Abstract
Tissue engineering strategies have utilized a wide spectrum of synthetic and naturally-derived scaffold materials. Synthetic scaffolds are better defined and offer the ability to precisely and reproducibly control their properties, while naturally-derived scaffolds typically have inherent biological and structural properties that may facilitate tissue growth and remodeling. More recently, efforts to design optimized biomaterial scaffolds have blurred the line between these two approaches. Naturally-derived scaffolds can be engineered through the manipulation of intrinsic properties of the pre-existing backbone (e.g., structural properties), as well as the addition of controllable functional components (e.g., biological properties). Chemical and physical processing techniques used to modify structural properties of synthetic scaffolds have been tailored and applied to naturally-derived materials. Such strategies include manipulation of mechanical properties, degradation, and porosity. Furthermore, bio-functional augmentation of natural scaffolds via incorporation of exogenous cells, proteins, peptides, or genes has been shown to enhance functional regeneration over endogenous response to the material itself. Moving forward, the regenerative mode of action of naturally-derived materials requires additional investigation. Elucidating such mechanisms will allow for the determination of critical design parameters to further enhance efficacy and capitalize on the full potential of naturally-derived scaffolds.
Keywords: Naturally-derived, Natural polymer, Protein-based, Native extracellular matrix (ECM), Tissue regeneration, Augmentation, Structural, Biofunctional
INTRODUCTION
Biomaterials are utilized in the field of tissue engineering and regenerative medicine as a temporary scaffold for migrating endogenous cells or as a delivery vehicle for exogenous cells and biological signals selected to promote tissue regeneration and restoration of function. Naturally-derived materials have been used in the body for centuries and for some applications continue to be more prevalent than their synthetic counterparts.51 While synthetic scaffolds offer tremendous ability for bottom up design of structural and biological properties, the inherent functionality and translational potential of naturally-derived materials continues to make them a highly attractive option.
Natural scaffolds used in tissue engineering are derived from various sources, ranging from plant to mammalian. These materials are often extracted polymers consisting of proteins (e.g., collagen, gelatin, silk, and fibrin) or polysaccharides (e.g., chitosan, alginate, hyaluronan, and chondroitin sulphate).80 However, some naturally-derived materials are sourced from a variety of extracellular matrices (ECMs) that have been gently processed in order to conserve desirable structural and biological properties. These native ECM materials (e.g., amniotic membrane) tend to exhibit high biocompatibility and, because the material mimics a physiological environment, are readily remodeled by cells in vivo following implantation.33 Naturally-derived scaffold materials also include a high density of cell adhesion ligands and contain a milieu of growth factors that could aid in tissue regeneration.4,66,102 Furthermore, minimally manipulated human tissues have an established history of clinical use and thus present a readily translatable strategy for tissue engineering.
Despite many advantages, naturally-derived scaffolds provide their own set of challenges. Natural materials are subject to considerable batch-to-batch variation and generally contain an ill-defined mixture of biological factors. 12,62,80 Consequently, traditional fabrication techniques fall short in controlling the properties as well as ensuring preserved bioactivity of these “black box” materials.8,45,120 This complexity of scaffold composition also acts to obscure therapeutic mechanisms of action, further challenging therapeutic optimization efforts.
To exploit the favorable properties of both material types, researchers have increasingly employed natural/synthetic polymer hybrids. One hybrid approach has focused on augmenting the bioactivity of synthetic scaffolds through the addition of natural elements to a synthetic backbone.20,77,137 An alternative approach involves augmenting the properties of naturally-derived scaffolds using material engineering principles normally applied to synthetic materials (Fig. 1). The following review highlights techniques used to modify the structural and biofunctional properties of naturally-derived materials for tissue engineering.
FIGURE 1.
Functional augmentation of naturally-derived scaffolds. The use of engineered natural matrices for tissue regeneration exploits favorable traits of both naturally-occurring and synthetic materials. Strategies to control the structural and biofunctional properties of natural scaffolds serve to improve the characterization, tunability, and bioactive extent of these materials.
STRUCTURAL PROPERTIES
The structural properties of a material play a central role in its efficacy as a tissue engineering scaffold. The ability to tailor the properties of a scaffold to more closely match those of the native ECM can facilitate more complete integration of the biomaterial with surrounding tissue. A crucial balance exists among biomaterial microstructure, mechanical strength, and degradation rate, with each parameter affecting how the implant interacts with the recipient host. A variety of engineering techniques, many of which will be discussed here, have been developed to augment the structural properties of naturally-derived materials (Table 1).
TABLE 1.
Augmentation of structural properties for naturally-derived materials
| Material | Augmentation strategy | References |
|---|---|---|
| Polysaccharide | ||
| Alginate | Crosslinking | 65 |
| Oxidation | 9,58,94 | |
| Gamma-irradiation | 112 | |
| Chitosan | Acetylation | 132 |
| Protein | ||
| Collagen | 3D Printing | 53 |
| Composite material | 15,19,31,119,136 | |
| Crosslinking | 27,44 | |
| Electrospinning | 99,119,136 | |
| Macromolecular crowding | 28 | |
| Plastic compression | 85 | |
| Elastin | Composite material | 19 |
| Crosslinking | 6,70 | |
| Electrospinning | 99 | |
| High pressure CO2 | 7 | |
| Fibrin | Crosslinking | 113 |
| GAG | Composite material | 15,136 |
| Crosslinking | 95 | |
| Electrospinning | 136 | |
| Gelatin | Composite Material | 89,135 |
| HA | Composite Material | 135 |
| Crosslinking | 43,71,95 | |
| Native ECM | ||
| DBM | Composite Material | 56,69,118 |
| SIS | Crosslinking | 120 |
Microstructure
In the design of tissue engineering scaffolds, there are many microstructural properties to consider, including porosity, pore size, and anisotropy. Bulk porosity is of interest because it affects cellular infiltration into and remodeling of the biomaterial. Often, a tradeoff exists between mechanical properties and porosity, whereby enhanced mechanical integrity is associated with a less porous structure. In fact, many of these microstructure properties are interrelated, such that fine-tuning a single parameter independently (e.g., the size or orientation of scaffold pores) is challenging, if not unfeasible. Sphere-templating, a technique using sacrificial spheres to enable precision of synthetic scaffold porosity, may be a way to control porosity in naturally-derived materials as well.82
The development of material composites can afford more control over microstructural parameters. For example, gelatin-hyaluronic acid (HA) composites have been prepared by freeze-drying and subsequent chemical crosslinking to provide tunable porosity, degradation rate, and compressive strength (Fig. 2a).135 For collagen-glycosaminoglycan (GAG) hybrid scaffolds comprising an anisotropic, porous core and a more dense outer shell, the tensile elastic modulus was augmented by increasing the thickness of the shell, without compromising porosity.15 Another method of enhancing porosity involves high pressure CO2 introduced during crosslinking, which has been used successfully with elastin hydrogels.7 Paradoxically, for hybrid tropoelastin-elastin hydrogels, both porosity and mechanical properties were increased using this approach.6
FIGURE 2.
