Abstract
Radiation caries have been reported to be correlated with radiotherapy-induced destruction of salivary function and changes in oral microbiota. There have been no published reports detailing patients who have remained radiation caries-free following radiotherapy for nasopharyngeal carcinoma. The aim of this study was to investigate the relationship between salivary function, oral microbiota and the absence of radiation caries. Twelve radiation caries-free patients and nine patients exhibiting radiation caries following irradiated nasopharyngeal carcinoma were selected. V40, the dose at which the volume of the contralateral parotid gland receives more than 40 Gy, was recorded. Stimulated saliva flow rate, pH values and buffering capacity were examined to assess salivary function. Stimulated saliva was used for molecular profiling by Denaturing Gradient Gel Electrophoresis. Mutans streptococci and Lactobacilli in saliva were also cultivated. There were no significant differences in V40 between radiation caries-free individuals and those with radiation caries. Compared with normal values, the radiation caries-free group had significantly decreased simulated saliva flow rate, while there were no significant differences in the saliva pH value and buffering capacity. Similar results were observed in the radiation caries group. There was no statistical difference in microbial diversity, composition and log CFU counts in cultivation from the radiation caries-free group and the radiation caries group. Eleven genera were detected in these two groups, among which Streptococcus spp. and Neisseria spp. had the highest distribution. Our results suggest that changes in salivary function and in salivary microbiota do not explain the absence of radiation caries in radiation caries-free individuals.
Introduction
Radiotherapy (RT) is a major treatment method used in nasopharyngeal carcinoma (NPC) patients [1]. However, the most common and severe sequela of RT is radiation caries [2–7]. Previous research has shown that nearly 90% of irradiated NPC patients suffered from radiation caries [4, 5, 8]. Radiation caries is a rapidly developing and highly destructive form of tooth decay, and tends to develop as quickly as four weeks after completion of RT [6]. In addition, it is worth noting that patients are at highest risk of developing radiation caries in the first year following RT [9, 10]. Destruction of salivary function and shift of oral microbiota have been reported as potential factors of the disease[11]. However, due to the potential multifactorial nature of the disease, there are still open question regarding other potential factors [6, 10, 12, 13]. Most of the prior research has focused on irradiated patients within the first year of RT [14, 15]. Little research has investigated irradiated patients more than one year after the completion of RT.
There are many reports on the correlation between the flow rate of saliva and radiation caries [16–19]. It is known that a significant decrease of saliva flow rate and the destruction of salivary function are commonly observed after RT [20, 21]. It has been reported that 85% of irradiated patients with a stimulated saliva flow rate of less than 0.5ml/min develop at least one carious lesion per year. The higher the rate of saliva secretion is, the lower the risk of radiation caries [21]. As a result, the practice of RT has adopted new techniques to reduce the decrease in salivary function. In recent years, intensity-modulated radiotherapy (IMRT), which can deliver higher RT doses to target volumes while sparing critical structures such as the parotid glands and submandibular glands, has been widely used in RT of NPC patients [22, 23]. It has been demonstrated that the effect on salivary function brought on IMRT is significantly less destructive than ordinary RT [22, 24]. Still, the incidence of radiation caries did not show significant decrease [25, 26].
Additionally, changes in the oral microbiota in oral cavity is considered to be yet another potential factor in the development of radiation caries [27]. In patients who have undergone RT, a higher number of acidogenic and aciduric flora was detected by traditional culturing techniques after RT than before [7, 14, 28]. In recent years, the rapid development of advanced molecular biology methods has provided important insights into the oral microbiome. Using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) fingerprinting, a culture-independent molecular fingerprinting technique, the predominant bacterial species can be rapidly assessed [29]. When this technique was applied to the oral microbiota of patients undergoing RT, a shift in the major taxa of oral microorganisms was observed [30]. Some researchers have also found that the total strepcocucci increased with decreased saliva flow rate [2]. However, it has also been reported that there is no significant shift of diversity, stability and microbial composition detected by PCR-DGGE within the first six months after IMRT [3]. Taken together, the current data do not show a clear relationship between the high risk of developing radiation caries and the changes in the oral microbiota within the first year following RT.
Most of the existing research on this topic are based on data from irradiated patients who finished their RT within the first year [3, 30, 31]. To our knowledge, there is currently no research focused on NPC patients who never developed new dental caries more than one year after IMRT. Thus, the purpose of this study is to access potential factors from radiation caries-free individuals. We hypothesize that the salivary function and the salivary microbiota are correlated to the absence of radiation caries after one year of RT.
Methods
Ethics statement
Approval for this study was received from the ethics committee of the West China Dental Hospital of Sichuan University (2011017). Informed and written consents were obtained from the patients who were recruited into the study from July 2012 to March 2014. All study subjects gave their voluntary written consent for taking part in this research.
Study design and subject selection
The present study employed a cross-sectional case-cohort design. Irradiated patients in the study were all NPC patients from West China Hospital in Sichuan Province, China, who were treated using the Precise X-ray accelerator (Sweden), and the plans of RT including dose at which the volume of parotid received were designed through ADAC Pinnacle3-9.0 (Philips, Netherland). All of patients had completed their IMRT at least one year before taking part in this study. Inclusion and exclusion criteria are displayed in Table 1.
Table 1. Admission criteria.
| Radiation caries-free (RCF) group | |
|---|---|
| Inclusion criteria | Exclusion criteria |
| -having at least 28 teeth in the oral cavity | -Receiving antibiotics within 3 months before the study |
| -finish RT at least one year | -priodontitis |
| -over eighteen years | -smoker |
| -having no newly decay of teeth after RT | |
| -Free of systemic disease(including systemic infection) | |
| -written informed consent |
|
|
Radiation caries (RC) group
| |
|
Inclusion criteria
|
Exclusion criteria
|
| -age, sex, after RT period comparable with RCF individuals | -receiving antibiotics within 3 months before the study |
| -having newly decay of teeth after RT(DMFT≥6) | -priodontitis |
| -free of systemic disease(including systemic infection) | -smoker |
| -written informed consent | |
History record
The bilateral size of the parotid gland, dose value of RT and V40, the volume of the contralateral parotid gland receiving more than 40Gy, were taken from the dental record held in the West China Dental Hospital of Sichuan University. V40 was shown to be the best predictive dose-value variable for salivary flow recovery [32].
