Abstract
Following an extensive review of the literature, we further analyze the published data to examine the health effects of indoor exposure to particulate matter (PM) of outdoor origin. We obtained data on all-cause, cardiovascular, and respiratory mortality per 10 μg/m3 increase in outdoor PM10 or PM2.5; the infiltration factors for buildings; and estimated time spent outdoors by individuals in the United States, Europe, China, and globally. These data were combined log-linear exposure–response model to estimate the all-cause, cardiovascular, and respiratory mortality of exposure to indoor PM pollution of outdoor origin. Indoor PM pollution of outdoor origin is a cause of considerable mortality, accounting for 81% to 89% of the total increase in mortality associated with exposure to outdoor PM pollution for the studied regions. The findings suggest that enhancing the capacity of buildings to protect occupants against exposure to outdoor PM pollution has significant potential to improve public health outcomes.
Introduction
Associations between exposure to particulate matter (PM) pollution and increased morbidity and mortality have been observed in both population-based [1–3] and cohort-based [4–9] research. PM is associated with negative health impacts, including cerebrovascular disease (stroke), respiratory infections, cardiopulmonary disorders, ischemic heart disease, and lung cancer [10–16]. Weichenthal et al. [17] examined the relationship between PM2.5 and non-accidental and cardiovascular mortality in the U.S. Agricultural Health Study cohort. Rural PM2.5 exposure may be associated with cardiovascular mortality in men; however, similar associations were not observed among women. Chen et al. [18] and Brook et al. [19] conducted population-based cohort studies, which indicated that long-term exposure to PM2.5 is associated with increased risk of mortality attributable to diabetes. Burnett et al. [13] developed a fine particulate mass-based relative risk model for estimating the global burden of disease attributable to ambient exposure to fine particulate matter, which was found to be a superior predictor of relative risk (RR) compared with seven other forms previously used in burden assessments. Wu et al. [14] examined the cardiopulmonary health effects of PM2.5 from different pollution sources in China. They repeatedly examined for a series of cardiopulmonary health indicators among a panel of 40 healthy university students, while simultaneously collecting daily ambient PM2.5 mass samples and measuring for 29 chemical constituents in the laboratory throughout the study. Their results indicated that different sources of PM2.5 may play important roles in different aspects of PM2.5 related cardiopulmonary health effects.
However, a limitation of most of these studies is that only outdoor PM pollution exposure was measured. In many buildings, high concentrations of ambient PM pollution enter the indoor environment [20–23], where people spend approximately 90% of their time [24]. There are two scenarios for personal exposure to outdoor PM pollution. The first is that individuals are directly exposed while outdoors. The second is that people remain indoors, but are exposed to particles that enter the building by means of infiltration or ventilation. The infiltration factor, defined as the fraction of the outdoor concentration that penetrates indoors and remains suspended, is a potential source of exposure variation but is often overlooked in epidemiological studies [25]. Infiltration depends on the air exchange rate, PM loss rate (the rate at which PM is removed from the air by deposition, filtration, and so forth), and penetration efficiency (the fraction of PM that penetrates the building envelope as outdoor air moves indoors) [26–27].
Many studies have examined the association between outdoor particles and mortality, and these existing epidemiological data are implicitly influenced by the fact that people spend approximately 90% of their time [24] indoors. Moreover, previous studies did not differentiate outdoor exposure from indoor exposure to particles of outdoor origin. Wilson et al. [28] discussed the legal and scientific importance of assessing personal exposure in terms of ambient particles outdoors and the fraction that infiltrates indoors. This discrete assessment of indoor exposure to indoor particles of outdoor origin is especially important for developing appropriate strategies for controlling indoor air quality. Therefore, the present study focuses on indoor exposure to particles of outdoor origin.
Although Wilson et al. [28] pointed out that it is important to estimate mortality associated with indoor exposure to particles of outdoor origin, to date, no such studies have been presented. For the purpose of controlling aerosol pollution, we are eager to learn from the epidemiological data, e.g. mortality derived from indoor exposure to particles of outdoor origin, in order to support strategies for managing indoor air quality (IAQ).
The study utilizes existing epidemiological data on the mortality of outdoor PM, combined with a physical model of aerosol mechanisms, to estimate mortality associated with indoor exposure to PM of outdoor origin. To the best of our knowledge, this is the first attempt to quantify this relationship for PM exposure.
Methods
Analytical model
Most epidemiological studies on the health effects of PM pollution use increased mortality or hospital admissions per 10 μg/m3 increase in PM exposure as the health endpoint [6–7, 29–31]. Daniels et al. [29] indicated that log-linear models are appropriate for assessing the effect of PM pollution on daily mortality. They examined the hypothesis of linearity in relation to PM-mortality by comparing the Akaike information criterion (AIC) values obtained under the linear-, threshold-, and spline dose-response models. Their results indicated that a log-linear model is preferable to the threshold and spline models, and so a log-linear analytical model is used here. This approach involves several assumptions: a) Outdoor air pollution is not affected by indoor sources; b) There are no interactions with indoor sources, including allergens and various chemicals; c) The health effects of particle exposure are a function of PM2.5 mass concentration. The toxicity of PM2.5 is assumed to differ only with mass exposure and not with PM2.5 composition [13, 32–33]. Based on these assumptions, the log-linear analytical model used for estimating the health effects of indoor exposure to outdoor-originated PM can be represented as:
| (1) |
Where Δlog M all, j is the increase in mortality due to the jth outcome associated with total PM exposure for each 10 μg/m3 increase in PM10 or PM2.5 outdoors. j represents three major health outcomes: all-cause, cardiovascular, and respiratory mortality.
ΔC out is the increase in outdoor PM10 or PM2.5 concentrations, which is set as 10 μg/m3.
ΔC out-in is the increase in outdoor-originated PM10 or PM2.5 concentrations found in the indoor environment.
t out is the duration of direct exposure to outdoor PM pollution.
t in is the duration of indoor exposure to PM of outdoor origin.
