Abstract
Purpose
Sepsis is a devastating condition with considerable mortality. The causes of long-term mortality are poorly understood. To test the hypothesis that septic patients are more susceptible to recurrent infections and death due to infectious complications, we investigated the outcomes of patients who survived sepsis, with regards to incidence of recurrent infections and mortality.
Material and Methods
A retrospective study of subjects admitted to the intensive care unit (ICU) for sepsis from 2001–2 who achieved 30-day survival (“SS”, N=78) and a control group of subjects admitted to the ICU for non-infectious conditions with a similar severity of illness (N=50) was performed. The primary endpoint was the number of recurrent infections in the first year post-hospitalization.
Results
The SS group had higher rates of infections following hospital discharge compared to controls. Using a multivariable model, having survived sepsis was the strongest predictor of the development of subsequent infections (rate ratio [RR] 2.83, P=0.0006), the need for re-hospitalization for infection in the year after the initial hospitalization (RR 3.78, P=0.0009), and post-discharge mortality (hazard ratio=3.61, P=0.003).
Conclusions
Critically ill patients who survive sepsis have an increased risk of recurrent infections in the year following their septic episode, which is associated with increased mortality.
Keywords: sepsis, immunosuppression, outcomes
Introduction
Sepsis is a devastating condition that remains a major cause of morbidity and mortality in the United States and worldwide(1, 2). With advances in supportive care, the in-hospital mortality rate has improved over time which has resulted in increasing numbers of sepsis survivors (2). Unfortunately, sepsis survivors have been found to have a number of poor outcomes long-term including impaired quality of life and mortality that extends beyond the standard 28-day in-hospital mortality endpoint (3). A large cohort study by Quartin and colleagues found that survivors of sepsis had an increased risk of death that persisted for up to 5 years following the initial septic event, even after accounting for their underlying medical co-morbidities (4). The mechanism of this increased long-term mortality following sepsisremains unclear.
In the past decade, the pathophysiology of the sepsis syndrome has shifted from a paradigm of uncontrolled inflammation to one of “sepsis-induced immunosuppression,” a state of immune dysregulation in which anti-inflammatory immunologic events develop concurrently or subsequently throughout the course of sepsis (5). A recently published post-mortem study found evidence of biochemical, flow cytometric, and immunohistochemical findings consistent with immunosuppression in patients who died in the ICU following sepsis compared with patients who died of nonsepsis etiologies(6). Furthermore, both the literature and anecdotal experience suggest that many patients survive sepsis only to succumb to other infections, often after a protracted hospital course or during a subsequent admission to the hospital (7, 8). Sepsis-induced immunosuppression has been postulated to contribute to sepsis-related mortality because of the inability to fight secondary infections in the post-septic period, (9) though the true clinical impact of this remains unclear. To our knowledge, no studies have examined the incidence and types of infections in patients who survive sepsis. We therefore performed a retrospective cohort study to determine whether critically ill patients who survive an episode of sepsis (sepsis survivors, SS) are more susceptible to recurrent infections and death due to infectious complications as compared to non-septic patients with a similar severity of critical illness.
Methods
Setting
The West Los Angeles Veteran Affairs (VA) Healthcare Center is a tertiary medical center with a Level 1 intensive care unit (ICU), a VA designation which includes a complex patient population and a full time intensivist. Approximately 800 patients are admitted annually. Since 1990, a computerized database designated as the patient registry has been kept for all ICU admissions at this facility. In addition to standard patient demographics, the registry contains prospectively collected data on admission and discharge diagnoses, admission Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and discharge locations. The VA also has an extensive electronic medical record (EMR; Computerized Patient Record System [CPRS] current version 1.0.27) with comprehensive data available since 1999.
Inclusion Criteria
We reviewed patient registry data between January 1, 2001, and December 31, 2002 and identified all patients admitted to the ICU with a diagnosis of sepsis that achieved 30-day survival (N=78). A separate contemporaneous control group of patients admitted to the ICU with non-infectious diagnoses and that achieved 30-day survival were matched using admission APACHE II scores within 2 points of the first 50 cases (N=50). Controls were unable to be matched for all 78 cases secondary to the lack of adequate numbers of control subjects with sufficiently elevated APACHE II scores that were within 2 points of the case subjects.
Exclusion Criteria
Patients were excluded if they had the following conditions or treatments: solid organ or bone marrow transplantation, the acquired immunodeficiency syndrome, hematologic malignancies, recent chemotherapy within 30 days, chronic corticosteroid use (equivalent of >10 mg/day of prednisone), or chronic immunosuppressive medications.
Criteria for Infection
Infections in the ICU were identified using chart review and based on criteria from the 2003 International Sepsis Forum Consensus Conference for the six most common infections: pneumonia, bloodstream infections, intravascular catheter-related sepsis, intra-abdominal infections, urosepsis, and surgical wound infections (10). To be considered an infection, “microbiologically confirmed” or “probable” criteria for infection had to be fulfilled. For other infections not included in the above categories and subsequent infections not in the ICU, infections were identified based on documentation by the treating physician of an infection as well as evidence of an intervention as a result of the infection (i.e., antimicrobial therapy or discontinuation of a catheter). Sites of infection and microorganisms identified were also collected for every subsequent infection in the one-year period after the initial hospitalization. Positive Candida cultures were not counted if isolated from respiratory samples or from the urine, in the absence of evidence of invasive disease or bloodstream infection.
Endpoints
Subjects’ records were reviewed and data abstracted onto an EXCEL® worksheet using a standardized case report form. Records were reviewed during their index ICU admission and for the ensuing year using the VA EMR. The primary endpoint was the number of recurrent infections in the first year post-hospitalization inclusive of both inpatient and outpatient documented infections. Secondary endpoints were the number of re-hospitalizations for infection and survival in the year post-hospitalization.
