Abstract
Background
Peanut allergy is one of the most severe class I food allergies with increasing prevalence. Especially lipophilic allergens, such as oleosins, were found to be associated with severe symptoms, but are usually underrepresented in diagnostic extracts. Therefore, this study focused on isolation, molecular characterization and assessment of the allergenicity of peanut oleosins.
Methods and Results
A comprehensive method adapted for the isolation of peanut oil bodies of high purity was developed comprising a stepwise removal of seed storage proteins from oil bodies. Further separation of the oil body constituents, including the allergens Ara h 10, Ara h 11, the presumed allergen oleosin 3 and additional oleosin variants was achieved by a single run on a preparative electrophoresis cell. Protein identification realized by N-terminal sequencing, peptide mass fingerprinting and homology search revealed the presence of oleosins, steroleosins and a caleosin. Immunoblot analysis with sera of peanut-allergic individuals illustrated the IgE-binding capacity of peanut-derived oleosins.
Conclusion
Our method is a novel way to isolate all known immunologically distinct peanut oleosins simultaneously. Moreover, we were able to provide evidence for the allergenicity of oleosins and thus identified peanut oleosins as probable candidates for component-resolved allergy diagnosis.
Introduction
The peanut (Arachis hypogaea) is one of the most cultivated oilseeds and a source of valuable edible oil, proteins and fiber. Peanuts contain 49% fat, 26% protein and 11% fiber [1]. However, one percent of the Western population is allergic to peanut, and the prevalence is still increasing [2]. Since diagnostic allergy testing is based on aqueous extracts of the respective allergenic source, potential allergenic proteins of lipophilic nature may be underrepresented as putative allergens [3]. Furthermore, there is a growing evidence that lipids trigger immune reactions, especially in conjunction with lipophilic proteins, pointing to the latter as potential allergens [4].
Peanut oleosins are made up of a highly conserved central hydrophobic domain and hydrophilic N- and C-termini, differing in the primary amino acid sequence. Oleosins are the major oil body (or oleosome) stabilizing proteins and assumed to be involved as enzymes in the germination process [5–7]. Oil bodies consist of a core of triacylglycerol surrounded by a single layer of phospholipids, embedded with the hydrophobic domain of the oleosins [8, 9]. Being triacylglycerol storage organelle in seeds, oleosomes can be found among a variety of oil-rich plants [8, 10, 11]. In contrast to peanut, the allergenic character of oleosins derived from sesame seeds and hazelnut and their association with serious symptoms has been clearly shown [3, 12, 13]. At least eight peanut-derived oleosins have been identified on the DNA level (www.uniprot.org) and proteomic approaches so far [14, 15]. Resulting from different molecular masses, due to the insertion of additional amino acid residues in the C-terminal domain, a classification into high- or low-Mr isoforms has been established [16, 17].
Two peanut (Ara chis h ypogaea) oleosins, Ara h 10, as well as an isoform, and Ara h 11 were approved by the WHO/IUIS-Allergen Nomenclature Subcommittee (www.allergen.org) as allergens in 2008, but the respective data have not yet been published (February 2015). Concerning the oleosin variant A (Uniprot accession no. Q9AXI1) Pons et al. [18] were able to show IgE-reactivity, but this finding has not been confirmed by others. Beside another isoform (oleosin variant B), which shows a sequence identity of 96% to variant A, an additional isoform, termed oleosin 5, was discovered by our group in 2004 (Uniprot accession no. Q6J1J8). Recently, Kobayashi et al. were able to identify an IgE-reactive epitope on a peptide of oleosin 3 (Uniprot accession no. Q647G3), obtained after enzymatic hydrolysis of peanut total protein, showing cross-reactivity with buckwheat [19].
Interestingly, Olszewski et al. were able to identify peanut-allergic patients reacting to oleosins present in refined peanut oil [20]. Peanut oil has been used as an essential ingredient in different skin care products like face creams, massage oils or ointments for the treatment of atopic eczema [21]. In the light of an ongoing controversial discussion concerning the skin as a possible sensitization route, it is important to know whether refined peanut-derived products (e.g. oil, lecithin) contain lipophilic allergens including oleosins [20–23]. Furthermore, the biotechnological advances of natural and reconstituted oleosomes have been explored regarding their chemical properties for a possible utilization in industry over the last years [6, 10, 24, 25]. Serving as natural emulsifiers or as carriers for active agents, different applications such as foodstuffs, personal care products and pharmaceuticals could be addressed in the future [26–28].
As the allergenicity of peanut oleosins is still a matter of debate, a comprehensive investigation of the molecular characteristics, the immunological features and the isolation conditions is consequently necessary to determine the allergenic potential of these proteins. The biggest problem to overcome is the low solubility of oleosins in aqueous solutions and organic solvents. Hence, the use of strong detergents is needed, which restricts the number of separation techniques considerably. In order to generate highly-sensitive analytical tools to detect oleosins in all kinds of matrices, these problems must first be solved.
In this study, we were able to establish a new method for the simultaneous isolation of all known immunologically distinct peanut oleosins.
Materials and Methods
Preparation of the peanut extract
Roasted peanuts (‘Seeberger Riesen’, Seeberger, Ulm, Germany) were bought in a local supermarket. Unroasted peanuts were purchased in a pet store. Peeled kernels were placed in a mortar, covered with liquid nitrogen and afterwards homogenized using a grinder (Moulinex, model AR100, Alencon, France) for 40 s. Ground peanuts (50 g) were suspended in 200 mL of 0.1 M potassium phosphate buffer, pH 7.2, containing 0.33 M sucrose and stirred for 30 min at room temperature.
Isolation of peanut oil bodies
After filtration, isolation of oil bodies was conducted according to the protocol of Millichip et al. [29] with the following modifications. The homogenate was placed in a 250 mL centrifugation bottle (Thermo Fisher Scientific, Waltham, MA, US) and centrifuged at 16,000 g (15 min, 20°C) (Herolab, model HiCen 21C, Wiesloch, Germany). The oil bodies, which formed a white fat pad floating on the supernatant, were dissolved in 5 mL of a freshly prepared 50 mM Tris/HCl buffer, pH 7.2, containing 9 M urea, by stirring for 10 min. The resuspension was again centrifuged (10,000 g, 15 min, 20°C) in a 50 mL centrifugation tube (Roth, Karlsruhe, Germany) and the fat pad was recovered. This step was repeated three more times. The obtained fat pad was further purified using detergent washing, ionic elution and integrity testing by hexane [30]. Briefly, the collected fat pad was dissolved in 20 mL detergent washing solution (0.1% Tween 20, 0.2 M sucrose, 5 mM sodium phosphate buffer, pH 7.5) and placed at the bottom of a 50 mL centrifugation tube. Afterwards, 10 mL phosphate buffer (10 mM, pH 7.5) was layered on top, and the suspension was centrifuged (10,000 g, 15 min, 20°C). Again, the floating oil bodies were recovered and suspended in 20 mL ionic elution buffer (2 M NaCl, 0.6 M sucrose, 10 mM phosphate buffer, pH 7.5), and overlaid with ionic elution buffer containing 0.25 M instead of 0.6 M sucrose. The centrifuged oleosomes were collected, mixed with 20 mL phosphate buffer (10 mM, pH 7.5) and 20 mL hexane. After centrifugation, the hexane layer was discarded and the oil bodies recovered once again.
