Abstract
Background
Chlorhexidine is a broad-spectrum antimicrobial commonly used to disinfect the skin of patients to reduce the risk of healthcare-associated infections. Because chlorhexidine is not sporicidal, it is not anticipated that it would have an impact on skin contamination with Clostridium difficile, the most important cause of healthcare-associated diarrhea. However, although chlorhexidine is not sporicidal as it is used in healthcare settings, it has been reported to kill spores of Bacillus species under altered physical and chemical conditions that disrupt the spore’s protective barriers (e.g., heat, ultrasonication, alcohol, or elevated pH). Here, we tested the hypothesis that similarly altered physical and chemical conditions result in enhanced sporicidal activity of chlorhexidine against C. difficile spores.
Principal Findings
C. difficile spores became susceptible to heat killing at 80°C within 15 minutes in the presence of chlorhexidine, as opposed to spores suspended in water which remained viable. The extent to which the spores were reduced was directly proportional to the concentration of chlorhexidine in solution, with no viable spores recovered after 15 minutes of incubation in 0.04%–0.0004% w/v chlorhexidine solutions at 80°C. Reduction of spores exposed to 4% w/v chlorhexidine solutions at moderate temperatures (37°C and 55°C) was enhanced by the presence of 70% ethanol. However, complete elimination of spores was not achieved until 3 hours of incubation at 55°C. Elevating the pH to ≥9.5 significantly enhanced the killing of spores in either aqueous or alcoholic chlorhexidine solutions.
Conclusions
Physical and chemical conditions that alter the protective barriers of C. difficile spores convey sporicidal activity to chlorhexidine. Further studies are necessary to identify additional agents that may allow chlorhexidine to reach its target within the spore.
Introduction
Chlorhexidine is a cationic bisbiguanide with activity against Gram-negative and Gram-positive bacteria, yeasts, and enveloped viruses [1–3]. Due to its broad-spectrum antimicrobial activity, chlorhexidine is used in a wide variety of disinfectant, antiseptic and preservative applications [2]. In healthcare settings, chlorhexidine is routinely used to disinfect the skin of patients prior to surgical procedures and catheter insertion to reduce the risk of healthcare-associated infections [4,5]. Furthermore, daily bathing of patients with chlorhexidine gluconate (CHG) has become increasingly prevalent because it has been shown to reduce the incidence of bloodstream infections and acquisition of multidrug-resistant organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) [6–10].
Clostridium difficile is an anaerobic, spore-forming bacterium that is the most important cause of healthcare-associated diarrhea [11,12]. Patients with C. difficile infection (CDI) shed spores in stool, resulting in contamination of their skin, clothing, and environmental surfaces [13]. C. difficile spores on skin are considered a major source of transmission because they can easily be acquired on the hands of healthcare workers [14–16]. In addition, skin contamination could contribute to recurrence of infection if spores are transferred to the hands of CDI patients and ingested. Thus, effective methods to reduce the burden of spores on skin could be helpful to reduce transmission and recurrence. Although showering reduced the burden of C. difficile spores on CDI patients’ skin to a modest degree through mechanical removal, bed baths using soap and water were ineffective [17]. Because chlorhexidine is not a sporicidal agent as applied in healthcare settings, it is not anticipated that CHG bathing, particularly when applied as a bed bath, would have an impact on the burden of spores on skin or on the incidence of CDI. However, Rupp et al. [18] recently reported that hospital-wide CHG patient bathing was associated with a significant reduction in the incidence of healthcare-associated CDI. The basis for this unexpected finding is unclear since the impact of bathing on the burden of spores on skin was not assessed.
Chlorhexidine is a membrane active compound that disrupts the protective barriers of vegetative organisms by interacting with the negative charges associated with the cell wall and plasma membrane [19,20]. Bacterial spores have several proteinaceous coats that surround and protect the dormant bacterial cell wall and membrane, making the antimicrobial properties of chlorhexidine ineffective against intact dormant spores [21,22]. Nonetheless, by applying physical and chemical conditions that degrade or allow penetration of the protective spore coats, chlorhexidine may reach its target within the spore [23–25]. For example, chlorhexidine does not kill dormant spores under ambient conditions, but as demonstrated in Bacillus species, sporicidal activity is observed at elevated temperatures. Bacillus spores suspended in 0.01% to 1% CHG solutions were reduced by 5 log10 colony forming units (CFU) after 6 minutes of exposure to temperatures of 98–100°C, as compared to a 1 log10CFU reduction in the absence of chlorhexidine [23]. In conjunction with sporicidal activity induced at elevated temperatures, several studies have reported that the presence of alcohol, elevated pH, and ultra-sonication enhance the sporicidal character of chlorhexidine at sub-lethal temperatures ranging from 37–55°C [23–25]. As a consequence, chlorhexidine has been recommended for cold liquid chemo-sterilization of thermolabile equipment and materials at moderate temperatures with the addition of an enhancing agent such as ultra-sonication, alcohol, or elevated pH [23].
