Abstract
N6-methyladenosine (m6A) is a modified base that has long been known to be present in noncoding RNAs, ribosomal RNA, polyadenylated RNA and at least one mammalian mRNA. However, our understanding of the prevalence of this modification has been fundamentally redefined by transcriptome-wide m6A mapping studies, which have shown that m6A is present in a large subset of the transcriptome in specific regions of mRNA. This suggests that mRNA may undergo post-transcriptional methylation to regulate its fate and function, analogous to methyl modifications in DNA. Thus, the pattern of methylation constitutes an mRNA ‘epitranscriptome’. The identification of adenosine methyltransferases (‘writers’), m6A demethylating enzymes (‘erasers’) and m6A binding proteins (‘readers’) is helping to define cellular pathways for the post-transcriptional regulation of mRNAs.
It is well established that DNA and proteins undergo dynamic chemical modifications that influence their function. For instance, methylation of cytosine residues in DNA to form 5-methylcytosine (5mC) can have widespread effects on gene expression by recruiting specific DNA-binding proteins1. Similarly, phosphorylation of proteins — a reversible chemical event initially thought to be restricted to just a few targets — is involved in nearly every aspect of cellular physiology2. However, until recently, mRNA had not been shown to be extensively subjected to such chemical modifications that might alter its function.
Two groups showed in 2012 that a large fraction of cellular mRNA contains adenosine residues that are methylated to form N6-methyladenosine (m6A)3, 4. In addition, the characterization of adenosine methyltransferases and m6A demethylating enzymes has provided insights into the pathways that dynamically regulate adenosine methylation in mRNA and how this process contributes to human disease3, 5, 6. Collectively, these studies demonstrate for the first time that mRNA is susceptible to dynamic, reversible chemical modification. Thus, analogous to methylation of DNA and phosphorylation of proteins, adenosine methylation in mRNA represents an additional layer of regulation that can potentially alter mRNA function and influence the way genes are expressed.
A flurry of recent discoveries has also pointed to important roles for m6A in regulating important cellular pathways and processes, highlighting the potentially broad role for m6A in regulating mRNA fate (Box 1). In this Review, we discuss the discovery of the m6A modification, including early studies that first revealed the existence of m6A as well as more recent studies that have profiled the features of m6A on a global scale. We also analyse the strengths and limitations of current methods used to study m6A and highlight improvements that are needed to move the field forward. Furthermore, we emphasize our current knowledge of adenosine methylation and demethylation pathways in mammalian systems and the insights that studies of these pathways have provided for the role of m6A in human disease. Last, we discuss the questions that are driving m6A research and the implications that their answers might have for our understanding of RNA biology.
Box 1: Physiological processes linked to m6A.
Obesity
Diverse mutations within intron 1 of human FTO have been found and are associated with increased body weight 105. Carriers of one or two copies of the mutant allele are on average 2.6 lb or 6.6 lb, respectively, heavier than individuals with normal alleles105. FTO–/– mice have lower body mass106, whereas FTO overexpression leads to increased food intake and obesity 107. The intronic mutations in humans likely influence FTO gene expression, although the underlying mechanisms remain poorly understood.
Synaptic signaling
FTO-knockout mice have impaired dopamine release, reduced dopaminergic receptor responses and an altered locomotor response to cocaine 42Select mRNAs involved in dopaminergic signalling pathways are hypermethylated in these mice, which may underlie their neurobiological and behavioural phenotypes. However, the consequences of mRNA hypermethylation on transcript stability and protein production are complex, and further studies are needed to determine the effects of FTO-induced demethylation in specific neuronal subtypes and signalling pathways.
Cancer
Recent genome-wide association studies have demonstrated that intron 1 FTO mutations are also risk factors for estrogen receptor negative breast cancer102. Additional studies have identified mutations within intron 8 of FTO which lead to increased melanoma risk independently of any effects on body mass index (BMI)108. These studies identify for the first time a set of FTO SNPs which are not associated with BMI but predispose to cancer. Although it remains to be determined how these intron 8 mutations affect the demethylase activity of FTO, they are postulated to influence FTO mRNA expression levels,.
Sperm development
Consistent with the high expression level of ALKBH5 in testes, ALKBH5 knockout mice exhibit reduced testicular size and abnormal morphology 6. They also have impaired spermatogenesis and increased apoptosis, which probably contributes to the reduced fertility observed in these mice. This phenotype may be explained in part by the altered expression level of genes in the spermatogenesis and P53 apoptotic pathways observed in ALKBH5–/– mice6; however, whether hypermethylation of these mRNAs underlies their alternative expression pattern remains to be determined.
Stem cell differentiation
m6A is required for self-renewal capacity of human embryonic stem cells and is found in critical pluripotency regulators63; dynamic changes in m6A levels are seen during stem cell differentiation63.
Circadian periods
The global methylation inhibitor 3-deazaadenosine (DAA) causes an extension of the normal circadian period both in cells and in mice. Moreover, MeRIP-Seq analysis shows that m6A decreases in several clock genes following DAA treatment in cells. This correlates with prolonged nuclear retention in clock transcripts Per2 and Arntl109. Similar results were also observed after METTL3 knockdown. Thus, adenosine methylation may influence the cyclic expression of mRNAs encoding clock genes.
Yeast meiosis
mRNA methylation in Saccharomyces cerevisiae selectively occurs during meiosis64 and is mediated by a core RNA methyltransferase complex. This complex, termed MIS, comprises Ime4 (an orthologue of mammalian METTL3), Mum2 (an orthologue of the mammalian Wilm's-tumor-1-associated protein WTAP) and Slz164. The MIS complex localizes to the nucleolus during meiosis, and is required for the proper time course of meiosis75.
Plant development
m6A contributes to plant embryonic development, and is required for normal growth patterns, apical dominance, and floral development 57, 110
Drosophila melanogaster oogenesis
m6A is required for Notch signaling during oogenesis based on phenotypes seen in D. melanogaster deficient in the adenosine methyltransferase IME459.
Serendipitous discovery of m6A
The finding that polyadenylated RNA contains m6A was a serendipitous discovery made by several groups that were characterizing the mRNA 5’ structure in mammalian cells in 19747, 8, and validated by others the following year9-12. The original goal of these studies was to characterize the methylation of the recently described 5’ cap of mRNAs. These studies were made possible because radioactive [3H]-methionine, the methyl source in most biochemical reactions, had become commercially available with high specific activity. [3H]-methionine could be applied to cells and is then metabolically incorporated into S-adenosylmethionine (SAM), the enzymatic cofactor that participates in many methylation reactions in the cell.
After digestion of the poly(A) RNA, it was clear that the radioactivity was not confined to the caps. Radioactivity was also detected in the mononucleotides, with the majority found in a modified nucleotide, N6-methyladenosine (m6A)7, 8. In most studies, m6A was the only modified base that was detected, although one study also reported the presence of 5-methyl-cytosine, although at a fourth of the abundance of m6A12, 13. Radioactive methyl groups were also found within what seemed to constitute an extended cap structure (Box 2)14. The finding of m6A in RNAs was not unprecedented, as m6A was known to be in bacterial tRNA and other noncoding RNAs15-19.
Box 2: Methyl code of the 5’ mRNA cap.
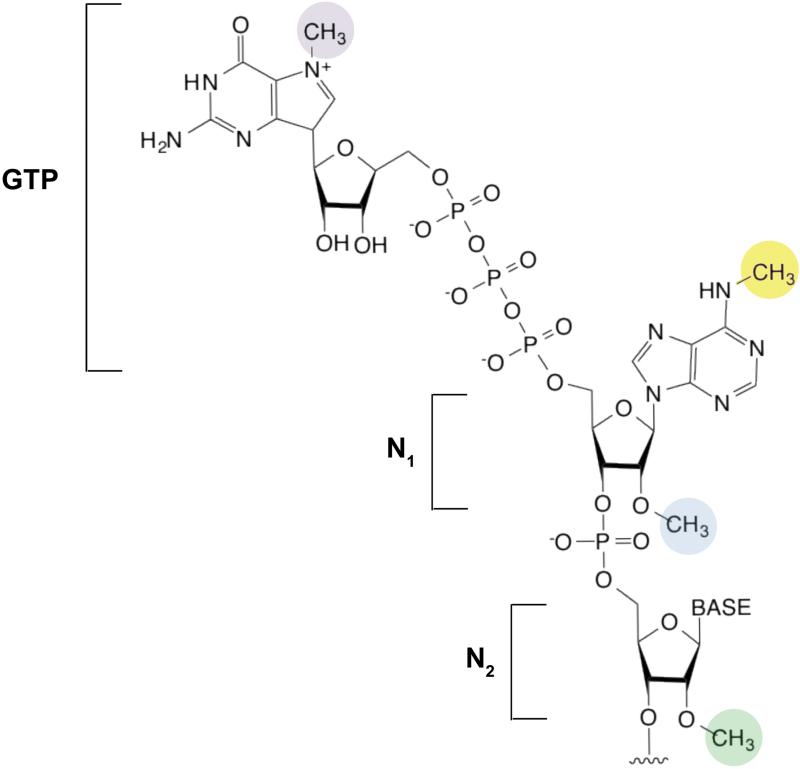
The mRNA cap is introduced in the nucleus by the attachment of a GTP to the first encoded nucleotide (N1) at the 5’ end of a transcript. The GTP is attached by an unusual linkage, in which the 5’ hydroxyl of the GTP is connected by three phosphates to the 5’ hydroxyl of the first nucleotide12, 111 (see the figure). The most well-characterized methyl group is found on the N7 position of the essential guanosine (purple). N7 methylation is required for mRNA export and subsequent recognition of the cap by the eIF4E translation initiation factor111. Lack of this methyl group markedly reduces translational efficiency in standard in vitro translation reactions112.
The first two nucleotides (N1 and N2) of the mRNA can also be methylated on the N1 2’ hydroxyl (blue), to form ‘cap-1’ or on both the N1 and N2 2’ hydroxyl (green) to form “cap-2.” 5’ caps which lack methyl groups on either N1 or N2 2’ hydroxyls are referred to as cap-0113. The first nucleotide in mRNA is frequently an adenosine, and this can be additionally methylated at the N6 position (yellow)14, 113. The N6 methylation appears to follow the 2’-O-methylation and is likely mediated by a methyltransferase other than METTL3114. Thus, when considering the cap as an extended structure comprising the m7G and the first encoded nucleotides, five different methylated forms of the cap can occur (m7GpppCm, m7GpppGm, m7GpppUm, m7GpppAm and m7Gpppm6Am). The function of these differentially methylated forms of the 5’ cap is unclear. However, mRNAs containing synthetic caps with m6A at the N1 position enhance translation beyond the level seen with caps containing nonmethylated nucleotides at the N1 position115. Additionally, structural studies suggest that eIF4E recognizes m7G but not the N1 nucleotide, and therefore its binding to the cap is unaffected by the methylation state of the first base116. Thus, other proteins may recognize the methylation states of the N1 and N2 nucleotides. The squiggly line refers to the position of the ribose sugar to which the base is attached.
