Abstract
Background
We performed an updated meta-analysis of randomized placebo-controlled trials testing memantine monotherapy for patients with Alzheimer’s disease (AD).
Methods
The meta-analysis included randomized controlled trials of memantine monotherapy for AD, omitting those in which patients were also administered a cholinesterase inhibitor. Cognitive function, activities of daily living, behavioral disturbances, global function, stage of dementia, drug discontinuation rate, and individual side effects were compared between memantine monotherapy and placebo groups. The primary outcomes were cognitive function and behavioral disturbances; the others were secondary outcomes.
Results
Nine studies including 2433 patients that met the study’s inclusion criteria were identified. Memantine monotherapy significantly improved cognitive function [standardized mean difference (SMD)=−0.27, 95% confidence interval (CI)=−0.39 to −0.14, p=0.0001], behavioral disturbances (SMD=−0.12, 95% CI=−0.22 to −0.01, p=0.03), activities of daily living (SMD=−0.09, 95% CI=−0.19 to −0.00, p=0.05), global function assessment (SMD=−0.18, 95% CI=−0.27 to −0.09, p=0.0001), and stage of dementia (SMD=−0.23, 95% CI=−0.33 to −0.12, p=0.0001) scores. Memantine was superior to placebo in terms of discontinuation because of inefficacy [risk ratio (RR)=0.36, 95% CI=0.17¬ to 0.74, p=0.006, number needed to harm (NNH)=non significant]. Moreover, memantine was associated with less agitation compared with placebo (RR=0.68, 95% CI=0.49 to 0.94, p=0.02, NNH=non significant). There were no significant differences in the rate of discontinuation because of all causes, all adverse events, and individual side effects other than agitation between the memantine monotherapy and placebo groups.
Conclusions
Memantine monotherapy improved cognition, behavior, activities of daily living, global function, and stage of dementia and was well-tolerated by AD patients. However, the effect size in terms of efficacy outcomes was small and thus there is limited evidence of clinical benefit.
Introduction
Dementia is not only a significant individual health problem but also a societal burden. Alzheimer’s Disease International reported that over 35 million individuals worldwide currently live with dementia (http://wwwalzcouk/research/world-report). Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive loss of cognitive function and other neurobehavioral symptoms. The pathology of AD includes the accumulation of extracellular senile plaques composed primarily of β-amyloid and intracellular neurofibrillary tangles comprising abnormally hyperphosphorylated tau, a microtubule-associated protein [1].
Currently, memantine is available in Japan for the treatment of AD and was approved for the treatment of moderate-to-severe AD by the United States Food and Drug Administration (FDA). Memantine is believed to exert its therapeutic effect by acting as a low-to-moderate affinity, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist that binds preferentially to open NMDA receptor-operated calcium channels [2, 3]. This activity-dependent binding blocks NMDA-mediated ion flux and ameliorates the deleterious effects of sustained, pathologically elevated levels of glutamate (excitotoxicity) that may lead to neuronal dysfunction [4]. The efficacy of memantine in the management of patients with AD, vascular dementia, and mixed dementia was assessed in a Cochrane meta-analysis including 12 randomized controlled trials (RCTs) [5]. The meta-analysis showed that memantine was superior to placebo in benefiting cognitive function for mild-to-moderate AD and moderate-to-severe AD [mild-to-moderate AD, using Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) [6], weighted mean difference (WMD) = −0.99, 95% confidence interval (CI) = −0.21 to −1.78, p = 0.013, I 2 = 0%, 3 studies, n = 1279; moderate-to-severe AD, using Severe Impairment Battery (SIB) [7], WMD = −2.97, 95% CI = −1.68 to −4.26, p = 0.00001, I 2 = 74%, 3 studies, n = 976]. However, the meta-analysis included both memantine monotherapy studies and memantine—cholinesterase inhibitor (ChEI) combination therapy studies. To clarify the clinical pharmacological characteristic of memantine, a meta-analysis should be conducted using data only from memantine monotherapy studies in patients with AD. A recent meta-analysis of 2 studies including 572 patients [8] suggested that memantine monotherapy did not significantly benefit moderate-to-severe AD as assessed by the Neuropsychiatric Inventory (NPI) [9] (WMD = −1.65, 95% CI = −4.78 to 1.49, p = 0.247, I 2 = 25.3%, 2 studies, n = not reported). However, apart from the small number of studies and patients, this meta-analysis did not evaluate other outcome measures such as cognitive function, activities of daily living, and global function assessment scores. To clarify whether memantine monotherapy is more efficacious than placebo for patients with AD, we have performed a meta-analysis of memantine monotherapy for AD including 9 studies with a total of 2433 patients.
Materials and Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10] (S1 PRISMA Checklist). We performed a systematic literature review using the PICO strategy (Patients: AD, Intervention: memantine monotherapy, Comparator: placebo or usual care, and Outcome: cognitive function, activities of daily living, behavioral disturbances, global function, stage of dementia, drug discontinuation rate, and individual side effects).
Inclusion Criteria, Search Strategy, Data Extraction, and Outcome Measures
We included RCTs of memantine monotherapy for patients with AD, omitting those in which patients were also administered a ChEI, and included studies that were not double blinded and/or placebo controlled (i.e., conventional treatment regimen) in order to obtain a larger patient population exposed to the drug. To identify relevant studies, we searched PubMed, the Cochrane Library databases, Google Scholar, EMBASE, CINAHL, and PsycINFO citations. There were no language restrictions, and we considered all studies published up to October 31, 2014. We used the following key words: “memantine” AND “Alzheimer’s disease” OR “Alzheimer disease.” Additional eligible studies were sought by searching the reference lists from primary articles and relevant reviews.
Two authors (S.M. and T.K.) of this meta-analysis scrutinized the patient inclusion and exclusion criteria for the identified studies. When data required for the meta-analysis were missing, the first and/or corresponding authors were contacted for additional information, including endpoint scores. All 3 authors of the current study independently extracted, checked, and entered the data into Review Manager (Version 5.2 for Windows, Cochrane Collaboration, http://ims.cochrane.org/revman). Discrepancies in different coding forms were resolved by discussions between authors (S.M. and T.K.)