Institutional Review Board
The study was approved by the VA Greater Los Angeles Healthcare System Institutional Review Board (PCC 2006-060932). The committee waived the need for informed consent since it involved analysis of existing data and posed minimal patient risk.
Statistical analysis
Statistical analysis was performed using SAS® software (version 9.2, SAS Institute Inc., Cary, NC, USA), and figures were created using GraphPad Prism® (version 5.0, GraphPad Software, San Diego California USA). Baseline variables were compared using Fisher’s exact test. Quantitative variables were compared using the independent two-sample t-test or Wilcoxon rank-sum test when appropriate. Uni- and multivariable Cox proportional hazards models of mortality were constructed along with survival plots using the Kaplan-Meier method.
Mean cumulative function plots for the recurrent events of post-discharge infections and rehospitalizations for infection were created using the method described by Nelson(11). Uni- and multivariable proportional rates/means models (also known as independent-incremental models) were created for the respective recurrent events(12–14). This is a semi-parametric method analogous to Cox proportional hazards models for survival in the context of recurring longitudinal outcomes data that represent a count process such as infections. Standard errors for the resulting model parameters were calculated using robust sandwich estimates for the covariance matrices, aggregating the score residuals per subject. First-order interaction terms were tested in final multivariable models, and none met statistical significance. Final models were validated by bootstrapping (1000 samples, resampling subjects with replacement). Variable selection for multivariable models was performed by backward selection based on Wald criteria.
Results
Baseline Patient Characteristics
We compared baseline characteristics for critically ill patients with sepsis (SS) and patients with critical illness without sepsis, who survived for 30 days after being admitted to the intensive care unit (Table 1). Subjects who were known to be immunocompromised at baseline, including patients with neutropenia, organ transplant, or active malignancy or taking immunosuppressive medications, were excluded so as to minimize the contribution of these conditions to secondary infections. Sepsis survivors (SS) were an older population (mean age 70 versus 64 years) and more often admitted from a nursing home. Survivors of sepsis were also more likely to have an indwelling catheter or a history of malignancy (predominantly prostate and skin), but other chronic co-morbidities were similar. A total of 25 sepsis patients had 14 urinary catheters, 12 gastrostomy tubes, 2 tracheostomy tubes, and 2 dialysis catheters. Sixty-three percent of SS had suffered an infection in the year prior to admission compared to 26% of controls. The mean APACHE II score obtained at admission, was well-matched at 21 points in both groups (SD 7 for SS, 6 for controls) demonstrating comparable severity of critical illness. Sepsis survivors had an increased use of mechanical ventilation and vasopressor therapy. The most common admission diagnoses in the control group were gastrointestinal bleed and cardiac conditions, including congestive heart failure, myocardial infarction, arrhythmias, and hypertensive emergency.
Table 1.
Baseline Characteristics
| Baseline Variable | Sepsis N=78 | Controls N=50 | p-value |
|---|---|---|---|
| Age, Mean (SD) | 70 (13) | 64 (14) | 0.01 |
| Race | |||
| Caucasian | 45 (58%) | 20 (40%) | 0.07 |
| African-american | 27 (35%) | 18 (36%) | 0.64 |
| Other | 6 (8%) | 12 (24%) | 0.02 |
| Male | 75 (96%) | 49 (98%) | 1.0 |
| Admission from | |||
| Home | 35 (50%) | 33 (66%) | 0.03 |
| Nursing Home | 35 (50%) | 6 (12%) | < 0.0001 |
| Other | 8 (10%) | 11 (22%) | 0.08 |
| Comorbidities | |||
| Cardiac | 61 (78%) | 42 (84%) | 0.5 |
| Neurological | 34 (44%) | 17 (34%) | 0.35 |
| Malignancy | 21 (27%) | 5 (10%) | 0.02 |
| Diabetes | 23 (29%) | 20 (40%) | 0.25 |
| Gastroinstestinal | 10 (13%) | 10 (20%) | 0.32 |
| Pulmonary | 29 (37%) | 12 (24%) | 0.13 |
| Indwelling catheter or foreign body | 25 (32%) | 4 (8%) | 0.002 |
| Renal | 20 (26%) | 17 (34%) | 0.32 |
| Infection in prior year | 49 (63%) | 13 (26%) | < 0.0001 |
| Substance abuse history | 22 (28%) | 22 (44%) | 0.09 |
| APACHE II Score, Mean (SD) | 21 (7) | 21 (6) | 0.94 |
| Mechanical ventilation | 30 (38%) | 9 (18%) | 0.02 |
| Vasopressor requirement | 26 (33%) | 3 (6%) | 0.0002 |
| Acute renal failure | 48 (62%) | 29 (58%) | 0.71 |
| Initiation of hemodialysis | 7 (9%) | 3 (6%) | 0.74 |
| Albumin, Mean (SD) | 2.5 (0.6) | 3.3 (0.7) | < 0.0001 |
| Mean glucose, Median [IQR] | 130 [118–161] | 131 [116–191] | 0.59 |
| pH, Mean (SD) | 7.37 (0.10) | 7.34 (0.15) | 0.15 |
| Lactate, Median [IQR] | 2.1 [1.1–3.3] | 2.0 [0.9–2.8] | 0.53 |
In Hospital Outcomes
SS had longer ICU and hospital lengths of stay as compared to controls. The median length of ICU stay for SS was 6 days compared to 3 days in the controls (p <0.0001), and the median length of hospital stay was 20 days compared to 7 days in the SS and control groups respectively (p<0.0001). In-hospital mortality for the 30-day survivors of sepsis was overall low at 5%. No control patients surviving 30 days subsequently expired in the hospital.
