | 0.73 ± 0.73 | 1.99 ± 1.13 | 156.00 | 0.69 ± 0.68 | 0.51 ± 0.16 | 10.50 |
LOD, limit of detectability; EtOH n = 10, Con n = 8; except for euthanasia groups (EtOH n = 5, Con n = 4); for serum units, pg/ml; for tissue units, pg/mg protein; numbers in bold indicate levels above the LOD.
Fig. 6.
Scatter plots of GRO/KC and IL-13 in the blood at baseline, binge, and euthanasia (EtOH n = 10, Con n = 8).
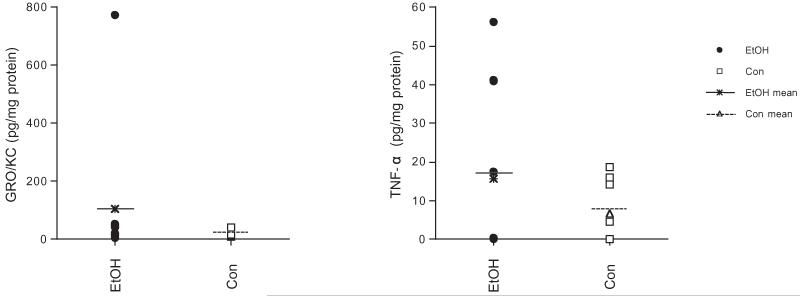
Fig. 7.
Scatter plots of GRO/KC and TNF-α in the hypothalamus (EtOH n = 10, Con n = 8).
Binge Ethanol Effects on Brain Cytokines
Only 2 of the 7 cytokines measured reached detectable levels in the brain: GRO/KC in the CC and Hyp, and TNF-α in the Hyp (Table 1, Fig. 7). Two-group-by-6-brain region ANOVAs on GRO/KC and TNF-α levels (average of the duplicated samples assessed) did not indicate significant group differences. Follow-up t-tests for GRO/KC in the CC and Hyp and TNF-α in the Hyp also failed to reveal significant group differences. To note, however, was the 1 animal in the EtOH group that demonstrated induction of GRO/KC in the hypothalamus to 774.86 pg/mg protein (compared with a group average of 104.60 ± 74.66 pg/mg protein, Fig. 7). This is especially notable because this animal also presented with the highest peak BAL (478.4 mg/dl, compared with a group average of 421.33 ± 15.04 mg/dl) and highest average BAL across 4 days (401 mg/dl, compared with a group average of 314.93 ± 16.42 mg/dl).
DISCUSSION
Human (Ende et al., 2006; Meyerhoff et al., 2004) and rodent (Lee et al., 2001; Zahr et al., 2009, 2010) studies indicate that the MRS signal from Cho is elevated in response to recent EtOH exposure. A potential interpretation for elevated brain Cho is neuroinflammation (e.g., De Stefano et al., 2005). In the alcohol literature, a provocative hypothesis suggests that EtOH-induced activation of the brain’s innate immune cells, microglia, may promote neuroinflammation and thereby lead to neurodegeneration (Crews et al., 2006). The mechanism proposed is an EtOH-induced elevation of liver and serum levels of pro-inflammatory cytokines, particularly TNF-α. Systemic TNF-α is the suggested carrier of the inflammatory signal to the brain (Qin et al., 2008), where it activates microglia to increase the synthesis of additional cytokines and create a positive feed-forward process of inflammation (Qin et al., 2004). Although promising, there is as yet little evidence for a role of EtOH-induced inflammation in brain toxicity in uncomplicated alcoholism (He and Crews, 2008). By contrast, there is substantial support for the presence of peripheral inflammation and cytokine production in diseases concomitant to alcoholism, especially liver disease (Leevy and Elbeshbeshy, 2005). There is also experimental support for microglial activation and increased pro-inflammatory cytokine production in animal models of thiamine deficiency (Karuppagounder et al., 2007; Todd and Butterworth, 1999; Vemuganti et al., 2006). The results presented here suggest that this single 4-day binge protocol, in the absence of liver pathology or thiamine deficiency, is insufficient to stimulate selective serum, liver, or brain cytokines.