Δlog M in, j estimates the increase in mortality due to the jth outcome associated with indoor exposure to outdoor-origin PM for each 10 μg/m3 increase in PM10 or PM2.5. A relatively low value of Δlog M in, j suggests low probability of morbidity or mortality. This implies that buildings adequately shield occupants against outdoor-origin PM pollution and, thus, investment in further reducing the indoor concentrations of such PM would have minimal benefits for public health outcomes. However, a high value of Δlog M in, j suggests high possibility of morbidity or mortality with increased exposure to outdoor-origin PM, emphasizing the importance of preventing ambient PM from entering the indoor environment.
When an epidemiologic study is performed, the observed mortality rate is lower than that in other places with higher infiltration factors, and this can be falsely interpreted that the dose-response slope (Δlog M in, j) is less steep. When this mortality estimate was combined with the lower exposure estimate (due to low infiltration) in Eq (1), we would get too low estimation for the mortality due to indoor exposure to outdoor PM. Therefore, the local infiltration factor should be used to adjust the observed mortality rate. We assume that the logarithms of the observed probabilities (or rates) of disease have the probability distributions as:
| (2) |
Where M is the probability of disease, a is a constant describing the background probability, b is a risk coefficient for the exposure, and C is the exposure concentration in the population.
The differences in the probability of disease are caused by the differences in exposure C. The observed C itself (the actual exposure concentration) is only the surrogate C obs, which in this case is the outdoor concentration of PM C out. With a given difference in the probability of disease between the exposed and non-exposed groups, a biased probability of disease could be described as:
| (3) |
where E is the exposed group, 0 is the non-exposed group, and obs is the biased observed variable (in contrast to the actual variable we would observe if all measurements were correct). Then the ratio of the biased and correct risk estimates is:
| (4) |
where i means different microenvironments and t i is the time spent in each microenvironment. Infiltration (F i) was used to denote the relative exposure concentrations in different microenvironments I (in this case of only indoor and outdoor microenvironments, F i is 1 for outdoor and equal to infiltration factor (F inf) for indoor), then
| (5) |
The observed b is biased downward if the population spends a lot of time in microenvironments with low infiltration factor. The Δlog M all, j can be calculated as follows:
| (6) |
Combine the Eq (1) and Eq (6), we can get:
| (7) |
Epidemiological data
The epidemiological data for all-cause, cardiovascular, and respiratory mortality attributable to outdoor PM exposure (PM10 or PM2.5) are based on meta-analyses published in the U.S., Europe, China, and globally between 2000 and 2012. All of the parameters used in the model are summarized in Table 1.
Table 1. Parameters used to evaluate of the effects on mortality of indoor exposure to particulates of outdoor origin.
| Location | Reference | Values | Remarks | |
|---|---|---|---|---|
| Overall world | Anderson et al. [31] | 0.9% (0.6%, 1.3%) | Mean (95%CI) all-cause mortality; PM2.5. | |
| 1.3% (0.5%, 2.2%) | Mean (95%CI) cardiovascular mortality; PM2.5. | |||
| 1.1% (0.2%, 2.0%) | Mean (95%CI) respiratory mortality; PM2.5. | |||
| United States | Daniels [29] | 0.54% (0.33%, 0.76%) | Mean (95%CI) all-cause mortality; PM10. | |
| Zanobetti and Schwartz [34] | 0.98% (0.75%, 1.22%) | Mean (95%CI) all-cause mortality; PM2.5. | ||
| 0.85% (0.46%, 1.24%) | Mean (95%CI) cardiovascular mortality; PM2.5. | |||
| 1.68% (1.04%, 2.33%) | Mean (95%CI) respiratory mortality; PM2.5. | |||
| Europe | Anderson et al. [31] | 0.6% (0.4%, 0.8%) | Mean (95%CI) all-cause mortality; PM10. | |
| 0.9% (0.5%, 1.3%) | Mean (95%CI) cardiovascular mortality; PM10. | |||
| 1.3% (0.5%, 2.0%) | Mean (95%CI) respiratory mortality; PM10. | |||
| China | Chen et al. [35] | 0.35% (0.18%, 0.52%) | Mean (95%CI) all-cause mortality; PM10. | |
| 0.44% (0.23%, 0.64%) | Mean (95%CI) cardiovascular mortality; PM10. | |||
| 0.56% (0.31%, 0.81%) | Mean (95%CI) respiratory mortality; PM10. | |||
| Cao et al. [36] | 0.20% (0.1%, 0.3%) | Mean (95%CI) all-cause mortality; PM2.5. | ||
| 0.3% (0.1%, 0.40%) | Mean (95%CI) cardiovascular mortality; PM2.5. | |||
| 0.4% (0.2%, 0.6%) | Mean (95%CI) respiratory mortality; PM2.5. | |||
| 10 μg/m3 | Each 10 μg/m3 increased in outdoor PM10 or PM2.5. |
We selected meta-analyses by Anderson et al. [31] that formed part of the World Health Organization’s “Systematic Review of Health Aspects of Air Pollution in Europe” project. The data were drawn from time-series (ecological and individual) estimates of the effects of PM10 on all-cause mortality in 33 European cities or regions. The majority of these estimates originated from multi-city studies conducted in France, Italy, and Spain in 2003–2004. The European exposure–response coefficient for all-cause mortality was 0.6% (95% CI: 0.4%, 0.8%), referring to the percentage change in the number of deaths with each 10 μg/m3 increase in outdoor PM10. The corresponding summary estimates for cardiovascular and respiratory mortality were 0.9% (95% CI: 0.5%, 1.3%) and 1.3% (95% CI: 0.5%, 2.0%) respectively.
