Abstract
Opioid-coded neural circuits play a substantial role in how individuals respond to drugs of abuse, and most individuals begin using such drugs during adolescence and within a social context. Several studies indicate that adolescent mice exhibit a heightened sensitivity to the effects of morphine, the prototypical opiate drug, when compared with adults, but it is unclear whether these developmental differences are related to aspects of motivated behavior. Moreover, exposure to opioids within the rodent brain can alter the expression of social behavior, yet little is known about whether this relationship changes as a function of development or genetic variation. In this study, we conducted a series of experiments to characterize the relationship between genetic background, adolescent development and morphine-induced changes in mouse social investigation (SI). At two time-points during adolescent development (postnatal day [PD] 25 and 45), social interactions of test mice of the gregarious C57BL/6J (B6) strain were more tolerant to the suppressive effects of morphine (ED50 = 0.97 and 2.17 mg/kg morphine, respectively) than test mice from the less social BALB/cJ (BALB) strain (ED50 = 0.61 and 0.91 mg/kg morphine, respectively). By contrast, this strain-dependent difference was not evident among adult mice on PD 90 (ED50 = 1.07 and 1.41 mg/kg morphine for BALB and B6 mice, respectively). An additional experiment demonstrated that the ability of morphine to alter social responsiveness was not directly related to drug-induced changes in locomotor behavior. Finally, administration of morphine to stimulus mice on PD 25 reduced social interaction of test mice only when individuals were from the B6 genetic background. Overall, these results indicate that alterations in endogenous opioid systems are related to changes in SI that occur during adolescence and that morphine administration may mimic the rewarding nature of SI.
Keywords: sociability, adolescence, social reward, mouse, opioid, mouse genetics, addiction, peers, motivation, drug abuse
INTRODUCTION
It is axiomatic that substance abuse is typically initiated during the teenage years. Popular drugs of abuse during adolescence include nicotine, alcohol and marijuana, but there has also been a recent rise in the non-medical use of opioid analgesics, such as OxyContin and Vicodin (Johnston et al., 2010). A substantial body of evidence indicates that several of these ‘gateway’ drugs engage opioid-coded neural circuits in the brain (Drews and Zimmer, 2010; Ghitza et al., 2010; Lopez-Moreno et al., 2010; Ray et al., 2010).
Extensive studies of rodent behavior indicate that exogenous opioids have a reward value, demonstrated primarily by self-administration (Belknap et al., 1993; Carney et al., 1991; Koob et al., 1984; Martin et al., 1997) and morphine conditioned place preference (MCPP) studies (Belzung and Barreau, 2000; Coudereau et al., 1997; Cunningham et al., 1992; Ribeiro Do Couto et al., 2006). Opioid receptor antagonists can modulate the behavioral responses of rodents to drugs of abuse, including nicotine (Liu et al., 2009), alcohol (Burattini et al., 2006; Foster et al., 2004; Varlinskaya and Spear, 2009) and cocaine (Burattini et al., 2008; Gerrits et al., 2005). Moreover, in studies employing gene knockout technologies, opioids have also been implicated in processes that mediate the rewarding properties of food, sex and addictive substances (Le Merrer et al., 2009).
In rodents, opioid sensitivity changes with maturation. For example, adolescent rodents are more sensitive than adults to the effects of morphine, the prototypical opiate, with respect to measures of analgesia (Ingram et al., 2007) and locomotor activity (Spear et al., 1982; White and Holtzman, 2005; White et al., 2008). With repeated administration of morphine, adolescent rodents lose this higher sensitivity (develop greater tolerance) relative to adults (Ingram et al., 2007). Comparisons of adolescent versus adult sensitivity to opioid withdrawal in the forced swim test show reduced adolescent immobility, indicating that the depressive symptoms of opioid withdrawal are less severe in adolescent mice relative to adult mice (Hodgson et al., 2009). Studies that have explored the reward aspects of opioid exposure are less clear. For example, both adolescent and adult rodents self-administer morphine (Doherty et al., 2009) and Oxycodone (Zhang et al., 2009), but adolescents administer significantly lower amounts of morphine than do adults, suggesting that adolescents may be more sensitive to the reward experience associated with exposure to this drug. However, the conditioned place preference (CPP) test, another measure of drug reward, does not reveal differences in reward sensitivities of adolescent and adult rats to morphine exposure (Campbell et al., 2000; Zheng et al., 2003). Indeed, adolescent sensitivity to opioids may be reflective of more general maturational process in brain reward systems, which is likely a key factor in the adolescent window of vulnerability to drug addiction (Crews et al., 2007), as illustrated by enhanced CPP responses and self-administration for substances such as nicotine and cocaine during adolescence (Doremus-Fitzwater et al., 2010).
There is a strong connection between the opioid system and the social environment. First, changes in the social environment can alter the rewarding properties of morphine. Using a two-bottle choice paradigm, rodents housed in isolation have been shown to consume more morphine compared to when they are socially housed, with individuals consuming more morphine after prolonged periods of isolation (Consorti et al., 1992) and reducing morphine consumption when placed back into social housing (Raz and Berger, 2010). In the opposite direction, social interactions are also affected by manipulations of endogenous opioid systems. For example, low-dose morphine treatment promotes social play in rats (Panksepp et al., 1985; Trezza and Vanderschuren, 2008), and morphine administration during isolation can counteract the heightened levels of social approach expressed by rodents that have experienced prolonged periods of social isolation (Hol et al., 1996). In addition, morphine can also directly reduce gregariousness in rodents when administered prior to a social encounter (Landauer and Balster, 1982; Meyerson, 1981; Panksepp et al., 1979; Shapiro et al., 1989). Taken together, these studies suggest a close relationship between opioid-mediated circuits and social interaction; that opioid reward pathways are responsive to changes in social environment and that social motivation responds to opioid exposure.
Given the high prevalence of drug experimentation among adolescent peer groups, rodent models can be extensively explored to elucidate the interplay between adolescent social processes and drug reward (Doremus-Fitzwater et al., 2010; Spear, 2000). An increase in social interest and gregariousness towards peers is a common feature of adolescent development. In rodents, the expression of social behaviors peaks during early adolescence and then becomes increasingly influenced by factors related to sex (Panksepp, 1981; Panksepp et al., 2007; Primus and Kellogg, 1989; Spear, 2000; Terranova et al., 1993; Varlinskaya and Spear, 2008). Moreover, transitions in sociability during adolescence are influenced by underlying genetic factors, with mice from the C57BL/6J (B6) strain exhibiting a more pro-social phenotype relative to the reduced social tendencies of BALB/cJ (BALB) mice (Panksepp et al., 2007). This strain-dependent difference is also expressed within the context of a social conditioned place preference (SCPP) procedure, in which adolescent B6 mice express a preference for social contact whereas BALB mice are socially indifferent (Panksepp and Lahvis, 2007). Despite the large body of work that has been devoted to understanding the relationship between endogenous opioids and social behavior and, separately, how social behavior changes with development, there have been very few studies that directly address the possibility that opioid systems mediate the developmental changes in sociability that occur during adolescence. By bringing together these research topics, we might elucidate the interactions between biological processes and social experience during adolescence that can ultimately lead to drug abuse in genetically susceptible individuals.
In the present study, we conducted a series of experiments using the BALB-B6 mouse model of sociability (Sankoorikal et al., 2006) to characterize the relationship between morphine administration and social investigation (SI) across adolescent development and early adulthood. To establish a general morphine dose-response curve for the expression of SI during early and late adolescence, a range of morphine doses were administered to male test mice prior to social reunion with a female (Experiment 1). Based on this dose-response relationship, a smaller range of morphine doses was utilized to evaluate strain- and sex- dependent differences in expression of SI during early and late adolescence as well as adulthood (Experiment 2). To control for the possibility that changes in social investigation that resulted from increased morphine exposure were indirect effects of drug-induced changes in locomotor activity, select doses of morphine were administered to adolescent mice, and locomotion was evaluated prior to and during SI testing (Experiment 3). The final experiment examined the social responsiveness of test mice toward morphine-injected stimulus mice (Experiment 4). Overall, we hypothesized that morphine exposure would reduce social motivation in test mice and administration of higher morphine doses would result in a progressive decline in social interaction behaviors. Since adolescent mice from the BALB and B6 strain derive different levels of reward from social contact (Panksepp and Lahvis, 2007), we also expected that the social behaviors of these two strains would exhibit a differential sensitivity to morphine treatment and that this strain-dependent difference would be particularly apparent during adolescence compared to adulthood.
