Abstract
Reliable data is fundamentally important for managing large carnivore populations, and vital for informing hunting quota levels if those populations are subject to trophy hunting. Camera-trapping and spoor counts can provide reliable population estimates for many carnivores, but governments typically lack the resources to implement such surveys over the spatial scales required to inform robust quota setting. It may therefore be prudent to shift focus away from estimating population size and instead focus on monitoring population trend. In this paper we assess the susceptibility of African leopards Panthera pardus to trophy hunting. This has management ramifications, particularly if the use of harvest composition is to be explored as a metric of population trend. We explore the susceptibility of different leopard age and sex cohorts to trophy hunting; first by examining their intrinsic susceptibility to encountering trophy hunters using camera-traps as surrogates, and second by assessing their extrinsic susceptibility using photographic questionnaire surveys to determine their attractiveness to hunters. We show that adult male and female leopards share similar incident rates to encountering hunters but adult males are the most susceptible to hunting due to hunter preference for large trophies. In contrast, sub-adult leopards rarely encounter hunters and are the least attractive trophies. We suggest that our findings be used as a foundation for the exploration of a harvest composition scheme in the Kwazulu-Natal and Limpopo provinces where post mortem information is collected from hunted leopards and submitted to the local provincial authorities.
Introduction
Trophy hunting is a popular wildlife management tool that has potential to contribute to species’ conservation [1, 2]. It can generate important revenue for landowners, contribute to national Gross Domestic Products (GDP), and hunters may enforce anti-poaching and land management approaches that protect wildlife and natural habitat [1]. Trophy hunting differs from other forms of harvest (e.g. for bushmeat or the traditional medicinal trade) in that offtake can be regulated and is typically selective, focusing on individuals with attractive secondary sexual attributes such as large horns, tusks or manes [3–5]. However, when poorly managed (e.g. when young animals are targeted or quotas are too high), trophy hunting can cause social disruption [6], the inheritance of undesirable traits [7] and localized population declines [8,2].
Ideally, trophy hunting quotas should be based on robust population estimates, but wildlife management authorities rarely possess such data because of time, funding and logistical constraints [9]. Accurately estimating population size is particularly challenging for wide-ranging, cryptic species such as large carnivores, which are also those most sensitive to the effects of trophy hunting [10–12]. Often the only data available are post-mortem reports that include the effort and success of hunts, and the sex and estimated age of harvested animals [13], but these age-sex ratios of harvested individuals can provide a useful index of population trend [14, 12]. However, an important caveat of such an approach is that relative susceptibility to hunting varies predictably among age and sex classes. Several factors may influence susceptibility to hunting, most notably the movement patterns of individual animals (‘intrinsic susceptibility’) [12, 15] and their attractiveness to hunters (‘extrinsic susceptibility’) [16]). Susceptibility could also be influenced by the configuration of home-ranges and their consequent accessibility by hunters. We use a combination of camera-trapping, radio-tracking and interview data to assess both the intrinsic and extrinsic susceptibility to hunting of different age and sex classes of African leopards.
Leopards are one of the most sought-after big game trophies; presently, 12 African countries are permitted by the Convention for the International Trade in Endangered Species (CITES) to export a collective 2648 leopard skins (Table 1) procured through trophy hunting each year [17]. Due to a widespread paucity of data on leopard numbers [18], most range states base leopard hunting quotas on expert guesstimates or an over-simplified model that correlated leopard density to rainfall [19] but ignored important factors such as anthropogenic mortality and prey availability [20]. This is of particular concern as leopards are demographically sensitive to over-harvesting, and an increased turnover of adult males may cause inflated rates of infanticide, which are already naturally high in leopard populations [21]. Moreover, leopards are important revenue generators for both photo safari operators [22] and hunters, with leopards contributing 8–20% of gross national trophy hunting income in East and southern Africa [1]. Many African countries already mandate trophy hunters to provide post-hunt data, which include photographic, morphometric and dental information that can be used for accurate ageing and sexing of harvested individuals [23]. We propose that with our estimates of susceptibility to hunting, this would be an important first step to explore the use of harvest composition as an index of leopard population trend. This is a method that has been used with success for the Puma Puma concolor in Wyoming, and we suggest a similar trial in Africa for leopard, a data deficient species across much of its range. As such, harvest composition has the potential to act as a valuable tool for monitoring leopard populations at meaningful management scales to inform conservation decisions.