The meta-analysis of Anderson et al. [31] was used to estimate all-cause, cardiovascular, and respiratory mortality attributable to outdoor PM2.5 exposure for the U.S., Canada, and globally in 2003–2004. Studies from North and South America as well as other areas of the world were identified in the database and used to conduct meta-analyses for each mortality group. The global exposure–response coefficients for all-cause, cardiovascular and respiratory mortality for a 10 μg/m3 increase in outdoor PM2.5 were 0.9% (95% CI: 0.6%, 1.3%), 1.3% (95% CI: 0.5%, 2.2%), and 1.1% (95% CI: 0.2%, 2.0%), respectively.
We used results from Daniels et al. [29] to estimate all-cause mortality due to outdoor PM10 exposure in the U.S. Their analysis used a database developed for the “National Morbidity, Mortality, and Air Pollution Study” for the 20 largest metropolitan areas in the U.S. over a 7-year period (1987–1994). These data were obtained from the Aerometric Information Retrieval System database maintained by the U.S. Environmental Protection Agency (EPA). Their estimates for all-cause, mortality with a 10 μg/m3 increase in outdoor PM10 in the U.S. were 0.54% (95% CI: 0.33%, 0.76%).
In the U.S., Zanobetti and Schwartz [34] conducted a national, multi-city time-series study of the acute effects of PM2.5 on risk of death from all causes, cardiovascular disease, myocardial infarction, stroke, and respiratory mortality for the years 1999–2005. They found a 0.98% increase (95% CI, 0.75%, 1.22%) in total mortality, a 0.85% increase (95% CI, 0.46%, 1.24%) in cardiovascular deaths, and a 1.68% increase (95% CI, 1.04%, 2.33%) in respiratory deaths for a 10 μg/m3 increase in two-day averaged PM2.5.
After reviewing studies published in both English and Chinese, on the health effects of PM10 in China, we chose the meta-analysis conducted by Chen et al. [35], on the association between PM10 and daily mortality in 16 Chinese cities between 1996 and 2008. Their results showed exposure–response coefficients of 0.35% (95% CI: 0.18%, 0.52%), 0.44% (95% CI: 0.23%, 0.64%), and 0.56% (95% CI: 0.31%, 0.81%) for all-cause, cardiovascular, and, respiratory mortality, respectively, resulting from a 10 μg/m3 increase in outdoor PM10 in China.
For China, we used results from Cao et al. [36] on the short-term association between PM2.5 constituents and daily mortality in Xi’an, a heavily polluted Chinese city. Those authors obtained daily mortality data and daily concentrations of PM2.5 for 1 January 2004 through 31 December 2008. Their results show that the exposure–response coefficients for all-cause, cardiovascular, and respiratory mortality for a 10 μg/m3 increase in outdoor PM2.5 in China were 0.20% (95% CI: 0.1%, 0.3%), 0.3% (95% CI: 0.1%, 0.4%), and 0.4% (95% CI: 0.2%, 0.6%), respectively.
Outdoor-originated particles in the indoor environment
The infiltration factor, F inf, is used to determine the relationship between outdoor PM concentration and indoor PM derived from outdoor sources.
The indoor PM2.5 concentration can be calculated as:
| (8) |
where C in is indoor PM mass concentration (μg/m3), C out is outdoor PM mass concentration (μg/m3), p is PM penetration rate, λ is hourly air exchange rate (h-1), β PM is particle deposition rate (h-1), and is the volume-averaged indoor PM2.5 source strength (μg/(h•m3)).
According to the steady-state assumption [37], the rates of penetration (P), deposition (β PM), and air exchange (λ) remain constant over a given time. Eq (8) can then be solved as:
| (9) |
The first item on the right side of Eq (9) represents the contribution of outdoor-originating particles, which is the infiltration definition, and the second item represents the contribution of indoor-emitted particles.
Therefore, infiltration factor is defined as:
| (10) |
The infiltration factor for PM2.5 is typically higher than that of PM10, due to the stronger effect of the deposition mechanism on gravity setting for coarse particles, which ultimately results in greater particle loss in the cracks of building envelopes and on indoor surfaces for PM10. Furthermore, since the infiltration factor is a function of a building’s crack geometry as well as its air exchange rate, particle deposition rate and penetration factor (the fractional penetration of particles from outdoors), the mean infiltration factors measured in these studies show considerable variation.
The literature was reviewed to determine the PM10 and PM2.5 infiltration factors reported for European, North American, Chinese, and global residences (see Table 2). We calculate mean infiltration factors of PM10 and PM2.5 in each region, and the maximum and minimum values for each region were also used in sensitivity analysis to study the effect of infiltration factor on mortality and particle infiltration (see Table 3). For the PM2.5 infiltration in China, there is no available data in literature, therefore, we calculated it according to the Eq (4). The maximum infiltration air exchange rate was 0.55 h-1 and the minimum infiltration air exchange rate was 0.06 h-1 according to Zhou and Zhao [44] study of Beijing region. The PM2.5 penetration rate was 0.8 and the deposition rate of PM2.5 was 0.09 h-1according to the systematic review by Chen and Zhao [26]. Therefore, the PM2.5 infiltration in China was set 0.69 (maximum) and 0.32 (minimum).
