Abstract
Purpose
To determine the characteristics, treatments and outcomes of patients with glioblastoma multiforme (GBM) or gliosarcoma (GS) and metastases outside of the central nervous system (CNS).
Methods
PubMed and Web of Science searches for peer-reviewed articles pertaining to GBM/ GS patients with metastatic dissemination were conducted using the keywords gliosarcoma, glioblastoma, GBM, metastasis, metastases and metastatic. Additionally, we performed hand search following the references from the selected papers. Cases with metastases to the CNS were excluded and evaluated in a separate study.
Results
109 articles published between 1928 and 2013 were eligible. They reported on 150 patients. We observed a remarkable increase in the number of cases per decade over time. Median overall survival from diagnosis of metastasis (OSM+) was 6.0 ± 0.8 months and median overall survival from initial diagnosis (OSID) 13 ± 2.4 months. On univariate analyses, gender, age, the histological subtype, the time interval between initial diagnosis and diagnosis of metastasis and pulmonary involvement did not influence OSM+. We did not observe any substantial treatment progress. A comparison of the present cohort with 84 GBM/ GS patients with exclusive CNS dissemination suggests that metastases outside the CNS are related to a slightly more favorable outcome.
Conclusions
The occurrence of extra-CNS metastasis from GBM/ GS is associated with a dismal prognosis, however it seems to compare slightly favorable to CNS dissemination. Crucial treatment progress has not been achieved over recent decades. A central registry should be considered to consecutively gain more information about the ideal therapeutic approach.
Introduction
Glioblastoma multiforme (GBM) and gliosarcoma (GS) rarely spread beyond the primary tumor site and dissemination outside the central nervous system (CNS) is even more uncommon. Based on older studies [1–4] Picirilli [5] and Lun [6] et al. estimated that the frequency of its occurrence ranges between 0.4% and 2.0%. Accordingly, the current literature on GBM/ GS with extra-CNS metastasis is mainly limited to single case reports or small case series. Although two systematic reviews aiming to summarize the available data were already published in 2008 (n = 128 patients) [5] and 2011 (n = 88 patients) [6] we still have a limited understanding of the disease. The results of these reviews are partly conflicting and thus a range of relevant questions have remained unanswered. For instance, Picirilli et al. identified a considerably larger patient number (+ 40 patients (45%)) than Lun et al. although their search period was shorter. In contrast, Lun et al. performed a much more detailed analysis of the cases. Outcome in terms of overall survival from initial diagnosis differed remarkably in both cohorts. In the meantime a considerable number of new cases of GBM/ GS have been reported and last but not least opinions about the optimal methodological approach to answer certain questions may differ. We now present an individual patient data (IPD) meta-analysis, which is based on a notably larger number of cases (150 patients) to update and complete the existing knowledge.
Methods
Aim of the study
The primary objective of this study was to assess clinical characteristics, treatments and outcomes of GBM/ GS patients with extra-CNS metastases. We aimed to include all cases reported in the literature until April 2013. The secondary objective was to evaluate potential prognostic factors for overall survival after the diagnosis of metastasis (OSM+) in an explorative manner.
Search strategy and selection criteria
Identification
In a first step we performed PubMed and Web of Science searches with predefined search terms. We did not use any time or language limitations. Key words were: (gliosarcoma OR glioblastoma OR GBM) AND (metastasis OR metastases OR metastatic). In total, the search engines delivered 1695 hits, which were imported in a reference management software (endnote.com X6.0.1). After removal of duplicates, the number of hits was reduced to 1688.
Screening
Titles and abstracts were reviewed by two authors (SP and KM). Minimal requirements for further consideration of a case were diagnosis of primary intracranial GBM/ GS and metastatic spread. GBM/ GS whose primary location was spinal were not eligible as they may show different clinical features [7]. Considering these basic inclusion criteria, 1288 publications were excluded after title screening and another 145 after abstract screening. Hand search following the references from the 255 remaining articles revealed 45 new, hitherto unknown publications, which were added to the pool of papers meriting closer investigation (n = 300). However, two publications had to be excluded from our analysis because the full-text articles were not available despite interlibrary loan.
