Abstract
The association between the disease-free interval (DFI) and survival after a locoregional recurrence (LRR) or second primary (SP) breast cancer remains uncertain. The objective of this study is to clarify this association to obtain more information on expected prognosis. Women first diagnosed with early breast cancer between 2003–2006 were selected from the Netherlands Cancer Registry. LRRs and SP tumours within five years of first diagnosis were examined. The five-year period was subsequently divided into three equal intervals. Prognostic significance of the DFI on survival after a LRR or SP tumour was determined using Kaplan-Meier estimates and multivariable Cox regression analysis. Follow-up was complete until January 1, 2014. A total of 37,278 women was included in the analysis. LRRs or SP tumours were diagnosed in 890 (2,4%) and 897 (2,4%) respectively. Longer DFI was strongly and independently related to an improved survival after a LRR (long versus short: HR 0.65, 95% CI 0.48–0.88; medium versus short HR 0.81, 95% CI 0.65–1.01). Other factors related to improved survival after LRR were younger age (<70 years) and surgical removal of the recurrence. No significant association was found between DFI and survival after SP tumours. This is the first study to explore the association between the DFI and survival after recurrence in a nationwide population-based cancer registry. The DFI before a LRR is an independent prognostic factor for survival, with a longer DFI predicting better prognosis.
Introduction
Breast cancer-related mortality is decreasing in many countries because of earlier diagnosis and improved treatment modalities [1,2]. After treatment patients receive follow-up care to improve life expectancy by detecting recurrence of breast cancer in an asymptomatic stage. Recurrence of breast cancer can be a local recurrence (LR), regional recurrence (RR), second primary (SP) tumour or distant metastasis (DM). Early detection and treatment of DM does not lead to better outcomes and is therefore not an aim of follow-up care. Presence of a LR and/or RR can be defined as locoregional recurrence (LRR). The majority of LRRs are diagnosed within five years of the primary tumour [3]. LRR rates vary between 3% for patients with stage T1N0 who underwent mastectomy to 13% for patients with nodal involvement and breast-conserving surgery [4–6]. SP breast cancer (diagnosed at least three months from the primary tumour diagnosis [7] and with different primary location and morphology) occurs in 2–6% of the patients [8–12].
The time between treatment of the primary tumour and the detection of a recurrence is known as the disease-free interval (DFI). The effect of the DFI on the prognosis after a LRR or SP tumour is not well established. Previous reports defined short DFI as the first five years after the primary tumour and long as the following years [13–16], or results were based on a single institution or small patient series [17–20]. If the distinction is made of a short DFI being within the first five years after diagnosis and a long DFI the following years, a long DFI is associated with favourable outcomes [13–16]. In a study with 391 women treated with breast-conserving surgery and radiotherapy Fredriksson et al. [21] also observed that a short DFI before a LR has a negative impact on survival. Yet based on 133 patients from two randomised trials Van Tienhoven et al. [3] found the DFI to be prognostic only for the time to subsequent recurrence and not an independent prognostic factor for survival. The aim of this study was to explore the relation between the DFI and survival after a LRR or SP tumour within five years of treatment of the primary breast cancer, based on a nationwide population.
Patients and Methods
Study population
Patients were selected from the Netherlands Cancer Registry (NCR), a nationwide population-based registry, which has recorded almost all newly diagnosed tumours since 1989. Notifications are obtained from the nationwide Pathology Automated Archives (PALGA). Specially trained registration clerks record the information on patient, tumour and treatment characteristics directly from the patient files, as well as data concerning recurrences within the first five years following primary breast cancer. The information from the patient records was anonymized and de-identified prior to analysis by the Comprehensive Cancer Centre the Netherlands (IKNL). The authors had consent from the Advisory Committee of the IKNL. The study did not need approval of the Medical Ethical Committee (METc), while there was no direct patient contact and according to local regulations in the Netherlands (WMO) only studies with high burden for patients have to be reviewed.
Women diagnosed with primary invasive breast cancer between 2003 and 2006 without DM, previous, or synchronous tumours (diagnosed within three months after the first tumour [7]), treated with curative intent and without neo-adjuvant systemic treatment were selected from the registry (N = 37,278). Patients were treated with curative intent if the tumour was surgically removed and no macroscopic residual disease was left. In case of microscopic residue, the patient should have had adjuvant treatment. Of the selected patients, 1,787 (4.8%) developed a LRR or SP tumour within the first five years following primary breast cancer treatment. Only first or synchronous LRRs or SP tumours were considered.
Statistical analyses
The DFI was measured from the date of final surgery of the primary tumour to the date of LRR or SP tumour diagnosis. To analyse the effect of the DFI on survival after a LRR or SP tumour, the five year period after the primary breast cancer was divided into three equal intervals: (1) patients with a LRR or SP tumour diagnosed within 608 days were considered to have a short DFI; (2) between 609–1217 days a medium DFI; and (3) after 1217 days a long DFI. Overall survival time was measured from the date of final surgery of the primary tumour to death or the end of the study period (January 1, 2014) and was used for the comparison with women without a recurrence. Survival after the LRR or SP tumour was used for the comparisons between the different DFI categories and determined from the date of diagnosis of the LRR or SP tumour until death or the end of the study period. Follow-up was complete by linkage with the Dutch municipal civil registry until January 1, 2014, after which surviving patients were censored.