Table 1. Country size, CITES quota size and mean trophy exports for the 2006–2010 period obtained from the CITES database.
| Country | Country size (km2) | Quota | 5 year mean export |
|---|---|---|---|
| Botswana | 600 370 | 130 | 44 ± 6.69 |
| Central African Republic | 622 984 | 40 | 23.6 ± 6.67 |
| Ethiopia £ | 1 127 127 | 500 | 0 £ |
| Kenya $ | 582 650 | 80 | 0 $ |
| Malawi* | 118 480 | 50 | 0* |
| Mozambique | 801 590 | 120 | 37 ± 2.81 |
| Namibia | 825 418 | 250 | 197.4 ± 32.46 |
| South Africa | 1 219 912 | 150 | 114.2 ± 11.73 |
| Tanzania | 945 203 | 500 | 280.4 ± 28.18 |
| Zambia | 752 614 | 300 | 68.8 ± 6.21 |
| Zimbabwe | 390 580 | 500 | 251.4 ± 11.72 |
| Uganda | 236 040 | 28 | 1 ± 1 |
£ Ethiopia has no records of exports/imports in the CITES database and at present leopards are not hunted there (Hans Bauer, personal.communication).
$ Kenya allow the export of leopard body parts but trophy hunting was outlawed in 1977.
*There were no leopard export records found in the CITES database for the 2006–2010 period in Malawi.
Methods
Ethics Statement
The GPS collar data used in this paper originates in part from previous research implemented by [24]. The original permission to radio-collar leopards on Phinda Game Reserve was granted by the provincial conservation authority, Ezemvelo Kwazulu-Natal Wildlife (permit number HO/4004/07), as well as by & Beyond, the reserve management on Phinda Game Reserve. Ethical clearance for collaring was also provided by the University of Kwazulu-Natal Ethics Committee (approval 051/12/Animal). We also examined Oxford University’s Central University Research Ethics Committee’s (CERU) ethics approval checklist in order to gauge whether our photographic survey work required further ethical audit. According to this checklist our work does not require further ethical audit. Specifically, the names of participants involved in our photographic survey were coded and identifiers removed. Participants were also informed that they would be anonymous and that the results emanating from the survey would be published in a peer-reviewed journal.
Study Area
We collected data on leopard population dynamics in Phinda Private Game Reserve (270 51’ 30” S, 320 19’ 00” E, hereafter Phinda) between April 2002 and December 2012. Phinda forms part of the Munyawana Conservancy (270km2) which is located in the Maputaland-Pondoland-Albany Biodiversity Hotspot in northern KwaZulu-Natal South Africa (Fig 1). Phinda receives an average of 550 mm of rainfall annually, which falls mainly between October and March. Phinda’s vegetation is dominated by several varieties of savanna, but broad-leafed woodland interspersed with grassland is the most common physiognomic form [24]. Forty-two large mammal species have been recorded on Phinda, including important leopard prey such as nyala Tragelaphus angasii, impala Aepyceros melampus and warthog Phacochoerus africanus [24]. The data used in this study originate from a long-term study on the species. The leopards on Phinda have experienced varied levels of persecution as attributed to trophy hunting and we feel it therefore comprises a useful study population for the exploration of hunting susceptibility.
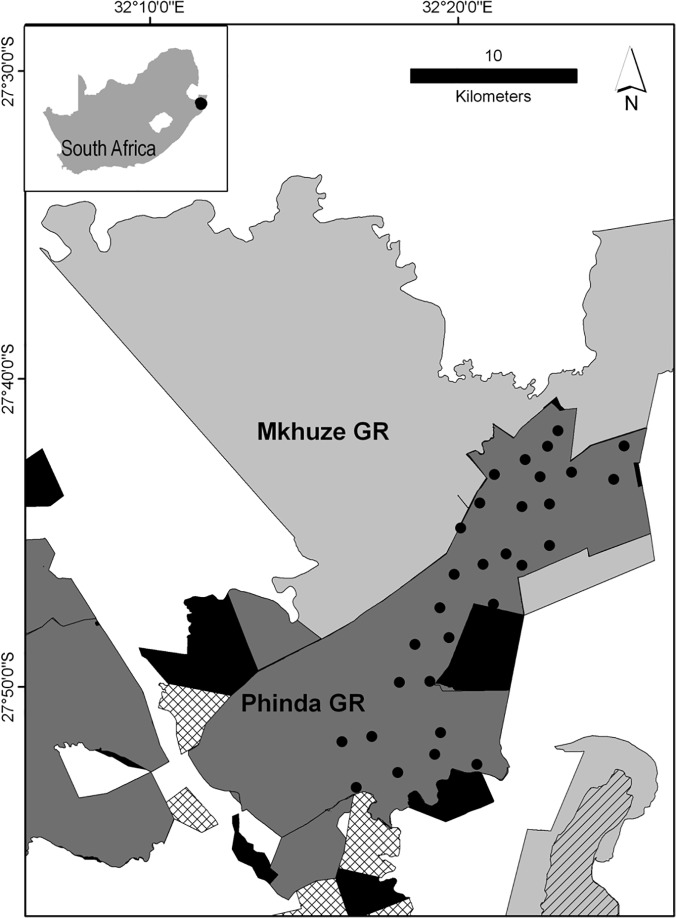
Fig 1. The location of the Phinda Private Game Reserve (Dark grey) with camera-trap stations (black) with the adjacent Mkhuze game reserve (light grey).
