Abstract
Introduction
The diffusion of multidrug-resistant (MDR) bacteria has created the need to identify risk factors for acquiring resistant pathogens in patients living in the community.
Objective
To analyze clinical features of patients with community-onset pneumonia due to MDR pathogens, to evaluate performance of existing scoring tools and to develop a bedside risk score for an early identification of these patients in the Emergency Department.
Patients and Methods
This was an open, observational, prospective study of consecutive patients with pneumonia, coming from the community, from January 2011 to January 2013. The new score was validated on an external cohort of 929 patients with pneumonia admitted in internal medicine departments participating at a multicenter prospective study in Spain.
Results
A total of 900 patients were included in the study. The final logistic regression model consisted of four variables: 1) one risk factor for HCAP, 2) bilateral pulmonary infiltration, 3) the presence of pleural effusion, and 4) the severity of respiratory impairment calculated by use of PaO2/FiO2 ratio. A new risk score, the ARUC score, was developed; compared to Aliberti, Shorr, and Shindo scores, this point score system has a good discrimination performance (AUC 0.76, 95% CI 0.71-0.82) and calibration (Hosmer-Lemeshow, χ2 = 7.64; p = 0.469). The new score outperformed HCAP definition in predicting etiology due to MDR organism. The performance of this bedside score was confirmed in the validation cohort (AUC 0.68, 95% CI 0.60-0.77).
Conclusion
Physicians working in ED should adopt simple risk scores, like ARUC score, to select the most appropriate antibiotic regimens. This individualized approach may help clinicians to identify those patients who need an empirical broad-spectrum antibiotic therapy.
Introduction
Pneumonia caused by multidrug-resistant (MDR) pathogens traditionally has been confined to the hospital setting, but the emergence of MDR bacteria that cause pneumonia in the community has created the need to identify risk factors for acquiring resistant pathogens by evaluating the contacts patients have with the healthcare environment [1–2]. Prior hospitalization, residency in a nursing home, attendance of hemodialysis centers, and receipt of domiciliary care are some of the risk factors for acquiring the resistant pathogens included in the healthcare-associated pneumonia (HCAP) definition [3,4,5,6]. Some studies have shown that the current definition of HCAP is no longer sufficient for early identification of patients with an increased risk of pneumonia due to MDR bacteria [1,5,7], and more recently three studies [8,9,10] have proposed new scores to discriminate patients with an increased risk of developing community-onset pneumonia due to MDR pathogens.
Considering the aforementioned, the aim of our study is a prospective evaluation of patients with community-onset pneumonia due to MDR pathogens, to compare this population to patients without a MDR etiology, and to define risk factors and outcomes of patients acquiring MDR bacteria. Furthermore, the aim is to evaluate the performance of existing scores [8,9,10] and to derive and to validate a new bedside risk score for an early identification of patients with community-onset pneumonia due to MDR pathogens in the Emergency Department (ED).
Patients and Methods
Study Design and Study Patients
This was an open, observational, prospective study of consecutive patients coming from the community who were admitted, from January 2011 to January 2013, in the Policlinico Umberto I of Rome, a teaching 1100-bed Hospital. The Institutional Review Board of the Policlinico Hospital approved the study. Patients >18 years of age who satisfied the criteria for pneumonia were included in the study. The patient enrollment process was: consecutive patients coming from the community and admitted to our hospital from the ED were initially screened. Two physicians (MF and AR) performed a screening twice per day in the ED looking for patients with a suspicion of pneumonia. Once patients were identified they signed an informed consent and then were followed during hospitalization until discharge or death. Patients taking immunosuppressive therapy, patients with hospital-acquired pneumonia (HAP), or discharged or dead within ≤48 hours were excluded from the final analysis.
Data on previous antibiotic therapy as well as other risk factors for MDR organisms were derived from the following sources: a) history taken from patients and/or relatives; b) discharge letters and summaries if patients were previously hospitalized in other facilities, and c) electronic charts if patients were previously hospitalized or seen in the clinics at the Policlinico Umberto I of Rome, Italy.
The following data were recorded: demographics; past medical history; severity of symptoms on admission; pneumonia severity index (PSI) and CURB-65 score (confusion, urea nitrogen, respiratory rate, blood pressure, >65 years of age) [11]; clinical, laboratory, and radiological findings on admission; microbiological data; empiric antibiotic therapy; hospital stay; in-hospital mortality (for a total of 133 variables).
Study Definitions
Pneumonia was defined, according to the standard definitions of the Centers for Disease Control and Prevention (CDC) [12]. Severe sepsis was defined as sepsis with sepsis-induced organ dysfunction or tissue hypoperfusion (manifesting as hypotension, elevated lactate, or decreased urine output); septic shock as severe sepsis plus persistently low blood pressure following the administration of intravenous fluids [13]. Pleural effusion was detected using by chest X-ray. Length of hospital stay (LOS) was calculated as the number of days from the date of admission to the date of discharge or death. We classified patients as having HCAP if they had attended a hospital or hemodialysis clinic or received intravenous chemotherapy in the past 30 days, had been admitted to an acute-care hospital for at least 2 days or had surgery in the past 90 days, or resided in a nursing home or long-term care facility. We classified patients as having hospital-acquired pneumonia (HAP) if they received their diagnosis after being hospitalized for more than 72 hours or within 10 days of leaving the hospital.