The patient, tumour, and treatment characteristics shown in Tables 1 and 2 were assessed for their influence on survival after a LRR or SP tumour using Kaplan-Meier estimates and the log-rank test for equality of survivor functions. To reveal differences in the characteristics between categorical variables, χ2 tests were used. Variables with probability values <0.1 on the log-rank test were included in the multivariable analysis. In the Cox proportional hazards model backward selection was used to find the variables with significant influence on survival after the LRR or SP tumour; p-values of <0.05 were considered statistically significant. This led to inclusion of the following variables: DFI category, type of recurrence, age, tumour grade according to Bloom and Richardson [22], size, lymph node status and hormone receptor status (ER and PR) of the primary tumour, and surgical removal of the recurrence. Interaction between the variables was tested by adding interaction terms into the model. The Cox proportional hazards assumption was tested both numerically and graphically. Stratification into short, medium and long DFIs and stratification for LRRs and SP tumours was performed to reveal possible differences in the influence of the covariates for the different categories.
Table 1. Patient and primary tumour characteristics, for LRRs stratified by length of the DFI and tested for differences between the DFI groups.
| Without LRR/SP | SP | LRR | Length of DFI | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short | Medium | Long | |||||||||||
| % | N | % | N | % | N | % | N | % | N | % | |||
| Total | 35,491 | 897 | 890 | 299 | 348 | 243 | |||||||
| Age category | 0.034 | ||||||||||||
| <70 | 77.3 | 713 | 79.5 | 690 | 77.5 | 221 | 73.9 | 267 | 76.7 | 202 | 83.1 | ||
| ≥70 | 22.7 | 184 | 20.5 | 200 | 22.5 | 78 | 26.1 | 81 | 23.3 | 41 | 16.9 | ||
| Histologic type | 0.048 | ||||||||||||
| Ductal | 79.4 | 672 | 74.9 | 740 | 83.1 | 263 | 88.0 | 288 | 82.8 | 189 | 77.8 | ||
| Lobular | 10.7 | 107 | 11.9 | 82 | 9.2 | 20 | 6.7 | 29 | 8.3 | 33 | 13.6 | ||
| Mixed | 4.2 | 54 | 6.0 | 27 | 3.0 | 5 | 1.7 | 13 | 3.7 | 9 | 3.7 | ||
| Other | 5.7 | 64 | 7.1 | 41 | 4.6 | 11 | 3.7 | 18 | 5.2 | 12 | 4.9 | ||
| Grade | <0.001 | ||||||||||||
| I,II | 66.9 | 644 | 76.6 | 440 | 54.0 | 115 | 41.8 | 167 | 52.5 | 158 | 71.2 | ||
| III | 33.1 | 197 | 23.4 | 375 | 46.0 | 160 | 58.2 | 151 | 47.5 | 64 | 28.8 | ||
| Unknown | 56 | 75 | 24 | 30 | 21 | ||||||||
| Tumour size | <0.001 | ||||||||||||
| ≤2 cm | 61.1 | 643 | 72.3 | 469 | 53.7 | 121 | 41.4 | 194 | 56.4 | 154 | 64.7 | ||
| >2 cm | 38.8 | 246 | 27.7 | 405 | 46.3 | 171 | 58.6 | 150 | 43.6 | 84 | 35.3 | ||
| Unknown | 8 | 16 | 7 | 4 | 5 | ||||||||
| Multifocal | 0.238 | ||||||||||||
| No | 84.8 | 558 | 81.8 | 507 | 79.1 | 184 | 80.7 | 187 | 75.7 | 136 | 81.9 | ||
| Yes | 15.2 | 124 | 18.2 | 134 | 20.9 | 44 | 19.3 | 60 | 24.3 | 30 | 18.1 | ||
| Unknown | 215 | 249 | 71 | 101 | 77 | ||||||||
| Lymph node status | 0.025 | ||||||||||||
| Negative | 61.3 | 660 | 74.7 | 503 | 58.1 | 155 | 53.1 | 189 | 56.3 | 159 | 66.8 | ||
| 1–3 positive | 27.5 | 166 | 18.8 | 244 | 28.2 | 90 | 30.8 | 100 | 29.8 | 54 | 22.7 | ||
| >3 positive | 11.2 | 58 | 6.6 | 119 | 13.7 | 47 | 16.1 | 47 | 14.0 | 25 | 10.5 | ||
| Unknown | 13 | 24 | 7 | 12 | 5 | ||||||||
| ER status | <0.001 | ||||||||||||
| Negative | 18.8 | 121 | 17.3 | 219 | 31.9 | 116 | 48.9 | 75 | 28.3 | 28 | 15.1 | ||
| Positive | 81.2 | 578 | 82.7 | 468 | 68.1 | 121 | 51.1 | 190 | 71.7 | 157 | 84.9 | ||
| Unknown | 198 | 203 | 62 | 83 | 58 | ||||||||
| PR status | <0.001 | ||||||||||||
| Negative | 33.7 | 223 | 31.9 | 330 | 48.7 | 156 | 66.7 | 120 | 46.2 | 54 | 29.5 | ||
| Positive | 66.3 | 475 | 68.1 | 347 | 51.3 | 78 | 33.3 | 140 | 53.8 | 129 | 70.5 | ||
| Unknown | 199 | 213 | 65 | 88 | 60 | ||||||||
| Her2-Neu status | 0.347 | ||||||||||||
| Negative | 85.2 | 346 | 88.7 | 278 | 82.7 | 92 | 81.4 | 113 | 80.7 | 73 | 88.0 | ||
| Positive | 14.8 | 44 | 11.3 | 58 | 17.3 | 21 | 18.6 | 27 | 19.3 | 10 | 12.0 | ||
| Unknown | 507 | 554 | 186 | 208 | 160 | ||||||||
Abbreviations: LRR = locoregional recurrence, SP = second primary, DFI = disease-free interval, ER = oestrogen receptor, PR = progesterone receptor, Her2-Neu = human epidermal growth factor receptor 2
Table 2. Primary treatment and recurrence characteristics, for LRRs stratified by length of the DFI and tested for differences between the DFI groups.
