Abstract
Cells and tissues counteract insults from exogenous or endogenous carcinogens through the expression of genes encoding antioxidants and phase II detoxifying enzymes regulated by antioxidant response element promoter regions. Nuclear factor-erythroid 2-related factor 2 plays a key role in regulating the antioxidant response elements-target gene expression. Hence, the Nrf2/ARE pathway represents a vital cellular defense mechanism against damage caused by oxidative stress and xenobiotics, and is recognized as a potential molecular target for discovering chemo-preventive agents. Using a stable antioxidant response element luciferase reporter cell line derived from human breast cancer MDA-MB-231 cells combined with a 96-well high-throughput screening system, we have identified a series of plant extracts from the family Lauraceae that harbor Nrf2-inducing effects. These extracts, including Litsea garrettii (ZK-08), Cinnamomum chartophyllum (ZK-02), C. mollifolium (ZK-04), C. camphora var. linaloolifera (ZK-05), and C. burmannii (ZK-10), promoted nuclear translocation of Nrf2, enhanced protein expression of Nrf2 and its target genes, and augmented intracellular glutathione levels. Cytoprotective activity of these extracts against two electrophilic toxicants, sodium arsenite and H2O2, was investigated. Treatment of human bronchial epithelial cells with extracts of ZK-02, ZK-05, and ZK-10 significantly improved cell survival in response to sodium arsenite and H2O2, while ZK-08 showed a protective effect against only H2O2. Importantly, their protective effects against insults from both sodium arsenite and H2O2 were Nrf2-dependent. Therefore, our data provide evidence that the selected plants from the family Lauraceae are potential sources for chemopreventive agents targeting the Nrf2/ARE pathway.
Keywords: Lauraceae, Cinnamomum, Litsea, plant extracts, Nrf2/ARE pathway, chemoprevention
Introduction
Despite the progress in early diagnosis and treatment, cancer is still a major public health problem around the world. Carcinogenesis is recognized as a multiple-step progress that is composed of three typical stages, which includes initiation, promotion, and progression [1]. Intervention of the carcinogenic processes with natural or synthetic chemicals is an effective approach to inhibit the incidence of cancer. Hence, the term “chemoprevention”, coined in the 1970s, is defined as the strategy of blocking or slowing the onset of premalignant tumors with relatively nontoxic chemicals (so-called chemopreventive agents). Chemopreventive agents are able to protect cells and tissues from exogenous or endogenous carcinogens. This might be achieved via the induction of phase II detoxifying and antioxidant enzymes, such as NQO1, γGCS, GST, and HO-1, a process mediated by the ARE located in the promoter region of these genes [2]. Nrf2 is a member of the cap “n” collar subfamily of transcription factors, and contains a basic leucine zipper DNA-binding domain [3]. Under basal conditions, Nrf2 is sequestered by Keap1 in the cytosol, and maintained in a low constitutive level through constant ubiquitination and degradation by the 26S proteasome. Upon the exposure of cells to chemopreventive agents, Nrf2 translocates into the nucleus, binds to the ARE sequence, and activates the transcription of many cytoprotective genes [4,5]. These ARE-containing target genes encode intracellular redox-balancing proteins and phase II detoxifying enzymes, including NQO1, γGCS, and HO-1, that are involved in GSH synthesis, the elimination of ROS, and xenobiotic metabolism [6]. Hence, the Nrf2/ARE pathway represents a vital cellular defense mechanism against damage caused by oxidative stress and xenobiotics, and facilitates cell survival under stressful conditions triggered by toxic insults. Studies with Nrf2-deficient mice highlight the protective role of the Nrf2/ARE pathway against oxidative stress and xenobiotics. Nrf2 null mice are more susceptible to xenobiotics and toxicants compared with wild-type littermate mice [7–10]. Based on the cytoprotective functions of the Nrf2/ARE pathway, it is concluded that Nrf2 activators have the potential to act as chemopreventive agents.
Characterization and isolation of Nrf2-inducing compounds from natural resources represents a rational strategy for chemoprevention. Presently, many natural products have been identified as Nrf2 activators, including SF (cruciferous vegetables) [7,11], curcumin (turmeric roots) [12,13], cinnamaldehyde (cinnamon) [14,15], resveratrol (giant knotweed plant, grapes, and others) [16], (-)-epigallocatechin-3-gallate (green tea) [17], zerumbone (ginger) [18], lycopene (tomato) [19], carnosol (rosemary) [20], and quercetin [21]. Besides these well-studied molecules, discovery of novel plant-derived ingredients with Nrf2-inducing effects is still a research hotspot, and some natural products, covering lignans [22], flavonoids [23,24], terpenoids [25,26], and quinones [27], have recently been identified to be activators of the Nrf2/ARE pathway. Hence, identifying Nrf2 activators from natural resources with a high potency, yet low toxicity profile, is the first critical step in our journey of achieving disease prevention. Cinnamonyl-based secondary metabolites, represented by cinnamaldehyde, are mainly distributed in the genus Cinnamomum (family Lauraceae), and have been verified as an important group of chemopreventive agents for the induction of the Nrf2/ARE pathway [14,15,28]. Considering the phytotaxonomy and biosynthesis, plants belonging to the same family or genus produce secondary metabolites containing similar substructures because of their similarity in the biosynthetic pathways [29]. Therefore, it can be expected that many molecules with similar structures to cinnamaldehyde are to be found within the family Lauraceae derived from homologous biosynthetic reactions, and that these molecules might be capable of activating the Nrf2/ARE pathway. Accordingly, the plants of the family Lauraceae have been collected, and their effects on the Nrf2/ARE pathway have been evaluated. In this study, the cytoprotective properties of the plant extracts from the family Lauraceae have been confirmed using cell-based models, demonstrating that these plants are potential sources for the purification of novel chemopreventive agents targeting the Nrf2/ARE pathway.