Use of composite scaffolds to tune structural properties. The combination of multiple components to produce a hybrid material can enable control over the structural properties of naturally-derived materials, (a) The porosity and degradation characteristics of gelatin/hyaluronic acid (GE/HA) scaffolds by controlling the ratio of these components (GE to HA ratios = 100:0 (GHO), 80:20 (GH2), 60:40 (GH4), 40:60 (GH6), and 20:80 (GH8)). (b) Incorporating collagen within tropoelastin-based electrospun materials increases fiber diameter and scaffold porosity (elastin to collagen ratios = 100:0 (100T) and 80:20 (80T20C). Figure reprinted with permission from (a) Zhang et al.,135 (b) Rnjak-Kovacina et al.99.
To gain superior control of microstructural features, researchers have modified electrospinning, a fabrication technique which transforms polymer solutions into nanofibrous scaffolds to allow for the processing of natural materials into interconnected fibers with physiologically relevant diameters.38,59 By varying parameters such as polymer concentration, voltages, air gap distances, delivery rates, and mandrel kinetics, scaffold properties including porosity, alignment, and fiber diameter can be tuned.42 Electrospinning of structural ECM components, such as collagen, gelatin, elastin, and fibrinogen, allows for enhanced control over the microstructure of these natural polymers while maintaining their bioactivity, as demonstrated by cell attachment, spreading, and proliferation on these nanofibers (Fig. 2b).99 Preservation of natural enzyme activity following electrospinning is achievable as well. In fact, enzymes immobilized on electrospun fibers were found to maintain high enzymatic activities in both aqueous and organic media.60
While electrospinning provides a method by which to extrude nano-sized fibers, it affords little control over the exact placement of these fibers. Other bio-fabrication techniques offer a high resolving power in positioning the deposition of natural polymers, allowing for standardization in the creation of these scaffolds. While some industrial microfabrication techniques involve caustic solvents that prevent the seeding of cells or growth factors during production, methods such as 3D printing or photolithography have been successfully adapted for natural scaffold formation. Bioprinting has been conducted using alginate, Matrigel, fibrin, collagen, and agarose, with resolutions ranging from millimeters down to placement of single cells.130 Composite materials have also been used in bioprinting to fabricate a strong scaffold that encourages cellular attachment and proliferation. Collagen-calcium phosphate composite solutions were inkjet printed to a shaped scaffold with controllable volumetric porosity for bone tissue engineering.53 With various techniques of handling natural polymers with high resolution, the field has moved towards creating large scaffolds with complex microstructures that can better emulate native tissues and microenvironments structurally and functionally.
Mechanical Properties
It is well understood that a scaffold’s mechanical properties play a primary role in its function, both structurally as well as biologically. At a cellular level, many naturally-derived materials are readily situated to participate in the transduction of mechanical stimuli due to adhesion ligands inherent in the protein backbone. At the tissue level, many biological materials exhibit increased stiffness when subjected to higher deformations.114 However, especially for load-bearing scenarios, polymer hydrogels are limited by their inability to recapitulate this phenomenon and may not provide sufficient mechanical strength for the regeneration of certain tissues. Thus, when tuning the mechanical properties of naturally-derived scaffolds for improved biomaterial performance, both structural and biological implications must be considered.
Enhancing the mechanical properties of naturally-derived hydrogels is often achieved by altering the crosslinking density. However, in addition to biomaterial strength, the degree of material crosslinking also impacts cellular infiltration and release of bioactive molecules from the hydrogel. Furthermore, it has been demonstrated that the elastic modulus of the matrix influences cell–matrix interactions and subsequent stem cell lineage specificity.32,36 By varying the elastic modulus (via altered crosslinking density) of alginate hydrogels, researchers were able to modulate cellular uptake and expression of non-viral genetic material; interestingly, gene expression exponentially increased with increasing shear modulus for a variety of scaffold materials.65 For HA polymer, material crosslinking was increased through the incorporation of synthetic functionalities, resulting in hydrogels of improved mechanical properties as well as growth factor retention.13,14,43,71 Despite the utility of crosslinking for enhancing mechanical properties, some methods of crosslinking involve harsh reagents such as gluteraldehyde and carbodiimides, which can compromise the bioactivity of associated growth factors and cells.27 Valentin et al. observed that carbodiimide-crosslinked SIS scaffolds exhibited poor degradation and remodeling in vivo. 120 One example of a gentler crosslinking method uses non-covalent crosslinking of fibrillar collagen to form hydrogels.122
Several techniques to manipulate the mechanical properties of naturally-derived scaffolds have been pioneered using collagen-based materials. Collagen type I is an enzymatically degradable protein abundant in the ECM as well as one of the most widely applied naturally-derived biomaterials for tissue regeneration. Fabrication techniques such as plastic compression have been employed to create dense collagen networks with improved mechanical strength.85 Use of macromolecular crowding during collagen fiber assembly can produce hydrogels of a more uniform pore size, smaller fiber diameter, and increased elastic modulus.28 Additionally, a variety of collagen composites allow further control over structural properties and have been used for tissue engineering of bone and bladder.31,81 Elastin, another native ECM protein, has been widely studied, particularly for engineering vascular tissues due to its high resilience and elasticity. Chemical crosslinking of natural elastin can increase the strength of resultant hydrogels.70 Additionally, incorporation of elastin within collagen-based scaffolds can achieve mechanical properties greater than those of purely collagen materials.19
In some cases, the addition of synthetic materials to natural scaffolds can allow for more control over structural properties. Demineralized bone matrix (DBM) has been explored for bone grafting, since its microstructure closely matches that of native bone. Although DBM is weakened during the demineralization process, several reports have shown that the inclusion of synthetic polymers with DBM resulted in increased construct mechanical integrity.56,69,118 Blends of natural and synthetic polymers have also been electrospun to create a bioactive scaffold with enhanced mechanical properties. GAG/collagen electrospun materials were used to facilitate dermal regeneration.136 The addition of hydroxyapatite to collagen nanofibers is shown to increase scaffold surface roughness, fiber diameter, and tensile strength, characteristics which may be advantageous for bone tissue engineering applications.119
Fibrin, a well-characterized, pro-angiogenic protein that serves as the provisional ECM during the natural healing cascade, is another example for which a synthetic component was utilized to enhance the mechanical properties of a natural material. Commercial fibrin products traditionally require high fibrinogen and thrombin concentrations (at least an order of magnitude higher than physiologic levels) to achieve mechanical stability. However, these elevated concentrations lead to faster polymerization and a denser fibrin network, effectively limiting subsequent cell infiltration.105 The native biology of fibrin polymerization, specifically fibrin knobs that have inherent binding affinity to fibrin holes, can be harnessed and modified to provide control over the resulting structural, mechanical, and degradation properties. In particular, augmentation of fibrin knobs with synthetic, polyethylene glycol-based functionalities led to altered polymerization characteristics, resulting in hydrogels of greater mechanical strength, slower enzymatic degradation, and enhanced porosity.113
Degradation Properties
Degradation, or breakdown of the scaffold in a biological environment, is an important structural parameter regulated by material qualities including microstructure, mechanics, and chemistry. Scaffold degradation concomitant with tissue formation is desirable for many tissue engineering applications. While the degradation products of naturally derived materials are often more favorable than those from synthetic materials, modulating the degradation rate of these scaffolds has been a challenge in the field of tissue engineering.