| Without LRR/SP | SP | LRR | Length of DFI | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short | Medium | Long | ||||||||||||
| % | N | % | N | % | N | % | N | % | N | % | ||||
| Number of surgeries | 0.684 | |||||||||||||
| 1 | 88.9 | 766 | 85.4 | 780 | 87.6 | 259 | 86.6 | 303 | 87.1 | 218 | 89.7 | |||
| 2 | 10.5 | 124 | 13.8 | 100 | 11.2 | 36 | 12.0 | 40 | 11.5 | 24 | 9.9 | |||
| ≥3 | 0.6 | 7 | 0.8 | 10 | 1.1 | 4 | 1.3 | 5 | 1.4 | 1 | 0.4 | |||
| Type of surgery | <0.001 | |||||||||||||
| Breast conserving | 56.3 | 517 | 57.6 | 395 | 44.4 | 95 | 31.8 | 156 | 44.8 | 144 | 59.3 | |||
| Non-breast conserving | 43.5 | 380 | 42.4 | 495 | 55.6 | 204 | 68.2 | 192 | 55.2 | 99 | 40.7 | |||
| Time from incidence to last surgery | 0.145 | |||||||||||||
| <30 days | 74.0 | 619 | 69.0 | 637 | 71.6 | 207 | 69.2 | 250 | 71.8 | 180 | 74.1 | |||
| 30–60 days | 22.0 | 223 | 24.9 | 207 | 23.3 | 69 | 23.1 | 82 | 23.6 | 56 | 23.0 | |||
| >60 days | 4.0 | 55 | 6.1 | 46 | 5.2 | 23 | 7.7 | 16 | 4.6 | 7 | 2.9 | |||
| Axillary lymph node dissection | 0.004 | |||||||||||||
| No | 49.4 | 545 | 60.8 | 425 | 47.8 | 126 | 42.1 | 162 | 46.6 | 137 | 56.4 | |||
| Yes | 50.7 | 352 | 39.2 | 465 | 52.2 | 173 | 57.9 | 186 | 53.4 | 106 | 43.6 | |||
| Chemotherapy | 0.003 | |||||||||||||
| No | 64.1 | 692 | 77.1 | 579 | 65.1 | 177 | 59.2 | 224 | 64.4 | 178 | 73.3 | |||
| Yes | 35.9 | 205 | 22.9 | 311 | 34.9 | 122 | 40.8 | 124 | 35.6 | 65 | 26.7 | |||
| Radiotherapy | <0.001 | |||||||||||||
| No | 34.3 | 313 | 34.9 | 447 | 50.2 | 184 | 61.5 | 174 | 50.0 | 89 | 36.6 | |||
| Yes | 65.7 | 584 | 65.1 | 443 | 49.8 | 115 | 38.5 | 174 | 50.0 | 154 | 63.4 | |||
| Hormone therapy | 0.054 | |||||||||||||
| No | 58.2 | 682 | 76.0 | 643 | 72.2 | 231 | 77.3 | 245 | 70.4 | 167 | 68.7 | |||
| Yes | 41.8 | 215 | 24.0 | 247 | 27.8 | 68 | 22.7 | 103 | 29.6 | 76 | 31.3 | |||
| Length of DFI | ||||||||||||||
| Short | na | 248 | 27.6 | na | na | na | na | |||||||
| Medium | 334 | 37.2 | ||||||||||||
| Long | 315 | 35.1 | ||||||||||||
| Type of recurrence | 0.378 | |||||||||||||
| LR | na | na | 584 | 65.6 | 184 | 61.5 | 233 | 67.0 | 167 | 68.7 | ||||
| RR | 240 | 27.0 | 88 | 29.4 | 93 | 26.7 | 59 | 24.3 | ||||||
| Synchronous LRRs | 66 | 7.4 | 27 | 9.0 | 22 | 6.3 | 17 | 7.0 | ||||||
| Surgery of LRR/SP | 0.001 | |||||||||||||
| No | na | 38 | 4.2 | 368 | 41.3 | 147 | 49.2 | 138 | 39.7 | 83 | 34.2 | |||
| Yes | 859 | 95.8 | 522 | 58.7 | 152 | 50.8 | 210 | 60.3 | 160 | 65.8 | ||||
| Chemotherapy of LRR/SP | 0.172 | |||||||||||||
| No | na | 705 | 78.6 | 625 | 70.2 | 198 | 66.2 | 250 | 71.8 | 177 | 72.8 | |||
| Yes | 192 | 21.4 | 265 | 29.8 | 101 | 33.8 | 98 | 28.2 | 66 | 27.2 | ||||
| Radiotherapy of LRR/SP | <0.001 | |||||||||||||
| No | na | 467 | 52.1 | 432 | 48.5 | 118 | 39.5 | 172 | 49.4 | 142 | 58.4 | |||
| Yes | 430 | 47.9 | 458 | 51.5 | 181 | 60.5 | 176 | 50.6 | 101 | 41.6 | ||||
| Hormone therapy of LRR/SP | 0.001 | |||||||||||||
| No | na | 597 | 66.6 | 562 | 63.1 | 213 | 71.2 | 211 | 60.6 | 138 | 56.8 | |||
| Yes | 300 | 33.4 | 328 | 36.9 | 86 | 28.8 | 137 | 39.4 | 105 | 43.2 | ||||
Abbreviations: LRR = locoregional recurrence, SP = second primary, LR = local recurrence, RR = regional recurrence, na = not applicable.
