Abstract
Background
DYT1 dystonia is a heritable, early-onset generalized movement disorder caused by a GAG deletion (ΔGAG) in the DYT1 gene. Neuroimaging studies and studies using mouse models suggest that DYT1 dystonia is associated with dopamine imbalance. However, whether dopamine imbalance is key to DYT1 or other forms of dystonia continues to be debated.
Methodology/Principal Findings
We used Dyt1 knock out (Dyt1 KO), Dyt1 ΔGAG knock-in (Dyt1 KI), and transgenic mice carrying one copy of the human DYT1 wild type allele (DYT1 hWT) or human ΔGAG mutant allele (DYT1 hMT). D1R, D2R, and Gα(olf) protein expression was analyzed by western blot in the frontal cortex, caudate-putamen and ventral midbrain in young adult (postnatal day 60; P60) male mice from all four lines; and in the frontal cortex and caudate putamen in juvenile (postnatal day 14; P14) male mice from the Dyt1 KI and KO lines. Dopamine receptor and Gα(olf) protein expression were significantly decreased in multiple brain regions of Dyt1 KI and Dyt1 KO mice and not significantly altered in the DYT1 hMT or DYT1 hWT mice at P60. The only significant change at P14 was a decrease in D1R expression in the caudate-putamen of the Dyt1 KO mice.
Conclusion/Significance
We found significant decreases in key proteins in the dopaminergic system in multiple brain regions of Dyt1 KO and Dyt1 KI mouse lines at P60. Deletion of one copy of the Dyt1 gene (KO mice) produced the most pronounced effects. These data offer evidence that impaired dopamine receptor signaling may be an early and significant contributor to DYT1 dystonia pathophysiology.
Introduction
Dystonia is the third most common movement disorder after essential tremor and Parkinson’s disease. It is a neurological disorder characterized by involuntary muscle contractions with debilitating, painful, twisting movements and contorted postures [1]. Although the vast majority of dystonia is sporadic, it can be inherited or may be a consequence of traumatic or vascular brain injury or side effect of medications [2,3].
DYT1 dystonia is an inherited form of early onset generalized dystonia. It is inherited in an autosomal dominant manner, and has relatively low penetrance [4,5]. A trinucleotide deletion in the TOR1A gene, which results in the loss of a glutamic acid residue in the C-terminus region of the torsinA protein is linked to DYT1 dystonia [6]. TorsinA is a member of AAA+ ATPase superfamily [6], associated with chaperone like functions in multiple processes including protein folding and degradation, cytoskeletal dynamics, membrane trafficking, vesicle fusion and transportation and secretion [7,8]. TorsinA mRNA is expressed in dopaminergic neurons of the substantia nigra pars compacta, granule and pyramidal neurons of the hippocampus, Purkinje and granule neurons of the cerebellum, and cholinergic neurons of the neostriatum in humans [9,10].
Although the pathophysiological basis of dystonia remains elusive, it is believed to involve dysfunction of motor circuits in the cerebral cortex, thalamus, cerebellum and basal ganglia [11–13]. In particular, the striatal dopaminergic system is considered to be involved [13]. Reduced striatal D2R binding was reported in humans with various forms of dystonia including DYT1 dystonia, idiopathic cervical dystonia, and nocturnal myoclonus [14–16]. In addition, decreased dopamine release was reported in the DYT1 hMT transgenic mice [17].
Recently mutations in the gene for guanine nucleotide binding protein alpha subunit [Gα(olf)], were found to be associated with primary dystonias, including some cases of early onset generalized dystonia [18]. Gα(olf) is enriched in the striatum, and it forms heterotrimeric complex with Gβ/Gγ7 [19]. These second messengers are coupled with dopamine D1 receptors, suggesting further involvement of the dopaminergic system in another form of dystonia.
Despite the compelling evidence described above, involvement of the dopaminergic system in dystonia remains a subject of debate. Moreover, whether changes in dopaminergic signaling begin early in development is also not clear. Here, we examined D1R, D2R and Gα(olf) protein expression in the frontal cortex, caudate putamen and ventral midbrain in four lines of DYT1 dystonia mouse models. We reasoned that a finding of dopaminergic dysfunction in multiple models of DYT1 dystonia could add additional support for involvement of the dopaminergic system in this form of dystonia.
Materials and Methods
Animals
The following mouse models were used: Heterozygous Dyt1 knock-out (Dyt1 KO), Dyt1 heterozygous ΔGAG knock-in (Dyt1 KI), hemizygous human DYT1 wild type transgenic (DYT1 hWT) and hemizygous human DYT1 ΔGAG transgenic (DYT1 hMT). The generation of the mouse lines has been described previously [20–22]. Homozygous Dyt1 KO and Dyt1 KI mice die within 2–3 days of birth, and only heterozygous mice are viable. DYT1 hWT and DYT1 hMT mice are hemizygous for the respective transgene (i.e. only one allele bore the transgene), and survive to adulthood [21]. For each line we bred heterozygous or hemizygous mice with wild type partners to produce wild type and hetero/hemizygous offspring. Offspring were genotyped at the time of weaning using methods described previously [20–22]. We used wild type littermates from each line as controls for that line. Mice were kept in temperature and humidity controlled rooms under a 12-h-light/dark cycle with free access to food and water in the laboratory animal facility at the Massachusetts General Hospital, Boston, MA. The experimental studies were approved by the Massachusetts General Hospital’s Institutional Animal Care and Use Committee (IACUC). All of the experimental procedures were in full compliance with institutional guidelines at the Massachusetts General Hospital and the NIH Guide for the Care and Use of Laboratory Animals. The experiments were performed by investigators blinded to the genotypes.