Microbiological Analysis and Empiric Antibiotic Therapy
Microbiological examinations performed on sputum, urine, and blood during the first 24 hours after admission and according to standards of practice were evaluated for assessment of microbial etiology. Results of pleural puncture, tracheobronchial aspirates, and bronchoalveolar lavage fluid, when performed, were also considered as well as serologic tests for Chlamydophila pneumoniae, L. pneumophila, or Mycoplasma pneumoniae. Clinical isolates obtained from patients were identified in the Microbiology Laboratory of our hospital using the automatic system VITEK 2 (bioMérieux Inc., Hazelwood, MO) [14]. Antibiotic resistance profiles, including the MIC for each isolate, were assessed using the abovementioned automatic system. Microbiological results were reviewed, and a microbiological cause was assigned independently by 2 of the investigators (MF and AR). The etiology was considered definite if 1 of the following criteria was met: positive blood culture in the absence of an apparent extrapulmonary focus; positive bacterial culture of pleural fluid; positive urinary antigen for Legionella pneumophila (Binax Now, Trinity Biotech); positive urinary antigen for Streptococcus pneumoniae (Binax Now, Emergo Europe); a bacterial yield in cultures of valid sputum (>25 polymorphonuclear cells and <10 epithelial cells per power field) of ≥106 colony forming units (CFU)/mL, tracheobronchial aspirates of ≥105 CFU/mL, bronchoalveolar lavage fluid of ≥104 CFU/mL, and protected specimen brush cultures of ≥103 CFU/mL; and occurrence of seroconversion (a 4-fold rise in immunoglobulin G [IgG] titers for Chlamydophila pneumoniae [1:512] or a rise in immunoglobulin M [IgM] titers for C. pneumoniae [1:32] and Mycoplasma pneumoniae [any titer]). When ≥ 2 microbiological causes were present, the patient was considered to have a polymicrobial infection. Patients for whom no microbiological tests were performed and patients with negative microbiological results were considered to have disease of an unknown etiology.
A patient was considered to have a MDR pathogens if one of the following was isolated: methicillin-resistant Staphylococcus aureus (MRSA), Stenotrophomonas maltophilia, extended spectrum β-lactamase (ESBL)–producing or carbapenem-resistant Enterobacteriaceae. For the remaining cases MDR was defined as isolation of a bacterial strain non-susceptible to at least one agent in three or more antimicrobial categories [15].
We defined empirical antibiotic therapy as antibiotics administered on the first day of therapy for pneumonia and considered an empirical antibiotic regimen as adherent current international guidelines [16–17]. We analyzed the appropriateness of antibiotic therapy for all patients with an etiologic diagnosis according to susceptibility test criteria for lower respiratory tract pathogens. We rated an antimicrobial treatment as inadequate if 1 or more of the organisms present were known to have intrinsic resistance or were found to be resistant through susceptibility testing.
Study Groups and Endpoints
Two study groups were identified among the study population according to the isolation of MDR bacteria: group “MDR+”, patients with isolation of ≥ 1 resistant pathogen, and group “MDR-“, patients without MDR isolation. The microbiological endpoint was the actual isolation of an MDR pathogen.
The primary objective of this study was an evaluation of the performance of three existing scoring systems and the derivation of a new scoring tool for the identification of patients with pneumonia due to MDR organisms.
The secondary objective is an external validation of our score in a multicenter Spanish population of patients with pneumonia.
External validation
The obtained score was validated on an external cohort of adult patients with pneumonia hospitalized in 59 IMDs of 53 Spanish hospitals participating at a prospective observational multicenter study, carried-out over a 2-week period, first week in February 2013 and second week in June 2013 (Estudio de Neumonia en Medicina Interna II). Study methods were reported in a previous study [18]. Briefly, all the participating IMDs reported data on all the adult patients treated for pneumonia at their departments during the study period through a web site. Collected data were systematically reviewed by the coordinating investigator (MG) before they were entered in the database.
Statistical analysis
Continuous variables are presented as mean±SD, and differences were evaluated by t-test. Categorical variables were expressed as count and percentages and compared by chi-square test or Fisher’s exact test, as appropriate.
We performed logistic regression on MDR isolation to determine the best predictors. We selected potentially useful baseline characteristic predictor variables by minimization of Akaike Information Criterion (AIC), given that the main target for analysis is prediction. For reasons of parsimony, we then discarded non-significant variables from the model minimizing AIC. Model stability and absence of collinearity was checked by computing Variance Inflaction Factors (VIF). A VIF < 3 usually indicates no issues with the model. Candidate variables included: demographic characteristics (age, sex), ≥2 comorbidities, pleural effusion, HCAP, PaO2/FiO2 <300, bilateral pulmonary infiltration, fever > 38°C. A risk score named ARUC score (Assessment of Risk of multidrUg resistant pathogens in Community-onset pneumonia) was developed. The resulting score values were derived by rounding the beta coefficients (logarithm of the odds-ratios).
We evaluated discrimination using receiver operating characteristic curves (ROC). We compared ROC curves for the different scores; we adjust the probability using the Sidak method. The calibration of the model was evaluated by the goodness-of-fit Hosmer-Lemeshow χ2 statistic.
We calculated sensitivity, specificity, negative and positive predictive values (with 95% confidence intervals) for the cut-off point of the score in order to predict the MDR status. We also calculated negative and positive likelihood ratios (with 95% confidence intervals). All tests were two-tailed, and a P value < 0.05 was considered significant. All computations were carried out with R version 3.0.2, SPSS 20.0 for Windows (SPSS Inc., Chicago, IL) and STATA v.12.
Results
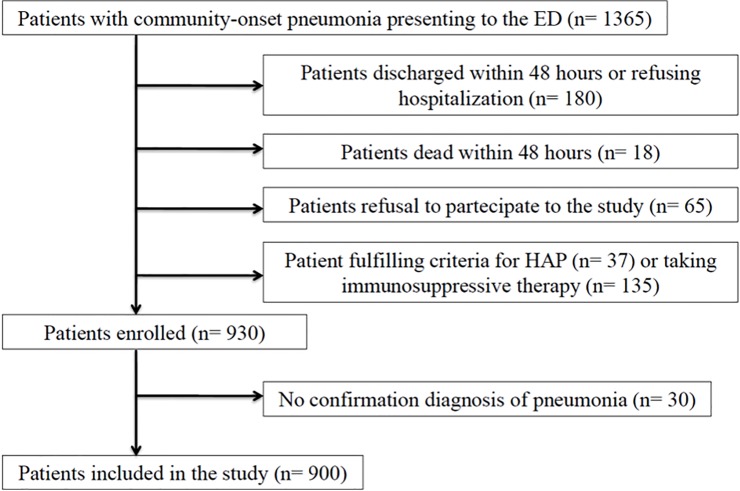
During the study period a total of 1,365 patients were assessed for eligibility, and 900 were finally included in the study: 536 patients had CAP and 364 HCAP. A consort diagram describing the study flow is presented in Fig 1.
Fig 1. Study flow diagram.
Legend. ED: emergency department; HAP: hospital-acquired pneumonia.