Delipidation of the oil bodies
The isolated oleosomes were placed in a 50 mL centrifugation tube and mixed with 3-fold excess of cold acetone to break up the oil body and release neutral lipids. After centrifugation (10,000 g, 5 min, 4°C), precipitated proteins were recovered and washed with acetone another three times. Proteins were then dried under nitrogen and stored at −80°C until use.
Purification of peanut oil body proteins
Oleosins were purified from the precipitated proteins using the Bio-Rad Model 491 Prep Cell (Bio-Rad, Hercules, CA, US) with continuous elution system. Electrophoresis was carried out applying the buffer conditions of the modified tricine-SDS-PAGE protocol of Haider et al. [31]. A 10% (w/v) polyacrylamide resolving gel (7.0 x 3.7 cm ID; 19:1, acrylamide-bis, Roth, Karlsruhe, Germany) was topped with a 4% stacking gel. Extracted oil body proteins (10 mg) were solubilized in 300 μL of a 0.1 M phosphate buffer containing 3% SDS. Afterwards, 500 μL 5x reducing SDS-PAGE sample buffer and 200 μL deionized water were added. The solution was mixed vigorously, heated for 5 min at 95°C and applied onto the gel column. Electrophoresis was performed with a constant current of 50 mA (180–350 V). After elution of the ionic front, fractions of 2 mL were collected at a flow rate of 0.5 mL/min using a Fast Protein Liquid Chromatography system (Äkta prime, Amersham Bioscience, Fairfield, CT, US). Protein elution was monitored at 280 nm, every 10th fractions was analyzed by SDS-PAGE and stained with the sensitive colloidal Coomassie staining protocol of Kang et al. [32] to determine the fractions containing the desired proteins. For the concentration of proteins (5:1), 1 mL of each fraction was transferred into an ultra-centrifugal device with a 3 kDa molecular weight cutoff (Merck, Darmstadt, Germany). Protein concentration was determined using the Bradford assay with BSA as standard (Thermo Fisher Scientific, Waltham, MA, US).
SDS-PAGE and Western blotting
SDS-PAGE was conducted according to Laemmli et al. [33] using the XCELL Mini Cell System (Novex, San Diego, CA, US) with 12% polyacrylamide gels (Life Technologies, Carlsbad, CA, US) under reducing conditions. After electrophoresis, proteins were visualized by staining with colloidal Coomassie. Immunoblotting was carried out using the semi-dry blotting technique [34]. Proteins were transferred at 0.8 mA/cm2 for 45 min onto a methanol-activated polyvinylidene fluoride (PVDF) membrane (pore size 0.45 μm, Millipore Corporation, Billerica, MA, US), which was blocked thereafter with TTBS (Tris buffered saline with Tween 20; 100 mM Tris, 100 mM NaCl, 2.5 mM MgCl2, 0.05% Tween 20, pH 7.4) supplemented with 2.5% skimmed milk powder. Membrane strips were incubated with diluted patients’ sera (1:10 in TTBS, with 2.5% skimmed milk) or anti-oleosin antibodies [35] (1:5,000 in TTBS, with 2.5% skimmed milk) overnight. Detection of bound antibodies was conducted using alkaline phosphatase-conjugated secondary antibodies, mouse anti-human IgE (Pharmingen, San Diego, CA, US, 1:10,000) or goat anti-rabbit IgG (Jackson Immuno Research, West Grove, PA, US, 1:5,000), diluted in TTBS for 2 h. Immunostaining was carried out according to Blake et al. [36]. Blotted proteins were stained with 0.1% Coomassie in 50% methanol and Gold solution (Sigma Aldrich, St. Louis, MO, US).
Protein sequencing
After electrophoresis, N-terminal microsequencing was performed as described elsewhere [37]. Protein transfer was achieved by semi-dry blotting onto PVDF membrane using 10 mM N-cyclohexyl-3-aminopropanesulfonic acid with 10% methanol (pH 11.0) as transfer buffer. Afterwards, the membrane was washed with deionized water and stained by 0.1% Coomassie in 50% methanol, destained in 50% methanol and air dried. Excised protein bands were sequenced using a Procise protein sequencer with online PTH (Phenylthiohydantoin) amino acid analyzer (PE Biosystems, Weiterstadt, Germany).
In gel digestion of proteins and peptide mass fingerprinting
Coomassie-stained protein bands were excised and prepared as described [38]. Briefly, excised and chopped bands were washed, destained and digested by trypsin (Trypsin Gold, Mass Spectrometry Grade, Promega, Mannheim, Germany) overnight. The ZipTip (C18, Millipore Corporation, Billerica, MA, US) eluates of the obtained tryptic fragments were mixed 1:1 (v/v) with a 12 mg/mL α-cyano-4-hydroxycinnamic acid matrix (Bruker Daltonics, Bremen, Germany), dissolved in a 2:1 (v/v) mixture of 100% acetonitrile/0.3% TFA, and spotted on the target. Tryptic mass fingerprinting was performed as described previously [39] using a Reflex III (Bruker Daltonics, Bremen, Germany) in reflector mode, while applying an acceleration voltage of 20 kV. External mass calibration was performed with peptide standard II (Bruker Daltonics, Bremen, Germany). Mascot Peptide Mass Fingerprint (http://matrixscience.com) and NCBInr database were used to identify digested fragments. For database search the following filters were applied: taxonomy on other green plants, peptide tolerance of ± 0.3 Da and up to one allowed missed cleavage. Variable modifications were the oxidation of methionine residues and N-terminal acetylation.
Human sera and rabbit antiserum
Sera of four patients with a positive clinical history of peanut allergy were used for the evaluation of IgE reactivity of purified peanut oleosins in immunoblot analysis. Sera of a healthy individual and an allergic patient (inhalant allergy) without symptoms after consumption of peanuts and without sensitization to peanut were used as controls. All patients who were known to be allergic to peanut refused to have oral challenge tests with foods, due to severe reactions in their history.