Eligibility
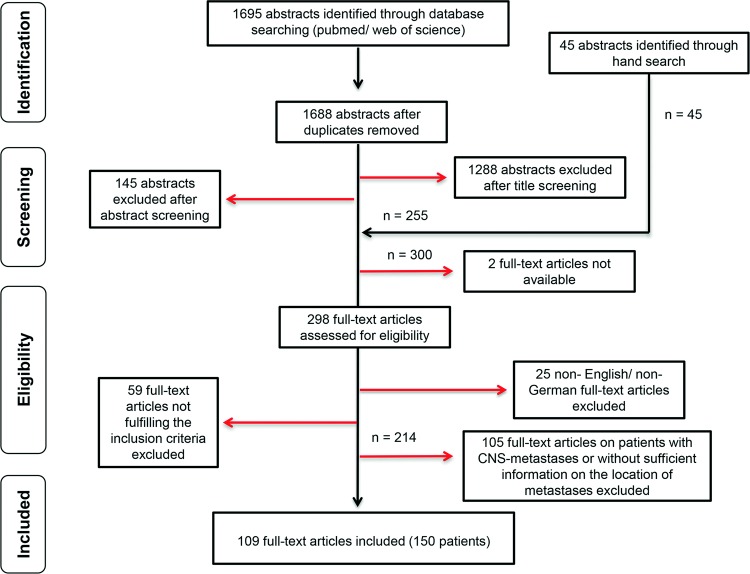
In total, we (SP and KM) evaluated 298 full-text articles for eligibility. Disagreements were resolved through discussion and consensus with a third author (AOvB). Twenty-five articles had to be excluded because they were drafted neither in English nor German and another 59 because they did not fulfill the inclusion criteria. In a second step, we excluded patients with CNS metastases and patients with unclear location of metastases (n = 105 publications). The cases with CNS metastases were analyzed separately and will be reported elsewhere. In total, we included 109 publications. The studies were published between 1928 and 2013 and reported on 150 patients. The procedure of publication retrieval and in- and exclusion of cases is displayed in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart (Fig 1) [8].
Fig 1. Procedure of publication retrieval and in- and exclusion of cases.
Data extraction
From the eligible articles, the following variables were recorded on a standard data extraction form:
- survival time divided into three periods:
- diagnosis of primary tumor to diagnosis of extra-CNS metastasis
- diagnosis of primary tumor to deaths or to last follow up assessment (OSID)
- diagnosis of extra-CNS metastasis to deaths or to last follow up assessment (OSM+)
year of publication
age at initial diagnosis
gender
histology
- site of metastasis, compiled into
- thorax and mediastinum (including lungs, pleura and heart)
- abdomen (including the peritoneal cavity, liver, intestines, spleen, kidney and adrenal gland)
- bones or bone marrow
- lymph nodes
- soft tissue and muscles
- skin
- parotid or thyroid gland
- other locations
- treatment after diagnosis of metastasis, categorized as
- not reported or best supportive care
- surgery only
- chemotherapy only
- radiotherapy only
- radiotherapy + chemotherapy
- surgery + radiotherapy
- surgery + chemotherapy
- surgery + chemotherapy + radiotherapy
Statistics
Overall survival rates from initial diagnosis (OSID) and diagnosis of metastasis (OSM+) were estimated using the Kaplan-Meier method. Survival plots (OSM+) relating to categorical variables were compared by means of the log rank test. Additionally, the influence of continuous variables on OSM+ was assessed using cox proportional hazards regression analysis. All analyses were conducted using SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Frequency of reported cases over time
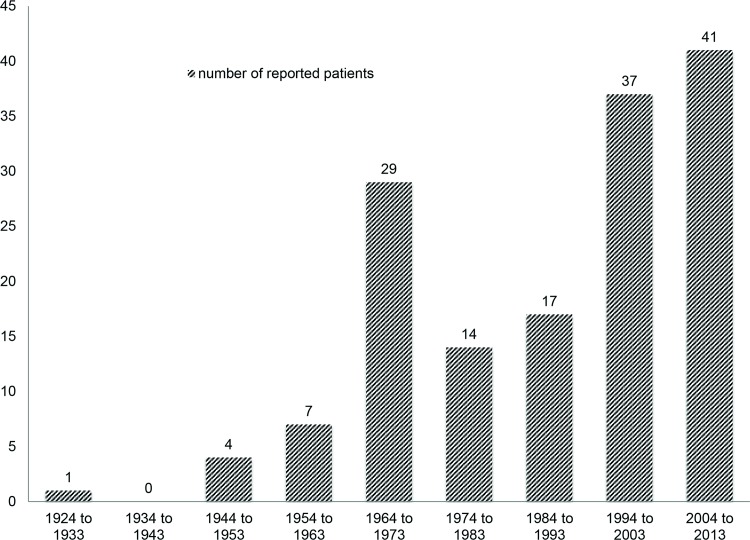
We observed an increase in the number of reported cases over recent decades. More than half of the 150 cases were published after 1993 (Fig 2).