Results
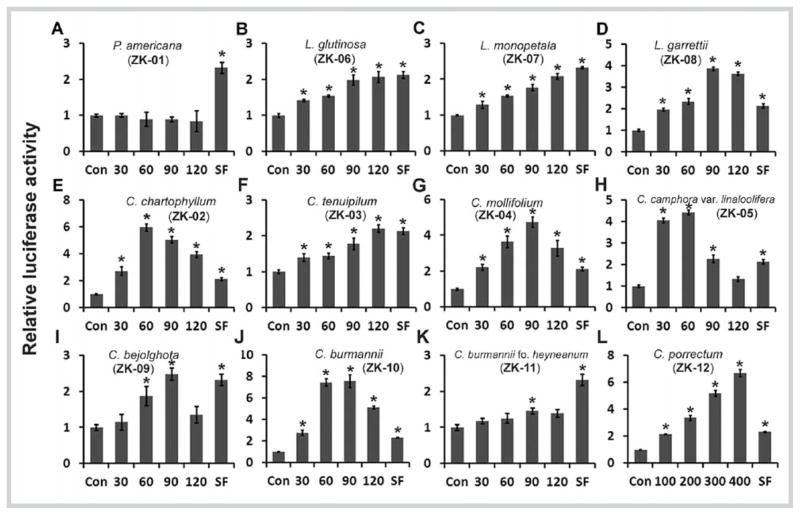
Twelve plant species of the family Lauraceae, covering the genera Persea, Litsea, and Cinnamomum, were extracted with EtOH and subjected to an ARE-luciferase reporter gene assay. Plant species, voucher ID, evaluated plant part, and extract yields are summarized in Table 1. To examine whether these plant extracts activate the Nrf2/ARE pathway, we carried out a high-throughput cell-based screening for plant extracts that are capable of inducing the ARE-dependent response using our established human breast cancer cell line MDA-MB-231-ARE-Luc [30]. The EtOH extracts of L. garrettii (ZK-08), C. chartophyllum (ZK-02), C. mollifolium (ZK-04), C. camphora var. linaloolifera (ZK-05), C. burmannii (ZK-10), and C. porrectum (ZK-12) potently increased the ARE-luciferase activity more than 3-fold over the control under the treatment of nontoxic doses (Fig. 1D,E,G,H,J, and L). Moderate inductions of ARE-luciferase activity (2- to 3-fold) were observed in cells treated with the extracts of L. glutinosa (ZK-06), L. monopetala (ZK-07), C. tenuipilum (ZK-03), and C. bejolghota (ZK-09) (Fig. 1B,C,F, and I). However, the extracts of P. americana (ZK-01) and C. burmannii f. heyneanum (ZK-11) did not induce the ARE-dependent luciferase activity (< 1.5-fold) (Fig. 1A and K). A noticeable decrease of the ARE-luciferase expression was observed with some extracts at higher doses (Fig. 1D,E, and G–J). This is likely due to the cytotoxicity detected when cells were treated with high doses of these extracts (Fig. 2S, Supporting Information)
Table 1.
Plants of the family Lauraceae tested in the present study.
| Genus | Plant species | Abbreviated ID | Voucher ID | Plant part | Extract yields (%) |
|---|---|---|---|---|---|
| Persea | P. americana Mill | ZK-01 | XSBN2011-ZK-01 | Aerial part | 8.50 |
| Litsea | L. glutinosa (Lour.) C.B. Rob. | ZK-06 | XSBN2011-ZK-06 | Aerial part | 4.27 |
| L. monopetala (Roxb.) Pers. | ZK-07 | XSBN2011-ZK-07 | Aerial part | 5.99 | |
| L. garrettii Gamble | ZK-08 | XSBN2011-ZK-08 | Aerial part | 6.46 | |
| Cinnamomum | C. chartophyllum H. W. Li | ZK-02 | XSBN2011-ZK-02 | Aerial part | 6.71 |
| C. tenuipilum Kosterm | ZK-03 | XSBN2011-ZK-03 | Aerial part | 8.00 | |
| C. mollifolium H. W. Li | ZK-04 | XSBN2011-ZK-04 | Aerial part | 6.27 | |
| C. camphora var. linaloolifera Y. Fujita | ZK-05 | XSBN2011-ZK-05 | Aerial part | 5.91 | |
| C. bejolghota (Buch.-Ham.) Sweet | ZK-09 | XSBN2011-ZK-09 | Aerial part | 8.10 | |
| C. burmannii (C.G. et Th. Nees) Bl. | ZK-10 | XSBN2011-ZK-10 | Aerial part | 7.22 | |
| C. burmannii fo. heyneanum (Nees) H.W. Li | ZK-11 | XSBN2011-ZK-11 | Leaf | 8.37 | |
| C. porrectum (Roxb.) Kosterm | ZK-12 | XSBN2011-ZK-12 | Aerial part | 6.72 |
Fig. 1.
Luciferase reporter gene assay of the plant extracts from the family Lauraceae. The stable MDA-MB-231 cells expressing antioxidant response element-luciferase were seeded in 96-well plates and treated with several doses of each plant extract (μg/mL) or sulforaphane (2.5 μM) for 16 hours before analysis of luciferase activity. Results are presented as the mean ± SD (n = 3). * P < 0.05 vs. “Con” group.