The primary mechanism of degradation of natural fibrous proteins is enzymatic. The rate of degradation is dependent upon the local enzyme concentration, so most protein-derived scaffolds are capable of degrading in physiological environments in a time frame appropriate for tissue healing.121 Nonetheless, in some cases, providing more control over biomaterial degradation is beneficial. For example, the degradation of silk fibroin scaffolds was enhanced by altering processing method, protein concentration, and pore size.63,125 Additionally, crosslinking of materials (e.g., collagen or non-sulfated GAG/hyaluronan) that may degrade too quickly for certain applications can provide for a more optimal degradation profile while increasing the mechanical integrity of the scaffold.44,95 Overall, a balance among the structural parameters is crucial for optimal performance of the regenerative scaffold. Ceramics such as beta-tricalcium phosphate (β-TCP) are notorious for fast resorption, often at the expense of mechanical integrity.98 However, by altering the particle size of the source powder, nanoscale β-TCP scaffolds demonstrated enhanced compressive strength compared to microscale β-TCP, while maintaining an appropriate degradation rate.74
For enhancing the degradation of synthetic polymers, biomimetic, enzymatically cleavable sequences (e.g., those found in collagenase and plasmin) or entire enzymatically degradable natural polymers can be introduced. These components are incorporated into the crosslinking of polymeric scaffolds to enable or further cell-mediated degradation, effectively tuning the rate of scaffold breakdown with that of cell infiltration.127 For example, the inclusion of naturally derived HA into PEG hydrogels promoted degradation of the hybrid biomaterial.90
Natural polysaccharides such as chitosan and alginate possess advantages such as ease of gelling, biocompatibility, and low immunogenicity. However, for these and other plant-based biomaterials that are not enzymatically degradable, structural modifications are needed to accelerate degradation for regenerative medicine applications. The hydrolytic degradation of chitosan has been modulated by varying the degree of acetylation.132 Similarly, alginate degradation is dependent on the often slow and passive dissociation of ionic crosslinks via hydrolysis.109 However, modification of the polymer structure by techniques such as irradiation and oxidation can increase the degradation rate. Gamma-irradiation decreases the alginate polymer molecular weight and has been shown to accelerate alginate degradation, tissue infiltration, and regeneration of bone in vivo compared to unmodified alginate.3,112 Alternatively, oxidation of a small portion of the alginate polymer results in a more open structure and facilitates hydrolysis without compromising the biocompatibility or ability to form crosslinks.9,10 Enhanced degradation of oxidized alginate hydrogels compared to irradiated and unmodified alginate has been observed in vitro and more recently in a rat critically sized segmental bone defect model.9,10,94,110 The ability to tune alginate hydrogel mechanical properties and degradation rate by varying the degree of irradiation and/or oxidation is advantageous for designing effective biomaterials for tissue regeneration.3,9,58,110
BIOLOGICAL PROPERTIES
In addition to engineering the structural properties of naturally-derived scaffolds, efforts to control the biofunctional aspects of these materials have received significant attention. Physical and biochemical cues of the biomaterial work in concert to drive cell behaviors including motility, proliferation, differentiation, and growth factor secretion.116 The tissue engineering scaffold aims to function as a regenerative niche, instructing recruited cell populations through the presentation of biological cues. Naturally-derived materials supply a beneficial environment for resident and recruited cells due to their inherent bioactivity. However, despite this intrinsic functionality, augmentation of natural scaffolds is often required in order to achieve tissue regeneration and restoration of function.97 This section will highlight biofunctional approaches including the incorporation of genes, peptides, proteins, and cells within natural biomaterials.