Subsequent Infections in the Year Post-Hospitalization
Given that the majority of subjects survived to hospital discharge, we examined long-term outcomes among the SS and non-septic survivors of critical illness (controls). SS had markedly higher rates of new infections following discharge compared to controls (Figure 1), with differences persisting out to 1 year. Over the first year, patients with sepsis developed post-discharge infections at an unadjusted rate ratio of 5.68 (95% CI 3.21–10.03, p<0.00001) compared to controls (Table 2). On average, the SS group had 3.22 infections per patient-year, compared to 0.6 infections per patient-year among controls. Overall, there were significant differences in the percentage of patients suffering a post-discharge subsequent infection (63% of SS compared to 30% of controls [p < 0.001]).
Figure 1.
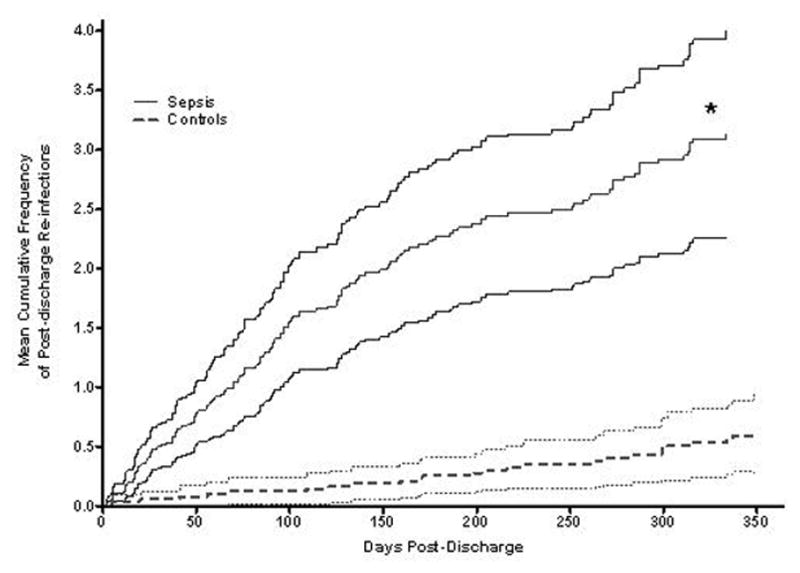
Mean cumulative frequencies of post-discharge infections were determined in the survivors of sepsis (“sepsis,” solid line) as compared to survivors of non-septic critical illness (“controls,” dashed line). The difference (unadjusted rate ratio = 5.68; *, p<0.00001) persisted out to one year. The lighter shaded lines represent 95% confidence intervals.
Table 2.
Predictive factors for Post-discharge Infections and Hospitalizations (Unadjusted)
| Post-Discharge Infections | Post-Discharge Hospitalizations | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rate Ratio | 95% CI | p-value | Rate Ratio | 95% CI | p-value | |
| Sepsis | 5.68 | 3.21–10.03 | < 0.0001 | 8.06 | 3.66–17.26 | < 0.0001 |
| Age (by Quartile) | ||||||
| 1st Quartile (34–54 years) | Ref | Ref | ||||
| 2nd Quartile (55–68 years) | 1.57 | 0.71–3.49 | 0.26 | 1.64 | 0.61–4.43 | 0.33 |
| 3rd Quartile (69–77 years) | 1.11 | 0.47–2.61 | 0.8 | 1.28 | 0.43–3.79 | 0.66 |
| 4th Quartile (78–98 years) | 2.73 | 1.27–5.89 | 0.01 | 3.35 | 1.26–8.81 | 0.02 |
| Race | ||||||
| White Race | Ref | Ref | ||||
| Black Race | 0.81 | 0.47–1.38 | 0.43 | 1.02 | 0.55–1.88 | 0.95 |
| Other Race | 0.40 | 0.17–0.93 | 0.03 | 0.33 | 0.09–1.17 | 0.09 |
| White Race vs Non-white (ref) | 1.44 | 0.87–2.40 | 0.16 | 1.21 | 0.66–2.21 | 0.53 |
| From | ||||||
| From Home | Ref | Ref | ||||
| From Nursing Home | 3.11 | 1.87–5.21 | < 0.0001 | 3.17 | 1.78–5.66 | < 0.0001 |
| From Other | 0.50 | 0.22–1.16 | 0.11 | 0.21 | 0.05–0.87 | 0.03 |
| From Nursing Home vs Not (ref) | 3.51 | 2.16–5.71 | < 0.0001 | 3.87 | 2.19–6.85 | < 0.0001 |
| ICU LOS (by Quartile) | ||||||
| 1st Quartile (2 days) | Ref | Ref | ||||
| 2nd Quartile (3–4 days) | 1.14 | 0.51–2.53 | 0.76 | 1.13 | 0.42–3.03 | 0.81 |
| 3rd Quartile (5–8 days) | 2.90 | 1.6–5.24 | 0.004 | 2.68 | 1.11–6.46 | 0.03 |
| 4th Quartile (9–147 days) | 2.11 | 1.01–4.40 | 0.04 | 1.89 | 0.73–4.88 | 0.19 |
| Hosp LOS (by Quartile) | ||||||
| 1st Quartile (2–5 days) | Ref | Ref | ||||
| 2nd Quartile (6–12 days) | 1.11 | 0.49–2.50 | 0.8 | 1.56 | 0.52–4.70 | 0.43 |
| 3rd Quartile (13–28 days) | 2.46 | 1.22–4.98 | 0.01 | 3.01 | 1.13–8.05 | 0.03 |
| 4th Quartile (31–147 days) | 3.02 | 1.42–6.40 | 0.004 | 2.97 | 1.04–8.44 | 0.04 |
| Cardiac | 1.14 | 0.59–2.23 | 0.69 | 1.69 | 0.76–3.73 | 0.20 |
| Neurologic | 2.00 | 1.19–3.37 | 0.009 | 2.38 | 1.30–4.35 | 0.005 |
| Malignancy | 0.92 | 0.46–1.82 | 0.8 | 0.76 | 0.37–1.55 | 0.45 |
| Diabetes | 0.73 | 0.41–1.30 | 0.28 | 0.62 | 0.34–1.16 | 0.14 |