However, these initial studies provided the first evidence that m6A is present in mammalian poly(A) RNA, as well as mRNA encoded by diverse viruses20-24. Subsequent studies showed that m6A is a prevalent nucleotide in poly(A) mRNA from nearly all higher eukaryotes and plants7, 8.
The early studies examined poly(A) RNA, not mRNA. However, since mRNA is highly enriched in poly(A) RNA preparations, it was tempting to speculate that the m6A in poly(A) RNA derived from mRNA. Based on this, m6A might potentially be present at a level of ~3-4 m6A residues per mRNA7-12. This measurement relies on the assumption, which is now known to be incorrect, that m6A is evenly distributed in all cellular transcripts. Nevertheless, these early investigators made the seminal proposal that post-transcriptional methylation of adenosine residues in mRNA might influence that fate of mRNA in cells, potentially analogous to the functional consequences of protein modifications.
Reluctance to accept m6A
Despite the interesting possibility that mRNA is regulated by adenosine methylation, there were very few studies that further investigated the original findings on m6A. The initial interest in m6A was largely abandoned due to considerable reluctance to accept the idea that this modification is biologically relevant. One concern was that m6A could have arisen from contamination from small amounts of known m6A sources25, such as ribosomal RNA26 and small nucleolar RNAs27, 28. Another factor was that these early studies used poly(A) RNA, which is often contaminated with mitochondrial mRNA, tRNA, and certain rRNAs owing to their poly(A) tracts29-31. Indeed, most preparations of poly(A) mRNA invariably contain ribosomal RNA bands. In some studies, rigorous gradient purification methods were used to remove this persistent contaminant32, which supported the idea that m6A is a constituent of mRNA. However, most studies did not use these approaches, questioning the relevance of studies using poly(A) mRNA. Indeed, it is now known that up to half of the RNA pool in poly(A) RNA fractions constitutes diverse noncoding RNA species33-36.
In addition to the concern about a contamination artifact, another concern was that mutation of specific m6A sites did not result in a change in RNA fate in cells. For example, point mutations that depleted m6A within the src transcript of Rous sarcoma virus did not affect src nuclear location, RNA expression levels, splicing, translation, packaging of the RNA into virions or infectivity of the mutant virus37, 38. These studies raised the possibility that m6A has a minimal impact on mRNAs.
Another problem was that few endogenous mRNAs were shown to contain m6A. If m6A was indeed highly prevalent, it should be seen in numerous cellular mRNAs. Although m6A was frequently detected in viral mRNAs39, the only endogenous mammalian mRNA shown to contain m6A was the prolactin mRNA40, 41. Therefore, it was plausible that m6A was preferentially introduced into foreign mRNAs. The prevalence of m6A in poly(A) mRNA might reflect the incorporation of m6A into a small number of hypermethylated cellular mRNAs.
Addressing these issues was a formidable challenge, as no methods existed that could globally detect m6A sites in the transcriptome. Identifying m6A is challenging, as m6A reverse transcribes to a T during reverse transcription, and because m6A is not susceptible to chemical treatments that might facilitate its detection. This inability to identify endogenous m6A-containing mRNAs further dampened interest in investigating the functional roles of this putative mRNA modification.
Improved detection of m6A
The major breakthrough that resulted in the acceptance of m6A as a highly prevalent mRNA modification was the development of novel tools and methods for detecting m6A in mRNAs (Box 3). For example, the m6A immunoblotting technique showed that m6A-containing poly(A) RNAs span a broad range of sizes, ranging from less than 500 bases to over 7 kb3. This resembles the distribution expected for mRNAs, suggesting that mRNAs are the m6A-containing species in these blots.
Box 3: Techniques for detecting m6A.
m6A immunoblotting. An important advance in establishing the prevalence of m6A in mRNA was the development of m6A-specific antibodies. The first m6A-specific antibody was described in 198827. It was initially used in studies to immunoprecipitate small nucleolar RNAs27 and was commercialized by Synaptic Systems (Gottingen, Germany). A second antibody was used to study m6A in bacterial DNA117. Both these antibodies only recognize oligonucleotides containing m6A. 3.
m6A antibodies can be used to generate m6A immunoblots, which allows m6A-containing RNAs to be identified based on their size. RNAs are fractionated by denaturing gel electrophoresis and transferred to a membrane; the blot is probed with an m6A-specific antibody. m6A immunoblots can also be used to determine if m6A is present in a specific transcript. For example, biotinylated oligonucleotides have been used to pull down mRNAs that hybridize to specific target mRNAs, followed by anti-m6A immunoblotting3, providing a simple way to probe the presence of m6A within individual endogenous mRNAs in cells.
m6A-sensitive ligase reaction. m6A can be detected in single target mRNAs based on the reduced ability of m6A-containing mRNA to template a ligation reaction118. The m6A-containing mRNA acts as a splint to catalyse a T4 DNA ligase-directed ligation of two oligonucleotides, which hybridize to the RNA so that the 3’ end of one of the oligonucleotides is complementary to the putative m6A in the RNA. If the 3’ nucleotide is a G, which can form a nonstandard G-A interaction, the ligation reaction is significantly slower than if the complementary nucleotide is an m6A instead of an A. The difference in the rates can be used to predict the m6A stoichiometry in the target RNA.
m6A-sensitive reverse transcription. Reverse transcription of m6A is markedly impaired when using Tth DNA polymerase, which can function as a reverse transcriptase119. The slowed reverse transcription can be detected by the reduced level of incorporated thymidine in a DNA primer119.
SCARLET. Another approach, termed SCARLET (site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography [TLC]), utilizes targeted nuclease digestion and radiolabeling steps combined with the well-known altered migration of m6A on thin-layer chromatography to detect m6A. This technique provides excellent measures of m6A stoichiometry and can potentially be used to recover any base in the transcriptome to determine whether it contains a modification. However, since detection of the m6A requires TLC, it cannot be adapted to a transcriptome-wide approach.
PacBio sequencing. The PacBio instrument uses a novel fluorescence detection technology which relies on ‘zero-mode waveguides121 to detect the altered kinetics of base incorporation opposite to an m6A relative to A during reverse transcription. This approach detects differences in the rate of incorporation of thymidine opposite m6A versus A when generating cDNA from an RNA template43, 122. However, this method requires complex instrumentation as well as purification of the specific target mRNA, and therefore will benefit from methodological simplification.
m6A immunoblots also allow changes in m6A levels to be measured. For example, the intensity of the m6A smear in the immunoblot differs markedly when comparing RNA derived from different tissues and cancer cell lines, with the highest m6A levels in the brain, heart and kidney3. m6A-containing mRNAs are low in the fetal brain, but increase during development, achieving a maximal level in the adult brain3. These data argued against initial theories that m6A might be a fixed feature of mRNA, like the 5’ cap modification, and suggested that it acts as a tissue-specific regulator of mRNA fate.
Finally, pulldown of the low density lipoprotein receptor 1, metabotropic glutamate receptor 1, and Dopamine receptor D1A transcripts followed by m6A immunoblotting analysis revealed that each of these mRNAs is endogenously methylated in cells3.
The methylated transcriptome
The methylated transcriptome was first defined using the MeRIP-Seq technique and an essentially equivalent approach, termed m6A-Seq (Box 4)3, 4. These are next-generation sequencing techniques that involve selective immunoprecipitation and sequencing of m6A-containing RNA fragments3. MeRIP-Seq led to the identification of 13,471 m6A peaks derived from 4,654 genes in the mouse brain. Analysis of HEK293T cell RNA led to the identification of 18,756 m6A peaks from 5,768 genes. Highly similar numbers were obtained in HepG2 cells, which showed 12,769 m6A peaks among 6,990 mRNAs4. These results provided the first unequivocal demonstration that m6A is a widespread mRNA modification, and thus laid to rest many of the previous doubts whether m6A was indeed a prevalent modification in mRNA.
Box 4: MeRIP-Seq profiling of m6A across the transcriptome.
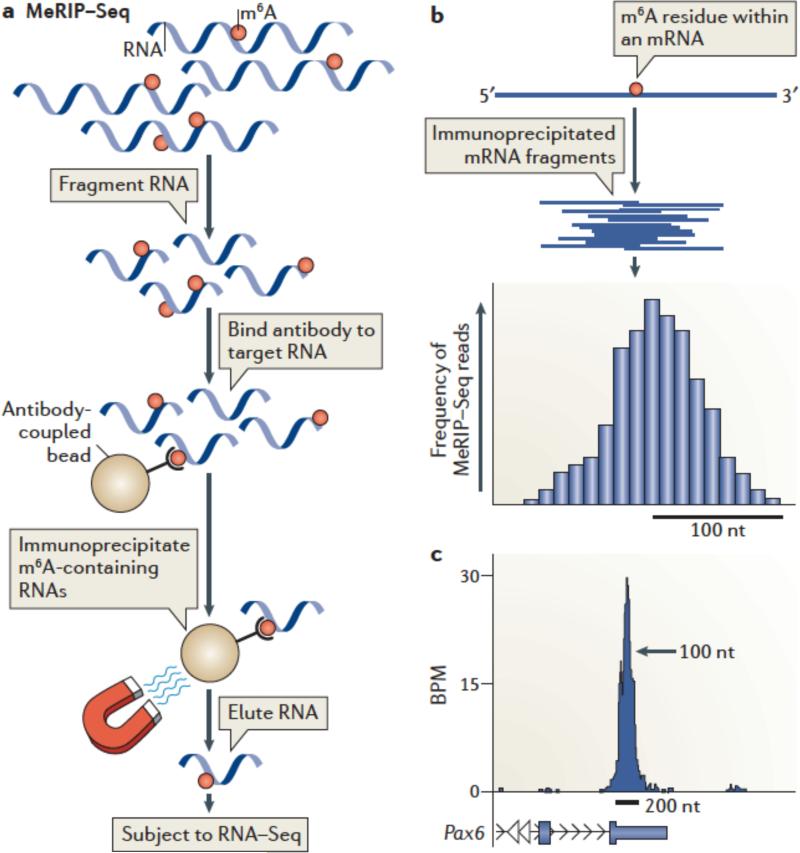
MeRIP-Seq3 enables transcriptome-wide profiling of m6A by subjecting m6A-containing RNA to next-generation sequencing. In MeRIP-Seq, RNA is fragmented into ~100-nt long fragments. Smaller fragments can also be generated to obtain finer resolution75 (see the figure, part a). m6A-containing RNAs are then immunoprecipitated with m6A-specific antibodies, which results in selective enrichment of RNA fragments containing m6A. After sequencing, the reads are mapped to the genome, and m6A peaks are identified. The pre-immunoprecipitation RNA fragments are also sequenced to provide an RNA-Seq dataset which is incorporated during peak calling.
Typically MeRIP-Seq experiments are performed with two different m6A-specific antibodies, and peaks found in both datasets are considered “true” m6A peaks. This is important, as antibodies can exhibit low-level RNA binding in an m6A-independent manner75. Only peaks found in both datasets are considered ‘true’ m6A peaks.