Previous studies have not examined the impact of altered physical and chemical conditions on activity of chlorhexidine against C. difficile spores. Therefore, we tested the hypothesis that similarly altered physical and chemical conditions result in enhanced sporicidal activity of chlorhexidine against C. difficile spores. Initially, we examined the effects of chlorhexidine gluconate (CHG) and chlorhexidine free base (CHX) on C. difficile spores at 80°C. Next, we determined the effects of ethanol and isopropanol on sub-lethal heat treatments in the presence of CHG. Finally, we assessed the impact of pH on the sporicidal character of chlorhexidine at elevated temperatures.
Materials and Methods
Clostridium difficile Strains
Two strains cultured from patients with CDI at the Cleveland VA Medical Center were studied. VA 17 is an epidemic (cdtB+) restriction endonuclease analysis (REA) BI strain and VA 11 is a non-epidemic (cdtB-) REA J strain. Both isolates are toxigenic (tcdA+, tcdB+) strains. The Institutional Review Board of the Cleveland VA Medical Center approved the study protocol for collection of all patient isolates. Informed consent was not obtained because the isolates were cultured from clinical samples with no collection of patient identifiers or interaction with subjects.
Preparation of Clostridium difficile Spores
C. difficile spores were prepared as previously described [26]. In brief, pre-reduced brain-heart infusion plates were spread with 100 μl of a 24-hour suspension of a culture of C. difficile and incubated for one week in a Whitley MG1000 anaerobic workstation (Microbiology International, Frederick, MD). Spores were harvested from 10 plates using sterile swabs and 8 mL of ice-cold, sterile, distilled water. Spores were washed five times by centrifuging at 15,000 x g for 5 min and re-suspending in distilled water. After washing, the spores were collected in 1 mL of 20% (w/v) HistoDenz and layered onto 20 mL of 50% (w/v) HistoDenz solution. The gradient was centrifuged at 15,000 x g for 15 min and the spore pellet was carefully collected from the bottom of the tube and washed with distilled water three times. Spores were stored at 4°C in sterile distilled water until use. Prior to testing, spore preps were confirmed by phase contrast microscopy and malachite green staining to be >99% dormant, bright-phase spores.
Heat Susceptibility of Clostridium difficile Spores Exposed to Chlorhexidine
Dormant C. difficile spores remain 100% viable when incubated at 80°C for up to 15 minutes [27]. Initial experiments were performed to assess the viability of C. difficile spores in the presence of chlorhexidine free base (Sigma-Aldrich, St. Louis, MO) or chlorhexidine gluconate (Sigma-Aldrich, St. Louis, MO) at 80°C. Solutions of chlorhexidine free base (CHX) and chlorhexidine gluconate (CHG) were prepared in sterile deionized water at concentrations of 0.4 (0.04% w/v), 0.04 (0.004% w/v), and 0.004 (0.0004% w/v) mg/mL. Ten microliter aliquots of C. difficile spores (5 log10CFU VA11 or VA17) were suspended in 1 mL of CHX, CHG, or sterile deionized water (positive control). Spore suspensions were incubated in an 80°C water bath for 5, 10, or 15 minutes. Vegetative C. difficile is acutely sensitive to chlorhexidine; therefore, to ensure that organisms were not inhibited from growing due solely to the presence of chlorhexidine, spores were incubated in water, CHX, and CHG preparations at room temperature (~22°C) to serve as verification of unrestricted outgrowth.
To quantify viable organisms, aliquots of the spore suspensions were neutralized 1:1 in Dey-Engley neutralizer (Becton Dickinson, Cockeysville, MD), then serially diluted and drop-plated onto pre-reduced cycloserine-cefoxitin-brucella agar containing 0.1% taurocholic acid and lysozyme 5 mg/L (CDBA) in a Whitley Workstation MG1000 anaerobic chamber (Microbiology International, Frederick, MD). For samples that yielded below the limit of detection following the serial dilution plating method, experiments were repeated and 1 mL of the neutralized sample was spread onto CDBA to detect low levels of C. difficile. Recovery of zero colony forming units after three spread plate trials was considered 100% reduction. To determine if carry-over of chlorhexidine was effectively neutralized and not affecting recovery of viable vegetative organisms, the American Society for Testing and Materials “Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents” was performed for the concentration of chlorhexidine in spread plate samples [28]. Following 48 hours of incubation at 37°C, log10CFU reductions were calculated by comparing the log10CFU recovered from chlorhexidine solutions to untreated controls (spores suspended in water). All experiments were repeated four times.