Overall, an etiologic diagnosis was obtained in 300 patients (33.3%). Pneumonia was caused by a MDR pathogen (group MDR+) in 99 cases (11%), while the remaining patients had no MDR pathogen isolation (group MDR-). Etiologic agents of pneumonia in our study population are described in Table 1.
Table 1. Etiology of 300 isolations in the study population.
| Isolation of MDR strain n = 99 patients | Isolation of non-MDR strain n = 201 patients | ||
|---|---|---|---|
| MRSA | 42 (42.4%) | Streptococcus pneumoniae | 69 (34.3%) |
| Pseudomonas aeruginosa | 11 (11.1%) | MSSA | 31 (15.4%) |
| Klebsiella pneumonia | 10 (10.1%) | Mycoplasma pneumoniae | 15 (7.5%) |
| Serratia marcescens | 8 (8.1%) | Pseudomonas aeruginosa | 15 (7.5%) |
| Escherichia coli | 7 (7.1%) | Klebsiella pneumoniae | 14 (7%) |
| MRSA + P. aeruginosa | 6 (6.1%) | Haemophilus influenzae | 13 (6.6%) |
| Acinetobacter baumannii | 6 (6.1%) | Clamydia pneumoniae | 10 (4.9%) |
| Enterobacter cloacae | 4 (4%) | Mycobacterium spp | 10 (4.9%) |
| E. cloacae + S. maltophilia | 2 (2%) | Legionella pneumophila | 10 (4.9%) |
| MRSA + A. baumannii | 2 (2%) | Enterobacter cloacae | 8 (4%) |
| Stenotrophomonas maltophilia | 1 (1%) | Escherichia coli | 6 (3%) |
Legend. MSSA: methicillin-sensitive Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; MDR: multidrug-resistant.
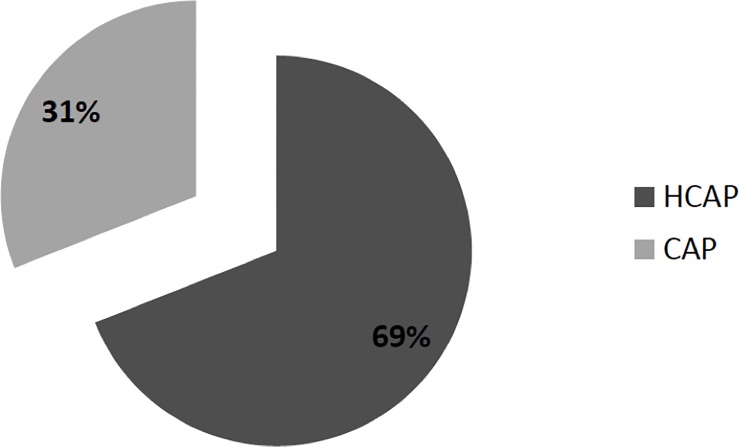
At least one microbiological test was performed in the 70% of patients enrolled. As described in Fig 2, the majority of patients with MDR isolates belonged to the HCAP group (68.7%) but a significant percentage (31.3%) was also encountered in the CAP group.
Fig 2. Distribution of MDR pathogens in CAP and HCAP populations.
Legend. MDR: multidrug-resistant; CAP: community-acquired pneumonia; HCAP: health-care associated pneumonia.
Comparison of demographics, clinical features and outcome of patients included in the MDR+ and MDR-groups are summarized in Table 2.
Table 2. Univariate analysis of MDR-group compared to patients without MDR isolation.
| Variables | MDR+ | MDR- | OR | 95% CI | p |
|---|---|---|---|---|---|
| n = 99 patients | n = 801 patients | ||||
| Age (median) | 78 | 82 | 1.02 | 1.00–1.03 | 0.029 |
| Male sex | 55 (61.2%) | 490 (55.5%) | 1.26 | 0.52–1.21 | 0.281 |
| PSI IV-V class | 98 (99%) | 637 (69.7%) | 2.71 | 1.78–4.15 | <0.001 |
| Adherence to guidelines | 31 (26%) | 421 (45.9%) | 0.41 | 0.26–0.62 | <0.001 |
| ≥ 2 comorbidities | 37 (37.4%) | 161 (20.1%) | 2.27 | 1.28–3.34 | <0.001 |
| Charlson Comorbidity Index (median) | 4.7 | 3.2 | 3.12 | 2.22–5.34 | <0.001 |
| Aliberti score ≥ 3 | 70 (70.7%) | 338 (42.2%) | 3.72 | 2.1–5.7 | <0.001 |
| Shorr score ≥ 1 | 69 (69.7%) | 303 (37.8%) | 4.1 | 2.56–6.11 | <0.001 |
| Shindo score ≥ 2 | 60 (60.6%) | 299 (37.3%) | 2.86 | 1.87–4.48 | <0.001 |
| Heart failure | 69 (69.7%) | 217 (27.1%) | 4.44 | 2.93–6.77 | <0.001 |
| Chronic hepatitis | 16 (16.1%) | 67 (8.3%) | 1.78 | 1.09–2.76 | 0.016 |
| Diabetes | 19 (19.2%) | 151 (18.8%) | 1.28 | 0.78–2.14 | 0.893 |
| Renal failure | 43 (43.4%) | 144 (17.9%) | 2.56 | 1.49–4.51 | <0.001 |
| COPD | 40 (40.4%) | 255 (31.8%) | 1.76 | 0.91–2.22 | 0.09 |
| Dementia | 21 (21.2%) | 180 (22.4%) | 1.08 | 0.59–1.45 | 0.898 |
| HCAP | 68 (68.7%) | 296 (36.9%) | 4.11 | 2.34–6.12 | <0.001 |
| Neoplasm | 21 (21.2%) | 170 (21.2%) | 0.29 | 0.12–0.87 | 1.0 |
| Pleural effusion | 56 (56.5%) | 329 (41.1%) | 2.72 | 1.79–3.84 | 0.005 |
| Malnutrition | 48 (48.5%) | 101 (12.6%) | 4.85 | 3.02–7.22 | <0.001 |
| PPI/H2 blockers | 54 (54.5%) | 239 (29.8%) | 2.82 | 1.89–4.71 | <0.001 |
| Previous surgery (30 days) | 13 (13.1%) | 19 (2.3%) | 5.9 | 2.81–12.1 | <0.001 |
| Bilateral pulmonary infiltration | 42 (42.4%) | 182 (22.7%) | 2.45 | 1.77–3.31 | <0.001 |
| Fever > 38°C | 48 (48.5%) | 437 (54.5%) | 0.28 | 0.12–0.67 | 0.285 |
| Increased ultrasensitive troponin | 32 (32.3%) | 215 (26.8%) | 1.34 | 1.02–2.12 | 0.04 |