Western blot analysis
Postnatal day 14 (P14; day of birth = P0) and P60 male mice from each line along with wild type male littermate controls were decapitated (mice were euthanized by anesthetic overdose which is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association) and the brains removed. Frontal cortex, caudate-putamen and ventral midbrain were micro-dissected based on anatomical landmarks. Samples of each region from the two hemispheres of a given brain were pooled such that there was only one sample for each brain region from one mouse. Our expertise in microdissection and isolation of different brain regions of the adult and developing mice [23–28], helps assure reliable sampling of the brain regions. We were also concerned that volumetric changes in the brains of transgenic mice could contribute variability to the sampling procedure. However, to the best of our knowledge, volumetric changes in the caudate-putamen, frontal cortex or midbrain do not exist in any of the mouse lines used here [20,21]. The tissue was homogenized in 200 μl of ice-cold lysis buffer containing 50mM Tris-Cl (pH 7.4), 175mM NaCl, 5mM EDTA with a protease inhibitor cocktail tablet (Roche, 05892791001) and sonicated for 10 sec. Triton X-100 was added to 1% w/v final concentration and the mixture was incubated on ice for 30 min and centrifuged at 10,000 × g for 15 min at 4°C. The protein concentration was measured using the Bradford assay with bovine serum albumin (BSA; Fisher Scientific, A7906) standard. The homogenates were mixed with SDS-PAGE loading buffer and boiled for 5 min, followed by 1-minute incubation on ice and 5 minutes of gentle centrifugation at 8,000g. The supernatant was stored at -20°C till the day of analysis. 40 μg of each sample was loaded on a 10% SDS-PAGE and the separated proteins were transferred to PVDF membrane. After blocking with 5% BSA or 5% milk in TBS-T buffer, which contains 20mM Tris-Cl (pH 7.6), 137 mM NaCl, 0.1% Tween 20, the membranes were incubated overnight at 4°C with rabbit anti-torsinA antibody (1:500; Abcam, ab34540) in 5% milk TBS-T buffer, rabbit anti-D2R antibody (1:500; Millipore, AB5084P) in 5% BSA TBS-T buffer (Abcam, Cambridge, MA), rabbit anti-D1R antibody (1:200; Santa Cruz, sc-14001) in 3% BSA TBS-T buffer, rabbit anti-Gα(olf), antibody (1:500; Abcam, ab74049) in 5% BSA TBS-T buffer, or rabbit anti-Gα(S) antibody (1:500; Santa Cruz, sc-823) in 3% BSA TBS-T buffer [29–35]. Membranes were washed three times and incubated with bovine anti-rabbit IgG-HRP (1:15000; Santa Cruz, sc-2370) in 5% BSA TBS-T at room temperature for 1 hour. β-actin was used as a loading control, and the membranes were also probed with HRP-conjugated β-actin antibody (1:5000; Santa Cruz, sc-4778). Immunoreactive bands were detected using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). The signals were captured by Alpha Innotech FluorChem FC2 and quantified with UN-SCAN-IT gel (Silk Scientific) software. Each western blot experiment was repeated three times.