The Effect of Alcohol on Heat Killing of Clostridium difficile Spores Exposed to Chlorhexidine
It has been previously demonstrated that alcohol enhances heat killing of Bacillus spores exposed to chlorhexidine [23–25]. To determine if heat killing of C. difficile spores is similarly enhanced by alcoholic chlorhexidine solutions, solutions of 4% (40mg/mL) CHG were prepared in sterile deionized water (aqueous CHG) or 70% ethanol (alcoholic CHG). Ten microliter aliquots of C. difficile spores (6 log10 CFU, VA17) were suspended in 1 mL of aqueous CHG, alcoholic CHG, or sterile deionized water (positive control). Spore suspensions were incubated at room temperature (20°C), 37°C, or 55°C in a water bath for 0, 1, 2, and 3 hours. At each time point, aliquots of the spore suspensions were neutralized 1:1 in Dey-Engley neutralizer (Becton Dickinson, Cockeysville, MD) and viable organisms were enumerated as described above in Heat Susceptibility of C. difficile Spores Exposed to Chlorhexidine. For samples that yielded below the limit of detection following the serial dilution plating method, experiments were repeated and 1 mL of the neutralized sample was spread onto CDBA to detect low levels of C. difficile. Recovery of zero colony forming units after three spread plate trials was considered 100% reduction. To determine if carry-over of chlorhexidine was effectively neutralized and not affecting recovery of viable vegetative organisms, the American Society for Testing and Materials “Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents” was performed for the concentration of chlorhexidine in spread plate samples [28]. Log10CFU reductions were calculated by comparing the log10CFU recovered from chlorhexidine solutions to untreated controls (spores suspended in water). All experiments were repeated three times.
Additionally, ethanol and isopropanol were comparatively assessed to determine whether the form of alcohol had an impact on heat killing of spores exposed to chlorhexidine. Solutions of CHG (4% and 0.04%) were prepared in water, 70% isopropanol, or 70% ethanol. Ten microliter aliquots of C. difficile spores (6 log10CFU, VA17) were suspended in 1 mL of aqueous CHG, alcoholic CHG (ethanol or isopropanol), or sterile deionized water (positive control). Spore suspensions were incubated at 55°C in a water bath for 0, 1, and 3 hours. At each time point, aliquots of the spore suspensions were neutralized 1:1 in Dey-Engley neutralizer and viable organisms were enumerated as described above in Heat Susceptibility of C. difficile Spores Exposed to Chlorhexidine. Log10CFU reductions were calculated by comparing the log10CFU recovered from chlorhexidine solutions to untreated controls (spores suspended in water). All experiments were repeated three times.
The Impact of pH on Heat Killing of Clostridium difficile Spores Exposed to Chlorhexidine
To determine the impact of pH on heat killing of spores exposed to chlorhexidine, 0.04% (0.4 mg/mL) solutions of CHG prepared in water, 70% isopropanol, or 70% ethanol were altered with either hydrochloric acid or sodium hydroxide to a final pH of 4.0, 9.5, or 11.5. Ten microliter aliquots of C. difficile spores (6 log10 CFU VA17) were suspended in 1 mL of pH altered aqueous CHG, alcoholic CHG (ethanol or isopropanol), or sterile deionized water (positive control). Spore suspensions were incubated at 55°C in a water bath for 0, 1, and 3 hours. At each time point, aliquots of the spore suspensions were neutralized 1:1 in Dey-Engley neutralizer (Becton Dickinson, Cockeysville, MD) and viable organisms were enumerated as described above in Heat Susceptibility of C. difficile Spores Exposed to Chlorhexidine. For samples that yielded below the limit of detection following the serial dilution plating method, experiments were repeated and 1 mL of the neutralized sample was spread onto CDBA to detect low levels of C. difficile. Recovery of zero colony forming units after three spread plate trials was considered 100% reduction. To determine if carry-over of chlorhexidine was effectively neutralized and not affecting recovery of viable vegetative organisms, the American Society for Testing and Materials “Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents” was performed for the concentration of chlorhexidine in spread plate samples [28]. Log10CFU reductions were calculated by comparing the log10CFU recovered from chlorhexidine solutions to untreated controls (spores suspended in water). All experiments were repeated three times.