Based on their potent induction of the transcriptional activity of Nrf2 in the high-throughput ARE-dependent luciferase assay, the plant extracts of ZK-08, ZK-02, ZK-04, ZK-05, and ZK-10 were further evaluated for their effects on the Nrf2/ARE pathway. First, we investigated the ability of these plant extracts (ZK-08, ZK-02, ZK-04, ZK-05, and ZK-10) to induce the expression of Nrf2 and its downstream genes NQO1 and γGCS in MDA-MB-231 cells. Immunoblot analyses were carried out to measure protein levels of Nrf2, NQO1, and γGCS after the cells were treated with several doses of extracts for 16 hours. The plant extracts of ZK-08, ZK-02, ZK-04, and ZK-05 upregulated the protein levels of Nrf2, NQO1, and γGCS in a dose-dependent manner (Fig. 2A–D). The plant extract of ZK-10 enhanced protein expression of Nrf2 and NQO1, but did not influence the γGCS protein level when cells were treated for 16 hours (Fig. 2E).
Fig. 2.
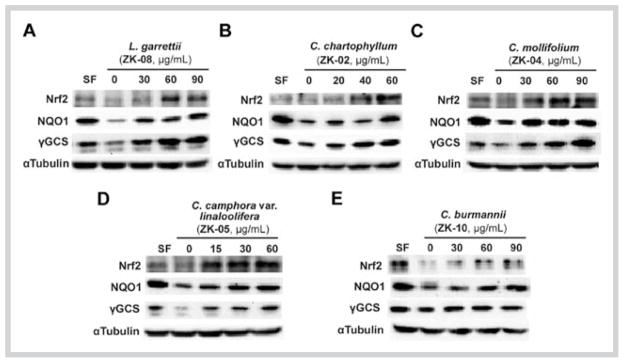
Effects of active plant extracts on protein levels of nuclear factor-erythroid 2-related factor 2 and its downstream genes in MDA-MB-231 cells. Total cell lysates from MDA-MB-231 cells treated with each plant extract or sulforaphane (2.5 μM) for 16 hours were subjected to immunoblot analysis with antinuclear factor-erythroid 2-related factor 2, anti-NAD(P)H quinone oxidoreductase 1, anti-γ-glutamylcysteine synthetase, and anti-tubulin antibodies. Data shown are representative of two independent experiments.
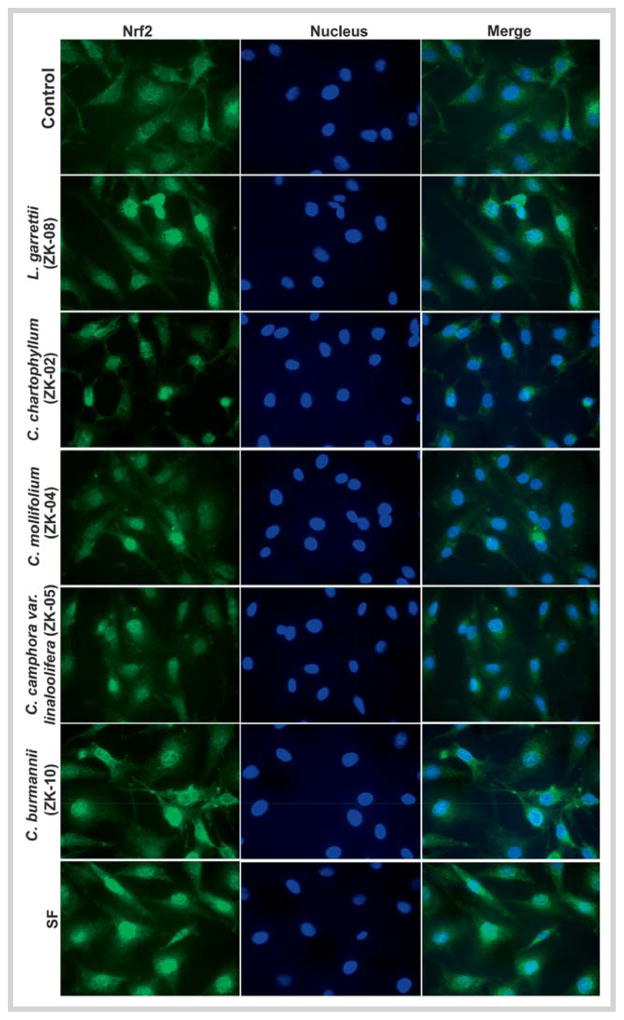
Next, an indirect immunofluorescence analysis was performed to detect Nrf2 localization in response to extract treatment. Not surprisingly, Nrf2 proteins were localized mainly in the cytoplasm in the untreated group, and were accumulated in the nucleus upon exposure to the plant extracts ZK-08, ZK-02, ZK-04, ZK-05, and ZK-10, as well as SF (Fig. 3).
Fig. 3.
Active Lauraceae plant extracts activated the nuclear translocation of nuclear factor-erythroid 2-related factor 2 in MDA-MB-231 cells. The concentrations of extracts were 60 μg/mL for ZK-02 and ZK-05, and 90 μg/mL for ZK-04, ZK-08, and ZK-10. The cells were treated with each active extract or sulforaphane (2.5 μM) for 8 hours, and then subjected to indirect fluorescence staining. Data shown are representative of two independent experiments. (Color figure available online only.)