| Multilobar pulmonary extension | 24 (24.2%) | 166 (20.7%) | 1.54 | 1.11–2.18 | 0.04 |
| PaO2/FiO2 < 300 | 71 (71.7%) | 265 (33.1%) | 3.79 | 2.42–4.91 | <0.001 |
| Inappropriate therapy | 83 (83.8%) | 19 (2.4%) | 277 | 134.7–434.1 | <0.001 |
| Quinolones or macrolide or cephalosporins in the previous 30 days | 71 (71.7%) | 272 (33.9%) | 5.14 | 3.34–8.81 | <0.001 |
| NIV | 7 (7%) | 20 (2.5%) | 2.81 | 1.31–5.9 | 0.022 |
| Platelets < 150.000 mm3 | 28 (28.3%) | 130 (13.5%) | 1.18 | 0.95–1.69 | 0.001 |
| SOFA score | 3.5 | 2.3 | 2.8 | 2.23–2.94 | <0.001 |
| Mean length of hospitalization (days) | 18.9 | 16.7 | 2.95 | 1.82–2.67 | 0.005 |
| Mean length of therapy (days) | 19.2 | 14.2 | 1.62 | 1.43–1.99 | 0.006 |
| ICU admission | 12 (12.2%) | 12 (1.5%) | 1.89 | 1.54–3.22 | 0.002 |
| Severe sepsis or septic shock | 25 (25.2%) | 74 (7.7%) | 2.63 | 2.34–3.78 | <0.001 |
| 30-day mortality | 40 (40.4%) | 89 (11.1%) | 2.72 | 1.62–3.82 | <0.001 |
| In-hospital mortality | 54 (54.5%) | 102 (12.7%) | 6.9 | 3.91–8.92 | <0.001 |
Legend. MDR: multidrug-resistant; PSI: pneumonia severity index; COPD: chronic obstructive pulmonary disease; HCAP: healthcare-associated pneumonia; PPI: proton pump inhibitors; NIV: non-invasive ventilation; SOFA: sequential organ failure assessment; ICU: intensive care unit.
The univariate analysis between patients with non-MDR etiology versus those without etiologic diagnosis is presented in S1 Table (see supplementary material). Compared to MDR-group, almost all patients included in the MDR+ group belonged to the high severity classes (IV or V) of PSI, received more frequently a previous antibiotic therapy with quinolones, macrolides or 3rd generation cephalosporins, had higher median Charlson score, were more likely to receive an inappropriate therapy, had higher mean duration of antibiotic therapy, higher mean LOS, and a significantly higher mortality rate.
Most patients included in the MDR+ group had an Aliberti score ≥3 (70.7% vs 42.2%, p<0.001), a Shorr score ≥1 (69.7% vs 37.8%, p<0.001), and a Shindo score ≥2 (60.6% vs 37.3%, p<0.001). As shown in Table 3, the final logistic regression model identified four variables significantly associated with MDR etiology including 1) one risk factor for HCAP, 2) bilateral pulmonary infiltration, 3) the presence of pleural effusion, and 4) the severity of respiratory impairment calculated by use of PaO2/FiO2 ratio.
Table 3. Multivariate analysis of factors associated with MDR isolation.
| Variables | p | OR | 95% CI | VIF |
|---|---|---|---|---|
| Bilateral Pulmonary Infiltration | 0.018 | 1.75 | 1.09–2.79 | 1.05 |
| Pleural effusion | <0.001 | 1.95 | 1.25–3.07 | 1.02 |
| At least one of HCAP criteria: Previous hospitalization (3 months), dialysis, i.v. therapy previous 30 days, residence in nursing home or long-term care facility | <0.001 | 2.52 | 1.57–4.09 | 1.07 |
| PaO2/FiO2 ratio < 300 | <0.001 | 4.08 | 2.55–6.67 | 1.03 |
Legend. MDR: multidrug-resistant; HCAP: health-care associated pneumonia.
Table 4summarizes the risk score (ARUC score) for MDR pneumonia and the designation of points. The total possible score ranged from 0 to 3.5.
Table 4. ARUC score for early identification of patients with MDR-pneumonia.
| HCAP criteria (at least one of the following): Previous hospitalization (3 months), dialysis, i.v. therapy previous 30 days, residence in nursing home or long-term care facility | + 1 pts |
| Bilateral Pulmonary Infiltration | + 0.5 pts |
| Pleural effusion | + 0.5 pts |
| PaO2/FiO2 < 300 | + 1.5 pts |
Legend. MDR: multidrug-resistant; HCAP: health-care associated pneumonia; COPD: chronic obstructive pulmonary disease.
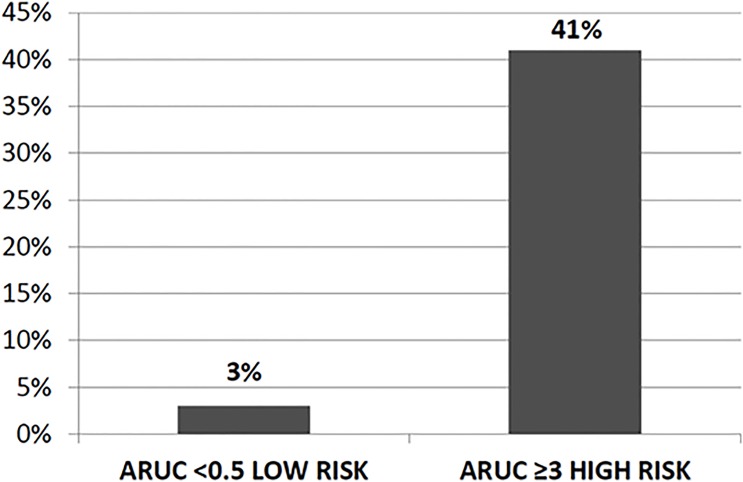
The prevalence of MDR pathogens rose with increasing score after stratifying the scores into Low (<0.5), and High (≥3) risk (see Fig 3).