Data Analysis
Data were analyzed using STATA 9.0 (StataCorp, College Station, TX). Continuous data were analyzed using unpaired t tests. The means of the data from experiments conducted are presented. Error bars indicate standard error.
Results
Heat Susceptibility of Clostridium difficile Spores Exposed to Chlorhexidine
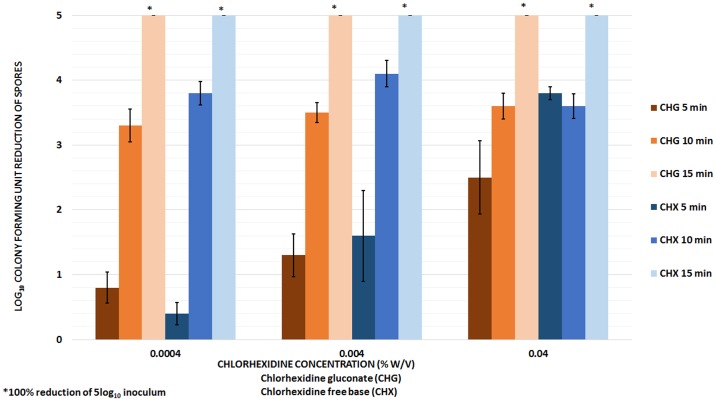
Fig 1 shows the mean log10CFU reduction of C. difficile spores exposed to CHG and CHX solutions at 80°C. There was no significant difference in the reductions achieved by the two strains of C. difficile spores assessed (VA11 and VA17); therefore, data for both strains were pooled for analysis (P >0.01 for each comparison). Spores suspended in sterile water were not killed by heating to 80°C for 15 minutes. Additionally, neutralization was shown to be effective for all concentrations of chlorhexidine assessed, therefore, killing of spores was not an artifact of inhibition of growing vegetative organisms due to carry-over of chlorhexidine onto culture media. There was no significant difference in the reductions achieved by equivalent concentrations of CHG or CHX solutions (P >0.01 for each concentration compared); therefore, in subsequent experiments CHG solutions were used because it is readily soluble in aqueous and alcoholic solvents. After 5 minutes of exposure to chlorhexidine (CHG or CHX) at 80°C, the killing effects of heat and chlorhexidine increased as the concentration of chlorhexidine was increased (<1log10CFU reduction for 0.004 mg/mL, >1 log10CFU reduction for 0.04 mg/mL, and >2 log10CFU reduction for 0.4 mg/mL). However, after 10 or 15 minutes of exposure to chlorhexidine at 80°C, similar reductions were achieved at each concentration (>3 log10CFU reduction after 10 minutes and ≥5 log10CFU reduction after 15 minutes). Complete elimination of spores was observed after 15 minutes of exposure to CHG or CHX solutions at 80°C.
Fig 1. Heat killing of Clostridium difficile spores exposed to chlorhexidine gluconate (CHG) and chlorhexidine free base (CHX) solutions.
The mean log10colony-forming unit (CFU) reduction of C. difficile spores exposed to CHG and CHX solutions at 80°C. After 5 minutes of exposure to CHG or CHX, heat killing increased as the concentration of chlorhexidine was increased. However, after 10 or 15 minutes of exposure to chlorhexidine at 80°C, similar reductions were achieved at each concentration. The means of the data from four experiments conducted are presented. Error bars indicate standard error.
The Effect of Alcohol on Heat Killing of Clostridium difficile Spores Exposed to Chlorhexidine
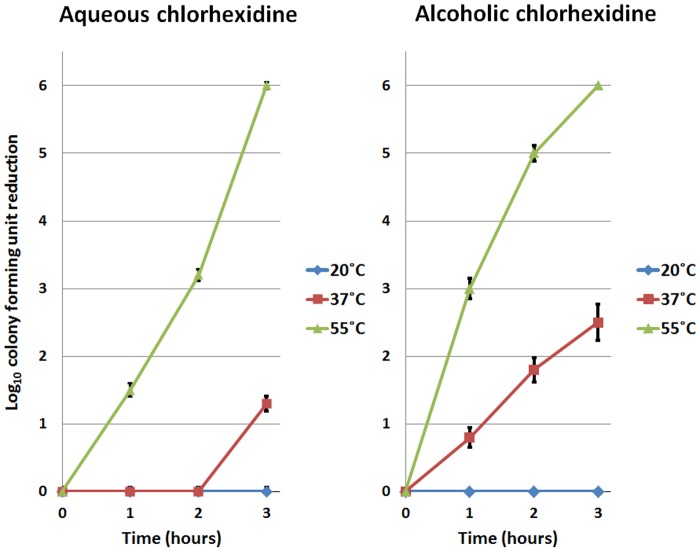
Fig 2 demonstrates that heat killing of C. difficile spores exposed to 4% (40 mg/mL) chlorhexidine was enhanced by the presence of 70% ethanol. No killing of spores was observed in aqueous or alcoholic chlorhexidine solutions at room temperature (20°C). At 37°C, spores exposed to aqueous chlorhexidine solution were reduced by ~1log10CFU after 3 hours of incubation. Alcohol enhanced spore killing at 37°C, reducing the incubation time from 3 hours to 1 hour to achieve ~1log10CFU reduction. Moreover, alcohol augmented the killing of spores after 3 hours of incubation at 37°C, increasing the reduction to >2log10CFU. At 55°C, alcohol boosted spore killing after 1 and 2 hours of incubation from 1.5 log10CFU (aqueous) to 3log10CFU (alcoholic), and 3log10CFU (aqueous) to 5log10CFU (alcoholic), respectively. Spores were 100% eliminated after 3 hours of exposure to either aqueous or alcoholic chlorhexidine solution at 55°C.