MeRIP-Seq generates m6A peaks rather than specific m6A sites; thus, the localization of an m6A residue in an mRNA can only be approximated. This is because MeRIP-Seq provides ~100 nt-long RNA fragments that can contain m6A anywhere within the fragment. Multiple different fragments that contain the same m6A residue will align as overlapping reads on the genome. The overlapping reads produce the appearance of a peak whose midpoint reflects the theoretical location of the m6A residue (see the figure, part b).
Prediction of the methylated m6A within a peak is possible, but challenging, because the nonrandom nature of RNA fragmentation123 leads to asymmetric peaks and thus difficulty in determining the peak midpoint. Closely clustered m6A residues can also result in broadening or asymmetry of m6A peaks. Nevertheless, the center of the peak often aligns with the location of the predicted m6A consensus G-m6A-C3, consistent with the idea that the peak derives from a m6A residue at the midpoint.
A typical m6A peak is ~200 nt wide at its base (see the figure, part c; showing UCSC Genome Browser plots of MeRIP-Seq reads of Pax6 mRNA. BPM= reads per base per million mapped reads Within the peak, reads cluster at the highest density near the middle of the peak, which is the presumed location of the m6A residue.
The image has been adapted, with permission, from REF. 3 © 2012 Elsevier.
However, m6A was not found in all mRNAs or just a few highly methylated mRNAs, but in ~25% of all transcripts. Thus, these studies ruled out earlier ideas that m6A might be an obligate feature of all mRNAs as a result of biogenesis or processing. Importantly, the distribution of m6A along the length of transcripts was nonrandom, suggesting that m6A has functional roles in mRNA3, 4. Furthermore, the sites of methylation in mRNAs were typically found in portions that showed higher evolutionary conservation than other regions3, further suggesting that m6A has conserved regulatory roles.
Importantly, although many transcripts were methylated in both brain and HEK293 cells, others were methylated in only one tissue3. This indicates that methylation is not exclusively regulated by cis-acting sequences in the mRNA and that trans-acting tissue-specific factors probably contribute to the specificity of methylation in different cell types. However, when a transcript was methylated in both tissues, the m6A peaks were typically localized to the same region of the transcript.
Interestingly, not all m6A peaks detected by MeRIP-Seq were found in mRNAs. In the brain for example, although 94.5% of m6A peaks were found within mRNAs, the rest mapped to several classes of (long) noncoding RNAs3. These data indicate that noncoding RNAs are also important targets of m6A.
MeRIP-Seq also demonstrated that different m6A peaks exhibit markedly distinct m6A stoichiometries. It is important not to view transcripts as either “methylated” or “unmethylated.” As with any modification in cells, some copies of a transcript will be methylated and some will be unmethylated. An indirect measure of stoichiometry can be obtained by normalizing the number of MeRIP-Seq reads within each peak to the transcript abundance3, 42. A rank of m6A peaks based on this method shows that m6A stoichiometry varies considerably, with the majority of m6A peaks likely exhibiting low stoichiometry3. This approach can be used to determine mRNAs with high stoichiometry m6A, which are therefore most likely to be influenced by methylation pathways in any given cell type.
Asymmetric m6A distribution in mRNAs
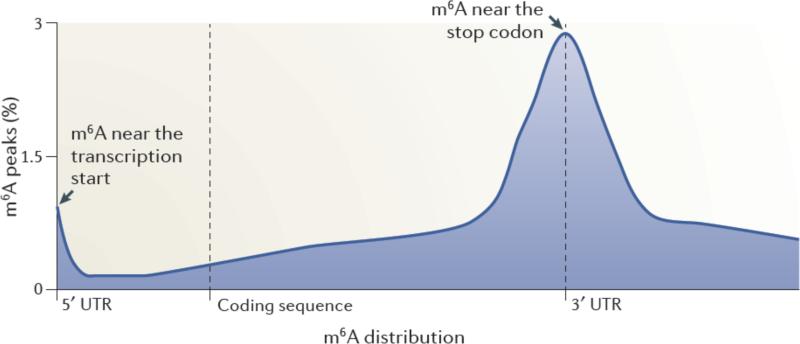
A striking result from the MeRIP-Seq analysis was the finding that m6A residues are enriched in specific regions of the mRNA3, 4 (Figure 1). The majority of m6A peaks are located in the vicinity of the stop codon, with a roughly even distribution both upstream and downstream of this site3. Additionally, a subset of mRNAs contains m6A residues in their 5’ untranslated regions (UTRs)3, 4. Typically, the 5’UTR of a transcript is relatively short; thus, the concentration of m6A residues within the 5’UTR is strikingly high compared with other regions of the transcript4. Interestingly, the fraction of cellular mRNAs that have m6A in the 5’UTR seems to exhibit tissue-specific differences4, 43, indicating that regulation by 5’UTR m6A residues may be more frequently utilized in certain tissues than others. Liver and liver-derived cells (HepG2) appear to have a higher proportion of 5’UTR m6A than do other tissues. Thus far, no tissues have been identified which completely lack m6A, although the overall abundance of m6A within RNA appears to vary across different tissue types3.
Figure 1. Spatially distinct pools of m6A in mRNAs.
Metagene analysis of MeRIP-Seq data shows that m6A is enriched in discrete regions of mRNA transcripts3. In this approach, each m6A peak in the transcriptome was mapped to a virtual transcript based on its position in the mRNA in which it is found. The metagene profile represents an overall frequency distribution of m6A residues along the entire mature transcript body. Shown is an idealized metagene profile based on published MeRIP-Seq datasets3. m6A residues can be found at the first encoded residue ,often as a dimethylated nucleotide (m6Am). m6A is also found at each of the other indicated positions in mRNAs, with a particularly prominent enrichment near the stop codon in mRNAs.
m6A residues are also found in the 3’UTR outside of the region adjacent to the stop codon3. However, these m6A residues were less abundant than the m6A residues near the stop codon. Furthermore, m6A residues were found within the coding sequence, but again, the relative abundance per unit length of the sequence was proportionately smaller than in other regions of the transcript. Finally, m6A was not found in poly(A) tails3, which is consistent with early metabolic labelling studies25.
The enrichment of m6A residues near the stop codon and the 5’UTR is markedly distinct from the binding properties of other known mRNA regulatory elements. For example, microRNA-binding sites are enriched at the 5’ and 3’ ends of the 3’UTR44. Many other mRNA-binding proteins bind to the 3’UTR of mRNAs45, which is also the most structured portion of the molecule46. Moreover, few mRNA-binding proteins show preferential binding to the 5’UTR45. Thus, the enrichment of m6A residues in the 5’UTR and around the stop codon is unusual compared with other mRNA regulatory elements, and suggests unique regulatory functions for this modification.
How is methylation targeted?
The asymmetric distribution of m6A residues in mRNA immediately raises the question of how this specificity is achieved. Analysis of the MeRIP-Seq-generated peaks using the motif-discovery algorithm FIRE47 identified enrichment of G-A-C and A-A-C consensus motifs within these peaks, with the former being the most prominent. Variants of this motif with preferences for residues 5’ and 3’ to the core motif were also found, including G-(G/A)-A*-C-U (* indicates putative m6A)3. These motifs were found in nearly 90% of all peaks3, and importantly, other motifs may exist for a smaller subset of m6A residues3, 4.
It is noteworthy that the motifs identified by bioinformatic analysis of MeRIP-Seq data were remarkably similar to the motifs predicted using biochemical approaches in the 1970s20, 24, 48-50. These experiments relied on the digestion of mRNAs using ribonucleases that selectively cleave RNA after specific nucleotides, and showed that the central adenosines in the GAC and in the less common AAC motifs are the methylation sites. Follow-up studies using in vitro methylation assays with purified nuclear extracts and synthetic RNAs showed that methylation only occurred on RNAs containing a GAC sequence, and not variants, such as GAU or UAC, or GACG51. Furthermore, mutation of GAC sequences to GAU was sufficient to block adenosine methylation38. The sequences discovered by FIRE analysis of the MeRIP-Seq dataset show that these m6A motifs apply to the majority of cellular m6A-containing mRNAs.
One of the immediate implications of finding very few methylation site motifs is that there are possibly only a few pathways that control and recognize methylation. A diversity of motifs would have suggested that there might be various methylating enzymes and m6A-binding proteins, each linked to a specific motif consensus site. This would be analogous to the known diversity of kinases, which generally are linked to specific phosphorylation consensus sites in the proteome. Thus, the relative consistency of m6A sites throughout the transcriptome suggests that there is a limited repertoire of readers, writers and erasers for this modification.
The presence of such a short methylation motif raises the question why so few are methylated in mRNAs? The most prominent m6A consensus motif, GAC, is found roughly every 64 nts in RNA, which suggests that an average 2000-nt long mRNA would have ~30 m6As distributed evenly along the length of the transcript. However, most transcripts lack an m6A altogether3, and in m6A-modified transcripts m6A is usually found the near the stop codon. Thus, the enrichment of m6A at specific GAC residues, especially residues near stop codons, suggests that additional factors determine whether a GAC is targeted for methylation. Structural features adjacent to the GAC sequence could be a possible determining factor. However, m6A peaks are typically found within unstructured regions of RNA4, rather than in loops or bulges of stem— loops. Thus, although the structure may contribute in some instances to specific methylation events, it does not seem to account for the overall distribution of m6A seen in the metagene analysis.
One mechanism that might allow region-specific methylation within mRNAs could be methyltransferase tethering. For example, tethering of a methyltransferase to 5’UTR elements or near the stop codon could account for the distribution of methylated GAC motifs in these regions. This model is not fully consistent with our understanding of the subcellular localization of the adenosine methyltransferase enzymes (see below) and does not explain m6As within the open reading frame, but could account for some of the defining features of m6A distribution in transcripts. Indeed, in agreement with a tethering model, MeRIP-Seq data indicate that most m6A peaks reflect a cluster of m6A residues3. Additionally, previous studies showed that m6A residues were clustered in the Rous sarcoma virus transcript39. These data are consistent with the idea that ‘hotspots’ of methylation occur in certain regions of mRNA, possibly as a result of a tethered methyltransferase. A global method for profiling m6A at single-nucleotide resolution will be important to determine whether the distribution of m6A in mammalian mRNAs is compatible with a clustering model.