Fig 2. Number of case reports on glioblastomas with extra-CNS metastases over the last decades.
Clinical characteristics
Gender was unknown in 2/150 cases (1.3%). 105/148 patients (70.9%) were male and 43/148 (29.1%) female. Median age at initial diagnosis was 42 years (range, 4–83 years). Age at initial diagnosis was reported for all patients. Histopathological diagnosis revealed GBM in 137/150 cases (91.3%), GS in 13/150 patients (8.7%). The time interval from initial diagnosis to the occurrence of metastases was reported in 71/150 patients (47.3%). In 7 of these 71 patients (9.9%) primary tumor and metastases were diagnosed simultaneously (Table 1). Median time interval from initial diagnosis to the diagnosis of metastases was 9.0 months (range, 0.0–81.0 months). Metastases affected chest and mediastinum (including lungs, pleura and heart) (52 cases, of those 52 patients 45 had lung metastases), abdomen (including the peritoneal cavity, liver, intestines, spleen, kidney and adrenal gland) (31 cases, of those 31 patients 23 had liver metastases), bones or bone marrow (53 cases), lymph nodes (51 cases), soft tissue and muscles (35 cases), skin (11 cases), parotid or thyroid gland (6 cases) and others (including eye and breast) in 4 cases. Most frequently involved were bones, lymph nodes and lungs. In a considerable number of patients metastasis affected more than one extra-cerebral organ. A wide diversity of different patterns of spread occurred (Table 2).
Table 1. Clinical characteristics and treatments after diagnosis of extra-CNS metastasis of 150 GBM/ GS patients reported in literature until April 2013.
| total | % | |
|---|---|---|
| Male | 105/148 | 70.9 |
| Female | 43/148 | 29.1 |
| Gender not specified | 2/150 | 1.3 |
| Children (≤ 18 years) | 11/150 | 7.3 |
| Adults (> 18 years) | 139/150 | 92.7 |
| GBM | 137/150 | 91.3 |
| GS | 13/150 | 8.7 |
| Primary metastases | 7/71 | 9.9 |
| Secondary metastases | 64/71 | 90.1 |
| Time of dissemination not specified | 79/150 | 52.7 |
| Surgery only | 17/60 | 28.3 |
| Radiotherapy only | 4/60 | 6.7 |
| Chemotherapy only | 8/60 | 13.3 |
| Combination of different treatments | 31/60 | 51.7 |
| Surgery + chemotherapy | 2/31 | 6.5 |
| Surgery + radiotherapy | 4/31 | 12.9 |
| Chemotherapy + radiotherapy | 15/31 | 48.4 |
| Surgery + chemotherapy + radiotherapy | 10/31 | 32.3 |
| Best supportive care only or treatment not specified | 90/150 | 60.0 |
| Death | 119/150 | 79.3 |
GBM: Glioblastoma multiforme (including one glioblastoma multiforme with an oligodendroglial component), GS: Gliosarcoma.