Oral examination
Before sampling, a questionnaire was issued to the subjects on their oral hygiene habits, dietary habits, clinical application of fluoride products, use of artificial saliva, and their subjective feeling of oral dryness [15]. Dental examinations for caries popularity were all carried out by the same research fellow, who adopted the WHO criteria for the diagnosis and coding of dental caries. Dental caries were scored by the sum of decayed, missing and filled teeth (DMFT). The plaque index, defined by Sillness and Loe [1964], was also recorded.
Measurement of salivary function
All saliva sample collection were conducted in the morning. Each subject was asked not to eat or drink for at least 2 hours prior to the sample collection. After resting for 5 minutes with no talking, subjects were asked to chew on a piece of paraffin wax for 30 seconds and expectorate directly into a graduated 50 mL sample collection tube on ice within 5 minutes[33]. The pH value of the stimulated saliva was measured using a pH meter (Metrohm632, Herisau, Switzerland) [28]. Buffering capacity was measured by the dip-slide technique (CRT bacteria, Ivo-clar Vivadent, Germany) according to Köhler and Bratthall[1997]and classified following the manufacturer’s instructions[34].
Microbial sampling
Twenty-one stimulated saliva samples were collected in total for genetic fingerprinting and culture. Samples were immediately transferred on ice to a microbiology laboratory (State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University).
Microbial cultivation
Samples were shaken (30 s) and diluted (10-1 to 10-5-fold). Brain heart infusion (BHI, Difco) with defibrinated 5% sheep blood agar, Mitis-Salivarius-Bacitracin (MSB) agar [35] and Rogosa-SL agar (4080; Difco) [28, 35, 36] were used for microbial culture. The diluted samples (10 μl) were plated on media that selected for Mutans Streptococcus (MS) and Lactobacilli (LB). Plates were incubated under 80% nitrogen, 10% carbon dioxide, and 10% hydrogen for 48 h, and colonies on each culture plate were counted. Colonies were collected and MS and LB were positively identified by Gram staining, microscopy, and sequencing. Colonies on each culture plate were manually counted, while the logarithm of the CFU values was calculated to estimate the distribution of both targeted microorganism in plaque and saliva, as well as for statistical analysis.
DNA extraction and PCR assay
Total bacterial genomic DNA was extracted from clinical samples using QIAamp DNA Micro Kit (Qiagen) using the manufacturer’s instructions. The concentration and purity of the extracted DNA was measured using a Nanodrop 2000 spectrometer (Thermo Fisher Scientific, Wilmington, USA). An approximately 300-bp internal fragment of the 16S rRNA gene was amplified using the universal primer set, Bac1 (5′-CGC CCG CCG CGC CCC GCG CCC GTC CCGCCG CCC CCG CCC GAC TAC GTG CCA GCA GCC-3′) and Bac2 (5′-GGACTA CCA GGGTAT CTA ATC C-3′)[37]. Each 50-μl PCR tube containeds 100 ng purified genomic DNA, 40 pmol of each primer, 200 μM of each dNTP, 4.0mM MgCl2, 5μl 10× PCR buffer, and 2.5 U Taq DNA polymerase (Invitrogen, California, USA). Cycling conditions were 94°C denaturation for 3 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 1 min, with a final extension time of 5 min at 72°C. PCR products were evaluated by 1% agarose gel electrophoresis [38].
A 40% [containing 2.8 M urea and 16% (vol/vol) formamide] to 60% [containing 4.2 M urea and 24% (vol/vol) formamide] linear DNA denaturing gradient was formed in 8% (wt/vol) polyacrylamide gels. The gels were submerged in 1× TAE buffer. Approximately 30 μl of PCR product was applied to each well and separated by electrophoresis for 16 h at 58°C using a fixed voltage of 60V in the Bio-Rad DCode System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Gels were stained for 15 min in 1× TAE buffer containing 0.5 μg/ml ethidium bromides, followed by 15 min of rinsing in 1×TAE buffer after electrophoresis. DGGE profile images were digitally recorded using the Molecular Imager Gel Documentation system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) [38].
DGGE profile analysis
DGGE profile analysis was performed based on the migration distances and intensity of each detected band by GelCompare II software version 6.5 (Applied Maths, Kortrijk, Belgium). Each gel was normalized according to a DGGE standard marker included in all the gels, which was generated using 5 species-specific strains (Lactobacillus acidophilus ATCC4356, Streptococcus mutans UA159, Actinomyces naeslundii 12104, Streptococcus gordonii ATCC 10558, and Streptococcus sanguinis ATCC10556). The background was subtracted using mathematical algorithms according to the spectral analysis of overall densitometric curves. A minimal profiling setting (1.0%) was used for band searching.
The diversity index of the oral or nasal microbiota was evaluated using the Shannon-Wiener index (H) and richness (S) based on the following equations:
| (1) |
| (2) |
Where p i is the ratio of the intensity of a single band to the total intensity of all bands within the same lane, and S is the total number of bands in each lane [39].
Similarities between profiles were analyzed by the Pearson product moment correlation coefficient of similarity [40], using the unweighted pair group method by means of arithmetic averages (UPGMA) [41]. Dendrograms of genetic similarity between samples were calculated.
Principal component analysis (PCA), which was used to calculate the dendrograms of each DGGE gel [42], was performed with Canoco 4.5. By PCA analysis, different data of the detailed DGGE patterns of each sample could be directly analyzed and reduced to one spot in a two dimensional space.