| Gastrointestinal | 0.57 | 0.23–1.41 | 0.22 | 0.75 | 0.31–1.83 | 0.52 |
| Pulmonary | 0.77 | 0.42–1.43 | 0.41 | 0.72 | 0.37–1.41 | 0.34 |
| Catheter/Foreign Body | 4.03 | 2.54–6.38 | < 0.0001 | 5.02 | 2.95–8.55 | < 0.0001 |
| Renal | 0.88 | 0.52–1.47 | 0.62 | 1.12 | 0.62–2.03 | 0.71 |
| Prior Infection | 2.78 | 1.63–4.73 | 0.0002 | 4.05 | 2.09–7.84 | < 0.0001 |
| Substance Abuse | 0.44 | 0.24–0.82 | 0.01 | 0.35 | 0.15–0.79 | 0.01 |
| APACHE (by Quartile) | ||||||
| 1st Quartile (8–16) | Ref | Ref | ||||
| 2nd Quartile (17–20) | 0.85 | 0.42–1.69 | 0.63 | 0.85 | 0.38–1.90 | 0.69 |
| 3rd Quartile (21–25) | 1.00 | 0.49–2.07 | 0.99 | 1.06 | 0.46–2.47 | 0.89 |
| 4th Quartile (26–42) | 1.42 | 0.68–2.95 | 0.35 | 0.96 | 0.40–2.27 | 0.92 |
| Mechanical Ventilation | 0.95 | 0.49–1.85 | 0.88 | 0.91 | 0.43–1.95 | 0.81 |
| Pressors | 2.13 | 1.14–3.97 | 0.02 | 1.71 | 0.85–3.42 | 0.13 |
| Acute renal failure | 1.28 | 0.75–2.18 | 0.36 | 0.99 | 0.54–1.83 | 0.99 |
| New hemodialysis | 1.10 | 0.34–3.61 | 0.87 | 1.30 | 0.38–4.44 | 0.68 |
| Albumin (by Quartile) | ||||||
| 1st Quartile (1.1–2.2 g/dL) | Ref | Ref | ||||
| 2nd Quartile (2.3–2.7 g/dL) | 1.11 | 0.58–2.13 | 0.75 | 0.73 | 0.34–1.55 | 0.41 |
| 3rd Quartile (2.8–3.3 g/dL) | 0.65 | 0.34–1.26 | 0.2 | 0.59 | 0.28–1.25 | 0.17 |
| 4th Quartile (3.4–4.7 g/dL) | 0.35 | 0.14–0.83 | 0.02 | 0.31 | 0.12–0.84 | 0.02 |
| Albumin (Continuous) | 0.52 | 0.38–0.69 | < 0.0001 | 0.51 | 0.35–0.75 | 0.0005 |
| Mean blood sugar (by Quartile) | ||||||
| 1st Quartile (82–116 mg/dL) | Ref | Ref | ||||
| 2nd Quartile (117–130 mg/dL) | 0.59 | 0.28–1.22 | 0.15 | 0.55 | 0.24–1.30 | 0.18 |
| 3rd Quartile (130–165 mg/dL) | 0.70 | 0.34–1.44 | 0.33 | 0.64 | 0.29–1.45 | 0.29 |
| 4th Quartile (166–528 mg/dL) | 0.69 | 0.37–1.30 | 0.26 | 0.56 | 0.26–1.22 | 0.15 |
| Lactate (by Quartile) | ||||||
| 1st Quartile (0.5–0.9 mmol/L) | Ref | Ref | ||||
| 2nd Quartile (1–1.9 mmol/L) | 0.96 | 0.47–1.95 | 0.91 | 1.00 | 0.41–2.42 | 1.00 |
| 3rd Quartile (2–3.2 mmol/L) | 1.30 | 0.65–2.61 | 0.45 | 0.94 | 0.42–2.10 | 0.88 |
| 4th Quartile (3.3–20.6 mmol/L) | 1.13 | 0.51–2.49 | 0.76 | 0.87 | 0.34–2.22 | 0.77 |
Predictive factors for post-discharge infections were measured based on univariable and multivariable proportional rates/means models. Risk factors for hospital readmission for infection were calculated based on univariable and multivariable proportional rates/means models.
Variable selection for multivariable models was performed by backward selection based on Wald criteria. ICU = intensive care unit; LOS = length of stay; APACHE = Acute Physiology and Chronic Health Evaluation.
We then analyzed predictive factors for post-discharge infections. Although being a survivor of sepsis was the most significant predictor of subsequent infections in univariable models, other significant predictors included being in the highest quartile of age (78–98 years), admission from a nursing home, a prolonged hospital stay (defined as ≥13 days which was the median length of stay), a neurological co-morbidity, the presence of an indwelling catheter/foreign body, an infection in the year prior to hospitalization, and decreased albumin (Table 2). A history of substance abuse was a negative predictor of post-discharge infections, which we speculated could be attributable to subjects with drug use being younger than other patients.