Table 2. Review of infiltration factors for PM10 and PM2.5 in the United States, Europe, and China.
| Reference | Values | Location | |
|---|---|---|---|
| PM10 infiltration | Ozkaynak et al. [38] | 0.51 | Riverside. USA |
| 0.52 | Riverside. USA | ||
| Ozkaynak et al. [39] | 0.60 | Riverside. USA | |
| Ott et al. [40] | 0.55 | Riverside. USA | |
| Lazaridis et al. [41] | 0.45 | Oslo. Norway | |
| Hoek et al. [42] | 0.17 | Helsinki, Finland | |
| 0.28 | Athens, Greece | ||
| 0.41 | Amsterdam, Netherlands | ||
| 0.27 | Birmingham, UK | ||
| Diapouli et al. [43] | 0.56 | Athens, Greece | |
| Zhou and Zhao [44] | 0.33 | Anshan | |
| 0.34 | Beijing | ||
| 0.42 | Fuzhou | ||
| 0.42 | Guangzhou | ||
| 0.38 | Hangzhou | ||
| 0.45 | Hong Kong | ||
| 0.29 | Lanzhou | ||
| 0.38 | Shanghai | ||
| 0.30 | Shenyang | ||
| 0.37 | Suzhou | ||
| 0.31 | Taiyuan | ||
| 0.33 | Tangshan | ||
| 0.34 | Tianjin | ||
| 0.30 | Urumqi | ||
| 0.37 | Wuhan | ||
| 0.35 | Xi'an | ||
| PM2.5 infiltration | Ozkaynak et al. [38] | 0.70 | Riverside. USA |
| 0.56 | Riverside. USA | ||
| Lee et al. [45] | 0.62 | Chongju. Korea | |
| Lachenmyer and Hidy [46] | 0.66 | Birmingham. USA | |
| Wallace et al. [47] | 0.48 | Seven cities. USA | |
| Williams et al. [47] | 0.45 | North Carolina. USA | |
| Reff et al. [48] | 0.51 | Three cities. USA | |
| Wallace and Williams [49] | 0.55 | North Carolina. USA | |
| Sarnat et al. [23] | 0.48 | L.A. USA | |
| Hoek et al. [42] | 0.63 | Three Cities, USA | |
| Ozkaynak et al. [39] | 0.71 | Riverside. USA | |
| Polidori et al. [50] | 0.47 | Los Angeles | |
| Allen et al. [27] | 0.62 | USA | |
| 0.47 | USA | ||
| 0.82 | USA | ||
| Meng et al. [51] | 0.56 | USA | |
| Haonninen et al. [52] | 0.70 | Athens. Greece | |
| 0.63 | Basle. Switzerland | ||
| 0.59 | Helsinki. Finland | ||
| 0.61 | Prague. Czech | ||
| Wichmann et al. [53] | 0.55 | Stockholm, Sweden | |
| Diapouli et al. [43] | 0.71 | Athens, Greece | |
| Calculated according to literature data | 0.69 | China | |
| 0.32 | China |
Table 3. Parameters used to evaluate the effects on human health of indoor exposure to particulates of outdoor origin.
| PM10 infiltration factor | PM2.5 infiltration factor | Tout (h) | ||
|---|---|---|---|---|
| United States | Mean | 0.55 | 0.58 | 1.8 |
| max | 0.60 | 0.82 | 2.7 | |
| min | 0.51 | 0.45 | 0.9 | |
| Europe | Mean | 0.36 | 0.63 | 1.8 |
| max | 0.56 | 0.71 | 2.7 | |
| min | 0.17 | 0.55 | 0.9 | |
| China | Mean | 0.36 | 0.51 | 1.6 |
| max | 0.45 | 0.69 | 2.4 | |
| min | 0.29 | 0.32 | 0.8 | |
| Global | Mean | 0.38 | 0.59 | 1.7 |
| max | 0.60 | 0.82 | 2.7 | |
| min | 0.17 | 0.32 | 0.8 |
Duration of exposure
The time spent by U.S. individuals in outdoor and indoor environments was determined from the National Human Activity Pattern Survey (NHAPS). NHAPS is a nationally representative survey (n = 9,386) conducted between September 1992 and October 1994 [24]. Based on self-reported time-activity budgets, people in the U.S. spend an average of 1.8 h outdoors per day [24]. Due to the lack of data on daily activity patterns for Europe, we extrapolated the results from the NHAPS to European populations.
Zhou and Zhao [44] reviewed time-activity patterns for both urban and rural Chinese residents and fitted log-normal distributions. The fitted distributions were then repeatedly sampled to generate a set of mean standard deviation (SD) pairs that could match the mean SD pattern of all individual studies in a mean SD plot. According to this analysis, Chinese adults spend an average of 1.6 (0.6, SD) hours outside per day.
No reports on global time-activity patterns were found in the literature, so we used 1.7 h, which is the mean of the values reported for the U.S. and China.
Sensitivity analysis was conducted to study the effect of time-activity pattern on mortality, which used a ±50% range of the mean time spent outside (see Table 3).
Results and Discussion
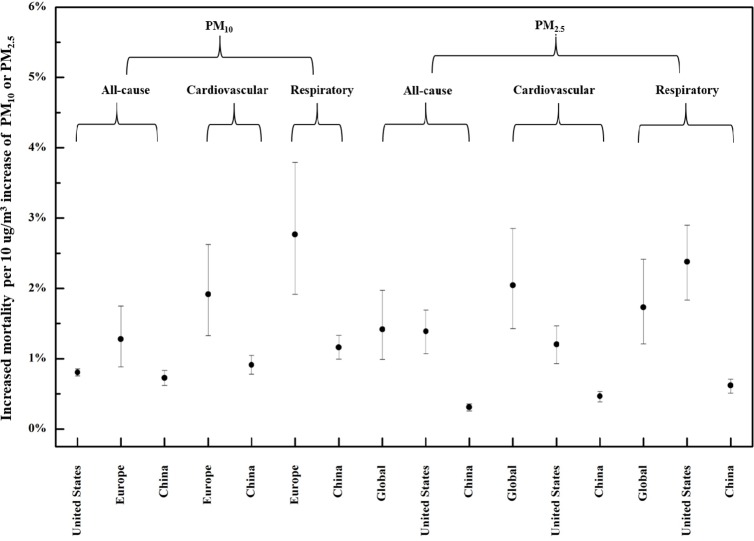
Fig 1 shows the increased mortality attributable to each 10 μg/m3 increase in indoor exposure to PM of outdoor origin. Each 10 μg/m3 increase in outdoor PM10 is predicted to result in 0.81%, 1.20%, and 0.73% increase in all-cause mortality in the U.S., Europe, and China, respectively. The mean increases in cardiovascular mortality are 1.8% and 0.91% for Europe and China respectively, whereas those for respiratory mortality are 2.60% and 1.16%. For each 10 μg/m3 increase in outdoor PM2.5, all-cause mortality is predicted to increase by 1.29%, 1.41%, and 0.32% in the globally, U.S. and China, compared with 1.86%, 1.22%, and 0.48%, respectively, for cardiovascular mortality, and 1.57%, 2.41% and 0.65% for respiratory mortality. Overall, the predicted increases in all-cause, cardiovascular, and respiratory mortality in China are considerably lower than in other regions.