16S rRNA gene sequencing
Distinct amplicons from DGGE gels were excised, and transferred to a 200 μl PCR tube containing 10 μl sterile nuclease-free water (Fisher Scientific, Wilmington, USA). After incubating at 4°C overnight, the recovered PCR products were reamplified with the same Bac1 and Bac2 primers lacking the GC clamp. The resulting PCR products were purified and sequenced on an ABI 3730XL automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). Obtained sequences were analyzed using ABI Sequence Analysis 5.2 (Applied Biosystems, Foster City, CA, USA), and determined by using the BLASTN search program at the NCBI Web site and HOMD (Version 10.1;http://www.homd.org/).16S rRNA sequence Identification. Sequences that had 98% higher similarity were considered positively identify their respective taxon.
Statistical analysis
Fisher’s exact test was used to compare the gender distribution and the frequencies of the sequenced bands representing distribution of major bacteria between the two groups. Nonparametric Mann-Whitney U tests were used for continuous dependent variables. The results for some continuous variables are presented as mean ± standard deviation (SD), range, and 95% confidence interval (CI). The level of significance was set at p<0.05. For all comparisons, the p-values are presented, even if they are not significant. All statistical analyses were performed with SPSS software version 19.0 (SPSS Inc., Chicago, IBM).
Results
Study subjects characteristics
Twelve radiation caries-free (RCF) patients and nine patients exhibiting radiation caries (RC) participated in this study. The study subjects had completed their RT 12 to 36 months before enrolling in this study. There were no significant differences in gender distribution, mean age, bilateral size of parotid gland, and V40 of the two groups (Table 2).
Table 2. Clinical features of the subject population.
| RCF group | RC group | |||
|---|---|---|---|---|
| n = 12 | n = 9 | |||
| Mean±SD | Range | Mean±SD | Range | |
| Age(years) a | 45.4±14.1 | (22–68) | 52.3±11.0 | (34–68) |
| Gender b | M = 58.3% | M = 7 | M = 66.7% | M = 5 |
| F = 41.6% | F = 5 | F = 33.3% | F = 4 | |
| Bilateral size of parotid gland L/R(cm 3) a | 44.31±18.63 | (25.23–76.30) | 43.92±15.63 | (26.59–75.06) |
| Dosage cGy a | 7033±115 | (7000–7400) | 7088±174 | (7000–7400) |
| V40(%) a | 33.41±4.82 | (23.51–40.72) | 36.40±6.37 | (24.79–42.76) |
aIndependent sample nonparametric Mann-Whitney U test was used;
bFisher’s Exact test was used.
Despite variation in volume of the parotid gland between individuals, they were all within the normal range (25.8–120cm3) [43, 44]. It has been reported that V40 should be less than 33% for complete saliva production recovery after 24 months [32]. In our cohort, eleven out of twelve RCF subjects had V40 over 33%, while eight out of nine subjects were in the RC group.
Oral health information
The general oral health of the study subjects is presented in Table 3. There are significant differences in the plaque index between the two groups (p = 0.038). None of the study subjects had clinical use of fluorine products and artificial saliva. The use of fluoride toothpaste was investigated through a questionnaire when the study subjects enrolling in the study. None of them had used fluoride mouthrinse (Table 4). Furthermore, no special food preferences were observed.
Table 3. Oral health information of RCF and RC subjects.
| RCF group n = 12 | RC group n = 9 | |||
|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | |
| Newly DMFT | 0 | 0 | 12.0±6.42 | (6–21) |
| Plaque index a | 1.06±0.42 | (0.35–1.59) | 1.52±0.49 | (0.79–2.09)* |
| Frequency of teeth brushing(times/day) | 2.08±0.42 | (2–3) | 1.78±0.44 | (1–2) |
| Clinical application of Fluorine production | No | - | No | - |
| Use of artificial saliva | No | - | No | - |
| Preference of food | No | - | No | - |
aIndependent sample nonparametric Mann-Whitney U test was used.
Table 4. Use of Toothpaste of RCF and RC subjects.
| Fluoride type/Concentration | Radiation caries-free group(n) | Radiation caries group(n) |
|---|---|---|
| Sodium fluoride /0.06%-0.14% | 10 | 8 |
| Sodium fluoride /0 | 2 | 1 |
Salivary function
The stimulated saliva flow rate, saliva pH value, and buffering capacity are showed in Table 5. Our data show that the stimulated saliva flow rate in the RCF group is far lower than the lowest normal standard, reported to be 2.0 ml/min [27, 45]. Although the mean stimulated saliva flow rate was higher in the RCF group than in the RC group, there was no statistically significant difference between the two groups. The pH value of saliva ranged from 6.77 to 7.41, which is in line with reported normal salivary pH range (5.3 to 7.8) [46]. Our results show that the pH and buffering capacity of saliva from both the RCF and RC groups fall within the normal range.
Table 5. Salivary function of subjects.
| Subjects | Stimulated saliva flow rate(ml/min) a | Saliva pH a | Buffering capacity No. of patients (%) | |||
|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | low | high | |
| RCF group n = 12 | 0.98±0.42 | (0.6–2) | 7.02±0.20 | (6.80–7.41) | 0 | 12(100) |
| RC group n = 9 | 0.66±0.27 | (0.05–0.9) | 6.96±0.19 | (6.77–7.40) | 0 | 9(100) |
aIndependent sample nonparametric Mann-Whitney U test was used.