METHODS
Animal husbandry
Mice from the BALB/cJ (BALB) and C57BL/6J (B6) strains were purchased from the Jackson Laboratories (Bar Harbor, ME), and bred in our colonies at the University of Wisconsin-Madison (UW; Experiments 1–2) and the Oregon Health and Science University (OHSU; Experiment 3) under tightly controlled temperature (21±1°C) and humidity (40–60%). The lighting conditions were 14:10 h light/dark (dark period from 1130–2130) and 12:12 h light/dark (dark period from 0930–2130) at UW and OHSU, respectively. New mice were routinely introduced to both breeding colonies and brother-sister mating was not conducted. Mice were housed at both facilities in standard polypropylene cages (290 × 180 × 130 mm) lined with 1/8” grain-size corncob bedding (The Andersons [UW]) or pelleted paper bedding (ECOfresh, Absorption Corp. [OHSU]), with free access to chow (Teklad Rodent Diet, Harlan [UW]; Lab Rodent Diet 5001, Purina Mills [OHSU]) and water. Pregnant females were isolated approximately 10–15 days post-coitus and pups were weaned on postnatal day (PD) 20–21. Pups from available litters were pooled, individuals from a single strain were randomly selected, and then reconstituted into mixed-sex groups (2 males and 2 females) for Experiment 1 or same-sex groups (4 mice) for Experiments 2–3. All experiments were conducted in strict accordance with the guidelines set forth by the institutional care and use committee at the University of Wisconsin – Madison and Oregon Health and Science University, and followed the national institutes of health (NIH) Guide for the Care and Use of Laboratory Animals. All animal husbandry was performed by our own laboratory personnel to maintain gentle and consistent handling of mice.
Social investigation testing: General methodology and analysis
Two individuals from each social group were selected as test mice, while the remaining 2 mice were designated as stimulus mice. With the exception of the 6 h of social isolation that preceded each test, all mice were maintained in their social groups from weaning age until the final test day. For the experiments 1 and 2, which involved multiple test days, each test mouse interacted with the same stimulus mouse in every test. While SI scores are highly sensitive to differences in the age of the test mice (Panksepp et al., 2007), differences between the degrees of dyad familiarity after 4 days versus 40 days of social housing is not expected to substantially moderate SI scores relative to differences in sex and age. In addition to engendering social familiarity, social housing promotes long-term social memory, which can last as long as 7 days when test mice are housed in groups (Kogan et al. 2000). By employing familiar mice, we also minimized the possible confounding effects of motivation to investigate a novel conspecific that may change during the development to sexual maturity.
On behavioral testing days, test and stimulus mice (Experiments 1–2), or test mice-only (Experiment 3) were weighed and socially isolated into clean cages without a nestlet. The duration of the social isolation period for all of the experiments was 6 h and occurred during the initial two-thirds of the dark phase of the light cycle (see Panksepp et al., 2008 for rationale). Cages containing test and stimulus mice were transported ≈5 m from the colony room to an experimental room illuminated by dim red light (30–40 lx) at least 30 min prior to testing. The top of the cage containing a test mouse was replaced with a transparent sheet of Plexiglas® and the cage was left undisturbed for 5 min. The test mouse then received a 200µl subcutaneous (s.c.) injection of morphine sulfate (Mallinckrodt Inc, St. Louis, MO) or vehicle (0.9% saline), and was subsequently returned to its cage undisturbed for 15 min (Experiment 1–2) or 17.5 min (Experiment 3) post-injection. This time period was chosen based on research indicating a rapid increase in brain morphine levels and concurrent behavioral changes after injection (Plomp et al., 1981; Kissin et al., 1991): for instance, 5 min after a 6mg/kg s.c. morphine injection, blood and brain morphine levels in rats are significantly elevated and an analgesic effect can be observed in a tail pinch test (Kissin et al., 1991). After the drug uptake period, the stimulus mouse was reunited with the familiar test mouse from the same home cage, and the ensuing social interaction was videotaped (Sony 3CCD digital camcorder) for 5 min and stored on a desktop computer (Dell™ Pentium® IV) for subsequent analysis.
Social investigation (SI) of the stimulus mouse by the test mouse was quantified using computer-assisted software (ButtonBox v.5.0, Behavioral Research Solutions, Madison, WI, USA). Two independent raters analyzed all of the SI tests, and all statistical testing and graphical presentations are based on the average of these duplicate measurements. One rater was blinded to the age and morphine dose administered to the mice. Inter-rater reliability for each experiment is presented in the legend of each figure in the Results section. Quantification of SI was based on the procedure described in Panksepp et al (2007). Briefly, behavioral variables included sniffing and snout contact toward the head/neck/mouth area, the flank area and the anogenital area, and pursuit within one body-length, as the stimulus mouse moved continuously throughout the cage. Social grooming behaviors were also tallied. We interpret these aspects of social interaction as social investigation/consumption of social reward (see Panksepp and Lahvis, 2007). The duration of time that the test mouse directed these behaviors at the stimulus mouse were summed into a composite measure of SI.
Experimental design
Experiment 1 - Morphine effects on mixed-sex social interactions in early- and late-adolescent BALB and B6 mice
Mice were weaned into mixed-sex, same-strain social groups. The 2 male mice in each social group served as test mice, while the 2 female mice served as stimulus mice. Each mouse was tested after a 6-h social isolation period on both PD 24–26 and PD 44–46. Test mice were injected with morphine sulfate (0.1, 0.25, 0.5, 1.0, 2.5, 5.0 or 10.0 mg/kg) or an isometric volume (200µl) of 0.9% saline. On the first testing day, the injected dose was pseudo-randomized and test mice never received same dose of morphine sulfate or saline on the second testing day. A female stimulus mouse was added to the cage of the male test mouse 15 min post-injection. Sample sizes were 10–16 test mice for each combination of genotype, dose and age, except for the highest dose (i.e., 10mg/kg), which consisted of a smaller sample size (4–6 mice). The total sample size for this experiment was 350 SI tests.
Experiment 2 - Morphine effects on same-sex social interactions in early-adolescent, late-adolescent, and adult BALB and B6 mice
Mice were weaned into same-sex, same-strain social groups. Two mice in each social group were selected as test mice and remained as test mice for the duration of the experiment. The other 2 mice in the social group served as stimulus mice. Each mouse was tested after a 6-h social isolation on each of the three testing periods (PD 24–26, PD 44–46 and PD 89–91). Test mice were injected with morphine sulfate (0.1, 0.25, 0.5, 1.0, 2.5 or 5.0 mg/kg) or 0.9% saline. Similar to Experiment 1, test mice received a different dose of morphine sulfate or saline for each of the three SI testing days and the selected dose was pseudo-randomized. A stimulus mouse was added to the cage of the test mouse 15 min post-injection. Sample sizes were 6–11 test mice for every combination of genotype, dose, age and sex, except for higher doses (2.5 and 5.0 mg/kg), which had sample sizes of 4–10 test mice. The total sample size was 698 SI tests.
Experiment 3 - Morphine effects on locomotor activity and same-sex social interactions in adolescent BALB and B6 mice
In this experiment, mice of each strain were again weaned into same-sex social groups. Two mice in each social group were selected as test mice, while the remaining 2 mice served as stimulus mice. Behavioral testing was conducted after a 6-h social isolation period on PD 25–32. Test mice were injected with morphine sulfate (0.25, 1.0 or 5.0 mg/kg) or 0.9% saline. Five min of video recording was taken at 12.5–17.5 min post-injection for subsequent locomotion analysis (see below). A stimulus mouse was added to the cage of the test mouse at 17.5 min post-injection for a 5-min SI test. Sample sizes were 3–8 test mice for every combination of genotype, dose and sex with a total sample size of 80 SI tests.
Video tracking of locomotor activity
The locomotor activity of test mice in Experiment 3 was monitored 12.5–17.5 min post-injection using video tracking software (TopScan v.2.0, Cleversys Inc., Reston, VA, USA). Test mouse locomotion was assessed with the ‘speed event rule’ setting available within the TopScan software package, which measures the time, duration, percentage of total time, latency, distance travelled and average velocity of movement only when a mouse exceeds a certain velocity threshold. For this experiment, a threshold of 1.0 mm/s was used to measure locomotion. Comparisons of the 1mm/s criterion with others (2.0, 5.0, 10.0, 20.0 and 50.0 mm/s) yielded very similar results (Pearson’s product-moment correlation, rp’s > 0.96 for each pair-wise comparison). In addition to locomotion, the video tracking program was used to generate tracings of the pathway taken by each mouse during the 5-min period prior to the SI test.
To assess the accuracy of the tracking software, locomotor activity was also hand-rated by dividing the cage into 8 regions (72.5 × 90mm) using a superimposed 2×4 grid. Locomotion was measured as the total number of grid crosses, which was operationally defined as the midline of the animal crossing any line dividing 2 regions. Grid crosses were tallied for test mice during both the tracking period (12.5– 17.5 min post-injection) and the social interaction test (17.5 – 22.5 min post-injection) using computer-assisted software. In order to measure locomotion within a social context, hand-rated locomotion measures were used to evaluate changes in test mouse locomotion, or ‘social arousal’, induced by the introduction of the stimulus mouse (similar to social facilitation, see Katz and Schmaltz, 1981). Social arousal was measured as the difference in the hand-rated locomotion score between the first minute of the SI test (17.5–18.5 min post-injection) and the minute prior to the SI test (16.5–17.5 min post-injection).