Risk factors for hospital readmission for infection
We next examined the risk of hospital readmission specifically for infection among sepsis survivors. The SS group was more likely to be readmitted with infection during the first year (Figure 2). The mean number of rehospitalizations for infections per patient-year was 1.45 for sepsis survivors and 0.18 for controls. Among candidate predictors, the initial presence of sepsis was the strongest predictor of re-hospitalization for infection with an unadjusted RR of 8.06 (p<0.0001), although other variables, including advanced age, admission from nursing home, prolonged hospital LOS, the presence of a catheter/indwelling foreign body, prior infection in the past year, and low albumin were also significant univariate predictors (Table 2). Malignancy and diabetes were not significant predictors while substance abuse was negatively associated.
Figure 2.
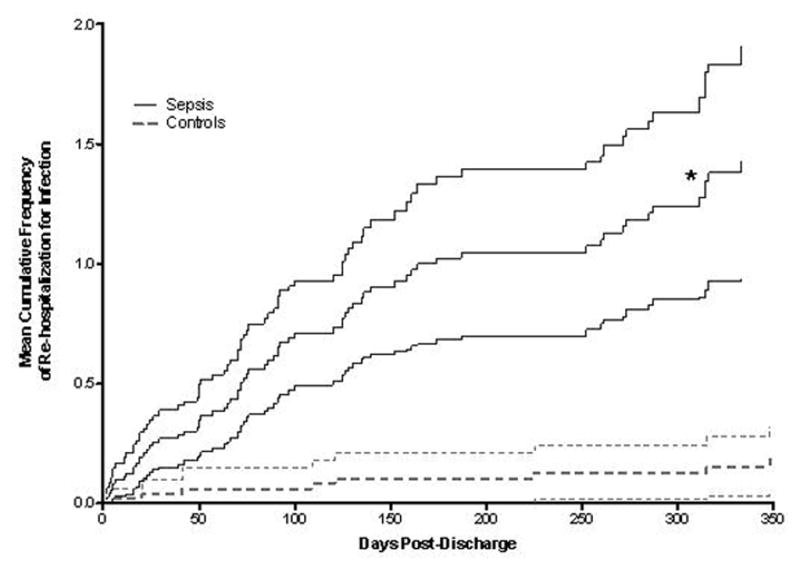
Mean cumulative frequencies of re-hospitalizations for infection were determined in survivors of sepsis (“sepsis,” solid line) as compared to survivors of non-septic critical illness (“controls,” dashed line). The difference (unadjusted rate ratio = 8, *, p<0.0001) persisted out to one year. The lighter shaded lines represent 95% confidence intervals.
Covariate analysis for recurrent post-discharge infections and hospitalization
We next adjusted for the significant covariates identified in the univariate model to determine the relative impact of sepsis on development of post-discharge infections. We found that having survived an episode of sepsis still remained the most significant factor associated with developing subsequent post-discharge infections (rate ratio [RR] = 2.83, p=0.0006) although prolonged hospitalization, advanced age, and admission from a nursing facility remained significant predictors (Table 3, left panel). After adjusting for other significant factors, we found that the presence of sepsis remained the strongest predictor for readmission for an infectious cause, with an adjusted RR of 3.78 (p<0.009; Table 3, right panel). Prolonged hospitalization, advanced age, and the presence of an indwelling catheter/foreign body were also significant predictors.
Table 3.
Adjusted post-discharge infections and re-hospitalizations for infections
| Post-discharge Infections | Re-hospitalizations for Infection | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rate Ratio | 95% CI | p-value | Rate Ratio | 95% CI | p-value | |
| Sepsis | 2.83 | 1.56–5.14 | 0.0006 | 3.78 | 1.73–8.27 | 0.0009 |
| 4th Quartile of Age | 1.64 | 1.03–2.61 | 0.04 | 1.92 | 1.17–3.14 | 0.03 |
| Catheter or Foreign Body | 1.7 | 0.93–3.13 | 0.09 | 2.44 | 1.29–4.62 | 0.006 |
| Prior Infection | 1.43 | 0.80–2.55 | 0.22 | 2.07 | 0.98–4.34 | 0.06 |
| Prolonged Hospitalization | 2.34 | 1.53–3.57 | <0.0001 | 1.96 | 1.18–3.26 | 0.01 |
| From Nursing Home | 1.78 | 0.99–3.18 | 0.05 | -- | -- | NS |
Sites of Infection and Microorganisms Identified
In addition to analyzing the rates of secondary infections, we also sought to determine whether SS had a qualitative difference in the types and microbiology of secondary infections. We observed that the type of infection in the SS was predominantly pneumonia, while controls had mostly urinary tract infections (Figure 3A). The microorganisms identified also differed. The SS group was more likely to develop infections with opportunistic pathogens such as Pseudomonas aeruginosa and Candida species, whereas no cases of Pseudomonas aeruginosa or Candida species occurred in the controls (Figure 3B).
Figure 3.