Fig 2. Enhancement of heat killing of Clostridium difficile spores exposed to alcoholic chlorhexidine gluconate (CHG) solutions.
The mean log10colony-forming unit (CFU) reduction of C. difficile spores exposed to 4% w/v CHG solution prepared in water or 70% ethanol. No killing of spores was observed in aqueous or alcoholic chlorhexidine solutions at 20°C. At 37°C, the presence of alcohol reduced the incubation time required to achieve an ~1 log10CFU reduction from 3 hours to 1 hour. At 55°C, alcohol boosted spore reductions from 1.5 log10CFU (aqueous) to 3log10CFU (alcoholic), and 3log10CFU (aqueous) to 5log10CFU (alcoholic) after 1 and 2 hours respectively. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
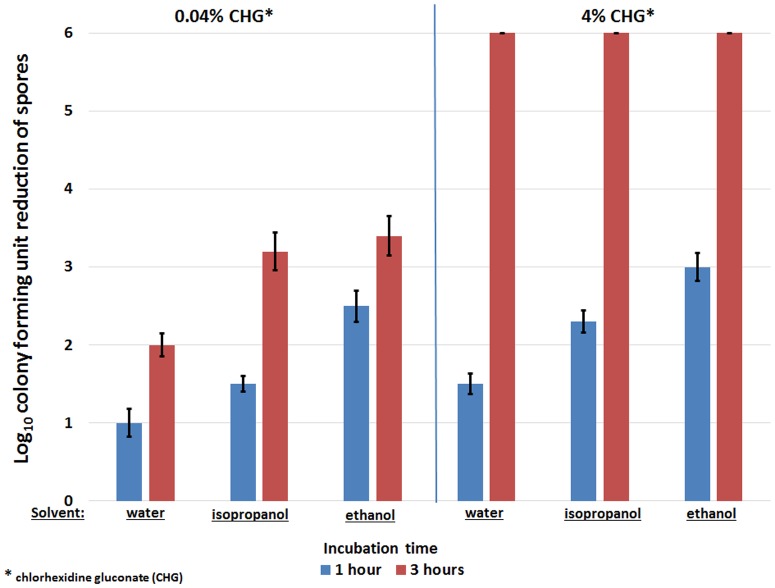
Fig 3 shows the difference in the mean log10CFU reductions of C. difficile spores achieved after 1 or 3 hours of exposure to CHG prepared in water, 70% isopropanol, or 70% ethanol at 55°C. CHG solutions prepared with ethanol significantly enhanced heat killing of spores after 1 hour of incubation compared to CHG solutions prepared in either isopropanol or water (P<0.01 compared to isopropanol; P<0.001 compared to water). When exposed to lower concentrations of CHG (0.04%), the presence of alcohol (isopropanol or ethanol) significantly enhanced reduction of spores after 1 or 3 hours of incubation compared to 0.04% CHG prepared in water. However, when the concentration of CHG was increased to 4%, the presence of alcohol (isopropanol and ethanol) only significantly enhanced heat killing of spores after 1 hour of incubation, because 100% reduction of spores was achieved by both aqueous and alcoholic CHG solutions after 3 hours of incubation.
Fig 3. Comparison of heat killing of Clostridium difficile spores in chlorhexidine gluconate (CHG) solutions prepared with isopropanol or ethanol.