The anti-oleosin antibody was obtained after the immunization of rabbits with a multi-antigenic peptide (MAP) with the following sequences: VQVHTPTTQRVDVPR and MADYVGQKTKDAGQQ taken from Q9AXI1 [35]. These sequences are located in the N- and C-terminal domain of oleosin 5 and oleosin variant A. Similarly, these sequences can be found in oleosin variant B, but with one replaced amino acid in each sequence. Additionally, MADYVGQKTKDAGQQ shows an identity of 76% to a sequence in both Ara h 10 isoforms and enables their detection.
Ethics Statement
The research was conducted according to the principles expressed in the Declaration of Helsinki, and approved by local ethics committee of the University of Luebeck (approval number 10–126). All patients gave a written informed consent.
The immunization of the animals was carried out in strict accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. Due to German animal protection law, the permission for animal experiments was given by the governmental animal welfare committee which in our district belongs to the Landesamt für Natur, Umwelt und Verbraucherschutz, Nordrhein-Westfalen (Seibertzstraße 1, 59821 Arnsberg, Nordrhein-Westfalen) (reference number: 23.8720 Nr. S-anzeige 11) which is the successor of the Bezirksregierung Arnsberg. In order to achieve enough blood it was necessary to sacrifice the rabbits by exsanguination after anesthesia. The anesthesia was performed with Ketaminhydrochlorid (Ketanest, Parke-Davis, Berlin, Germany) and Xylazin (Rompun, Bayer, Leverkusen, Germany). Before performing the injection of the antigen-adjuvant emulsion in 4 volumes of approximately 200 μl per injection near the dorsal shoulders, the site was locally anesthetized with Lidocainhydrochlorid (Xylocain, Astra Zeneca, Södertälje, Sweden). Local anesthesia with Lidocainhydrochlorid of the ear was also performed before blood sampling from the rabbit (500 μl from the ear vein).
Results and Discussion
Isolation of oil body proteins
Oleosins are the most abundant oil body proteins, comprising of a hydrophobic domain which is embedded into the oil body matrix, and an amphipathic N- and C-terminal domain covering the entire surface of the oleosome [14]. It has been suggested that at neutral pH the positively charged amino acids of the amphipathic domains are directed towards the negatively charged head groups of the phospholipids, whereas the negatively charged amino acids are directed towards the exterior [40, 41]. Thus, the steric hindrance and the negative charge prevent the coalescence of oil bodies [17, 42]. However, the electrostatic repulsion depends on both, the pH and the ionic strength of the aqueous environment [43, 44]. At a pH close to the isoelectric point of the oleosomes (between pH 5–6) aggregation starts due to an attenuation of the electrostatic repulsion [43–45]. The same effect can be observed with increasing salt concentrations as cations (e.g. Na+, Ca2+) are able to shield the electrostatic repulsion of the negatively charged amino acids on the oil body surface [44, 46]. However, this oleosome aggregation seems to be reversible [42].
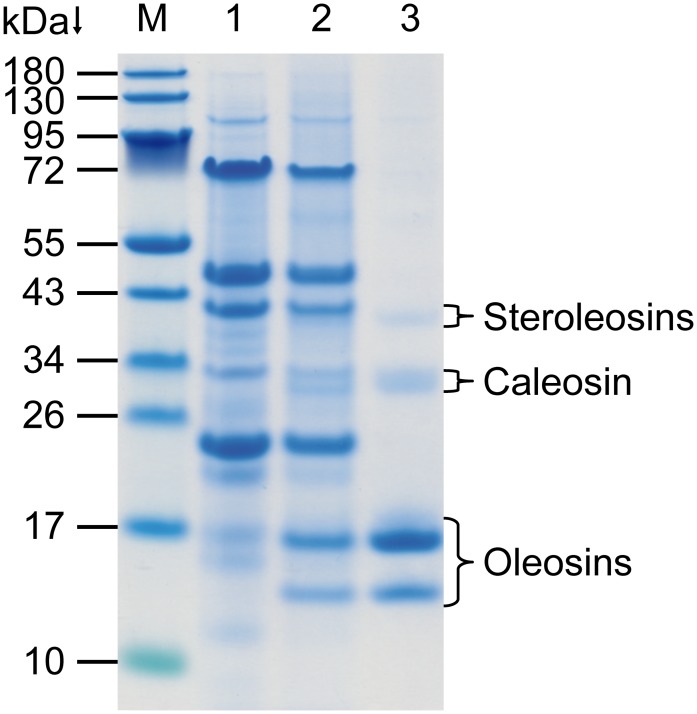
Isolation and purification of oil bodies from unroasted peanut seeds were conducted for structural characterization by centrifugation-flotation, treatment with urea, detergent washing, ionic elution and integrity testing by use of hexane (Fig 1). In particular, the neutral pH of the solutions used provided a fully accessible surface of the individual oil bodies and supports the separation of non-specifically associated proteins in this step by step procedure. Additional washing with cold acetone facilitated the isolation of oil body proteins by way of the oil body disintegration and lipid removal. The fat pad obtained after the first centrifugation of the total extract (Fig 1, lane 2) differs from the protein composition of the initial extract (Fig 1, lane 1) especially between 14–17 kDa. Owing to the specific molecular masses these proteins are assumed to be oleosins [14, 15]. Further washing removed most of the seed storage proteins (globulins and albumins) and intensified these oleosin bands along with three pale protein bands of about 30 kDa and 40 kDa, confirming that these proteins are constituents of the oleosomes (Fig 1, lane 3). This finding is in accordance with the recent discovery of minor oil body proteins called caleosins and steroleosins in diverse species, comprising of a molecular mass of about 30 kDa and 40 kDa, respectively [47–49].
Fig 1. Purification steps of the oil body isolation from unroasted peanuts shown by SDS-PAGE.
M, molecular mass marker; lane 1, total extract; lane 2, fat pad obtained after centrifugation of the total extract; lane 3, purified oil bodies.