Fig 3. Risk stratification of MDR isolation on the basis of ARUC score.
Legend. MDR: multidrug-resistant.
When the score was <0.5 the prevalence of MDR pathogens was very low (3%), while the prevalence climbed to 41% when the score was ≥3.
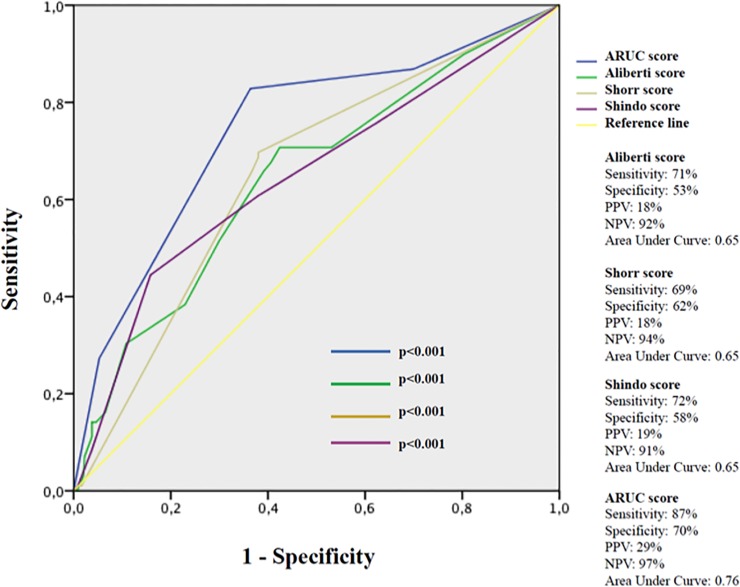
As a screening test, a negative ARUC score (<0.5) had a good sensitivity (75%) and a strong NPP (95%), while a positive score ≥3 had a high specificity (92%) and a strong PPV (93%). Fig 4compares sensitivity, specificity, PPV, and NPV of ARUC, Aliberti, Shorr, and Shindo scores.
Fig 4. ROC curves of ARUC, Aliberti, Shorr and Shindo scores.
Legend. PPV: positive predictive value; NPV: negative predictive value.
The area under curve (AUC) of our model was higher (0.76, 95% Confidence Interval [CI] 0.71–0.82), than that of Aliberti (AUC = 0.65, 95%CI 0.59–0.71), Shorr (AUC = 0.65, 95%CI 0.60–0.70), and Shindo scores (AUC = 0.65, 95%CI 0.59–0.71). The goodness-of-fit Hosmer-Lemeshow χ2 was 7.64 (p = 0.469) for ARUC score, 7.45 (p = 0.489) for Shorr score, 10.25 (p = 0.248) for Aliberti score, and 11.91 (p = 0.155) for Shindo score.
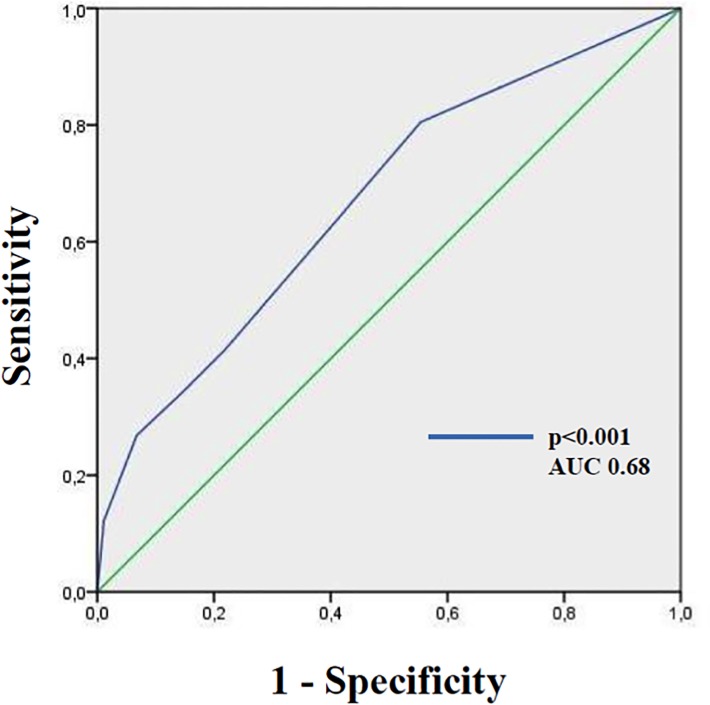
The performance of the score was evaluated on 929 patients with pneumonia with a median age of 80 (IQR 70–87) years. Most of patients had multiple comorbidities and 35% were classified as having HCAP. An attempt to microbiological diagnosis was made in 857 patients with an overall rate of etiological diagnosis of 23.2%. In 49 out of 199 patients (24.6%) with microbiological diagnosis a MDR pathogen was isolated (5.2% of the overall cohort). Clinical and microbiological characteristics of the validation cohort are listed in S2 Table (see supplementary material). The distribution of ARUC score’s variables in the validation cohort is detailed in S3 Table (see supplementary material). Overall, the 4% of population belonged to the low risk class of ARUC score, while the 39% belonged to the high risk class. A positive ARUC score (≥0.5) showed a sensitivity of 85% and a specificity of 50%, a PPV of 28% and a NPV of 94% to predict isolation of MDR pathogen. As reported in Fig 5, the AUC of this model was 0.68 (95% CI 0.60–0.77).
Fig 5. ROC curves of ARUC score on the Spanish population.
Discussion
The relevant and novel findings of this study are that 1) in the context of patients with community-onset pneumonia exists a proportion of patients who develop an infection due to MDR pathogens, 2) MDR isolation is associated with inappropriate empirical antibiotic therapy and accounts for an unacceptable related mortality, 3) HCAP definition alone is not sufficient to discriminate patients at risk for MDR pathogens, and 4) the ARUC score, a new prediction rule, appears as a useful tool to help physicians in an early recognition of patients living in the community with pneumonia due to MDR pathogens.