Table 2. Patterns of extracerebral metastasis of 150 GBM/ GS patients reported in literature until April 2013.
| Total | % | Patterns of extracerebral metastasis | |||
|---|---|---|---|---|---|
| Bone | Lymph node | Lung | Other site | ||
| 25/150 | 16.7 | • | |||
| 20/150 | 13.3 | • | |||
| 32/150 | 21.3 | • | |||
| 13/150 | 8.7 | • | |||
| 12/150 | 8.0 | • | • | ||
| 12/150 | 8.0 | • | • | ||
| 11/150 | 7.3 | • | • | ||
| 6/150 | 4.0 | • | • | • | • |
| 5/150 | 3.3 | • | • | ||
| 4/150 | 2.7 | • | • | • | |
| 3/150 | 2.0 | • | • | ||
| 3/150 | 2.0 | • | • | • | |
| 2/150 | 1.3 | • | • | ||
| 2/150 | 1.3 | • | • | • | |
| 0/150 | 0.0 | • | • | • | |
Treatments after diagnosis of extra-CNS metastasis
The treatment after the diagnosis of extra-CNS metastasis varied widely. Complete treatment details were provided for 60/150 cases (40%). In 90/150 cases (60%) treatment details were not reported or patients exclusively received best supportive care. Seventeen patients were treated with surgery only, four with radiotherapy only, and eight with chemotherapy only. Thirty-one patients underwent a combination of different treatments (surgery + chemotherapy, n = 2; surgery + radiotherapy, n = 4; chemotherapy + radiotherapy, n = 15; surgery + chemotherapy + radiotherapy, n = 10). In total, 33 patients underwent surgery, 33 chemo- and 33 radiotherapy (Table 1).
Overall survival from initial diagnosis and from diagnosis of extra-CNS metastasis
110/150 cases provided sufficient information to estimate overall survival from initial diagnosis (OSID) whereas 42/150 cases supplied the relevant data to calculate overall survival from diagnosis of metastasis (OSM+). The latter group was used for any further analysis (Table 3 as well as Figs 3 and 4). Median OSID was 13 ± 2.4 months and median OSM+ 6.0 ± 0.8 months.
Table 3. Evaluation of potential risk factors for overall survival after diagnosis of extra-CNS metastases (OSM+) using Kaplan Meier method and log rank test.
| subgroup | n | Deaths | Median OSM+ (months) | SE | 95% CI | p |
|---|---|---|---|---|---|---|
| male | 30 | 28 | 6.0 | 0.9 | 4.2–7.8 | 0.935 |
| female | 12 | 12 | 5.0 | 2.6 | 0.0–10.1 | |
| ≤ 42 years at ID | 22 | 21 | 5.0 | 0.8 | 3.5–6.5 | 0.686 |
| > 42 years at ID | 20 | 19 | 6.0 | 1.7 | 2.7–9.3 | |
| < 18 years at ID | 4 | 4 | 1.3 | 2.0 | 0.0–5.1 | 0.048 |
| ≥ 18 years at ID | 38 | 36 | 6.0 | 0.8 | 4.5–7.5 | |
| GBM | 37 | 35 | 6.0 | 0.7 | 4.5–7.5 | 0.264 |
| GS | 5 | 5 | 1.5 | 0.5 | 0.4–2.6 | |
| time between ID and DoM ≤ 9 months | 16 | 15 | 4.0 | 1.3 | 1.4–6.6 | 0.866 |
| time between ID and DoM > 9 months | 15 | 15 | 7.0 | 1.3 | 4.5–9.5 | |
| pulmonary involvement at DoM | 5 | 5 | 1.5 | 1.5 | 0.0–4.5 | 0.156 |
| no pulmonary involvement at DoM | 37 | 35 | 6.0 | 0.7 | 4.5–7.5 |
n: number of patients, OSM+: overall survival after the diagnosis of metastasis, SE: standard error (months), p: p-value, log-rank test, CI: confidence interval (months), ID: initial diagnosis, GBM: glioblastoma multiforme, GS: gliosarcoma, DoM: diagnosis of metastasis.