Data Synthesis and Statistical Analysis
Each outcome measure reported here was used in at least 3 out of the 9 included studies. The primary outcome measures for efficacy were cognitive function and behavioral disturbances associated with AD. Cognitive function was measured using the SIB, ADAS-cog, Standardized Mini-Mental State Examination (SMMSE) [11], or MMSE [12]. Two studies [13, 14] used 2 cognitive function scales (SIB and MMSE) and 1 study [15] used the SIB, ADAS-cog, and MMSE scales. Because SIB was the most common instrument used in the studies included in the current meta-analysis for evaluating cognitive function, we used data of SIB when multiple scales were used for this outcome (S1 Appendix). Behavioral disturbances were measured using the NPI and Behavioral Pathology in Alzheimer’s Disease Rating Scale (Behave-AD) scores [16]. Secondary outcome measures included activities of daily living [Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory (modified for more severe dementia) (ADCS-ADLsev) [17], Alzheimer’s Disease Cooperative Study-Activities of Daily Living 19 Items (ADCS-ADL19) scale scores [17, 18], Alzheimer’s Disease Cooperative Study-Activities of Daily Living 23 Items (ADCS-ADL23) scale scores [18], and Bristol Activities of Daily Living Scale (BADLS) scores [19]], global function assessment [Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC-Plus) [20]], stage of dementia assessment[Functional Assessment Staging instrument (FAST) [21]], rate of drug discontinuation from any cause, discontinuation because of adverse events, and discontinuation because of inefficacy. In addition, we pooled the drug side effects data.
We based our analyses on intent-to-treat (ITT) or modified ITT data (i.e., at least 1 dose or at least 1 follow-up assessment). However, we analyzed the complete set of data to ensure that as much information as possible was provided ([22]: SMMSE, NPI, and BADLS scores; [15]: SIB, ADAS-cog, MMSE, and NPI).
The meta-analysis was performed using Review Manager. To combine studies, we used the random-effects model by DerSimonian and Laird [23]. The random-effects model is more conservative than the fixed-effects model and produces a wider CI. For continuous data, we calculated Hedges’ g standardized mean difference (SMD) effect sizes and used the cut-off values for small, medium, and large effect sizes (0.2, 0.5, and 0.8, respectively) set out by Cohen [24]. If only 95% CI was reported, we converted the 95% CI to standard deviation [25]. For dichotomous data, the risk ratio (RR) was estimated along with 95% CIs. When the random-effects model showed significant differences between groups, the number needed to harm (NNH) was calculated. The NNH values were then derived from the risk difference (RD) using the formula NNH = 1/RD. We explored study heterogeneity using the I 2 statistic, with values of 50% or higher considered to reflect considerable heterogeneity [26]. In cases with I 2 values ≥50% for primary outcome measures, we conducted sensitivity analyses to determine the reasons for heterogeneity. Funnel plots were inspected visually to assess the possibility of publication bias. We also assessed the methodological qualities of the articles included in the meta-analysis on the basis of the Cochrane risk of bias criteria (Cochrane Collaboration; http://www.cochrane.org/).
Results
Study Characteristics
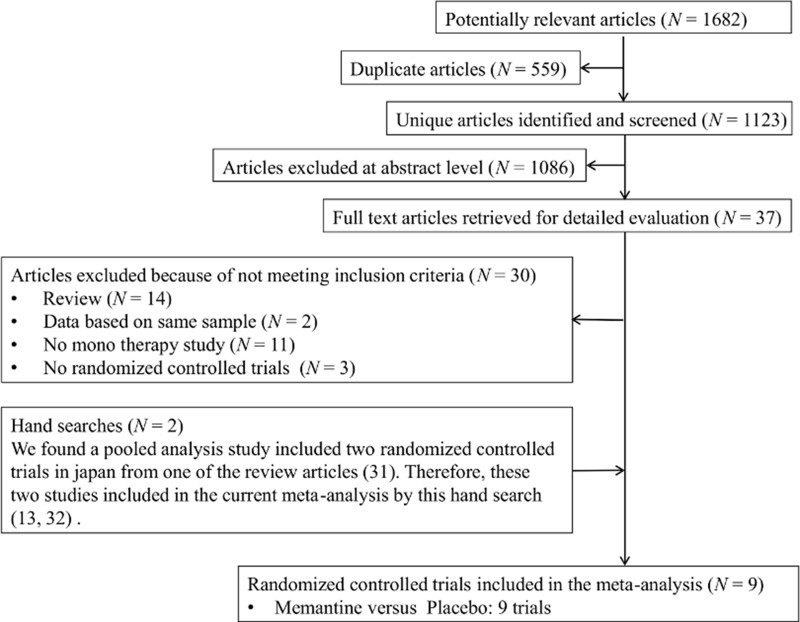
The search yielded a total of 1647 references, of which 542 were duplicates (Fig 1). Seven RCTs testing memantine monotherapy for AD [14, 15, 22, 27–30] were included in the current meta-analysis. We excluded 1068 references after reviewing the title and abstract because these articles did not meet our criteria, and a further 30 references were excluded after full-text reviews because they were review articles (14 articles), were duplicative studies (2 articles), involved combination therapy with ChEI and memantine (11 articles), or were non-RCTs (3 articles). From a review article [31], we found a pooled analysis of 2 RCTs from Japan [13, 32]; these 2 studies were included in the current meta-analysis.
Fig 1. Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) flow diagram.