Binge Ethanol Effects on the Levels of Thiamine and Its Phosphate Derivates
The current findings suggest that a single 4-day binge EtOH exposure is insufficient to modulate the levels of thiamine or its phosphate derivatives. Although the relationship between alcoholism and thiamine deficiency is well established (e.g., Harper, 2006), few alcohol studies report thiamine levels, likely because reliable quantification of thiamine and its phosphate derivates is difficult (Mancinelli et al., 2003). In a previous study where thiamine and its derivates were measured, HPLC was performed on unprocessed plasma. We demonstrated that 24 weeks of vaporized EtOH exposure can reduce the levels of thiamine and TMP in EtOH-exposed compared with Con animals (Zahr et al., 2009). The levels of TDP, however, were nearly undetectable, likely reflecting the fact that “80% of the total thiamine pool in whole blood is stored in erythrocytes mostly as TDP” (Mancinelli et al., 2003). In an attempt to provide a more accurate reflection of thiamine body stores, the current assay was developed to evaluate the levels of thiamine and its derivates in RBCs as previously described (Mancinelli et al., 2003). While there were no between-group differences in the levels of thiamine, TMP, or TDP, the significantly higher levels of thiamine and TDP in the current study compared with the previous study (Zahr et al., 2009) likely reflect the accuracy achieved when measuring thiamines in isolated RBCs (and especially in a calibrated hematocrit) rather than in unprocessed plasma.
Binge Ethanol Effects on Liver Enzyme Levels
The levels of ALP were higher in the EtOH group than in the Con group at the binge time-point. Elevations in ALP can indicate blockage of bile flow (cholestasis) (Meyer-Sabellek et al., 1988). However, although cholestasis can be caused by a wide variety of conditions (e.g., hepatitis, congestive heart failure, hyperthyroidism), the diagnosis of cholestasis requires elevations in additional liver enzyme values, classically GGT and bilirubin (Szalay, 2001), and would be apparent upon histological analysis. Furthermore, although elevations in ALP may indicate hepatitis, alcohol-induced hepatitis and cirrhosis are typically associated with elevations in other liver enzymes. Specifically, elevations in AST and ALT are presumed necessary for the diagnosis of alcohol-induced hepatitis or cirrhosis (Levitsky and Mailliard, 2004). Altogether, the minor elevations in ALP in the EtOH group are not indicative of clinically significant alcoholic liver disease.
Binge Ethanol Effects on Liver Histopathology
Histopathology of the left lateral lobe of livers from alcoholized animals did not provide evidence for cirrhosis, which would minimally require observable hepatocellular parenchymal loss, or centrilobular, portal-portal, or central-portal fibrosis. Likewise, the absence of hepatocellular swelling and/or necrosis indicates the absence of hepatitis. In a previous study, 24 weeks of EtOH exposure via vapor chamber resulted in only mild steatosis (Zahr et al., 2009). In the current study, only 1 of the EtOH livers scored a 2 (i.e., mild) for the presence of microvesicular lipidosis, indicating that although group differences were statistically significant, the presence of microvesicular lipidosis in the EtOH livers, with an average score of 0.75 ± 0.21, is insufficient to diagnose steatosis. Similarly, while statistically significant, the minimal presence of portocentric fibrosis in the EtOH livers (average score = 0.6 ± 0.22) probably does not represent true liver pathology.