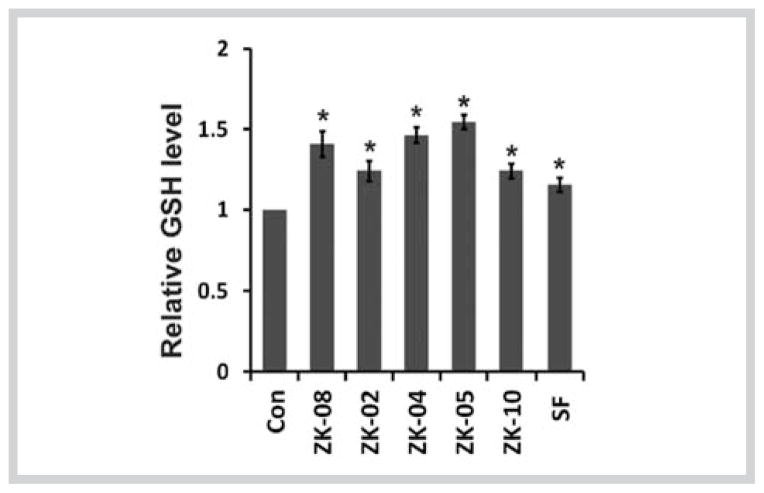
Nrf2-mediated cell protection against oxidative stress is partially achieved by regulating the intracellular redox status [31]. To confirm the induction of the Nrf2/ARE pathway by the plant extracts, we compared intracellular reduced GSH levels in cells left untreated or treated with each plant extract. In normal HBE cells, treatment with each plant extract resulted in a 1.2- to 1.5-fold increase in the reduced GSH level (Fig. 4), implying that these plant extracts augmented the cellular redox capacity.
Fig. 4.
Active Lauraceae plant extracts upregulated intracellular reduced glutathione levels in human bronchial epithelial cells. The concentrations of extracts were 60 μg/mL for ZK-02 and ZK-05, and 90 μg/mL for ZK-04, ZK-08, and ZK-10. The cells were treated with each active plant extract or sulforaphane (2.5 μM) for 24 hours. The reduced gluthatione concentration was measured by the QuantiChrom glutathione assay kit. Data presented are the mean ± SD (n = 3).* P < 0.05 vs. “Con” group.
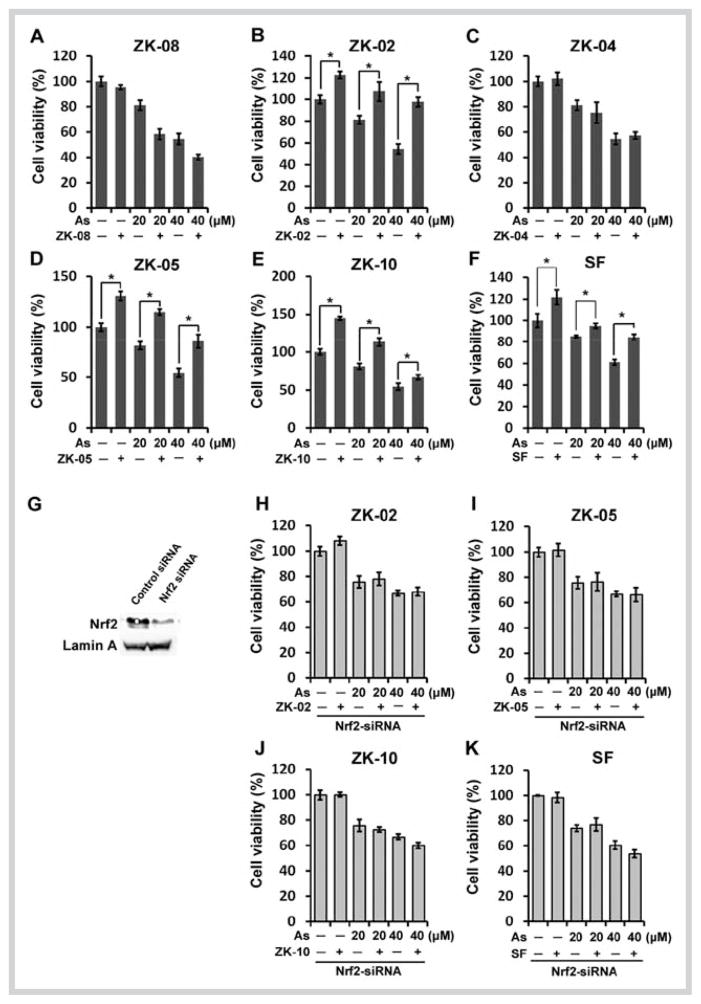
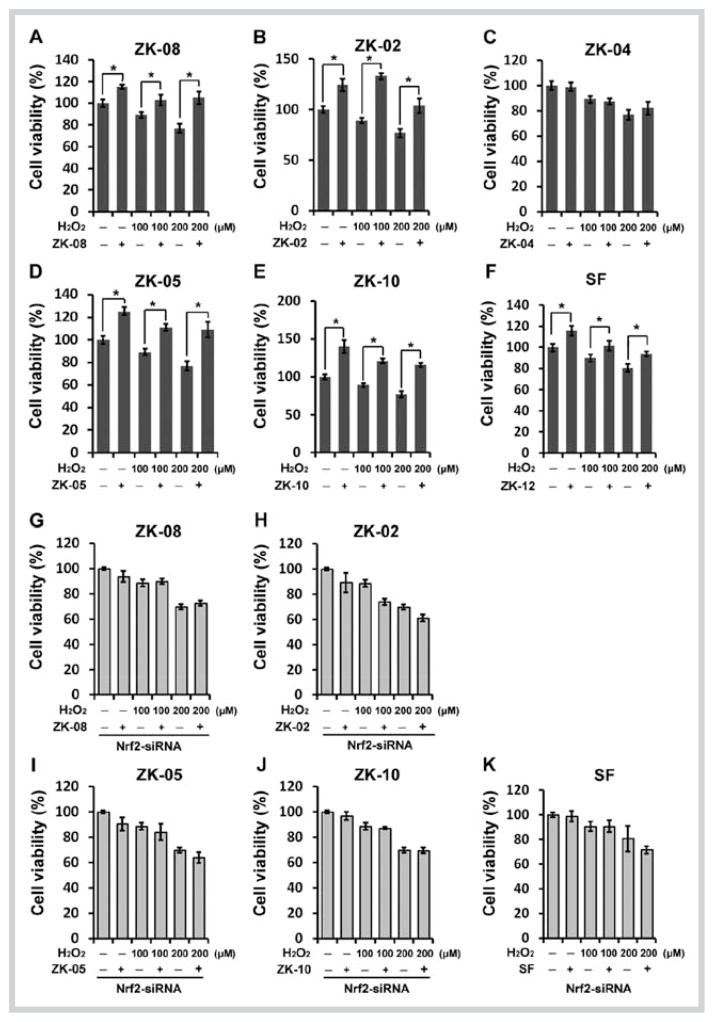
Next, we investigated the cytoprotective effect of these plant extracts against the environmental toxicant and human carcinogen arsenic in the form of As(III). Since the lung is one of the target organs for arsenic-induced cancer, an immortalized primary lung epithelial cell line HBE was used for this study. The HBE cells were either left untreated or were pretreated with several doses of each plant extract for 8 hours, and further co-treated with 20 or 40 μM As(III) for an additional 24 hours before measuring the cell viability using an MTT assay. Pretreatment followed by co-treatment with the extracts of ZK-02, ZK-05, and ZK-10 evidently improved cell survival in response to 20 or 40 μM As(III) treatments (Fig. 5B and D–E). Nevertheless, no protective effects against As(III)-induced cytotoxicity were observed after exposure of HBE cells to the extract of ZK-08 or ZK-04 (Fig. 5A and C). To further evaluate whether protection conferred by the active extracts (ZK-02, ZK-05, and ZK-10) against As(III)-induced toxicity was Nrf2-dependent, we knocked down Nrf2 in HBE cells utilizing siRNA and subsequently measured the cell viability. Immunoblot analysis verified the ability of Nrf2-siRNA to downregulate Nrf2 expression (Fig. 5G). Plant extracts can no longer confer cyto-protection in the cells whose Nrf2 expression was knocked down by Nrf2-siRNA (Fig. 5H–J). Taken together, these results indicated that protection from the plant extracts against As(III)-induced toxicity required the activation of the Nrf2/ARE pathway. We further analyzed the effects of these