Experiment 4- Social response of BALB and B6 test mice to stimulus mice treated with morphine during early-adolescence, late-adolescence, and adulthood
Similar to experiment 2, mice were weaned into groups of same-sex, same-strain social groups. This experiment attempted to assess whether the SI phenotype of test mice was sensitive to the drug-state of the respective stimulus mice. Stimulus mice were injected with 0.25 or 5.0 mg/kg morphine sulfate, or 0.9% saline 15 min prior to being reunited with an untreated test mouse. All other aspects of SI testing were similar to the experimental protocol described above. Sample sizes were 6–11 test mice for every combination of genotype, dose, age and sex, with a total sample size of 325 SI tests.
Statistical analysis
Before generating dose-response curves, we conducted multi-factor Analysis of Variance (ANOVA) with different combinations of genotype, morphine sulfate dose, sex and age as independent variables depending on the experiment. SI values were normalized with an exponential transformation. Dose-response curves were then generated (Prism v.5.0, Irvine, CA, USA) for each strain using a 4 parameter, non-linear regression technique that allowed for a variable slope and normalized the data relative to a 100% response for the maximum group mean for each genotype. ED50 values were compared between BALB and B6 mice using F-tests. Locomotor activity was evaluated with a 3-factor ANOVA (with genotype, morphine sulfate dose and sex as independent variables). Post-hoc comparisons were conducted as necessary using Tukey’s HSD tests and orthogonal contrasts that were controlled for type I error (JMP v.8.0.1, SAS Institute Inc., Cary, NC, USA). Statistical significance was set at a level of P<0.05.
RESULTS
Experiment 1 - Morphine effects on male mouse social investigation towards a female during early (PD 25) and late (PD 45) adolescence
Consistent with prior studies (Sankorikaal et al., 2006; Panksepp et al., 2007), expression of the SI phenotype was dependent on the genetic background and age of male test mice. B6 mice expressed more robust SI responses than age-matched BALB mice (effect of genotype, F[1,343] = 141.1, P<0.001). The overall magnitude of SI was greater on PD 45 versus PD 25 (effect of age, F[1,343] = 29.0, P<0.001) for both strains. For all treatments across all experiments, we did not find consistent differences between individual SI behaviors and the composite SI score.
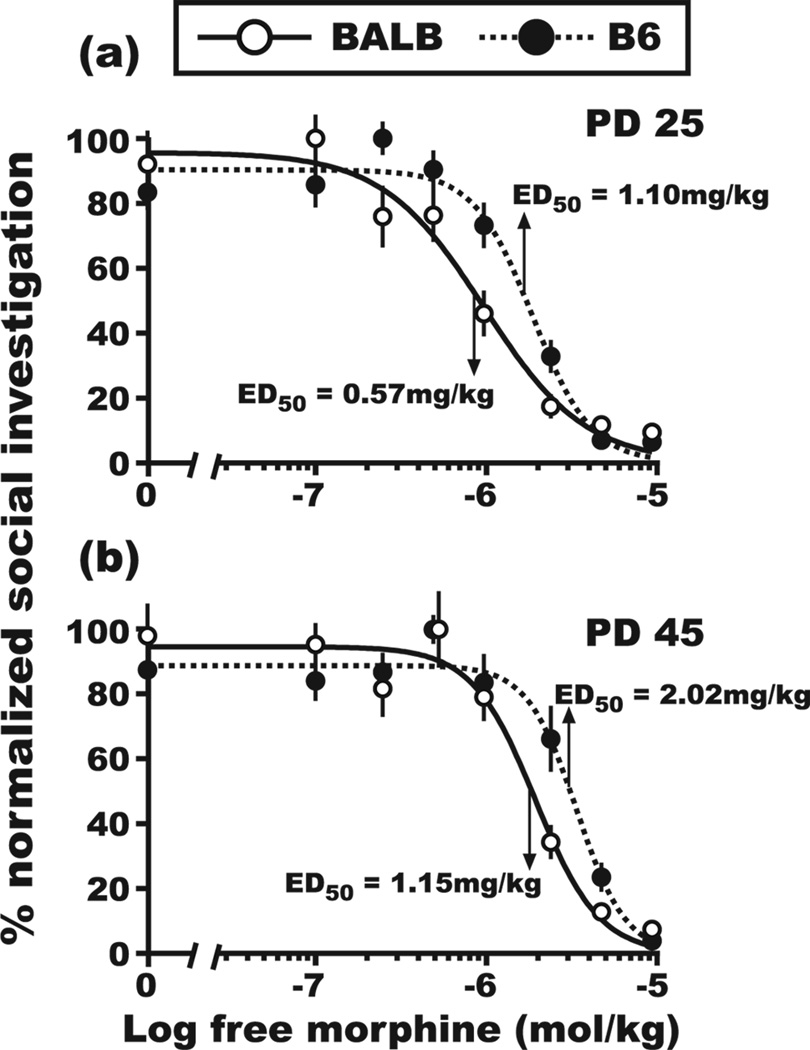
Exposure to morphine reduced social interaction behaviors of test mice from both the BALB and B6 genetic backgrounds (effect of morphine dose, F[7,337] = 106.1, P<0.001), irrespective of age. However, the effective dose of morphine sulfate that engendered a decrease in SI was different for each mouse strain (genotype × morphine dose interaction, F[7,337] = 5.3, P<0.001). In BALB test mice, a 1mg/kg dose of morphine sulfate reduced SI compared to the vehicle control group (orthogonal contrast, F[1,313] = 14.8, P<0.001; mean SI response ± SEM, saline − 91 ± 6.9 s, 1.0 mg/kg − 59 ± 6.4 s), but the same dose was ineffective in B6 test mice (orthogonal contrast, F[1,313] = 1.7, NS; saline − 126 ± 6.6 s, 1.0 mg/kg − 116 ± 8.5 s). Morphine-induced suppression of SI in B6 test mice required a 2.5mg/kg dose of morphine sulfate (orthogonal contrast, F[1,313] = 43.1, P<0.001; saline − 126 ± 6.6 s, 2.5 mg/kg − 71 ± 9.7 s). The SI phenotype of BALB test mice was more sensitive to morphine administration, an effect illustrated by a strain-dependent difference in the ED50 (ED50 at PD25; BALB- 0.57mg/kg, B6- 1.10 mg/kg) for morphine-induced suppression of SI at PD 25 (Fig. 1a; F[1,172] = 11.4, P<0.001) and PD 45 (Fig. 1b; F[1,159] = 11.5, P<0.001). Age-dependent sensitivity of the SI phenotype to morphine administration was indicated by an approximate 2-fold rightward shift in the ED50 for test mice at PD 45 (ED50 at PD45; BALB- 1.15 mg/kg, B6- 2.02 mg/kg) and was observed for test mice from both strains (Fig. 1; age × morphine dose interaction, F[7,337] = 5.7, P<0.01).
Figure 1. Dose-response curves for morphine-induced suppression of social investigation during mixed-sex social interactions.
Four-parameter, non-linear regression models were used to calculate the ED50 for morphine-induced suppression of SI during (a) early (PD 25) and (b) late (PD 45) adolescence for male BALB and B6 mice that were reunited with a female. SI values were normalized by assigning 100% to the maximum group mean for SI in each strain. SI values for the saline-injected group at PD 25 were 82 ± 9.2 s and 120 ± 7.9 s for BALB and B6 mice, respectively. SI values for the saline-injected group at PD 45 were 100 ± 10.1 s and 132 ± 10.3 s for BALB and B6 mice, respectively. The ED50 values for BALB and B6 mice were significantly different at each of the two developmental time points. Morphine doses are reported with the sulfate conjugate subtracted from the active compound. Inter-rater reliability, rp = 0.96, d.f. = 331. All SI data are reported as the mean ± SEM.
Experiment 2 - Morphine effects on social investigation within the context of same-sex dyads during early adolescence (PD 25), late adolescence (PD 45) and adulthood (PD 90)
Similar to the mixed-sex social interactions of Experiment 1, B6 test mice expressed greater SI responses than mice from the BALB strain (effect of genotype, F[1,693] =121.3 P<0.001; mean SI response ± SEM for saline treated mice; BALB – 74 ± 4.3 s, B6 – 100 ± 5.7 s) despite the same-sex social pairing. Notably, this strain-dependent difference was evident at PD 25 and 45 but was not maintained at PD 90 (genotype × age interaction, F[6,688] =16.4, P<0.001). SI expression for both strains was sensitive to the sex of the interacting mice. For instance, during late-adolescence (PD 45) and adulthood (PD 90), female test mice generally exhibited a more robust SI response than male test mice (age × sex interaction, F[2,692] =3.2, P<0.05). Consistent with previous studies, we did not find a difference in the degree of social interaction among early adolescent (PD 25) males and females. Importantly, suppression of SI by morphine sulfate did not vary with the sex of the test mouse at any age (morphine dose × sex interaction; PD 25 F[6,248] = 0.9, NS, PD45- F[6,202] = 0.9, NS, P90- F[6, 202] = 1.5, NS).