Binge Ethanol Effects on Blood and Liver Cytokines
Levels of a specific set of 7 cytokines were not affected in blood or liver by a single episode of binge EtOH exposure. These findings are consistent with the fact that the constitutive synthesis of inflammatory cytokines in most tissues is inconsequential and only dramatically upregulated upon exposure to severe pathology (e.g., Frost et al., 2005). Unstimulated human blood mononuclear cells from healthy volunteers secrete very low levels of cytokine proteins (e.g., IL-2 = 27 ± 22, IL-4 = 2 ± 4, IL-6 = 49 ± 56, IFN-γ = 12 ± 11, TNF-α = 15 ± 18 pg/ml in 16 donors, Sullivan et al., 2000). Similarly, low cytokine levels are detected in the serum of healthy human controls (e.g., IL-1β = 9.4 ± 2.0, IL-6 = 16.0 ± 7.1 pg/ml, n = 6, Fukuma et al., 1996). While in vitro work has clearly demonstrated the sensitivity of the immune system to alcohol, the direction of change (up- or down-regulation) can depend on the cell type studied (e.g., Choi et al., 2005; mouse mixed glial cultures; Fernandez-Lizarbe et al., 2009; rat or mouse mixed glial cultures; Verma et al., 1993; human monocytes). In the following 3 examples, EtOH reduced cytokine responsiveness. Monocytes harvested from healthy human volunteers and stimulated by 1 μg/ml LPS revealed that 100 mM EtOH significantly down-regulated TNF-α protein production from ~18 to ~7 ng/106 monocytes/ml (Szabo et al., 1996). In a mouse study, EtOH treatment (6 g/kg gavage) significantly reduced LPS (500 μg via tail vein)-stimulated serum TNF-α protein levels from ~1,000 to under 100 pg/ml (Dai and Pruett, 2006). EtOH (4 g/kg) by oral gavage 4 or 26 hours prior to cytokine analysis did not change constitutive mRNA levels of IL-6, TNF-α, or IL-1β in the gastrocnemius muscle or spleen and additionally did not affect mRNA levels of IL-6 or IL-1β in the livers of male Sprague–Dawley rats; however, TNF-α mRNA levels in livers were reduced by EtOH treatment (Frost et al., 2005).
Even in patients with liver disease, cytokine responsiveness can be variable. There were no differences in the levels of 3 cytokines evaluated in serum collected from control (n = 12; IL-6 = 5.95 ± 1.67, IL-10 = 8.22 ± 10.69, TNF-α = 6.14 ± 1.91 pg/ml), noncirrhotic alcoholic (n = 26; IL-6 = 6.69 ± 4.48, IL-10 = 14.42 ± 30.34, TNF-α = 10.30 ± 11.24 pg/ml), and cirrhotic alcoholic (n = 10; IL-6 = 9.99 ± 10.59, IL-10 = 48.77 ± 130.81, TNF-α = 18.27 ± 16.05) individuals (Garcia-Valdecasas-Campelo et al., 2007). Only 8 of 16 monocyte samples from peripheral blood of patients with alcoholic hepatitis revealed detectable spontaneous TNF-α activity; LPS stimulation elevated TNF-α levels to 25.3 ± 3.7 in the alcoholic hepatitis patients and to 10.9 ± 2.4 units/ml in the healthy controls (McClain and Cohen, 1989). From another study using human plasma from patients with alcoholic hepatitis, “the measured levels of the bioactive form of TNF-α were widely variable among the patients. TNF-α was undetectable in 6 patients, and in the other 5, the values were 13, 18, 133, 283, and 370 pg/ml. Of the 2 patients who died, the levels of TNF-α were undetectable in 1 and 18 pg/ml in the other” (Tilg et al., 2003). For the other cytokines measured in the blood of these patients with alcoholic hepatitis, levels for IL-1β averaged 100 pg/ml, and neither IL-4 nor IFN-γ exceeded 10 pg/ml (Tilg et al., 2003).
In experimental procedures, animals are often challenged with LPS in addition to EtOH administration to produce a robust immune response. In Wistar rats, hepatic mRNA levels of TNF-α and IL-1β were not induced by 6 weeks of a nutritionally adequate high fat/low carbohydrate liquid diet containing 34.5% of calories from EtOH but were nearly tripled in the presence of 0.1 mg/kg per day of LPS (Jarvelainen et al., 1999). Similarly, mice given 35% EtOH-derived calories alone for 14 days did not reveal alterations in the plasma protein levels of TNF-α, IL-12, or MCP-1; likewise, liver IL-1β, TNF-α, and IL-6 mRNA levels were unchanged by EtOH treatment alone but approximately tripled in the presence of 25 μg LPS injected intraperitoneally (IP) 3 hours before tissue harvesting (Fleming et al., 2001). Together, these data support the current findings that a single episode of binge EtOH exposure in the absence of liver pathology or LPS challenge is insufficient to change the levels of circulating and liver cytokines.