Private game ranches (black) and cattle farms (hatched) are also provided for reference.
Likelihood of a leopard encountering a hunter (intrinsic susceptibility)
Leopards are usually hunted from a stationary hide or blind positioned 50–80 m from a bait (typically the carcass of a locally common prey species) hung in a tree [25]. We used camera-trap data of known collared individuals to estimate the relative frequency that different age and sex classes of leopards encounter blinds. Hunters typically drag baits along game trails or dirt roads for 500–1000 m to lure an animal to a blind [25]. The likelihood of an individual responding to a lure may vary in a population [26], but we demonstrated that response rates (as determined by photographic captures) were similar among leopard age and sex classes at our study site [27]. We were therefore confident that the absence of a bait at camera-trap stations was unlikely to affect our cohort-specific encounter rates.
We undertook four 40-day camera-trap surveys in 2005, 2007, 2009 and 2011. All surveys took place during the dry season. We deployed 30 paired stations, comprising 35-mm Deercam Dc300 (Deercam, Park Falls, WI, USA) and Panthera IV digital camera-traps, across 140 km2 [28]. Hunters usually set 3–5 baits during a leopard hunt and the mean size of property on which leopard hunts are conducted in KwaZulu-Natal is roughly 25 km2 (Ezemvelo KwaZulu-Natal Wildlife, unpublished data). Hence, the density of camera-traps in our study area was comparable to the density and spacing of baits deployed during a typical leopard hunt. The distribution of camera-traps also ensured that each leopard was capable of being captured (i.e. ≥4 stations were present in the mean female leopard home-range recorded on Phinda; mean = 29.4±1.5 km2; [29]). Camera-traps were checked every 2–3 days to replace batteries and film, and to download images. Individual leopards were distinguished using their unique pelage patterns [30] and assigned to one of three demographic cohorts based on their morphological characteristics (presence of testes in males, dewlap development, facial scarring and dental wear) [23]: 1) adult males≥3 years; 2) adult females ≥3 years, and 3) or sub-adults of 1–3 years (transients), (Table 2). We grouped male and female sub-adults due to small samples sizes, and because we found no difference in the mean numbers of photographs recorded between sub-adult sexes (Two-sample t-test; d.f = 1; t = 0.88; p = 0.54). If a leopard changed cohort from one survey to the next (i.e. a sub-adult became an adult), it was re-assigned to the appropriate cohort [31].
Table 2. Number of daily telemetry locations, photographic events and the relative time spent in the study area by radio-collared leopards in Phinda Private Game Reserve, South Africa (2005–2011).
| Year | Cohort | Collared individuals | Total number of telemetry locations | Telemetry locations inside study area | Mean relative time inside study area | Number of captures (collared population) | Number of captures (total population) |
|---|---|---|---|---|---|---|---|
| 2005 | ♂ | 4 | 217 | 157 | 72.35 | 14 | 16 |
| ♀ | 5 | 175 | 166 | 94.86 | 18 | 21 | |
| Subs | 2 | 55 | 52 | 94.55 | 2 | 2 | |
| 2007 | ♂ | 1 | 14 | 14 | 100.00 | 2 | 10 |
| ♀ | 4 | 138 | 120 | 86.96 | 11 | 12 | |
| Subs | 6 | 114 | 77 | 67.54 | 12 | 16 | |
| 2009 | ♂ | 0 | NA | NA | NA | 0 | 27 |
| ♀ | 6 | 96 | 88 | 91.67 | 20 | 21 | |
| Subs | 2 | 21 | 20 | 95.24 | 1 | 5 | |
| 2011 | ♂ | 2 | 179 | 144 | 80.45 | 8 | 24 |
| ♀ | 3 | 164 | 158 | 96.34 | 6 | 23 | |
| Subs | 4 | 139 | 90 | 64.75 | 2 | 8 | |
| Total | 39 | 1312 | 1086 | 84.20 | 96 | 185 |
For each of the four camera-trap surveys, we investigated how many times each member of a cohort encountered a camera-trap station, correcting for the proportion of time they spent in the survey area (Table 2). A sample of leopards was radio-collared throughout the study (see [24] for full details of capture and immobilization); hence, we used radio-telemetry data to estimate the proportion of time leopards spent in the survey area. In order to provide sufficient spatial data, we present three months of radio-telemetry data, with each 40-day camera-trap survey in the middle of the three months. We used only one location per individual per day to ensure data from VHF and GPS collared leopards were comparable [32]. We added a buffer equal to the mean camera-trap spacing (1.67 km) to the outermost camera-traps, and divided the number of telemetry locations recorded for each leopard inside the buffered survey area by the total number of telemetry locations collected over the three-month radio-tracking period.