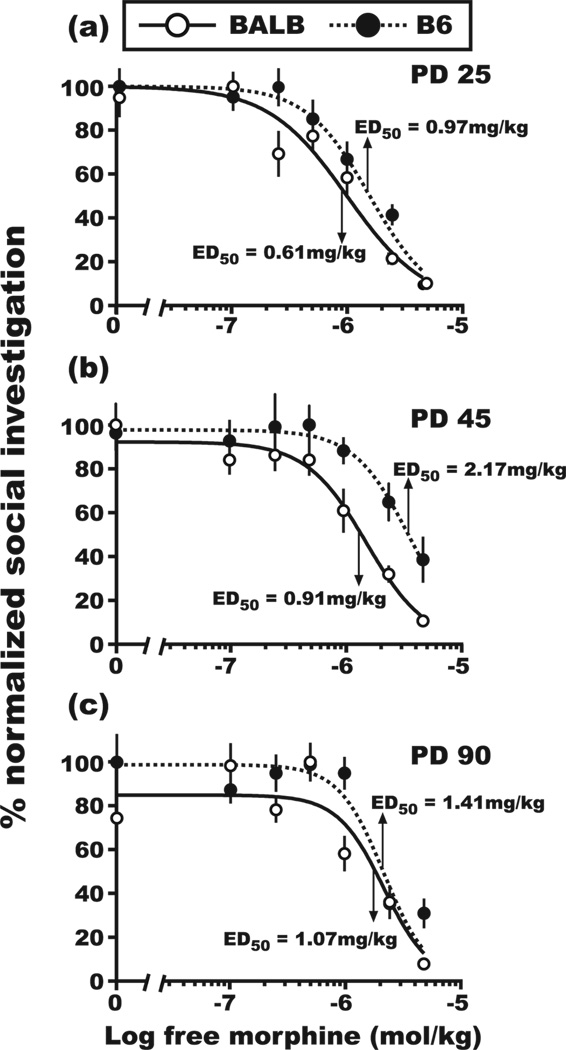
Within this same-sex SI experiment, administration of morphine again had an overall suppressive influence on the SI phenotype of test mice (effect of morphine dose, F[6,688] =111.7, P<0.001), but this effect was dependent on both the genetic background and age of the test mouse (genotype × age × morphine dose interaction, F[12,682] = 2.1, P<0.05). This 3-way statistical interaction was visualized by comparing normalized morphine dose-response curves for BALB and B6 test mice across the 3 different test ages (Fig. 2). Using non-linear regression analysis, the respective ED50 for morphine-induced suppression of SI was found to be lower for BALB test mice relative to B6 test mice at PD 25 (Fig. 2a; F[1,254] = 5.8, P<0.05) and PD 45 (Fig. 2b; F[1,203] = 11.0, P<0.01), but not at PD 90 (Fig. 2c; F[1,221] = 1.5, NS). Thus, consistent with the results of Experiment 1, the BALB SI phenotype was more sensitive to morphine administration, compared to mice from the B6 strain, during adolescent development, but not in adulthood (i.e., PD 90).
Figure 2. Dose-response curves for morphine-induced suppression of social investigation during same-sex social interactions.
Four-parameter, non-linear regression models were used to calculate the ED50 for morphine-induced suppression of SI during (a) early adolescence (PD 25), (b) late adolescence (PD 45) and (c) adulthood (PD 90) for BALB and B6 mice that were reunited with a same-sex conspecific. SI values and morphine doses are reported in the same manner as Figure 1. SI values for the saline-injected group at PD 25 were 67 ± 6.4 s and 106 ± 8.8 s for BALB and B6 mice, respectively. SI values for the saline-injected group at PD 45 were 89 ± 8.5 s and 109 ± 8.9 s for BALB and B6 mice, respectively. SI values for the saline-injected group at PD 90 were 71 ± 7.2 s and 86 ± 11.1 s for BALB and B6 mice, respectively. The ED50 values for BALB and B6 mice were significantly different at each of the two developmental time points during adolescence, but not during adulthood. Inter-rater reliability, rp = 0.92, d.f. = 671. All SI data are reported as the mean ± SEM.
Experiment 3 - Morphine effects on social investigation and locomotion in adolescent BALB and B6 mice
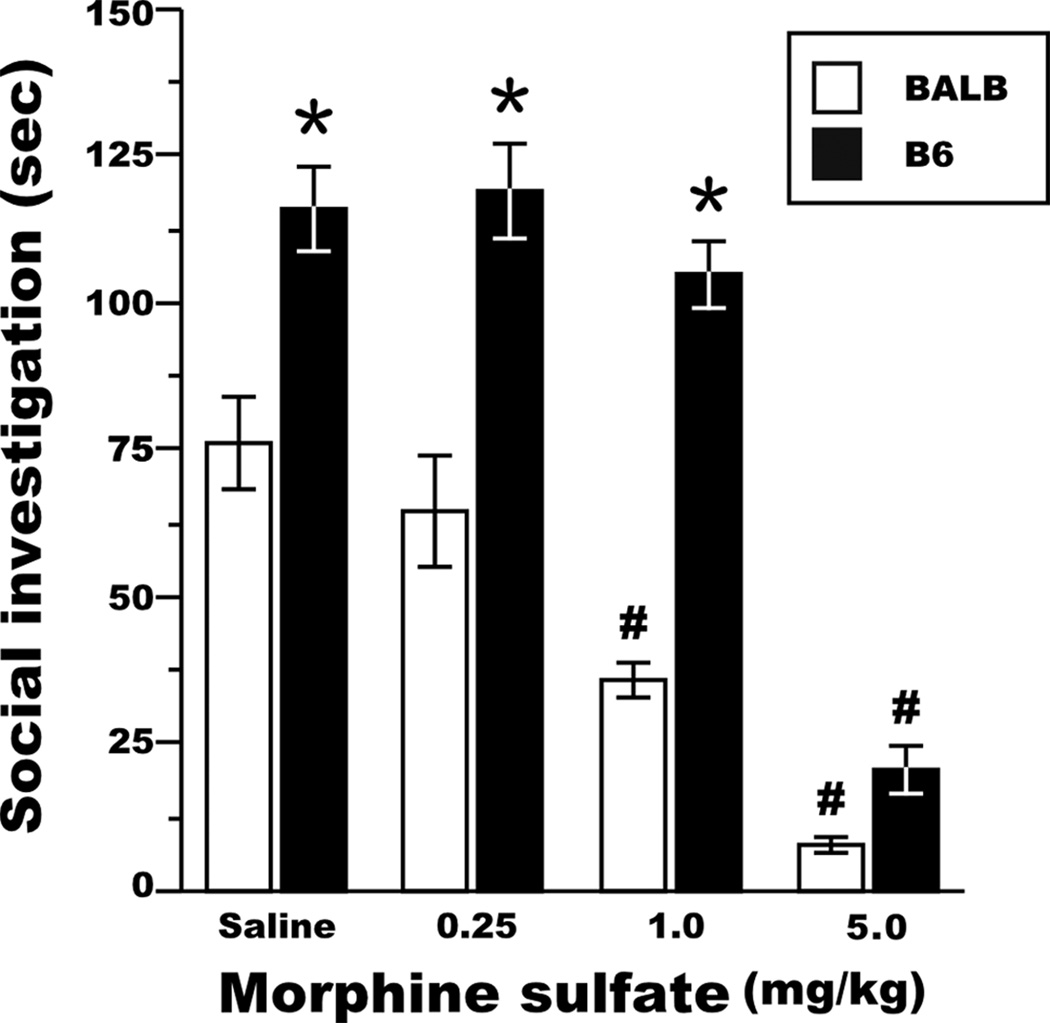
One explanation for the diminished SI response associated with morphine exposure in experiments 1 and 2 is the possibility that morphine reduced locomotion and the decrease in SI was a secondary effect. We first addressed the possibility that dose-dependent decreases in SI resulted from diminished locomotor activity, by measuring locomotor activity in the 5 min prior to the introduction of the stimulus mouse. In this third experiment, the primary findings regarding the effects of genetic background and morphine administration on SI were reproduced in PD 25–32 mice (Fig 3). Total social investigation did not differ between male and female test mice (effect of sex, F[1,78] = 0.0, NS) and the effects of morphine treatment on SI did not vary by the sex of test mice (morphine dose × sex interaction, F[3,76] = 1.3, NS). B6 test mice exhibited longer SI responses than mice from the BALB strain (effect of genotype, F [1,77] = 92.6, P<0.001) and the SI phenotype for mice from both strains was mitigated by the administration of morphine (effect of morphine dose, F[3,75] = 62.2, P<0.001). As in the previous experiments, the SI responses of BALB and B6 test mice exhibited a differential sensitivity to morphine administration (genotype × morphine dose interaction, F[3,75] = 7.3, P<0.001), in which BALB test mice exhibited a suppression of SI following a 1mg/kg dose of morphine sulfate (Fig. 3; orthogonal contrast, F[1,63] = 15.4, P<0.001) and B6 test mice expressed reduced SI after administration of 5mg/kg morphine sulfate (orthogonal contrast, F[1,63] = 107.6, P<0.001).