Purification of oil body proteins
An additional problem beyond the close molecular masses which must be overcome while purifying oleosins is their tendency to interact with each other due to their hydrophobic nature. The interaction leads to the formation of self or mixed aggregates, even when applying strong denaturing conditions, and prevents a separation with powerful HPLC procedures, leading to co-elution and elution of micellar aggregates [29, 50]. High ionic strength even facilitates the dimerization and oligomerization reactions [50, 51]. In general, the use of strong detergents to prevent aggregation and to keep oleosins in solution is crucial. Experiments performed by Kim et al. demonstrated that a phosphate buffer (well known for its solubilization properties), supplemented with SB 3–10 (zwitterionic detergent), NP-40 or dodecyl-maltoside (both non-ionic detergents) had the most significant effects on the solubility of oleosins, whereas other kinds of detergents (e.g. Brij 56, CHAPS) were less effective [52, 53]. In some cases the addition of chaotropes like urea and thiourea or 10% glycerol further enhanced the solubility of oleosins [52, 53]. Similarly, the anionic detergent SDS is able to solubilize crude oil body proteins and to impede the dimerization processes, even when the solution is stored several months (own observations). Since the application of detergents on HPLC systems causes often problems, purification of the oil body proteins was conducted using a continuous-elution electrophoresis cell combined with the low salt concentration protocol developed by Haider et al. [31].
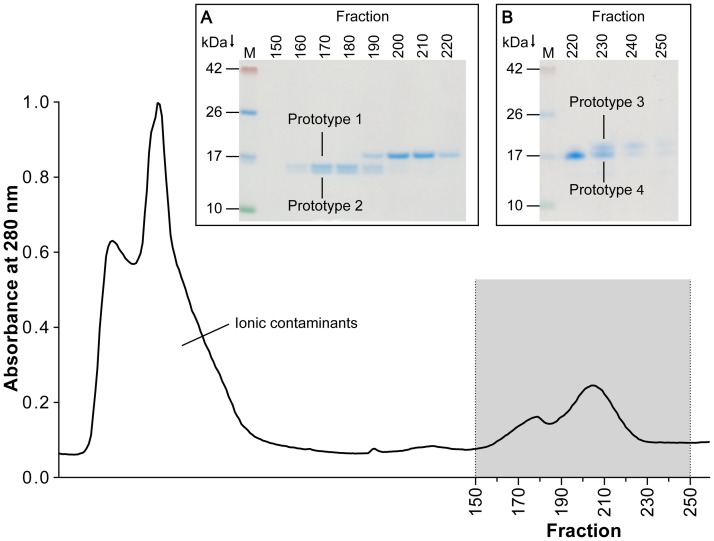
After elution of the ionic front and dye, a peak occurring at fraction 160 indicated the elution of two oleosins with molecular masses of roughly 15.5 kDa and 16 kDa as shown by SDS-PAGE analysis (Fig 2, insert A). A progressive transition between these proteins and an additional protein of approximately 17 kDa was monitored at fraction 190, whereas successive fractions only contained the protein of higher molecular weight. A further transition of proteins was observable only after concentrating fractions 220-250 (5:1) (Fig 2, insert B), showing a protein comprising of a molecular mass of about 17.5 kDa. In contrast to the findings of Pons et al. [49], our results show that the oleosin variants A and B (17.5 kDa band) are not the major peanut oleosins and indicate an almost equal distribution of all oleosins among the oleosome. Previously published data also support our results [15].
Fig 2. Representative elution profile of oil body proteins separated by preparative electrophoresis monitored at 280 nm.
Electrophoresis was performed with extracted oil body proteins on a 10% polyacrylamide resolving gel, and fractions of 2 mL were collected. Fractions corresponding to the main peaks were visualized by SDS-PAGE on 12% polyacrylamide gels with Coomassie staining (Insert). M, marker; (A) Fraction 150–220 (B) Enriched (5:1) fractions 220–250, for prototype classification see Table 1.
Isolation of single compounds by using altered conditions was not successful (data not shown) owing to the limited resolution of the Prep Cell. Nevertheless, the separation achieved was sufficient to identify the different oleosins in the fractions by peptide mass fingerprinting, N-terminal sequencing and specific antibodies. Thus, the requisite to investigate the specificity of patient-IgE is given.
A classification of peanut oleosins into high and low molecular isoforms according to Tzen [16] has been arranged by Jolivet et al. [14]. Although this clustering might be appropriate for a basic molecular and immunological differentiation of oleosins [54], it does not reflect the immunogenic properties of a group of eight peanut-derived oleosins. Therefore, we propose a new classification model (Table 1) based on the sequence identity, contributing to the fact that similar sequences are recognized equally by patients’ IgE. This model clusters the peanut oleosins in four distinct groups of immunogenic related compounds called ‘prototypes’.
Table 1. Classification of peanut oleosins.
| Name | Uniprot accession no. | Isoform [14] | Number of amino acids |
|---|---|---|---|
| Prototype 1 | |||
| Ara h 10.0101 | Q647G5 | H-oleosin | 169 |
| Ara h 10.0102 | Q647G4 | H-oleosin | 150 |
| Prototype 2 | |||
| Ara h 11.0101 | Q45W87 | L-oleosin | 137 |