We used a General Linear Model (GLM) to assess cohort-specific encounter rates to camera-traps. We examined the effects that cohort, year and the relative time spent by individual leopards in the survey area had on the total number of captures recorded for each collared leopard. The relative time each leopard spent within the survey area was used as the offset parameter in the model and we censored any animal with <10 telemetry locations for a given 3-month period. To test the robustness of using a minimum threshold of ≥10 locations for each leopard, we created a second model using only animals with ≥30 locations and compared those parameter estimates to the original model. Models were fitted with a negative binomial distribution using the MASS package in R as this provides a better fit for over-dispersed count data when compared to the Poisson distribution [33]. We created four candidate models incorporating cohort, year and offset parameters and assessed them according to Aikaike’s Information Criterion [33].
We hypothesized that adult male leopards would be recorded on camera-traps with a higher frequency than adult females and sub-adults due to (a) males ranging more widely [34, 35] and (b) potential avoidance of trails and roads by females, which are frequently patrolled by adult males [36, 37]. We also hypothesized that due to their use of temporary home ranges [38], sub-adult leopards would be photographed at a lower rate than adults.
Attractiveness of leopards to hunters (extrinsic susceptibility)
We examined the relative attractiveness of leopard cohorts to trophy hunters using a structured questionnaire survey. Survey participants (professional hunters—PH) were randomly selected from the membership lists of national professional hunting associations from the main leopard hunting countries [23]. The questionnaire contained 25 side-profile photographs (minimum 300 dpi) of different known-age and sex leopards from a long-term study in the Sabi Sand Game Reserve, South Africa (S1 Fig). Hunters were asked whether they would choose to shoot a leopard, and on which day during a 14-day hunting safari they would make this decision (14 days is the typical duration for leopard hunts in East and southern Africa; [39]). Responses were placed on an ordinal scale (a) 3 = willing to shoot on the first day of a 14-day safari; (b) 2 = willing to shoot on the seventh day of a 14-day safari; (c) 1 = willing to shoot on the fourteenth day of a 14-day safari (d) 0 = unwilling to shoot the leopard at any stage of the hunt. Scores therefore reflected relative trophy preference, with leopards scoring closer to three more attractive to hunters than those closer to zero. We used the same demographic cohorts used in the camera-trap analysis to build two GLMs: 1) the first examined leopard attributes, i.e. the effects of leopard age and sex on the average score that each photograph achieved. The scores averaged across respondents were treated as continuous. A normal errors model was used; inspection of diagnostic plots indicated no deviation from the assumption of normality and homogeneity of residuals. 2) The second explored GLM the effect of hunter attributes, i.e. the effects of hunter experience, in terms of the number of years that the PH had guided hunts, the number of leopard hunts they had guided, and the number of countries in which they had hunted leopards. For this analysis the average score assigned to photographs across the sample of hunters was used as the response, also using a normal errors model.
We hypothesized that photographs of adult male leopards would be rated more highly higher than those of adult female and sub-adults due to their larger body size [23]. We also hypothesized that more experienced hunters would exercise greater caution in deciding to shoot animals, and therefore record lower scores than less experienced hunters.
All statistical analyses were performed in the R statistical environment [40] and results are provided with means ( ± S.E) and standard error as a measure of precision.
Results
Intrinsic susceptibility
On average, we obtained 19.3±3.9 photographs of adult male leopards, 19.3±0.1 of adult female leopards, and 7.8±2.6 of subadults during each of the four surveys (Table 1). Of these, 35±17%, 75±37%, and 55±28% were of collared individuals respectively. We used 119±7 telemetry locations (range = 14–217) from 9.8±0.7 individuals (range = 8–11) per survey to construct our GLM. We compared four possible candidate models, and used a model which omitted the influence of year as it was not a significant predictor of captures, and yielded a lower AIC score (AIC = 155.63 compared to AIC = 160.31; Table 3) compared to a model with a year effect. Exponentiation of our GLM coefficients indicated that adult male (exponentiated estimate = 3.27 (95% lower = 1.40; 95% upper = 11.30)) and adult female leopards (exponentiated estimate = 4.00 (95% lower = 2.26; 95% upper = 4.69)) were similarly likely to encounter a camera-trap, when accounting for the proportion of time they spent in the survey area (Table 4). Sub-adults were less likely to encounter a camera-trap (exponentiated estimate = 1.64; 95% lower = 0.57; 95% upper = 4.55)) than were either adult males or adult females. Our GLM including only animals with ≥30 telemetry locations performed similarly to the original model, except for sub-adult parameter estimates, which were near significant (S1 Table).