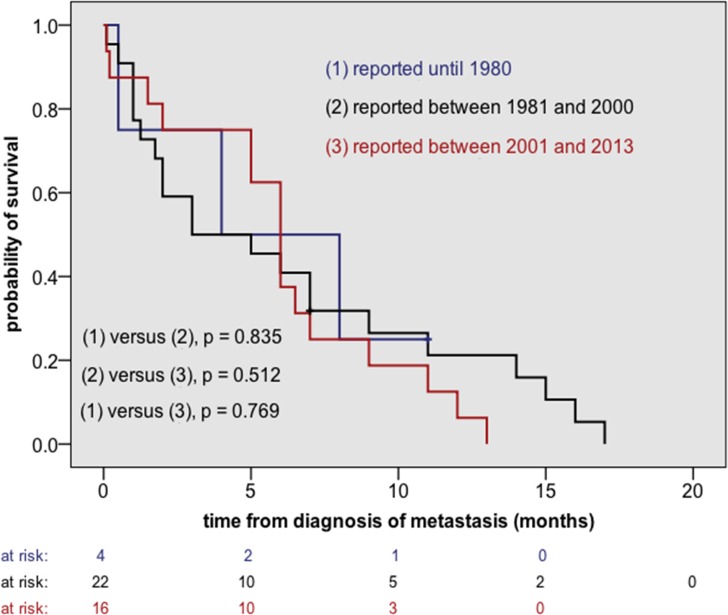
Fig 3. Overall survival after the diagnosis of metastasis (OSM+) according to the period of publication.
A substantial treatment progress has not been achieved over recent decades.
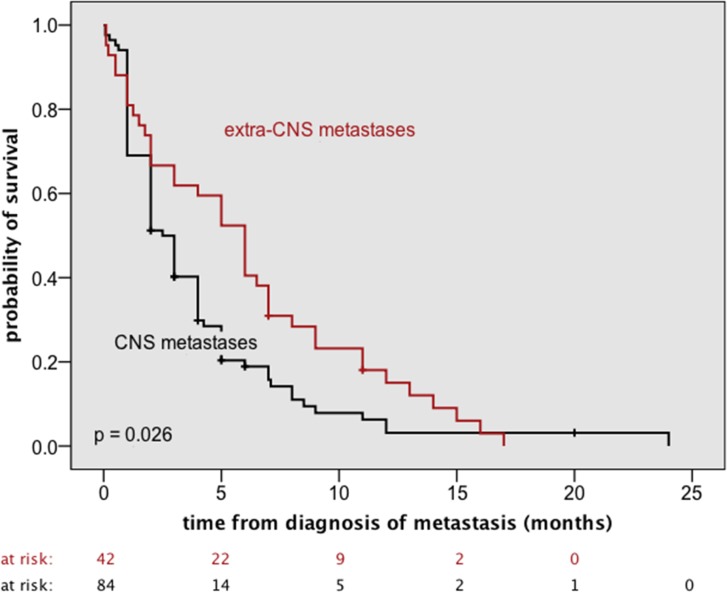
Fig 4. Comparison of OSM+ of the present cohort (42 patients) with OSM+ of a cohort of GBM/ GS patients with exclusive CNS dissemination (84 patients).
Detailed information on characteristics and treatments of these 84 patients and on data collection has been reported previously [9].
Potential prognostic factors for OSM+
On univariate analysis using Kaplan Meier method and log rank test OSM+ was not influenced by gender, age at initial diagnosis (cut-off 42 years), histology (GBM versus GS), the time interval between initial diagnosis and metastatic spread (cut-off 9 months) and pulmonary involvement at diagnosis of metastatic disease (Table 3). In contrast, adults showed a more favorable outcome than children, however, the number of patients aged < 18 years was very limited (n = 4) (Table 3). Moreover, we did not find a substantial difference in OSM+ between patients reported before 1981, between 1981 and 2000 and after 2000 (Fig 3).
On univariate cox regression analyses age at initial diagnosis (p = 0.852, hazard ratio = 1.002 per year, 95% CI: 0.981–1.024) and the time period between initial diagnosis and diagnosis of metastasis (p = 0.972, hazard ratio = 1.000 per months, 95% CI: 0.980–1.020) did not impact on OSM+.
Finally we compared OSM+ of the present cohort with OSM+ of a cohort of GBM/ GS patients with exclusive CNS dissemination. Detailed information on characteristics and treatments of these patients and on data collection has been reported previously [9]. Median OSM+ was 2.5 ± 0.4 months for the patients with CNS metastases and 6.0 ± 0.8 months for the patients without (Fig 4).
Discussion
Is extra-cerebrospinal metastasis from GBM/ GS an increasing phenomenon?