Figure 3. Strain- and dose-dependent suppression of adolescent mouse social investigation by morphine.
B6 test mice expressed more SI compared to BALB mice across all doses of morphine sulfate. The SI phenotype was suppressed by a 1mg/kg and 5mg/kg morphine sulfate dose in BALB and B6 test mice, respectively. * P<0.0001 for between-strain comparisons at a specific dose; # P<0.005 for within-strain comparisons to the next-lowest dose. Inter-rater reliability, rp = 0.97, d.f. = 79. All data are presented as the mean ± SEM.
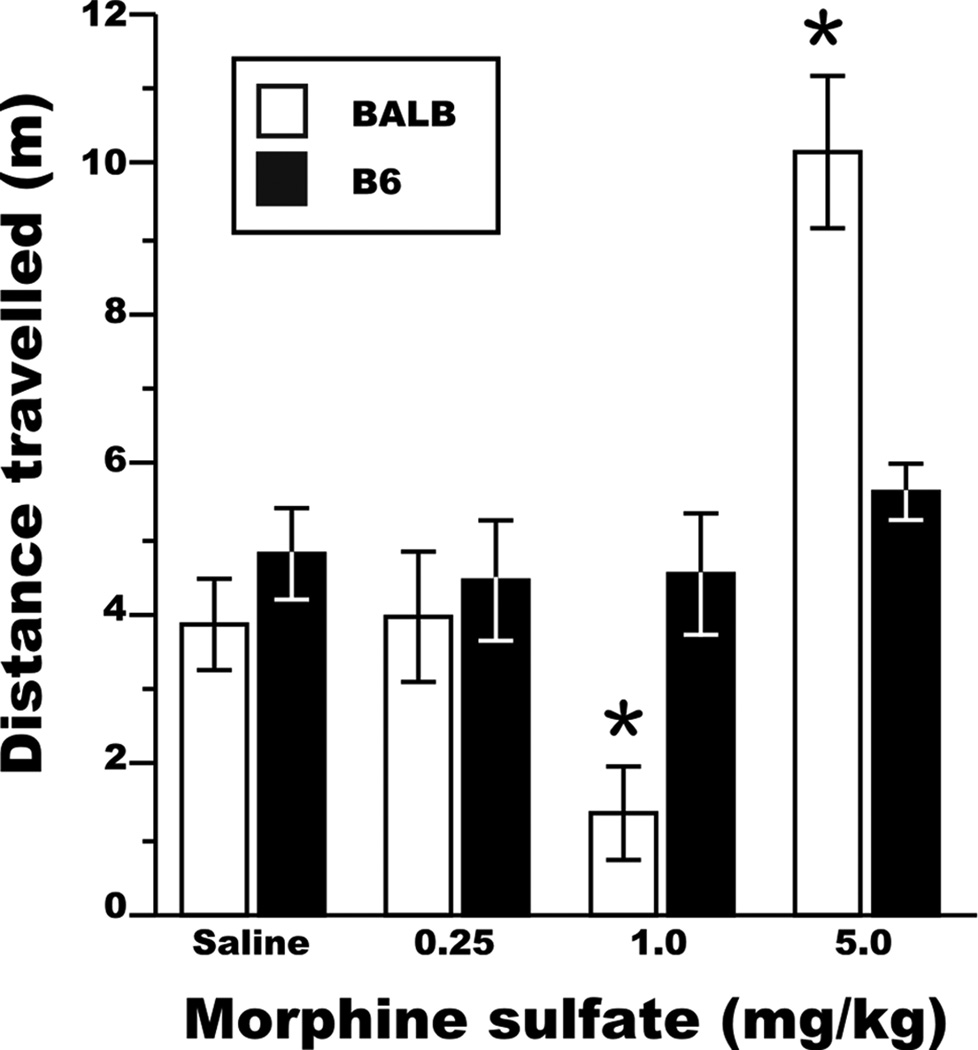
To evaluate whether morphine administration had an influence on the locomotor activity of test mice, video-tracking and hand-rated analyses of mouse spatial behavior were conducted prior to and during SI testing. There was a strong correlation between the hand-rated scoring and the video-tracking assessment of locomotion during the 5-min period pre-SI testing (rp = 0.98, d.f. = 55, P<0.001), which demonstrated the reliability of the video-tracking software. We found that locomotion, as measured by the total distance traveled during the 5 min pre-SI testing, was dependent on an interaction between the dose of morphine sulfate administered and the genetic background of the test mouse (Fig. 4; genotype × morphine dose interaction, F[3,70] = 7.8, P<0.001). BALB and B6 test mice injected with vehicle exhibited similar levels of locomotion (orthogonal contrast, F[1,58] = 0.6, NS; mean distance ± SEM, BALB - 3.87 ± 0.9 m, B6 - 4.81 ± 0.7 m). Locomotion was similar in male and female test mice (effect of sex, F[1,72] = 0.2, NS), and the effect of morphine on locomotion did not vary by the sex of the test mouse (morphine dose × sex interaction, F[3,70] = 0.2, NS). However, while the locomotion of B6 mice remained constant across different doses of morphine sulfate, BALB test mice moved less after administration of the 1mg/kg dose (orthogonal contrast, F[1,58] = 7.5, P<0.01) and more after receiving the 5mg/kg dose (orthogonal contrast, F[1,58] = 14.9, P<0.001), relative to mice from the B6 strain.
Figure 4. Strain-dependent influence of morphine administration on the locomotor activity of adolescent mice.
No differences in locomotor activity were observed between saline-treated mice from the BALB and B6 strains, and B6 locomotor activity did not change as a function of morphine administration. BALB mice expressed lower levels of locomotor activity after receiving the 1mg/kg dose and elevated levels of locomotor activity after receiving the 5mg/kg dose. * P<0.005 for between-strain comparisons at a specific dose. All data are expressed as the mean ± SEM.
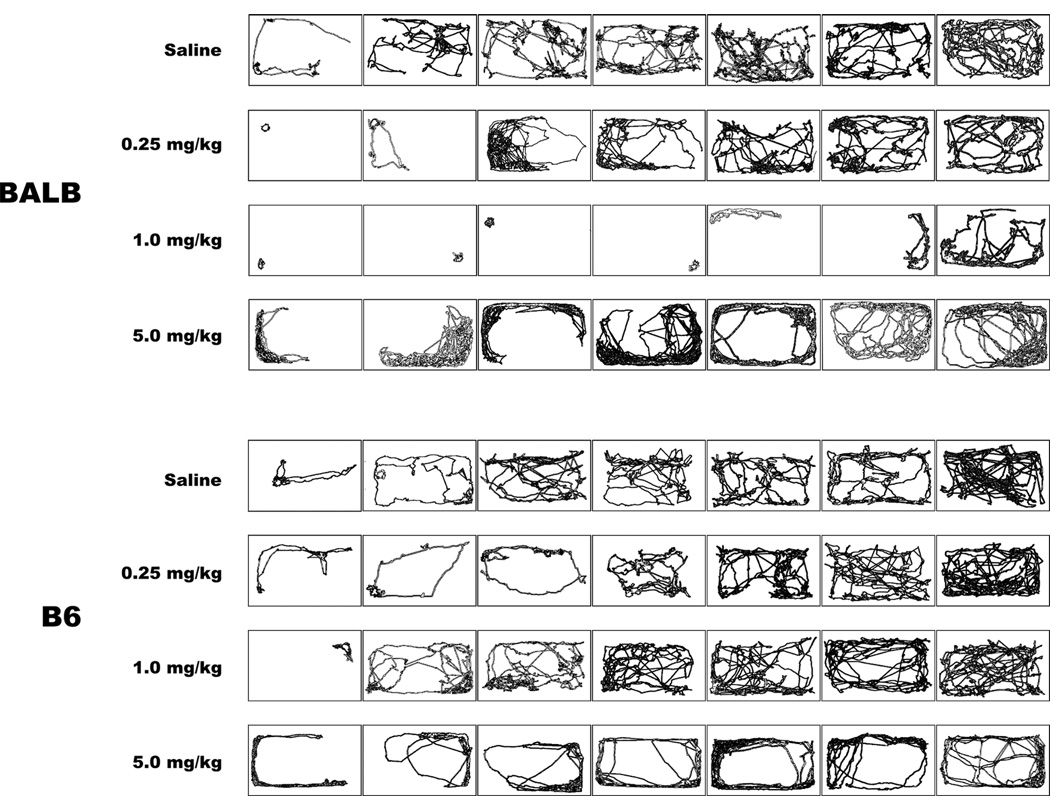
As illustrated in Figure 5, BALB and B6 mice administered 5mg/kg morphine sulfate exhibited a distinct qualitative pattern of movement that was not observed in mice that received lower doses. Test mice moved in a stereotypic pattern along the outer edge of the cage, often doubling back and retracing the same wall or cage corner. B6 mice that received lower doses of morphine sulfate (0.25 and 1.0 mg/kg) and BALB mice receiving 0.25 mg/kg morphine sulfate were similar to saline-treated mice, exhibiting no clear spatial pattern with respect to locomotor activity. When administered 1mg/kg morphine sulfate, BALB mice expressed very little spatial displacement over the 5-min period, consistent with the locomotion results generated by the video-tracking software (Fig. 5).