| Isoform of Ara h 11.0101 | Q45W86 | L-oleosin | 137 |
| Prototype 3 | |||
| Oleosin variant A | Q9AXI1 | H-oleosin | 176 |
| Oleosin variant B | Q9AXI0 | H-oleosin | 176 |
| Oleosin 5 | Q6J1J8 | H-oleosin1 | 176 |
| Prototype 4 | |||
| Oleosin 3 | Q647G3 | L-oleosin | 166 |
1 not classified by Jolivet et al. [14], but in analogy to the oleosin variants A and B
Characterization of the oleosins by peptide mass fingerprinting and N-terminal sequencing
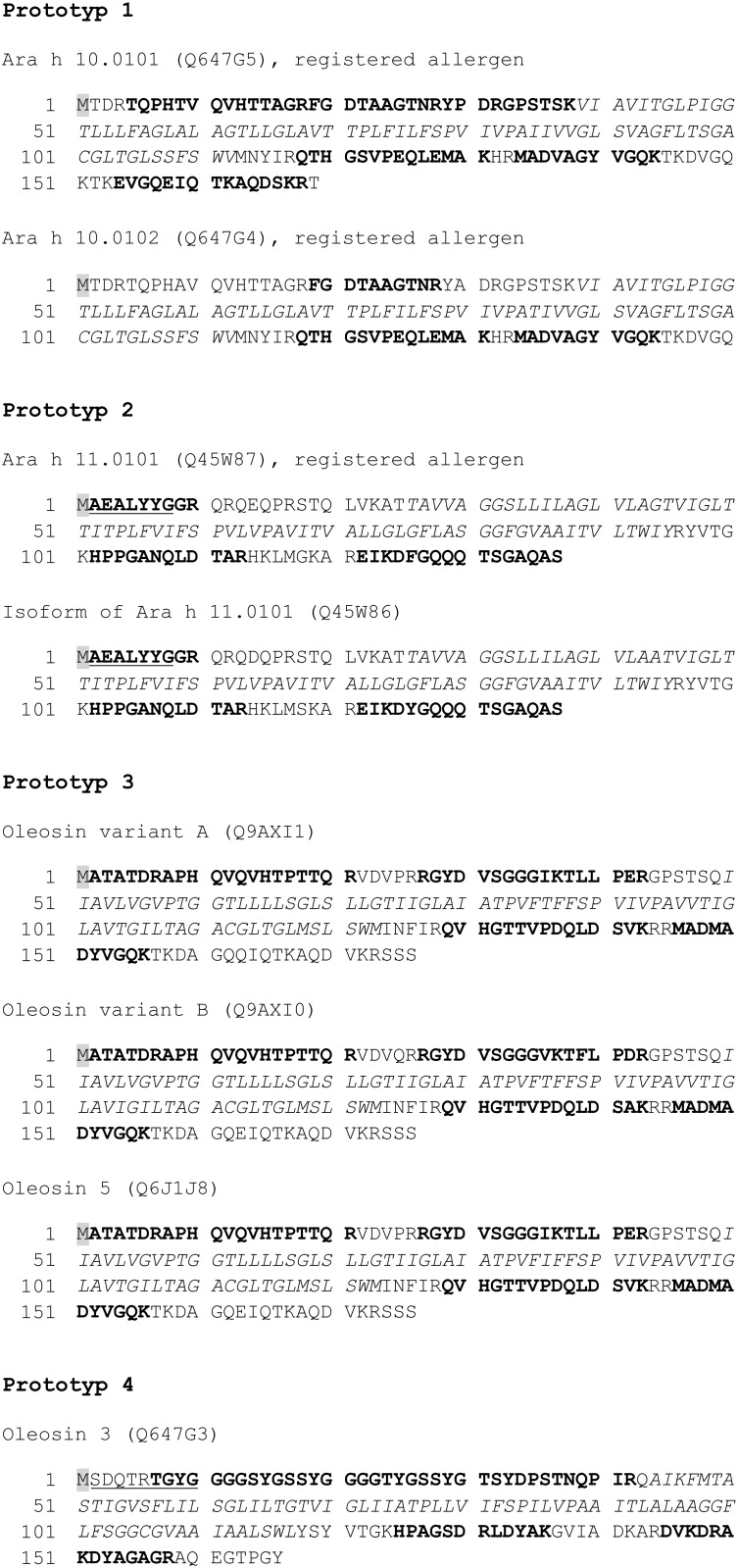
Using tryptic mass fingerprint analysis and N-terminal sequencing including homology search, we were able to identify oleosins from each prototype (Fig 3). Due to the cleavage by trypsin exclusively in the C-terminal region of arginine and lysine residues, the hydrophobic central domain is inaccessible for enzymatic digestion due to a lack of cleavage sites [55]. Thus, cleavage is only possible in the hydrophilic C- and N-terminal region of the oleosins, reducing the overall coverage of the protein to approximately 50%. However, a certain number of peptide fragments resulting from specific cleavage has been identified for each oleosin of the different prototypes. In addition to that, acetylation of the N-terminal domain of oleosins was observed by MALDI-TOF-MS (mass difference: 42 Da), except for prototype 1 and prototype 4. In contrast to Lin et al. [56], oleosin fragments without acetylation were also observable among the different prototypes, but to a much lesser extent. Thus, N-terminal sequencing of the prototypes 2 and 4 was successful, revealing the sequences AEALYYG and SDQTRTGYG, respectively (Fig 3). However, the N-terminal methionine residue was neither detected with MALDI-TOF-MS nor N-terminal sequencing, which corresponds to previously published data of sesame oleosins [56]. A translational modification of the N-terminal domain of Ara h 10 has been described [14], but the peptide fragment carrying a possible acetylation was not detected in our experiments. Acetylation of the N-terminal domain is a common posttranslational modification in eukaryotic organisms [57, 58], regulating enzymatic activities [59], increasing thermal stability [60] and preventing ubiquitin-dependent degradation [60, 61]. Since oleosomes are designated for long-term storage of lipids, the preservation of oleosins is a key element to maintain the integrity of oil bodies.
Fig 3. Identification of peanut oleosins using peptide mass fingerprinting and N-terminal sequencing.
Peptides resulting from tryptic digestion were searched against the NCBInr database using the Mascot search engine. Bold letters indicate identified sequences by MALDI-TOF-MS, whereas underlined letters mark sequences obtained after N-terminal sequencing. The hydrophobic domains, not accessible to tryptic cleavage, are written in italics. The methionine residue, highlighted in grey, was not observed.
Moreover, we were able to prove the existence of a peanut caleosin by a proteomic approach for the first time (S1 Fig) and verified the presence of two peanut steroleosins (Uniprot accession no. A7LB59, A7LB60) (S2 Fig).
Immunological recognition of peanut oleosins
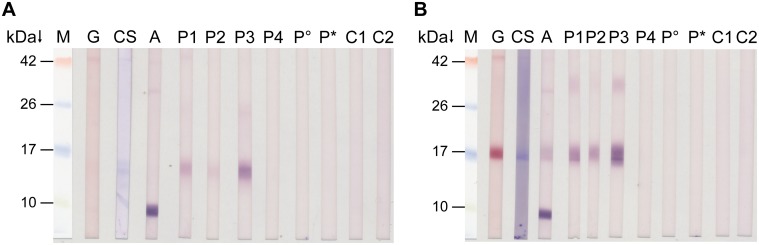
For the assessment of the allergenic properties of the oleosin prototypes, roasted peanuts were chosen, following the developed isolation strategy, because in the western countries peanuts are usually consumed after roasting. Pooled fractions of prototype 1/prototype 2 and prototype 3/prototype 4 were subjected to SDS-PAGE and subsequent Western blotting (10 μg/cm). Sera from four peanut-allergic individuals were screened for IgE reactivity (Fig 4). Interestingly, all four prototypes were recognized exclusively by the sera of three patients (Fig 4A and 4B, lane P1–P3) with a clinical history of severe anaphylactic reactions to peanut. The symptoms included anaphylactic shock, cardiac problems, generalized urticarial, dyspnea, hypotonia, angioedema, laryngeal edema, flush, with minor differences between the three patients. The fourth peanut-allergic patient with moderate to severe symptoms did not display a reaction to any oleosin prototype at all (Fig 4A and 4B, lane P4). This can be explained by the fact that in this case the peanut allergy is pollen-associated, which is due to the cross-reactivity between birch pollen and peanut, mainly via the Bet v 1-homologue Ara h 8.