plant extracts on H2O2-induced toxicity. Consistent with the results of the active extracts ZK-02, ZK-05, and ZK-10 in As(III)-induced toxicity, these four extracts also exerted protective effects against HBE cell damage induced by 100 and 200 μM H2O2 (Fig. 6B and D–E). Notably, the extract of ZK-08 defended cells against H2O2- but not As(III)-induced toxicity (Fig. 6A). The extract of ZK-04 failed to attenuate H2O2-induced toxicity (Fig. 6C), which is consistent with its ineffectiveness against As(III)-induced toxicity. To validate that the protection conferred against H2O2-induced cell death was attributed to the activation of the Nrf2/ARE pathway, a similar cell viability analysis was performed in HBE cells transfected with Nrf2-siRNA. As shown in Fig. 6G–J, the protective effect of the active extract ZK-08, ZK-02, ZK-05, or ZK-10 was lost, verifying that Nrf2 is responsible for the protective effect of each extract.
Fig. 5.
Active Lauraceae plant extracts protected human bronchial epithelial cells against sodium arsenite-induced toxicity. A–F Cell survival in human bronchial epithelial cells untreated or pretreated with ZK-08 (60 μg/mL), ZK-02 (40 μg/mL), ZK-04 (60 μg/mL), ZK-05 (30 μg/mL), ZK-10 (60 μg/mL), and sulforaphane (3.0 μM) for 8 hours, and then treated with 20 or 40 μM sodium arsenite in the absence or presence of half of the pretreated doses of Lauraceae extracts and sulforaphane (2.0 μM) for 24 hours. G Immunoblot analysis of nuclear factor-erythroid 2-related factor 2 protein level in human bronchial epithelial cells 36 hours after transfection with nuclear factor-erythroid 2-related factor 2-small interfering RNA. H–K Cell survival was performed as described in A–F in human bronchial epithelial cells transfected with nuclear factor-erythroid 2-related factor 2-small interfering RNA. Values shown are the mean ± SD of experiments run in triplicate. * P < 0.05 vs. plant extract-treated group.
Fig. 6.
Active Lauraceae plant extracts protected human bronchial epithelial cells against H2O2-induced toxicity. A–F Cell survival in human bronchial epithelial cells untreated or pretreated with ZK-08 (60 μg/mL), ZK-02 (40 μg/mL), ZK-04 (60 μg/mL), ZK-05 (30 μg/mL), ZK-10 (60 μg/mL), and sulforaphane (3.0 μM) for 8 hours, and then treated with 100 or 200 μM H2O2 in the absence or presence of half of the pretreated doses of Lauraceae extracts and sulforaphane (2.0 μM) for 24 hours. G–K Cell survival was performed as described in A–F in human bronchial epithelial cells transfected with nuclear factor-erythroid 2-related factor 2-small interfering RNA. Values shown are the mean ± SD of experiments run in triplicate. *P < 0.05 vs. plant extract-treated group.