Table 3. Four candidate models with the number of leopard photographic events as the response variable evaluated using AIC criteria.
| Model parameter | Parameters | DF | AIC |
|---|---|---|---|
| Events ~ Cohort + Offset | 2 | 4 | 155.63 |
| Events ~ Cohort + Year + Offset | 3 | 7 | 160.31 |
| Events ~ Cohort | 1 | 5 | 162.10 |
| Events ~ Year + Offset | 2 | 4 | 162.31 |
Table 4. Parameter estimates from a General Linear Model (GLM) examining the intrinsic susceptibility of leopard cohorts to trophy hunting, measured by encounter rates of leopards to camera-traps, which are used as surrogates for hunters.
| Coefficients | Estimate | 2.5% CI | 97.5% CI | Exponentiated estimate | Std.error | z-value | Pr (>|z|) |
|---|---|---|---|---|---|---|---|
| Intercept (Adult females) | -3.42 | -3.79 | -3.06 | 3.27 | 0.19 | -18.36 | <0.005 |
| Adult males | 0.2 | -0.48 | 0.87 | 4.00 | 0.35 | 0.58 | 0.56 |
| Sub-adults | -0.69 | -1.38 | -0.03 | 1.64 | 0.34 | -2.01 | 0.04 |
Residual deviance: 46.24 on 36 d.f
AIC: 155.63
Theta: 3.42
Extrinsic susceptibility
The questionnaire survey was completed by 39 professional hunters that had hunted leopards in Botswana, South Africa, Namibia, Tanzania, Zambia and Zimbabwe (there were no missing responses). Our GLM assessing the relative attractiveness of cohorts to hunters suggested that adult males were the most attractive cohort to hunters (exponentiated estimate = 14.30; 95% lower = 3.86; 95% upper = 52.98))), followed by females (exponentiated estimate = 3.71; 95% lower = 2,05; 95% upper = 6,75)) while sub-adults were least attractive (exponentiated estimate = 2.44; 95% lower = 0.64; 95% upper = 9.30; Table 5). Adult males (mean = 2.66±0.12) scored significantly higher than adult females (mean = 1.31±0.30) and sub-adults (mean = 0.90±0.17; Tukey test p = <0.05).
Table 5. Parameter estimates from a General Linear Model (GLM) examining the extrinsic susceptibility of leopard cohorts to trophy hunting, derived from scores of the willingness of hunters to shoot leopards presented in photographic questionnaire survey.
| Coefficients | Estimate | 2.5% CI | 97.5% CI | Exponentiated estimate | Std.error | t-value | Pr (>|z|) |
|---|---|---|---|---|---|---|---|
| Intercept (Adult females) | 1.31 | 0.72 | 1.91 | 3.71 | 0.29 | 4.6 | <0.005 |
| Adult males | 1.35 | 0.63 | 2.06 | 14.30 | 0.34 | 3.91 | <0.005 |
| Sub-adults | -0.42 | -1.16 | 0.32 | 2.44 | 0.36 | -1.17 | 0.25 |
Residual deviance: 8.96 on 22 d.f
Our GLM examining hunter experience revealed that hunters who had hunted for longer scored significantly lower than less experienced hunters (F(1,33) = 9.18, p = <0.005). Similarly hunters which guided more hunts scored lower than those with fewer hunts (F(2,33) = 3.73, p = 0.05). There was little evidence that the number of countries hunted in affected the scores (F(2,33) = 0.11; p = 0.90).
Discussion
Our results support the hypothesis that leopards exhibit varying susceptibility to trophy hunting. They suggest that adult male and female leopards share a similar risk of encountering a trophy hunter, if we can assume that the likelihood of encountering a camera is a useful surrogate, but that adult males are more attractive as trophies and are thus more susceptible to harvesting. Sub-adult leopards are the least susceptible cohort as they would seldom encounter a hunter and, even if encountered, are less attractive as trophies. Our findings differ from susceptibility indices estimated for puma Puma concolor, which suggest transient males are the most susceptible to harvesting [10]. This highlights the need for species-specific susceptibility estimates, which take into account all factors likely to influence the relative vulnerability of different age and sex classes (e.g. the biology of the target species, harvest methods, etc.). Interestingly, we did not record a marked male-bias in capture rates as documented by other camera-trap studies on leopard [36, 37] and jaguar Panthera onca [41, 42]. We attribute this to the concentration of camera-traps in our survey area, which appear to have captured the movements of adult females satisfactorily [28].
The lower encounter rates recorded for sub-adults are likely due to their spatial patterns. Dispersing sub-adult carnivores (including leopard; [43]) often establish small, temporary home ranges in which they settle for a few months before making a considerable foray. For example [44] found that dispersing Californian pumas occupied home ranges as small as 2% of the size of adult male ranges, sometimes for several months. Such confined movement would strongly reduce the probability of a sub-adult encountering a camera-trap. Exploratory forays by dispersing sub-adults following the abandonment of a temporary home range also make them prone to using areas outside the area sampled by cameras [29].