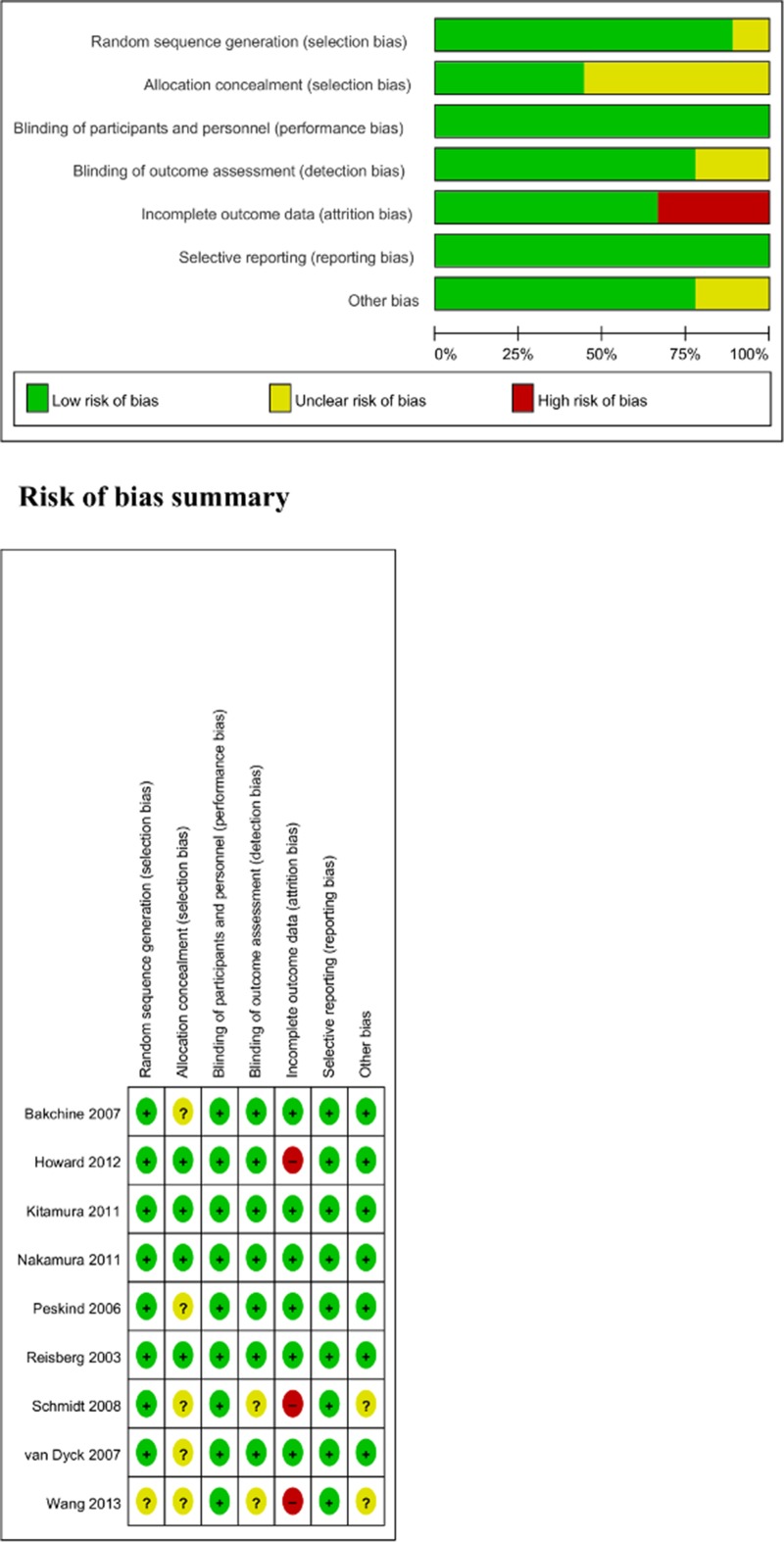
In total, we identified 9 RCTs including 2433 patients with AD that met our inclusion criteria [13–15, 22, 27–30, 32]. The mean study duration was 31 weeks, with 6 trials lasting 24 weeks, 2 trials lasting 52 weeks, and 1 trial lasting 28 weeks. The total number of subjects in each study ranged from 26 to 470 patients. The mean age of the study population was 76 years. Eight out of 9 studies were sponsored by pharmaceutical companies and 2 out of 9 were published in Japanese [13, 32]. The studies were conducted in 1 or multiple countries: 3 were conducted in the United States, 2 in Japan, 1 in Austria, 1 in the United Kingdom, 1 in China, and 1 in multiple countries (Austria, Belgium, Denmark, Finland, France, Greece, Lithuania, the Netherlands, Poland, Spain, Sweden, and the United Kingdom). The characteristics of the trials included in our study are shown in Table 1. We evaluated the methodological quality of all studies using the Cochrane risk of bias criteria (Fig 2). All the studies [13–15, 22, 27–30, 32] were double blind and randomized. One study [15] did not mention the method of randomization. Five studies [14, 15, 27, 29, 30] did not mention the method of allocation concealment. Two studies [15, 30] did not mention the method of blinding outcome assessment. Finally, 3 studies [15, 22, 30] reported the complete analysis.
Table 1. Characteristics of included trials.