The mean log10colony-forming unit (CFU) reductions of C. difficile spores achieved after 1 or 3 hours of exposure to 0.04% or 4% w/v CHG prepared in water, 70% isopropanol, or 70% ethanol at 55°C. CHG solutions prepared with ethanol significantly enhanced heat killing of spores after 1 hour of incubation compared to CHG solutions prepared in either isopropanol or water (P <0.01 compared to isopropanol; P <0.001 compared to water). After 3 hours of incubation in 0.04% w/v CHG, both isopropanol and ethanol enhanced reduction of spores compared to aqueous CHG solution; however, at increased CHG concentrations (4% w/v), spores were completely eliminated by both aqueous and alcoholic preparations. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
The Impact of pH on Heat Killing of Clostridium difficile Spores Exposed to Chlorhexidine
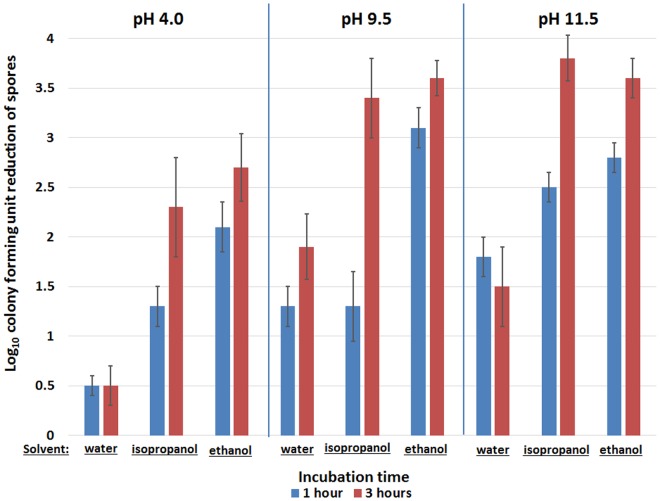
Fig 4 demonstrates the effect of pH on heat killing of spores (55°C) exposed to aqueous and alcoholic CHG solutions (0.04% chlorhexidine gluconate). Elevating the pH to ≥9.5 significantly enhanced the killing of spores in either aqueous or alcoholic CHG solutions after 1 and 3 hours of incubation (P<0.001 for each comparison to pH 4.0). In aqueous CHG solutions, increasing the pH to ≥9.5 enhanced heat killing of spores by ≥1log10CFU after 1 or 3 hours of incubation. Similarly, after 3 hours of incubation, increasing the pH to ≥9.5 enhanced heat killing of spores by ≥1log10CFU in CHG solutions prepared in isopropanol or ethanol.
Fig 4. The Impact of pH on heat killing of Clostridium difficile spores exposed to chlorhexidine gluconate (CHG).
The mean log10colony-forming unit (CFU) reductions of C. difficile spores achieved after 1 or 3 hours of incubation in pH altered aqueous and alcoholic CHG solutions (0.04% w/v). Elevating the pH to ≥9.5 significantly enhanced the killing of spores in either aqueous or alcoholic CHG solutions (P <0.001 for each comparison to pH 4.0). In aqueous or alcoholic CHG solutions, increasing the pH to ≥9.5 enhanced heat killing of spores by ≥1log10CFU after 3 hours of incubation. The means of the data from experiments conducted in triplicate are presented. Error bars indicate standard error.
Discussion
We found that in the presence of chlorhexidine (CHG or CHX), C. difficile spores became susceptible to heat killing at 80°C within 15 minutes, as opposed to spores suspended in water, which remained viable after 15 minutes of incubation at 80°C. The extent to which the spores were reduced was directly proportional to the concentration of chlorhexidine in solution. No viable spores were recovered after 15 minutes of incubation in 0.04%- 0.0004% w/v chlorhexidine solutions at 80°C. Reduction of spores exposed to 4% w/v chlorhexidine solutions at moderate temperatures (37°C and 55°C) was enhanced by the presence of 70% ethanol, but complete elimination of spores was not achieved until 3 hours of incubation at 55°C. Ethanol was superior to isopropanol for enhancement of heat killing at 55°C after 1 hour of incubation, but after 3 hours of incubation isopropanol and ethanol provided equivalent enhancement of heat killing. Elevating the pH to ≥9.5 significantly enhanced the killing of spores in either aqueous or alcoholic chlorhexidine solutions. These data suggest that the altered physical and chemical conditions that result in enhanced sporicidal activity of chlorhexidine against Bacillus spp. spores result in similar enhancement of sporicidal activity against C. difficile spores.