Fig 1. Mortality attributable to indoor exposure to particulates of outdoor origin.
For PM10, the predicted increases in all-cause, cardiovascular, and respiratory mortality were all higher in Europe than in the U.S. Although the infiltration factor for PM10 in European countries was lower than in the U.S. and China in this study, the total increase in mortality is actually predicted to be higher in Europe, which dominates the influence on total increased mortality.
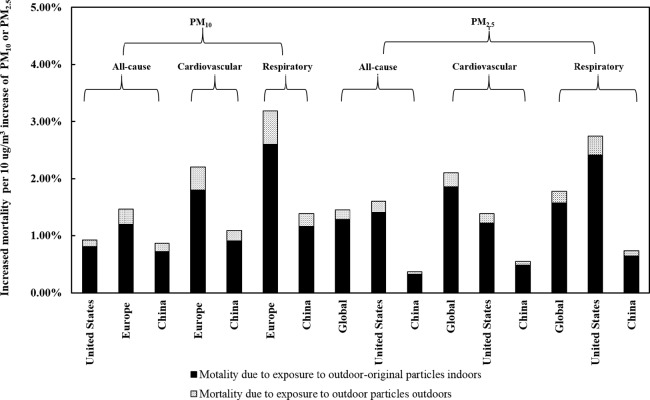
Fig 2 shows increased mortality attributed to direct exposure to outdoor particles for every 10 μg/m3 increase in PM pollution, compared with that predicted for indoor exposure to outdoor-originated PM. Increased mortality resulting from indoor exposure accounts for 81–89% of the total increase in mortality.
Fig 2. Comparison of mortality due to direct exposure to outdoor particles versus indoor exposure to particulates of outdoor origin.
The results of this study suggest that it is important to account for indoor exposure to outdoor-originated particles in the relationship between air pollution and health. PM infiltration and time-activity patterns are both important factors in human exposure, so a sensitivity analysis was conducted to determine their effects on mortality.
Influence of infiltration
Since particle infiltration differs even between studies conducted in the same region, we conducted an extensive review of the literature, from which infiltration values are shown in Table 2. We selected the maximum and minimum infiltration values for use in the sensitivity analysis, shown in Fig 1.
For each 10 μg/m3 increase in outdoor PM10 a range of indoor infiltration values is calculated; based on these data, there is no remarkable increase in all-cause mortality associated indoor exposure in the U.S. and China (increase of 0.10–0.21%); however, all-cause mortality in Europe showed a much larger increase of approximately 0.87%. Similarly to the findings for all-cause mortality, considerable increases were observed in mean cardiovascular and respiratory mortality for Europe but not for China. For each 10 μg/m3 increase in outdoor PM2.5, all-cause, cardiovascular, and respiratory mortalities due to indoor exposure all showed little increase in mean mortality in China; in the U.S, all-cause and cardiovascular mortalities due to indoor exposure in mean mortality increased 0.62% and 0.54%, respectively, the exception was respiratory mortality in the U.S., which showed increases of 1.07% in respiratory mortality. The each 10 μg/m3 increase in outdoor PM2.5, all-cause, cardiovascular, and respiratory mortalities due to indoor exposure increase 0.98%, 1.42% and 1.20% respectively for the global region, which is large compare with U.S. and China. There are two explanations for this finding. One is that the mortality values of all three forms of mortality are very small, and are therefore not sensitive to the infiltration value; another is that the infiltration we reviewed in the literature is relatively comprehensive, whereas the infiltration values in the different studies differ slightly.
Influence of exposure time
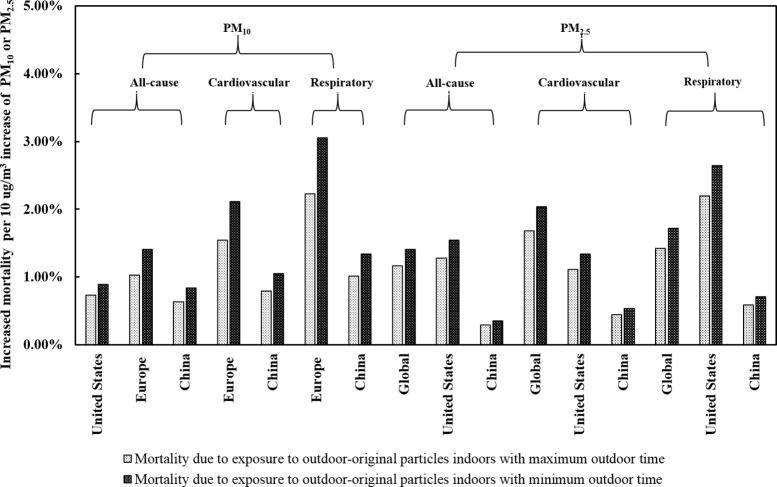
Since personal activity patterns can be highly variable, the exposure duration in indoor and outdoor environments may show frequent fluctuation. We therefore varied the duration of outdoor activity within a range of 50% when conducting the sensitivity analysis. The results are shown in Fig 3.
Fig 3. Comparison of mortality due to indoor exposure to particles of outdoor origin, according to maximum/minimum duration of outdoor exposure.