Discussion
Carcinogenesis is a complex process in which normal cells are transformed into malignant cancer cells, caused by the exposure of carcinogens. Chemicals that activate signaling pathways involved in the removal of carcinogen metabolism, neutralization of ROS, and repair of DNA damage could be used for chemoprevention to eliminate insult to normal cells, and prevent or reverse carcinogenesis [32]. Induction of phase II detoxifying and antioxidant enzymes, such as NQO1, GST, γGCS, and HO-1, are essential for the elimination of exogenous or endogenous carcinogens [33]. It has been established that the ARE is the key regulatory element for the expression of these aforementioned genes, and the transcription of these genes is activated by the binding of Nrf2 to their respective ARE sequence contained within the promoter region. Indeed, activation of the Nrf2/ARE pathway has been regarded as an effective strategy for preventing or reversing carcinogenesis [2], which has been verified in Nrf2 null mice [7,10,34].
Cinnamon, which originates from the bark of eight Cinnamomum species, has been used in traditional Chinese medicine for improving immunity, promoting blood circulation, and relieving pain. Cinnamon powder is sold as a dietary supplement in many countries to promote sugar metabolism, and maintain heart and circulatory health. Other plants from the family Lauraceae were adopted as antidiabetic and anti-inflammatory drugs in Chinese folk medicine. The benefits reported from traditional uses of these plants might be derived from the activation of the Nrf2/ARE pathway, because of the presence of cinnamonyl-based ingredients [14,15,28,35,36]. Here, we identified several extracts of the family Lauraceae as Nrf2 activators. These plant extracts enhanced the expression of Nrf2 and its downstream antioxidant and phase II enzymes, such as NQO1 and γGCS, and augmented the cellular redox capacity by upregulating GSH levels. More importantly, they were capable of protecting HBE cells against As(III) and H2O2-induced cytotoxicity in an Nrf2-dependent manner.
These plant extracts, with the exception of ZK-01 and ZK-11, induced ARE-dependent luciferase activity (Fig. 1). We further evaluated the plant extracts with Nrf2-inducing effects of greater than 3-fold using immunoblot analysis and immunofluorescence techniques (Figs. 2 and 3). These results convincingly indicated that the plant extracts ZK-08, ZK-02, ZK-04, ZK-05, and ZK-10 were capable of activating the Nrf2/ARE pathway. Importantly, the Nrf2-inducing effects of these plants were not attributed to cinnamaldehyde, as verified by the TLC profile assuring the absence of cinnamaldehyde in these plant extracts (Figs. 3S and 4S, Supporting Information). These results imply that novel Nrf2 activators, other than cinnamaldehyde, exist in these plants. Previous phytochemical investigations of the genera Litsea and Cinnamomum led to the isolation of flavonoids, alkaloids, terpenoids, phenols, and lactone-type ingredients [37]. Taken together, results from the current study combined with analysis of reported structures from these two genera support the notion that these plants contain Nrf2 activators.
GSH, as the most abundant thiol-containing molecule in most cells, plays important roles in the cellular antioxidant defense system [38]. Similar to SF, the plant extracts enhanced the cellular reducing capacity by elevating GSH levels (Fig. 4). Increased GSH levels maintain a reducing environment within cells, and prevent DNA damage and apoptosis caused by insults from oxidants and xenobiotics [39].
In this study, we have demonstrated the effectiveness of plant extracts (ZK-02, ZK-05, and ZK-10) in conferring cytoprotection against As(III) acute toxicity via induction of the Nrf2/ARE pathway (Fig. 5). Arsenic is a human carcinogen, and chronic exposure to arsenic results in the formation of skin, lung, and bladder cancer [40,41]. It has been shown that arsenic exerts its carcinogenic effect through promotion of ROS and RNS generation, DNA damage, and mutagenesis [42–45]. Pretreatment with plant extracts ZK-02, ZK-05, or ZK-10 enhanced cell survival in response to As(III) treatment in a cytotoxicity protection assay. Furthermore, the protective role of these plant extracts is Nrf2-dependent, and their function in suppressing the As(III)-induced toxicity was abolished when the expression of Nrf2 was blocked by Nrf2-siRNA. This result demonstrates the important role of the Nrf2/ARE pathway for eukaryotic cells counteracting oxidative stress, inflammation, and apoptosis caused by arsenic insults [30,34,46,47]. Therefore, the coordinated induction of Nrf2 downstream events including upregulation of phase II detoxifying enzymes and enhancement of cellular reducing capacity, confers protection against As(III)-induced toxicity in HBE cells.
Consistent with the results in the As(III)-toxicity model, the plant extracts ZK-08, ZK-02, ZK-05, and ZK-10 suppressed cytotoxicity in an Nrf2-dependent manner when HBE cells were treated with H2O2 (Fig. 6). ROS, such as H2O2, hydroxyl radicals, and the superoxide ion, are highly reactive, causing damage to cells by oxidizing DNA, lipids, and proteins [48]. They are thereby regarded as potentially mutagenic and carcinogenic substances because of their detrimental behavior on macromolecules [49,50]. Since the elucidation of the pivotal role of the Nrf2/ARE pathway in cellular redox homeostasis, there has been mounting evidence supporting its action in eliminating the harmful effects of ROS [51–54]. Consistently, our study demonstrated that upregulation of Nrf2 and its downstream genes by plant extracts promoted cell viability in response to H2O2 treatment.