Although extra-cerebrospinal metastasis from GBM/ GS is without doubt a rare phenomenon, the frequency of case reports on the topic has steadily increased over past decades (Fig 2). The reasons for this may be complex including both a rising awareness of the issue in the medical community associated with adaption and extension of staging and significant advances in imaging diagnostics. Moreover, improved local tumor control and prolonged survival, the benefits of combined treatment [10], may have increased the probability of metastatic spread in- and outside the CNS and of occult metastases becoming symptomatic [11, 12].
Which GBM/ GS patients are at risk for extra-cerebrospinal metastasis?
Interestingly, our data suggests that younger GBM/ GS patients possibly have a predisposition for extra-cerebrospinal metastasis. This observation might be explicable by the fact that younger patients do survive longer [13–16]. Median age in our cohort was 42 years. In the cohort of Lun et al. (n = 88 patients) it was only 38 years [6]. In contrast, median age in a large historical control with non-metastatic glioblastoma patients was 56 years (Stupp et al., 2005, n = 573 patients) [10].
Has any therapeutic progress been achieved over the last decades?
Moreover, we were interested in whether any advances had been achieved in the treatment of GBM/ GS with extra-cerebrospinal metastases over the last few decades. To answer this question, patients were divided into three groups according to the years of publication of the corresponding articles (≤ 1980, 1981–2000, ≥ 2001). Using the Kaplan-Meier method and the log rank test, we did not detect any substantial difference in OSM+ between the three groups (Fig 3), indicating that notable treatment advances had not been achieved over recent decades. Of note, our findings are in contradiction with the results of Lun et al. who reported a progressive lengthening of the interval from detection of extra-cranial metastasis to death from 1940 to 2009, at a rate of 0.7 months per decade. However, Lun et al. evaluated less patients (n = 88) and they used a different methodological approach (linear regression). To use linear regression the authors had to simplify their dataset. Survival intervals were used without identifying the censorship status, which was justified by the small number of censored observations. Interestingly, Lun et al. reported a median OSM+ of only 1.5 months for the total of their patients [6]. Median OSM+ in our cohort was notably longer (6.0 ± 0.8 months).
Evaluation of potential non-treatment related prognostic factors for OSM+
We assessed the potential prognostic relevance of some simple clinical parameters. Similar to Lun et al. [6], we failed to demonstrate an influence of gender. Age is consistently a strong prognostic factor for non-metastatic glioblastoma [13–16]. Moreover, it has been widely recognized, that there are crucial molecular and clinical differences between adult and pediatric glioblastomas [17]. Hence, we furthermore assessed the impact of age on OSM+. We used the median age in our cohort (42 years) and the threshold to adulthood (18 years) as cut-offs for the Kaplan Meier method and log rank test. There was no significant difference in OSM+ between patients over 42 years and patients under 42 years. In contrast, children performed poorer than adults. However, the number of children in our analysis was extremely small (n = 4). On univariate cox proportional hazards regression analysis age (as a continuous variable) did not impact OSM+. In summary, our findings do not suggest that age is a major prognostic factor for OSM+ and support the conclusions previously drawn by Lun et al. [6]. In an older analysis patients with GS and GBM had essentially identical outcomes [18]. Our findings suggest that the assumption, that both entities share a similar prognosis, remains applicable when focusing on the setting of extra-cerebrospinal dissemination. Piccirilli et al. stated that patients with extra-CNS metastasis from GBM with a sarcomatous component had a worse prognosis than patients with other gliomas [5]. In the present dataset we identified only two patients with this particular histological GBM subtype. One of them died two months after diagnosis of metastasis. OSM+ of the other patient was not reported [19, 20]. Therefore and given the comprehensive nature of our literature search, we doubt whether the above mentioned statement can be accepted. To the best of our knowledge a potential correlation between the time interval from initial diagnosis of the primary tumor to the diagnosis of extra-cerebrospinal metastases and prognosis has never been assessed before. In our cohort, this time period did not have an influence on OSM+. Lun et al. stated that lung metastasis stood out as having the worst prognosis [6]. In contrast, we were unable to confirm this finding, however, this may be due to the small patient number in our analysis (n = 5 patients with pulmonary involvement at initial diagnosis of metastasis) as for statistical reasons, we did not include patients in which lung metastasis occurred at a later point in time (see also “particular statistical limitations”).
Is it useful to draw any distinction between CNS and extra-CNS metastasis?