For each 10 μg/m3 increase in outdoor PM10, changes in the duration of outdoor exposure are associated with remarkable changes in all-cause mortality in the U.S., Europe, and China, respectively, which derive from a reciprocal shift in the duration of indoor exposure. The mean increases in all-cause mortality associated with indoor exposure range from 0.73% to 0.89%, 1.03% to 1.41%, and 0.63% to 0.84% for the United States, Europe, and China, respectively. The mean increases in cardiovascular mortality range from 1.54% to 2.11%, and from 0.79% to 1.05% for Europe and China respectively. The mean increases in respiratory mortality range from 2.23% to 3.05%, and from 1.01% to 1.34% for Europe and China respectively.
For each 10 μg/m3 increase in outdoor PM2.5, the change in duration of outdoor exposure also shows a remarkable increase in all-cause mortality and respiratory and cardiovascular mortality associated with indoor exposure. Mean all-cause, cardiovascular and respiratory mortality due to indoor exposure to outdoor-originated PM2.5 show approximately increases of 0.24%, 0.35% and 0.30% globally and 0.26%, 0.22% and 0.44% for the United States when outdoor activity time decreased by two-thirds. However, when outdoor activity time decreases by two-thirds, all-cause, cardiovascular and respiratory mortality due to indoor exposure to outdoor-originated PM2.5 in China increase no more than 0.12% for each 10 μg/m3 increase in outdoor PM2.5.
These results indicate that outdoor-derived PM has considerable effects on human health arising from exposure within the indoor environment. Enhancing the capacity of buildings to exclude outdoor particles, and the installation of air purifiers in indoor environments are both important measures for protection of public health.
Limitations
This study has several limitations. Since most studies on the effects of outdoor particles on human health were conducted in the U.S., Europe, and China, the data used in our models may not be representative of all regions of the world, especially Africa, South America, and Oceania, where almost no data are available the published references.
This study was also limited by a lack of infiltration factor data for much of Asia (except China), Africa, South America, and Oceania in our study (work sites and bars/restaurants). However, since detailed analysis of measured data shows that the infiltration factor is not strongly region-dependent [21–23,37–38,41–42,45–46,48–49,52,54–57], the assumed infiltration factors used in this study are not likely to deviate significantly from the actual measurements. Rare infiltration factors were studied at the same time for most of the epidemiologic studies, so further study need to be conducted to obtain the detailed and systematic infiltration factors. This is also what we want to do in the future work. For accurately estimate as possible, we conducted the literature review to determine the PM10 and PM2.5 infiltration factors for European, North American, Chinese, and global residences, try to apply local infiltration factors we could obtain for the corresponding regions (see Table 2). We also calculated mean infiltration factors of PM10 and PM2.5 in each region, and the maximum and minimum values for each region were further used in sensitivity analysis to study the effect of infiltration factor on mortality and particle infiltration. We think such treatment of infiltration factors represents the state of the art approach for such study.
Parameters such as particle size, the crack geometry of building envelopes, differences in indoor/outdoor air pressure, and the efficiency of mechanical filtration can affect particle infiltration; however, such factors are highly complex and require further study. After analyzing these factors, we were able to account for the relationship between mortality and indoor exposure to PM of outdoor origin.
Finally, although Daniels et al. [29] concluded that log-linear models are appropriate for assessing the effect of PM pollution on daily mortality, and many studies have employed such models to analyze epidemiological data [6–7, 29–31], the exact nature of this relationship has not been conclusively established. The assumption of linearity is a potential limitation of the model applied in this study, and warrants further research.
Perspectives
The limited scientific knowledge on the health effects of exposure to airborne particles in the indoor environment represents a major barrier to establishing limit values or guidelines that protect public health [58]. One explanation for the lack of studies on the health effects of indoor exposure to outdoor-originated PM is the expense, in addition to the logistical and technological constraints inherent in measuring direct personal PM exposure. This study introduces an innovative method for quantifying the relationship between outdoor PM and indoor PM originating from outdoors, based on a known set of physical principles. For example, based on existing knowledge of aerosol physics, we know that this relationship is affected by the particle penetration factor through building envelopes, in conjunction with the rates of indoor particle deposition and air exchange. Reducing the need for conducting personal PM exposure measurements may facilitate further studies on this topic so that guidelines on indoor exposure to ambient PM can be established.
This method for estimating mortality derived from indoor exposure to particles of outdoor origin has some disadvantages (e.g., the composition issue). Wilson et al. [28] suggested a reasonable, epidemiological approach to estimate such mortality. For the purpose of controlling aerosol particle pollution, we are eager to learn from the epidemiological data, e.g. mortality derived from indoor exposure to particles of outdoor origin, from the method suggested by Wilson et al. [28] in order to support strategies for managing indoor air quality. However, to date, no such studies have been presented. Therefore, through this paper, we also wish to encourage epidemiologists to further consider this important issue.
