To the Editor
The epidemic of Ebola virus disease (EVD) in West Africa has caused clinical illness and deaths among persons with reported ages ranging from less than 1 year to more than 100 years. Most published estimates of key epidemiologic parameters have been based on patients of all ages1,2 and have thus been dominated by cases in which patients are 16 years of age or older, and as of January 5, 2015, these cases accounted for 79% of the confirmed and probable cases for which age has been reported.
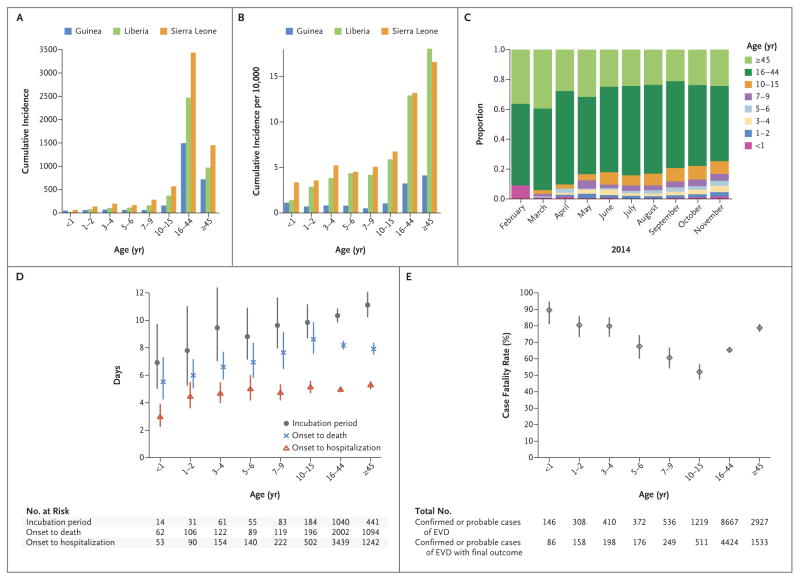
Here we investigate the progression and out come of EVD in confirmed and probable pediatric cases reported from Guinea, Liberia, and Sierra Leone, stratified according to age. The absolute and per capita case incidence of EVD among children younger than 16 years of age has been significantly and consistently lower than the incidence among adults in all three countries (Fig. 1A, 1B, and 1C). This pattern is similar to that observed in past EVD outbreaks.3,4 However, because the current epidemic is so large, it provides an opportunity to explore the ways in which epidemiologic and clinical parameters vary according to age. Although the age distribution of confirmed, probable, and suspected cases is similar in all three countries (Fig. S3 in the Supplementary Appendix, available with the full text of this letter at NEJM.org), the proportion of pediatric cases (those younger than 16 years of age) among all cases increased over the course of 2014 (Fig. 1C, and Fig. S4 in the Supplementary Appendix).
Figure 1. Age-Group–Specific Incidence of Ebola Virus Disease in West Africa, Incubation Period, Intervals from Onset to Death and Onset to Hospitalization, and Case Fatality Rate.
Panel A shows the cumulative incidence of confirmed and probable cases of Ebola virus disease (EVD) according to age group and country. Panel B shows the cumulative incidence of confirmed and probable cases per 10,000 population according to age group and country. Panel C shows the overall age distribution for confirmed and probable cases according to month of symptom onset. Panel D shows the estimated average incubation period, the interval between symptom onset and death, and the interval between symptom onset and hospitalization according to age among persons with confirmed or probable EVD (with vertical lines indicating 95% confidence intervals [CIs]). The numbers represent the sample sizes in each age group. Panel E shows the estimated case fatality rate according to age among persons with confirmed or probable EVD (with 95% CIs), with representation of the total number of confirmed or probable cases of EVD cases in each age group and the total number of confirmed or probable cases of EVD for which there was information on the final outcome in each age group.
The mean incubation period (the average time from infection until symptom onset) was shortest, on average, in the youngest children, with means ranging from 6.9 days (95% confidence interval [CI], 5.1 to 9.5) in 14 children younger than 1 year of age to 9.8 days (95% CI, 8.7 to 11.1) in 184 children 10 to 15 years of age (Fig. 1D, and Table S1 and Fig. S5 in the Supplementary Appendix). Younger children also had shorter times from symptom onset to hospitalization and from symptom onset to death (Fig. 1D, and Fig. S6 and S7 and Tables S2 and S3 in the Supplementary Appendix). There was no clear evidence that age affected the distribution of the intervals between symptom onset and hospital discharge, between hospitalization and death, between hospitalization and hospital discharge, or between symptom onset and onward transmission (Fig. S8 to S11 and Tables S4 to S7 in the Supplementary Appendix).
Almost all children with EVD who were younger than 1 year of age had fever (92%) before clinical presentation, and children younger than 16 years of age were more likely than adults to present with fever (P<0.001) (Table S8 and Fig. S13 in the Supplementary Appendix). Children were less likely than adults (i.e., persons 16 years of age or older) to report pain in the abdomen, chest, joints, or muscles, difficulty breathing or swallowing, and hiccups between symptom onset and clinical presentation (P<0.001); however, this finding may reflect the difficulty young children have in reporting such symptoms rather than a different symptom profile (Table S8 and Fig. S12 in the Supplementary Appendix). The case fatality rate (CFR) was lowest among children between 10 and 15 years of age and highest among those 4 years of age or younger (Fig. 1E, and Fig. S14 and S15 and Table S9 in the Supplementary Appendix). The CFR for persons younger than 45 years of age (most of whom are 5 to 44 years of age) was lower than that among those 45 years of age or older (Fig. 1E), a finding that is in line with that of an earlier report.5
The shorter incubation period in children, the relatively high risk of death among children younger than 5 years of age (as compared with older children), and the more rapid progression to death highlight the importance of including children among case contacts for follow-up, of examining children for early signs of disease during active case finding, and of explaining the risk of EVD to parents, guardians, and caregivers. All persons in whom EVD is suspected, but especially children, need the earliest possible referral for diagnostic testing, and children need age-appropriate treatment. The causes of the relatively rapid disease progression and relatively high CFR in the youngest children requires further investigation.
Supplementary Material
Acknowledgments
The study was conducted in support of the response to the Ebola outbreak in Guinea, Liberia, and Sierra Leone and is based on data routinely collected by national and international staff in conjunction with WHO.
Supported by the Medical Research Council, the Bill and Melinda Gates Foundation, the Models of Infectious Disease Agent Study of the National Institute of General Medical Sciences, National Institutes of Health, the Health Protection Research Units of the National Institute for Health Research, the European Union PREDEMICS consortium, the Wellcome Trust, and Fogarty International Center. Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.WHO Ebola Response Team. Ebola virus disease in West Africa — the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Ebola Response Team. West African Ebola epidemic after one year — slowing but not yet under control. N Engl J Med. 2015;372:584–7. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Outbreak of Ebola haemorrhagic fever, Uganda August 2000–January 2001. Wkly Epidemiol Rec. 2001;76:41–6. [PubMed] [Google Scholar]
- 4.Mupere E, Kaducu OF, Yoti Z. Ebola haemorrhagic fever among hospitalised children and adolescents in northern Uganda: epidemiologic and clinical observations. Afr Health Sci. 2001;1:60–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Bah EI, Lamah M-C, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–7. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.