The relative attractiveness of leopards as trophies appears to be solely a function of their size. Leopards exhibit striking sexual size dimorphism, with adult males typically 60% larger than females [23, 45] and it was thus unsurprising that adult males were preferred by hunters. Adult females and sub-adults, in contrast, are similarly sized [23] and hunters showed no preference for either class. Our results support the findings of [23] which suggested hunters could distinguish mature males but not females and sub-adults. Worryingly, many hunters (87% of respondents) report willingness to hunt a female at some stage during a hunt, even though this is illegal in most countries [17]. Using genetic data, [46] similarly showed that females comprised 27% of 77 leopard trophies hunted in Tanzania between 1995 and 1998, even though only males can be hunted there legally. The hunting of adult females has important ramifications for population viability as they are the key reproductive unit [47] and are typically more difficult to replace than adult males, due to male-biased dispersal [10]. Stipulating (and strictly enforcing) that only mature, adult males can be hunted will essentially eliminate the possibility of hunters mistakenly harvesting females. Restricting offtakes to males aged ≥7 years would further improve the sustainability of trophy hunting, as by this age male leopards have had the opportunity to rear at least one litter to independence which is sufficient to ensure population persistence [8]. We acknowledge that susceptibility is also likely to be affected by a measure of catch per unit effort over a typical 14 day hunt. A professional hunting guide is therefore also likely to influence his/her client, based upon previous experience of how many leopards they saw over previous hunts.
Conclusions
The sustainable management of leopard hunting has previously been hampered by a lack of reliable population data and, as a consequence, quotas are typically set in an arbitrary fashion, sometimes leading to population declines [17].Our findings suggest that African leopards exhibit varying levels of susceptibility to trophy hunting. We suggest that harvest composition may therefore have potential as an index of leopard population trend, especially as the most susceptible cohorts are likely to be removed from populations first (this indeed was the case with puma in Wyoming [10]). In our case, a proportional increase in adult female and sub-adult in the harvest composition would suggest a declining or over-harvest population since the most vulnerable cohort (adult male) should be depleted first. Thus, wildlife agencies can use this to facilitate the adaptive management of leopard hunting, adjusting quotas based on the sex and age of individuals harvested. Similar approaches have been applied successfully in the past to manage the trophy hunting of lions Panthera leo [48] and pumas [10]. For leopards, detailed photographs showing the relative body dimensions and tooth wear (S2 Fig) can be used to age trophies [23], and a small tissue sample collected to molecularly validate gender [49]. It requires that hunters submit accurate and complete data for every leopard harvested, but this can be enforced by authorities using penalties such as a reduction in future quotas or even the confiscation of trophies.
Supporting Information
Respondents were asked whether they would choose to shoot a leopard, and on which day during a 14-day hunting safari they would make this decision: (a) 3 = willing to shoot on the first day of a 14-day safari; (b) 2 = willing to shoot on the seventh day of a 14-day safari; (c) 1 = willing to shoot on the fourteenth day of a 14-day safari (d) 0 = unwilling to shoot the leopard at any stage of the hunt. All photographs are of known sex and age leopards from the Sabi Sand Game Reserve, South Africa.
(PDF)
(TIF)
Model input and structure remained constant for comparison to our model using ≥10 telemetry locations.
(DOCX)
Data used for the examination of intrinsic leopard susceptibility.
(CSV)
Data used for the examination of hunter attributes as part of extrinsic leopard susceptibility.
(CSV)
Data used for the examination of extrinsic susceptibility of leopards to trophy hunting.
(CSV)
Critical code for running leopard susceptibility models in R.
(DOCX)
Acknowledgments
We would like to thank Albert and Didy Hartog, the Timbo-Stichting Foundation and Panthera for funding the study and the provision of materials to perform this research. &Beyond is thanked for access to research sites. The manuscript benefitted from the constructive comments of Matt Hayward and two anonymous reviewers. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by the Panthera Foundation, Albert and Didi Hartog and the Timbo-Stitching Foundation. However the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lindsey PA, Roulet PA, Romañach SS (2007) Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa. Biol Cons 134: 455–469. [Google Scholar]
- 2. Loveridge AJ, Searle AW, Murindagomo F, Macdonald DW (2007) The impact of sport-hunting on the population dynamics of an African lion population in a protected area. Biol Cons 134:548–558. [Google Scholar]
- 3. Crosmary WG, Loveridge AJ, Ndaimani H, Lebel S, Booth V, Côté SD, et al. (2013) Trophy hunting in Africa: long‐term trends in antelope horn size. Anim Conserv 16: 648–660. [Google Scholar]
- 4. Whitman K, Starfield AM, Quadling HS, Packer C (2004) Sustainable trophy hunting of African lions. Nature 428: 175–178. [DOI] [PubMed] [Google Scholar]
- 5. Leader-Williams N, Smith RJ, Walpole MJ (2001) Elephant hunting and conservation. Science 293:2203–2204. [DOI] [PubMed] [Google Scholar]
- 6. Milner-Gulland EJ, Bukreeva OM, Coulson T, Lushchekina AA, Kholodova MV, Bekenov AB, et al. (2003) Reproductive collapse in a harem-breeding ungulate. Nature 422:135 [DOI] [PubMed] [Google Scholar]
- 7. Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M (2003) Undesirable evolutionary consequences of trophy hunting. Nature 426: 655–658. [DOI] [PubMed] [Google Scholar]
- 8. Packer C, Kosmala M, Cooley HS, Brink H, Pintea L, Garshelis D, et al. (2009) Sport hunting, predator control and conservation of large carnivores. PLOS ONE 4: 1–8. 10.1371/journal.pone.0005941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark T, Rutherford M, Casey D (2005) Coexisting with large carnivores: Lessons from Greater Yellowstone New York: Island Press. [Google Scholar]
- 10. Anderson CR, Lindzey FG (2005) Experimental evaluation of population trend and harvest composition in a Wyoming cougar population. Wildlife Soc B 33:179–188. [Google Scholar]
- 11. Lee J, Taylor M (1994) Aspects of the Polar Bear Harvest in the Northwest Territories, Canada. Bears: Their Biology and Management 9: 237–243. [Google Scholar]
- 12.Barnhurst D (1986) Vulnerability of cougars to hunting. M.S thesis. Utah State University, Logan.