Acknowledgments
This study was funded by the special fund of the Key Laboratory of Eco Planning & Green Building, Ministry of Education (Tsinghua University), China; and by Tsinghua National Laboratory for Information Science and Technology (TNList) Cross-discipline Foundation.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the special fund of the Key Laboratory of Eco Planning & Green Building, Ministry of Education (Tsinghua University), China and Tsinghua National Laboratory for Information Science and Technology (TNList) Cross-discipline Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lave LB, Seskin EP. Air pollution and human health. Science. 1970;169: 723–733. [DOI] [PubMed] [Google Scholar]
- 2. Evans JS, Tosteson T, Kinney PL. Cross-sectional mortality studies and air pollution risk assessment. Environ Int. 1984;10: 55–83. [Google Scholar]
- 3. Özkaynak H, Thurston GD. Associations between 1980 U.S. mortality rates and alternative measures of airborne particle concentration. Risk Anal. 1980;7: 449–461. [DOI] [PubMed] [Google Scholar]
- 4. Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six US cities. New England journal of medicine. 1993; 329(24): 1753–1759. [DOI] [PubMed] [Google Scholar]
- 5. Pope CA III, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, et al. Particulate air pollution as a predictor of mortality in a prospective study of US adults. American journal of respiratory and critical care medicine. 1995;151: 669–674. [DOI] [PubMed] [Google Scholar]
- 6. Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002; 287(9): 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 8. Krewski D, Burnett RT, Goldberg MS, Hoover K, Siemiatycki J, Jerrett M, et al. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of particulate air pollution and mortality. Cambridge, MA: Health Effects Institute; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Jerrett M, Burnett RT, Ma R, Pope CA III, Krewski D, Newbold KB, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005; 16(6): 727–736. [DOI] [PubMed] [Google Scholar]
- 10. Van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environmental health perspectives. 2010;118(6): 847 10.1289/ehp.0901623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brauer M, Amann M, Burnett RT, Cohen A, Dentener F, Ezzati M, et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environmental science & technology. 2012; 46(2): 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet. 2013;380(9859): 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnett RT, Pope CA, Ezzati M, Olives C, Lim SS, Mehta S, et al. An Integrated Risk Function for Estimating the Global Burden of Disease Attributable to Ambient Fine Particulate Matter Exposure.” Environmental Health Perspectives. 2014;122(4): 397–403. 10.1289/ehp.1307049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Deng F, Wei H, Huang J, Wang X, Hao Y, et al. Association of Cardiopulmonary Health Effects with Source-Appointed Ambient Fine Particulate in Beijing, China: A Combined Analysis from the Healthy Volunteer Natural Relocation (HVNR) Study. Environmental science & technology. 2014;48(6): 3438–3448 [DOI] [PubMed] [Google Scholar]
- 15. Pope CA, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke shape of the exposure-response relationship. Circulation. 2009; 120(11): 941–948. 10.1161/CIRCULATIONAHA.109.857888 [DOI] [PubMed] [Google Scholar]
- 16. Anenberg SC, Horowitz LW, Tong DQ, West J. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environmental health perspectives. 2010;118(9): 1189–1195. 10.1289/ehp.0901220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weichenthal S, Villeneuve PJ, Burnett RT, van Donkelaar A, Martin RV, Jones RR, et al. Long-Term Exposure to Fine Particulate Matter: Association with Nonaccidental and Cardiovascular Mortality in the Agricultural Health Study Cohort. Environ Health Perspect. 2014;122(6): 609–615. 10.1289/ehp.1307277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environmental health perspectives. 2013;121(7): 804–810. 10.1289/ehp.1205958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes care. 2013;36(10): 3313–3320. 10.2337/dc12-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abt E, Suh HH, Catalano P, Koutrakis P. Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environmental science & technology. 2000;34(17): 3579–3587. [Google Scholar]
- 21. Long CM, Suh HH, Catalano PJ, Koutrakis P. Using time- and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ Sci Technol. 2001;35: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 22. Allen R, Larson T, Sheppard, L, Wallace L, Liu LJS. Use of real-time light scattering data to estimate the contribution of infiltrated and indoor-generated particles to indoor air. Environ Sci Technol. 2003;37: 3484–3492. [DOI] [PubMed] [Google Scholar]
- 23. Sarnat SE, Coull BA, Ruiz PA, Koutrakis P, Suh HH. The influences of ambient particle composition and size on particle infiltration in Los Angeles, CA, residences. J Air Waste Manage Assoc. 2006;56: 186–196. [DOI] [PubMed] [Google Scholar]
- 24. Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of exposure analysis and environmental epidemiology. 2001;11(3): 231–252. [DOI] [PubMed] [Google Scholar]
- 25. Sarnat JA, Wilson WE, Strand M, Brook J, Wyzga R, Lumley T. Panel discussion review: session one—exposure assessment and related errors in air pollution epidemiologic studies. J Expo Sci Env Epid. 2007;17:S75–S82. [DOI] [PubMed] [Google Scholar]
- 26. Chen C and Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ. 2011;45: 275–288. [Google Scholar]
- 27. Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, et al. Modeling the residential infiltration of outdoor PM2.5 in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environmental health perspectives. 2012;120(6): 824 10.1289/ehp.1104447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: Why and how. J Air Waste Manage Assoc. 2000;50: 1167–1183. [DOI] [PubMed] [Google Scholar]
- 29. Daniels MJ, Dominici F, Samet JM, Zeger SL. Estimating particulate matter-mortality dose-response curves and threshold levels: an analysis of daily time-series for the 20 largest US cities. Am J Epidemiol. 2000;152: 397–406. [DOI] [PubMed] [Google Scholar]
- 30. Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manage Assoc. 2002;52: 470–484. [DOI] [PubMed] [Google Scholar]
- 31.Anderson HR, Atkinson RW, Peacock JL, Marston L, Konstantinou K. Meta-analysis of time-series studies and panel studies of Particulate Matter (PM) and Ozone (O3). Report of a WHO task group. 2004. Available: http://www.euro.who.int/__data/assets/pdf_file/0004/74731/e82792.pdf.
- 32. Smith KR. Biofuels, air pollution, and health: a global review. Plenum Press; 1987. [Google Scholar]
- 33. Stanek LW, Sacks JD, Dutton SJ, Dubois JJB. Attributing health effects to apportioned components and sources of particulate matter: an evaluation of collective results. Atmos Environ. 2011; 45:5655–5663. [Google Scholar]
- 34. Zanobetti A and Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6): 898–903. 10.1289/ehp.0800108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen R, Kan H, Chen B, Huang W, Bai Z, Song G, et al. Association of Particulate Air Pollution With Daily Mortality The China Air Pollution and Health Effects Study. American journal of epidemiology. 2012. June 8 10.1093/aje/kwr425 [DOI] [PubMed] [Google Scholar]
- 36. Cao J, Xu H, Xu Q, Chen B, Kan H. Fine particulate matter constituents and cardiopulmonary mortality in a heavily polluted Chinese city. Environmental health perspectives. 2012;120(3): 373–378. 10.1289/ehp.1103671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dockery DW and Spengler JD. Indoor-outdoor relationships of respirable sulfates and particles. Atmos Environ. 1981;15: 335–343. [Google Scholar]
- 38.Ozkaynak H, Xue J, Weker R, Butler D, Spengler J. The Particle TEAM (PTEAM) study: analysis of the data. Volume Ⅲ. Draft Final Report. Contract #68-02-4544. US EPA. Research Triangle Park, NC.1993.