The first key point of our study is that patients with community-onset pneumonia due to MDR pathogens have a high 30-day mortality rate (40.4%) and a high likelihood to receive an inappropriate initial antibiotic therapy (83.8%). Aliberti and coworkers, analyzing a population of 935 patients, observed that patients with risk factors for MDR had higher mortality rate than those who had not (22% vs 10%). Compared to abovementioned study, we found a higher proportion of MDR isolates (11%) and also higher mortality rates. The high mortality observed in our patients appears directly related to a high frequency of empirical inadequate antibiotic therapy. Moreover, another factor which may also explain the high mortality rate is the fact that most of our patients were old and critically ill and that we focused our analysis in patients who were hospitalized in medical wards for at least 48 hours; thus patients with mild or moderate pneumonia were excluded from the study.
Some reports describe the increasing prevalence of MDR organisms in subjects presenting de novo to the hospital [1,4,6,9,10,19,20,21]. The concept of HCAP, which was proposed in 2005 [16], was meant to serve as a tool to help clinicians to stratify individuals with high likelihood to have pneumonia due to a resistant pathogen, like MRSA, P. aeruginosa or other MDR gram-negative bacilli. Many studies have documented that HCAP is associated with increased mortality and higher risk of etiology due to MDR pathogens [1–4,6,8–10,19–22], and a prospective interventional study on elderly patients with pneumonia showed clinical benefit of an empirical broad-spectrum antibiotic therapy [23]. However, some authors have expressed concern that antibiotic treatment decisions driven by the concept of HCAP might lead to excessive prescription and abuse of broad-spectrum anti-infectives [5,7], leading to unnecessary costs and promote resistance. Our study confirms that HCAP per se has limited value in segregating subjects with potentially resistant infections: as a matter of fact a significant percentage of cases of pneumonia caused by MDR-bacteria (31.3%) were encountered in the CAP group [24, 25, 26].
Since HCAP definition alone is not sufficient to predict the risk for MDR pathogens, the development of risk stratification scoring tools provide to physicians an easy-to-use mechanism to determine which patients presenting with pneumonia may require broad-spectrum antibiotic coverage. We evaluated the performance of 3 existing scores and developed a new risk score (ARUC score) for the assessment of MDR pathogens in patients with community-onset pneumonia. Both C statistic of the model and goodness-of-fit Hosmer-Lemeshow χ2 statistic confirmed a good discriminatory power of ARUC score in the early identification of patients with pneumonia due to MDR organisms. Of importance a score <0.5 had a good sensitivity and a strong NPP, while a score ≥3 had a high specificity and strong PPV. These results highlight the role of ARUC score in selecting patients at lowest and highest risk for carrying MDR pathogens.
The strength of ARUC score may be that it is derived from a population of severely ill elderly patients, and in the majority of our patients were contemporary present at least two or more recognized risk factors for MDR; thus we had the chance to explore additional factors possibly related to an infection by a MDR strain. In addition ARUC score takes in account not only predisposing host factors for MDR but also clinical-radiological parameters evaluating the severity of illness, such as PaO2/FiO2 ratio, bilateral pulmonary infiltration and the presence of pleural effusion. A moderately good performance of this bedside score was confirmed in a multicenter Spanish population of elderly patients with pneumonia.
Another message of our article is that pneumonia due to MDR bacteria may be more severe than other causes, and is more frequently associated with higher degree of respiratory impairment expressed as PaO2/FiO2 ratio. This is not surprising since the involved species (S. aureus, P. aeuginosa and other MDR Enterobacteriaceae) are particularly virulent and frequently cause bilateral pneumonia; moreover, initial outpatient antibiotics usually prescribed in patients with CAP (e.g. macrolides, cephalosporins, or fluoroquinolones) are ineffective in patients with MDR etiology and this may lead to a more complicated clinical course. As matter of fact, Khawaja and coworkers found that the microbes causing severe CAP were different from the usual spectrum: Staphylococcus aureus and Pseudomonas aeruginosa were the common causative pathogens and were associated with high mortality [27].
Our study has important limitations: culture data were not always obtained in every case of suspected pneumonia, which introduces a selection bias. Thus percentage of MDR bacteria may be underestimated, and we cannot exclude that a proportion of patients who died without definite diagnosis had a MDR etiology. Another limitation is that the ARUC score was derived by comparing the MDR population to the composite group of patients without MDR etiology or with unknown etiology; however, we did not find significant differences between these latter groups in term of baseline characteristics and outcomes, and this finding reduces the potential bias of this analysis. Finally, since ARUC score showed a good but not optimal discrimination power (AUC 0.68 in the validation cohort) future large studies are needed to assess the better scoring tool for the early identification of patients with pneumonia due to MDR pathogens.
In conclusion a rapid identification of patients with infection due to MDR pathogens seems crucial to reduce mortality for community-onset pneumonia. Physicians working in ED should adopt simple risk scores, like ARUC score, to select the most appropriate antibiotic regimens. This individualized approach may help clinicians to identify those patients who need an empirical broad-spectrum antibiotic therapy [28].
Supporting Information
PSI: pneumonia severity index; MRSA: methicillin-resistant Staphylococcus aureus.
(DOCX)
PSI: pneumonia severity index; MRSA: methicillin-resistant Staphylococcus aureus.
(DOC)
HCAP: healthcare-associated pneumonia; COPD: chronic obstructive pneumonia disease.