Genes
Genetic strategies applied towards tissue engineering consist of polynucleotide material encoding for a protein of interest which is presented in vivo for uptake by endogenous cell populations.126 Gene functionalization affords a more sustained and directed approach over that of protein delivery due to its greater stability in vivo and the ability to control protein expression through promoter and vector selection. Viral and non-viral genetic functionalization strategies have been applied to the regeneration of tissues ranging from musculoskeletal to the nervous system.26,76 Viral strategies are more generally efficient, yet harbor risks of immune response and uncontrolled placement of gene insertion into the host genome.18 Non-viral approaches have a higher safety profile and lower cost, but suffer from poor efficiency.39 Efforts to enhance the cellular uptake of nucleic acids from naturally-derived scaffolds include the use of amino acids and cationic polymers.17,30 Elangovan et al. observed enhanced bone regeneration upon loading a collagen scaffold with cationic polyethylenimine-plasmid DNA complexes encoding for platelet-derived growth factor (PDGF).30
Strides have been taken to control the spatiotemporal delivery of genetic material from naturally-derived vehicles.104,134 Aims to better target genes to sites of interest have motivated the use of less conventional delivery systems and, consequently, the development of novel immobilization approaches. Ito et al. freeze-dried recombinant adeno-associated viral (rAAV) vectors encoding for receptor activator of nuclear factor κB ligand (RANKL) and vascular endothelial growth factor (VEGF) onto femoral allografts, effectively improving their vascularization and remodeling in vivo.54 Informed by the role of heparan sulfate proteoglycans for cellular recognition of AAV serotype 2 (rAAV2), a heparinized coating was conjugated to small intestine submucosa (SIS) matrix to enable rAAV2 binding to this atypical gene delivery platform.133
Peptides
One the most common augmentation strategies for tissue engineering scaffolds is the incorporation of peptides. Despite the presence of biomolecules and growth factors inherent to many naturally-derived materials, there are several reasons to guide bioactivity via peptide modulation. Studies conducted using well defined surfaces in vitro have drawn attention to the impact of peptide density and sequence on a variety of cell responses including motility, proliferation, and differentiation.79 Such experiments are often performed using synthetic scaffolds, but have strong implications for naturally-derived materials of non-mammalian background (e.g., polysaccharides) that are of low intrinsic biofunctionality when interacting within mammalian systems.67
Peptides are primarily incorporated into naturally-derived scaffolds via chemical conjugation, a process facilitated by a variety of reactive groups present on the surface or within the bulk of these materials.97 Incorporated sequences generally consist of functional domains identified from ECM proteins and growth factors. Arguably the most commonly leveraged peptide sequence in tissue engineering is Arg-Gly-Asp (RGD). RGD was first identified as a signaling domain within fibronectin, but is present in several ECM proteins and has been found to facilitate cell adhesion through its interaction with roughly half of currently known integrins.46 Adhesion characteristics, including ligand topography and associated integrins, have the capacity to alter cell morphology and phenotype.101 In efforts to fine-tune this interaction, strategies to reduce the availability of adhesive sites have also been implemented.88
Peptides can be used to direct tissue regeneration through interaction with specific integrins and receptors that encourage the adhesion of particular cell types or elicit a change in cell behavior. As more is understood about the utility of these peptides, work to incorporate them into the material backbone, rather than attachment to a pre-existing backbone, has grown in popularity. This strategy, which employs the use of recombinant proteins, also serves to reduce the batch-to-batch variability that results from using proteins derived from digested tissue. Elastin-, resilin-, and silk-based biomaterials have been created using this approach for fabricating a better defined, more potent scaffold.111 Tejeda-Montes et al. modulated the bioactive peptide domain appended to pre-crosslinked elastin-based molecules to guide the behavior of stem cells adhered to the resulting elastin-like recombinamer membrane.117
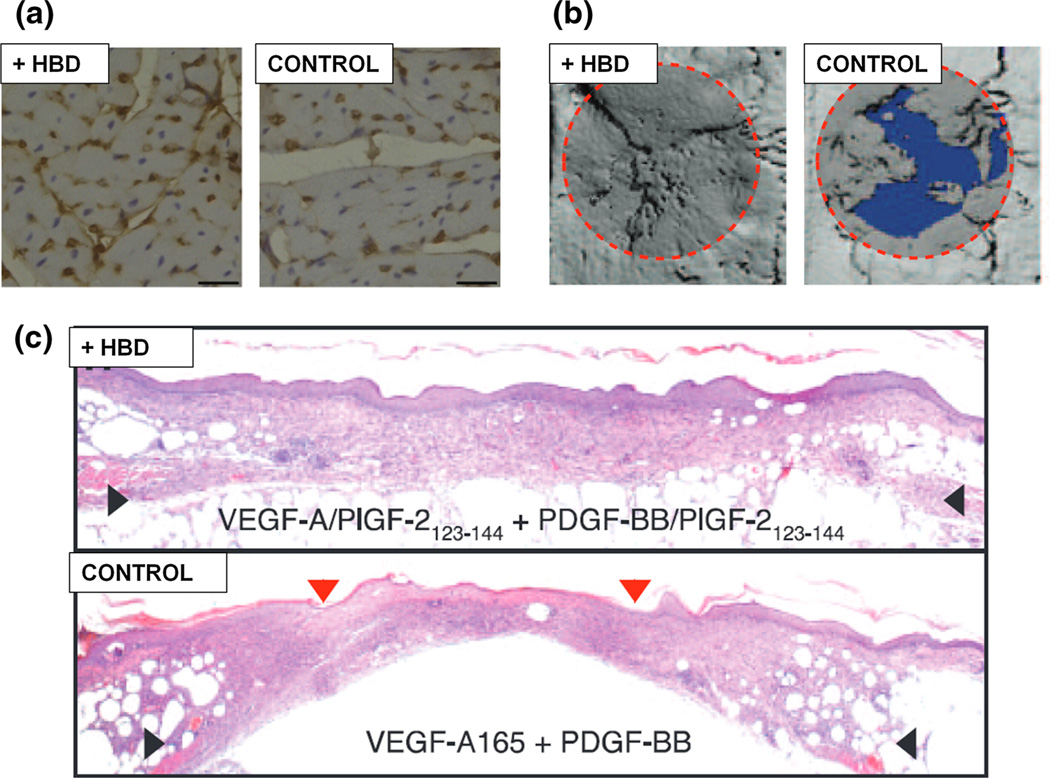
A final class of peptides worth noting are those that act via protein, rather than cellular, interaction. One such example is the use of heparin-binding domains (HBDs), a technique inspired by the interactions between ECM proteins and factors with the highly sulfated GAG heparin (Fig. 3). Heparin resides in the native ECM where it’s known to reversibly bind to a host of growth factors, particularly those that are positively charged including bone morphogenetic protein (BMP) and fibroblast growth factor (FGF).23,91,96 In addition to growth factors, ECM proteins such as fibronectin, vitronectin, fibrinogen, laminin, and collagen also contain HBDs. Incorporation of HBDs within natural matrices has been shown to promote the regeneration of tissues including bone and myocardium.23,41 Using the consensus sequence of physiological HBDs, Rajangam et al. engineered a novel heparin-binding motif capable of self-assembly into nanofibers and promoting angiogenesis upon delivery in vivo.24
FIGURE 3.
Biofunctional augmentation using heparin-binding domains. The biofunctional properties of naturally-derived matrices are often augmented through the co-delivery of bioactive factors. Several strategies target electrostatic interactions between the biomaterial and a growth factor of interest in order to direct biomolecule availability and activity. Scaffold/factor binding within growth factor delivery systems can be modulated through the addition of heparin-binding domains (HBDs), a technique shown to promote the regeneration of tissues including cardiac (a), bone (b), and skin (c). Figure reprinted with permission from (a) Guo et al.,41 (b, c) Martino et al.84.