Our findings have several important implications. First, it is impractical to imply that the sporicidal effects of chlorhexidine, alcohol, and high temperatures (or long exposures to more moderate temperatures) would be a promising approach for disinfection of C. difficile spores from skin or environmental surfaces. However, we can postulate that more benign physical or chemical agents that cause similar denaturation of the spore’s coat might provide a means to enhance the sporicidal activity of chlorhexidine. Future studies may provide insight into alternative means to allow chlorhexidine to reach its target within the dormant spore. Second, as previously demonstrated, the sporicidal activity of chlorhexidine was increased under basic conditions (pH ≥9.5). One potential explanation for this observation is that a basic environment may serve as an additional form of denaturation. It is well documented that base is an effective protein denaturant [29]. Alternatively, under basic conditions chlorhexidine is largely non-ionized [25]. Non-ionized forms of molecules have been shown to more readily permeate the spore’s protective coats [22, 25]. Finally, our findings suggest some potential mechanisms by which CHG bathing as currently practiced could reduce the burden of spores on skin. Previous studies have shown that chlorhexidine has a persistent effect for up to 24 hours after application to the skin [30, 31]. We can postulate that the elevated temperature of skin in combination with the persistent effect of high concentrations of chlorhexidine may reduce spores over extended periods of time. Future studies are necessary to determine the impact of CHG bathing on levels of spores on skin of CDI patients.
Our study has some limitations. First, there is ambiguity in the literature regarding the effect of chlorhexidine on the germination and outgrowth of spores [5, 32]. In the present study we did not determine whether the combination of chlorhexidine and altered physical and chemical conditions induced or inhibited spore germination. Further studies are necessary to ascertain whether germination was stimulated or halted in effected spores. However, spores were neutralized and exposed to rich nutrient media containing specific C. difficile germinants post treatment. Consequently, the killing effects observed were permanent even under germination stimulation conditions. Additionally, uninhibited outgrowth was confirmed by recovery of viable organisms after exposure to each individual physical or chemical condition assessed (i.e. chlorhexidine alone, heat alone, etc.). Second, the effect of organic load on the efficacy of chlorhexidine’s induced sporicidal activity was not assessed. However, previous studies on Bacillus spores showed that organic load did not reduce the killing efficacy of chlorhexidine under altered chemical and physical conditions [23]. Lastly, elevated temperatures were required to actuate chlorhexidine’s sporicidal character, with or without the addition of a secondary enhancement condition (i.e. the presence of alcohol or elevated pH). Further research is necessary to elucidate the changes that heat imparts on the dormant spore which create an opportunistic environment for chlorhexidine’s activity.
Supporting Information
Raw data and statistical analysis for Fig 1.
(XLSX)
Raw data and statistical analysis for Fig 2.
(XLSX)
Raw data and statistical analysis for Fig 3.
(XLSX)
Raw data and statistical analysis for Fig 4.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Merit Review grant from the Department of Veterans Affairs to CJD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Davies GEJ, Francis AR, Martin FL, Swain G. 1,6-Di-4’-chlorophenyl-diguanidohexane (Hibitane). Laboratory investigation of a new anti-bacterial agent of high potency. Br J Pharmacol. 1954;9: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardner JF, Gray KG. Antiseptics for skin preparation prior to procedures In: Block SS, editor. Disinfection, Sterilisation and Preservation. Philadelphia, Pennsylvania: Lea and Febiger; 1983. pp. 251–270. [Google Scholar]
- 3. Gorman SP, Jones DS, Loftus AM. The sporicidal activity and inactivation of chlorhexidine gluconate in aqueous and alcoholic solution. J Appl Bacteriol. 1987;63: 193–198. [DOI] [PubMed] [Google Scholar]
- 4. Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46: 274–281. 10.1086/524736 [DOI] [PubMed] [Google Scholar]
- 5. Denton GW. Chlorhexidine In: Block SS, editor. Disinfection, Sterilization, and Preservation. 5th ed Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2001. pp. 321–336. [Google Scholar]
- 6. Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167: 2073–2079. [DOI] [PubMed] [Google Scholar]
- 7. Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37: 1858–1865. 10.1097/CCM.0b013e31819ffe6d [DOI] [PubMed] [Google Scholar]
- 8. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30: 959–963. 10.1086/605925 [DOI] [PubMed] [Google Scholar]