Clustering of DGGE profiles
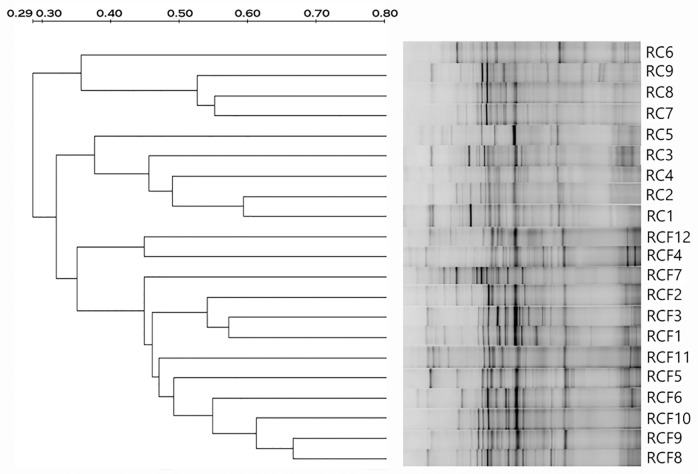
We compared the DGGE profiles 16S ribosomal genes amplified from the saliva of RCF and RC subjects. We used the Pearson product moment correlation coefficient and dendrograms to analysis similarity between salivary microbes in both groups (Fig 1). The dendrogram resulting from Dice cluster analysis of saliva samples show three distinct clusters with four RC subjects in cluster I (36% similarity), the remainder of the RC group in cluster II (38% similarity), and all the RCF subjects in cluster III (35% similarity).
Fig 1. Clustering analysis results of all DGGE profiles demonstrated graphically as an UPGMA dendrogram.
Percentage similarity is shown on the scale above the dendrogram.
Analysis of DGGE banding patterns
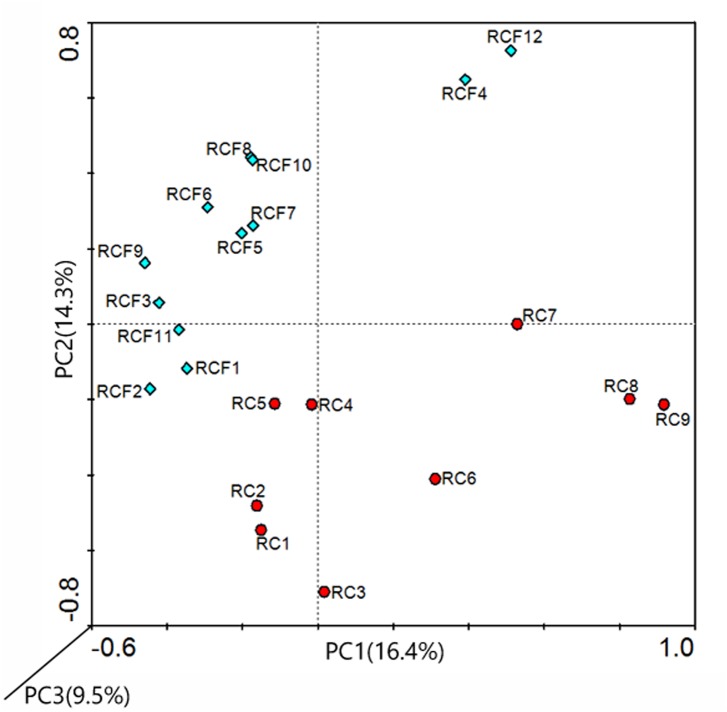
DGGE banding patterns were examined by computer-aided analyses based on densitometric curves. The number of peaks and their intensity in the denstiometric curve of the gel reflect the number and relative abundance of the predominant 16S rRNA gene sequences. PCA is classified as dimensioning mechanics among grouping mechanics. It is worth noting that all DGGE patterns available were involved to perform PCA analysis. Basing on the relatedness, it produces two-dimensional plots where the entries are spread. Additionally, the construction is more subjective than dendrogram. In our study, a clear separation between spots and an evenly distributed spots were seen, and a low and almost mean percentage of total variation was revealed by the three principal components (PC) (only PC 1 and PC 2 were adopted to the analysis) (Fig 2).
Fig 2. PCA analysis of samples.
Principal components were calculated for each lane of each DGGE gel with group-specific 16S rDNA fragments. RCF group (blue dots); RC group (red dots).
The diversity indices (H, S and E H) of microbial samples from saliva are presented in Table 6. No significant differences in diversity indices were found in the stimulated saliva samples. Although the RCF group showed higher Shannon-Wiener index and richness than the RC group, we did not detect statistical differences in H, S and E H between the two groups.
Table 6. Comparison of the diversity indices calculated from DGGE profiles.
| Diversity Indices a | RCF group | RC group |
|---|---|---|
| H | 2.54±0.16 | 2.52±0.14 |
| S | 12.90±2.16 | 12.49±1.78 |
| E H | 16.49±3.01 | 16.12±2.63 |
H: Shannon-Wiener index, S: Richness, E H: Evenness;
aIndependent sample nonparametric Mann-Whitney U test was used.
Sequence analysis
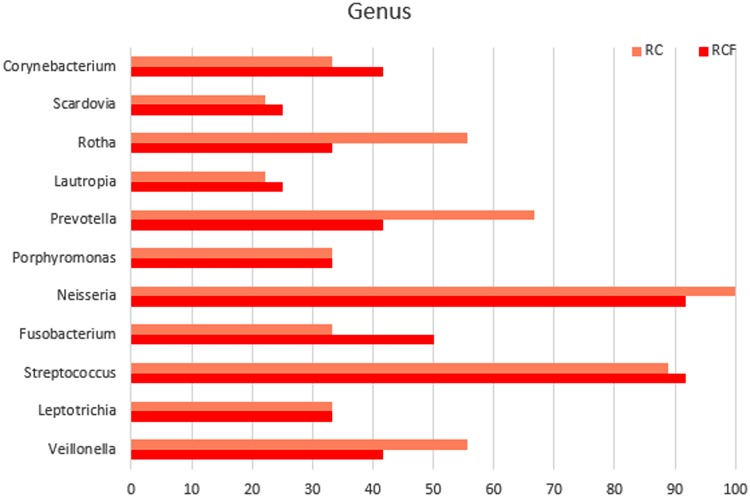
In total, 175 distinct amplicons were excised from the DGGE gels. Eleven genera were identified (Fig 3), and the frequencies of their occurrence in different types of samples analyzed between groups. Streptococcus and Neisseria had the highest distribution. Scardovia and Lautropia were present at very low distribution (about 20%). Similar distributions were detected in the RC group. Among all genera, there were no ‘gender-specific’ (present in one gender but absent in the other) or ‘gender-associated’ (differentially distributed yet present in both gender) genera detected (data not shown). No ‘radiation-caries-specific’ genera (present only in either the RCF or the RC populations) was detected either.