| Study | Total n | Patients | Diagnosis | Duration | Age (years) (mean ± SD) | Male, % | Race (%) | Drug | n | Dose (mg/day) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reisberg 2003 (USA), industry | 252 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 28 weeks | 76.1 ± 8.07 | MEM: 28, PLA: 37 | MEM: White 89, Black 4, Other 7, PLA: White 91, Black 5, Other 4 | MEM | 126 | MEM 20 mg | MEM > PLA: SIB, ADCS-ADL (sev), FAST, MEM = PLA: CIBIC-Plus, MMSE, GDS, NPI |
| Inclusion: age ≥50 years, MMSE 3–14, GDS 5–6, FAST ≥ 6a, MRI or CT consistent with a diagnosis of probable AD (within 12 months) | |||||||||||
| Exclusion: any neurodegenerative disorder or MDD other than AD, modified HIS <4, clinically significant coexisting medical conditions or laboratory abnormalities, receiving specific concomitant medications (anticonvulsant, antiparkinsonian, hypnotic, anxiolytic, neuroleptic, cholinomimetic, or any other investigational compounds) | PLA | 126 | PLA | ||||||||
| Peskind 2006 (USA), industry | 403 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA | 24 weeks | MEM: 78.0 ± 7.3, PLA: 77.0 ± 8.2 | MEM: 40, PLA: 43 | MEM: White 92, Other 8, PLA: White 91, Other 9 | MEM | 201 | MEM 20 mg (fixed dose) | MEM > PLA: ADAS-cog, CIBIC-Plus, NPI, MEM = PLA: ADCS-ADL23 |
| Inclusion: MMSE 10–22, age ≥50 years, MRI or CT consistent with a diagnosis of probable AD (within 12 months), MADRS <22 | PLA | 202 | PLA | ||||||||
| Exclusion: significant and active pulmonary, gastrointestinal, renal, hepatic, endocrine, or cardiovascular disease; clinically significant B12 or folate deficiency; evidence of any psychiatric or neurological disorder other than probable AD; HIS <4; screening; delusions or delirium (DSM-IV); treatment with a depot neuroleptic within 6 months of screening; previous treatment with MEM; treatment within 30 days of screening with a ChEI or any investigational drug | |||||||||||
| Study | Total n | Patients | Diagnosis | Duration | Age (years) (mean ± SD) | Male, % | Race (%) | Drug | n | Dose (mg/day) | Outcomes |
| van Dyck 2007 (USA), industry | 350 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA | 24 weeks | MEM: 78.1 ± 8.2, PLA: 78.3 ± 7.6 | MEM: 27.5, PLA: 29.7 | MEM: White 80, Other 20, PLA: White 82, Other 18 | MEM | 178 | MEM 20 mg | MEM = PLA: SIB, ADCS-ADL19, CIBIC-Plus, NPI, BGP, FAST |
| Inclusion: MMSE 5–14, age ≥50 years, MRI or CT consistent with a diagnosis of probable AD (within 12 months), stable dose of following concomitant medication were allowed: antihypertensives, anti-inflammatories, diuretics, laxatives, antidepressants, atypical antipsychotics, and tocopherol | PLA | 172 | PLA | ||||||||
| Exclusion: significant and active pulmonary, gastrointestinal, renal, hepatic, endocrine, or cardiovascular disease; clinically significant B12 or folate deficiency; evidence of any psychiatric or neurological disorder other than probable AD; HIS <4; delusions or delirium (DSM-IV); active malignancy; history of substance abuse within 10 years; treatment with a depot neuroleptic within 6 months of screening; previous treatment with MEM; treatment within 30 days of screening with a ChEI or any investigational drug | |||||||||||
| Bakchine 2007 (Austria, Belgium, Denmark, Finland, France, Greece, Lithuania, the Netherlands, Poland, Spain, Sweden, and United Kingdom), industry | 470 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 24 weeks | MEM: 74.0 ± 7.4, PLA: 73.3 ± 6.9 | MEM: 35, PLA: 40 | MEM: White 100, PLA: White 100 | MEM | 318 | MEM 20 mg (fixed dose) | MEM = PLA: ADAS-cog, CIBIC-Plus, ADCS-ADL23, NPI |
| Inclusion: MMSE 11–23, age ≥50 years, MRI or CT consistent with a diagnosis of probable AD (within 12 months); SSRIs, estrogens, anti-inflammatory drugs, β-blockers, insulin, and H2 blockers were allowed if the dose and medication had been stable for at least 3 months and were kept stable during the study; vitamin E, coenzyme Q, and atypical antipsychotics were allowed if the dose and medication had been stable for at least 30 days and kept stable during the study; atypical antipsychotics were not to be taken 3 days before a visit | PLA | 152 | PLA | ||||||||
| Exclusion: any neurodegenerative disorder or MDD other than AD; modified HIS <4; significant coexisting medical conditions or laboratory abnormalities; receiving anticonvulsants, antiparkinsonian agents, classical and depot antipsychotics, anxiolytics, hypnotics, non-SSRI antidepressants, ChEI | |||||||||||
| Study | Total n | Patients | Diagnosis | Duration | Age (years) (mean ± SD) | Male, % | Race (%) | Drug | n | Dose (mg/day) | Outcomes |
| Schmidt 2008 (Austria), industry | 36 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 52 weeks | MEM: 76.5 ± 4.8, PLA: 75.8 ± 5.7 | MEM: 27.8, PLA: 44.4 | NR | MEM | 18 | MEM 20 mg | NR |
| Inclusion: age ≥50 years; HIS ≥4; MMSE 14–22; patients who had either failed to respond to ChEI or experienced severe side effects leading to termination of such treatment and had MMSE scores >14, which, at the time of study conduct, had excluded them from other approved antidementia treatment once ChEI had been stopped; generally good health; ChEI had to be terminated at least 4 weeks before screening; low-dose atypical neuroleptics, SSRI, non-centrally active antihypertensives, anti-inflammatory drugs, platelet antiaggregants and anticoagulants, laxatives, diuretics, and sedatives/hypnotics were permitted to continue on stable dose at least 3 months before screening | PLA | 18 | PLA | ||||||||