Proteins
Studies have highlighted advantages of whole protein and multi-domain peptide functionalization over the use of isolated peptide sequences. In fact, these elucidations are not entirely unexpected, as the relationship between peptide functionality and presentation context has long been acknowledged.25,92 Conveying full protein functionality via a system of peptide sequences hinges on a comprehensive understanding of the functional domains, and their corresponding orientations, within a protein of interest. For this reason, whole protein incorporation into tissue engineering scaffolds remains a widely used technique. Heparin is a commonly incorporated biologic, as its incorporation into natural matrices provides additional control over growth factor sequestration without compromising the biocompatibility, injectability, mechanical strength, or degradation of the scaffold.57 The conjugation of heparin to natural matrices has been shown to improve growth factor bioactivity and release, resulting in enhanced tissue regeneration.61,131 Recently, synthetic heparin-functionalized microparticles have demonstrated the ability to sequester positively charged growth factors at unmatched concentrations and subsequently promote their sustained release.47 Synthetic elements have been incorporated into natural materials in the form of fibers and particles to aid in growth factor release for several applications. These natural/synthetic hybrid scaffolds can facilitate the sequential release of biologics due to differences in material properties, a strategy which has been employed for the regeneration of tissues including bone, cartilage, and brain.72,89,124
The incorporation of growth factors to augment tissue repair has been used even in instances where the naturally-derived scaffold being delivered has inherent growth factor content. Despite DBM containing several BMPs, its efficacy for bone defect repair is enhanced when delivered in conjunction with BMP.55,93 SIS matrix, which contains detectable levels of FGF and VEGF growth factors in addition to its structural protein content, has been functionalized with exogenous amounts of either protein to enhance abdominal wall defect repair.48,75,123,129 Matrices produced from platelet-rich plasma (PRP) are rich in the host of growth factors, yet have been combined with recombinant proteins including nerve growth factor (NGF) and BMP.22,138 PRP scaffolds, consisting of platelet-derived factors (e.g., PDGF, VEGF, transforming growth factor-β (TGF-β), and insulin-like growth factor (IGF)) present at physiological ratios within a fibrin network, are capable of stimulating cell proliferation, ECM production, and angiogenesis.5,29 This technique of mimicking physiological context for growth factor presentation extends beyond the use of blood-derived biomaterials, prompting strategies which target the capacity of ECM interaction to facilitate factor signaling. Martino et al. capitalized on such potential through the delivery of growth factor variants, engineered via fusion of an HBD with super affinity for ECM proteins, which displayed improved efficacy for the treatment of chronic wound and bone repair (Fig. 3b, c).84 Similarly, engineering FGF to contain a collagen-binding domain is shown to promote nerve regeneration.78
Cells
The functionalization of natural materials using cellular components has been explored for a variety of applications. This prevalence of investigation is largely attributed to the biochemical and topographic cues present in naturally-derived materials, making them advantageous for cell delivery due to the presence of a pre-existing instructive niche.86 A substantial portion of this work has capitalized on use of the cell secretome, which can be modulated using genetic engineering. Cells have been genetically modified for growth factor secretion (e.g., BMP, VEGF, and NGF) ex vivo, seeded on or within naturally-derived scaffolds, and subsequently implanted.21,37,49,108 There is recent interest in the use of genetically modified mesenchymal stem cells (MSCs) to aid in the transplantation of xenogeneic materials.73 When delivered in the absence of a scaffold, galactosyl epitope knock-out (Gal-KO) pig MSCs attenuated immune response and improved bone regeneration.68,106 This strategy could serve to improve the host response to native ECM scaffolds. Allogenic and xenogenic ECM materials, even after decellularization, are capable of eliciting an immune response due to the presence of ECM proteins, which have been shown to stimulate the migration of neutrophils and macrophages.1,50,107
In addition to genetic modification, cellular components have the capacity to augment regeneration via inherent properties. MSCs can enhance biointegration of naturally-derived scaffolds through their immunomodulatory properties.64,87 Delivery of MSCs within a fibrin carrier is shown to promote the repair of myocardial, calvarial, and chronic cutaneous wounds.35,52,83,103 For orthopedic applications alone, the benefit of MSC delivery has been observed in conjunction with a variety of scaffolds including hydrogels, bioceramics, DBM, and allograft tissue.11,34,40 Improved regeneration in MSC delivery studies is often due to their release of trophic factors, rather than differentiation of implanted cells into the tissue type of interest, as the long-term survival of these cells in vivo is generally poor.2,16 Cells have also served to augment materials through physical interaction. They can interact with natural scaffolds through the exertion of forces, whereby altering the conformation and composition of surface proteins.100,115,128
CONCLUSION
Naturally-derived scaffolds are advantageous for tissue regeneration due to their availability, biocompatibility, and inherent bioactivity. Despite the desirable qualities of natural materials in their raw form, the engineering of such products in order to better control in vivo behavior and enhance therapeutic efficacy remains critical. Measures to manipulate their properties through structural and biofunctional augmentation have been extensively examined. Many strategies, including the addition of synthetic and biologic components, have improved the regenerative capacity of natural materials. The lion’s share of this success has been realized for polymeric (i.e., single protein or polysaccharide based) scaffolds. The relative simplicity of these systems, in comparison to more “black box” ECM products, has positioned them as natural starting points for characterization and augmentation efforts. While engineering approaches for native ECM scaffolds have been effective, the vast potential of these materials remains untapped, as employed strategies as well as our mechanistic understanding are in their infancy. Looking forward, rapid advancements in fabrication techniques and comprehension of regenerative biology will enable creation of highly efficacious natural material-based regenerative constructs for a wide range of clinical applications.
Contributor Information
Ashley B. Allen, Email: aallen37@gatech.edu.
Lauren B. Priddy, Email: lbpriddy@gatech.edu.
Mon-Tzu. A. Li, Email: a.li@gatech.edu.