Fig 3. Distribution of taxonomy profiles according to 16S rDNA V3–V5 region sequence analysis.
Microbial cultivation results
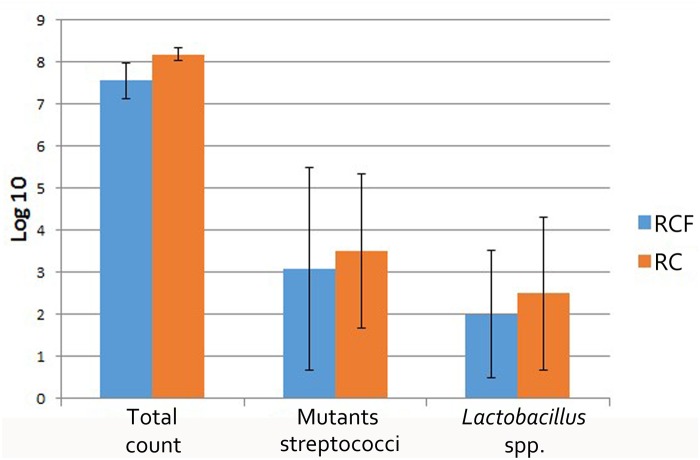
The log CFU counts of total cultured bacteria, LB and MS also presented in Fig 4. Although the average log CFU of total cultured bacteria, LB and MS in the RCF group is lower than that from the RC group, there was no significant difference between these two groups.
Fig 4. Mean±SD log10 number of microorganism in subjects with RCF (n = 12)and RC (n = 9).
Discussion
We initially hypothesized that salivary function and salivary microbiota are potentially related with the absence of radiation caries one year or more after RT. To our knowledge, this is the first paper to report on these characteristics in RCF irradiated NPC patients. Most existing research focused on RC patients within the first year post-RT, during which they have the highest risk of suffering from radiation caries [14, 28]. In this study, all participants have completed IMRT for at least one year.
In this study, salivary function was measured through stimulated flow rate, saliva pH and buffering capacity [23]. Previous researches has demonstrated that IMRT-treated patients still commonly experienced loss of salivary function [24]. The mean stimulated salivary flow rate in healthy control groups has been previously reported to be above 2.0ml/min [28, 45]. However, in our study, the mean stimulated salivary flow rate in the RCF group was significant lower than normal, as was the flow rate in the RC group. This findings is in agreement with several publications which have reported that the mean stimulated saliva flow rate of irradiated patients do not recover to their baseline even one or two years after IMRT [23, 47].
Notably, we did not detect any significant decreases in pH value and buffering capacity of the stimulated saliva when compared with normal values. The pH value of stimulated saliva in our study ranged from 6.77 to 7.41, which is in line with reported ‘normal’ pH between 5.3 and 7.8 [46]. It is interesting to note that, some researchers have reported a decrease in salivary pH from about 7.0 to 5.0 in combination with a loss in buffering capacity immediately after RT [18, 48, 49]. As salivary flow gradually increases with time after RT, the secreted saliva also became less acidic. The mean salivary pH improves to 6.9 after year 1 and recovered to 7.2 at 2 years after IMRT. Additionally, the proportion of RT patients showing saliva with medium-to-high buffering capacity increases to 70.6% at 1 year post-RT and to 85.7% at 2 years post-RT [23]. Hence, a new buffering system may become established approximately one year after IMRT. However, since the salivary flow rate is not yet recovered in our study subjects, who are 12–36 months post-RT, it is possible to conclude from the given data that salivary function in RCF individuals is still damaged. A similar conclusion may be drawn from the RC group.
It has been reported that the oral microbiota of irradiated patients intrinsically changes with their salivary function[50]. The diversity index and major species were analyzed to characterize the oral microbiota. The Shannon-Wiener index (H), richness (S) and evenness (E H) values characterizing salivary microbes from the RCF group and RC groups showed no statistical differences, suggesting that similar microbial diversity is found in these two groups. However, these data only compare the diversity between a caries-free group and a caries bearing group. Whether caries-bearing individuals exhibit higher [51, 52] or lower [53, 54] microbial diversity than caries-free subjects is still debated. Our results conflicted with previously published reports, possibly because all the participants in those studies had not treated by RT and had healthy salivary function. However, our data only showed a weak correlation between oral microbial diversity with the absence of radiation caries.
In our study, eleven genera (Streptococcus, Neisseria, Scardovia, Porphyromonas, Fusobacteria, Lautropia, Veillonella, Capnocytophaga, Rithia, Leptotrichia and Prevotella) of microbes were found and Streptococcus spp. and Neisseria spp. represented over 80% of samples. Likewise, a similar distribution has been shown in previous surveys [3, 31, 55]. Veillonella, Capnocytophaga, Neisseria, Rithia, Leptotrichia and Prevotella were detected in both irradiated groups, which matched the results of previous studies [3, 31]. Other genera, such as Granulicatella and Gemella, were detected by Hu et al. partly because they had planted in oral cavity before RT began. Porphyromonas and Fusobacteria were observed in both groups partly because they had been shown to be healthy oral genera in previous studies [56]. Scardovia has been shown to be significantly increased in cavitated dentin lesions of children [57]. It is reasonable to expect factors such as age, diet and life-style to affect oral microbial community structures [58]. In spite of differences between individuals, there were no statistical differences in the distribution of taxa in both the RCF and RC groups. Alternatively, a similar composition of major species may explain the same diversity index found in RCF and RC subjects, due in part to the same destruction of salivary function brought on by radiotherapy [2, 59]. However, contrary to our hypothesis, our data showed a weak correlation between major microbial species with the absence of radiation caries.
