Abstract
A substantive literature has accumulated implicating sphingolipids, in particular ceramides, as mediators of insulin resistance in metabolic syndrome. Thanks to recent technical advances in mouse genetics and lipidomics, two independent laboratories identify the same sphingolipid, C16:0-ceramide, as principal mediator of obesity-related insulin resistance.
The term “metabolic syndrome” defines a constellation of distinct clinical entities that present together in aging populations of wealthy and newly developed nations. Insulin resistance, obesity, hyperlipidemia, and hypertension represent core abnormalities of metabolic syndrome. This syndrome accelerates progression of major diseases such as atherosclerotic vascular disease and type 2 diabetes, leading to increased morbidity and mortality. Refinement of criteria to improve our understanding of progression of these comorbid conditions is an active area of ongoing investigation (Eckel et al., 2010).
Much evidence supports altered sphingolipid metabolism and, more specifically, enhanced ceramide generation as integral to progression of type 2 diabetes and insulin resistance, contributing to metabolic syndrome (Chavez and Summers, 2012). In addition to ceramide, glycosphingolipid metabolism may be deregulated in type 2 diabetes (Hla and Dannenberg, 2012). Thus, molecular description of specific sphingolipid species-mediating disease pathophysiology is critical. As estimates of the number of bioactive sphingolipid mediators range from 4,000 to 60,000 (Merrill, 2011), this is indeed a daunting challenge. Now, two Cell Metabolism articles conclude that a specific ceramide species, C16:0-ceramide, mediates the key pathophysiology of insulin resistance (Turpin et al., 2014; Raichur et al., 2014).
Sphingolipids serve two distinct and interrelated purposes in eukaryotes: they act as building blocks of biologic membranes and as bioactive lipids. Although sphingosine 1-phosphate was originally considered a classic second messenger, it is now recognized as an extracellularly acting lipid mediator signaling via G protein-coupled receptors to regulate multiple organ systems (Hla and Dannenberg, 2012). In contrast, ceramides, often generated after stress, mediate complex intracellular functions in a topographically restricted manner. For instance, plasma membrane stress in some cells (i.e., endothelium, hepatocytes, oocytes) releases acid sphingomyelinase from secretory vesicles, generating cell-surface ceramide within seconds, whereas unrepaired DNA damage in numerous cells stimulates prolonged de novo ceramide synthesis via ceramide synthase in mitochondrial membranes, which, either as an intact molecule or after processed degradation to hexadecenal, triggers mitochondrial outer membrane permeabilization commitment to apoptosis (Chipuk et al., 2012).
Two complementary papers published in Cell Metabolism independently conclude that C16:0-ceramide mediates the pathophysiology of insulin resistance (Turpin et al., 2014; Raichur et al., 2014) (Figure 1). These studies were made possible by recent cloning of the six mammalian ceramide synthases (CerS1–6), which acylate sphinganine to distinct dihydroceramides or in some instances free sphingosine to ceramides (salvage pathway) (Chavez and Summers, 2012); development of mass spectrometry-based lipidomic analysis of long-chain ceramide (C16:0–C20:0) and very-long-chain ceramide (C22:0–C24:1) species (Merrill, 2011); and a growing awareness that different ceramide species manifest specific biologic properties. A developing consensus is that while C16:0-ceramide is proapoptotic, the C24-ceramide series confers antiapoptotic and proliferative functions (Park et al., 2014). These differences may reflect the impact of different ceramide species on biophysical properties of membranes, as C16:0-ceramide is the only ceramide species so far shown to be capable of inducing generation of ceramide-rich platforms, large (1–5 μM) nonbilayer structures in mammalian membranes into which select proteins insert and oligomerize for transmembrane signal transmission (Stancevic and Kolesnick, 2010).
Figure 1. Impact of High-Fat Diet-Induced C16:0-Ceramide on Metabolic Dysregulation.