REFERENCES
- 1.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, Mecham RP, Senior RM. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemo-taxis. J. Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 2.Allen AB, Gazit Z, Su S, Stevens HY, Guldberg RE. In vivo bioluminescent tracking of mesenchymal stem cells within large hydrogel constructs. Tissue Eng. Part C Methods. 2014;20:806–816. doi: 10.1089/ten.tec.2013.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003;82:903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 4.Andree B, Bar A, Haverich A, Hilfiker A. Small intestinal submucosa segments as matrix for tissue engineering: review. Tissue Eng. Part B Rev. 2013;19:279–291. doi: 10.1089/ten.TEB.2012.0583. [DOI] [PubMed] [Google Scholar]
- 5.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 6.Annabi N, Mithieux SM, Weiss AS, Deh-ghani F. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high pressure CO2. Biomaterials. 2010;31:1655–1665. doi: 10.1016/j.biomaterials.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Annabi N, Mithieux SM, Weiss AS, Deh-ghani F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials. 2009;30:1–7. doi: 10.1016/j.biomaterials.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Ann. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 11.Breitbart EA, Meade S, Azad V, Yeh S, Al-Zube L, Lee YS, Benevenia J, Arinzeh TL, Lin SS. Mesenchymal stem cells accelerate bone allograft incorporation in the presence of diabetes mellitus. J. Orthop. Res. 2010;28:942–949. doi: 10.1002/jor.21065. [DOI] [PubMed] [Google Scholar]
- 12.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014;163:268–285. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Caliari SR, Ramirez MA, Harley BA. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials. 2011;32:8990–8998. doi: 10.1016/j.biomaterials.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 17.Casettari L, Vllasaliu D, Lam JK, Soliman M, Ilium L. Biomedical applications of amino acid-modified chitosans: a review. Biomaterials. 2012;33:7565–7583. doi: 10.1016/j.biomaterials.2012.06.104. [DOI] [PubMed] [Google Scholar]
- 18.Cavazzana-Calvo M, Thrasher A, Mavilio F. The future of gene therapy. Nature. 2004;427:779–781. doi: 10.1038/427779a. [DOI] [PubMed] [Google Scholar]
- 19.Caves JM, Cui W, Wen J, Kumar VA, Haller CA, Chaikof EL. Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair. Biomaterials. 2011;32:5371–5379. doi: 10.1016/j.biomaterials.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan BK, Wippich CC, Wu CJ, Sivasankar PM, Schmidt G. Robust and semi-interpenetrating hydrogels from poly(ethylene glycol) and collagen for elastomeric tissue scaffolds. Macromol. Biosci. 2012;12:1490–1501. doi: 10.1002/mabi.201200234. [DOI] [PubMed] [Google Scholar]
- 21.Chen BS, Xie H, Zhang SL, Geng HQ, Zhou JM, Pan J, Chen F. Tissue engineering of bladder using vascular endothelial growth factor gene-modified endothelial progenitor cells. Int. J. Artif. Organs. 2011;34:1137–1146. doi: 10.5301/ijao.5000069. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Lu X, Li S, Sun Q, Li W, Song D. Sustained delivery of BMP-2 and platelet-rich plasma-released growth factors contributes to osteogenesis of human adipose-derived stem cells. Orthopedics. 2012;35:e1402–e1409. doi: 10.3928/01477447-20120822-29. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Lee JY, Park JH, Park JB, Suh JS, Choi YS, Lee SJ, Chung CP, Park YJ. The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role on osteogenesis. Biomaterials. 2010;31:7226–7238. doi: 10.1016/j.biomaterials.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Chow LW, Bitton R, Webber MJ, Carvajal D, Shull KR, Sharma AK, Stupp SI. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials. 2011;32:1574–1582. doi: 10.1016/j.biomaterials.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins C, Osborne LD, Guilluy C, Chen Z, O’Brien ET, 3rd, Reader JS, Burridge K, Superfine R, Tzima E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat Commun. 2014;5:3984. doi: 10.1038/ncomms4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliver Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 27.De Paoli Lacerda SH, Ingber B, Rosenzweig N. Structure-release rate correlation in collagen gels containing fluorescent drug analog. Biomaterials. 2005;26:7164–7172. doi: 10.1016/j.biomaterials.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Dewavrin J-Y, Hamzavi N, Shim VPW, Raghunath M. Tuning the architecture of 3D collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 29.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, Van Dyke TE. Platelet-rich plasma: growth factors pro- and anti-inflammatory properties. J Periodontal. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 30.Elangovan S, D’Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR, Salem AK. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials. 2014;35:737–747. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelhardt EM, Micol LA, Houis S, Wurm FM, Hilborn J, Hubbell JA, Frey P. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 2011;32:3969–3976. doi: 10.1016/j.biomaterials.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Erdbrugger W, Konertz W, Dohmen PM, Posner S, Ellerbrok H, Brodde OE, Robenek H, Modersohn D, Pruss A, Holinski S, Stein-Konertz M, Pauli G. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo . Tissue Eng. 2006;12:2059–2068. doi: 10.1089/ten.2006.12.2059. [DOI] [PubMed] [Google Scholar]
- 34.Evans CH. Advances in regenerative orthopedics. Mayo Clin. Proc. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 36.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34:1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 40.Gu H, Xiong Z, Yin X, Li B, Mei N, G Li, Wang C. Bone regeneration in a rabbit ulna defect model: use of allogeneic adipose-derived stem cells with low immunogenicity. Cell Tissue Res. 2014;358:453–464. doi: 10.1007/s00441-014-1952-3. [DOI] [PubMed] [Google Scholar]
- 41.Guo HD, Cui GH, Yang JJ, Wang C, Zhu J, Zhang LS, Jiang J, Shao SJ. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2012;424:105–111. doi: 10.1016/j.bbrc.2012.06.080. [DOI] [PubMed] [Google Scholar]
- 42.Haghi AK, Akbari M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi A. 2007;204:1830–1834. [Google Scholar]
- 43.Hahn SK, Kim JS, Shimobouji T. Injectable hyaluronic acid microhydrogels for controlled release formulation of erythropoietin. J. Biomed. Mater. Res. A. 2007;80:916–924. doi: 10.1002/jbm.a.30997. [DOI] [PubMed] [Google Scholar]
- 44.Harley BA, Spilker MH, Wu JW, Asano K, Hsu HP, Spector M, Yannas IV. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153–165. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- 45.Harvey AL. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 47.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morpho-genetic protein-2. Biomaterials. 2014;35:7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J. Clin. Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howes EL, Harvey SC. The strength of the healing wound in relation to the holding strength of the catgut suture. New Engl J. Med. 1929;200:1285–1291. [Google Scholar]