In this study, we also adapted traditional method to culture LB and MS from stimulated saliva. Interestingly, the number of MS we found is consistent with previously published figures, while the number of LB is not in agreement [28, 60]. The log CFU counts of LB were lower than the previously reported 4 or 5. However, as discussed above, most of the previous reports focused on irradiated patients within the first 6 months after ordinary RT [27, 28] or within the first year after ordinary RT[14]. From the sixth month after RT, LB partly returns to its baseline composition [12]. In this study, we used both traditional microbial culture as well as molecular biology techniques to minimize the bias in each assay. However, the limitation of DGGE should not be ignored and levels of certain microbes in the samples may be below the detection limit of PCR (102 cells) [61]or DGGE (103 cells) [62]. The visible bands are considered to be from species that represent more than 1% of the sample microbiota [63]. Our data showed that the number of LB was below 103. This may explain why LB was not detected when microbial diversity was investigated with DGGE. In agreement with previous reports, LB was absent in studies employing the Human Oral Microbe Identification Microarray (HOMIM) [52, 64]. As the same quantity of LB and MS were detected in both the RCF and RC groups, we observed a weak correlation with the absence of radiation caries, and the role of LB and MS in the process of developing radiation caries may have been overestimated.
In our study, we found lower levels of plaque index (PL) in RCF group participants than RC group participants (p = 0.038). Since PL is a direct assessment of the oral hygiene level, a significant difference in PL indicates that the RCF group has better oral hygiene than the RC group. However, the cross-section nature of this project did not allow for any causative statements. Therefore, the question of whether bad oral hygiene was a causative factor in foregoes radiation caries development or whether it is a result of radiation caries could not be addressed.
Conclusion
In conclusion, in this study we find no clear correlation between the characteristics of patient salivary microbiota and radiation caries one year after IMRT. Our results suggest that salivary function in irradiated patients does not recovered fully after 12–36 months, but the pH value and buffering capacity of saliva return to normal after one year or more following IMRT. However, population-based longitudinal studies are required to reveal the factors for the absence of radiation caries.
Acknowledgments
We gratefully acknowledge the support of Prof. Ping Li and his colleagues in the Department of Oncology, West China Hospital, China. We also thank Prof. Sen Bo and his colleagues in the Radiotherapy Department of West China Hospital for their informative advice.
Data Availability
All the data are represented in the figures and tables of the manuscript.
Funding Statement
This investigation was supported by the National Science & Technology Pillar Program during the 12th Five-year Plan Period (No. 20911BAZ03171), the Chinese National Natural Science Fund for Young Scholars (No. 31200389), and the Chinese National Natural Science Fund (No. 81371135). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. The Lancet, 2008. 371(9625): p. 1695–1709. 10.1016/S0140-6736(08)60728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudney JD, Pan Y, Chen R. Streptococcal diversity in oral biofilms with respect to salivary function. Archives of Oral Biology, 2003. 48(7): p. 475–493. [DOI] [PubMed] [Google Scholar]
- 3. Shao ZY, Tang ZS, Yan C, Jiang YT, Ma R, Liu Z, et al. Effects of Intensity-modulated Radiotherapy on Human Oral Microflora. Journal of Radiation Research, 2011. 52(6): p. 834–839. [DOI] [PubMed] [Google Scholar]
- 4. Silverman S Jr. Oral cancer: complications of therapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 1999. 88(2): p. 122–126. [DOI] [PubMed] [Google Scholar]
- 5. Jham BC, Freire ARdS. Oral complications of radiotherapy in the head and neck. Revista Brasileira de Otorrinolaringologia, 2006. 72(5): p. 704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguiar GP, Jham BC, Magalhães CS, Sensi LG, Freire AR. A review of the biological and clinical aspects of radiation caries. J Contemp Dent Pract, 2009. 10(4): p. 83–89. [PubMed] [Google Scholar]
- 7. Epstein JB, Chin EA, Jacobson JJ, Rishiraj B, Le N. The relationships among fluoride, cariogenic oral flora, and salivary flow rate during radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1998. 86(3): p. 286–92. [DOI] [PubMed] [Google Scholar]
- 8. Lizi E. A case for a dental surgeon at regional radiotherapy centres. British dental journal, 1992. 173(1): p. 24–26. [DOI] [PubMed] [Google Scholar]
- 9. Kielbassa AM, Meyer-Lueckel H. Die Auswirkungen von Speichelersatzmitteln und Mundspullosungen auf Dentin. SCHWEIZER MONATSSCHRIFT FUR ZAHNMEDIZIN, 2001. 111(9): p. 1060–1074. [PubMed] [Google Scholar]
- 10. Dreizen S, Brown LR, Daly TE, Drane JB. Prevention of Xerostomia-Related Dental Caries in Irradiated Cancer Patients. Journal of Dental Research, 1977. 56(2): p. 99–104. [DOI] [PubMed] [Google Scholar]
- 11. Vissink A, Burlage FR, Spijkervet FKL, Jansma J, Coppes RP. Prevention and Treatment of the Consequences of Head and Neck Radiotherapy. Critical Reviews in Oral Biology & Medicine, 2003. 14(3): p. 213–225. 10.2147/OTT.S46933 [DOI] [PubMed] [Google Scholar]
- 12. Brown LR, Dreizen S, Daly TE, Drane JB, Handler S, Riggan LJ, et al. Interrelations of oral microorganisms, immunoglobulins, and dental caries following radiotherapy. Journal of dental research, 1978. 57(9): p. 882–893. [DOI] [PubMed] [Google Scholar]