Statistical analysis
Intensity of each band from each western blot for each experimental group (i.e. wild type and transgenic) in each experiment was normalized to the intensity of the β-actin band (loading control) for that blot. Thus, expression of each protein [D1R, D2R or Gα(olf)] was analyzed in independent and separate blots for every brain region in every mouse line and at each age. Mean±SEM values of normalized band intensities were calculated for each protein by using 3–4 replications. Each brain sample was considered as one replication. Thus, we had 3 to 4 replications for each protein (n = 3 to 4). The data were analyzed using One-Way ANOVA, followed by Bonferroni post hoc analysis (Prism 6 Software). A p-value smaller than 0.05 was considered to be statistically significant.
Results
D1R expression
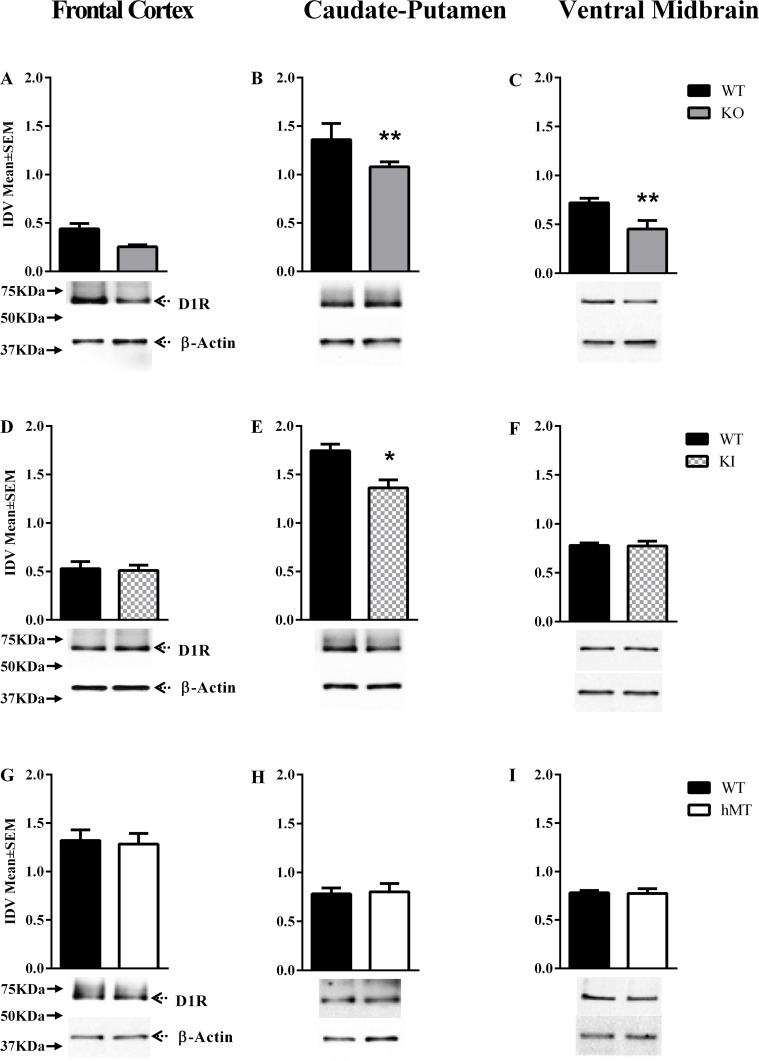
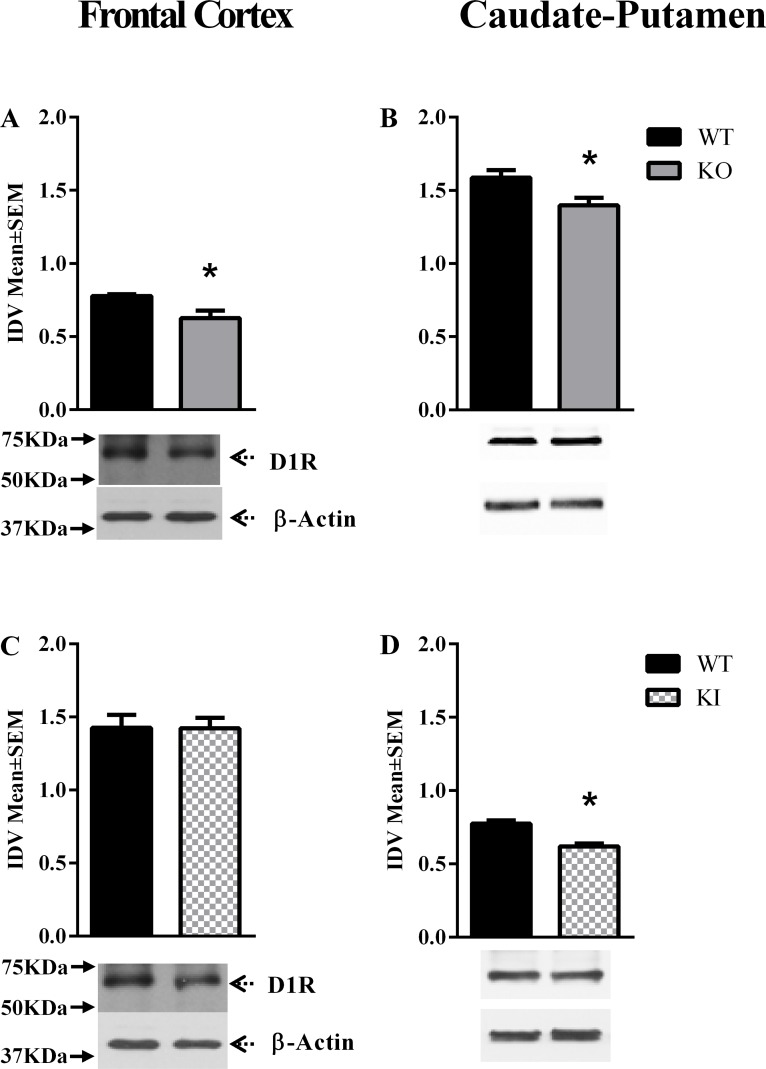
D1R expression in the frontal cortex, caudate putamen and ventral midbrain showed significant changes in the Dyt1 KO [F = (5,12) = 63.88, P < 0.0001] and Dyt1 KI [F = (5,17) = 61.15, P < 0.0001] lines of mice at P60 [Table 1]. Post hoc analysis revealed significant reductions in D1R expression in the caudate-putamen and ventral midbrain regions in the Dyt1 KO mice [Mean±SEM; caudate-putamen: WT = 1.36±0.10, KO = 1.08±0.03, p = 0.0084; ventral midbrain: WT = 0.72±0.03, KO = 0.45±0.05, p = 0.012; Fig 1A–1C] and only in the caudate-putamen in the Dyt1 KI mice [Mean±SEM; caudate-putamen: WT = 1.75±0.07, KI = 1.36±0.08, p = 0.002; Fig 1E]. D1R expression was not significantly altered in the DYT1 hMT or hWT mice [Fig 1G–1I, and S1 Table]. D1R expression at P14 [Table 2] showed significant changes only in the Dyt1 KO line [F = (3,12) = 107.4, P < 0.0001, Fig 2B]. Post-hoc analysis revealed significant reductions only in the caudate putamen of this mouse line [Mean±SEM; WT = 1.59±0.05, KO = 1.40±0.05, p = 0.023, Fig 2B and S4 Table]. We did not analyze DYT1 hWT or hMT lines of mice at P14.
Table 1. A summary of the changes in D1R, D2R and Gα(olf) expression at P60.
| P60 D1R | P60 D2R | P60 Gα(olf) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FC | CP | vMB | FC | CP | vMB | FC | CP | vMB | |
| KO | – | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | – | ↓ |
| KI | – | ↓ | – | ↓ | – | – | – | – | – |
| hWT | – | – | – | – | – | – | N/A | N/A | N/A |
| hMT | – | – | – | – | – | – | N/A | N/A | N/A |
A summary of the changes in D1R, D2R and Gα(olf) expression at postnatal day 60 (P60) in the frontal cortex (FC), caudate-putamen (CP) and ventral midbrain (vMB) in the Dyt1 KO (KO), Dyt1 KI (KI), human wild-type torsinA (hWT) and human mutant torsinA (hMT) lines of mouse.
↓ indicates statistically significant reductions in expression compared to wild-type control mice.
—indicates no statistically significant difference.
N/A differences were not analyzed.
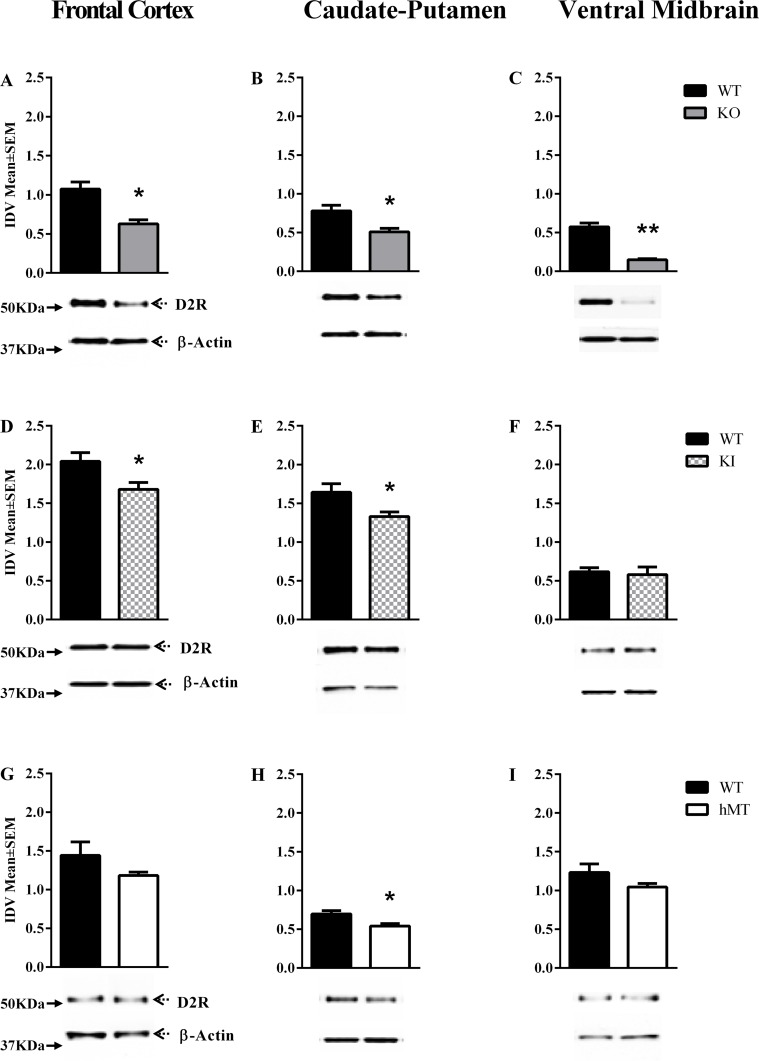
Fig 1. D1R expression at P60.