Missing values from the variables included in the Cox proportional hazards model were multiple imputed using chained equations [23, 24]. The HER2neu status had a relatively high percentage of missing values (60%) while it has only been registered on a nation-wide basis since 2004. Missing values were considered to occur randomly, which validates the use of imputation. All analyses were performed using STATA version 12 software.
Results
Characteristics
Of the 1,787 patients with a recurrence, 33% developed a LR, 13% a RR, 4% synchronous LRRs and 50% SP breast cancer. Almost all of the SP tumours (95%) were contralateral. Median length of the DFI after treatment of the primary tumour was 2.5 years. Compared to women with LRRs, women with SP tumours had less often ductal primary tumours, had a smaller primary tumour with a lower grade, less nodal involvement and more often a positive hormone status (both ER and PR).
Treatment
The treatment of the LRR appeared to diverge from the treatment of the primary tumour (Table 3). Of the women with a LRR, 59% received surgery; 76% of the women with a LR and 15% with a RR. The patients without surgery for their LRR were treated with radiotherapy in 59% of the cases. In contrast, the treatment of SP tumours only diverged from the treatment of the primary tumour with regard to the same type of therapy (Table 4). The SP tumours were surgically removed in 96% of the cases. Differences in treatment of LRRs were also found when comparing the DFI groups: women with a LRR and a short DFI received less surgery, more radiotherapy and less hormone therapy compared to women with a medium or long DFI (Table 2).
Table 3. Treatment of the primary tumour against the treatment of LRRs.
| Primary tumour | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast-conserving surgery | Chemotherapy | Radiotherapy | Hormone therapy | ||||||
| LRR | No | Yes | No | Yes | No | Yes | No | Yes | |
| (n = 506) | (n = 395) | (n = 589) | (n = 312) | (n = 447) | (n = 454) | (n = 649) | (n = 252) | ||
| Surgery | No | 29%* | 13%* | 24%* | 18%* | 24%* | 18%* | 28%* | 13%* |
| (n = 385) | |||||||||
| Yes | 27%* | 32%* | 41%* | 17%* | 26%* | 32%* | 44%* | 15%* | |
| (n = 516) | |||||||||
| Chemotherapy | No | 40% | 30% | 51%* | 19%* | 38%* | 32%* | 49%* | 21%* |
| (n = 682) | |||||||||
| Yes | 16% | 14% | 14%* | 16%* | 12%* | 17%* | 23%* | 7%* | |
| (n = 219) | |||||||||
| Radiotherapy | No | 19%* | 30%* | 33%* | 15%* | 17%* | 32%* | 37% | 12% |
| (n = 457) | |||||||||
| Yes | 37%* | 15%* | 32%* | 20%* | 33%* | 18%* | 36% | 16% | |
| (n = 444) | |||||||||
| Hormone therapy | No | 34% | 29% | 36%* | 27%* | 29%* | 34%* | 49%* | 14%* |
| (n = 589) | |||||||||
| Yes | 22% | 15% | 29%* | 8%* | 21%* | 16%* | 23%* | 14%* | |
| (n = 312) | |||||||||
* indicates a significant difference Abbreviations: LRR = locoregional recurrence.