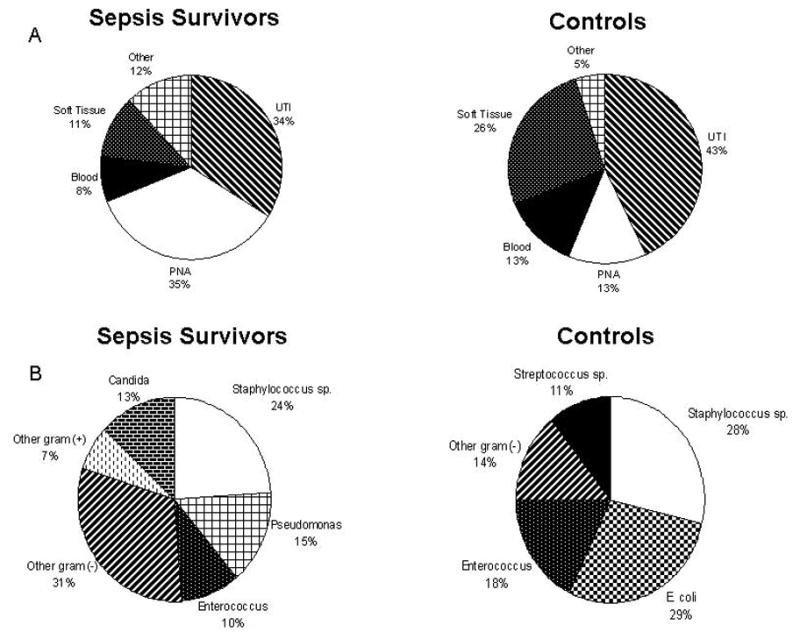
Comparison of the sites of recurrent infection (A) and microorganisms implicated (B) in survivors of sepsis as compared to survivors of non-septic critical illness (controls). An increased rate of pneumonia and opportunistic pathogens were observed in the sepsis survivors as compared to control subjects.
Overall Survival
Finally, we examined long-term survival in both groups and found that the SS group had a higher subsequent one-year mortality than controls (52.7% versus 12%; P<0.0001). (Figure 4). Using uni- and multivariable Cox models of mortality, sepsis was found to be the strongest predictor for post-discharge mortality with a hazard ratio (HR) for death of 3.61 (Table 4). Given that vasopressor use was highly associated with sepsis, a separate model which did not take vasopressor use into account led to an even higher HR of 4.36 (P=0.0004) (Table 5).
Figure 4.
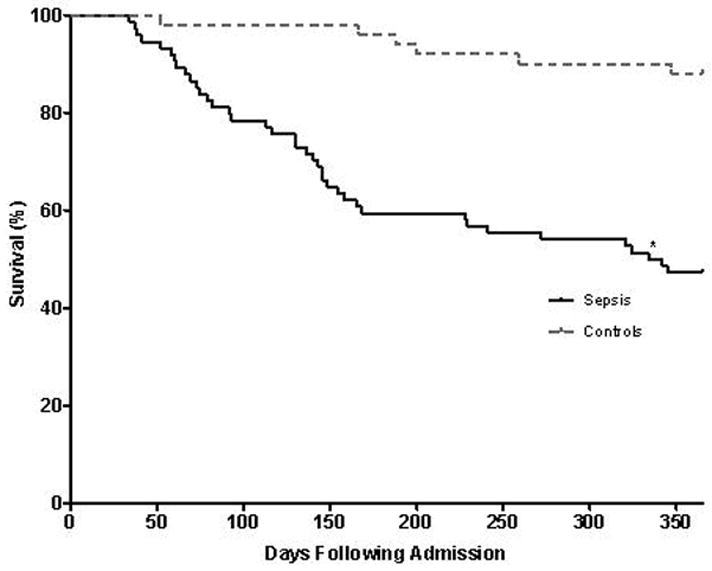
The Kaplan-Meier curve of one-year survival is shown for sepsis survivors as compared to controls. The sepsis survivors (“sepsis,” solid line) had significantly lower survival (higher mortality) compared to survivors of non-septic critical illness (“controls,” dashed line). *, p-value <0.0001.
Table 4.
Post-discharge Mortality (Adjusted) – Model I
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Sepsis | 3.61 | 1.57–8.31 | 0.003 |
| Age (Continuous per decade of life) | 1.046 | 1.020–1.072 | 0.0005 |
| Neurologic | 2.06 | 1.05–4.05 | 0.04 |
| Malignancy | 1.64 | 0.89–3.01 | 0.11 |
| Prior Infection | 1.78 | 0.92–3.45 | 0.09 |
| Vasopressor Use | 1.78 | 0.92–3.15 | 0.09 |
The adjusted post discharge hazard ratio for mortality was calculated based on univariable and multivariable Cox models of mortality. Variable selection for multivariable models was performed by backward selection based on Wald criteria.
Table 5.
Post-discharge Mortality (Adjusted) – Model II
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Sepsis | 4.36 | 1.93–9.84 | 0.0004 |
| Age (Continuous per decade of life) | 1.047 | 1.022–1.073 | 0.0002 |
| Neurologic | 2.09 | 1.05–4.17 | 0.04 |
| Malignancy | 1.58 | 0.84–2.98 | 0.16 |
| Prior Infection | 1.79 | 0.92–3.49 | 0.09 |
| Vasopressor Use | - | - | - |
The adjusted post discharge hazard ratio for mortality was calculated based on univariable and multivariable Cox models of mortality. Vasopressor use, which was highly associated with sepsis, was excluded in this model.
Causes of death were known for 69% of SS and 40% of controls. For those with a known cause of death, SS were much more likely to die of an infectious complication than controls (73% versus 11%; p=0.002; data not shown).
Discussion
Sepsis continues to be a significant cause of death worldwide, but an understanding of the mechanisms responsible for mortality remains elusive. Most studies of sepsis have examined predominantly short-term outcomes with the few studies examining long-term outcomes largely focused on mortality and quality of life(3, 15–17). While these studies are crucial to understanding the long-term consequences of sepsis, why sepsis patients are at an increased long-term risk of death remains poorly understood.