Fig 4. Western blot of oleosin prototypes isolated from roasted peanuts and purified by preparative electrophoresis.
Fractions obtained by preparative electrophoresis containing the prototypes 1/2 (A) and prototypes 3/4 (B) were pooled, subjected to SDS-PAGE and subsequent immunoblotting. M, molecular mass marker; G, Gold staining; CS, Coomassie staining; A, polyclonal anti-oleosin antibody; P1–P4, allergic patients’ sera; P°, allergic individual (not to peanut) with respiratory symptoms; P*, healthy control; C1, secondary antibody control (mouse anti-human IgE antibody); C2, secondary antibody control (goat anti-rabbit IgG antibody).
Apart from that, detection of proteins of approximately 10 kDa (Fig 4A and 4B, lane A) and 34 kDa has also been observed (Fig 4B, lane A, P1, P3). This shows on the one hand the tendency to decompose into smaller fragments as examined previously [49] and on the other hand the potential to form dimers and oligomers as reported for oleosins from diverse species [49, 51, 62]. Using autoradiography Pons et al. [49] detected a 6 kDa fragment, resulting from the cleavage at the C- or N-termini, but they were not able to observe the corresponding counterpart. Since our anti-oleosin antibody recognized a protein band at 10 kDa, we suggest that this fragment could be the corresponding protein fragment (Fig 4A and 4B, lane A). However, it does not seem to possess immunogenic features for the peanut-allergic patients, as no reaction has been detected so far. The weak reaction to an additional higher molecular mass band at about 34 kDa (Fig 4B, P1 and P3) is likely due to the formation of self or mixed dimers, even occurring when oleosins were stored in detergent-containing solutions.
Although only a small number of peanut-allergic individuals could be tested, we were able to show that, in addition to the accepted allergens (prototype 1 and 2), other oleosins (prototype 3 and 4) are potential allergens possibly associated with severe reactions.
Conclusion
To summarize the major results of this study, a method has been developed that allows the separation of all peanut oleosins described so far. In addition, further oil body constituents, a caleosin and two steroleosins, were isolated and identified. Immunoblot analyses revealed the binding of IgE antibodies in sera of peanut-allergic patients to all prototypes, providing evidence for the allergenicity of the corresponding oleosins. More patients with different clinical phenotypes need to be investigated to expand the knowledge on oleosin allergenicity.
The future component-resolved diagnostics will be performed with recombinant proteins due to obvious advantages of this production technique. The purified natural oleosins provide an excellent tool for the authentication of the recombinant surrogates.
Concerning applications in the food, cosmetic or pharmaceutical industry, a hypoallergenic oleosin respective oleosome would provide an enormous benefit. However, since the testing of the allergenicity of oleosins has just begun, there is a lack of data with respect to the safe use of oleosins for industrial purposes. Aside from that, genetic engineering is used to change the properties of both, the hydrophobic domain and the amphipathic domains by varying amino acid sequences or truncating the central hydrophobic domain to produce specialized surfactants [63]. The overall effect on the allergenicity of these modified oleosins is not predictable and needs to be further investigated in order to establish a safe tool for the industrial use.
Supporting Information
(TIF)
(TIF)
Acknowledgments
We acknowledge Prof. Otto Holst from the Division of Structural Biochemistry and Dr. Gabriele Schramm from the Division of Experimental Pneumology at the Research Center Borstel for the critical and fruitful discussions concerning this project. We also wish to thank Prof. Monika Raulf and Dr. Eva Zahradnik from the Department of Allergology/Immunology at the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-University Bochum for generous support of production of anti-oleosin antibodies. The authors wish to express special thanks to Daniel Rosero for excellent technical assistance and to Rainer Bartels for the N-terminal sequencing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the German Research Foundation (http://www.dfg.de/) Grant no. JA 1007/2-1. Receiver of funding: UJ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Souci S, Fachmann W, Kraut H. Food composition and nutrition tables. Stuttgart: CRC Press Inc; 2000. 1014–5 p. [Google Scholar]
- 2. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. The Journal of allergy and clinical immunology. 2010;125(1):191–7 10.1016/j.jaci.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 3. Zuidmeer-Jongejan L, Fernandez-Rivas M, Winter MG, Akkerdaas JH, Summers C, Lebens A, et al. Oil body-associated hazelnut allergens including oleosins are underrepresented in diagnostic extracts but associated with severe symptoms. Clinical and translational allergy. 2014;4(1):4 10.1186/2045-7022-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bublin M, Eiwegger T, Breiteneder H. Do lipids influence the allergic sensitization process? The Journal of allergy and clinical immunology. 2014;134(3):521–9. 10.1016/j.jaci.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimada TL, Hara-Nishimura I. Oil-body-membrane proteins and their physiological functions in plants. Biol Pharm Bull. 2010;33(3):360–3. [DOI] [PubMed] [Google Scholar]
- 6. Maurer S, Waschatko G, Schach D, Zielbauer BI, Dahl J, Weidner T, et al. The role of intact oleosin for stabilization and function of oleosomes. The journal of physical chemistry B. 2013;117(44):13872–83. 10.1021/jp403893n [DOI] [PubMed] [Google Scholar]
- 7. Parthibane V, Rajakumari S, Venkateshwari V, Iyappan R, Rajasekharan R. Oleosin is bifunctional enzyme that has both monoacylglycerol acyltransferase and phospholipase activities. The Journal of biological chemistry. 2012;287(3):1946–54. 10.1074/jbc.M111.309955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tzen J, Cao Y, Laurent P, Ratnayake C, Huang A. Lipids, proteins, and structure of seed oil bodies from diverse species. Plant physiology. 1993;101(1):267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frandsen GI, Mundy J, Tzen JT. Oil bodies and their associated proteins, oleosin and caleosin. Physiologia plantarum. 2001;112(3):301–7. [DOI] [PubMed] [Google Scholar]