Table 4. Treatment of the primary tumour against the treatment of SP tumours.
| Primary tumour | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast-conserving surgery | Chemotherapy | Radiotherapy | Hormone therapy | ||||||
| SP | No | Yes | No | Yes | No | Yes | No | Yes | |
| (n = 299) | (n = 446) | (n = 585) | (n = 160) | (n = 248) | (n = 497) | (n = 585) | (n = 160) | ||
| Surgery | No | 3%* | 1%* | 4% | 1% | 3%* | 2%* | 3% | 1% |
| (n = 34) | |||||||||
| Yes | 39%* | 56%* | 73% | 22% | 32%* | 63%* | 73% | 23% | |
| (n = 711) | |||||||||
| Chemotherapy | No | 34% | 44% | 64%* | 14%* | 29% | 50% | 60%* | 19%* |
| (n = 551) | |||||||||
| Yes | 8% | 13% | 13%* | 9%* | 6% | 15% | 16%* | 5%* | |
| (n = 194) | |||||||||
| Radiotherapy | No | 33%* | 19%* | 39% | 13% | 28%* | 25%* | 37%* | 15%* |
| (n = 373) | |||||||||
| Yes | 9%* | 38%* | 38% | 10% | 7%* | 41%* | 39%* | 9%* | |
| (n = 372) | |||||||||
| Hormone therapy | No | 27% | 39% | 51% | 15% | 22% | 45% | 53%* | 14%* |
| (n = 477) | |||||||||
| Yes | 15% | 18% | 26% | 7% | 13% | 20% | 24%* | 10%* | |
| (n = 268) | |||||||||
* indicates a significant difference. Abbreviations: SP = second primary.
Survival
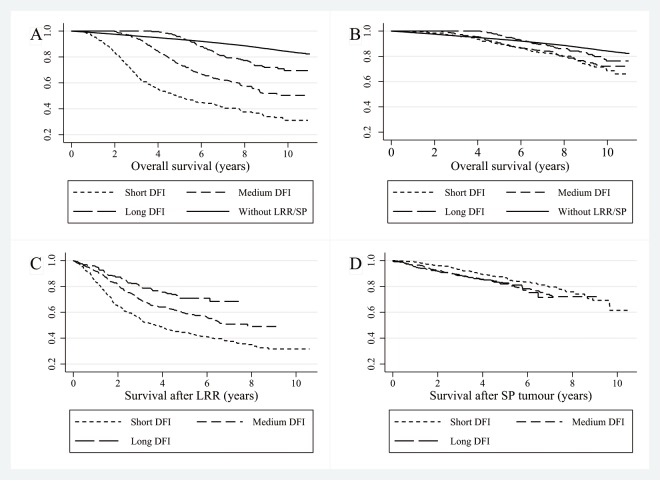
Ten year overall survival was 84% for women without recurrence, 49% for women with a LRR and 72% for women with a SP tumour. Ten year overall survival for patients with a LRR and a short DFI was 31%, 50% for medium and 70% for women with long DFIs (Fig 1A). Patients with a SP tumour demonstrated higher ten year overall survival rates with 69% for short DFIs, 72% for medium and 76% for long DFIs (Fig 1B). Table 5 shows the multivariable Cox regression analyses. Compared to short DFIs, women with a medium DFI and a LRR showed better survival after recurrence (Fig 1C) (Hazard Ratio (HR) 0.81, 95% CI 0.65–1.01) as well as women with a long DFI (HR 0.65, 95% CI 0.48–0.88). No significant association was found for the DFI and survival after SP tumours after adding covariates (Fig 1D).
Fig 1. Kaplan-Meier curves divided in short, medium and long DFIs for (A) overall survival in patients with a LRR, (B) overall survival in patients with a SP, (C) survival after recurrence in patients with a LRR, and (D) survival after recurrence for patients with a SP tumour.
Abbreviations: DFI = disease-free interval, LRR = locoregional recurrence, SP = second primary.
Table 5. Multivariable Cox regression analysis after multiple imputation.
| LRR (n = 890) | SP (n = 745) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Mutivariable | Univariable | Multivariable | ||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| DFI | |||||||||||||
| Short | Ref. | Ref. | Ref. | Ref. | |||||||||
| Medium | 0.63 | 0.51–0.78 | <0.001 | 0.81 | 0.65–1.01 | 0.066 | 1.26 | 0.90–1.78 | 0.184 | 1.14 | 0.80–1.61 | 0.475 | |
| Long | 0.39 | 0.29–0.52 | <0.001 | 0.65 | 0.48–0.88 | 0.005 | 1.37 | 0.93–2.01 | 0.113 | 1.42 | 0.96–2.09 | 0.079 | |
| Type of recurrence | |||||||||||||
| LR | Ref. | Ref. | na | na | |||||||||
| RR | 1.44 | 1.17–1.78 | <0.001 | 0.83 | 0.64–1.07 | 0.144 | |||||||
| Synchronous LRRs | 2.03 | 1.47–2.83 | <0.001 | 1.99 | 1.41–2.79 | <0.001 | |||||||
| Age | |||||||||||||
| <70 years | Ref. | Ref. | Ref. | Ref. | |||||||||
| ≥70 years | 2.28 | 1.86–2.79 | <0.001 | 2.29* | 1.85–2.84 | <0.001 | 2.59* | 1.93–3.48 | <0.001 | 2.28* | 1.67–3.12 | 0.002 | |
| Grade | |||||||||||||
| I, II | Ref. | Ref. | Ref. | Ref. | |||||||||
| III | 2.61* | 2.12–3.21 | <0.001 | 1.42 | 1.10–1.82 | 0.006 | 1.45 | 1.05–2.00 | 0.023 | 1.12 | 0.75–1.67 | 0.570 | |
| Tumour size | |||||||||||||
| ≤2 cm | Ref. | Ref. | Ref. | Ref. | |||||||||
| >2 cm | 2.60 | 2.13–3.18 | <0.001 | 1.47 | 1.19–1.83 | <0.001 | 1.76 | 1.32–2.36 | <0.001 | 1.42 | 1.03–1.96 | 0.033 | |
| Lymph node status | |||||||||||||
| Negative | Ref. | Ref. | Ref. | Ref. | |||||||||
| 1–3 positive | 1.71 | 1.37–2.14 | <0.001 | 1.15 | 0.86–1.54 | 0.355 | 1.66 | 1.19–2.32 | 0.003 | 1.36 | 0.92–2.02 | 0.123 | |
| >3 positive | 4.06 | 3.17–5.20 | <0.001 | 2.06 | 1.48–2.88 | <0.001 | 1.89 | 1.17–3.06 | 0.010 | 1.05 | 0.61–1.82 | 0.864 | |
| Hormone receptor status | |||||||||||||
| Other | Ref. | Ref. | Ref. | Ref. | |||||||||
| ER/PR negative | 2.49 | 2.02–3.06 | <0.001 | 1.64 | 1.29–2.08 | <0.001 | 1.45 | 0.99–2.13 | 0.055 | 1.30 | 0.82–2.05 | 0.268 | |
| Lymph node dissection | |||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | |||||||||
| Yes | 2.09 | 2.02–3.06 | <0.001 | 1.40 | 1.05–1.86 | 0.023 | 1.80* | 1.36–2.39 | <0.001 | 1.28 | 0.90–1.83 | 0.170 | |
| Radiotherapy of LRR/SP | |||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | |||||||||
| Yes | 1.09 | 0.90–1.32 | 0.381 | 0.94 | 0.76–1.16 | 0.575 | 0.73 | 0.55–0.97 | 0.032 | 1.04 | 0.76–1.41 | 0.812 | |
| Surgery of LRR/SP | |||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | |||||||||
| Yes | 0.42* | 0.35–0.51 | <0.001 | 0.49* | 0.39–0.61 | <0.001 | 0.13* | 0.09–0.19 | <0.001 | 0.17* | 0.11–0.27 | <0.001 | |