| Exclusion: primary diagnosis of psychiatric disorders other than AD; cerebrovascular disease; any unstable medical condition; using anticonvulsants, antiparkinsonian agents, barbiturates, Ginkgo biloba and nootropics, systemic corticosteroids, and insulin | |||||||||||
| Kitamura 2011 (Japan), industry | 315 | AD: Outpatient (100%) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 24 weeks | MEM (20 mg): 73.2 ± 9.9, MEM (10 mg): 73.2 ± 9.6, PLA: 73.6 ± 8.9 | MEM (20 mg): 26, MEM (10 mg): 35, PLA: 31 | Japanese: 100% | MEM (20 mg) | 100 | MEM 20 mg (fixed dose) | MEM > PLA: SIB (20 mg), MMSE(20 mg), FAST (20 mg), MEM = PLA: ADCS-ADL19, SIB (10 mg), CIBIC-Plus, NPI, MMSE (10 mg), FAST (10 mg) |
| Inclusion: age ≥50 years, MMSE 5–14, FAST 6a–7a | MEM (10 mg) | 107 | MEM 10 mg (fixed dose) | ||||||||
| Exclusion: MDD (DSM-IV) other dementia with AD, other severe neurological disorder | PLA | 108 | PLA | ||||||||
| Study | Total n | Patients | Diagnosis | Duration | Age (mean ± SD) | Male, % | Race (%) | Drug | n | Dose (mg/day) | Outcomes |
| Nakamura 2011 (Japan), industry | 432 | AD: Outpatient (100%) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 24 weeks | MEM: 74.4 ± 8.5, PLA: 74.9 ± 8.4 | MEM: 36.2, PLA: 35.1 | Japanese: 100% | MEM | 221 | MEM 20 mg (fixed dose) | MEM > PLA: SIB, Behave-AD, MEM = PLA: CIBIC-Plus, AST, MENFIS |
| Inclusion: age ≥50 years, MMSE 5–14, FAST 6a–7a | PLA | 211 | PLA | ||||||||
| Exclusion: MDD (DSM-IV) other dementia with AD, other severe neurological disorder | |||||||||||
| Howard 2012 (United Kingdom), no industry | 149 | AD: Outpatient (NR) | Probable or possible AD: NINCDS-ADRDA criteria | 52 weeks | MEM: 76.2 ± 8.9, PLA: 7.7 ± 8.0 | MEM: 39, PLA: 36 | MEM: White 96, Black 3, Other 1, PLA: White 97, Black 3, Other 0 | MEM | 76 | MEM 20 mg | MEM > PLA: SMMSE, BADLS, MEM = PLA: NPI, DEMQOL-proxy, GHQ-12 |
| Inclusion: continuously treatment with DON for at least 3 months (treatment with DON 10 mg for at least the previous 6 weeks), SMMSE 5–13, each eligible patient’s prescribing clinician was considering a change in drug treatment on the basis of NICE guidelines at the time, discussions with the patient and caregivers, and the physician’s clinical judgment | PLA | 73 | PLA | ||||||||
| Exclusion: severe or unstable medical conditions, current prescription of MEM, contraindications or previous adverse or allergic reactions to trial drugs | |||||||||||
| Wang 2013 (China), industry | 26 | AD: Outpatient (NR) | Probable AD: NINCDS-ADRDA and DSM-IV criteria | 24 weeks | MEM: 65.7 ± 12.5, PLA: 64.7 ± 11.5 | MEM: 36, PLA: 36 | NR | MEM | 13 | MEM 20 mg | MEM > PLA: SIB, MEM = PLA: MMSE, ADAS-cog, NPI |
| Inclusion: age 50–90 years, MMSE 4–20, HIS ≥4, BP 160–95/95–60 | PLA | 13 | PLA | ||||||||
| Exclusion: diabetes, renal impairment, significant systemic condition, psychiatric disorder, seizures, traumatic brain injuries, using approved or investigational antidementia drugs in the previous 3 months |
AD: Alzheimer’s Disease, ADAS-cog: Alzheimer’s Disease Assessment Scale cognitive subscale, ADCS-ADL (sev): Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory (modified for more severe dementia), Behave-AD: Behavioral Pathology in Alzheimer’s Disease Rating Scale, BGP: Behavioral Rating Scale for Geriatric Patients, BP: Blood pressure, ChEI: Cholinesterase Inhibitors, CIBIC-Plus: Clinician’s Interview-Based Impression of Change Plus Caregiver Input, CT: computed tomography, DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders-4th edition-text revision, FAST: Functional Assessment Staging instrument, GDS: Global Deterioration Scale, GHQ-12: General Health Questionnaire 12, HIS: Hachinski Ischemic Score, MADRS: Montgomery Asberg Depression Rating Scale, MDD: Major Depressive Disorder, MEM: memantine, MENFIS: Mental Function Impairment Scale, MMSE: Mini-Mental State Examination, MRI: magnetic resonance imaging, NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, NPI: Neuropsychiatric Inventory, NR: Not reported, PET: positron emission tomography, PLA: placebo, SD: standard deviation, SIB: Severe Impairment Battery, SMMSE: Standardized Mini-Mental State Examination, SSRI: Selective Serotonin Reuptake Inhibitors.
Fig 2. Risk of bias assessment.
Results of the Meta-Analysis for Primary Outcomes
Memantine monotherapy improved cognitive function scores (SMD = −0.27, 95% CI = −0.39 to −0.14, Z = 4.25, p = 0.0001, I 2 = 52%, 9 comparisons, n = 2409; Fig 3) and behavioral disturbance scores (SMD = −0.12, 95% CI = −0.22 to −0.01, Z = 2.24, p = 0.03, I 2 = 30%, 9 comparisons, n = 2358; Fig 4). Visual inspection of the funnel plots for primary outcomes did not suggest the presence of publication bias (S2 Appendix).
Fig 3. Forest plot of cognitive function (9 comparisons, n = 2409).
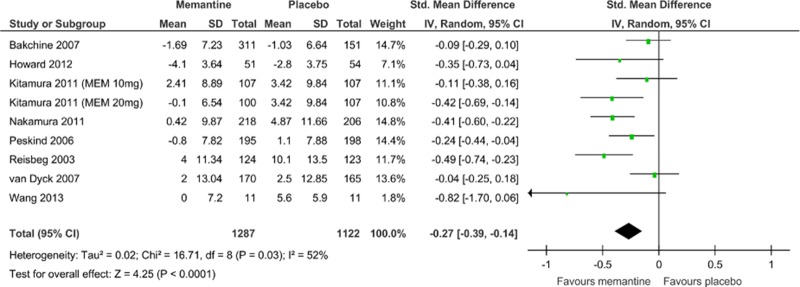
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Fig 4. Forest plot of behavioral disturbances (9 comparisons, n = 2358).
