Abstract
Background
Attention-Deficit/Hyperactivity Disorder (ADHD) frequently co-occurs with externalizing disorders, but a clear understanding of the etiologic underpinnings is hampered by the limited understanding of the co-development of the traits from childhood into early adulthood.
Methods
Using a birth cohort of 2600 twins, the Swedish Twin study of Child and Adolescent Development study, assessed at ages 8-9, 13-14, 16-17 and 19-20, we investigated the co-development of ADHD and externalizing behavior from childhood to adulthood. The analyses examined ADHD-like and externalizing traits, as rated by twins and their parents using the Attention Problems scale and Externalizing scale of the Child Behavior Checklist, and estimated cross-lagged effects (one trait at one time-point predicting the other at the next). The covariation between the traits were decomposed into stable (effects carried over from the prior time-points) and innovative (new effects for each time-point) sources; each source was further decomposed into additive genetics, shared and non-shared environment.
Results
The analysis suggested that externalizing traits in middle childhood (age 8-9) predicted ADHD-like traits in early adolescence (age 13-14), whereas the reverse association was non-significant. In contrast, ADHD-like traits in mid-adolescence (age 16-17) predicted externalizing traits in early adulthood (age 19-20). The correlation between ADHD-like and externalizing traits increased over time. At all time-points innovative sources contributed substantially to maintained comorbidity. Genetic effects explained 67% of the covariation at each time-point; importantly, nearly 50% of these effects were innovative.
Conclusions
This study challenges the belief that ADHD generally precedes externalizing behaviors; rather, change in the etiologic factors across the development is the rule. The effects were due to both new genetic and environmental factors emerging up to young adulthood. Clinicians and researchers needs to consider complex etiologic and developmental models for the comorbidity between ADHD and externalizing behaviors.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder characterized by developmentally inappropriate and impairing levels of inattention, hyperactivity and impulsivity (Biederman & Faraone, 2005). ADHD frequently co-occurs with externalizing disorders; 30-50% of individuals meeting the criteria for ADHD also fulfill the criteria for Conduct Disorder (CD) or Oppositional Defiant Disorder (ODD) (Angold, Costello, & Erkanli, 1999; Biederman, Newcorn, & Sprich, 1991; Singh, 2008), and population-based studies suggest that ADHD-like and externalizing traits show considerable covariation in the general population (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). Previous research has shown that ADHD is present in about 24-45% of adult prison inmates (Young & Thome, 2011), but little is known about the developmental trajectories leading to this serious outcome.
Although externalizing problems (e.g., ODD and CD symptoms) may occur early in life they are often assumed to be preceded by ADHD symptoms. Previous research has therefore mainly explored ADHD as a risk for later development of externalizing traits. Longitudinal studies of children with ADHD into adolescence and adult life suggest that externalizing outcomes such as antisocial personality disorder, criminality and substance abuse is more frequent among people with ADHD compared to children without psychopathology (Barkley, Fischer, Smallish, & Fletcher, 2004; Klein et al., 2012; Mannuzza, Klein, Abikoff, & Moulton, 2004; Satterfield et al., 2007). Some studies have shown that childhood ADHD predicts externalizing outcomes, even in the absence of co-occurring ODD and CD symptoms in childhood (Elkins, McGue, & Iacono, 2007), whereas other suggest that the elevated risk for externalizing outcomes disappears after controlling for co-morbid CD (Lahey, Loeber, Burke, Rathouz, & McBurnett, 2002; Lee, Humphreys, Flory, Liu, & Glass, 2011; van Lier, van der Ende, Koot, & Verhulst, 2007). To our knowledge, only two studies has explored if externalizing traits influence later ADHD-like traits; a study of clinic-referred boys reporting that childhood CD predicted later ADHD symptoms, when early levels of ADHD were controlled, but not vice versa (Lahey et al., 2002), and a study where screening positive for conduct problems in boys aged 6 to 7 did not predict hyperactivity ratings at age 16 to 18 when controlling for a positive screening for conduct problems in childhood (Taylor, Chadwick, Heptinstall, & Danckaerts, 1996). Clearly, the dynamic relationship between ADHD-like and externalizing traits has not yet been adequately described, in particular during different developmental periods from childhood to adulthood.