Abbreviations: HR = hazard rate, CI = Confidence Interval, DFI = disease-free interval, LR = local recurrence, RR = regional recurrence, LRR = locoregional recurrence, SP = second primary, ER = oestrogen receptor, PR = progesterone receptor, na = not applicable.
* indicates most influencing factors.
Convergence of the imputed dataset was adequate and the results of the Cox proportional hazards model on the multiple imputed data were comparable to those of the complete case analysis. Continuous DFI (expressed in days) also displayed significant influence in the multivariable analysis for LRR (HR 0.9997 per day), but did not result in a better model fit. There was no interaction identified for the complete model. The Cox proportional hazards assumption was not violated when tested both numerically and graphically. The variables that influenced survival after recurrence the most were age category and whether or not the LRR or SP tumour had been surgically removed.
Discussion
To the best of our knowledge, this is the first time the relation between the DFI and survival after a LRR or SP tumour has been explored on a large scale in a nationwide population-based registry. The DFI before a LRR is a prognostic factor for overall survival and survival after a LRR, with longer DFIs showing longer survival. Besides the DFI, age and surgical removal of the recurrence were significantly associated with survival after recurrence. After adding covariables in the multivariable analysis, length of the DFI before a LRR remained of significant influence on survival after the LRR, which suggests that it is an independent prognostic factor. No significant independent association was found for the DFI and survival after SP tumours. Patients with a short DFI and a LRR show on average less differentiated cells (grade III) and a larger primary tumour compared to the patients with medium and long DFIs, which suggests a more biological aggressive form of breast cancer. Moreover, short DFIs are significantly associated with subsequent DM [16, 25]. Another possible explanation is that patients with a short DFI still had to recover fully from the treatment given for the primary tumour. In the multivariable analysis, the effect of the DFI on survival after a SP tumour was not significant. SP tumours are new breast cancers independent of the primary tumour and the prognosis is therefore also independent of the DFI after the primary tumour.
Consistent with previous studies, age <70, good differentiation of the tumour cells (grade I,II), smaller tumour size, negative lymph nodes, positive hormone status, no axillary lymph node dissection, radiotherapy and surgical removal of the recurrence have been identified to be associated with better survival [16–18, 26]. Both chemotherapy and hormone therapy are known to have a positive influence on survival after primary breast cancer [19, 27]. Yet after adding covariates in the proportional hazards models chemotherapy and hormone therapy were not of significant influence on survival after a LRR or a SP tumour. In the univariable analysis women who received chemotherapy or hormone therapy showed worse survival compared to those without (HR 1.61, 95% CI 1.37–1.90 for chemotherapy; HR 1.45, 95% CI 1.23–1.73 for hormone therapy). Recurrence of the breast cancer, possibly even during the adjuvant treatment period, might suggest resistance to the treatment [28, 29]. The SP tumours were in 95% of the cases contralateral. Most of the patients with a SP tumour (96%) had undergone surgery, which is associated with higher survival after the SP tumour. However, this positive effect cannot solely be contributed to the influence of the surgery, but also the selection of patients that are eligible for surgery. Of the patients with a LRR, 41% did not receive surgery for their recurrence. In case of a short DFI this proportion was 49%. However, this could be explained by the higher amount of non-breast conserving surgeries for the primary tumour in this group (68% received mastectomy). Typical reasons for withholding from surgical treatment include diffuse infiltration, inflammatory changes, subsequent DM, high age or patient preference [30, 31]. Besides less surgery, women with a short DFI also received less hormone therapy for their LRR (Table 2). Consistent with previous studies we found that women with hormone negative primary tumours have on average a shorter DFI, which resulted in less hormone therapy in this group [32]. The cohort represented patients who were diagnosed in the period 2003–2006 and treated at that time, which could explain the lower percentage of hormone and chemotherapy compared to current treatment (data not shown). Another reason is the exclusion of patients who received neo-adjuvant systemic therapy (4% of the patients), while it is uncertain with which stage the patients should be included in the model. LRRs and SP tumours show differences in characteristics and prognosis and therefore need a different approach. In some studies the distinction between SP tumours and LRR is not made [14, 16, 33], which might overestimate the prognosis after a LRR.