Enzymes that mediate m6Amethylation
Identifying the enzymes that catalyse adenosine methylation will be an important advance for delineating the regulation of mRNA methylation in cells. Several enzymes have been identified that seem to mediate physiological adenosine methylation in mRNA:
•The METTL3–METTL14 complex
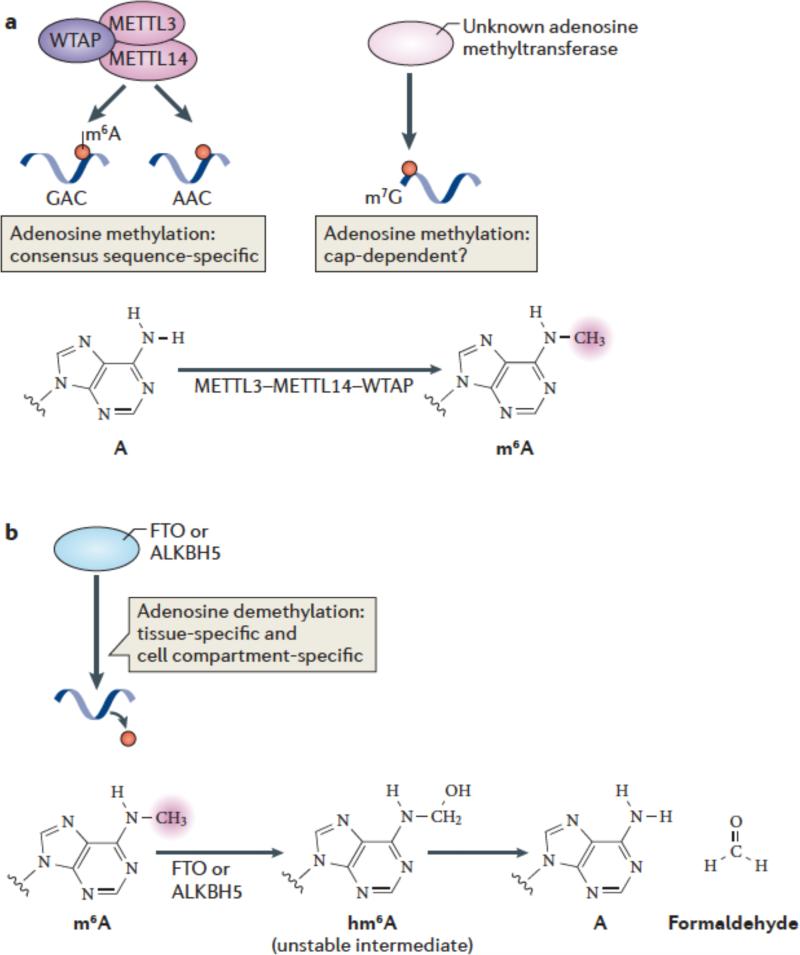
Early studies identified a large (> 1 MDa) multiprotein complex that mediates adenosine methylation52. Nuclear preparations were capable of methylating RNA in a SAM-dependent manner in vitro, and the substrate specificity matched the m6A consensus motif established by biochemical methods51. Thus, mRNAs containing GAC were more efficiently methylated than mRNAs containing AAC, whereas other sequences, such as GAU, were typically unmethylated (Figure 2a). Importantly, this complex did not induce the formation of N,N-dimethyladenosine51, unlike various Lys methyltransferases, which can produce ‘higher order’ methylated forms of Lys, such as di- and tri-methylated Lys53. Subsequent efforts focused on purifying the catalytic subunit of the methyltransferase complex54. The catalytic component was detected by its ability to crosslink to [3H]-SAM, and was designated METTL3 (methyltransferase-like 3; also known as MT-A70 Purification of the enzyme showed that it exhibited the predicted specificity towards GAC and AAC sequences in single-stranded RNA55, 56. Cloning of this enzyme revealed that it has a classic SAM-binding methyltransferase domain, consistent with its function54. Homologues in plants (MTA)57, S. cerevisiae (IME4)58 and Drosophila (IME4)59 have also been identified.
Figure 2. m6A methylation and demethylation pathways.
(a) METTL3 and METTL14 are two homologous m6A methyltransferases that synergize to methylate adenosines in RNA. WTAP is an additional component of this complex which lacks methyltransferase activity but which has a strong influence on m6A formation by interacting with METTL3/14. The central adenosines in the GAC and AAC motifs are the methylation sites of these enzymes. Additional methyltransferases may also contribute to m6A formation in cells, such as those which direct N6 methylation of adenosines associated with the cap structure. The generation of N6-methyladenosine (m6A) is shown.
(b) FTO and ALKBH5, the two mammalian m6A mRNA demethylases identified to date, catalyze methyl group removal from the N6 position of adenosine residues. FTO catalyzes oxidation of m6A in a reaction that requires O2, ascorbate, Fe(II) and 2-oxoglutarate. Oxidation of m6A generates CO2, succinate, and Fe(III). The product of this reaction is N6-hydroxymethyladenosine, an unstable intermediate that spontaneously decomposes to adenosine and formaldehyde. The squiggly lines refer to the position of the ribose sugar to which the base is attached.
Recent studies have begun to clarify other components of the METTL3 multiprotein complex. A proteome-wide analysis of protein complexes identified a single interactor of METTL3, METTL1460. METTL14 is highly similar to METTL3 and is a predicted methyltransferase61-63. Subsequent studies confirmed that both METTL3 and METTL14 form complexes in cells and that purified METTL14 also selectively methylates GAC sequences in vitro61-63. Furthermore, the methyltransferase activity of both METTL3 and METTL14 are synergistically enhanced when they are mixed62, 63. It is not clear why two methyltransferases are present in the methyltransferase complex, but it is possible that these highly similar enzymes can function independently or each have unique forms of regulation.
Importantly, knockdown of either METTL3 or METTL14 results in reduced m6A peaks in more than half of all m6A-containing mRNAs in mouse embryonic stem cells63, confirming that METTL3 and METTL14 physiologically target mRNAs for methylation.
In addition to METTL14, Wilms tumor 1 (WT1)-associated protein (WTAP) was found to associate with METTL3/14. The importance of this interaction was first established in yeast and Arabidopsis, in which the WTAP homologs Mum2 and AtFIP37, respectively, were shown to associate with METTL3 and were required for efficient methylation of mRNA57, 64. Analysis of mammalian WTAP showed that it too is required in mammalian cells for cellular mRNA methylation61, 62. Although WTAP binds to WT1, this tumor suppressor-oncogene is not involved in mRNA methylation as knockdown of WT1 does not affect cellular m6A levels in mRNA61. WTAP seems to interact with numerous cellular proteins, thus, despite its name, many of its functions are unrelated to WT165. WTAP, which lacks methyltransferase domains or activity, was shown to bind to the METTL3—METTL14 complex61, 62, and induce its localization to nuclear speckles61. Thus, WTAP may facilitate mRNA methylation by translocating METTL3—METTL14 to nuclear speckles.
Where in the cell does methylation occur? Because of the readily detectable levels of METTL3, METTL14 and WTAP in nuclear speckles54, 61, this is likely to be an important site for methylation. A nuclear site for methylation is also supported by early studies that showed that nuclear extracts contain methyltransferase activity51, 66. Additionally, WTAP knockdown prevents METTL3 localization to speckles and reduces cellular m6A levels61. The localization of METTL3 in speckles suggests that this protein may methylate speckle-associated RNAs, such as snRNAs and pre-mRNA, and function during splicing reactions (see below). Additional support for nuclear mRNA methylation comes from PAR-CLIP analysis of METTL3-binding sites in the transcriptome, which revealed an exceptionally high number of binding sites in intronic mRNA sequences61.
However, early studies also detected methyltransferase activity in cytosolic extracts, which supports the idea that at least some methylation could occurs in the cytoplasm51. Cytoplasmic methylation would imply a role for m6A in the control of mRNAs fates that do not affect splicing or nuclear export. Furthermore, the localization of METTL3 seen using different antibodies showed a granular cytoplasmic staining or perinuclear staining67, and immunostaining for the Drosophila homologue, IME4, indicated some immunoreactivity in the cytoplasm59. Notably, METTL3 is expressed as both long and short alternatively spliced isoforms, which remain to be characterized68, 69. Conceivably, some of the variability in the staining and localization may reflect these isoforms.
The distribution of m6A within transcripts also supports the idea that mRNA methylation might occur in the cytosol. MeRIP-Seq analysis of m6A localization in the transcriptome shows that m6A residues are highly enriched around stop codons. Cellular recognition of stop codons is mediated solely by ribosomes bound to release factors70. Thus, stop codon-bound ribosomes could in principle tether a methyltransferase, resulting in stop codon-adjacent adenosine methylation. Importantly, ribosomes bound to stop codons have key regulatory roles in controlling nonsense-mediated decay and potentially other processes71. As ribosome function is predominantly cytosolic72, a ribosome-mediated methylation pathway would indicate a cytosolic location for methylation, at least for stop-codon proximal m6A residues.
Could other methyltransferases drive m6A formation?
The possibility that additional methyltransferases have a role in adenosine methylation is supported by the fact that a small number of m6A peaks do not seem to contain the AAC or GAC consensus site3, 4. Furthermore, m6A can be found at the first position in many mRNAs after the m7G cap, resulting in a m7Gpppm6Am structure (Figure 2a, see Box 2 for other methylated forms of the 5’ cap). This adenosine contains two methyl modifications: one at the 2’ position of the ribose ring, and the other at the N6 position of adenosine (see Supplementary Table 1). Importantly, the residues following the methylated adenosine can be any base50. Because of the lack of a cytosine following and a guanosine immediately preceding the adenosine residue, these m6A residues differ from the GAC and AAC consensus motifs. Thus, methylation of these noncanonical m6A motifs might be mediated by other methyltransferases.
There are numerous candidate methyltransferases that could mediate the methylation of these residues. METTL3 is part of a superfamily of at least three different types of mammalian methyltransferases of unclear function73. The adenosine methyltransferase activity that targets the U6 small nuclear RNA, which is different from METTL328, could potentially target certain m6A residues in mRNA. Thus, these or other enzymes could contribute to mRNA methylation. Interestingly, analysis of cellular extracts from mammalian cells produced two distinct adenosine methyltransferase activities, each of which could be partially purified into distinct fractions74. One fraction, similarly to METTL3, methylated diverse RNA templates; however, a separate activity selectively labelled RNA containing a 5’ cap74. As most studies of adenosine methylation used noncapped RNAs as substrates, the activity of the cap-dependent adenosine methyltransferase might have been missed and additional methyltransferasesmay also contribute to m6A formation in cells.
m6A demethylation pathways
An important recent discovery was the finding that endogenous enzymes can demethylate m6A. The presence of m6A erasers suggests that the effects of m6A can potentially be reversed, and that cellular demethylation pathways might be a mechanism of dynamic regulation of m6A within mRNAs. Notably, these erasers are not expressed in yeast, pointing to the potential role of passive RNA degradation as a mechanism to remove m6A in certain cell types75. Whether m6A demethylases preferentially target mRNA or whether they target rRNA and snRNA has not yet been established; however, early evidence points to a role for these enzymes in mRNA demethylation.
FTO
The first enzyme identified as an m6A demethylase was FTO (fat mass and obesity-associated protein)5. FTO has a controversial history as numerous enzymatic activities have been ascribed to the enzyme. Bioinformatic analysis showed that FTO is a member of the superfamily of Fe(II)/2-oxoglutarate (2-OG)-dependent oxygenases, which typically mediate oxygen transfer to specific target molecules76, 77. FTO has highest homology to the AlkB subfamily of Fe(II)/2-OG-dependent oxygenases, which comprises eight family members in humans and has known functions in nucleotide base demethylation reactions. These reactions occur by hydroxylation of methyl substituents on bases, forming a hydroxymethyl substituent78, 79. Hydroxymethyl substituents are highly unstable when connected to nitrogen atoms in a nucleotide base, and spontaneously decompose to formaldehyde, resulting in demethylation (Figure 2b) Thus, based on homology, FTO was thought to be potentially involved in similar hydroxylation—demethylation reactions.