Figure 5. Traced pathways of individual mice following morphine administration.
BALB and B6 mice displayed distinct, qualitative patterns of locomotion after receiving 5mg/kg morphine sulfate relative to the other morphine sulfate doses and saline treatment. BALB mice that received 1mg/kg morphine sulfate were relatively motionless prior to the SI test. Traced pathways were chosen randomly and ordered based on total amount of spatial displacement.
To determine whether the level of locomotion prior to the SI test was maintained following the introduction of the stimulus animal, we compared SI scores following morphine administration with social arousal scores (see experiment 3 under the method and materials for details). Social arousal might be expressed as an increase in test mouse locomotion resulting from to the addition of a stimulus mouse to the cage, but not necessarily directed toward the stimulus mouse. Such an increase in social arousal would not correlate with SI behavior.
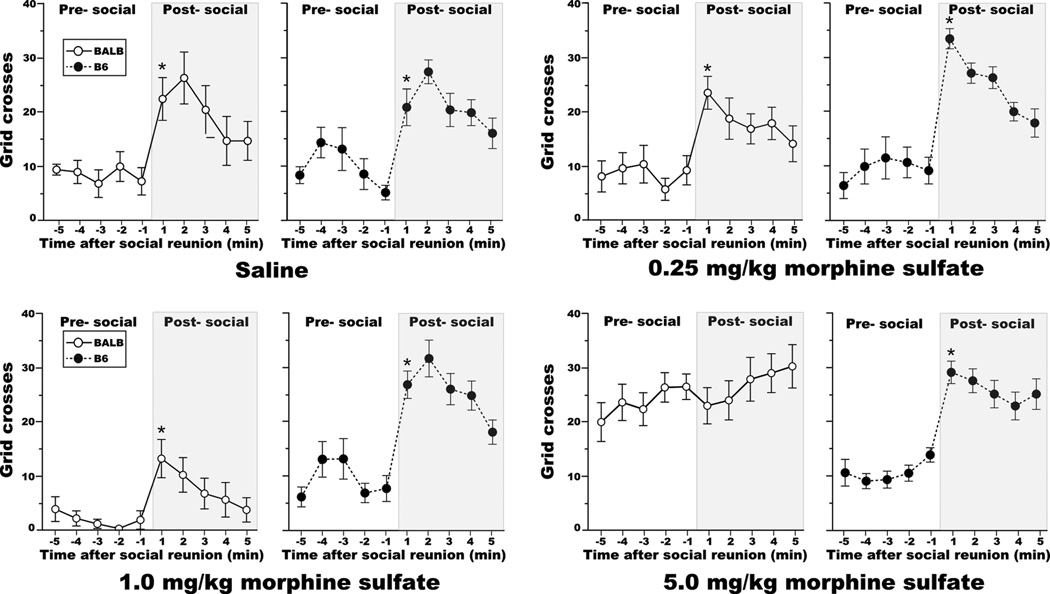
Manual scoring of locomotion during the beginning of the SI test demonstrated that movement of a test mouse could serve as an index of behavioral arousal in response to social reunion (Figure 6). Social arousal, was similar for saline-treated test mice from the BALB and B6 strains (orthogonal contrast, F[1,58] = 0.011, NS; difference score ± SEM; BALB - +15 ± 3.9 grid crosses, B6 - +16 ± 3.3 grid crosses). Interestingly, a similar level of social arousal was found for B6 test mice after administration of 5mg/kg morphine sulfate (orthogonal contrast, F[1,58] = 0.012, NS), a dose that effectively suppressed SI (see Fig. 3). By contrast, social arousal was reduced in BALB test mice following administration of 5mg/kg morphine sulfate (orthogonal contrast, F[1,58] = 11.84, P<0.005; saline - +15 ± 3.9 grid crosses, 5mg/kg - −4 ± 3.7 grid crosses). Despite exhibiting low levels of locomotion before social reunion, BALB test mice that received the 1mg/kg morphine sulfate dose expressed levels of social arousal comparable to BALB mice that were treated with saline (orthogonal contrast, F[1,58] = 0.48, NS). Overall, the amount of locomotion during the SI test did not predict the expression of SI (rp = 0.01, df = 73, NS).
Figure 6. Socially induced increases in the locomotor activity of adolescent mice.
Saline-treated BALB and B6 test mice exhibited similar increases in locomotor activity after the introduction of a stimulus mouse. Increases in locomotor activity upon social reunion were found for all experimental groups except BALB test mice that received the 5mg/kg morphine sulfate dose. * P<0.005 for comparisons between the minute prior to and after social reunion. All data are presented as the mean ± SEM.
Experiment 4- Social response of BALB and B6 test mice to stimulus mice treated with morphine during early-adolescence, late-adolescence, and adulthood
To assess the hypothesis that test mice are sensitive to the ‘drug state’ of the mice they interact with, the SI responses of untreated test mice towards stimulus mice treated with saline or morphine sulfate (0.25 or 5.0 mg/kg) was measured. Consistent with the findings for saline-treated test mice (see above), a genotype-by-age interaction on the SI phenotype was found for test mice that investigated a saline-treated stimulus mouse (Fig. 7, genotype × age interaction, F[4,322] = 5.2, P<0.01). Interestingly, an effect of morphine-treated stimulus mice on the SI phenotype of test mice was also age- and strain- dependent. B6 test mice expressed reduced SI towards stimulus mice only when they had been administered 5mg/kg morphine sulfate and were tested on PD 25 (Fig. 7; genotype × age × dose interaction, F[4,320] = 2.4, P<0.05), while BALB test mice expressed no changes in SI toward morphine-treated stimulus mice at any age.
Figure 7. Strain-, age- and dose-dependent differences in social investigation of a morphine-treated stimulus mouse.
B6 test mice expressed less SI toward stimulus mice that received 5mg/kg morphine sulfate compared to stimulus mice the received saline or 0.25 mg/kg morphine sulfate during (a) early adolescence (PD 25), but not during (b) late adolescence (PD 45) or (c) adulthood (PD 90). There were no dose-dependent differences in SI for mice from the BALB genetic background at any of the developmental time points. * P<0.05 for the 5mg/kg dose compared to the other conditions. Inter-rater reliability, rp = 0.90, d.f. = 319. All SI data are reported as the mean ± SEM.
DISCUSSION
The social behaviors of adolescent BALB and B6 mice respond to morphine exposure in a dose-dependent fashion. ED50 values ranged from 0.57 mg/kg (BALB mice, PD 25) to 2.17 mg/kg (B6 mice, PD 45). Similar to previous studies that examined the effects of morphine on rodent social behavior (Landauer and Balster, 1982; Meyerson, 1981; Panksepp et al., 1979; Shapiro et al., 1989), the general pattern uncovered in the present study was that higher doses of morphine (1.0–10.0 mg/kg) lead to a reduction in sociability. By contrast, an increase in sociability between mice was not observed following the administration of low doses of morphine, as has been previously described for rats (Panksepp et al., 1985; Trezza and Vanderschuren, 2008). Overall, our present findings implicate opioid-coded neural circuits in adolescent social motivation even for mice from a strain (BALB) that does not express social reward in a SCPP paradigm (Panksepp and Lahvis, 2007).
The SI phenotype of BALB mice was more sensitive to morphine exposure compared to mice from the gregarious B6 strain. This heightened sensitivity was replicated in 3 independent experiments that included different experimental (e.g., same-sex vs. mixed-sex social pairings, isolated vs. non-isolated stimulus mice) and animal husbandry conditions (e.g., different circadian lighting schedules, different mouse chow). Although the experiments were not designed to make direct comparisons between them, the consistency of this effect suggests that the increased sensitivity of BALB social behavior to morphine is a very robust phenomenon.