- 39. Ozkaynak H, Xue J, Spengler J, Wallace L, Pellizzari E, Jenkins P. Personal exposure to airborne particles and metals results from the Particle TEAM study in Riverside, California. J Expos Anal Environ Epidemiol. 1996;6: 57–78. [PubMed] [Google Scholar]
- 40. Ott W, Wallace L, Mage D. Predicting particulate (PM10) personal exposure distributions using a random component superposition statistical model. J Air Waste Manage Assoc. 2000;50:1390–1406. [DOI] [PubMed] [Google Scholar]
- 41. Lazaridis M, Aleksandropoulou V, Smolik J, Hansen JE, Glytsos T, Kalogerakis N. Physico-chemical characterization of indoor/outdoor particulate matter in two residential houses in Oslo, Norway: measurements overview and physical properties–URBAN–AEROSOL Project. Indoor air. 2006;16(4): 282–295. [DOI] [PubMed] [Google Scholar]
- 42. Hoek G, Kos G, Harrison R, de Hartog J, Meliefste K, ten Brink H, et al. Indoor–outdoor relationships of particle number and mass in four European cities. Atmospheric Environment. 2008;42(1): 156–169. [Google Scholar]
- 43. Diapouli E, Chaloulakou A, Koutrakis P. Estimating the concentration of indoor particles of outdoor origin: A review. Journal of the Air and Waste Management Association. 2013;63(10): 1113–1129. [DOI] [PubMed] [Google Scholar]
- 44. Zhou B, Zhao B. Population inhalation exposure to polycyclic aromatic hydrocarbons and associated lung cancer risk in Beijing region: Contributions of indoor and outdoor sources and exposures. Atmospheric Environment. 2012;62: 472–480. [Google Scholar]
- 45. Lee HS, Kang BW, Cheong JP, Lee SK. Relationships between indoor and outdoor or quality during the summer season in Korea. Atmos Environ. 1997;31: 1689–1693. [Google Scholar]
- 46. Lachenmyer C, Hidy GM. Urban measurements of outdoor/indoor PM concentrations and personal 2.5 Exposure in the Deep South. Part I. pilot study of mass concentrations for nonsmoking subjects. Aerosol Sci Technol. 2000;32: 34–51. [Google Scholar]
- 47. Wallace LA, Mitchell H, O’Connor GT, Neas L, Lippmann M, Kattan M, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reff A, Turpin BJ, Porcja RJ Giovennetti R, Cui W, Weisel CP, et al. Functional group characterization of indoor, outdoor, and personal PM2. 5: results from RIOPA. Indoor Air. 2005;15(1): 53–61. [DOI] [PubMed] [Google Scholar]
- 49. Wallace A, William R. Use of personal-indoor-outdoor sulfur concentrations to estimate the infiltration factor and outdoor exposure factor for individual homes and persons. Environ Sci Technol. 2005;39: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 50. Polidori A, Arhami M, Sioutas C, Delfino RJ, Allen R. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles basin. J Air Waste Manage Assoc. 2007;57:366–379. [DOI] [PubMed] [Google Scholar]
- 51. Meng QY, Turpin BJ, Korn L, Weise CP, Mosrandi M, Colome S, Zhang J, et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: Analyses of RIOPA data. J Expos Anal Environ Epidemiol. 2005;15:17–28. [DOI] [PubMed] [Google Scholar]
- 52. Hanninen OO, Lebret E, Ilacqua V, Katsouyanni K, Künzli N, Srám RJ, et al. Infiltration of ambient PM2.5 and levels of indoor generated non-ETS PM2.5 in residences of four European cities. Atmos. Environ. 2004;38: 6411–6423. [Google Scholar]
- 53. Wichmann J, Lind T, Nilsson MA, and Bellander T. PM2.5, soot and NO2 indoor-outdoor relationships at homes, pre-schools and schools in Stockholm, Sweden. Atmos Environ. 2010;44:4536–4544. [Google Scholar]
- 54. Landis MS, Norris GA, Williams RW, Weinstein JP. Personal exposures to PM2.5 mass and trace elements in Baltimore, MD, USA. Atmos Environ. 2001;35: 6511–6524. [Google Scholar]
- 55. Williams R, Suggs J, Rea A, Sheldon L, Rodes C, Thornburg J. The Research Triangle Park particulate matter panel study: modeling ambient source contribution to personal and residential PM mass concentrations. Atmos Environ. 2003;37: 5365–5378. [Google Scholar]
- 56. Meng QY, Turpin BJ, Lee JH, Polidori A, Weisel CP, Morandi M, et al. How does infiltration behavior modify the composition of ambient PM2. 5 in indoor spaces? An analysis of RIOPA data. Environmental science and technology. 2007;41(21): 7315–7321. [DOI] [PubMed] [Google Scholar]
- 57. Meng QY, Spector D, Colome S, Turpin B. Determinants of indoor and personal exposure to PM2.5 of indoor and outdoor origin during the RIOPA study. Atmos Environ. 2009;43: 5750–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider T, Sundell J, Bischof W, Bohgard M, Cherrie JW, Clausen PA, et al. ‘EUROPART’. Airborne particles in the indoor environment. A European interdisciplinary review of scientific evidence on associations between exposure to particles in buildings and health effects. Indoor Air. 2003;3(1): 38–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.