D1R expression in the frontal cortex, caudate-putamen and ventral midbrain of postnatal day 60 (P60; A-I) mice. In each panel, the upper band shows D1R and the lower band shows β-actin, which was used as a loading control. The bar graphs in each panel represent D1R band intensity (mean ± SEM) normalized to intensity of the loading control (integrated density value; IDV). The names of the mouse lines (Dyt1 KO, Dyt1 KI, hMT) are indicated to the right. (*p<0.05, **p<0.01; n = 3 or 4).
Table 2. A summary of the changes in D1R, D2R and Gα(olf) expression at P14.
| D1R | D2R | Gα(olf) | ||||
|---|---|---|---|---|---|---|
| FC | CP | FC | CP | FC | CP | |
| KO | – | ↓ | – | – | – | – |
| KI | – | – | – | – | – | – |
A summary of the changes in D1R, D2R and Gα(olf) expression at postnatal day 14 (P14) in the frontal cortex (FC) and caudate-putamen (CP) in the Dyt1 KO (KO) and Dyt1 KI (KI) lines of mouse.
↓ indicates statistically significant reductions in expression compared to wild-type control mice.
—indicates no statistically significant difference.
Fig 2. D1R expression at P14.
D1R expression in the frontal cortex and caudate-putamen of postnatal day 14 (P14; A- D) mice. In each panel, the upper band shows D1R and the lower band shows β-actin, which was used as a loading control. The bar graphs in each panel represent D1R band intensity (mean ± SEM) normalized to intensity of the loading control (integrated density value; IDV). The names of the mouse lines (Dyt1 KO, Dyt1 KI) are indicated to the right. (*p<0.05, n = 3 or 4).
Gα(olf) expression
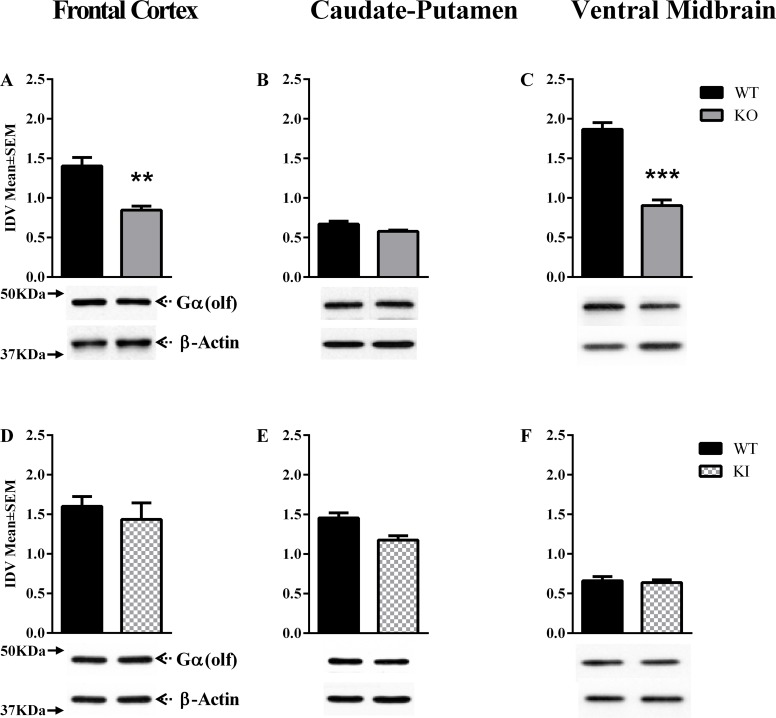
Only the Dyt1 KO mice showed significant changes in Gα(olf) expression at P60 [F = (5,12) = 51.60, P < 0.0001, Table 1]. Post hoc analysis indicated a significant reduction in the frontal cortex and ventral midbrain [Mean± SEM; frontal cortex: WT = 1.40±0.85, KO = 0.84±0.05, p = 0.0003; ventral midbrain: WT = 1.87±0.08, KO = 0.90±0.07, p = 0.0001; Fig 3A–3C]. Gα(olf) levels did not show significant changes in the Dyt1 KI, DYT1 hMT or hWT mice [S2 Table]. We examined Gα(olf) expression in the Dyt1 KI and KO mice at P14 [Table 2]. However, neither line of mouse showed significant changes in any of the brain regions examined [S5 Table].
Fig 3. Gα(olf) and Gα(s) expression at P60.
Gα(olf) and Gα(s) expression in the frontal cortex, caudate-putamen and ventral midbrain of Dyt1 KO, and, Dyt1 KI mice at postnatal day 60 (P60; A-F). In each panel, the upper band shows Gα(olf) (A-F), and the lower band shows β-actin, which was used as a loading control. The bar graphs in each panel represent Gα(olf) band intensity (mean ± SEM) normalized to intensity of loading control (integrated density value; IDV). The names of the mouse lines (Dyt1 KO, Dyt1 KI) are indicated to the right (**p<0.01, ***p<0.001; n = 3 or 4).