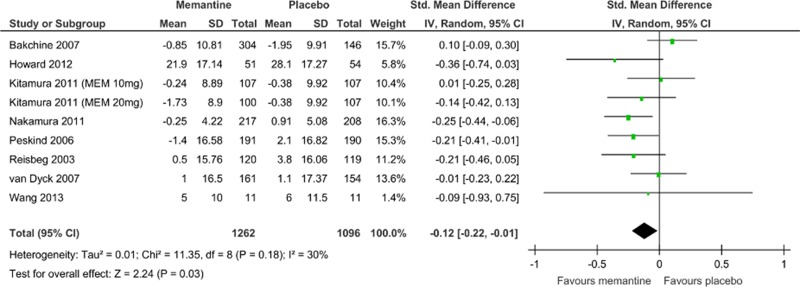
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Sensitivity Analyses of Primary Outcomes
There was significant heterogeneity in cognitive function scores among the studies (I 2 = 52%; Fig 3). Therefore, we performed a sensitivity analysis to determine the confounding factors (Table 2), but did not detect any robust causes for the heterogeneity.
Table 2. Sensitivity analysis of the efficacy of memantine monotherapy (cognitive function).
| Variable | Subgroup | N | n | I 2 | SMD | 95% CI | p value | Test for subgroup differences |
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease stage | Mild to moderate | 2 | 855 | 8 | -0.17 | -0.31 to -0.02 | 0.02 | I 2 = 43.8%, p = 0.18 |
| Moderate to severe | 7 | 1554 | 55 | -0.31 | -0.47 to -0.15 | 0.0001 | ||
| Neuropsychological test | ADAS-cog | 2 | 855 | 8 | -0.17 | -0.31 to -0.02 | 0.02 | I 2 = 0%, p = 0.39 |
| SMMSE | 1 | 105 | NA | -0.35 | -0.73 to 0.04 | 0.08 | ||
| SIB | 6 | 1449 | 62 | -0.31 | -0.49 to -0.13 | 0.0007 | ||
| Sample size | Total n ≥ 200 | 7 | 2282 | 60 | -0.25 | -0.38 to -0.12 | 0.0002 | I 2 = 0%, p = 0.37 |
| Total n < 200 | 2 | 127 | 0 | -0.43 | -0.78 to -0.07 | 0.02 | ||
| Memantine dose | Memantine 10 mg | 1 | 214 | NA | -0.11 | -0.38 to 0.16 | 0.43 | I 2 = 29.5%, p = 0.23 |
| Memantine 20 mg | 8 | 2195 | 55 | -0.29 | -0.42 to -0.15 | 0.0001 | ||
| Method of analysis | Intention to treat | 7 | 2282 | 60 | -0.25 | -0.38 to -0.12 | 0.0002 | I 2 = 0%, p = 0.37 |
| Observed case | 2 | 127 | 0 | -0.43 | -0.78 to -0.07 | 0.02 | ||
| Sponsorship | Industry-sponsored | 1 | 105 | NA | -0.35 | -0.73 to 0.04 | 0.08 | I 2 = 0%, p = 0.68 |
| Non-industry-sponsored | 8 | 2304 | 57 | -0.26 | -0.40 to -0.13 | 0.0001 | ||
| Duration | ≥28 weeks | 8 | 2304 | 57 | -0.26 | -0.40 to -0.13 | 0.0001 | I 2 = 0%, p = 0.68 |
| <28 weeks | 1 | 105 | NA | -0.35 | -0.73 to 0.04 | 0.08 |
ADAS-cog: Alzheimer’s Disease Assessment Scale cognitive subscale, CI: confidence interval, N: number of comparisons, n: number of patients NA: not applicable, SIB: Severe Impairment Battery, SMD: standardized mean difference, SMMSE: Standardized Mini-Mental State Examination
Results of Meta-analysis for Secondary Outcomes
Memantine monotherapy significantly improved activities of daily living scores (SMD = −0.09, 95% CI = −0.19 to −0.00, Z = 1.97, p = 0.05, I 2 = 8%, 7 comparisons, n = 1954; Fig 5), global function assessment scores (SMD = −0.18, 95% CI = −0.27 to −0.09, Z = 4.02, p = 0.0001, I 2 = 13%, 7 comparisons, n = 2270; Fig 6), and stage of dementia assessment scores (SMD = −0.23, 95% CI = −0.33 to −0.12, Z = 4.22, p = 0.0001, I 2 = 0%, 5 comparisons, n = 1376; Fig 7). The data in each treatment group were simulated with no publication bias (data not shown).
Fig 5. Forest plot of activity of daily living (7 comparisons, n = 1954).
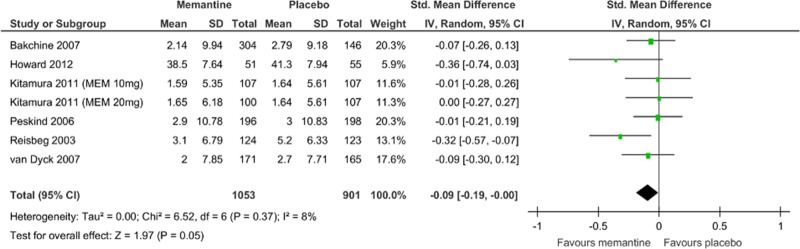
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Fig 6. Forest plot of global function assessment (7 comparisons, n = 2270).
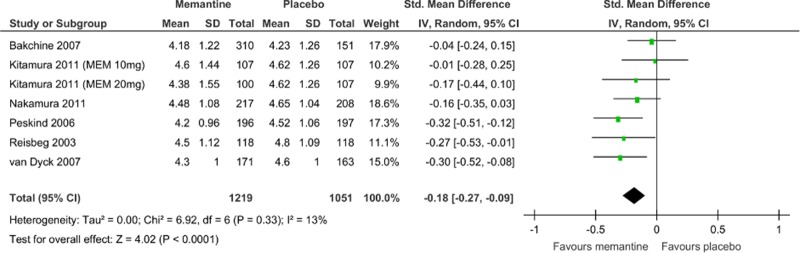
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Fig 7. Forest plot of stage of dementia (5 comparisons, n = 1376).