Twin studies of ADHD-like traits among children and adolescents have consistently showed strong genetic influences, with heritability estimates around 60–90% (Burt, 2009; Faraone et al., 2005) whereas both genetic and shared environmental influences seem to be important for externalizing traits, especially during childhood (Burt, 2009; Rhee & Waldman, 2002). There is also evidence that the overlap between ADHD-like and externalizing traits, such as ODD and CD symptoms, are mainly of genetic origin (Dick, Viken, Kaprio, Pulkkinen, & Rose, 2005; Knopik et al., 2013; Nadder, Rutter, Silberg, Maes, & Eaves, 2002; Tuvblad, Zheng, Raine, & Baker, 2009); however, not all studies have reached that conclusion (Burt, Krueger, McGue, & Iacono, 2001).
Longitudinal twin studies have suggested that continuity in ADHD-like (Chang, Lichtenstein, Asherson, & Larsson, 2013) and externalizing traits (Wichers et al., 2013) is mainly due to genetic effects operating across time, and that developmental change in these traits is attributable to new genetic factors that emerge from childhood to early adulthood. Less is known about how stable and new factors contribute to comorbidity over time; in fact, no prior twin study has explored how the comorbidity between ADHD-like and externalizing traits is maintained across the development from childhood to early adulthood. To investigate this we analyzed data collected at four waves from age 8 to age 20 in a Swedish population-based twin study, the Twin study of CHild and Adolescent Development (TCHAD; Lichtenstein, Tuvblad, Larsson, & Carlstrom, 2007).We aimed to; (1) explore the longitudinal direction of effects between ADHD-like and externalizing traits by simultaneously estimating the longitudinal phenotypic associations between the two traits when controlling for the preexisting associations. (2) Decompose the covariation between ADHD-like and externalizing traits at each time-point into components of stability (i.e., comorbidity maintained by stable sources) and innovation (i.e., comorbidity due to new sources).(3) Decompose these sources into their genetic and environmental etiologies.
Methods
Sample
TCHAD is a longitudinal cohort of all twins born in Sweden between May 1985 and December 1986. In total 1480 twin pairs have been invited, of which 2604 individuals (88.0%) from 1310 twin pairs (88.5%; 521 MZ [monozygotic] and 789 DZ [dizygotic] twin pairs) were included in the current study. Zygosity was determined using DNA testing or questions regarding physical similarity. Data was collected at ages 8-9 (middle childhood), 13-14 (early adolescence), 16-17 (late adolescence) and 19-20 (young adulthood). Response rates were 75%, 73%, 74% and 78% for parents (all time-points), and 78%, 82% and 59% for the twins (early adolescence to young adulthood)(Kendler, Gardner, & Lichtenstein, 2008; Lichtenstein et al., 2007). In young adulthood the twins were contacted to give consent before parents were approached with the questionnaire. The study was approved by the regional ethics committee at Karolinska Institutet, Stockholm, Sweden. Informed consent was not required since Swedish rules states that response to the questionnaire constitutes consent.