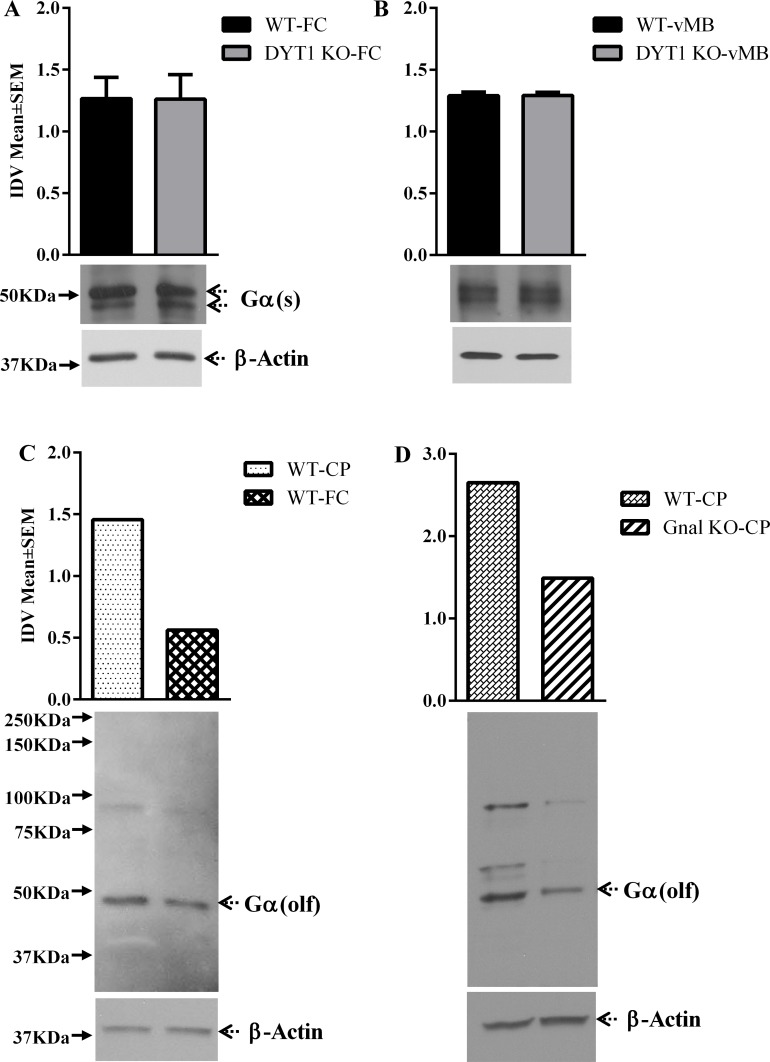
Specificity of the Gα(olf) antibody was verified by the manufacturer using a peptide containing amino acids 60–90 of human Gα(olf) protein. The peptide used as the immunogen for this antibody has no homology with Gα(s). Since Gα(olf) and Gα(s) share homology, and since Gα(s) is not associated with dystonia, it became necessary to verify that the changes in Gα(olf) reported above were not somehow influenced by potential changes in Gα(s). Toward that end we performed additional studies using a Gα(s) polyclonal antibody (Santa Cruz, sc-823), which recognizes a 15–20 amino acid epitope that maps to a 50 amino acid stretch (aa 100–150) of the Gα(s) protein (accession number P63092). The short and long forms of Gα(s) only differ between each other at residues 71–72 and 73–86. Since this difference lies outside the amino acid sequence used to raise the antibody, the antibody detects both forms of the protein. We examined Gα(s) protein expression in every brain region of the Dyt1 KO mice in which significant reductions in Gα(olf) protein were found. Our data show that expression levels of neither the short nor the long form of Gα(s) protein were significantly different between the WT and the Dyt1 KO in any brain region examined [F = (5,12) = 3.099, P > 0.05, Mean± SEM; frontal cortex: WT = 1.27±0.17, KO = 1.26±0.20; ventral midbrain: WT = 1.29±0.03, KO = 1.29±0.03 Fig 4A and 4B].
Fig 4. Gα(s) and Gα(olf) expression at P60.
Gα(s) and Gα(olf) expression in the frontal cortex, caudate-putamen and ventral midbrain of Dyt1 KO and Gnal KO mice at postnatal day 60 (P60; A-D). In each panel, the upper band shows Gα(s) (Fig 4A and 4B), and Gα(olf) (Fig 4C and 4D), and the lower band shows β-actin, which was used as a loading control. The bar graphs in each panel represent Gα(s), and Gα(olf) band intensity (mean ± SEM) normalized to intensity of loading control (integrated density value; IDV). The names of the mouse lines (Dyt1 KO, and Gnal KO) are indicated to the right.
We performed two additional studies to confirm specificity of the Gα(olf) antibody. First, we compared expression levels of Gα(olf) in the caudate putamen and frontal cortex of wild type P60 mice. Consistent with earlier reports using different Gα(olf) antibodies [36,37], Gα(olf) expression detected by the antibody used here was higher in the caudate putamen compared to the frontal cortex [Fig 4C]. Next, we examined Gα(olf) expression in samples of the caudate putamen obtained from adult heterozygous Gnal knockout mice [38]. We found a 56% reduction in Gα(olf) expression in the heterozygous samples [Fig 4D]. Based on these observations we suggest that the Gα(olf) antibody used here is capable of detecting the mouse Gα(olf) protein.
Although the Gα(olf) antibody identified a single prominent 50 KDa band, consistent with the molecular weight of the Gα(olf) protein, in the frontal cortex sample (Fig 4C), additional bands were recognized in the caudate putamen samples (Fig 4C and 4D). Virtually all the bands, not only the 50 KDa band, showed reduced intensity in the caudate putamen of the Gnal knockout mouse (Fig 4D). We suggest that the additional bands identified by this antibody represent some form of posttranslational modification of the Gα(olf) protein, especially in the caudate putamen, and that the reduction in the Gα(olf) protein in the Gnal knockout mouse not only leads to a decrease in the 50 KDa species but also in the other forms of the Gα(olf) protein.
D2R expression
D2R expression was significantly altered at P60 [Table 1] in the Dyt1 KO [F = (5,12) = 26.88, P < 0.0001], and Dyt1 KI lines (F = (5,18) = 44.60, P < 0.0001). Post hoc analysis indicated significant reductions in the frontal cortex, caudate-putamen and ventral midbrain of the Dyt1 KO line [Mean± SEM; frontal cortex: WT = 1.08±0.09, KO = 0.63±0.05, p < 0.0005; caudate-putamen: WT = 0.78± 0.07, KO = 0.51± 0.05, p < 0.021; ventral midbrain: WT = 0.57±0.05, KO = 0.15±0.01, p < 0.001; Fig 5A–5C], as well as the frontal cortex,of the Dyt1 KI line [Mean± SEM; frontal cortex: WT = 2.04± 0.11, KI = 1.68±0.09, p < 0.031 Fig 5D]. Whereas the P60 DYT1 hMT and hWT mice did not display any significant changes in D2R expression in any brain region [S3 Table] Similarly, we did not find significant changes in D2R expression in any brain region of the Dyt1 KI or KO mice at P14 [Table 2 and S6 Table].