The advantage of using the large cohort from the population-based NCR—including almost all breast cancer patients diagnosed between 2003 and 2006—is the reflection of daily practice. However, only limited information was available regarding the characteristics of the recurrences. The median overall survival in this study is 8.1 years, which could be considered short in a tumour with such a high ten year survival rate. Still, the balance between sufficiently long follow-up and relevance of the results for the present clinical situation needs to be considered. The changes and improvements in the treatment of breast cancer in more recent years can, by definition, not be reflected in a study with a long follow-up. Since only first or synchronous recurrences are registered by the NCR, no correction is possible for subsequent recurrences. DM after breast cancer is considered responsible for most of the morbidity and virtually all mortality associated with breast cancer. If a LRR was followed by a DM, this would drastically influence prognosis [34, 35]. Therefore, an improvement of the registry would be to register not only first but also possible subsequent recurrences. Furthermore, knowledge about cause-specific death can help improve future research. Because of the observational design and a lack of detailed information on breast cancer-specific survival and comorbidity, results should be interpreted with caution. However, Doyle et al. [36] found no difference in cause-specific and overall survival after a recurrence in the first five years and only a 3% difference after ten years.
Conclusion
This is the first study to explore the association between the DFI and survival after recurrence in a nationwide population-based registry and it contributes to insight into prognosis after LRR or SP breast cancer. The results of this study confirm that the DFI before a LRR is an independent prognostic factor for survival after a LRR, with longer DFIs showing longer survival. Furthermore, age and surgical removal of the recurrence greatly impact survival after recurrence. However, no significant independent association was found for the DFI and survival after SP tumours.
Acknowledgments
We would like to thank the registrars of the Netherlands Cancer Registry for their effort in gathering the data essential to this study.
Data Availability
Data are from the Netherlands Cancer Registry, please contact the authors at a.witteveen@utwente.nl.
Funding Statement
The authors have no support or funding to report.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh J, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49: 1374–403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 2. Lu W, Jansen L, Post W, Bonnema J, van de Velde J, de Bock G. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res. Treat. 2009;114: 403–12. 10.1007/s10549-008-0023-4 [DOI] [PubMed] [Google Scholar]
- 3. van Tienhoven G, Voogd A, Peterse J, Nielsen M, Andersen K, Mignolet F, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). Eur. J. Cancer. 1999;35: 32–38. [DOI] [PubMed] [Google Scholar]
- 4.Hastings J, Iganej S, Huang C, Huang R, Slezak J. Risk Factors for Locoregional Recurrence After Mastectomy in Stage T1 N0 Breast Cancer. Am J Clin Oncol. 2013: 1–6. [DOI] [PubMed]
- 5. Kwast A, Groothuis-Oudshoorn K, Grandjean I, Ho V, Voogd A, Menke-Pluymers M, et al. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res. 2012;135: 271–80. 10.1007/s10549-012-2160-z [DOI] [PubMed] [Google Scholar]
- 6. Min S, Lee S, Shin K, Park I, Jung S, Lee K, et al. Locoregional recurrence of breast cancer in patients treated with breast conservation surgery and radiotherapy following neoadjuvant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;81: e697–705. 10.1016/j.ijrobp.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 7.Nederlandse Kankerregistratie. Codeerhandleiding Nederlandse Kankerregistratie. 2013.