Binge Ethanol Effects on Brain Cytokines
The findings of this experiment suggest that a single bout of binge EtOH treatment (intragastric EtOH given every 8 hours for 4 days, with BALs averaging 314.93 ± 16.42 mg/dl across the 4 days) is insufficient to induce the expression of 7 cytokines in 6 different brain regions of the wild-type Wistar rat. This negative finding is in contrast to evidence for a neuroinflammatory response when EtOH is given intermittently. For example, inflammatory markers COX-2 and iNOS were identified in neocortex, hippocampus, and cerebellum of adolescent male Wistar rats provided with 25% EtOH (3.0 g/kg, IP) for 2 consecutive days at 48-hour intervals over 14 days (Pascual et al., 2007). In adolescent female Wistar rats, although the entire brain was examined for OX-6 immunohistochemical staining implying the presence of activated microglia following doses of 20% EtOH (2 or 3 g/kg administered by gavage 3 times per day for 2 days, followed by a period of 5 days of abstinence, repeated 3 times), microglial activation was only observed in the dentate gyrus of hippocampus (Ward et al., 2009). Adult male Wistar rats intermittently (2 days per week off EtOH) but not continuously exposed to 10% EtOH as the only source of fluid for 5.5 months showed an increase in the number of microglia in the molecular layer of the cerebellar vermis as revealed by tomato lectin staining (Riikonen et al., 2002). In a continuous exposure protocol, quantitative immunohistochemical evaluation of the aging rat brain exposed to chronic EtOH (liquid diet containing 35% EtOH for 40 weeks) did not reveal changes in the number of microglial cells in the molecular layer of the cerebellar cortex (Dlugos and Pentney, 2001). By contrast, one study revealed robust COX-2 induction in perirhinal and piriform cortices, dentate gyrus, and tenia tecta with only a single bout of binge EtOH treatment (i.e., ~1.7 g/kg 15% EtOH every 6 hours for 4 days) (Knapp and Crews, 1999). A single bout may have been sufficient to induce COX-2 because the animals used were Sprague–Dawley rather than Wistar rats. Another reason that increased COX-2 expression may have been observed in the Knapp and Crews (1999) study is because the BALs achieved were high (500 ± 117 and 490 ± 34 mg/dl 1 hour before and after treatment on Day 2, and 500 ± 36 and 592 ± 10 mg/dl 1 hour before and after treatment on Day 4). This is in agreement with the induction of GRO/KC to 774.86 pg/mg protein in the hypothalamus of the animal in this study that reached the highest peak BAL (i.e., 478.4 mg/dl) in 4 days of treatment.