To address this question, we compared the characteristics and outcomes of critically ill patients with and without sepsis who survived 30 days after ICU admission, and who were matched for severity of illness based upon initial APACHE II scores. Consistent with earlier studies(3, 4), we found that patients with sepsis who survive the initial 30 days of critical illness still have significantly higher mortality rates compared to non-septic survivors of critical illness (Figure 4). Not surprisingly, the patients admitted with sepsis were different from critically ill patients without sepsis. Sepsis patients were, on average, 6 years older than critically ill controls. In addition, they were more likely to have been admitted from a nursing home, to have an underlying malignancy (mostly skin and prostate, as hematologic malignancies were excluded), to have a history of infection in the year prior, and to have an indwelling catheter or foreign body. After admission to the ICU, they were also more likely to require mechanical ventilation and vasopressors. We therefore controlled for these potentially confounding variables through the statistical models as outlined above. Interestingly, after controlling for these confounding variables, we found that patients who survived sepsis remained at increased risk for recurrent infections. Indeed, having survived sepsis remained the strongest predictor of recurrent infections post-discharge, re-hospitalizations for infection, and post-discharge mortality after multivariable analysis.
These findings highlight the significant impact of late infectious complications to the long-term outcomes of patients who survive sepsis. At the present time, it is unclear whether patients who acquire sepsis are simply inherently at risk for infectious complications, or whether sepsis imposes additional risk for subsequent infections. However, recent studies suggest that patients with sepsis appear to develop a state of relative immunosuppression secondary to a variety of molecular and cellular mechanisms, including dysfunction of monocytes, dendritic cells, neutrophils, and lymphocytes as outlined in a recent review(5). This suggests that after a septic episode, hosts may be at increased risk for further infectious complications. Despite this, little data exists regarding the clinical impact of sepsis-induced immunosuppression. Our study attempts to address this issue by comparing long-term outcomes of SS with survivors of non-sepsis critical illness. While this study was not designed to prove a causal relationship between sepsis and the development of secondary infections, the results do suggest that the presence of sepsis leads to long-term infectious complications independent of other confounding factors such as advanced age. The increased numbers of infections extended out to one year beyond the initial hospitalization, resulting in a higher number of re-hospitalizations for infection among survivors of sepsis. Importantly, this association remained after controlling for multiple variables that differed between SS and controls, and having survived a bout of sepsis remained the most significant predictor of subsequent post-discharge infections and readmissions for infectious causes. Furthermore, there was a markedly increased risk of death due to infection among SS. Collectively, these results underscore the importance of recurrent infections as a cause of long-term morbidity and mortality in this patient population.
The timing of the recurrent infections also differed in the two groups. Almost immediately after hospital discharge, SS began developing secondary infections. (Figure 1). The sites of infections and infecting microorganisms also differed between the two groups (Figure 3) with SS having higher rates of pneumonia and infections with more opportunistic pathogens (e.g., Pseudomonas aeruginosa, Candida sp.). These findings parallel findings in animal models of sepsis-induced immunosuppression in which they were found to be susceptible to pneumonias and less virulent nosocomial pathogens such as Pseudomonas sp. (18, 19). Although undoubtedly antimicrobial therapy for the initial septic episode placed selection pressure in favor of these organisms, hosts with otherwise healthy immune responses are not generally at risk for developing invasive disease with these pathogens. Infections caused by Clostridium difficile, which is associated with antibiotic treatment, comprised a small percentage (1.8%) of the post-discharge infections among the sepsis survivors. Rather, the patients initially admitted to the ICU for non-infectious reasons ultimately developed infections by more virulent organisms such as Staphylococcus, Streptococcus, and E. coli. However, further investigation is warranted regarding the role that broad-spectrum antibiotics may play in altering the host microbiome and colonization patterns, thereby increasing the likelihood of developing certain types of infections in a susceptible host.
There are limitations to our study. It is a retrospective study performed in the early 2000s in a VA population largely composed of elderly males; hence, further studies are needed to determine whether the findings are applicable to a more general population. Although there have been changes in supportive care of critically ill patients with sepsis including lung-protective ventilatory strategies (in patients with acute respiratory distress syndrome) and early goal directed therapy, we would anticipate that these developments would increase the numbers of long-term survivors of sepsis rather than directly impact the development of infectious complications in subjects who have already survived sepsis. Given the study design, we were unable to obtain data on transfusions of blood products, which has been reported to be associated with immunosuppression.(20, 21) However, the most common admitting diagnosis for the non-septic critically ill controls was gastrointestinal bleeding; therefore, one would anticipate that the non-septic controls would be more likely to receive blood products than the sepsis group. Also, we chose to match the controls based on initial severity of illness using the admission APACHE II score, which is a well-validated ICU scoring system that includes several baseline patient parameters, such as age and history of chronic organ insufficiency (22). While there were differences in some baseline characteristics between the sepsis and non-sepsis groups, none of these were found increase the risk of repeated infection or hospitalization more than sepsis.. Importantly, the two groups were similar with regards to other co-morbidities that could contribute to infection susceptibility and poor outcomes, including cardiopulmonary, renal, and neurologic disease and diabetes (23). In addition, the development of renal failure, which has prognostic value in sepsis (24), was similar between the two groups. Nonetheless, without a prospective study examining specific measures of immune function and development of secondary infections, it is difficult to definitively prove the presence of sepsis-induced immunosuppression and if it has a direct causal relationship to secondary infections.