Initial studies first demonstrating that FTO could mediate oxidative demethylation of nucleotide bases showed demethylation of 3-methyl thymidine (3mT) in the context of single-stranded DNA77. A subsequent study demonstrated that FTO could also demethylate 3-methyl uracil (m3U) in single-stranded RNA80. Interestingly, m3U is a minor constituent of ribosomal RNA81, potentially linking FTO to ribosome function.
Furthermore, m6A in RNA has been shown to be an additional substrate for FTO. One study showed that FTO demethylated m6A with a catalytic efficiency that was substantially higher than the activity of FTO towards 3mU5. Overexpression of FTO in HeLa cells reduced the level of m6A in purified poly(A) RNA by ~18%, whereas FTO knockdown increased m6A levels by 23% in poly(A) mRNA. Consistent with this, another study showed that FTO overexpression greatly reduced the levels of m6A in cellular RNA3, and the size of bands produced were consistent with mRNA being a target of this enzyme. Thus, m6A is a physiologic target of FTO.
Definitive evidence for the specificity of FTO towards mRNA came from MeRIP-Seq analysis of FTO-knockout brain tissue. Surprisingly, MeRIP-Seq data showed that m6A levels are generally unaffected in most mRNAs in FTO knockout brain42. However, for a small subset of mRNAs, m6A peaks are markedly higher in the FTO-knockout tissue than in wild-type42. Thus, these data demonstrate that FTO demethylates mRNAs, although only a few m6A-containing mRNAs are targeted. At present it is unknown why certain mRNAs become targeted by FTO. Conceivably, sequence or structural context might account for this selectivity, but further studies are needed to determine the role of such cis-acting elements in targeting FTO to specific m6A residues.
FTO was originally proposed to target m6A in nuclear RNAs5. Nuclear m6A-containing RNAs include certain noncoding RNAs, U6 snRNA, rRNA, and pre-mRNAs. The proposed selectivity towards nuclear RNA was based on immunostaining results showing that FTO is in nuclear speckles5. However, more recent results show that FTO is also found in the cytoplasm in various cell types, including dopaminergic neurons, hypothalamic neurons and mouse embryonic fibroblasts42, 82, 83. Thus, FTO may also target cytosolic mRNAs, which would allow FTO to have roles in regulating cytosolic mRNA processing events.
The activity of FTO may involve the formation of oxidized m6A intermediates. Demethylation of m6A involves a hydroxylation reaction to form N6-hydroxymethyl-adenosine (hm6A) (see Supplementary Table 1). This modification is highly labile and spontaneously decomposes to adenosine within a few hours. In vitro oxidative demethylation reactions using FTO also produces other oxidized species, such as N6-formyl-adenosine (f6A), in analogy to the diverse oxidized forms of cytosine generated by the FTO-homolog Tet proteins84. It is not clear yet whether these labile oxidized intermediates of m6A have specific functions.
Studies of FTO target specificity and function will be facilitated by small-molecule inhibitors of FTO that will enable temporal regulation of the specific m6A residues. Rhein, a bioactive component of rhubarb, is a moderately selective FTO inhibitor85. The development of potent and selective inhibitors may be useful for treatment of type 2 diabetes, obesity, and other physiological processes linked to FTO.
ALKBH5
Another recently described m6A demethylase is ALKBH5, an FTO homologue of the AlkB family6. A systematic test of each of the nine mammalian AlkB homologues showed that ALKBH5 catalyses the demethylation of an m6A-containing RNA with nearly as high a rate as FTO, and showed specificity for demethylation of m6A over various other methylated nucleotides in single-stranded RNA6.
ALKBH5 seems to affect mRNA export pathways. In ALKBH5 knockdown cells, increased levels of poly(A) mRNA were detected in the nucleus6, suggesting that ALKBH5 influences the expression of protein regulators of mRNA export. A more speculative hypothesis is that the mRNAs retained in the nucleus could be those that are hypermethylated in ALKBH5 knockdown cells. Although this idea is intriguing, it will require the demonstration that the retained, hypermethylated mRNAs in the nucleus are direct ALKBH5 targets.
ALKBH5-knockout mice grow to adulthood, but with a marked increase in the levels of apoptotic cells in the testes6, indicating a defect in spermatogenesis. The restriction of the knockout phenotype to testes is consistent with the distribution of the enzyme, which is primarily expressed in testes, with lower expression levels in the spleen and lung. It is intriguing that phenotypes associated with m6A-deficiency in lower organisms relate to gametogenesis and meiosis57, 59, 64, 75, suggesting a possibly evolutionarily conserved function for m6A in these processes.
It will be important to determine if ALKBH5 demethylates mRNA, noncoding RNAs, or both. ALKBH5 knockdown increased m6A levels in poly(A) mRNA by ~9%, whereas ~50X overexpression of ALKBH5 reduced m6A levels by ~29%6. The subtle changes in m6A levels in cellular poly(A) mRNA suggests that this enzyme, similarly to FTO42, only demethylates certain m6A residues in mRNAs. Selected mRNAs were tested for their susceptibility to ALKBH5-mediated demethylation, which showed that in some cases, the m6A level increased in ALKBH5 knockdown cells6. The identification of the specific ALKBH5 targets using MeRIP-Seq will be useful for characterizing the specificity of this enzyme.
What is the difference between these two FTO and ALKBH5? Currently, the pathways that activate or inhibit these enzymes are unknown. However, ALKBH5 appears to be markedly enriched in the nucleus6, unlike FTO, which is readily detected in the cytosol42, 82, 83. Thus, ALKBH5 may target nuclear RNAs, whereas FTO may be capable of targeting mature mRNAs. The tissue distribution of these enzymes is another major difference. Whereas FTO is highly brain enriched77, ALKBH5 is predominantly expressed in testis, with substantially lower levels in other tissues6. Thus, ALKBH5 may confer demethylase activity in tissues lacking FTO, and vice versa. MeRIP-Seq analysis of the specific targets of ALKBH5 and FTO will address whether these enzymes have redundant targets or if they each target different types of mRNAs.
Mechanisms of m6A action
Based on the localization of methyltransferase and demethylase enzymes, m6A may be introduced and removed in either the nucleus or the cytosol. Similarly, m6A may have a different effect on an mRNA, depending on whether it is detected in the nucleus or the cytosol. Currently, several functions have been ascribed to m6A that begin to tease apart these compartment-specific m6A roles.
Protein recruitment
m6A is likely to have a role in facilitating RNA—protein interactions. In principle, methylation of adenosine could either block or induce RNA—protein interactions (Figure 3a). To date, several m6A-binding proteins have been identified from mammalian cellular extracts using RNA pulldown approaches followed by mass spectrometry4, 75, 86. These include the mammalian proteins YTHDF1, YTHDF2 and YTHDF3, each of which contain a YTH RNA-binding domain87. A YTH-domain containing protein, MRB1, was also found to be a m6A-binding protein in yeast75. The RNA-binding protein HuR (also known as ELAVL1) was also detected in m6A-RNA pulldowns from mammalian lysates4. Several other proteins were shown to bind m6A using yeast extracts 75. However, since these studies used a pulldown approach, it was not clear which of these proteins directly bind to m6A or are instead part of a m6A-binding ribonucleoprotein complex. Also, it is not clear if these proteins bind m6A in living cells.
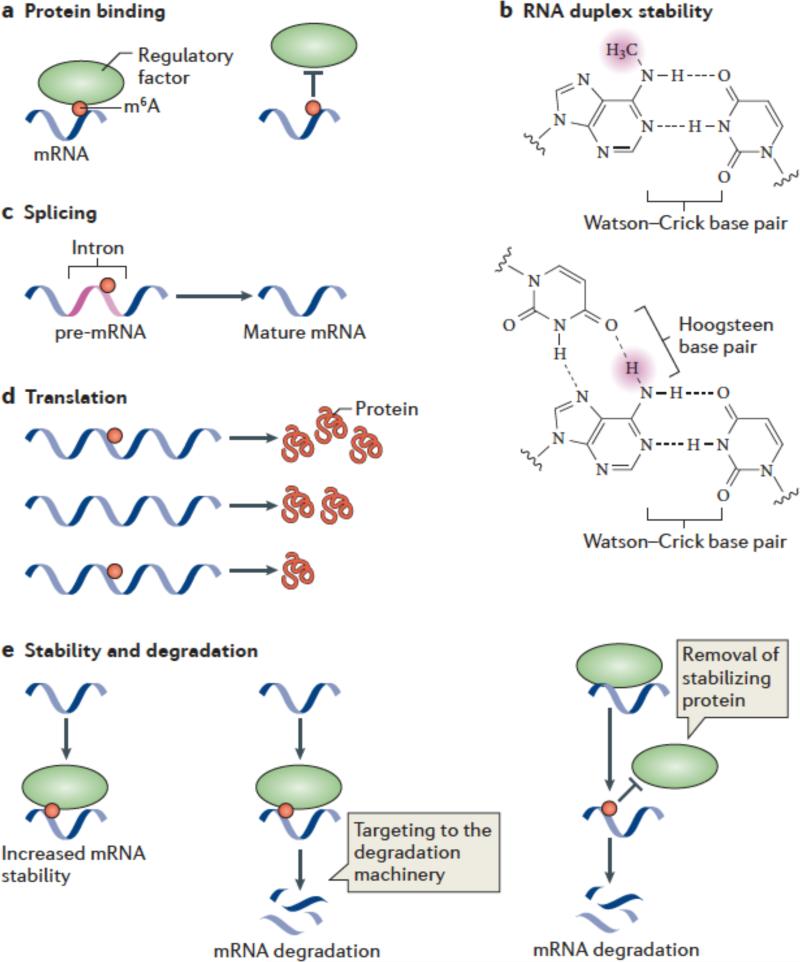
Figure 3. Mechanisms and functions of m6A.
(a,b) Several mechanisms have been ascribed to m6A. m6A is likely to have a role in facilitating or blocking RNA—protein interactions (a). m6A can also affect RNA by altering RNA structure or folding (b). In standard Watson-Crick base pairing, m6A is capable of pairing with uridine. Since a free proton at the N6 position is still available for hydrogen bonding, N6-methyladenosine behaves like adenosine in its ability to base pair to uridine. The squiggly line refers to the position of the ribose sugar to which the base is attached.
m6A interferes with the formation of base triples. As is shown in this U•A-U base triple, two free protons are required at the N6 position for Hoogsteen base pairing on one face and Watson-Crick base pairing on the other. The presence of a methyl group in place of one of these protons blocks base-triple formation. Thus, m6A can disrupt RNA structures dependent on base triples.
c-e) Great efforts are underway to determine the effects of m6A on mRNA fate and function. It has been suggested that m6A is targeted to specific intronic regions in order to influence splicing efficiency (c). Adenosine methylation may also lead to increased or reduced translation compared with unmethylated transcripts (d). m6A might promote mRNA degradation by recruiting proteins which target mRNAs toward the cellular degradation machinery. Alternatively, m6A might stabilize mRNAs by binding to proteins that promote transcript stability.