(DOC)
Acknowledgments
We would like to thank all the members of the ENEMI II study group:
Alejandro De La Sierra Iserte, Anna Sangil Betriu, Medicina Interna, Hospital Universitario Mutua De Terrasa Barcelona. Angela Felip Benach, Josep Anton Capdevila Morell, Medicina Interna, Hospital De Mataro, Barcelona. Antonio Zapatero Gaviria, Juan Hinojosa Mena-Bernal, Medicina Interna, Hospital Universitario De Fuelabrada, Madrid. Antonio Jimeno Carruez, Luis Angel Sánchez Muñoz, Medicina Interna, Hospital Clínico Universitario De Valladolid. Arturo Noguerado Asensi Y Raquel Carrillo Gomez, Medicina Interna Cantoblanco-H.U. La Paz. Arturo Artero Mora Y Francesc Puchades Gimeno, Medicina Interna, Hospital Universitario Dr Peset, Valencia. Carlos Tornero Esteban, Simona Cioaia, Medicina Interna, Hospital Francisco De Borja Gandia, Valencia. Cárlos San Roman Teran, Sonsoles Fernández Sepúlveda, Medicina Interna, Hospital Comarcal De La Axarquia, Malaga. Cármen Suárez Fernández, Cármen Saez Bejar, Medicina Interna, Hospital Universitario De La Princesa, Madrid. Domingo Bofill Montoro, Merce Cardona Ribera, Medicina Interna, Hospital De Tortosa Verge De La Cinta, Tarragona. Elpidio Calvo, Alba Ibáñez Botella, Medicina Interna, Hospital Clínico San Carlos, Madrid. Ernest Bragulat, Francisco José Castro Bohórquez, Urgencias Y Medicina Interna, Hospital Parc Sanitari San Joan De Deu, Barcelona. Esteban Pascual Pablo, Medicina Interna, Hospital Santiago Apostol, Burgos. Fernando Cuadra García Tenorio Julio González Moraleja, Medicina Interna Hospital Virgen De La Salud, Toledo. Francisco Pasquau Liaño, Concepcion Amador Prous, Medicina Interna, Hospital Marina Baixa De Villajoyosa, Alicante. Francisco Javier Santolaria Fernandez, Jose Juan Viña Rodriguez Medicina Interna, Hospital Universitario De Canarias, St. Cruz De Tenerife. Gonzalo García De Casasola Sánchez, José Luis Pérez Quero, Medicina Interna, Hospital Universitario Infanta Cristina, Madrid. Jesús Millán Cortés Núñez, Miguel Angel Artacho Rodriguez, Maria Gomez Antunez, Jesus Garcia Castano, Pablo Demelo Rodriguez, Elena Trigo Esteban, MariaJesus Granda Martin, Medicina Interna, Hospital General Universitario Gregorio Maranon, Madrid. Joan Brugues Tarradelles, Esteban Reynaga Sosa, Medicina Interna, Hospital General De Vic, Barcelona. Joan Colomer I Pairés, Cristina Soler I Ferrer, Medicina Interna, Hospital Santa Caterina, Girona. Joaquin Vila Plana, Jordi Grau Amorós, Medicina Interna, Hospital Minicipal De Badalona. Joaquin Lopez Alvarez, Eduardo Montero Ruiz, Medicina Interna, Hospital Universitario Principe De Asturia, Madrid. Jordi Casademont, Olga H Torres Bonafonte, Medicina Interna, Hospital Sant Pau, Barcelona. Jorge Francisco Gómez Cerezo, Inés Suárez García, Medicina Interna, Hospitla Del Norte, Madrid. Jose López Miranda, Rafael Martinez Fernandez, Medicina Interna, Hospital Universitario Reina Sofía, Cordoba. Manuel De Toro Santos, Mª Dolores Díaz López, Medicina Interna, Complexo Hospitalario Sta María Nai-Cabaleiro Goas, Ourense. Josep Masferrer Serra, Jose Felipe Morales Martin, Medicina Interna, Hospital Comarcal Valdeorras, Ourense. Pilar Román Sánchez, Medicina Interna, Hospital General De Requena, Valencia. Maria Victoria Egurbide Arberas, Ramiro De La Prieta Lopez, Medicina Interna, Hospital Universitario De Cruces, Vizcaya. Miguel Torres Salinas, Javier Ramos Lazaro, Medicina Interna, Fundacio Hospital Del Esperit Sant, Barcelona. Miguel F Carrascosa, Medicina Interna, Hospital De Laredo, Santander. Paloma Geijo Martinez, Medicina Interna, Hospital General Virgen Luz, Cuenca. Pascual Sesma Sánchez, Hortensia Álvarez Díaz, Medicina Interna, Complejo Hospitalario Arquitecto Marcide-Novoa Sts, A Coruna. Rafael Perez Vidal, Omar El Boutrouki, Medicina Interna, Centre Hospitalari De Manresa, Althaia. Valentín Cuervas-Mons Martínez, Pedro Durán Del Campo, Medicina Interna, Hospital Universitario Puerta De Hierro, Madrid. Valentín Del Villar Sordo, Mario Del Valle Sánchez, Medicina Interna, Hospital Santa Barbara, Soria. Fernando Escolar Castellón, Teresa Rubio Obanos, Medicina Interna, Hospital Reina Sofía, Navarra. Antoni Castro, Simona Iftimie, Medicina Interna, Hospital San Juan De Reus, Tarragona. Juan Luis Rodriguez Calderon, Jose Pablo Robles Ruiz, Medicina Interna, Hospital San Rafael, Madrid. Carmelo Perea Perea, Beatriz Rueda Rodriguez, Medicina Interna, Hospital Militar Central Gómez Ulla, Madrid. Eduardo Aguilar Cortés, Maria Ruiz Mariscal, Medicina Interna, Hospital De Alcañiz, Teruel. Faustino Herrero Huertas, Gandia Herrero Marina, Medicina Interna, Hospital Morales Meseguer, Murcia. Javier Solera Santos, Mª Elena De Tomas Labat, Medicina Interna, Hospital General De Albacete, Albacete. Joaquim Oristrell Salvà, Susana Herranz Martínez, Medicina Interna, Consorci Hospitalari Del Parc Taulí, Barcelona. Jose Miguel Aguirre Errasti, Laura Bolea Laderas, Carlota Lopez Lapuerta, Medicina Interna, Hospital Miguel Servet, Zaragoza. Jose Hernandez Quero, Antonio Diez Ruiz, Medicina Interna, Hospital San Cecilio, Granada. Jose Luis Beato Perez, Medicina Interna, Hospital Hellin, Albacete. Jose Maria Pascual Izuel, Alberto Belda Mira, Medicina Interna, Hospital De Sagunto, Valencia. Pedro González Santos, Juan Luis Carrillo Linares, Medicina Interna, Complejo Hospitalario Virgen De La Victoria, Malaga. Pere Comas Casanova, Francesc Báguena Escolano, Medicina Interna, Hospital De Igualada, Barcelona.