Fig 5. D2R expression at P60.
D2R expression in the frontal cortex, caudate-putamen and ventral midbrain of postnatal day 60 (P60; A-I) mice. In each panel, the upper band shows D2R and the lower band shows β-actin, which was used as a loading control. The bar graphs in each panel represent D2R band intensity (mean ± SEM) normalized to intensity of loading control (integrated density value; IDV). The names of the mouse lines (Dyt1 KO, Dyt1 KI, hMT) are indicated to the right (*p<0.05, **p<0.01; n = 3 or 4).
Relationship among genotype, developmental stage, brain region and dopamine receptor expression
The use of Dyt1 KI and Dyt1 KO lines of mice and the availability of juvenile (P14) and young adult (P60) developmental time periods offered us the opportunity to evaluate the relative impact of the loss of torsinA (Dyt1 KO) versus the presence of the mutant torsinA (Dyt1 KI), and the impact of developmental stage (P14 versus P60) on the expression of D1R and D2R proteins in the frontal cortex and the caudate-putamen. The reductions in D2R protein expression in the frontal cortex and caudate-putamen were greater in magnitude in the Dyt1 KO mice compared to the Dyt1 KI mice at P60 [Table 3]. At P14, only the Dyt1 KO caudate-putamen showed significant changes (reductions) in the D1R protein [Table 3]. Thus, genotype, developmental stage and brain region appeared to impact D1R and D2R expression. Since the hWT and hMT lines were not analyzed at P14, similar comparisons were not performed for those lines.
Table 3. A comparison of the magnitude of reductions in D1R and D2R protein expression.
| D1R | D2R | |
|---|---|---|
| P60—FC | No change | KO > KI |
| P60—CP | KO = KI | KO > KI |
| P14—FC | No change | No change |
| P14—CP | KO > KI | No change |
A comparison of the magnitude of reductions in D1R and D2R protein expression in the frontal cortex (FC) and caudate putamen (CP) of postnatal day 60 (P60) and postnatal day 14 (P14) Dyt1 KO (KO) and Dyt1 KI (KI) mice.
Discussion
Our data show that dopamine receptor and Gα(olf) protein expression are significantly decreased in multiple brain regions of Dyt1 KI and Dyt1 KO mice whereas the expression is not altered in the hMT or hWT mice at P60 [Table 1]. In the juvenile mice (P14) significant decreases occurred in D1R in the caudate-putamen of the Dyt1 KO mice [Table 2]. Gα(olf) expression did not show significant changes in any brain region in either mouse line at P14 [Table 2]. The ventral midbrain showed significant decreases in the expression of D1R and D2R in the Dyt1 KO line at P60. Our data demonstrate that impairment of dopaminergic neurotransmission is a common theme in two of the four mouse models of DYT1 dystonia examined here, and that reduction in the expression of D1R but not D2R may begin early in the juvenile period. Interestingly, there was no evidence of significantly increased expression of D1R, D2R or Gα(olf) in any of the regions investigated, at either postnatal age, and in any of the mouse lines examined, indicating that a common feature of the DYT1 phenotype is attenuation, rather than enhancement of dopamine receptor signaling.
Although the effects of the loss of one wild type Dyt1 allele in the Dyt1 KO mouse and the introduction of one copy of the ΔGAG allele in the Dyt1 KI mouse produced comparable effects on D1R and D2R expression at P60 in terms of the direction of the change (decreased expression of both proteins and in both genotypes; Table 1), there were differences between the two mouse lines in terms of the magnitude of the effects [Table 3]. Thus, at P60, the Dyt1 KO line showed greater decreases compared to the Dyt1 KI line [Table 3] in D2R expression levels in the frontal cortex. In other words, loss of the wild type torsinA and introduction of the mutant torsinA produced a difference in the magnitude of the effect on D1R and D2R expression at P60. Molecular insights into the mechanisms of such differential effects were not possible. However, both the D1R and D2R mediated pathways, the direct and indirect striatal pathways, respectively may be compromised in DYT1 dystonia, as suggested in earlier reports from other laboratories [39–41]
There were differences in the presence or absence of an effect on D1R expression between Dyt1 KO and Dyt1 KI lines in a brain region-dependent manner at P14 [Table 3]. Thus, D1R expression in the caudate-putamen was decreased in the Dyt1 KO line and was unaffected in the Dyt1 KI line. Interestingly, D2R expression was unaffected in either mouse line in either brain region at P14 [Table 2]. Thus, loss of the wild type torsinA and introduction of the mutant torsinA produced markedly different effects on dopamine receptor expression at P14, suggesting that the developmental stage is an important variable in modifying the effects of torsinA on dopamine receptor expression.
Here we wish to emphasize two points from the literature. First, a patch-clamp study of the DYT1 hMT mouse line showed significant impairment of D2R signaling in striatal cholinergic interneurons at P10. Since the cholinergic interneurons constitute a very small proportion of striatal cells (<1% of neurons), it is possible that our western blot data did not detect potential changes in D2R expression in the DYT1 hMT line or other lines at P14. Second, it would have been interesting to correlate changes in D1R expression at P14 with behavioral changes at early developmental stages in a genotype-phenotype correlation. However, unfortunately, for the mouse lines used in this study phenotype analysis during development (e.g. P14) has not been performed. The only relevant behavioral analysis may be the one in Dyt1 KI (heterozygous) mice at 3- and 6-months of age, where a deficit in beam walking was noted at 6-months but not at 3-months [20].
That ontogenetic factors may modify torsinA’s effects on dopamine receptor expression is not unexpected. For example, expression of D1R and D2R mRNA and protein, relative distribution of receptor binding sites, and behavioral consequences of receptor activation show brain region-specific ontogenetic changes [42,43]. Although these developmental changes may not fully correlate with one another (e.g. changes in mRNA and protein expression for the same receptor may not correlate with each other), it appears that dopamine receptor expression and function undergo significant region-specific remodeling during development. The cell biological mechanisms underlying the developmental changes likely include mRNA and protein trafficking, membrane recycling and protein conformational changes in response to the changing functional demands on the neurotransmitter signaling machinery of the developing animal. TorsinA is thought to be involved in all these cellular processes [44–46], suggesting impairment of one or more of these processes in our mouse models as a contributor to the decrease in D1R, D2R or Gα(olf) proteins.