In terms of OSM+, the present cohort compared favorable with a cohort of 84 GBM/ GS patients with exclusive CNS dissemination. Characteristics and treatments of these patients were reported in detail in a previous article of our working group [9]. As our data do not suggest that the occurrence of (additional) extra-CNS metastasis remarkably worsens prognosis in comparison with exclusive intra-CNS disease, aggressive treatment instead of best supportive care may still be justified in selected patients.
Which is the best treatment approach in the setting of extra-CNS metastasis?
The question of whether GBM/ GS patients with extra-CNS metastasis are best cared for with aggressive treatment or best supportive care is important and one needs to balance between treatment efficacy in terms of survival, quality of life and toxicity. Lun et al. observed, that patients treated with surgery + radiation + chemotherapy + cerebrospinal fluid shunting had the longest average survival interval from metastasis to death when compared to patients undergoing less intense treatments [6]. Moreover extra-CNS metastasis often affects younger patients in good general condition. Hence, from an ethical point of view, an aggressive treatment approach may be justified whenever feasible. However, one has to keep in mind that currently a survival benefit cannot be proven statistically on the basis of the data available (for details, see particular statistical limitations).
Limitations inherent to IPD meta-analyses
There are several limitations inherent to IPD meta-analyses. First, there certainly is a selection bias, because the cases reported in the literature might have been published due to their rare or uncommon presentation and outcomes. Second, not all data regarding the patient tumor and treatment characteristics was available for each individual patient. Occasionally the time course of the disease could not be reconstructed. This is reflected by different patient numbers in patient characteristics and survival analyses.
Particular statistical limitations
In this study, the potential benefit of radio- or chemotherapy and other treatment-related factors for survival was not investigated. The reason for this is that the patients in our cohort received individualized treatments implying that these factors were unknown at the beginning of survival time, i.e. at diagnosis of metastasis. To investigate a variable that is still elusive at the beginning of survival time or that changes over time, time-dependent Cox regression must be used. For example, if we wish to know whether cancer patients’ cumulative dose of chemotherapy affects the length of time until the tumor progresses, we cannot stipulate the cumulative dose as a known quantity at the outset. Patients who survive longer will generally receive a higher total dose. However, this high cumulative dose is not the cause of longer disease control. To allow for this, the cumulative dose must be included in Cox regression as a time-dependent variable. Time-dependent Cox regression is a procedure that requires particularly detailed information about the starting date of therapy, which is generally not provided by case series/ reports extracted from literature [21].
Moreover, we restrained from using a multivariate Cox proportional regression model to reassess the total of potential risk factors for OSM+. First, the number of suitable patients (death and complete information on all risk factors) was too small (n = 30) to include all of them simultaneously in the model and second the assumption of proportional hazards, a necessary prerequisite for Cox regression could not be upheld for all factors after visual comparison of the respective Kaplan-Meier plots [21].
Conclusions
The increasing number of reported GBM/ GS cases with extra-CNS metastasis over time underscores the need to draw a comprehensive picture. These tumors are associated with a dismal prognosis whereby crucial treatment progress is not evident. A central registry should be considered to consecutively gain more information about the ideal treatment approach.
Supporting Information
(DOC)
No.: number, OS ID: overall survival from initial diagnosis, OS M+: overall survival from diagnosis of metastasis.
(XLSX)
(DOCX)
Acknowledgments
We are indebted to the authors of articles, who provided the data to this study that otherwise would not have been possible. We would like to thank Mrs. Christiane Hofmann for assisting the literature search.
Data Availability
Our data were extracted from pubmed and web of science databases.