Our present findings are consistent with a study that reported less morphine self-administration in BALB mice compared to age-matched B6 mice, which is an indication of heightened reward sensitivity in BALB mice (Belknap et al., 1993). In rodents, social contact and social isolation result in an increase or decrease in opioid receptor occupancy, respectively (Bertrand et al., 1997; Panksepp and Bishop, 1981; Vanderschuren et al., 1995a,b). In this context, one intriguing possibility is that the experience of reward, mediated by opioid receptor occupancy, is effectively attained at lower doses of morphine exposure in adolescent BALB mice compared to age-matched B6 mice. Under this scenario, adolescent B6 mice may need to engage in more social interaction than BALB mice (Panksepp et al., 2007) to attain a similar level of opioid receptor occupancy in brain regions relevant to motivation and reward.
An alternative explanation for the heightened sensitivity of BALB mice to the suppressive influence of morphine on SI (relative to B6 mice) is that endogenous opioid systems are not normally activated during the expression of SI by BALB mice, and that administration of morphine induces a novel state of reward typically not achieved during a social encounter. Previous work demonstrated that adolescent B6 mice express greater amounts of social behavior compared to BALB mice or the related strain BALB/cBYJ (Brodkin et al., 2004; Moy et al., 2007; Panksepp et al., 2007), and it was suggested that social reward is the underlying proximate mechanism in explaining this strain-dependent difference (Panksepp and Lahvis, 2007). Whether endogenous opioids play a functional role in generating this strain-dependent difference is unknown, but it is interesting to note that the differential opiate sensitivity found in the present study was expressed specifically during adolescence (and not adulthood), which is precisely when BALB and B6 mice differ the most in terms of sociability (Panksepp et al., 2007). The hypothesis that social contact does not activate endogenous opioid systems in the adolescent BALB brain could be addressed in the future by using a combination of experimental approaches (i.e., measuring endogenous opioid release and receptor distributions).
A third possible explanation for the strain-dependent difference of morphine on the SI phenotype is that opiate uptake and/or metabolism is different between BALB and B6 mice. This explanation is unlikely, however, because the pharmacokinetics of morphine administration in late-adolescent (PD 49) BALB and B6 mice do not differ (Belknap et al., 1998), and do not appear to change between adolescence and adulthood (see Hodgson et al., 2009).
A persistent concern in studies of social behavior is whether observed differences between experimental groups are a direct or a secondary effect of the experimental manipulation on social behavior. In this regard, it could be helpful to compare dose-response sensitivity to morphine across several behavioral phenotypes to determine if there are other, more sensitive endpoints that may contribute to a reduction in SI. When studied in outbred mice, the ED50 for the analgesic effect of morphine on ‘phasic pain’ (tail-flick and hot plate tests) and ‘tonic pain’ (formalin test) responses ranged from 1.6–4.0 mg/kg (Elliott et al., 1994; Hunskaar et al., 1985; Rosow et al., 1980). Sensitivities of inbred mice from several different genetic backgrounds to morphine-mediated analgesia in a tail-flick (ED50 = 2.6 – 11.0 mg/kg; Liang et al., 2006) or hotplate test (ED50: 2.0 – 24.0 mg/kg; Elmer et al., 1998) are higher than the effective levels of morphine exposure that are reported here. Similar to the results for the SI phenotype in this study, BALB mice have been found to be more sensitive than B6 mice to morphine-induced analgesia in tail flick (ED50: BALB = 2.63 mg/kg, B6 = 5.63 mg/kg; Liang et al., 2006) and hot plate tests (ED50s: BALB = 3.8 ± 1.4 mg/kg, B6 = 16.4 ± 3.5 mg/kg; Elmer et al., 1998). The ED50 values for morphine-induced suppression of SI found in this study were considerably lower than those found for analgesia in both mouse strains. Thus, it is unlikely that the suppressive influence of morphine on the SI phenotype is directly attributable to the analgesic properties of morphine.
Morphine administration can also influence the locomotor activity of rodents, which in turn could affect the expression of SI. For example, if the direction or velocity of locomotion of a morphine-treated test mouse differs from that of the stimulus mouse, it could result in diminished or enhanced levels of SI. To address the possibility that the morphine-SI relationship is simply a byproduct of changes in locomotion, we evaluated locomotion prior to and during the SI test. In contrast to a previous study that demonstrated high levels of morphine-induced locomotion in the B6 strain (Belknap et al., 1998), we found that the locomotor behavior of B6 mice prior to the SI test did not change as a function of morphine administration. The insensitivity of B6 locomotion to morphine in our study can be explained by the brief period (15 minutes) between injection and testing. Peak effects on locomotion are observed at about 1 h post-injection for a 3-mg/kg dose (Murphy et al., 2001). Our mice were tested for SI and locomotion approximately 15 minutes after injection. BALB locomotion, however, was substantially diminished following 1mg/kg morphine sulfate and substantially enhanced after a 5mg/kg dose.
Importantly, there was not a direct relationship between morphine-induced changes in SI and locomotion for mice from either genetic background. For instance, B6 test mice exhibited a progressive decline in SI as they were administered higher doses of morphine without changes in locomotion, whereas the SI phenotype of BALB test mice continuously declined despite a substantial biphasic effect on locomotor activity after receiving higher doses of morphine. These patterns support the contention that the effect of morphine on the expression of SI is dissociable from its effect on locomotion.
The pattern of movement also varied by morphine dose in both BALB and B6 mice. Mice in both strains treated with the 5-mg/kg dose of morphine sulfate moved along the outer wall of the test cage. These thigmotaxic movements can indicate anxiety-like experiences in mice and can typically be suppressed by anxiolytic drugs (Treit and Fundytus, 1988). However, recent evidence suggests that the irregular locomotor behaviors that are expressed by morphine-treated mice are not a result of anxiety (Hodgson et al. 2010a). Thigmotaxic behaviors of nocturnal rodents that are relevant to anxiety are typically induced under bright light conditions within a novel cage structure. The experiment described here was conducted under dim red illumination within a familiar cage.
Social arousal, measured as the change in locomotion with the introduction of a stimulus mouse was largely unaffected by genetic background or morphine dose. With the exception of BALB test mice that were injected with a 5mg/kg dose of morphine sulfate, mice from both strains expressed a similar increase in undirected locomotion when the stimulus mouse was added to the cage. For BALB test mice that received the 5mg/kg dose, locomotion was already substantially elevated in the period prior to social reunion, and thus the lack of additional activity typically elicited during social arousal could be due to a ‘ceiling’ effect. Importantly, despite the low levels of SI exhibited by BALB test mice after receiving a 1mg/kg dose of morphine sulfate, BALB undirected locomotor activity at this dose was responsive to social reunion. Taken together these observations indicate that social responsiveness (arousal), social investigation, and locomotor behavior are dissociable in adolescent BALB and B6 mice, and that each phenotype is affected differentially by morphine administration.
It is interesting to note that only early-adolescent (PD 25) B6 test mice were responsive to the drug state of the stimulus mouse. This behavioral responsiveness requires (1) that B6 mice can detect the difference between mice that are administered morphine versus saline and (2) that this difference can influence its social behavior. The enhanced sensitivity of mice from the B6 strain to changes in the state of a conspecific has also been demonstrated in a behavioral paradigm where B6 mice, but not BALB mice, were responsive to conspecific distress (Chen et al., 2009). In addition, B6 mice that have never been directly exposed to morphine can be sensitized to morphine-induced hyper-locomotion through exposure to morphine-treated littermates (Hodgson et al., 2010b). Interestingly, this social responsiveness was expressed by adolescents, but not by adults. One explanation for the differential response of B6 mice during early adolescence is that a morphine dependent state-change in the stimulus mouse diminishes the ability of that mouse to socially interact with the test mouse. Perhaps the early adolescent B6 mouse requires a high degree of reciprocity from the social interaction, which is diminished when the companion is affected by morphine. This concept is especially compelling if both members of the dyad must be capable of affective responsiveness to social stimuli to maintain the social interaction (Lahvis et al., 2010; Lahvis and Black, 2010). One might speculate that the diminished SI responses towards morphine-injected stimulus mice generalize to drugs other than opiates. Future studies could examine the locomotor and bioacoustics features of such interactions, to determine whether a stimulus mouse responds to the solicitations of a test mouse following treatment with morphine or other psychoactive drugs.
The strain-dependent difference in sensitivity to the suppressive influence of morphine on the SI phenotype suggests that discrete genetic factors modulate how opioid-coded neural circuits interact with social motivation. Perhaps more intriguing, however, is the finding that these genetic influences occur during a defined window of adolescent development. Endogenous opioid peptides and receptor densities generally peak by the third postnatal week and levels are maintained in adulthood (McDowell and Kitchen, 1987), while dopamine (DA) receptor 1 densities in the medial prefrontal cortex, which has been implicated in the rewarding effects of drugs, peak during adolescence and then decline in adulthood (Brenhouse et al., 2008). The relatively high densities of both receptor types during adolescence could explain the heightened sensitivity to morphine expressed by mice at this age (Brenhouse et al., 2008). The SI phenotype of early adolescent BALB mice (PD 25) was the most sensitive to morphine administration, irrespective of whether stimulus mice were opposite or same-sex conspecifics (ED50 = 0.57 and 0.61 mg/kg morphine, respectively), relative to older BALB test mice and B6 test mice at all time points. This observation suggests that relative differences in individual sensitivities to opiates can be highly variable and dynamic during adolescent development. This might be explained by maturation of the opioid reward circuitry that may be differentially influenced by the BALB versus B6 genetic background. Recognition that individual social motivations undergo changes in sensitivity to drugs of abuse may be highly relevant to the generation of new clinical treatments for addiction.