Despite the limitations, we feel that our findings contribute important epidemiologic information about the long-term infectious risks of survivors of sepsis and critical illness. With the abundance of immunologic data supporting the presence of sepsis-induced immunosuppression and the increased long-term mortality in sepsis survivors, there has yet to be a study evaluating the magnitude of this phenomenon in the clinical setting, whether in the short-term or long-term. This is of extreme importance in this era of critical care medicine as the numbers of patients who survive sepsis will steadily increase with continued improvements in supportive care. From our and other groups’ clinical experience, patients now rarely succumb to their initial bout of sepsis – or even their initial ICU admission - but instead die after a protracted course, often due to a recurrent infection(7, 8). Our study emphasizes that despite improvements in short-term ICU care, patients who survive sepsis remain at risk for long-term infectious complications. This reinforces the importance of developing diagnostic studies that can aid in assessing immune function in the post-septic period as well as therapies directed towards overcoming the immune dysregulation caused by sepsis.
Conclusion
Sepsis is an independent predictor of recurrent infections, hospitalization for infectious causes, and death in the post-septic period. Prospective studies of SS are needed with concurrent measurements of immune function parameters to predict and ultimately prevent the growing problem of recurrent infections.
Acknowledgments
Research funded by: NIH 5K08HL81229 to JCD.
Abbreviation List
- AIDS
Acquired immunodeficiency syndrome
- APACHE
Acute Physiology and Chronic Health Evaluation
- CPRS
Computerized Patient Record System
- EMR
Electronic medical record
- ICU
Intensive Care Unit
- IL-1
Interleukin-1
- LOS
Length of stay
- MRSA
Methicillin resistant Staphylococcus aureus
- SS
Sepsis Survivors
- TNF
Tumor necrosis factor antagonists
- VA
Veterans Affairs
Footnotes
Author contributions:
Dr. Wang contributed to study design, primary data gathering, data interpretation, and drafting and revision of the manuscript.
Dr. Derhovanessian contributed to the statistical analysis of the data, creation of the tables and figures, and revision of the methods section of the manuscript.
Dr. De Cruz contributed to preparation of figures, revision and submission of the final manuscript.
Dr. Belperio contributed to analysis and interpretation of the data as well as revision of the manuscript.
Dr. Soo Hoo contributed to the study design, supervision of the primary data gathering and supervision of the statistical analysis, and manuscript review and revision.
Dr. Deng contributed to the drafting and revision of the manuscript, data analysis and interpretation, as well as the original study design and premise.
Financial/nonfinancial disclosures: Drs. Wang, Derhovanessian, De Cruz, Belperio, Soo Hoo, and Deng report no potential conflicts of interest that exist with any companies or organizations whose products of services may be discussed in this article.
This work was performed at the West Los Angeles Veterans Affairs Medical Center and at the David Geffen School of Medicine at UCLA in Los Angeles, California.
COI disclosure: none for TW, AD, SD, JAB, JCD, and GSH.
Contributor Information
Tisha Wang, Email: tiwang@mednet.ucla.edu.
Ariss Derhovanessian, Email: aderhovanessian@mednet.ucla.edu.
Sharon De Cruz, Email: sdecruz@mednet.ucla.edu.
John A. Belperio, Email: jbelperio@mednet.ucla.edu.
Jane C. Deng, Email: jdeng@mednet.ucla.edu.
Guy Soo Hoo, Email: guy.soohoo@va.gov.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 4.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 5.Wang TS, Deng JC. Molecular and cellular aspects of sepsis-induced immunosuppression. J Mol Med. 2008;86:495–506. doi: 10.1007/s00109-007-0300-4. [DOI] [PubMed] [Google Scholar]
- 6.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA: the journal of the American Medical Association. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- 8.Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ. Monocyte deactivation--rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 9.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 11.Nelson WB. Confidence limits for recurrence data -- applied to cost or number of repairs. Technometrics. 1995;37:147–157. [Google Scholar]
- 12.Lawless JF, CN Some simple robust methods for the analysis of recurrent events. Technometrics. 1995;37:158–168. [Google Scholar]
- 13.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Statis Soc B. 2000;62:711–730. [Google Scholar]
- 14.Pepe MSJC. Some graphical displays and marginal regression analyses for recurrent failure times and time dependent covariates. J Am Statist Ass. 1993;88:811–820. [Google Scholar]
- 15.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 16.Laupland KB, Zygun DA, Doig CJ, Bagshaw SM, Svenson LW, Fick GH. One-year mortality of bloodstream infection-associated sepsis and septic shock among patients presenting to a regional critical care system. Intensive Care Med. 2005;31:213–219. doi: 10.1007/s00134-004-2544-6. [DOI] [PubMed] [Google Scholar]
- 17.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 18.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GH, Reddy RC, Newstead MW, Tateda K, Kyasapura BL, Standiford TJ. Intrapulmonary TNF gene therapy reverses sepsis-induced suppression of lung antibacterial host defense. J Immunol. 2000;165:6496–6503. doi: 10.4049/jimmunol.165.11.6496. [DOI] [PubMed] [Google Scholar]
- 20.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Current opinion in hematology. 1994;1:457–461. [PubMed] [Google Scholar]
- 21.Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology. 2005;10(Suppl 1):208–214. doi: 10.1080/10245330512331390447. [DOI] [PubMed] [Google Scholar]
- 22.Keegan MT, Gajic O, Afessa B. Severity of illness scoring systems in the intensive care unit. Crit Care Med. 2011;39:163–169. doi: 10.1097/CCM.0b013e3181f96f81. [DOI] [PubMed] [Google Scholar]
- 23.Laupland KB, Gregson DB, Zygun DA, Doig CJ, Mortis G, Church DL. Severe bloodstream infections: a population-based assessment. Crit Care Med. 2004;32:992–997. doi: 10.1097/01.ccm.0000119424.31648.1e. [DOI] [PubMed] [Google Scholar]
- 24.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]