- 10. Adams GG, Imran S, Wang S, Mohammad A, Kok MS, Gray DA, et al. Extraction, isolation and characterisation of oil bodies from pumpkin seeds for therapeutic use. Food chemistry. 2012;134(4):1919–25. 10.1016/j.foodchem.2012.03.114 [DOI] [PubMed] [Google Scholar]
- 11. Rodelas AJD, Regalado ES, Bela-Ong DB, Garcia RN, Laurena AC, Mendoza EMT. Isolation and characterization of the oil bodies and oleosin of Coconut (Cocos nucifera L.). Philippine Agricultural Scientist. 2008;91(4):389–94. [Google Scholar]
- 12. Leduc V, Moneret-Vautrin DA, Tzen JT, Morisset M, Guerin L, Kanny G. Identification of oleosins as major allergens in sesame seed allergic patients. Allergy. 2006;61(3):349–56. [DOI] [PubMed] [Google Scholar]
- 13. Akkerdaas JH, Schocker F, Vieths S, Versteeg S, Zuidmeer L, Hefle SL, et al. Cloning of oleosin, a putative new hazelnut allergen, using a hazelnut cDNA library. Mol Nutr Food Res. 2006;50(1):18–23. [DOI] [PubMed] [Google Scholar]
- 14. Jolivet P, Acevedo F, Boulard C, d'Andrea S, Faure JD, Kohli A, et al. Crop seed oil bodies: from challenges in protein identification to an emerging picture of the oil body proteome. Proteomics. 2013;13(12–13):1836–49. 10.1002/pmic.201300198 [DOI] [PubMed] [Google Scholar]
- 15. White BL, Gökce E, Nepomuceno AI, Muddiman DC, Sanders TH, Davis JP. Comparative proteomic analysis and IgE binding properties of peanut seed and testa (skin). Journal of agricultural and food chemistry. 2013;61(16):3957–68. 10.1021/jf400184y [DOI] [PubMed] [Google Scholar]
- 16. Tzen JT, Lai Y-K, Chan K-L, Huang AH. Oleosin isoforms of high and low molecular weights are present in the oil bodies of diverse seed species. Plant physiology. 1990;94(3):1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tai SSK, Chen MCM, Peng CC, Tzen JTC. Gene family of oleosin isoforms and their structural stabilization in sesame seed oil bodies. Biosci Biotech Bioch. 2002;66(10):2146–53. [DOI] [PubMed] [Google Scholar]
- 18. Pons L, Chery C, Romano A, Namour F, Artesani MC, Gueant JL. The 18 kDa peanut oleosin is a candidate allergen for IgE-mediated reactions to peanuts. Allergy. 2002;57 Suppl 72:88–93. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi S, Katsuyama S, Wagatsuma T, Okada S, Tanabe S. Identification of a new IgE-binding epitope of peanut oleosin that cross-reacts with buckwheat. Bioscience, biotechnology, and biochemistry. 2012;76(6):1182–8. [DOI] [PubMed] [Google Scholar]
- 20. Olszewski A, Pons L, Moutete F, Aimone-Gastin I, Kanny G, Moneret-Vautrin DA, et al. Isolation and characterization of proteic allergens in refined peanut oil. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1998;28(7):850–9. [DOI] [PubMed] [Google Scholar]
- 21. Ring J, Mohrenschlager M. Allergy to peanut oil-clinically relevant? Journal of the European Academy of Dermatology and Venereology: JEADV. 2007;21(4):452–5. [DOI] [PubMed] [Google Scholar]
- 22. Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of P, Children Study T. Factors associated with the development of peanut allergy in childhood. The New England journal of medicine. 2003;348(11):977–85. [DOI] [PubMed] [Google Scholar]
- 23. Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2005;35(6):757–66. [DOI] [PubMed] [Google Scholar]
- 24. Peng CC, Lin IP, Lin CK, Tzen JT. Size and stability of reconstituted sesame oil bodies. Biotechnology progress. 2003;19(5):1623–6. [DOI] [PubMed] [Google Scholar]
- 25. Iwanaga D, Gray DA, Fisk ID, Decker EA, Weiss J, McClements DJ. Extraction and characterization of oil bodies from soy beans: a natural source of pre-emulsified soybean oil. Journal of agricultural and food chemistry. 2007;55(21):8711–6. [DOI] [PubMed] [Google Scholar]
- 26. Bhatla SC, Kaushik V, Yadav MK. Use of oil bodies and oleosins in recombinant protein production and other biotechnological applications. Biotechnology advances. 2010;28(3):293–300. 10.1016/j.biotechadv.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 27. Chang MT, Tsai TR, Lee CY, Wei YS, Chen YJ, Chen CR, et al. Elevating bioavailability of curcumin via encapsulation with a novel formulation of artificial oil bodies. Journal of agricultural and food chemistry. 2013;61(40):9666–71. 10.1021/jf4019195 [DOI] [PubMed] [Google Scholar]
- 28. Chiang CJ, Chen CJ, Lin LJ, Chang CH, Chao YP. Selective delivery of cargo entities to tumor cells by nanoscale artificial oil bodies. Journal of agricultural and food chemistry. 2010;58(22):11695–702. 10.1021/jf102944c [DOI] [PubMed] [Google Scholar]
- 29. Millichip M, Tatham AS, Jackson F, Griffiths G, Shewry PR, Stobart AK. Purification and characterization of oil-bodies (oleosomes) and oil-body boundary proteins (oleosins) from the developing cotyledons of sunflower (Helianthus annuus L.). The Biochemical journal. 1996;314 (Pt 1):333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzen JT, Peng CC, Cheng DJ, Chen EC, Chiu JM. A new method for seed oil body purification and examination of oil body integrity following germination. Journal of biochemistry. 1997;121(4):762–8. [DOI] [PubMed] [Google Scholar]
- 31. Haider SR, Reid HJ, Sharp BL. Modification of tricine-SDS-PAGE for online and offline analysis of phosphoproteins by ICP-MS. Analytical and bioanalytical chemistry. 2010;397(2):655–64. 10.1007/s00216-010-3588-9 [DOI] [PubMed] [Google Scholar]
- 32. Kang DH, Gho YS, Suh MK, Kang CH. Highly sensitive and fast protein detection with coomassie brilliant blue in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. B Kor Chem Soc. 2002;23(11):1511–2. [Google Scholar]
- 33. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. [DOI] [PubMed] [Google Scholar]
- 34. Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polycrylamide to nitrocellulose. Journal of biochemical and biophysical methods. 1984;10(3):203–9. [DOI] [PubMed] [Google Scholar]
- 35.Riecken S. Untersuchungen zum Allergenrepertoire der Erdnuss: Molekulare Charakterisierung von Ara h 7, Ara h 8, Oleosin und LTP PhD Thesis. 2008:69.