In summary, these findings strongly suggest that plants from the family Lauraceae contain ingredients that activate the Nrf2/ARE pathway, and are potential resources for discovering novel chemopreventive agents. Future directions call for an activity-guided purification and structure elucidation of these specific Nrf2-inducing compounds within these extracts.
Materials and Methods
Plant material and extraction
The plants of the family Lauraceae were collected from Xishuangbanna, Yunnan Province, China, in September 2011, and identified by Prof. Lan Xiang, School of Pharmaceutical Sciences, Shandong University. These plants were identified by comparison of their characteristics in plant morphology and taxonomy with those described in Flora of China. Voucher specimens (voucher ID, see Table 1) of the plants have been deposited at the Laboratory of Pharmacognosy, School of Pharmaceutical Sciences, Shandong University.
Crushed aerial parts or leaves of plant materials (50 g) were extracted under reflux for 2 hours with EtOH (Tianjin Fuyu Chemical) (2 × 500 mL), and then EtOH was removed under reduced pressure. The yield of each extract is presented as a percentage of weight of dried plant material, and is summarized in Table 1.
Cell cultures
Human breast carcinoma MDA-MB-231 cells (ATCC) were maintained in Eagle’s MEM (Cellgro) supplemented with 10% FBS (Atlanta Biologicals), 2 mM HEPES (Gibco), and 6 ng/mL bovine insulin (Sigma). HBE cells (ATCC) were cultured in MEM supplemented with 10% FBS. All mammalian cells were incubated at 37°C in a humidified incubator containing 5% CO2.
Cell viability
As(III) (Sigma) and H2O2 (Mallinckrodt Chemicals)-induced toxicity was determined using an MTT (Sigma) assay. HBE cells (4.0 × 104 cells/well) were seeded in a 96-well plate and treated with several doses of extracts for 8 hours, and then co-treated with extracts and As(III) or H2O2 for an additional 24 hours. After the addition of 20 μL 2.0 mg/mL MTT solution, the cells were incubated at 37°C for 3 hours, and absorbance was measured at 570 nm on the Synergy 2 plate reader (BioTeK). All samples were carried out in triplicate for each experiment and the data represent the mean ± SD.
Luciferase reporter gene assay
The MDA-MB-231-ARE-Luc, a stable ARE luciferase reporter cell line previously established in our lab, was used for identification of the plant extracts with Nrf2-inducing effects [30]. The cells were seeded in a 96-well plate and treated with several doses of plant extracts, as well as the positive control SF (2.5 μM, Fig. 1S, Supporting Information) (Sigma, catalog # S6317, ≥ -% purity) for 16 hours. Then, cells were lysed in passive lysis buffer (Promega), and the luciferase activity was measured using an assay buffer [25 mM glycylglycine (Sigma), 15 mM MgSO4 (Sigma), 500 μM ATP (Sigma), 250 μM luciferin (Thermo Scientific), and 250 μM CoA (Sigma)] in the Synergy 2 plate reader (BioTeK). The reporter gene assay was carried out in triplicate, and the data are presented as the mean ± SD.
Immunoblot analysis
Antibodies for Nrf2, NQO1, GCS, and α-tubulin were purchased from Santa Cruz Biotechnology. MDA-MB-231 cells were seeded in D35 dishes and treated with plant extracts for 16 hours. To detect the protein expression in total cell lysates, cells were washed with PBS buffer and lysed in sample buffer [50 mM Tris-HCl (pH 6.8), 2% SDS (J.T. Baker), 10% glycerol (Sigma), 100 mM dithiothreitol (Sigma), 0.1% bromophenol blue (Fisher Biotech)]. After sonication, samples were electrophoresed through SDS-polyacrylamide gels and transferred onto nitrocellulose membranes for immunoblot analysis.
Indirect fluorescence staining
Cells were grown on glass coverslips in D35 dishes. After treatment with extracts for 8 hours, cells were fixed with methanol and washed with PBS. Cells were then incubated with an anti-Nrf2 antibody and Alexa Fluor 488 anti-rabbit IgG antibody (Invitrogen-Molecular Probes), as well as Hoechst 33342 dye. Fluorescent images were visualized with the Zeiss Observer.Z1 microscope with the Slidebook computer program (Intelligent Imaging Inovations).
Glutathione assay
Intracellular reduced GSH concentration was measured using the QuantiChrom GSH assay kit from BioAssay Systems. All the procedures were carried out according to the manufacturer’s instructions. All samples were carried out in triplicate for each experiment and the value from the untreated group was set as 1. The data represent the mean ± SD.
Statistical analysis
ANOVA and post hoc multiple comparison Bonferroni test were used to determine the significant difference between two groups. Results are presented as the mean ± SD. P < 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
This work was supported by the NNSF of China, China Postdoctoral Science Foundation (Nos. 201104602 and 20100471536), Postdoctoral Innovation Foundation of Shandong Province (No. 201002018) to T.S., NNSF (81228023) and NIH grants R01ES015010 and R01CA154377 to D.D.Z., and R21 CA166926 to G.T.W. and D.D.Z.