- 13. Kielbassa A, Munz I, Bruggmoser G, Schulte-Mönting J. Effect of demineralization and remineralization on microhardness of irradiated dentin. The Journal of clinical dentistry, 2001. 13(3): p. 104–110. [PubMed] [Google Scholar]
- 14. Al-Nawas B, Grotz KA. Prospective study of the long term change of the oral flora after radiation therapy. Support Care Cancer, 2006. 14(3): p. 291–6. [DOI] [PubMed] [Google Scholar]
- 15. Meng L, Liu J, Peng B, Fan M, Nie M, Chen Z, et al. The persistence of Streptococcus mutans in nasopharyngeal carcinoma patients after radiotherapy. Caries Res, 2005. 39(6): p. 484–9. [DOI] [PubMed] [Google Scholar]
- 16. Jansma J, Vissink A, Bouma J, Vermey A, Panders AK, Gravenmade EJ. A survey of prevention and treatment regimens for oral sequelae resulting from head and neck radiotherapy used in Dutch radiotherapy institutes. Int J Radiat Oncol Biol Phys, 1992. 24(2): p. 359–67. [DOI] [PubMed] [Google Scholar]
- 17. Epstein JB, Scully C. The role of saliva in oral health and the causes and effects of xerostomia. J Can Dent Assoc, 1992. 58(3): p. 217–21. [PubMed] [Google Scholar]
- 18. Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Lückel H. Radiation-related damage to dentition. The Lancet Oncology, 2006. 7(4): p. 326–335. [DOI] [PubMed] [Google Scholar]
- 19. Shannon I, Starcke E, Wescott W. Effect of radiotherapy on whole saliva flow. Journal of dental research, 1977. 56(6): p. 693–693. [DOI] [PubMed] [Google Scholar]
- 20. Joyston-Bechal S. Prevention of dental diseases following radiotherapy and chemotherapy. International dental journal, 1992. 42(1): p. 47–53. [PubMed] [Google Scholar]
- 21. Spak C, Johnson G, Ekstrand J. Caries incidence, salivary flow rate and efficacy of fluoride gel treatment in irradiated patients. Caries research, 1994. 28(5): p. 388–393. [DOI] [PubMed] [Google Scholar]
- 22. Chao K, Majhail N, Huang Cj, Simpson JR, Perez CA, Haughey B, et al. Intensity-modulated radiation therapy reduces late salivary toxicity without compromising tumor control in patients with oropharyngeal carcinoma: a comparison with conventional techniques. Radiotherapy and oncology, 2001. 61(3): p. 275–280. [DOI] [PubMed] [Google Scholar]
- 23. Kwong DL, Pow EH, Sham JS, McMillan AS, Leung LH, Leung WK, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: a prospective study on disease control and preservation of salivary function. Cancer, 2004. 101(7): p. 1584–93. [DOI] [PubMed] [Google Scholar]
- 24. Marta GN, Silva V, de Andrade Carvalho H, de Arruda FF, Hanna SA, Gadia R, et al. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiotherapy and Oncology, 2014. 110(1): p. 9–15. 10.1016/j.radonc.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 25. Kielbassa AM, Schendera A, Schulte-Monting J. Microradiographic and microscopic studies on in situ induced initial caries in irradiated and nonirradiated dental enamel. Caries Res, 2000. 34(1): p. 41–7. [DOI] [PubMed] [Google Scholar]
- 26. Kielbassa AM, Hellwig E, Meyer-Lueckel H. Effects of irradiation on in situ remineralization of human and bovine enamel demineralized in vitro. Caries Res, 2006. 40(2): p. 130–5. [DOI] [PubMed] [Google Scholar]
- 27. AlmståhI A, Wikström M, Stenberg I, Jakobsson A, Fagerberg-Mohlin B. Oral microbiota associated with hyposalivation of different origins. Oral microbiology and immunology, 2003. 18(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 28. Almstahl A, Wikstrom M, Fagerberg-Mohlin B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis, 2008. 14(6): p. 541–9. 10.1111/j.1601-0825.2007.01416.x [DOI] [PubMed] [Google Scholar]
- 29. Gafan GP, Lucas VS, Roberts GJ, Petrie A, Wilson M, Spratt DA. Statistical analyses of complex denaturing gradient gel electrophoresis profiles. J Clin Microbiol, 2005. 43(8): p. 3971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu YJ, Wang Q, Jiang YT, Ma R, Xia WW, Tang ZS, et al. Characterization of oral bacterial diversity of irradiated patients by high-throughput sequencing. Int J Oral Sci, 2013. 5(1): p. 21–5. 10.1038/ijos.2013.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu YJ, Shao ZY, Wang Q, Jiang YT, Ma R, Tang ZS, et al. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS One, 2013. 8(2): p. e56343 10.1371/journal.pone.0056343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortholan C, Chamorey E, Benezery K, Thariat J, Dassonville O, Poissonnet G, et al. Modeling of salivary production recovery after radiotherapy using mixed models: determination of optimal dose constraint for IMRT planning and construction of convenient tools to predict salivary function. Int J Radiat Oncol Biol Phys, 2009. 73(1): p. 178–86. 10.1016/j.ijrobp.2008.03.068 [DOI] [PubMed] [Google Scholar]
- 33. Almstahl A, Wikstrom M. Oral microflora in subjects with reduced salivary secretion. Journal of dental research, 1999. 78(8): p. 1410–1416. [DOI] [PubMed] [Google Scholar]
- 34. Campus G, Cagetti MG, Sale S, Carta G, Lingstrom P. Cariogram validity in schoolchildren: a two-year follow-up study. Caries Res, 2012. 46(1): p. 16–22. 10.1159/000334932 [DOI] [PubMed] [Google Scholar]
- 35. Gold OG, Jordan H, Van Houte J. A selective medium for Streptococcus mutans. Archives of Oral Biology, 1973. 18(11): p. 1357–1364. [DOI] [PubMed] [Google Scholar]