- 8. Panet-Raymond V, Truong P, McDonald R, Alexander C, Ross L, Ryhorchuk A, et al. True recurrence versus new primary: an analysis of ipsilateral breast tumor recurrences after breast-conserving therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;81: 409–17. 10.1016/j.ijrobp.2010.05.063 [DOI] [PubMed] [Google Scholar]
- 9. Grantzau T, Mellemkjær L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother. Oncol. 2013;106: 42–9. 10.1016/j.radonc.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Yadav B, Sharma S, Patel F, Ghoshal S, Kapoor R. Second primary in the contralateral breast after treatment of breast cancer. Radiother. Oncol. 2008;86: 171–6. [DOI] [PubMed] [Google Scholar]
- 11. Smith T, Lee D, Turner B. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses. Int. J. Radiat. Oncol. Biol. Phys. 2000;48: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 12. Vichapat V, Garmo H, Holmberg L, Fentiman I, Tutt A, Gillett C, et al. Prognosis of metachronous contralateral breast cancer: importance of stage, age and interval time between the two diagnoses. Breast Cancer Res. Treat. 2011;130: 609–18. 10.1007/s10549-011-1618-8 [DOI] [PubMed] [Google Scholar]
- 13. Hölzel D, Emeny R, Engel J. True local recurrences do not metastasize. Cancer Metastasis Rev. 2011;30: 161–76. 10.1007/s10555-011-9275-2 [DOI] [PubMed] [Google Scholar]
- 14. van der Sangen M, van de Poll-Franse L, Roumen R, Rutten H, Coebergh J, Vreugdenhil G, et al. The prognosis of patients with local recurrence more than five years after breast conservation therapy for invasive breast carcinoma. Eur. J. Surg. Oncol. 2006;32: 34–8. [DOI] [PubMed] [Google Scholar]
- 15. Davies E. Metastatic disease of the breast and local recurrence of breast cancer. Surg., vol. 2013;31: 41–45. [Google Scholar]
- 16. van Laar C, van der Sangen M, Poortmans P, Nieuwenhuijzen G, Roukema J, Roumen R, et al. Local recurrence following breast-conserving treatment in women aged 40 years or younger: Trends in risk and the impact on prognosis in a population-based cohort of 1143 patients. Eur. J. Cancer. 2013;49: 3093–101. 10.1016/j.ejca.2013.05.030 [DOI] [PubMed] [Google Scholar]
- 17. Ciatto S, Miccinesi G, Zappa M, Marco Z, Guido M. Prognostic impact of the early detection of metachronous contralateral breast cancer. Eur. J. Cancer. 2004;40: 1496–501. [DOI] [PubMed] [Google Scholar]
- 18. Voogd A, van Oost F, Rutgers E, Elkhuizen P, van Geel A, Scheijmans L, et al. Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur. J. Cancer. 2005;41: 2637–44. [DOI] [PubMed] [Google Scholar]
- 19. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365: 1687–717. [DOI] [PubMed] [Google Scholar]
- 20. Neri A, Marrelli D, Rossi S, De Stefano A, Mariani F, De Marco G, et al. Breast cancer local recurrence: risk factors and prognostic relevance of early time to recurrence. World J. Surg. 2007;31: 36–45. [DOI] [PubMed] [Google Scholar]
- 21. Fredriksson I, Liljegren G, Arnesson L, Emdin S, Palm-Sjövall M, Fornander T, et al. Local recurrence in the breast after conservative surgery—a study of prognosis and prognostic factors in 391 women. Eur. J. Cancer. 2002;38: 1860–70. [DOI] [PubMed] [Google Scholar]
- 22. Bloom H, Richardson W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br. J. Cancer. 1957;11: 359–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White I, Royston P, Wood A. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011;30: 377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 24. Spratt M, Carpenter J, Sterne J, Carlin J, Heron J, Henderson J, et al. Strategies for multiple imputation in longitudinal studies. Am. J. Epidemiol. 2010;172: 478–87. 10.1093/aje/kwq137 [DOI] [PubMed] [Google Scholar]
- 25. Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106: 35–41. [DOI] [PubMed] [Google Scholar]
- 26. Dawood S, Broglio K, Ensor J, Hortobagyi G, Giordano S. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann. Oncol. 2010;21: 2169–74. 10.1093/annonc/mdq220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378: 771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Yang B. Friend or foe: the role of microRNA in chemotherapy resistance. Acta Pharmacol. Sin. 2013;34: 870–9. 10.1038/aps.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osborne C, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011;3: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monteiro Grillo I, Jorge M, Marques Vidal P, Ortiz M, Ravasco P. The effect of locoregional recurrence on survival and distant metastasis after conservative treatment for invasive breast carcinoma. Clin. Oncol. 2005;17: 111–117. [DOI] [PubMed] [Google Scholar]
- 31. Tanis E, van de Velde C, Bartelink H, van de Vijver M, Putter H, van der Hage J. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur. J. Cancer. 2012;12: 1751–6. 10.1016/j.ejca.2012.02.051 [DOI] [PubMed] [Google Scholar]
- 32. Livi L, Meattini I, Saieva C, Franzese C, Di Cataldo V, Greto D, et al. Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer. 2012;13: 3236–43. 10.1002/cncr.26647 [DOI] [PubMed] [Google Scholar]
- 33. Anderson S, Wapnir I, Dignam J, Fisher B, Mamounas E, Jeong J, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J. Clin. Oncol. 2009;27: 2466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwast A, Voogd A, Menke-Pluijmers M, Linn S, Sonke G, Kiemeney L, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014. [DOI] [PubMed]
- 35. Bantema-Joppe E, van den Heuvel E, de Munck L, de Bock G, Smit W, Timmer P, et al. Impact of primary local treatment on the development of distant metastases or death through locoregional recurrence in young breast cancer patients. Breast Cancer Res. Treat. 2013;140: 577–85. 10.1007/s10549-013-2650-7 [DOI] [PubMed] [Google Scholar]
- 36. Doyle T, Schultz D, Peters C, Harris E, Solin L. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001;51: 74–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the Netherlands Cancer Registry, please contact the authors at a.witteveen@utwente.nl.