We observed a decrease in D1R but not D2R expression at P14, whereas both the receptors were decreased at P60 in multiple brain regions in the KO and KI lines. The relative abundance of the D1R versus D2R shows significant ontogenetic changes [25,42,43], which could underlie the differential susceptibilities of the two proteins during the juvenile period to changes in torsinA expression or function. Another consideration is the potential compensatory effects of torsinB, which shares 70% homology with torsinA [6]. We have shown previously that torsinA expression in the brain is high during development and low at maturity, whereas torsinB expression shows the opposite trend (low during development and high at maturity) [24]. However, the expression of torsinB in the mouse models used in the present study is not fully characterized. Therefore, a role for torsinB in the modulation of the effects of torsinA on dopamine receptor protein expression remains speculative.
Introduction of the human ΔGAG allele (hMT) and the human wild type DYT1 (hWT) produced no significant effects on D1R, D2R or Gα(olf) protein expression in any brain region at P60. Earlier studies using electrophysiological methods showed functional deficits in dopamine D2R in the striatum of the hMT line [40] and behavioral analyses showed motor learning deficits in this mouse line [21]. The lack of significant changes in the expression of any of the proteins in the present study may be due to the global nature of the present analyses (i.e. protein expression analysis rather than functional analysis of the receptors).
Expression of the Gα(olf) protein was significantly decreased in the frontal cortex and ventral midbrain of the Dyt1 KO line. Recent evidence suggests that mutations in the Gα(olf) gene (GNAL) are associated with generalized dystonia [18]. Gα(olf) is enriched in neurons of the caudate-putamen. However, we did not observe significant changes in Gα(olf) expression in the caudate-putamen in any of the mouse lines.
Finally D1R and D2R expression was affected in the midbrain region only in the Dyt1 KO line. This may represent an example of region-specificity of the effects of the loss of torsinA. Alternatively, since the expression of D1R and D2R is relatively low in the ventral midbrain compared to the other brain regions, it is possible that our techniques are not sensitive enough to detect additional changes.
In summary our data show that dopamine D1R, D2R and Gα(olf) protein expression is vulnerable to the loss of torsinA as well as introduction of the mouse ΔGAG mutation. The Dyt1 KO and Dyt1 KI lines showed the most marked reductions in the expression of the proteins. The decrease in D1R occurred in the juvenile period (P14) and preceded the decreases in D2R and Gα(olf) expression, the latter occurring only at P60. Thus, changes in D1R signaling may occur early in postnatal development predisposing the individual carrying the Dyt1 mutation to dystonia beginning at an early age. Our findings represent the first comprehensive study of the changes in dopamine receptor signaling mechanisms in DYT1 dystonia.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the staff of the Massachusetts General Hospital’s Center for Comparative Medicine for expert assistance with mouse colony management, and Dr. Michelle Ehrlich, Ph.D. of the Mount Sinai School of Medicine, New York, NY for providing us with tissue samples from heterozygous Gnal knockout and wild type mice.
Data Availability
All the data underlying the findings described in this paper are available within the paper itself and in the Supporting Data tables [S1 to S6 Tables].
Funding Statement
Support was provided by the Brian Jackson Dystonia Research and Discovery Fund and NINDS P01NS037409.
References
- 1. Fahn S (1988) Concept and classification of dystonia. Adv Neurol 50: 1–8. [PubMed] [Google Scholar]
- 2. Keepers GA, Casey DE (1987) Prediction of neuroleptic-induced dystonia. J Clin Psychopharmacol 7: 342–345. [PubMed] [Google Scholar]
- 3. Marrero-Gonzalez PC, Ruano OL, Catalano G, Catalano MC (2014) Dystonia associated with lamotrigine therapy: a case report and review of the literature. Curr Drug Saf 9: 60–62. [DOI] [PubMed] [Google Scholar]
- 4. Bressman SB, Greene PE (2000) Dystonia. Curr Treat Options Neurol 2: 275–285. [DOI] [PubMed] [Google Scholar]
- 5. Fahn S (1991) The genetics of idiopathic torsion dystonia. Int J Neurol 25–26: 70–80. [PubMed] [Google Scholar]
- 6. Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. (1997) The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet 17: 40–48. [DOI] [PubMed] [Google Scholar]
- 7. Granata A, Schiavo G, Warner TT (2009) TorsinA and dystonia: from nuclear envelope to synapse. J Neurochem 109: 1596–1609. 10.1111/j.1471-4159.2009.06095.x [DOI] [PubMed] [Google Scholar]
- 8. Warner TT, Granata A, Schiavo G (2010) TorsinA and DYT1 dystonia: a synaptopathy? Biochem Soc Trans 38: 452–456. 10.1042/BST0380452 [DOI] [PubMed] [Google Scholar]
- 9. Augood SJ, Martin DM, Ozelius LJ, Breakefield XO, Penney JB Jr, Standaert DG (1999) Distribution of the mRNAs encoding torsinA and torsinB in the normal adult human brain. Ann Neurol 46: 761–769. [PubMed] [Google Scholar]
- 10. Augood SJ, Penney JB Jr, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, et al. (1998) Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann Neurol 43: 669–673. [DOI] [PubMed] [Google Scholar]
- 11. Fuller J, Prescott IA, Moro E, Toda H, Lozano A, Hutchison WD (2013) Pallidal deep brain stimulation for a case of hemidystonia secondary to a striatal stroke. Stereotact Funct Neurosurg 91: 190–197. 10.1159/000345113 [DOI] [PubMed] [Google Scholar]
- 12. Nishimura K, Uehara T, Toyoda K (2014) Early-onset dystonia after supplementary motor area infarction. J Stroke Cerebrovasc Dis 23: 1267–1268. 10.1016/j.jstrokecerebrovasdis.2013.09.028 [DOI] [PubMed] [Google Scholar]
- 13. Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ (2012) The cerebellum in dystonia—help or hindrance? Clin Neurophysiol 123: 65–70. 10.1016/j.clinph.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 14. Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. (2005) Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology 64: 347–349. [DOI] [PubMed] [Google Scholar]
- 15. Naumann M, Pirker W, Reiners K, Lange KW, Becker G, Brucke T (1998) Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: a SPECT study using [123I] epidepride and [123I] beta-CIT. Mov Disord 13: 319–323. [DOI] [PubMed] [Google Scholar]