Overall, the present study demonstrates that the endogenous opioid system, as indicated by sensitivity of social interaction to morphine administration, changes with adolescent maturation. Morphine-induced changes in SI are dependent upon the genetic background, suggesting that specific alleles contribute differentially to the interaction between opioid signaling and social behavior. The heightened sensitivity of the adolescent BALB SI phenotype to morphine administration, as it relates to reward in general and social reward in particular, will require additional attention within the context of CPP experiments.
ACKNOWLEDGEMENTS
Funding: This work was supported by an NIH research grant (R01DA022543) and a program-project grant (P30 HD03352) to the Waisman Center. JBP was supported by NIH training grants to the Neuroscience Training Program (T32GM07507) and the Training Program in Emotion Research (T32MH018931) at UW-Madison.
We thank Shannon Nelson, QiLiang Chen, Caitlin Jacowski and Rebecca Foster for their contributions to this project. We are also thankful to Dr. Kim Neve for his advice regarding dose response curve fitting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Belknap JK, Crabbe JC, Riggan J, O'Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:352–358. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Riggan J, Cross S, Young ER, Gallaher EJ, Crabbe JC. Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol Biochem Behav. 1998;59(2):353–360. doi: 10.1016/s0091-3057(97)00421-8. [DOI] [PubMed] [Google Scholar]
- Belzung C, Barreau S. Differences in drug-induced place conditioning between BALB/c and C57Bl/6 mice. Pharmacol Biochem Behav. 2000;65(3):419–423. doi: 10.1016/s0091-3057(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V. Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience. 1997;80(1):17–20. doi: 10.1016/s0306-4522(97)00136-x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002(1–2):151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139(3):877–887. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine- and sucrose-seeking behaviour in response to associated stimuli in rats. Int J Neuropsychopharmacol. 2008;11(1):103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68(4):487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2(8):477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4(2):e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consorti D, Castellano C, Oliverio A, Pavone F. Length of social deprivation differently affects free-choice morphine consumption in C57BL/6J mice. Funct Neurol. 1992;7(4):299–303. [PubMed] [Google Scholar]
- Coudereau JP, Debray M, Monier C, Bourre JM, Frances H. Isolation impairs place preference conditioning to morphine but not aversive learning in mice. Psychopharmacology (Berl) 1997;130(2):117–123. doi: 10.1007/s002130050218. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107(2–3):385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92(1):164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews E, Zimmer A. Modulation of alcohol and nicotine responses through the endogenous opioid system. Prog Neurobiol. 2010;90(1):1–15. doi: 10.1016/j.pneurobio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Elliott K, Hynansky A, Inturrisi CE. Dextromethorphan attenuates and reverses analgesic tolerance to morphine. Pain. 1994;59(3):361–368. doi: 10.1016/0304-3959(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75(1):129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, et al. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29(2):269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Kuzmin AV, van Ree JM. Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol. 2005;15(3):297–303. doi: 10.1016/j.euroneuro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, et al. Brain Mu-Opioid Receptor Binding Predicts Treatment Outcome in Cocaine-Abusing Outpatients. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84(1–2):52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Buckman SG, Wellman PJ, Eitan S. Morphine-induced stereotyped thigmotaxis could appear as enhanced fear and anxiety in some behavioural tests. J Psychopharmacol. 2010a;24(6):875–880. doi: 10.1177/0269881108100797. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Roberts KW, Wellman PJ, Eitan S. Socially induced morphine pseudosensitization in adolescent mice. Behav Pharmacol. 2010b;21(2):112–120. doi: 10.1097/FBP.0b013e328337be25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol T, Ruven S, Van Ree JM, Spruijt BM. Chronic administration of Org2766 and morphine counteracts isolation-induced increase in social interest: implication of endogenous opioid systems. Neuropeptides. 1996;30(3):283–291. doi: 10.1016/s0143-4179(96)90074-8. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14(1):69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32(3):600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. (NIH Publication No. 10-7583) Bethesda, MD: National Institute on Drug Abuse; 2010. [Google Scholar]
- Katz RJ, Schmaltz K. Contribution of social factors to opiate-induced activation in the mouse. Neuropharmacology. 1981;20(4):381–383. doi: 10.1016/0028-3908(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Kissin I, Brown PT, Robinson CA, Bradley EL., Jr Acute tolerance in morphine analgesia: continuous infusion and single injection in rats. Anesthesiology. 1991;74(1):166–171. doi: 10.1097/00000542-199101000-00025. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10(1):47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther. 1984;229(2):481–486. [PubMed] [Google Scholar]
- Lahvis GP, Alleva E, Scattoni ML. Translating Mouse Vocalizations: Prosody and Frequency Modulation. Genes Brain Behav. 2010a doi: 10.1111/j.1601-183X.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvisz GP, Black LM. Social Interactions in the Clinic and the Cage: Toward a More Valid Mouse Model of Autism. 2010:153-192b. [Google Scholar]
- Landauer MR, Balster RL. Opiate effects on social investigatory behavior of male mice. Pharmacol Biochem Behav. 1982;17(6):1181–1186. doi: 10.1016/0091-3057(82)90117-4. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121(3):232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, et al. Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology (Berl) 2009;202(4):589–598. doi: 10.1007/s00213-008-1335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Lopez-Jimenez A, Gorriti MA, de Fonseca FR. Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Curr Drug Targets. 2010;11(4):406–428. doi: 10.2174/138945010790980312. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Harris LS, Smith JE. Potent reinforcing effects of dihydroetorphine in rats. Eur J Pharmacol. 1997;324(2–3):141–145. doi: 10.1016/s0014-2999(97)00074-5. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors and pharmacology. Brain Res. 1987;434(4):397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Meyerson BJ. Comparison of the effects of beta-endorphin and morphine on exploratory and socio-sexual behaviour in the male rat. Eur J Pharmacol. 1981;69(4):453–463. doi: 10.1016/0014-2999(81)90449-0. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J Neurochem. 2001;79(3):626–635. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14(4):327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Bishop P. An autoradiographic map of (3H)diprenorphine binding in rat brain: effects of social interaction. Brain Res Bull. 1981;7(4):405–410. doi: 10.1016/0361-9230(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6(7):661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Najam N, Soares F. Morphine reduces social cohesion in rats. Pharmacol Biochem Behav. 1979;11(2):131–134. doi: 10.1016/0091-3057(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P. Opiates and play dominance in juvenile rats. Behav Neurosci. 1985;99(3):441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2(4):e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Wong JC, Kennedy BC, Lahvis GP. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195(2):239–245. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp GJ, Maes RA, van Ree JM. Disposition of morphine in rat brain: relationship to biological activity. J Pharmacol Exp Ther. 1981;217(1):181–188. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9(1):13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Raz S, Berger BD. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol. 2010;21(1):39–46. doi: 10.1097/FBP.0b013e32833470bd. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185(4):459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Rosow CE, Miller JM, Pelikan EW, Cochin J. Opiates and thermoregulation in mice. I. Agonists. J Pharmacol Exp Ther. 1980;213(2):273–283. [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59(5):415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiol Behav. 1989;46(4):719–723. doi: 10.1016/0031-9384(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4(3):279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. delta-Opioid modulation of social interactions in juvenile mice weaned at different ages. Physiol Behav. 2001;73(3):393–400. doi: 10.1016/s0031-9384(01)00447-4. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26(8):467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31(4):959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008;18(7):519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Kitchen I, Gerrits MA, Spruijt BM, Van Ree JM. Morphine treatment during juvenile isolation increases social activity and opioid peptides release in the adult rat. Brain Res. 1999;830(1):16–23. doi: 10.1016/s0006-8993(99)01330-x. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, et al. Endogenous opioids and reward. Eur J Pharmacol. 2000;405(1–3):89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social isolation and social interaction alter regional brain opioid receptor binding in rats. Eur Neuropsychopharmacol. 1995a;5(2):119–127. doi: 10.1016/0924-977X(95)00010-M. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 1995b;680(1–2):148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33(6):991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528(1–3):119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89(2):188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34(4):912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Ke X, Tan B, Luo X, Xu W, Yang X, et al. Susceptibility to morphine place conditioning: relationship with stress-induced locomotion and novelty-seeking behavior in juvenile and adult rats. Pharmacol Biochem Behav. 2003;75(4):929–935. doi: 10.1016/s0091-3057(03)00172-2. [DOI] [PubMed] [Google Scholar]