- 13. Litvaitis JA, Kane DM (1994) Relationship of hunting technique and hunter selectivity to composition of black bear harvest. Wild Soc Bull 22:604–606. [Google Scholar]
- 14. Bunnefeld N, Baines D, Newborn D, Milner‐Gulland EJ (2009) Factors affecting unintentional harvesting selectivity in a monomorphic species. J Anim Ecol 78: 485–492. 10.1111/j.1365-2656.2008.01500.x [DOI] [PubMed] [Google Scholar]
- 15. Beecham J (1980) Some Population Characteristics of Two Black Bear Populations in Idaho. Bears: Their Biology and Management 4:201–204. [Google Scholar]
- 16. Mysterud A (2011) Selective harvesting of large mammals: how often does it result in directional selection? J App Ecol 48: 827–834. [Google Scholar]
- 17. Balme GA, Hunter LTB, Goodman P, Ferguson H, Craigie J, Slotow R (2010) An adaptive management approach to trophy hunting of leopards (Panthera pardus): a case study from Kwazulu-Natal, South Africa In Macdonald D.W. & Loveridge A.J. (eds) The biology and conservation of wild felids. Oxford: Oxford University Press. [Google Scholar]
- 18.Balme GA, Lindsey PA, Swanepoel LH, Hunter LT (2013) Failure of research to address the rangewide conservation needs of large carnivores: leopards in South Africa as a case study. Cons Lett. 10.1111/conl.12028 [DOI]
- 19.Martin RB, de Meulenaer T (1988) Survey of the Status of the Leopard (Panthera pardus) in Sub-Saharan Africa. Secretariat for the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Lausanne: Switzerland.
- 20. Marker LL, Dickman AJ (2005) Factors affecting leopard (Panthera pardus) spatial ecology, with particular reference to Namibian farmlands. S Afr J Wild Res 35: 105–115. [Google Scholar]
- 21. Balme GA, Hunter LT (2013) Why leopards commit infanticide. Anim Behav 86: 791–799. [Google Scholar]
- 22. Di Minin E, Fraser I, Slotow R, MacMillan DC (2012) Understanding heterogeneous preference of tourists for big game species: implications for conservation and management. Anim Conserv 16: 249–258. [Google Scholar]
- 23. Balme GA, Hunter LTB, Braczkowski AR (2012) Applicability of Age-Based Hunting Regulations for African Leopards. PLOS ONE 7: e35209 doi: 10.1371/ journal.pone.0035209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balme GA, Hunter LTB, Slotow R (2007) Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: prey catchability versus abundance.Anim Behav 74: 589–598. [Google Scholar]
- 25.Grant TL (2012) Leopard population density, home range size and movement patterns in a mixed landuse area of the Mangwe District of Zimbabwe. PhD thesis, Rhodes University: Grahamstown.
- 26. Ferreira SM, Funston PJ (2010) Estimating lion population variables: prey and disease effects in Kruger National Park, S Afr J Wild Res 37: 194–206. [Google Scholar]
- 27.Braczkowski AR, Balme GA, Dickman A, Fattebert J, Dickerson T, Johnson P, et al. (under review) Effects of lures on the rigour and precision of density estimates from a camera-trap survey of a protected leopard Panthera pardus population. PLOS ONE.
- 28. Balme GA, Hunter LTB, Slotow R (2009a) Evaluating methods for counting cryptic carnivores. J Wild Manage 73: 433–441. [Google Scholar]
- 29.Fattebert J (2013). Spatial ecology of a leopard population recovering from over-harvest. PhD thesis. University of Kwazulu-Natal,Kwazulu-Natal.