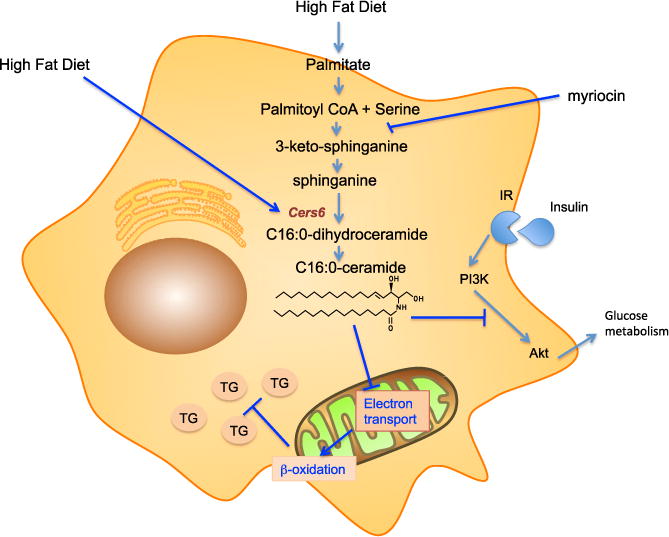
Sphingolipid synthesis begins with condensation of palmitoyl-CoA with serine forming 3-ketosphinganine, which is reduced to dihydrosphingosine (a.k.a. sphinganine) and thereafter acylated to dihydroceramide. Further reduction of the sphingoid base sphinganine backbone by formation of a trans-double bond yields ceramide, a central molecule in sphingolipid metabolism. Transfer of a phosphocholine headgroup from phosphatidylcholine to ceramide yields sphingomyelin, the most abundant mammalian sphingolipid, concentrated in the external plasma membrane, while glycosylation in Golgi begins the formation of a plethora of glycosphingolipids with complex carbohydrate headgroups. Alternately, ceramide deacylation by ceramidase frees up sphingosine for compartmentalized phosphorylation yielding sphingosine 1-phosphate. In the context of insulin resistance, high-fat diet selectively upregulates expression of CerS6, the C16:0-ceramide synthetic enzyme, and concomitantly provides increased amounts of the CerS6 substrate palmitate, enhancing C16:0-ceramide production. C16:0-ceramide induces insulin resistance by antagonizing insulin receptor (IR)-stimulated PI3K/Akt signaling, which attenuates glucose metabolism. In addition, Akt normally stimulates lipid metabolism by SREBP-dependent mechanisms, which would presumably be inhibited, although not directly investigated in the current manuscripts. C16:0-ceramide also appears to directly inhibit mitochondrial electron transport, by an as-yet-unknown mechanism, thereby indirectly suppressing β-oxidation. Inhibition of β-oxidation reduces fatty acid disposal, promoting accumulation of excessive TG in lipid droplets.
Both teams use mouse genetics to address insulin resistance mechanisms. Turpin et al. (2014) show that wild-type mice on a high-fat diet (HFD) manifest increased long-chain ceramides and selectively increased expression of CerS6, the enzyme responsible for mammalian C16:0-ceramide synthesis. CerS6 knockout mice, which accommodate loss of C16:0-ceramide by upregulating C24-ceramides, among others, display minor abnormalities at baseline, yet are protected from HFD-induced obesity and glucose intolerance. Protection from diet-induced obesity (DIO) is manifest as reduction in body weight, adiposity, adipocyte size, serum leptin, macrophage infiltration, and proinflammatory gene expression in white adipose tissue (WAT). Concomitantly, CerS6 knockouts display decreased circulating insulin, and increased glucose tolerance, insulin sensitivity, and activation of insulin effector signaling kinases: Akt and its target GSK3β in liver.
Turpin et al. (2014) went the extra distance, making tissue-specific Cers6 knockouts in macrophages and brown adipose tissue (BAT). Whereas CerS6 deletion in macrophages had little impact on the panoply of abnormalities observed in DIO, ~50% loss of CerS6 in BAT reduced DIO and improved energy expenditure. Adipocytes isolated from CerS6 knockout mice displayed increased mitochondrial β-oxidative capacity, while glycolysis was unaltered. Liver-specific CerS6 knockouts displayed only subtle protection from HFD-induced weight gain, yet significantly improved glucose intolerance. Furthermore, isolated hepatocytes from this strain displayed protection from palmitate-induced attenuation of insulin-stimulated Akt activation. Altogether, these studies support a critical role for CerS6-derived C16:0-ceramide in obesity-related metabolic syndrome.