*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
The rates of discontinuation because of all causes (Fig 8) and all adverse events (Fig 9) were similar between pooled memantine monotherapy and placebo groups. There was a significantly lower rate of discontinuation because of inefficacy in the pooled memantine monotherapy group (RR = 0.36, 95% CI = 0.17 to 0.74, p = 0.006, I 2 = 0%, 4 comparisons, n = 1372; NNH was not significant; Fig 10). Memantine monotherapy was also associated with a lower incidence of agitation compared with placebo (RR = 0.68, 95% CI = 0.49 to 0.94, p = 0.02, I 2 = 7%, 6 comparisons, n = 2222; NNH was not significant; S3 Appendix). No significant differences were found between groups in the incidence of all adverse events, serious adverse events, insomnia, anxiety, depression, falls, influenza-like symptoms/upper respiratory infections, dizziness, headache, urinary tract infection, peripheral edema, diarrhea, constipation, rhinitis, and death (S3 Appendix).
Fig 8. Forest plot of discontinuation due to all causes (9 studies, n = 2433).
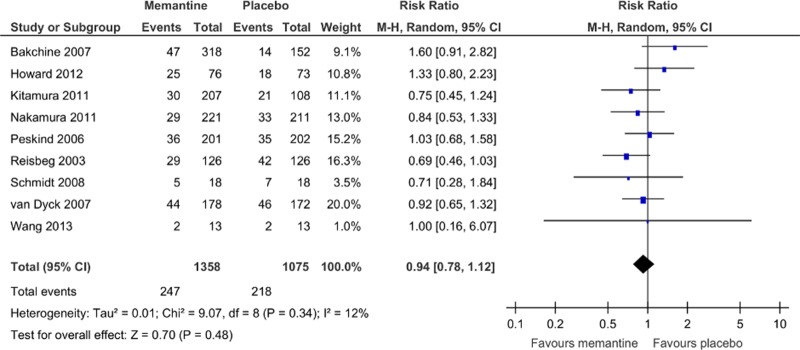
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Fig 9. Forest plot of discontinuation due to adverse events (7 studies, n = 2371).
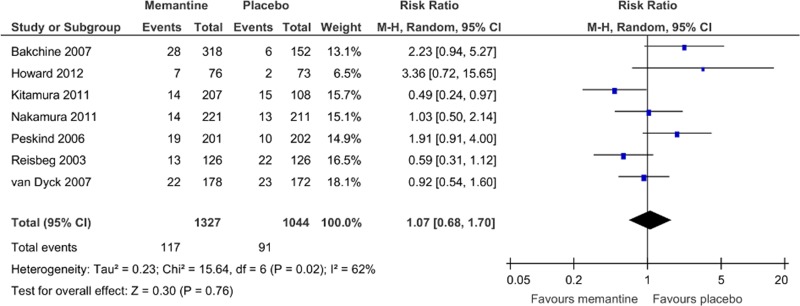
*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Fig 10. Forest plot of discontinuation due to inefficacy (4 studies, n = 1372).

*Negative SMD values favor memantine; positive SMD values favor placebo.†RR < 1 favors memantine; RR > 1 favors placebo.
Discussion
This study provides an updated, comprehensive meta-analysis of RCTs testing the efficacy of memantine monotherapy for AD. The main results indicate that memantine improves cognitive function, AD-associated behavioral disturbances, activities of daily living, global function assessment, and stage of dementia compared with placebo. However, as these effect sizes were small (SMD = −0.09 to −0.27), they suggest a limited clinical benefit. We also performed sensitivity analyses for several factors; however, we could not detect the causes of heterogeneity.
The pooled memantine treatment group exhibited a lower rate of discontinuation because of inefficacy and a lower incidence of agitation compared with the pooled placebo group. Moreover, there were no significant differences in the rates of discontinuation because of all causes, all adverse events, and individual side effects other than agitation between the memantine monotherapy and placebo groups. Therefore, memantine monotherapy does not appear to worsen the symptoms of AD and is well tolerated, exhibiting a potential beneficial role in the symptom treatment of AD.
However, these conclusions must be considered in light of several limitations. The first limitation is that we did not exclude the publication bias. The Cochrane Handbook states that tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta-analysis, because when there are fewer studies, the power of the tests is too low to distinguish chance from real asymmetry [25]. Although the funnel plot for primary and secondary outcomes did not suggest the presence of publication bias, the number of studies included in the meta-analysis was slightly small to allow for a definitive interpretation. A second limitation is that our meta-analysis includes gray literature studies such as supported by pharmaceutical companies; we included these articles because they were published in peer-reviewed journals and represented the majority of the articles retrieved. The only non-industry-sponsored study [22] showed that memantine was marginally superior to placebo in cognitive function. Moreover, there was no significant subgroup difference between industry-sponsored and non-industry-sponsored studies (I 2 = 0%, p = 0.68). A third limitation is that patients with dementia are known to have poor drug compliance [33], reducing the measured effectiveness. Finally, several studies included in this meta-analysis did not report any available data on symptom scales (disease severity) and safety outcomes; therefore, the outcome results for efficacy and safety did not include data from all 9 studies.
Conclusions
Our results suggest that memantine monotherapy is beneficial for the treatment of AD as assessed by multiple scales evaluating cognition, behavioral disturbances, activities of daily living, global function, and stage of dementia. Furthermore, memantine monotherapy appears to be well tolerated. However, the effect size in terms of efficacy outcomes was small and thus there is limited evidence of clinical benefit.
Supporting Information
(PDF)
(PDF)
(PDF)
*Negative SMD values favor memantine; positive SMD values favor placebo. †RR < 1 favors memantine; RR > 1 favors placebo.