YTHDF1, YTHDF2 and YTHDF3 binding to m6A has been validated in vitro86, 88. The binding affinity was relatively weak, ranging from 400 nM t~1.2 μM86, 88. Although this binding is relatively weak compared with established sequence-specific RNA-protein interactions89, it is possible that these proteins function by binding transiently to m6A.
Do these proteins bind m6A in living cells? Transcriptome-wide methods for monitoring binding sites of RNA-binding proteins in living cells, such as HITS-CLIP, iCLIP, and PAR-CLIP90-92 will be essential for assessing the physiologic targets of these RNA-binding proteins. These approaches involve crosslinking of mRNA with target proteins in live cells, followed by the recovery of the target proteins with bound RNA fragments. Analysis of the bound RNA enables the identification of the binding sites within the mRNA at near single nucleotide resolution90. The major criteria for establishing that a putative m6A-binding protein is a bona fide interactor is that the binding sites of the protein need to precisely match m6A residues in the transcriptome. An m6A-binding protein might physiologically function to bind to m6A in rRNA or in ncRNAs (such as U6), and not mRNA; therefore overexpression of an m6A-binding protein can lead to nonphysiological binding to mRNA. For this reason, putative m6A-binding proteins and their binding partners should be assessed by analysis of the endogenous protein.
Analysis of the binding sites of the endogenous protein will indicate its physiological binding partners. Comparing the binding sites to m6A peaks obtained by MeRIP-Seq is problematic, as these peaks are ~200 nt-wide regions. Thus, alignments using MeRIP-Seq data will not show if the RNA-binding protein directly binds to the m6A in the mRNA. Single-nucleotide resolution m6A mapping techniques will therefore be needed to confidently identify m6A-binding proteins. Additionally, it is important to demonstrate that any putative m6A-binding protein shows decreased binding to mRNA in METTL3/14-deficient cells.
What kind of binding selectivity can be achieved with such a small modification like a methyl group? Based on our understanding of protein binding to methylated amino acids, it seems that considerable specificity can be achieved. For example, 53BP1, which binds methyl-Arg, exhibits a >20-fold increase in affinity between the nonmethylated and methylated form of a peptide ligand, 52.9 μM and >1,000 μm, respectively93. Similarly, L3MBT1, which binds methyl-Lys in histone proteins, exhibits a ~80-fold increase in affinity between the nonmethylated and methylated forms (6 μM and >500 μM respectively)94. Thus, the methyl group on adenosine is may introduce >20-fold binding selectivity.
Conformational changes
m6A can also affect RNA by altering RNA structure or folding. The two hydrogen bonds that constitute the A•U basepair still form if the adenosine is methylated, but they are slightly destabilized95 (Figure 3b). Although the overall effect of methylation on the stability of an RNA duplex is subtle, it could influence certain types of interactions in which duplex stability is particularly important, such as microRNA—mRNA interactions96. Thus, subtle alterations in binding and basepair stability could contribute to some of the effects of m6A in cells.
A more pronounced effect of adenosine methylation would occur on A-A•U or U-A•U base triples. This interaction involves a Hoogsteen basepair between an adenosine or uracil on the adenosine base in a A•U Watson-Crick basepair (Figure 3b). This interaction will not form if the N6 position is methylated as both hydrogens on this nitrogen are H-bond donors in the base triple. Thus, the formation of A-A•U base triples is blocked by methylation. A-U•A base triples have been seen in several mammalian noncoding RNAs, including MENβ and MALAT197. Interestingly, MALAT1 also contains m6A on the basis of MeRIP-Seq analysis3. Thus, methylation could act as a trigger to disrupt RNA structures that depend on hydrogen bonding of both N6 hydrogens on adenosine.
m6A effects on mRNA fate and function
The mechanisms through which m6A regulates cellular mRNAs are still in the process of being uncovered. However, studies to date have provided important insights into our understanding of how m6A contributes to the processing, localization, and functional roles of mRNAs in cells.
Effects on mRNA splicing
One of the earliest proposed roles for m6A was as a regulator of splicing (Figure 3c). This was initially based on studies that characterized the m6A content of the pre-mRNA in the nucleus, compared to the m6A content in mature cytoplasmic mRNA. In these studies, pre-mRNA was found to be methylated at ~4 m6A residues per mRNA, whereas the mature mRNA was methylated at ~2 m6A residues per mRNA32. These studies documented that methylation occurs in the nucleus, and suggested that the removal of introns resulted in the loss of total m6A content per mRNA.
More recent studies support this idea. PAR-CLIP analysis shows that the vast majority of METTL3-binding sites in transcripts occurs in introns61. Thus, intronic sequences may be a major target of nuclear methylation. The localization of METTL3 and METTL14 to nuclear speckles also supported the idea that m6A is linked to splicing, and these sites may enable intronic methylation.
MeRIP-Seq does not fully support the concept that methylation is highly targeted to introns. MeRIP-Seq studies showed that m6A was preferentially found in exons, and only ~5-7% of m6A peaks in introns. However, MeRIP-Seq studies to date have been performed on steady state cellular RNA, which contains a low concentration of the highly labile pre-mRNA and introns. Thus, m6A may be introduced into introns at a relatively high level, which is then followed by rapid intron excision and degradation. Although the prevalence and distribution of m6A within intronic sequences needs to be more thoroughly examined by performing MeRIP-Seq on premRNA, one possibility is that m6A is targeted to specific intronic regions in order to influence splicing efficiency.
The function of m6A in splicing seems to be linked to regulating isoform diversity by alternative splicing. mRNAs that exhibit multiple isoforms due to alternative splicing are significantly more likely to contain m6A and exhibit METTL3 binding than mRNAs that are expressed as only one spliced isoform4, 61. Additionally, m6A is more likely to be found in introns and exons that undergo alternative splicing4. Thus, m6A appears to contribute to alternative splicing of mRNA. However, a mechanistic relationship between the presence of m6A and splicing events is not yet established.
Regulation of mRNA translation
There are conflicting results as to whether m6A affects protein translation in vitro. Early studies using a rabbit reticulocyte lysate system showed that translation of m6A-containing DHFR mRNA was increased by about 50% compared with mRNA98. However, more recent studies using both in vitro translation as well as transfection of reporter mRNAs into cells have shown that adenosine methylation leads to reduced translation compared with unmethylated transcripts99 (Figure 3d). Thus, the effects of m6A on protein production do not seem to be uniform among different mRNAs. One possibility is that this effect is determined in part by other cis-acting factors within the transcript, or perhaps that the location of m6A within an mRNA influences its ability to interact with specific trans-acting factors which mediate its effects on translation.
Effects on mRNA expression and degradation
Analysis of differential mRNA expression levels in control and METTL3 knockdown cells suggested that m6A stabilizes mRNA4 (Figure 3e). Loss of methylation correlated with reduced expression levels of transcripts that contain m6A4. This effect was most prominent for mRNAs that contain m6A in introns. These studies support the general idea that m6A is needed for normal mRNA expression levels. A possible explanation for these data is that m6A is needed for proper splicing of mRNA, which when disrupted results in impaired splicing and subsequent mRNA degradation.
Studies using FTO-knockout mice provide an opportunity to determine how altered m6A levels influence mRNA abundance and protein production. In these mice, five mRNAs that have been tested had increased m6A peaks (that is, that are hypermethylated) and also exhibited slightly increased mRNA levels, but there was no consistent effect on the level of the encoded proteins: in some cases, protein levels were decreased, whereas for other transcripts the protein levels were slightly increased or were unchanged42.
Furthermore, other reports suggest that m6A promotes mRNA degradation63, 86. One study focused on YTHDF2, a mammalian protein that has been identified to bind to m6A using pulldown approaches86. YTHDF2 was shown to promote mRNA degradation of thousands of cellular transcripts86. Consistent with this idea, YTHDF2 is found in a subset of cellular P-bodies. Additionally, PAR-CLIP studies probing the binding sites of overexpressed epitope-tagged YTHDF2 identified target mRNAs that are also known to contain m6A. In some transcripts, the PAR-CLIP site seems to overlap with the m6A peak86. Further analysis will be required to define the specific identity and number of m6A-containing mRNAs that are subjected to YTHDF2 regulation in cells.
Another study used METTL3 and METTL14 knockdown to identify mRNAs that were methylated by these methyltransferases, and monitored the stability of these mRNAs in mouse embryonic stem cells63. In knockdown cells, many METTL3- and METTL14-target mRNAs showed a modest increase in stability, suggesting that m6A functioned to induce mRNA stability. The authors proposed that m6A displaces HuR, a known mRNA stabilizer. Indeed, in vitro HuR binding to its typical U-rich binding site was impaired by adjacent m6A residues63. Previous studies had suggested that HuR might be an m6A-binding protein4; however, this more recent analysis argued that m6A reduces HuR binding, thereby facilitating mRNA degradation.
In both of these studies, the effect of m6A on mRNA stability was measured by monitoring total mRNA levels. Since every mRNA exists as a pool of both methylated and nonmethylated forms, it will be important to directly follow the fate of only the mRNA molecules that contain m6A. This is important, as the stoichiometry of m6A in the vast majority of m6A-containing mRNAs is likely to be very low. These experiments will be useful to distinguish between the functional roles of YTHDF2 and HuR in mRNA degradation.
Relationship between m6A and microRNAs
Analysis of the MeRIP-Seq dataset revealed a strong correlation between the presence of m6A and microRNA-binding sites3. 67% of 3’UTRs that contain m6A peaks also contain at least one TargetScan-predicted microRNA-binding site. Because ~30% of genes have microRNA-binding sites in their 3’UTRs100, this is a significantly enhanced association. Importantly, the m6A peak and microRNA site did not overlap. In general, the m6A peaks are most abundant near the stop codon and generally decrease in frequency along the 3’UTR length, whereas the microRNA target sites are enriched at the 5’ and 3’ end of 3’UTRs44.
The strong association between m6A in 3’UTRs and the presence of microRNA-binding sites suggests an interplay between mRNA methylation and microRNAs. It is possible that the presence of a microRNA-binding site induces pathways that lead to mRNA methylation. Indeed, in the brain, mRNAs containing brain-enriched microRNA-binding sites were more likely to have an m6A than mRNAs that contain sites for microRNAs that are not abundant in brain3. Further studies will be necessary to determine whether microRNAs contribute to the induction of methylation at upstream regions of the transcript. Additionally, it will be important to determine whether adenosine methylation contributes to the actions of microRNA-induced mRNA silencing.
Future directions
The results of transcriptome-wide m6A mapping techniques have resolved many of the longstanding concerns regarding the physiological relevance of m6A and have renewed interest in this mRNA modification. Currently, an understanding of the function of m6A and how mRNA methylation is regulated remain poorly understood, despite many important recent advances. Resolving these issues will require efforts on several different fronts.