- 30. Miththapala S, Seidensticker J, Phillips LG, Fernando SBU, Smallwood JA (1989) Identification of individual leopards (Panthera pardus kotiya) using spot pattern variation. J Zool 218: 527–536. [Google Scholar]
- 31. Noyce KV, Garshelis DL, Coy PL (2001) Differential Vulnerability of Black Bears to Trap and Camera Sampling and Resulting Biases in Mark-Recapture Estimates. Ursus 12: 211–226. [Google Scholar]
- 32. Balme GA, Slotow R, Hunter LTB (2010) Edge effects and the impact of non‐protected areas in carnivore conservation: leopards in the Phinda–Mkhuze Complex, South Africa. Anim Conserv 13: 315–323. [Google Scholar]
- 33. Venables WN, Ripley BD (2002) Modern Applied Statistics with S. New York: Springer. [Google Scholar]
- 34. Mizutani F, Jewell PA (1998) Home‐range and movements of leopards (Panthera pardus) on a livestock ranch in Kenya. J Zool 244: 269–286. [Google Scholar]
- 35. Bailey TN (1993) The African leopard: ecology and behavior of a solitary felid New York: Columbia University Press. [Google Scholar]
- 36. Maputla NW, Chimimba CT, Ferreira SM (2013) Calibrating a camera trap–based biased mark–recapture sampling design to survey the leopard population in the N'wanetsi concession, Kruger National Park, South Africa. Afr J Ecol 51: 422–430. [Google Scholar]
- 37. Martins Q, Harris S (2013) Movement, activity and hunting behaviour of leopards in the Cederberg mountains, South Africa. Afr J Ecol 51: 571–579. [Google Scholar]
- 38. Sunquist ME (1983) Dispersal of three radiotagged leopards. J Mammal 64: 337–341. [Google Scholar]
- 39. Booth V (2009) A comparison of the prices of hunting in Southern and Eastern Africa Joint publication of FAO and CIC, Budapest: CIC website. Available: www.cic-wildlife.org/fileadmin/Press/Technical_Series/EN/7.pdf via the internet. Accessed: 2011 Aug. [Google Scholar]
- 40. R Development Core Team (2008) R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna: Austria. [Google Scholar]
- 41. Conde DA, Colchero F, Zarza H, Christensen NL Jr, Sexton JO, Manterola C, et al. (2010) Sex matters: Modeling male and female habitat differences for jaguar conservation. Biol Conserv 143: 1980–1988. [Google Scholar]
- 42.Miller CM, Miller B (2005) Jaguar density in La Selva Maya. Unpublished report. Wildlife Conservation Society, Gallon Jug, Belize.
- 43. Fattebert J, Dickerson T, Balme G, Slotow R, Hunter LTB (2013) Long-distance natal dispersal in leopard reveals potential for a three-country metapopulation. S Afr J Wild Res 43: 61–67. [Google Scholar]
- 44. Beier P (1995) Dispersal of juvenile cougars in fragmented habitat. J Wild Manage 59: 228–237. [Google Scholar]
- 45. Hunter L (2011) A field guide to the carnivores of the world London: New Holland Publishers. [Google Scholar]
- 46. Spong G, Hellborg L, Creel S (2000) Sex ratio of leopards taken in trophy hunting: genetic data from Tanzania. Conserv Genet 1: 169–171. [Google Scholar]
- 47.Daly B, Power J, Camacho G, Traylor-Holzer K, Barber S, Catterall S, et al. (2005) Leopard Panthera pardus population and habitat viability assessment. Proceedings of a workshop of the Conservation Breeding Specialist Group World Conservation Union IUCN Species Survival Commission. Endangered Wildlife Trust, Johannesburg.
- 48.Lindsey PA, Balme GA, Funston P, Henschel P, Hunter LTB, Madzikanda H, et al. (2013)The trophy hunting of African lions: current extent and management of the practice and interventions necessary to improve sustainability. PLOS ONE: e73808. 10.1371/journal.pone.0073808 [DOI] [PMC free article] [PubMed]
- 49. Dutta T, Sharma S, Maldonado JE, Wood TC, Seidensticker J (2012) A reliable method for individual identification and gender determination of wild leopards (Panthera pardus fusca) using non-invasive samples. Conserv Genet Resources 4:665–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Respondents were asked whether they would choose to shoot a leopard, and on which day during a 14-day hunting safari they would make this decision: (a) 3 = willing to shoot on the first day of a 14-day safari; (b) 2 = willing to shoot on the seventh day of a 14-day safari; (c) 1 = willing to shoot on the fourteenth day of a 14-day safari (d) 0 = unwilling to shoot the leopard at any stage of the hunt. All photographs are of known sex and age leopards from the Sabi Sand Game Reserve, South Africa.
(PDF)
(TIF)
Model input and structure remained constant for comparison to our model using ≥10 telemetry locations.
(DOCX)
Data used for the examination of intrinsic leopard susceptibility.
(CSV)
Data used for the examination of hunter attributes as part of extrinsic leopard susceptibility.
(CSV)
Data used for the examination of extrinsic susceptibility of leopards to trophy hunting.
(CSV)
Critical code for running leopard susceptibility models in R.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.