Abbreviations
- ARE
antioxidant response element
- As(III)
sodium arsenite
- ATCC
American Type Culture Collection
- FBS
fetal bovine serum
- GCLM
glutamate-cysteine ligase, modifier subunit
- γGCS
γ-glutamylcysteine synthetase
- GSH
glutathione
- GST
glutathione S-transferase
- HBE
human bronchial epithelial
- HO-1
heme oxygenase-1
- Keap1
kelch-like ECH-associated protein 1
- MEM
minimal essential medium
- MTT
3-(4,5-dimthylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
nuclear factor-erythroid 2-related factor 2
- PBS
phosphate buffered salt
- ROS
reactive oxygen species
- SF
sulforaphane
- siRNA
small interfering RNA
Footnotes
Conflict of Interest
There are no conflicts of interest to declare.
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
References
- 1.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Sign. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 3.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Tao S, Lian F, Chau BT, Chen J, Sun G, Fang D, Lantz RC, Zhang DD. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol Appl Pharmacol. 2012;265:292–299. doi: 10.1016/j.taap.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu KC, Zhang Y, Klaassen CD. Nrf2 protects against diquat-induced liver and lung injury. Free Radic Res. 2012;46:1220–1229. doi: 10.3109/10715762.2012.700709. [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juge N, Mithen R, Traka M. Molecular basis for chemoprevention by sul-foraphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 13.Garg R, Gupta S, Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo [a] pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 14.Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15:3338–3355. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskó G, Pacher P. Endothelial Nrf2 activation: a new target for resveratrol? Am J Physiol Heart Circ Physiol. 2010;299:H10–H12. doi: 10.1152/ajpheart.00436.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572:245–250. doi: 10.1016/j.febslet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- 20.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, de Galarreta CMR, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 21.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med. 2012;52:1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Hu Q, Zhang DD, Wang L, Lou H, Ren D. Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem Toxicol. 2012;50:1927–1932. doi: 10.1016/j.fct.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Izumi Y, Matsumura A, Wakita S, Akagi K, Fukuda H, Kume T, Irie K, Takada-Takatori Y, Sugimoto H, Hashimoto T. Isolation, identification, and biological evaluation of Nrf2-ARE activator from the leaves of green perilla (Perilla frutescens var. crispa f. viridis) Free Radic Biol Med. 2012;53:669–679. doi: 10.1016/j.freeradbiomed.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Saw CL, Yang AY, Cheng DC, Boyanapalli SS, Su ZY, Khor TO, Gao S, Wang J, Jiang ZH, Kong AN. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25:1574–1580. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischedick JT, Standiford M, Johnson DA, De Vos RC, Todorović S, Banjanac T, Verpoorte R, Johnson JA. Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 2012;78:1725–1730. doi: 10.1055/s-0032-1315241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura T, Shinkai Y, Jiang HY, Iwamoto N, Sumi D, Taguchi K, Yamamoto M, Jinno H, Tanaka-Kagawa T, Cho AK. Initial response and cellular protection through the Keap1/Nrf2 system during the exposure of primary mouse hepatocytes to 1, 2-naphthoquinone. Chem Res Toxicol. 2011;24:559–567. doi: 10.1021/tx100427p. [DOI] [PubMed] [Google Scholar]
- 28.Wondrak GT, Cabello CM, Villeneuve NF, Zhang S, Ley S, Li Y, Sun Z, Zhang DD. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic Biol Med. 2008;45:385–395. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewick PM. Medicinal natural products: a biosynthetic approach. 2. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- 30.Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen W, Li J, Lou H, Wong PK, Zhang DD. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ Health Perspect. 2008;116:1154–1161. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey C, Thimmulappa R, Singh A, Blake D, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 33.Talalay P, De Long M, Prochaska HJ. Molecular mechanisms in protection against carcinogenesis. New York: Plenum Press; 1987. [Google Scholar]
- 34.Tao S, Zheng Y, Lau A, Jaramillo MC, Chau BT, Lantz RC, Wong PK, Wondrak GT, Zhang DD. Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid Redox Signal. 2013;19:1647–1661. doi: 10.1089/ars.2012.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, Holmgren A, Westwell AD. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med. 2010;48:98–111. doi: 10.1016/j.freeradbiomed.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Haan JB. Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes. 2011;60:2683–2684. doi: 10.2337/db11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Combined Chemical Dictionary. Boca Raton: Chapman and Hall/CRC Press; [Accessed May, 2013]. Online database available at: http://www.chemnetbase.com/ [Google Scholar]
- 38.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 39.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Ke Q, Costa M, Shi X. Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem. 2004;255:57–66. doi: 10.1023/b:mcbi.0000007261.04684.78. [DOI] [PubMed] [Google Scholar]
- 41.Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 42.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kB activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 43.Lynn S, Gurr JR, Lai HT, Jan KY. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ Res. 2000;86:514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Jan KY. DNA damage in arsenite-and cadmium-treated bovine aortic endothelial cells. Free Radic Biol Med. 2000;28:55–63. doi: 10.1016/s0891-5849(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 45.Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 46.Jiang T, Huang Z, Chan JY, Zhang DD. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol Appl Pharmacol. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XJ, Sun Z, Chen W, Eblin KE, Gandolfi JA, Zhang DD. Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity. Toxicol Appl Pharmacol. 2007;225:206–213. doi: 10.1016/j.taap.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 49.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14–21. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 51.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 52.Frohlich D, McCabe M, Arnold R, Day M. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 53.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chartoumpekis D, Ziros PG, Psyrogiannis A, Kyriazopoulou V, Papavassiliou AG, Habeos IG. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem Biophys Res Commun. 2010;396:463–466. doi: 10.1016/j.bbrc.2010.04.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.