An important next step will be to develop approaches for higher resolution mapping of m6A in the mammalian transcriptome. This will allow researchers to selectively mutate specific adenosine residues and determine how methylation regulates the fate of an mRNA. Furthermore, precise m6A mapping is needed to define the specific m6A residues that are bound to putative m6A-binding proteins. MeRIP-Seq involves immunoprecipitation of ~100-nt long fragments, which therefore results in peaks that are approximately 200 nt in resolution. The use of smaller RNA fragments has recently been described, which provides greater resolution75. This approach was used to map m6A sites in yeast, and its applicability to more complex transcriptomes has not yet been established. Other approaches to achieve finer resolution can be envisaged, for example by crosslinking cellular RNA to m6A antibodies and analysing the crosslinking site using methods that have been highly optimized for HITS-CLIP and related techniques90-92. Last, the inclusion of control RNAs with known m6A stoichiometry could be used as standards to obtain absolute measures of m6A stoichiometry. These strategies will be essential to more precisely define the specific modified m6A residues in the transcriptome and the degree of methylation at specific adenosines within a transcript.
A major goal will be to determine how m6A affects the fate of mRNA. In addition, there is a need to understand whether all m6As have the same function, or whether their distribution within a transcript dictates their role in mRNA processing. A crucial step in this process will be the identification of high-affinity m6A-binding proteins, and the demonstration that endogenous protein binds mRNAs at m6A residues. This requires, in part, mapping of m6A at higher resolution than is currently available. The demonstration that m6A-binding proteins are indeed binding m6A should in turn enable the characterization of the mechanisms through which m6A regulates mRNAs.
One of the exciting aspects of m6A is its potential to be reversed by FTO or ALKBH5. As mutations in FTO are linked to various diseases and altered neuronal function 42, 101, 102, the identification of physiological signaling pathways that trigger m6A demethylation are particularly important. However, evidence for dynamic reversibility of m6A in mRNA has not been established. The recent identification of mRNAs that are targeted by FTO42 is a first step in deciphering these demethylation pathways.
Similarly, it is not clear why certain mRNAs are targeted for methylation. This is an outstanding puzzle, as all mRNAs contain the G-A*-C consensus motif, yet only a fraction of cellular mRNAs seem to be targeted for methylation. Thus, the factors that determine whether an mRNA will be methylated are unknown. Identifying cis-acting elements in mRNAs that render an mRNA susceptible to methylation will be important for ultimately deciphering the methylation specificity in cells.
As mentioned above, m6A stoichiometry varies greatly among different mRNAs. The factor that makes an mRNA a target for high stoichiometry methylation remains unclear, but is likely to be the trigger that shunts an mRNA into an m6A-regulated processing pathway. Insights are likely to come from a more comprehensive assessment of the components of the methyltransferase complexes. Although some components have been identified, such as WTAP-1, the identification of the remaining components will likely provide insight into the basis for methylation specificity.
It remains unclear whether specific signalling pathways use mRNA methylation as an effector mechanism. Conceivably, signalling mechanisms could recruit methyltransferases or demethylases to alter m6A levels and thereby influence mRNA fate. mRNA methylation is already known to have highly specific effects on cellular processes. For example, increased adenosine methyltransferase activity is correlated with cellular transformation103. Furthermore, in yeast, induction of meiosis coincides with increased expression of the METTL3 homologue IME4 and concomitant m6A accumulation58, 104. IME4 also affects the developmental response to nutrient starvation in yeast64. These studies show that regulated adenosine methylation influences cellular processes. Ultimately, by identifying the signalling pathways that rely on mRNA methylation and demethylation, we will gain a better appreciation for the breadth of physiological roles that involve m6A.
Supplementary Material
Summary.
In 2012, two independent studies demonstrated that N6-methyladenosine (m6A) is a widespread base modification in the mammalian transcriptome which exhibits a unique enrichment near the stop codon and in the UTRs of mRNAs.
Recent studies have identified METTL3, METTL14, and WTAP as components of an m6A methyltransferase complex. Further characterization of this complex will be needed to understand the dynamics and specificity of adenosine methylation in various classes of cellular RNA.
FTO and ALKBH5 are the two m6A demethylating enzymes identified to date. Based on studies of the mRNA targeting specificity and tissue-specific expression patterns of these enzymes, however, it is likely that additional m6A demethylases exist.
m6A likely functions by recruiting m6A binding proteins which influence RNA processing and regulation. Although a small number of m6A binding proteins have been identified, much work remains to understand the full repertoire of m6A binding proteins and how they contribute to mRNA regulation.
Although many functions likely exist for m6A, studies so far suggest that it plays a role in splicing regulation and mRNA stability
Acknowledgements
We thank O. Elemento and members of the Jaffrey laboratory for helpful comments and suggestions. This work was supported by NIH grant R01 DA037150 (S.R.J.) and the Revson Senior Fellowship in Biomedical Sciences to K.M.
Glossary
- rRNA
RNAs that are a highly abundant species of cellular RNA that function in complex with ribosomes to mediate mRNA translation.
- snRNA (Small nuclear RNA)
RNAs that associate with specific proteins and are frequently involved in pre-mRNA processing events such as splicing.
- non-coding RNAs (ncRNAs)
RNAs that are not translated into proteins. ncRNAs include functional RNAs such as transfer RNAs, microRNAs and long ncRNAs.
- Nuclear speckles
Small, dynamic, subnuclear structures that are enriched in pre-mRNA splicing factors.
- Fe(II)/2-oxoglutaratedependent oxygenases
A family of proteins that catalyzes cellular oxidative reactions, most notably hydroxylation. Several members of this family are involved in nucleic acid demethylation.
- Zero-mode waveguides
Nanostructures with highly confined optical observation volumes.
Biography
Kate Meyer received her Ph.D. in neuroscience at Northwestern University in Chicago, USA and is currently a postdoctoral researcher in the laboratory of Samie R. Jaffrey. Her current research focuses on the function of m6A in mRNA regulatory pathways.
Samie R. Jaffrey is a professor of pharmacology at Weill Cornell Medical College, New York, USA. He received his M.D. and Ph.D. at Johns Hopkins University and completed his postdoctoral studies in the laboratory of Solomon H. Snyder at Johns Hopkins University. His laboratory studies dynamic regulation of mRNA methylation and the role of m6A in cellular mRNA regulation. His laboratory also develops novel tools for imaging RNAs in living cells in order to study RNA trafficking and RNA processing events.
REFERENCES
- 1.Lewis JD, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–14. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T, Scott JD. Protein phosphorylation in signaling--50 years and counting. Trends Biochem Sci. 2005;30:286–90. doi: 10.1016/j.tibs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–46. doi: 10.1016/j.cell.2012.05.003. [Provides the first demonstration that m6A is a widespread modification in mammalian mRNAs and reveals that m6A is highly enriched surrounding stop codons and in UTRs. Also identifies many methylated noncoding RNAs which were not previously known to contain m6A.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6. doi: 10.1038/nature11112. [Demonstrates, together with reference 3, that m6A is a pervasive feature of the transcriptome which exhibits a unique distribution within mRNAs. Identifies YTHDF2-3 and HuR as potential m6A binding proteins.] [DOI] [PubMed] [Google Scholar]
- 5.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7. doi: 10.1038/nchembio.687. [Reveals that the obesity-associated protein, FTO, is capable of demethylating m6A residues in mRNA and points to the reversibility of this modification.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng G, et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry RP, Kelley DE. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. [Google Scholar]
- 8.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–5. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavi S, Shatkin AJ. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975;72:2012–6. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei CM, Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975;72:318–22. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuichi Y, et al. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975;72:1904–8. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JM, Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 13.Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–68. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei C, Gershowitz A, Moss B. N6, O2'-dimethyladenosine a novel methylated ribonucleoside next to the 5' terminal of animal cell and virus mRNAs. Nature. 1975;257:251–3. doi: 10.1038/257251a0. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt W, Arnold HH, Kersten H. Biosynthetic pathway of ribothymidine in B. subtilis and M. lysodeikticus involving different coenzymes for transfer RNA and ribosomal RNA. Nucleic Acids Res. 1975;2:1043–51. doi: 10.1093/nar/2.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Weisblum B. Systematic difference in the methylation of ribosomal ribonucleic acid from gram-positive and gram-negative bacteria. J Bacteriol. 1975;123:771–4. doi: 10.1128/jb.123.2.771-774.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munns TW, Sims HF, Liszewski MK. Immunospecific retention of oligonucleotides possessing N6-methyladenosine and 7-methylguanosine. J Biol Chem. 1977;252:3102–4. [PubMed] [Google Scholar]
- 18.Epstein P, Reddy R, Henning D, Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980;255:8901–6. [PubMed] [Google Scholar]
- 19.Harada F, Kato N, Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980;95:1332–40. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- 20.Dimock K, Stoltzfus CM. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977;16:471–8. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- 21.Beemon K, Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977;113:165–79. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- 22.Furuichi Y, Shatkin AJ, Stavnezer E, Bishop JM. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975;257:618–20. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- 23.Sommer S, et al. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3:749–65. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canaani D, Kahana C, Lavi S, Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979;6:2879–99. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry RP, Kelley DE, Friderici K, Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell. 1975;4:387–94. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- 26.Choi YC, Busch H. Modified nucleotides in T1 RNase oligonucleotides of 18S ribosomal RNA of the Novikoff hepatoma. Biochemistry. 1978;17:2551–60. doi: 10.1021/bi00606a015. [DOI] [PubMed] [Google Scholar]
- 27.Bringmann P, Luhrmann R. Antibodies specific for N6-methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett. 1987;213:309–15. doi: 10.1016/0014-5793(87)81512-0. [DOI] [PubMed] [Google Scholar]
- 28.Shimba S, Bokar JA, Rottman F, Reddy R. Accurate and efficient N-6-adenosine methylation in spliceosomal U6 small nuclear RNA by HeLa cell extract in vitro. Nucleic Acids Res. 1995;23:2421–6. doi: 10.1093/nar/23.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlman S, Abelson HT, Penman S. Mitochondrial protein synthesis: RNA with the properties of Eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1973;70:350–3. doi: 10.1073/pnas.70.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaike T, Suzuki T, Ueda T. Polyadenylation in mammalian mitochondria: insights from recent studies. Biochim Biophys Acta. 2008;1779:266–9. doi: 10.1016/j.bbagrm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Slomovic S, Laufer D, Geiger D, Schuster G. Polyadenylation of ribosomal RNA in human cells. Nucleic Acids Res. 2006;34:2966–75. doi: 10.1093/nar/gkl357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salditt-Georgieff M, et al. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976;7:227–37. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- 33.Okazaki Y, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 34.Numata K, et al. Identification of putative noncoding RNAs among the RIKEN mouse full-length cDNA collection. Genome Res. 2003;13:1301–6. doi: 10.1101/gr.1011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravasi T, et al. Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Res. 2006;16:11–9. doi: 10.1101/gr.4200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquier A. The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nat Rev Genet. 2009;10:833–44. doi: 10.1038/nrg2683. [DOI] [PubMed] [Google Scholar]
- 37.Csepany T, Lin A, Baldick CJ, Jr., Beemon K. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990;265:20117–22. [PubMed] [Google Scholar]
- 38.Kane SE, Beemon K. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J Biol Chem. 1987;262:3422–7. [PubMed] [Google Scholar]