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
The ENEMI-II study was partially supported by the Programa de Centros de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias CB06/06/0058. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors received no other specific funding for this work.
References
- 1. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for healthcare-associated pneumonia. Arch Intern Med 2008;168:2205–10. 10.1001/archinte.168.20.2205 [DOI] [PubMed] [Google Scholar]
- 2. Falcone M, Shindo Y, Venditti M, Kollef MH. Healthcare-associated pneumonia: diagnostic criteria and distinction from community-acquired pneumonia. Int J Infect Dis 2011; 15:e545–50. 10.1016/j.ijid.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 3. Venditti M, Falcone M, Corrao S, Licata G, Serra P, and the Study Group of the Italian Society of Internal Medicine. Comparison of the outcomes of patients hospitalized with community-acquired, health care–associated, and hospital-acquired pneumonia. Ann Intern Med 2009; 150: 19–26. [DOI] [PubMed] [Google Scholar]
- 4. Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005;128:3854–62. [DOI] [PubMed] [Google Scholar]
- 5. Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis 2010;10:279–87. 10.1016/S1473-3099(10)70032-3 [DOI] [PubMed] [Google Scholar]
- 6. Falcone M, Venditti M, Corrao S, Serra P, for the Italian Society of Internal Medicine (SIMI) Study Group. Role of multidrug-resistant pathogens in health-care-associated pneumonia. Lancet Infect Dis 2011; 11: 11–12. 10.1016/S1473-3099(10)70301-7 [DOI] [PubMed] [Google Scholar]
- 7. Ewig S, Welte T, Torres A. Is healthcare-associated pneumonia a distinct entity needing specific therapy? Curr Opin Infect Dis. 2012;25:166–75. 10.1097/QCO.0b013e32835023fb [DOI] [PubMed] [Google Scholar]
- 8. Aliberti S, Di Pasquale M, Zanaboni AM, Cosentini R, Brambilla AM, Seghezzi S, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis. 2012;54:470–8 10.1093/cid/cir840 [DOI] [PubMed] [Google Scholar]
- 9. Shorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, et al. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis 2012;54:193–8. 10.1093/cid/cir813 [DOI] [PubMed] [Google Scholar]
- 10. Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk Factors for Drug-Resistant Pathogens in Community-Acquired and Healthcare-Associated Pneumonia. Am J Respir Crit Care Med. 2013. 15;188:985–95. 10.1164/rccm.201301-0079OC [DOI] [PubMed] [Google Scholar]
- 11. Falcone M, Corrao S, Venditti M, Serra P, Licata G. Performance of PSI, CURB-65, and SCAP scores in predicting the outcome of patients with community-acquired and healthcare-associated pneumonia. Intern Emerg Med. 2011; 6: 431–436. 10.1007/s11739-011-0521-y [DOI] [PubMed] [Google Scholar]
- 12. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008; 36:309–332. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 13. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Visser MR, Bogaards L, Rozenberg-Arska M, Verhoef J. Comparison of the autoSCAN-W/A and Vitek Automicrobic systems for identification and susceptibility testing of bacteria. Eur J Clin Microbiol Infect Dis. 1992;11:979–84. [DOI] [PubMed] [Google Scholar]
- 15. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 16. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 17. Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect 2011;17 Suppl 6:E1–59. 10.1111/j.1469-0691.2011.03672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giannella M, Pinilla B, Capdevila JA, Martínez Alarcón J, Muñoz P, López Álvarez J, et al. ; Estudio de Neumonía En Medicina Interna study Group from the Sociedad Española de Medicina Interna. Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clin Microbiol Infect. 2012; 18:786–94. 10.1111/j.1469-0691.2011.03757.x [DOI] [PubMed] [Google Scholar]
- 19. Orsi GB, Falcone M, Venditti M. Surveillance and management of multidrug-resistant microrganisms. Expert Rev Anti Infect Ther 2011; 9: 653–79. 10.1586/eri.11.77 [DOI] [PubMed] [Google Scholar]
- 20. Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis. 2013. Jun 6;13(1):268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aliberti S, Cilloniz C, Chalmers JD, Zanaboni AM, Cosentini R, Tarsia P, et al. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax. 2013 Jun 17 (ahead of print) [DOI] [PubMed]
- 22. Falcone M, Blasi F, Menichetti F, Pea F, Violi F. Pneumonia in older frail patients: an up to date. Intern Emerg Med 2012; 7:415–24. [DOI] [PubMed] [Google Scholar]
- 23. Falcone M, Corrao S, Licata G, Serra P, Venditti M, on behalf of the Italian Society of Internal Medicine (SIMI). Clinical impact of broad-spectrum empirical antibiotic therapy in patients with Healthcare-Associated Pneumonia: a multicenter interventional study. Intern Emerg Med 2012; 7: 523–31. 10.1007/s11739-012-0795-8 [DOI] [PubMed] [Google Scholar]
- 24.Corrao S, Venditti M, Argano C, Russo A, Falcone M. Healthcare-Associated Pneumonia and Multidrug-Resistant Bacteria: Do We Have a Convincing Answer? Clin Infect Dis. 2014 Feb 9. [Epub ahead of print] [DOI] [PubMed]
- 25.Falcone M, Russo A, Venditti M. CAP and HCAP are different? An unresolved question. Thorax. 2014 Jan 28. [Epub ahead of print] [DOI] [PubMed]
- 26.Russo A, Falcone M, Venditti M. Early identification of severe community-onset pneumonia in "frail elderly patient". Intern Emerg Med. 2013 Nov 28. [Epub ahead of print] [DOI] [PubMed]
- 27. Khawaja A, Zubairi AB, Durrani FK, Zafar A. Etiology and outcome of severe community acquired pneumonia in immunocompetent adults. BMC Infect Dis. 2013; 20; 13:94 10.1186/1471-2334-13-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maruyama T, Fujisawa T, Okuno M, Toyoshima H, Tsutsui K, Maeda H, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57:1373–83. 10.1093/cid/cit571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PSI: pneumonia severity index; MRSA: methicillin-resistant Staphylococcus aureus.
(DOCX)
PSI: pneumonia severity index; MRSA: methicillin-resistant Staphylococcus aureus.
(DOC)
HCAP: healthcare-associated pneumonia; COPD: chronic obstructive pneumonia disease.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files