Funding Statement
The authors acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bouillot-Eimer S, Loiseau H, Vital A. Subcutaneous tumoral seeding from a glioblastoma following stereotactic biopsy: case report and review of the literature. Clinical neuropathology. 2005. Nov-Dec;24(6):247–51. PubMed PMID: . [PubMed] [Google Scholar]
- 2. Gamis AS, Egelhoff J, Roloson G, Young J, Woods GM, Newman R, et al. Diffuse bony metastases at presentation in a child with glioblastoma multiforme. A case report. Cancer. 1990. Jul 1;66(1):180–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3. Pasquier B, Pasquier D, N'Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980. Jan 1;45(1):112–25. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4. Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. Journal of neurosurgery. 1969. Jul;31(1):50–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5. Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori. 2008. Jan-Feb;94(1):40–51. PubMed PMID: 18468334. [DOI] [PubMed] [Google Scholar]
- 6. Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. Journal of neuro-oncology. 2011. Nov;105(2):261–73. PubMed PMID: 10.1007/s11060-011-0575-8 [DOI] [PubMed] [Google Scholar]
- 7. Tendulkar RD, Pai Panandiker AS, Wu S, Kun LE, Broniscer A, Sanford RA, et al. Irradiation of pediatric high-grade spinal cord tumors. International journal of radiation oncology, biology, physics. 2010. Dec 1;78(5):1451–6. PubMed PMID: 10.1016/j.ijrobp.2009.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009. Oct;62(10):1006–12. PubMed PMID: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 9. Pietschmann S, von Bueren AO, Henke G, Kerber MJ, Kortmann RD, Muller K. An individual patient data meta-analysis on characteristics, treatments and outcomes of the glioblastoma/gliosarcoma patients with central nervous system metastases reported in literature until 2013. Journal of neuro-oncology. 2014. Aug 27. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005. Mar 10;352(10):987–96. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11. Kalokhe G, Grimm SA, Chandler JP, Helenowski I, Rademaker A, Raizer JJ. Metastatic glioblastoma: case presentations and a review of the literature. Journal of neuro-oncology. 2012. Mar;107(1):21–7. PubMed PMID: 10.1007/s11060-011-0731-1 [DOI] [PubMed] [Google Scholar]
- 12. Shahideh M, Fallah A, Munoz DG, Loch Macdonald R. Systematic review of primary intracranial glioblastoma multiforme with symptomatic spinal metastases, with two illustrative patients. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2012. Aug;19(8):1080–6. PubMed PMID: 10.1016/j.jocn.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 13. Bertolini F, Zunarelli E, Baraldi C, Valentini A, Del Giovane C, Depenni R, et al. Survival in patients with newly diagnosed conventional glioblastoma: a modified prognostic score based on a single-institution series. Tumori. 2012. Nov;98(6):756–61. PubMed PMID: 10.1700/1217.13500 [DOI] [PubMed] [Google Scholar]
- 14. Fujii O, Soejima T, Kuwatsuka Y, Harada A, Ota Y, Tsujino K, et al. Supratentorial glioblastoma treated with radiotherapy: use of the Radiation Therapy Oncology Group recursive partitioning analysis grouping for predicting survival. Japanese journal of clinical oncology. 2010. Aug;40(8):726–31. PubMed PMID: 10.1093/jjco/hyq051 [DOI] [PubMed] [Google Scholar]
- 15. Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro-oncology. 2004. Jul;6(3):227–35. PubMed PMID: . Pubmed Central PMCID: 1871999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006. Jun 1;24(16):2563–9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 17. MacDonald TJ, Aguilera D, Kramm CM. Treatment of high-grade glioma in children and adolescents. Neuro-oncology. 2011. Oct;13(10):1049–58. PubMed PMID: Pubmed Central PMCID: 3177659. 10.1093/neuonc/nor092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galanis E, Buckner JC, Dinapoli RP, Scheithauer BW, Jenkins RB, Wang CH, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. Journal of neurosurgery. 1998. Sep;89(3):425–30. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19. Garret R. Glioblastoma and fibrosarcoma of the brain with extracranial metastases. Cancer. 1958. Sep-Oct;11(5):888–94. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20. Yokoyama H, Ono H, Mori K, Kishikawa M, Kihara M. Extracranial metastasis of glioblastoma with sarcomatous component. Surgical neurology. 1985. Dec;24(6):641–5. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 21. Zwiener I, Blettner M, Hommel G. Survival analysis: part 15 of a series on evaluation of scientific publications. Deutsches Arzteblatt international. 2011. Mar;108(10):163–9. PubMed PMID: Pubmed Central PMCID: 3071962. 10.3238/arztebl.2010.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
No.: number, OS ID: overall survival from initial diagnosis, OS M+: overall survival from diagnosis of metastasis.
(XLSX)
(DOCX)
Data Availability Statement
Our data were extracted from pubmed and web of science databases.