There are currently few studies that report brain cytokine levels in response to alcoholism or EtOH exposure in the absence of hepatopathology or thiamine depletion. In human brain tissue, the levels of MCP-1 measured in homogenates of the VTA, substantia nigra, hippocampus, and amygdala were found to be elevated in alcoholics relative to controls (He and Crews, 2008). However, 4 of the 8 alcoholic brains examined were harvested from alcoholics who died of complications related to liver disease. Given extensive evidence that liver disease is associated with cytokine induction (e.g., McClain et al., 2004), this study does not clearly demonstrate that EtOH per se induced cytokine production. In adult male Sprague–Dawley rats, intermittent EtOH exposure (5 hours a day for 5 days) blunted the TNF-α mRNA response to LPS (0.75 μg/kg, intravenously) by at least 40% in the hypothalamus and pituitary (Seo et al., 2004). Two studies reveal brain cytokine induction in response to EtOH alone: Qin and colleagues (2008) and Valles and colleagues (2004). Qin and colleagues (2008) used daily doses of intragastric EtOH (5 g/kg, 10%) for 10 days given to adult male C57BL/6J mice. This procedure resulted in whole brain induction of TNF-α (from ~27 in controls to ~50 pg/mg protein in EtOH-treated animals) and MCP-1 (from ~2 in controls to ~5 pg/mg protein in EtOH-treated animals) (Qin et al., 2008). This model may have induced brain TNF-α production for several reasons: mice may be more susceptible to brain TNF-α induction than rats; the 1 daily dose could be perceived as an intermittent exposure protocol given the 24-hour interval between doses; and analysis of a whole brain homogenate may have revealed a TNF-α response that is not evident in the regions chosen for analysis in this study. It is notable that in this study, despite lack of statistical differences between groups, levels of TNF-α were detectable in the Hyp of the EtOH but not the Con group. In the Valles and colleagues (2004) study, adult female Wistar rats were fed with the Lieber–DeCarli diet containing 5% EtOH for 5 months. BALs achieved were 24.5 ± 6 mM, and the cerebral cortex showed increases in IL-1β protein production from ~100 in controls to ~200 pg/mg protein in EtOH-treated animals (Valles et al., 2004). Again, these positive findings may be attributed to analysis of cerebral cortex homogenates rather than regional examination of cytokine levels.
Limitations
A potential limitation of this study is the number of animals used. An n = 8 in the EtOH group is at least as many animals as has previously been used in rodent studies measuring cytokine protein levels (e.g., n = 4, Valles et al., 2004; n = 6, Qin et al., 2008) but may not be sufficient for statistical power to detect group differences in the current experiment. Another consideration is that sectioning of the brain into multiple regions may have reduced the ability of the assay to quantify cytokines above the limits of detection. Adequate tissue was available for the liver assay, however, and none of the cytokines analyzed were detected in liver tissue. Finally, it is acknowledged that the key cytokine previously identified as EtOH responsive in both human and animal studies, i.e., MCP-1, was not quantified in this study.
CONCLUSION
The current protocol used is the same model previously used including species, strain, route, dose and administration schedule when Cho levels were found to be elevated in the brains of EtOH compared with Con animals (Zahr et al., 2010). We conducted this study to determine whether the elevations in brain Cho previously observed in the rat hippocampus could be attributed to neuroinflammation, a possibility that would be supported by induction of selective cytokines. However, the single 4-day episode of binge EtOH exposure did not induce the levels of any of 7 cytokines measured in serum, liver, and 6 brain regions of EtOH-exposed animals. These negative findings do not entirely rule out neuroinflammation as an interpretation for increased brain Cho in response to EtOH, as it is possible that other cytokines (e.g., MCP-1) in other brain regions not examined herein may have been elevated. Alternatively, it is possible that (i) longer EtOH exposure protocols (e.g., Valles et al., 2004), (ii) higher BALs (e.g., Knapp and Crews, 1999), (iii) models of intermittent EtOH exposure (e.g., Qin et al., 2008), (iv) different sampling times, or (v) different species or strains are necessary to observe changes in the levels of circulating, liver, or brain cytokines. Optional interpretations for the increase in Cho that need to be explored include but are not limited to demyelination (Mader et al., 2008) or cell membrane disruption because of changes in the contributions of free Cho, phosphocholine, or glycerophosphocholine on the MRS-detectable Cho signal (Griffin et al., 2001; Le Bourhis et al., 1986).
ACKNOWLEDGMENTS
We thank Dr. Saroj Das at AniLytics, Inc. for her willingness to attempt a novel assay and establish reliable thiamine, TMP, and TDP measurements. We appreciate Dr. Carsten Alt’s expertise in all things related to cytokines: measurements and interpretations of findings. We are grateful for the time and energy Oliver Hsu, Shara Vinco, Evan Nunez, and Juan Orduna (SRI International) committed to the experiment. SRI International internal research funding, AA013521-INIA, AA005965.