- 16. Staedt J, Stoppe G, Kogler A, Riemann H, Hajak G, Munz DL, et al. (1995) Nocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine D2-receptor alteration. Eur Arch Psychiatry Clin Neurosci 245: 8–10. [DOI] [PubMed] [Google Scholar]
- 17. Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG (2007) Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem 102: 783–788. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. (2013) Mutations in GNAL cause primary torsion dystonia. Nat Genet 45: 88–92. 10.1038/ng.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vemula SR, Puschmann A, Xiao J, Zhao Y, Rudzinska M, Frei KP, et al. (2013) Role of Galpha(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet 22: 2510–2519. 10.1093/hmg/ddt102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, et al. (2005) Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol 196: 452–463. [DOI] [PubMed] [Google Scholar]
- 21. Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, et al. (2005) Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J Neurosci 25: 5351–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yokoi F, Dang MT, Mitsui S, Li J, Li Y (2008) Motor deficits and hyperactivity in cerebral cortex-specific Dyt1 conditional knockout mice. J Biochem 143: 39–47. [DOI] [PubMed] [Google Scholar]
- 23. Bhide PG, Day M, Sapp E, Schwarz C, Sheth A, Kim J, et al. (1996) Expression of normal and mutant huntingtin in the developing brain. J Neurosci 16: 5523–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasudevan A, Breakefield XO, Bhide PG (2006) Developmental patterns of torsinA and torsinB expression. Brain Res 1073–1074: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Araki KY, Sims JR, Bhide PG (2007) Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res 1156: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG (2008) Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci 11: 429–439. 10.1038/nn2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarthy DM, Zhang X, Darnell SB, Sangrey GR, Yanagawa Y, Sadri-Vakili G, et al. (2011) Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J Neurosci 31: 13400–13411. 10.1523/JNEUROSCI.2944-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG (2012) Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J Neurosci 32: 9410–9418. 10.1523/JNEUROSCI.1041-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boundy VA, Luedtke RR, Molinoff PB (1993) Development of polyclonal anti-D2 dopamine receptor antibodies to fusion proteins: inhibition of D2 receptor-G protein interaction. J Neurochem 60: 2181–2191. [DOI] [PubMed] [Google Scholar]
- 30. Mengual E, Pickel VM (2002) Ultrastructural immunocytochemical localization of the dopamine D2 receptor and tyrosine hydroxylase in the rat ventral pallidum. Synapse 43: 151–162. [DOI] [PubMed] [Google Scholar]
- 31. Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C (2008) Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol 74: 59–69. 10.1124/mol.107.043885 [DOI] [PubMed] [Google Scholar]
- 32. Cao S, Hewett JW, Yokoi F, Lu J, Buckley AC, Burdette AJ, et al. (2010) Chemical enhancement of torsinA function in cell and animal models of torsion dystonia. Dis Model Mech 3: 386–396. 10.1242/dmm.003715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yokoi F, Yang G, Li J, DeAndrade MP, Zhou T, Li Y (2010) Earlier onset of motor deficits in mice with double mutations in Dyt1 and Sgce. J Biochem 148: 459–466. 10.1093/jb/mvq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Yokoi F, Jin YH, DeAndrade MP, Hashimoto K, Standaert DG, et al. (2011) Altered dendritic morphology of Purkinje cells in Dyt1 DeltaGAG knock-in and purkinje cell-specific Dyt1 conditional knockout mice. PLoS One 6: e18357 10.1371/journal.pone.0018357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Yokoi F, Parsons DS, Standaert DG, Li Y (2012) Alteration of striatal dopaminergic neurotransmission in a mouse model of DYT11 myoclonus-dystonia. PLoS One 7: e33669 10.1371/journal.pone.0033669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, et al. (1993) G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci 13: 2237–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sako W, Morigaki R, Nagahiro S, Kaji R, Goto S (2010) Olfactory type G-protein alpha subunit in striosome-matrix dopamine systems in adult mice. Neuroscience 170: 497–502. 10.1016/j.neuroscience.2010.06.072 [DOI] [PubMed] [Google Scholar]
- 38. Belluscio L, Gold GH, Nemes A, Axel R (1998) Mice deficient in G(olf) are anosmic. Neuron 20: 69–81. [DOI] [PubMed] [Google Scholar]
- 39. Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, et al. (2002) Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology 59: 445–448. [DOI] [PubMed] [Google Scholar]
- 40. Sciamanna G, Tassone A, Martella G, Mandolesi G, Puglisi F, Cuomo D, et al. (2011) Developmental profile of the aberrant dopamine D2 receptor response in striatal cholinergic interneurons in DYT1 dystonia. PLoS One 6: e24261 10.1371/journal.pone.0024261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yokoi F, Dang MT, Liu J, Gandre JR, Kwon K, Yuen R, et al. (2015) Decreased dopamine receptor 1 activity and impaired motor-skill transfer in Dyt1 DeltaGAG heterozygous knock-in mice. Behav Brain Res 279: 202–210. 10.1016/j.bbr.2014.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37: 167–169. [DOI] [PubMed] [Google Scholar]
- 43. Chen YI, Choi JK, Xu H, Ren J, Andersen SL, Jenkins BG (2010) Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev Neurosci 32: 125–138. 10.1159/000286215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Breakefield XO, Blood AJ, Li YQ, Hallett M, Hanson PI, Standaert DG (2008) The pathophysiological basis of dystonias. Nature Reviews Neuroscience 9: 222–234. 10.1038/nrn2337 [DOI] [PubMed] [Google Scholar]
- 45. Breakefield XO, Kamm C, Hanson PI (2001) TorsinA: Movement at many levels. Neuron 31: 9–12. [DOI] [PubMed] [Google Scholar]
- 46. Hewett J, Ziefer P, Bergeron D, Naismith T, Boston H, Slater D, et al. (2003) TorsinA in PC12 cells: Localization in the endoplasmic reticulum and response to stress. Journal of Neuroscience Research 72: 158–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All the data underlying the findings described in this paper are available within the paper itself and in the Supporting Data tables [S1 to S6 Tables].