- 52.Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen. Med. 2009;4:527–538. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O’Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat. Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang YS, Choi CH, Cho YB, Kang MK, Jang CH. Recombinant human BMP-2 enhances osteogenesis of demineralized bone matrix in experimental mastoid obliteration. Acta Otolaryngol. 2014;134:785–790. doi: 10.3109/00016489.2014.900702. [DOI] [PubMed] [Google Scholar]
- 56.Jayasuriya AC, Ebraheim NA. Evaluation of bone matrix and demineralized bone matrix incorporated PLGA matrices for bone repair. J. Mater. Sci.-Mater. M. 2009;20:1637–1644. doi: 10.1007/s10856-009-3738-9. [DOI] [PubMed] [Google Scholar]
- 57.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeon O, Samorezov JE, Alsberg E. Single and dual crosslinked oxidized methacrylated alginate/PEG hydro-gels for bioadhesive applications. Acta Biomater. 2014;10:47–55. doi: 10.1016/j.actbio.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji J, Bar-On B, Wagner HD. Mechanics of electrospun collagen and hydroxyapatite/collagen nanofibers. J. Mech. Behav. Biomed. Mater. 2012;13:185–193. doi: 10.1016/j.jmbbm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Jia H, Zhu G, Vugrinovich B, Kataphinan W, Reneker DH, Wang P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol. Prog. 2002;18:1027–1032. doi: 10.1021/bp020042m. [DOI] [PubMed] [Google Scholar]
- 61.Jiang T, Wang G, Qiu J, Luo L, Zhang G. Heparinized poly(vinyl alcohol)—small intestinal submucosa composite membrane for coronary covered stents. Biomed. Mater. 2009;4:025012. doi: 10.1088/1748-6041/4/2/025012. [DOI] [PubMed] [Google Scholar]
- 62.Johnson TD, Dequach JA, Gaetani R, Ungerleider J, Elhag D, Nigam V, Behfar A, Christ-man KL. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014;2014:60283D. doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 64.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 65.Kong HJ, Liu JD, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat. Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 66.Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int. Wound J. 2013;10:493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krishna OD, Kiick KL. Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94:32–48. doi: 10.1002/bip.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar G, Hara H, Long C, Shaikh H, Ayares D, Cooper DK, Ezzelarab M. Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties. Cytotherapy. 2012;14:494–504. doi: 10.3109/14653249.2011.651529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurkalli BGS, Gurevitch O, Sosnik A, Cohn D, Slavin S. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr. Stem Cell Res. T. 2010;5:49–56. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 70.Leach JB, Wolinsky JB, Stone PJ, Wong JY. Crosslinked alpha-elastin biomaterials: towards a processable elastin mimetic scaffold. Acta Biomater. 2005;1:155–164. doi: 10.1016/j.actbio.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Lee F, Chung JE, Kurisawa M. An injectable hyaluronic acid-tyramine hydrogel system for protein delivery. Control Release. 2009;134:186–193. doi: 10.1016/j.jconrel.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Lee HJ, Koh WG. Hydrogel micropattern-incorporated fibrous scaffolds capable of sequential growth factor delivery for enhanced osteogenesis of hMSCs. ACS Appl. Mater. Interfaces. 2014;6:9338–9348. doi: 10.1021/am501714k. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Ezzelarab MB, Ayares D, Cooper DK. The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Stem Cell Rev. 2014;10:79–85. doi: 10.1007/s12015-013-9478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin KL, Chen L, Qu HY, Lu JX, Chang J. Improvement of mechanical properties of macroporous beta-tricalcium phosphate bioceramic scaffolds with uniform and interconnected pore structures. Ceram. Int. 2011;37:2397–2403. [Google Scholar]
- 75.Liu Z, Feng X, Wang H, Ma J, Liu W, Cui D, Gu Y, Tang R. Carbon nanotubes as VEGF carriers to improve the early vascularization of porcine small intestinal submucosa in abdominal wall defect repair. Int. J. Nanomed. 2014;9:1275–1286. doi: 10.2147/IJN.S58626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu CH, Chang YH, Lin SY, Li KC, Hu YC. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol. Adv. 2013;31:1695–1706. doi: 10.1016/j.biotechadv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 78.Ma F, Xiao Z, Chen B, Hou X, Dai J, Xu R. Linear ordered collagen scaffolds loaded with collagen-binding basic fibroblast growth factor facilitate recovery of sciatic nerve injury in rats. Tissue Eng. Part A. 2014;20:1253–1262. doi: 10.1089/ten.tea.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000;113(Pt 10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 80.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, Nazhat SN. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 82.Marshall AJ, Ratner BD. Quantitative characterization of sphere-templated porous biomaterials. AIChE J. 2005;51:1221–1232. [Google Scholar]
- 83.Martinez EC, Vu DT, Wang J, Lilyanna S, Ling LH, Gan SU, Tan AL, Phan TT, Lee CN, Kofidis T. Grafts enriched with subamnion-cord-lining mesenchymal stem cell angiogenic spheroids induce post-ishemic myocardial revascularization and preserve cardiac function in failing rat hearts. Stem Cells Dev. 2013;22:3087–3099. doi: 10.1089/scd.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 85.Mueller-Rath R, Gavenis K, Andereya S, Mumme T, Albrand M, Stoffel M, Weichert D, Schneider U. Condensed cellular seeded collagen gel as an improved biomaterial for tissue engineering of articular cartilage. Bio-Med. Mater. Eng. 2010;20:317–328. doi: 10.3233/BME-2010-0645. [DOI] [PubMed] [Google Scholar]
- 86.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat. Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 88.Neff JA, Tresco PA, Caldwell KD. Surface modification for controlled studies of cell-ligand interactions. Biomaterials. 1999;20:2377–2393. doi: 10.1016/s0142-9612(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 89.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, Mikos AG. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J. Biomed. Mater. Res. A. 2009;88:889–897. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 90.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 91.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 92.Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- 93.Pietrzak WS, Woodell-May J, McDonald N. Assay of bone morphogenetic protein-2, -4, and -7 in human demineralized bone matrix. J. Craniofac. Surg. 2006;17:84–90. doi: 10.1097/01.scs.0000179745.91165.73. [DOI] [PubMed] [Google Scholar]
- 94.Priddy LB, Chaudhuri O, Stevens HY, Krishnan L, Uhrig BA, Willett NJ, Guldberg RE. Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects. Acta Biomater. 2014;10:4390–4399. doi: 10.1016/j.actbio.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010;35:403–440. [Google Scholar]