- 36. Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136(1):175–9. [DOI] [PubMed] [Google Scholar]
- 37. Petersen A, Dresselhaus T, Grobe K, Becker WM. Proteome analysis of maize pollen for allergy-relevant components. Proteomics. 2006;6(23):6317–25. [DOI] [PubMed] [Google Scholar]
- 38. Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–8. [DOI] [PubMed] [Google Scholar]
- 39. Petersen A, Suck R, Lindner B, Georgieva D, Ernst M, Notbohm H, et al. Phl p 3: structural and immunological characterization of a major allergen of timothy grass pollen. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2006;36(6):840–9. [DOI] [PubMed] [Google Scholar]
- 40. Huang AH. Oil bodies and oleosins in seeds. Annual review of plant biology. 1992;43(1):177–200. [Google Scholar]
- 41. Alexander LG, Sessions RB, Clarke AR, Tatham AS, Shewry PR, Napier JA. Characterization and modelling of the hydrophobic domain of a sunflower oleosin. Planta. 2002;214(4):546–51. [DOI] [PubMed] [Google Scholar]
- 42. Tzen J, Lie G, Huang A. Characterization of the charged components and their topology on the surface of plant seed oil bodies. Journal of Biological Chemistry. 1992;267(22):15626–34. [PubMed] [Google Scholar]
- 43. Nikiforidis CV, Kiosseoglou V. Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil-in-water emulsion. Journal of agricultural and food chemistry. 2009;57(12):5591–6. 10.1021/jf900771v [DOI] [PubMed] [Google Scholar]
- 44. Waschatko G, Schiedt B, Vilgis TA, Junghans A. Soybean oleosomes behavior at the air–water interface. The Journal of Physical Chemistry B. 2012;116(35):10832–41. 10.1021/jp211871v [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Ono T. Simple extraction method of non-allergenic intact soybean oil bodies that are thermally stable in an aqueous medium. Journal of agricultural and food chemistry. 2010;58(12):7402–7. 10.1021/jf1006159 [DOI] [PubMed] [Google Scholar]
- 46. White D, Fisk I, Mitchell J, Wolf B, Hill S, Gray D. Sunflower-seed oil body emulsions: rheology and stability assessment of a natural emulsion. Food Hydrocolloids. 2008;22(7):1224–32. [Google Scholar]
- 47. Chen EC, Tai SS, Peng CC, Tzen JT. Identification of three novel unique proteins in seed oil bodies of sesame. Plant & cell physiology. 1998;39(9):935–41. [DOI] [PubMed] [Google Scholar]
- 48. Jolivet P, Roux E, D'Andrea S, Davanture M, Negroni L, Zivy M, et al. Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant physiology and biochemistry: PPB / Societe francaise de physiologie vegetale. 2004;42(6):501–9. [DOI] [PubMed] [Google Scholar]
- 49. Pons L, Chery C, Mrabet N, Schohn H, Lapicque F, Gueant JL. Purification and cloning of two high molecular mass isoforms of peanut seed oleosin encoded by cDNAs of equal sizes. Plant physiology and biochemistry: PPB / Societe francaise de physiologie vegetale. 2005;43(7):659–68. [DOI] [PubMed] [Google Scholar]
- 50. Pons L, Olszewski A, Gueant JL. Characterization of the oligomeric behavior of a 16.5 kDa peanut oleosin by chromatography and electrophoresis of the iodinated form. Journal of chromatography B, Biomedical sciences and applications. 1998;706(1):131–40. [DOI] [PubMed] [Google Scholar]
- 51. Li M, Smith LJ, Clark DC, Wilson R, Murphy DJ. Secondary structures of a new class of lipid body proteins from oilseeds. The Journal of biological chemistry. 1992;267(12):8245–53. [PubMed] [Google Scholar]
- 52. Kim H, Kim S-Y, Han NS, Tao BY. Solubilization conditions for hydrophobic membrane protein, oleosin, in soybeans. Biotechnology and Bioprocess Engineering. 2007;12(5):542–7. [Google Scholar]
- 53. Cabanos C, Katayama H, Tanaka A, Utsumi S, Maruyama N. Expression and purification of peanut oleosins in insect cells. The protein journal. 2011;30(7):457–63. 10.1007/s10930-011-9351-z [DOI] [PubMed] [Google Scholar]
- 54. Tzen JTC, Chuang RLC, Chen JCF, Wu LSH. Coexistence of both oleosin isoforms on the surface of seed oil bodies and their individual stabilization to the organelles. Journal of biochemistry. 1998;123(2):318–23. [DOI] [PubMed] [Google Scholar]
- 55. Olsen JV, Ong SE, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Molecular & cellular proteomics: MCP. 2004;3(6):608–14. [DOI] [PubMed] [Google Scholar]
- 56. Lin LJ, Liao PC, Yang HH, Tzen JT. Determination and analyses of the N-termini of oil-body proteins, steroleosin, caleosin and oleosin. Plant physiology and biochemistry: PPB / Societe francaise de physiologie vegetale. 2005;43(8):770–6. [DOI] [PubMed] [Google Scholar]
- 57. Martinez A, Traverso JA, Valot B, Ferro M, Espagne C, Ephritikhine G, et al. Extent of N-terminal modifications in cytosolic proteins from eukaryotes. Proteomics. 2008;8(14):2809–31. 10.1002/pmic.200701191 [DOI] [PubMed] [Google Scholar]
- 58. Driessen H, De Jong W, Tesser G, Bloemendal H. The mechanism of N-terminal acetylation of protein. Critical Reviews in Biochemistry and Molecular Biology. 1985;18(4):281–325. [DOI] [PubMed] [Google Scholar]
- 59. Smyth DG, Massey DE, Zakarian S, Finnie MD. Endorphins are stored in biologically active and inactive forms: isolation of alpha-N-acetyl peptides. Nature. 1979;279(5710):252–4. [DOI] [PubMed] [Google Scholar]
- 60. Permyakov SE, Vologzhannikova AA, Emelyanenko VI, Knyazeva EL, Kazakov AS, Lapteva YS, et al. The impact of alpha-N-acetylation on structural and functional status of parvalbumin. Cell Calcium. 2012;52(5):366–76. 10.1016/j.ceca.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 61. Hershko A, Heller H, Eytan E, Kaklij G, Rose IA. Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(22):7021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee K, Ratnayake C, Huang AH. Genetic dissection of the co-expression of genes encoding the two isoforms of oleosins in the oil bodies of maize kernel. The Plant journal: for cell and molecular biology. 1995;7(4):603–11. [DOI] [PubMed] [Google Scholar]
- 63. Vargo KB, Sood N, Moeller TD, Heiney PA, Hammer DA. Spherical micelles assembled from variants of recombinant oleosin. Langmuir. 2014;30(38):11292–300. 10.1021/la502664e [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.