REFERENCES
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29(11 Suppl):166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med. 1993;29:737–745. doi: 10.1002/mrm.1910290605. [DOI] [PubMed] [Google Scholar]
- Choi DK, Lee H, Jeong J, Lim B, Suk K. Differential effects of ethanol on glial signal transduction initiated by lipopolysaccharide and interferon-gamma. J Neurosci Res. 2005;82:225–231. doi: 10.1002/jnr.20647. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J Immunotoxicol. 2006;3:217–225. doi: 10.1080/15476910601080156. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Bartolozzi ML, Guidi L, Stromillo ML, Federico A. Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis. J Neurol Sci. 2005;233:203–208. doi: 10.1016/j.jns.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Dlugos CA, Pentney RJ. Quantitative immunocytochemistry of glia in the cerebellar cortex of old ethanol-fed rats. Alcohol. 2001;23:63–69. doi: 10.1016/s0741-8329(00)00143-9. [DOI] [PubMed] [Google Scholar]
- Ende G, Walter S, Welzel H, Demirakca T, Wokrina T, Ruf M, Ulrich M, Diehl A, Henn FA, Mann K. Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. Neuroimage. 2006;32:740–746. doi: 10.1016/j.neuroimage.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579–589. [PubMed] [Google Scholar]
- Frost RA, Nystrom G, Burrows PV, Lang CH. Temporal differences in the ability of ethanol to modulate endotoxin-induced increases in inflammatory cytokines in muscle under in vivo conditions. Alcohol Clin Exp Res. 2005;29:1247–1256. doi: 10.1097/01.alc.0000171935.06914.5d. [DOI] [PubMed] [Google Scholar]
- Fukuma H, Morshed SA, Watanabe S, Uchida N, Ezaki T, Minami A, Matsuoka H, Hirabayashi S, Nakatsu T, Nishioka M. Increased expression of cytokines in liver and serum in patients with extrahepatic diseases. J Gastroenterol. 1996;31:538–545. doi: 10.1007/BF02355054. [DOI] [PubMed] [Google Scholar]
- Gallin J, Snyderman R. Inflammation: Basic Principles and Clinical Correlates. 3rd edn. Lippincott William and Wilkins; Philadelphia: 1999. [Google Scholar]
- Garcia-Valdecasas-Campelo E, Gonzalez-Reimers E, Santolaria-Fernandez F, De La Vega-Prieto MJ, Milena-Abril A, Sanchez-Perez MJ, Martinez-Riera A, Rodriguez-Rodriguez E. Brain atrophy in alcoholics: relationship with alcohol intake; liver disease; nutritional status, and inflammation. Alcohol Alcohol. 2007;42:533–538. doi: 10.1093/alcalc/agm065. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Mann CJ, Scott J, Shoulders CC, Nicholson JK. Choline containing metabolites during cell transfection: an insight into magnetic resonance spectroscopy detectable changes. FEBS Lett. 2001;509:263–266. doi: 10.1016/s0014-5793(01)03175-1. [DOI] [PubMed] [Google Scholar]
- Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur. J Neurol. 2006;13:1078–1082. doi: 10.1111/j.1468-1331.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol. 2009;44:141–147. doi: 10.1093/alcalc/agn120. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503–1510. doi: 10.1002/hep.510290508. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol Dis. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–643. [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Le Bourhis B, Beauge F, Aufrere G, Nordmann R. Membrane fluidity and alcohol dependence. Alcohol Clin Exp Res. 1986;10:337–342. doi: 10.1111/j.1530-0277.1986.tb05100.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Holburn GE, Price RR. In vivo and in vitro proton NMR spectroscopic studies of thiamine-deficient rat brains. J Magn Reson Imaging. 2001;13:163–166. doi: 10.1002/1522-2586(200102)13:2<163::aid-jmri1025>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lee H, Jeong J, Son E, Mosa A, Cho GJ, Choi WS, Ha JH, Kim IK, Lee MG, Kim CY, Suk K. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J Neuroimmunol. 2004;156:88–95. doi: 10.1016/j.jneuroim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Leevy CB, Elbeshbeshy HA. Immunology of alcoholic liver disease. Clin Liver Dis. 2005;9:55–66. doi: 10.1016/j.cld.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Levitsky J, Mailliard ME. Diagnosis and therapy of alcoholic liver disease. Semin Liver Dis. 2004;24:233–247. doi: 10.1055/s-2004-832937. [DOI] [PubMed] [Google Scholar]