- 96.Rabenstein DL. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 97.Renth AN, Detamore MS. Leveraging, “raw materials” as building blocks and bioactive signals in regenerative medicine. Tissue Eng. Part B Rev. 2012;18:341–362. doi: 10.1089/ten.teb.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 99.Rnjak-Kovacina J, Wise SG, Li Z, Maitz PK, Young CJ, Wang Y, Weiss AS. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8:3714–3722. doi: 10.1016/j.actbio.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 100.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J. Cell. Physiol. 2005;203:465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 101.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 102.Ruoslahti E. The RGD story: a personal account. Matrix Biol. 2003;22:459–465. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 103.Schantz JT, Hutmacher DW, Lam CX, Brinkmann M, Wong KM, Lim TC, Chou N, Guldberg RE, Teoh SH. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo . Tissue Eng. 2003;9(Suppl 1):S127–S139. doi: 10.1089/10763270360697030. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt C, Bezuidenhout D, Zilla P, Davies NH. A slow-release fibrin matrix increases adeno-associated virus transduction of wound repair cells in vivo. J Biomater Appl. 2014;28:1408–1418. doi: 10.1177/0885328213510331. [DOI] [PubMed] [Google Scholar]
- 105.Schmoekel HG, Weber FE, Schense JC, Gratz KW, Schawalder P, Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 106.Schubert T, Poilvache H, Galli C, Gianello P, Dufrane D. Galactosyl-knock-out engineered pig as a xenogenic donor source of adipose MSCs for bone regeneration. Biomaterials. 2013;34:3279–3289. doi: 10.1016/j.biomaterials.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 107.Senior RM, Hinek A, Griffin GL, Pipoly DJ, Crouch EC, Mecham RP. Neutrophils show chemotaxis to type-Iv collagen Its 7s domain contain a 67-Kd type-Iv collagen binding-protein with lectin properties. Am. J. Resp. Cell Mol. 1989;1:479–187. doi: 10.1165/ajrcmb/1.6.479. [DOI] [PubMed] [Google Scholar]
- 108.Sheyn D, Ruthemann M, Mizrahi O, Kallai I, Zilberman Y, Tawackoli W, Kanim LEA, Zhao L, Bae H, Pelled G, Snedeker JG, Gazit D. Genetically modified mesenchymal stem cells induce mechanically stable posterior spine fusion. Tissue Eng. Pt. A. 2010;16:3679–3686. doi: 10.1089/ten.tea.2009.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shoichet MS, Li RH, White ML, Winn SR. Stability of hydrogels used in cell encapsulation: an in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 1996;50:374–381. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 110.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 111.Silva R, Fabry B, Boccaccini AR. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 2014;35:6727–6738. doi: 10.1016/j.biomaterials.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 112.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 113.Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH. Engineering fibrin polymers through engagement of alternative polymerization mechanisms. Biomaterials. 2012;33:535–544. doi: 10.1016/j.biomaterials.2011.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 115.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc. Natl. Acad. Sci. USA. 2008;105:6537–6542. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tejeda-Montes E, Smith KH, Poch M, Lopez-Bosque MJ, Martin L, Alonso M, Engel E, Mata A. Engineering membrane scaffolds with both physical and biomolecular signaling. Acta Biomater. 2012;8:998–1009. doi: 10.1016/j.actbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Tejeda-Montes E, Smith KH, Rebollo E, Gomez R, Alonso M, Rodriguez-Cabello JC, Engel E, Mata A. Bioactive membranes for bone regeneration applications: effect of physical and biomolecular signals on mesenchymal stem cell behavior. Acta Biomater. 2014;10:134–141. doi: 10.1016/j.actbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Thomas CB, Maxson S, Burg KJ. Preparation and characterization of a composite of demineralized bone matrix fragments and polylactide beads for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2011;22:589–610. doi: 10.1163/092050610X488232. [DOI] [PubMed] [Google Scholar]
- 119.Thomas V, Dean DR, Jose MV, Mathew B, Chowdhury S, Vohra YK. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules. 2007;8:631–637. doi: 10.1021/bm060879w. [DOI] [PubMed] [Google Scholar]
- 120.Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng. Part A. 2009;15:1687–1694. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Amerongen MJ, Harmsen MC, Petersen AH, Kors G, van Luyn MJ. The enzymatic degradation of scaffolds and their replacement by vascularized extracellular matrix in the murine myocardium. Biomaterials. 2006;27:2247–2257. doi: 10.1016/j.biomaterials.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, Lai DM, Yang B, Jiang ZP, C Zhang Y, Zhou J, Lai W, Chen S. Reconstruction of abdominal wall defects using small intestinal submucosa coated with gelatin hydrogel incorporating basic fibroblast growth factor. Acta Cir. Bras. 2014;29:252–260. doi: 10.1590/s0102-86502014000400006. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Cooke MJ, Sachewsky N, Morshead CM, Shoichet MS. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J. Control Release. 2013;172:1–11. doi: 10.1016/j.jconrel.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wegman F, Oner FC, Dhert WJ, Alblas J. Non-viral gene therapy for bone tissue engineering. Biotechnol. Genet. Eng. Rev. 2013;29:206–220. doi: 10.1080/02648725.2013.801227. [DOI] [PubMed] [Google Scholar]
- 127.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 128.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur. J. Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 129.Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33:2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wust S, Muller R, Hofmann S. Controlled positioning of cells in biomaterials-approaches towards 3D tissue printing. J. Funct. Biomater. 2011;2:119–154. doi: 10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. Pt. A. 2010;16:1225–1233. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 132.Yang YM, Hu W, Wang XD, Gu XS. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vitro. J. Mater. Sci. Mater. Med. 2007;18:2117–2121. doi: 10.1007/s10856-007-3013-x. [DOI] [PubMed] [Google Scholar]
- 133.Yue TW, Chien WC, Tseng SJ, Tang SC. EDC/NHS-mediated heparinization of small intestinal submucosa for recombinant adeno-associated virus serotype 2 binding and transduction. Biomaterials. 2007;28:2350–2357. doi: 10.1016/j.biomaterials.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 134.Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials. 2004;25:147–157. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 135.Zhang F, He C, Cao L, Feng W, Wang H, Mo X, Wang J. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int. J. Biol. Macromol. 2011;48:474–481. doi: 10.1016/j.ijbiomac.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 136.Zhong S, Teo WE, Zhu X, Beuerman R, Ramakrishna S, Yung LY. Formation of collagen-glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules. 2005;6:2998–3004. doi: 10.1021/bm050318p. [DOI] [PubMed] [Google Scholar]
- 137.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajon A, Vaquero J. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010;12:522–537. doi: 10.3109/14653241003615164. [DOI] [PubMed] [Google Scholar]