Raichur et al. (2014) used heterozygous CerS2 knockout mice, which manifest reduction of C24 series ceramides and compensatory upregulation of C16:0-cer-amide. Such compensatory reregulation of ceramide species to maintain overall ceramide levels is a developing theme. On normal chow, CerS2+/− mice were indistinguishable from wild-type mice; however, on HFD CerS2+/− mice displayed increased liver weight, triglycerides, macrophage infiltration, circulating liver enzymes, and plasma cholesterol, indicating liver damage. These mice also displayed slightly impaired glucose tolerance, high fasting and fed insulin levels, increased circulating insulin upon glucose tolerance testing, decreased insulin sensitivity during insulin tolerance testing, decreased ambulatory activity, and increased fat to lean mass, accompanied by a small reduction in insulin-induced Akt phosphorylation. Altogether, this resembles a phenotype opposite to the CerS6 knockout. Critically, myriocin, a specific serine-palmitoyl transferase inhibitor, reduces ceramide species and abrogates CerS2+/− insulin resistance, indicating it is not loss of the C24 series that mediates insulin resistance, but rather C16:0-ceramide upregulation.
Detailed analysis of genes involved in triglyceride synthesis ruled out transcriptional regulation as the mechanism of triglyceride accumulation in CerS2+/− mice, nor was there reduced lipoprotein synthesis. To address mechanism, Raichur et al. (2014) resorted to primary hepatocyte culture. Indeed CerS2+/− hepatocytes display the insulin-resistant phenotype ex vivo. Further, they accumulate mediumchain acylcarnitines implicated in cardiovascular pathology (Shah et al., 2012) and exhibit markedly diminished rates of fatty acid oxidation, basal respiration, ATP turnover, and maximal and spare respiratory activity. Analysis of electron transport chain complexes revealed reduced Complex II and IV activity. Further, hepatocytes isolated from myriocin-treated wild-type mice showed increased Complex IV activity. Consistent with C16:0-ceramide as mediator of metabolic syndrome, adenoviral CerS6 overexpression in wild-type C57BL6/J hepatocytes reduced Complex II activity, leading to increased triglyceride accumulation and reduced insulin-stimulated Akt phosphorylation. In contrast to studies using CerS6 knockouts, CerS2+/− mice do not display altered body weight or energy expenditure, consistent with C16:0-ceramide metabolic effects as primarily exerted in liver.
Novel insights developed from these studies are likely relevant to human metabolic syndrome. Raichur et al. (2014) report that genome-wide analyses reveal a human CerS2 polymorphism, a single amino acid substitution at CerS2 position 115, that associates strongly with insulin resistance markers. Delineation of this polymorphism vis-à-vis metabolic syndrome is underway. Even more specifically, Turpin et al. (2014) report data from 439 human subjects across a broad spectrum of body masses. While visceral and subcutaneous WAT express CerS1, CerS2, CerS4, CerS5, and CerS6, only CerS6 expression positively correlated with obesity and insulin resistance. Further, in a small cohort of lean versus obese human subjects, long-chain ceramide, in particular C16:0-ceramide, was increased in obese visceral WAT, while most very-long-chain ceramides were not, strengthening association of metabolic syndrome with C16:0-ceramide.
We have only recently entered the era of sphingolipid therapeutics. Fingolimod/Gilenya, a sphingosine 1-phosphate receptor functional antagonist, was approved in 2010 as an orally available, first-line therapy for relapsing-remitting multiple sclerosis. In August 2014, the FDA approved the glucosylceramide synthase inhibitor Cerdelga (Eliglustat) for treatment of Gaucher’s disease, the first highly effective non-enzyme-replacement therapy for an inherited sphingolipidosis. Numerous other small-molecule sphingolipid therapeutics are in development and clinical trials. We believe this newfound therapeutic landscape supports the possibility of specific CerS6/C16:0-ceramide inhibition as a potential treatment of insulin resistance and metabolic syndrome.
References
- Chavez JA, Summers SAA. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. Lancet. 2010;375:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- Hla T, Dannenberg AJ. Cell Metab. 2012;16:420–434. doi: 10.1016/j.cmet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH., Jr Chem Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Park WJ, Futerman AH. Biochim Biophys Acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, et al. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Shah SH, Kraus WE, Newgard CB. Circulation. 2012;126:1110–1120. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancevic B, Kolesnick R. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, et al. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]