- Mader I, Rauer S, Gall P, Klose U. (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. Eur J Radiol. 2008;67:250–257. doi: 10.1016/j.ejrad.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mancinelli R, Ceccanti M, Guiducci MS, Sasso GF, Sebastiani G, Attilia ML, Allen JP. Simultaneous liquid chromatographic assessment of thiamine, thiamine monophosphate and thiamine diphosphate in human erythrocytes: a study on alcoholics. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789:355–363. doi: 10.1016/s1570-0232(03)00139-9. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497–G502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Sabellek W, Sinha P, Kottgen E. Alkaline phosphatase. Laboratory and clinical implications. J Chromatogr. 1988;429:419–444. [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A. Intermittent ethanol exposure increases the number of cerebellar microglia. Alcohol Alcohol. 2002;37:421–426. doi: 10.1093/alcalc/37.5.421. [DOI] [PubMed] [Google Scholar]
- Seo DO, Lee S, Rivier C. Prolonged exposure to intermittent alcohol vapors decreases the ACTH as well as hypothalamic nitric oxide and cytokine responses to endotoxemia. Alcohol Clin Exp Res. 2004;28:848–854. doi: 10.1097/01.alc.0000128230.82909.a5. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Cutilli J, Piliero LM, Ghavimi-Alagha D, Starr SE, Campbell DE, Douglas SD. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2000;7:920–924. doi: 10.1128/cdli.7.6.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Szalay F. Treatment of primary biliary cirrhosis. J Physiol Paris. 2001;95:407–412. doi: 10.1016/s0928-4257(01)00055-9. [DOI] [PubMed] [Google Scholar]
- Tallaksen CM, Bell H, Bohmer T. The concentration of thiamin and thiamin phosphate esters in patients with alcoholic liver cirrhosis. Alcohol Alcohol. 1992a;27:523–530. [PubMed] [Google Scholar]
- Tallaksen CM, Bohmer T, Bell H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol Clin Exp Res. 1992b;16:320–325. doi: 10.1111/j.1530-0277.1992.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Narayanan S, De Stefano N, Arnaoutelis R, Antel SB, Francis SJ, Santos AC, Lapierre Y, Arnold DL. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol. 2002;249:1382–1390. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- Technical Bulletin. Charles River Laboratories; Wilmington, MA: 1999. [Google Scholar]
- Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, Mookerjee RP, Graziadei I, Datz C, Trauner M, Schuppan D, Obrist P, Vogel W, Williams R. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419–425. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia. 1999;25:190–198. doi: 10.1002/(sici)1098-1136(19990115)25:2<190::aid-glia9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ, Renau-Piqueras J, Guerri C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Au Duong M, Audoin B, Le Fur Y, Confort-Gouny S, Malikova I, Soulier E, Viout P, Ali-Cherif A, Pelletier J, Cozzone PJ, Ranjeva JP. Relationships between gray matter metabolic abnormalities and white matter inflammation in patients at the very early stage of MS: a MRSI study. J Neurol. 2007;254:914–923. doi: 10.1007/s00415-006-0474-7. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Kalluri H, Yi JH, Bowen KK, Hazell AS. Gene expression changes in thalamus and inferior colliculus associated with inflammation, cellular stress, metabolism and structural damage in thiamine deficiency. Eur J Neurosci. 2006;23:1172–1188. doi: 10.1111/j.1460-9568.2006.04651.x. [DOI] [PubMed] [Google Scholar]
- Verma BK, Fogarasi M, Szabo G. Down-regulation of tumor necrosis factor alpha activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–22. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak M, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry. 2010;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–1442. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]