- 36. Dal Bello F, Hertel C. Oral cavity as natural reservoir for intestinal lactobacilli. Systematic and applied microbiology, 2006. 29(1): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Ismail AI, Ge Y, Tellez M, Sohn W. Similarity of bacterial populations in saliva from African-American mother-child dyads. Journal of clinical microbiology, 2007. 45(9): p. 3082–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang R, Kaplan A, Guo L, Shi W, Zhou X, Lux R, et al. The influence of iron availability on human salivary microbial community composition. Microbial ecology, 2012. 64(1): p. 152–161. 10.1007/s00248-012-0013-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang W, Long X, Huo X, Chen Y, Lou K. 16S rRNA-Based PCR-DGGE Analysis of Actinomycete Communities in Fields with Continuous Cotton Cropping in Xinjiang, China. Microbial ecology, 2013. 66(2): p. 385–393. 10.1007/s00248-012-0160-5 [DOI] [PubMed] [Google Scholar]
- 40. Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, et al. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Applied and environmental microbiology, 2001. 67(10): p. 4742–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tao Y, Zhou Y, Ouyang Y, Lin H. Dynamics of oral microbial community profiling during severe early childhood caries development monitored by PCR-DGGE. Archives of oral biology, 2013. 58(9): p. 1129–1138. 10.1016/j.archoralbio.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 42. Boon N, Windt W, Verstraete W, Top EM. Evaluation of nested PCR–DGGE (denaturing gradient gel electrophoresis) with group-specific 16S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiology Ecology, 2002. 39(2): p. 101–112. 10.1111/j.1574-6941.2002.tb00911.x [DOI] [PubMed] [Google Scholar]
- 43. Ono K, Morimoto Y, Inoue H, Masuda W, Tanaka T, Inenaga K. Relationship of the unstimulated whole saliva flow rate and salivary gland size estimated by magnetic resonance image in healthy young humans. Arch Oral Biol, 2006. 51(4): p. 345–9. [DOI] [PubMed] [Google Scholar]
- 44. Roesink JM, Terhaard CH, Moerland MA, van Iersel F, Battermann JJ. CT-based parotid gland location: implications for preservation of parotid function. Radiotherapy and Oncology, 2000. 55(2): p. 131–133. [DOI] [PubMed] [Google Scholar]
- 45. Eliasson L, Almstahl A, Lingstrom P, Wikstrom M, Carlen A. Minor gland saliva flow rate and proteins in subjects with hyposalivation due to Sjogren's syndrome and radiation therapy. Arch Oral Biol, 2005. 50(3): p. 293–9. [DOI] [PubMed] [Google Scholar]
- 46. Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. The Journal of prosthetic dentistry, 2001. 85(2): p. 162–169. [DOI] [PubMed] [Google Scholar]
- 47. Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys, 2006. 66(4): p. 981–91. [DOI] [PubMed] [Google Scholar]
- 48. Ben-Aryeh H, Gutman D, Szargel R, Laufer D. Effects of irradiation on saliva in cancer patients. International journal of oral surgery, 1975. 4(5): p. 205–210. [DOI] [PubMed] [Google Scholar]
- 49. Dreizen S, Brown LR, Handler S, Levy BM. Radiation-induced xerostomia in cancer patients. Effect on salivary and serum electrolytes. Cancer, 1976. 38(1): p. 273–278. [DOI] [PubMed] [Google Scholar]
- 50. Puy CL. The role of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal, 2006. 11: p. E449–55. [PubMed] [Google Scholar]
- 51. Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis, 2012. 18(6): p. 595–601. 10.1111/j.1601-0825.2012.01915.x [DOI] [PubMed] [Google Scholar]
- 52. Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J, 2012. 6(1): p. 1–10. 10.1038/ismej.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Ge Y, Saxena D, Caufield PW. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J Clin Microbiol, 2007. 45(1): p. 81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Y, Ku CYS, Xu J, Saxena D, Caufield PW. Survey of Oral Microbial Diversity using PCR-based Denaturing Gradient Gel Electrophoresis. Journal of Dental Research, 2005. 84(6): p. 559–564. [DOI] [PubMed] [Google Scholar]
- 55. Keijser B, Zaura E, Huse S, Van Der Vossen J, Schuren F, Montijn R, et al. Pyrosequencing analysis of the oral microflora of healthy adults. Journal of Dental Research, 2008. 87(11): p. 1016–1020. [DOI] [PubMed] [Google Scholar]
- 56. Zarco M, Vess T, Ginsburg G. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral diseases, 2012. 18(2): p. 109–120. 10.1111/j.1601-0825.2011.01851.x [DOI] [PubMed] [Google Scholar]
- 57. Jiang W, Ling Z, Lin X, Chen Y, Zhang J, Yu J, et al. Pyrosequencing Analysis of Oral Microbiota Shifting in Various Caries States in Childhood. Microbial ecology, 2014. 67(4): p. 962–969. 10.1007/s00248-014-0372-y [DOI] [PubMed] [Google Scholar]
- 58. Belstrom D, Fiehn NE, Nielsen CH, Holmstrup P, Kirkby N, Klepac-Ceraj V, et al. Altered Bacterial Profiles in Saliva from Adults with Caries Lesions: A Case-Cohort Study. Caries Res, 2014. 48(5): p. 368–375. 10.1159/000357502 [DOI] [PubMed] [Google Scholar]
- 59. Haffajee AD, Teles RP, Patel MR, Song X, Veiga N, Socransky SS. Factors affecting human supragingival biofilm composition. I. Plaque mass. J Periodontal Res, 2009. 44(4): p. 511–9. 10.1111/j.1600-0765.2008.01154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Almståhl A, Wikström M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Archives of oral biology, 2003. 48(5): p. 337–344. [DOI] [PubMed] [Google Scholar]
- 61. Watanabe K, Frommel T. Detection of Porphyromonas gingivalis in oral plaque samples by use of the polymerase chain reaction. Journal of dental research, 1993. 72(6): p. 1040–1044. [DOI] [PubMed] [Google Scholar]
- 62. Cocolin L, Bisson L, Mills D. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiology Letters, 2000. 189(1): p. 81–87. [DOI] [PubMed] [Google Scholar]
- 63. Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and environmental microbiology, 1993. 59(3): p. 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol, 2010. 60(3): p. 677–90. 10.1007/s00248-010-9712-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are represented in the figures and tables of the manuscript.