- 9. Vernon MO, Hayden MK, Trick WE, Hayes RA, Bloom DW, Weinstein RA, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006;166: 306–312. [DOI] [PubMed] [Google Scholar]
- 10. Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2010;145: 240–246. 10.1001/archsurg.2010.5 [DOI] [PubMed] [Google Scholar]
- 11. Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353: 2442–2449. [DOI] [PubMed] [Google Scholar]
- 12. Poxton IR, McCoubrey J, Blair G. The pathogenicity of Clostridium difficile . Clin Microbiol Infect. 2001;7: 421–427. [DOI] [PubMed] [Google Scholar]
- 13. Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26: 338–344. 10.1097/QCO.0b013e3283630f04 [DOI] [PubMed] [Google Scholar]
- 14. Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile . Infect Control Hosp Epidemiol. 2009;30: 939–944. 10.1086/605322 [DOI] [PubMed] [Google Scholar]
- 15. Pop-Vicas A, Baier R. Healthcare workers' hands and Clostridium difficile spores: making progress? Infect Control Hosp Epidemiol. 2014;35: 16–17. 10.1086/674397 [DOI] [PubMed] [Google Scholar]
- 16. Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Brun-Buisson C. Contamination of healthcare workers' hands with Clostridium difficile spores after caring for patients with C.difficile infection. Infect Control Hosp Epidemiol. 2014;35: 10–15. 10.1086/674396 [DOI] [PubMed] [Google Scholar]
- 17. Jury LA, Guerrero DM, Burant CJ, Cadnum JL, Donskey CJ. Effectiveness of routine patient bathing to decrease the burden of spores on the skin of patients with Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32: 181–184. 10.1086/657911 [DOI] [PubMed] [Google Scholar]
- 18. Rupp ME, Cavalieri RJ, Lyden E, Kucera J, Martin M, Fitzgerald T, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33: 1094–1100. 10.1086/668024 [DOI] [PubMed] [Google Scholar]
- 19. Schindler PR, Teuber M. Ultrastructural study of Salmonella typhimurium treated with membrane-active agents: specific reaction dansylchloride with cell envelope components. J Bacteriol. 1978;135(1): 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hugo WB, Longworth AR. Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol. 1964;16: 655–662. [DOI] [PubMed] [Google Scholar]
- 21. Setlow P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007;15(4): 172–180. [DOI] [PubMed] [Google Scholar]
- 22. Leggett MJ, McDonnell G, Denyer SP, Setlow P, Maillard JY. Bacterial spore structures and their protective role in biocide resistance. J Appl Microbiol. 2012;113(3): 485–498. 10.1111/j.1365-2672.2012.05336.x [DOI] [PubMed] [Google Scholar]
- 23. Gorman SP, Jones DS, Loftus AM. The sporicidal activity and inactivation of chlorhexidine gluconate in aqueous and alcoholic solution. J Appl Bacteriol. 1987;63(2): 183–188. [DOI] [PubMed] [Google Scholar]
- 24. Gorman SP, Jones DS, Loftus AM. The synergistic effect of direct and indirect ultrasonic energy and chlorhexidine gluconate on spores of Bacillus subtilis. Int J Pharm. 1990;65: 127–132 [Google Scholar]
- 25. Jones DS, Loftus AM, Gorman SP. Physical factors affecting the sporicidal activity of chlorhexidine gluconate. Int J Pharm. 1995;119: 247–250. [Google Scholar]
- 26. Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190(7): 2505–2512. 10.1128/JB.01765-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez-Palacios A, Lejeune JT. Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile . Appl Environ Microbiol. 2011;77(9): 3085–3091. 10.1128/AEM.01589-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Society for Testing and Materials. Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents (Method E 1054–08). West Conshohocken, PA: American Society for Testing and Materials; 2008. [Google Scholar]
- 29. Macheboeuf M, Robert B. Studies on the denaturation of proteins. I. Auto-oxidation of proteins in alkaline medium. Bull Soc Chim Biol. 1953;35(5–6): 399–411. [PubMed] [Google Scholar]
- 30. Beausoleil CM, Paulson DS, Bogert A, Lewis GS. In vivo evaluation of the persistant and residual antimicrobial properties of three hand-scrub and hand-rub regimes in a simulated surgical environment. J Hosp Infect. 2012;81(4): 283–287. 10.1016/j.jhin.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 31. Nishihara Y, Kajiura T, Yokota K, Kobayashi H, Okubo T. A comparative clinical study focusing on the antimicrobial efficacies of chlorhexidine gluconate alcohol for patient skin preparations. J Infus Nurs. 2012;35(1): 44–50. 10.1097/NAN.0b013e31823d79ba [DOI] [PubMed] [Google Scholar]
- 32. Russell AD. Chlorhexidine: antibacterial action and bacterial resistance. Infection. 1986;14(5): 212–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data and statistical analysis for Fig 1.
(XLSX)
Raw data and statistical analysis for Fig 2.
(XLSX)
Raw data and statistical analysis for Fig 3.
(XLSX)
Raw data and statistical analysis for Fig 4.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.