(PDF)
Acknowledgments
We thank Dr. Robert Howard (Department of Old Age Psychiatry and Psychopathology, King’s College London) and Dr. Patrick Phillips (MRC Clinical Trials Unit at UCL Institute of Clinical Trials & Methodology) for providing information for the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Ittner LM, Gotz J. Amyloid-beta and tau—a toxic pas de deux in Alzheimer's disease. Nature reviews Neuroscience. 2011;12(2):65–72. Epub 2011/01/05. 10.1038/nrn2967 [DOI] [PubMed] [Google Scholar]
- 2. Kishi T, Iwata N. NMDA receptor antagonists interventions in schizophrenia: Meta-analysis of randomized, placebo-controlled trials. Journal of psychiatric research. 2013;47(9):1143–9. Epub 2013/05/23. 10.1016/j.jpsychires.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 3. Berman K, Brodaty H, Withall A, Seeher K. Pharmacologic treatment of apathy in dementia. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2012;20(2):104–22. Epub 2011/08/16. 10.1097/JGP.0b013e31822001a6 [DOI] [PubMed] [Google Scholar]
- 4. Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. International journal of geriatric psychiatry. 2003;18(Suppl 1):S23–32. Epub 2003/09/16. 10.1002/gps.938 [DOI] [PubMed] [Google Scholar]
- 5. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. The Cochrane database of systematic reviews. 2006;(2):CD003154 10.1002/14651858.CD003154.pub5 [DOI] [PubMed] [Google Scholar]
- 6. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. The American journal of psychiatry. 1984;141(11):1356–64. Epub 1984/11/01. [DOI] [PubMed] [Google Scholar]
- 7. Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Archives of neurology. 1994;51(1):41–5. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 8. Lockhart IA, Orme ME, Mitchell SA. The efficacy of licensed-indication use of donepezil and memantine monotherapies for treating behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: systematic review and meta-analysis. Dementia and geriatric cognitive disorders extra. 2011;1(1):212–27. Epub 2011/12/14. 10.1159/000330032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. Epub 1994/12/01. 7991117 [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535 Epub 2009/07/23. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molloy DW, Standish TI. A guide to the standardized Mini-Mental State Examination. International psychogeriatrics / IPA. 1997;9 Suppl 1:87–94; discussion 143–50. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–98. Epub 1975/11/01. [DOI] [PubMed] [Google Scholar]
- 13. Kitamura S, Homma A, Nakamura Y. Late phase II study of mementine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer's disease. Jpn J Geriatr Psychiat. 2011;22(4):453–63. [Google Scholar]
- 14. Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2006;14(8):704–15. Epub 2006/07/25. 10.1097/01.JGP.0000224350.82719.83 [DOI] [PubMed] [Google Scholar]
- 15. Wang T, Huang Q, Reiman EM, Chen K, Li X, Li G, et al. Effects of memantine on clinical ratings, fluorodeoxyglucose positron emission tomography measurements, and cerebrospinal fluid assays in patients with moderate to severe Alzheimer dementia: a 24-week, randomized, clinical trial. Journal of clinical psychopharmacology. 2013;33(5):636–42. Epub 2013/08/21. 10.1097/JCP.0b013e31829a876a [DOI] [PubMed] [Google Scholar]
- 16. Asada T, Homma A, Kimura M, Uno M. Study on the reliability of the Japanese version of the BEHAVE-AD. Jpn J Geriatr Psychiat. 1999;10(7):825–34. [Google Scholar]
- 17. Galasko D, Schmitt F, Thomas R, Jin S, Bennett D, Alzheimer's Disease Cooperative S. Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease. Journal of the International Neuropsychological Society: JINS. 2005;11(4):446–53. Epub 2005/10/08. [DOI] [PubMed] [Google Scholar]
- 18. Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer disease and associated disorders. 1997;11 Suppl 2:S33–9. Epub 1997/01/01. [PubMed] [Google Scholar]
- 19. Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and ageing. 1996;25(2):113–20. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 20. Olin JT, Schneider LS, Doody RS, Clark CM, Ferris SH, Morris JC, et al. Clinical evaluation of global change in Alzheimer's disease: identifying consensus. Journal of geriatric psychiatry and neurology. 1996;9(4):176–80. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 21. Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer's disease: reliability, validity, and ordinality. International psychogeriatrics / IPA. 1992;4 Suppl 1:55–69. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 22. Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. The New England journal of medicine. 2012;366(10):893–903. Epub 2012/03/09. 10.1056/NEJMoa1106668 [DOI] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. [DOI] [PubMed] [Google Scholar]
- 24. J C. Statistical power analysis in the behavioral sciences. Hillsdale NJ: Erlbaum; 1988. [Google Scholar]
- 25. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration. 2011;www.cochrane-handbook.org. [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: results of a randomised, double-blind, placebo-controlled 6-month study. Journal of Alzheimer's disease: JAD. 2007;11(4):471–9. Epub 2007/07/28. [DOI] [PubMed] [Google Scholar]
- 28. Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, et al. Memantine in moderate-to-severe Alzheimer's disease. The New England journal of medicine. 2003;348(14):1333–41. Epub 2003/04/04. 10.1056/NEJMoa013128 [DOI] [PubMed] [Google Scholar]
- 29. van Dyck CH, Tariot PN, Meyers B, Malca Resnick E, Memantine MEMMDSG. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer disease and associated disorders. 2007;21(2):136–43. Epub 2007/06/05. 10.1097/WAD.0b013e318065c495 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt R, Ropele S, Pendl B, Ofner P, Enzinger C, Schmidt H, et al. Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine. Journal of neurology, neurosurgery, and psychiatry. 2008;79(12):1312–7. Epub 2008/07/01. 10.1136/jnnp.2007.141648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D. Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert opinion on pharmacotherapy. 2014;15(7):913–25. Epub 2014/03/29. 10.1517/14656566.2014.902446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakamura Y, Homma A, Kitamura S, Yoshimura I. Phase III study of mementine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer's disease. Jpn J Geriatr Psychiat. 2011;22(4):464–73. [Google Scholar]
- 33. Boada M, Arranz FJ. Transdermal is better than oral: observational research of the satisfaction of caregivers of patients with Alzheimer's disease treated with rivastigmine. Dementia and geriatric cognitive disorders. 2013;35(1–2):23–33. Epub 2013/01/12. 10.1159/000345989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
*Negative SMD values favor memantine; positive SMD values favor placebo. †RR < 1 favors memantine; RR > 1 favors placebo.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.