- 39.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol. 1985;5:2298–306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll SM, Narayan P, Rottman FM. N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol Cell Biol. 1990;10:4456–65. doi: 10.1128/mcb.10.9.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horowitz S, Horowitz A, Nilsen TW, Munns TW, Rottman FM. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1984;81:5667–71. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hess ME, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042–8. doi: 10.1038/nn.3449. [DOI] [PubMed] [Google Scholar]
- 43.Saletore Y, et al. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol. 2012;13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimson A, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baltz AG, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46:674–90. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Li F, et al. Global analysis of RNA secondary structure in two metazoans. Cell Rep. 2012;1:69–82. doi: 10.1016/j.celrep.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Elemento O, Slonim N, Tavazoie S. A universal framework for regulatory element discovery across all genomes and data types. Mol Cell. 2007;28:337–50. doi: 10.1016/j.molcel.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei CM, Gershowitz A, Moss B. 5'-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976;15:397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]
- 49.Wei CM, Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977;16:1672–6. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- 50.Schibler U, Kelley DE, Perry RP. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol. 1977;115:695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- 51.Harper JE, Miceli SM, Roberts RJ, Manley JL. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 1990;18:5735–41. doi: 10.1093/nar/18.19.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem. 1994;269:17697–704. [PubMed] [Google Scholar]
- 53.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–49. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 54.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–47. [Identifies METTL3 as a key component of the m6A methyltransferase complex.] [PMC free article] [PubMed] [Google Scholar]
- 55.Rottman FM, Bokar JA, Narayan P, Shambaugh ME, Ludwiczak R. N6-adenosine methylation in mRNA: substrate specificity and enzyme complexity. Biochimie. 1994;76:1109–14. doi: 10.1016/0300-9084(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 56.Narayan P, Ludwiczak RL, Goodwin EC, Rottman FM. Context effects on N6-adenosine methylation sites in prolactin mRNA. Nucleic Acids Res. 1994;22:419–26. doi: 10.1093/nar/22.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–88. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–18. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc Natl Acad Sci U S A. 2011;108:14855–60. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Havugimana PC, et al. A census of human soluble protein complexes. Cell. 2012;150:1068–81. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ping XL, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014 doi: 10.1038/cr.2014.3. [Identifies, together with reference 62, WTAP as additional components of the mammalian m6A mRNA methyltransferase complex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N(6)-adenosine methylation. Nat Chem Biol. 2014;10:93–5. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, et al. N(6)-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–8. doi: 10.1038/ncb2902. [Indentifies, together with references 61 and 62, METTL14 as an adenosine methyltransferase and a component of the m6A mRNA methyltransferase complex. Reference 63 also shows that that m6A in mouse embryonic stem cells is required for stem cell differentiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. doi: 10.1371/journal.pgen.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horiuchi K, et al. Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayan P, Rottman FM. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988;242:1159–62. doi: 10.1126/science.3187541. [DOI] [PubMed] [Google Scholar]
- 67.Uhlen M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 68.Leach RA, Tuck MT. Methionine depletion induces transcription of the mRNA (N6-adenosine)methyltransferase. Int J Biochem Cell Biol. 2001;33:1116–28. doi: 10.1016/s1357-2725(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 69.Leach RA, Tuck MT. Expression of the mRNA (N6-adenosine)-methyltransferase S-adenosyl-L-methionine binding subunit mRNA in cultured cells. Int J Biochem Cell Biol. 2001;33:984–99. doi: 10.1016/s1357-2725(01)00071-1. [DOI] [PubMed] [Google Scholar]
- 70.Petry S, Weixlbaumer A, Ramakrishnan V. The termination of translation. Curr Opin Struct Biol. 2008;18:70–7. doi: 10.1016/j.sbi.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Czaplinski K, et al. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 1998;12:1665–77. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahlberg JE, Lund E. Does protein synthesis occur in the nucleus? Curr Opin Cell Biol. 2004;16:335–8. doi: 10.1016/j.ceb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MTA70 subunit of the human mRNA:m(6)A methyltransferase. J Mol Evol. 2002;55:431–44. doi: 10.1007/s00239-002-2339-8. [DOI] [PubMed] [Google Scholar]
- 74.Tuck MT. Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem J. 1992;288(Pt 1):233–40. doi: 10.1042/bj2880233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz S, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–21. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-Pulido L, Andrade-Navarro MA. The FTO (fat mass and obesity associated) gene codes for a novel member of the non-heme dioxygenase superfamily. BMC Biochem. 2007;8:23. doi: 10.1186/1471-2091-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerken T, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–72. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falnes PO, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–82. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 79.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–8. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 80.Jia G, et al. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–9. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maden BE, Corbett ME, Heeney PA, Pugh K, Ajuh PM. Classical and novel approaches to the detection and localization of the numerous modified nucleotides in eukaryotic ribosomal RNA. Biochimie. 1995;77:22–9. doi: 10.1016/0300-9084(96)88100-4. [DOI] [PubMed] [Google Scholar]
- 82.Cheung MK, Gulati P, O'Rahilly S, Yeo GS. FTO expression is regulated by availability of essential amino acids. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.77. [DOI] [PubMed] [Google Scholar]
- 83.Vujovic P, et al. Fasting induced cytoplasmic Fto expression in some neurons of rat hypothalamus. PLoS One. 2013;8:e63694. doi: 10.1371/journal.pone.0063694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–3. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen B, et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134:17963–71. doi: 10.1021/ja3064149. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20. doi: 10.1038/nature12730. [Shows that the YTHDF2 m6A-binding protein can destabilize target mRNAs by recruiting them to cellular mRNA decay sites.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, et al. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285:14701–10. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu Y, et al. FTO-mediated formation of N(6)-hydroxymethyladenosine and N(6)-formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang X, Li H, Huang Y, Liu S. The dataset for protein-RNA binding affinity. Protein Sci. 2013 doi: 10.1002/pro.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang C, Darnell RB. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat Biotechnol. 2011;29:607–14. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konig J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17:909–15. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugimoto Y, et al. Analysis of CLIP and iCLIP methods for nucleotide-resolution studies of protein-RNA interactions. Genome Biol. 2012;13:R67. doi: 10.1186/gb-2012-13-8-r67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Botuyan MV, et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–73. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, et al. Structural basis for lower lysine methylation state-specific readout by MBT repeats of L3MBTL1 and an engineered PHD finger. Mol Cell. 2007;28:677–91. doi: 10.1016/j.molcel.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kierzek E, Kierzek R. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 2003;31:4472–80. doi: 10.1093/nar/gkg633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hibio N, Hino K, Shimizu E, Nagata Y, Ui-Tei K. Stability of miRNA 5'terminal and seed regions is correlated with experimentally observed miRNA-mediated silencing efficacy. Sci Rep. 2012;2:996. doi: 10.1038/srep00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown JA, Valenstein ML, Yario TA, Tycowski KT, Steitz JA. Formation of triple-helical structures by the 3'-end sequences of MALAT1 and MENbeta noncoding RNAs. Proc Natl Acad Sci U S A. 2012;109:19202–7. doi: 10.1073/pnas.1217338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heilman KL, Leach RA, Tuck MT. Internal 6-methyladenine residues increase the in vitro translation efficiency of dihydrofolate reductase messenger RNA. Int J Biochem Cell Biol. 1996;28:823–9. doi: 10.1016/1357-2725(96)00014-3. [DOI] [PubMed] [Google Scholar]
- 99.Kariko K, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 101.Yeo GS. FTO and obesity: a problem for a billion people. J Neuroendocrinol. 2012;24:393–4. doi: 10.1111/j.1365-2826.2011.02254.x. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Closas M, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45:392–8. 398e1–2. doi: 10.1038/ng.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tuck MT, James CB, Kelder B, Kopchick JJ. Elevation of internal 6-methyladenine mRNA methyltransferase activity after cellular transformation. Cancer Lett. 1996;103:107–13. doi: 10.1016/0304-3835(96)04203-6. [DOI] [PubMed] [Google Scholar]
- 104.Bodi Z, Button JD, Grierson D, Fray RG. Yeast targets for mRNA methylation. Nucleic Acids Res. 2010;38:5327–35. doi: 10.1093/nar/gkq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev. 2008;9:246–50. doi: 10.1111/j.1467-789X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 106.Fischer J, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–8. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 107.Church C, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–92. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iles MM, et al. A variant in FTO shows association with melanoma risk not due to BMI. Nat Genet. 2013;45:428–32. 432e1. doi: 10.1038/ng.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fustin J-M, et al. RNA-Methylation-Dependent RNA Processing Controls the Speed of the Circadian Clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [Demonstrates that inhibition of adenosine methylation alters the circadian period of target mRNAs and disrupts mRNA processing.] [DOI] [PubMed] [Google Scholar]
- 110.Bodi Z, et al. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3' End and Reduced Levels Cause Developmental Defects. Front Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gu M, Lima CD. Processing the message: structural insights into capping and decapping mRNA. Curr Opin Struct Biol. 2005;15:99–106. doi: 10.1016/j.sbi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 112.Muthukrishnan S, Both GW, Furuichi Y, Shatkin AJ. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975;255:33–7. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- 113.Shatkin AJ, et al. 5'-Terminal caps, cap-binding proteins and eukaryotic mRNA function. Biochem Soc Symp. 1982;47:129–43. [PubMed] [Google Scholar]
- 114.Schibler U, Perry RP. The 5'-termini of heterogeneous nuclear RNA: a comparison among molecules of different sizes and ages. Nucleic Acids Res. 1977;4:4133–49. doi: 10.1093/nar/4.12.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishikawa M, Murai R, Hagiwara H, Hoshino T, Suyama K. Preparation of eukaryotic mRNA having differently methylated adenosine at the 5'-terminus and the effect of the methyl group in translation. Nucleic Acids Symp Ser (Oxf) 2009:129–30. doi: 10.1093/nass/nrp065. [DOI] [PubMed] [Google Scholar]
- 116.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cocrystal structure of the messenger RNA 5' cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997;89:951–61. doi: 10.1016/s0092-8674(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 117.Kong H, et al. Functional analysis of putative restriction-modification system genes in the Helicobacter pylori J99 genome. Nucleic Acids Res. 2000;28:3216–23. doi: 10.1093/nar/28.17.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dai Q, et al. Identification of recognition residues for ligation-based detection and quantitation of pseudouridine and N6-methyladenosine. Nucleic Acids Res. 2007;35:6322–9. doi: 10.1093/nar/gkm657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harcourt EM, Ehrenschwender T, Batista PJ, Chang HY, Kool ET. Identification of a selective polymerase enables detection of N(6)-methyladenosine in RNA. J Am Chem Soc. 2013;135:19079–82. doi: 10.1021/ja4105792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu N, et al. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–56. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levene MJ, et al. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–6. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- 122.Vilfan ID, et al. Analysis of RNA base modification and structural rearrangement by single-molecule real-time detection of reverse transcription. J Nanobiotechnology. 2013;11:8. doi: 10.1186/1477-3155-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.