Measures
Ratings of ADHD-like traits came from the Attention Problems scale (AP), ratings of externalizing traits came from the Externalizing scale (Ext), both scales are from the Achenbach System of Empirically Based Assessment (Achenbach, 1991a, 1991b; Achenbach & Rescorla, 2003). In middle childhood the Child Behavior Checklist (CBCL; Achenbach, 1991a) was used for parent-ratings; in early and late adolescence CBCL was used for parent-rating and Youth Self-Report (Achenbach, 1991b) was used for self-ratings; in young adulthood the Adult Behavior Checklist (Achenbach & Rescorla, 2003) was used for parent-ratings and Adult Self-Report (Achenbach & Rescorla, 2003) was used for self-ratings. Parents and twins were asked to rate the behavior within the past six months. Each construct (AP and Ext) consisted of several questions, each rated on a three-point scale (0 = not true; 1 = sometimes true; 2 = often true). The scales were the sum of the item scores; there was no item overlap across the two scales. Consistent with previous research, we used the AP-scale as a measure of ADHD (Chang et al., 2013), and the combination of two subscales of aggressive and delinquency/rule-breaking behaviors as the measure of Ext (Wichers et al., 2013). Both scales had a slightly skewed distribution and were therefore log-transformed before analysis.
Statistical analyses
The analysis is based on the assumptions in the twin method (Neale & Cardon, 1992); MZ twins share 100% of their co-segregating genes, while DZ twins on average share 50%. Furthermore, MZ and DZ twin pairs are assumed affected by their shared environment to the same extent. The variance within, and covariance between, phenotypes is partitioned into additive genetic sources (A), shared (between twins in a pair) environmental sources (C), and environmental sources unique to each twin (E). To assess the appropriateness of performing analysis on the present data we performed a series of tests of equality of means and covariance matrices between twin order and zygosity (see eAppendix A for details). These analyses showed that models with more restrictions always was preferable to the less restricted (eTable 1), therefore we considered the data to be appropriate for analysis. The most restricted model (Model 5; eTable 1) was used for calculating correlations within and between twins in pairs.
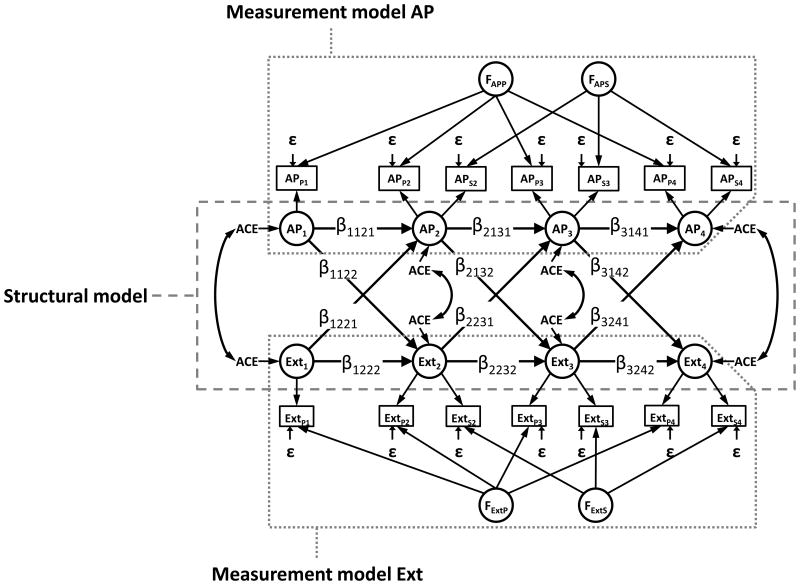
To model the co-development of AP and Ext we used a cross-lagged model (Burt, McGue, Krueger, & Iacono, 2005) including the four time-points (Figure 1). In line with previous research (Chang et al., 2013; Kendler et al., 2008; Wichers et al., 2013), we used a measurement model that combines parent- and self-ratings to generate indexes of unobserved latent factors (AP1-AP4 and Ext1-Ext4) reflecting the shared view across raters. The measurement part of the model included rater-specific latent variables (FAPP, parent-rated AP; FAPS, self-rated AP; FExtP parent-rated Ext; FExtS self-rated Ext) to handle rater-bias. Each measurement had an error term (ε) to remove bias due to time-specific effects. Analyses of AP and Ext have earlier found to similar results between genders (Chang et al., 2013; Wichers et al., 2013); thus, we did not fit gender-specific models. However, each measure was adjusted for gender.
Figure 1.
The model. Note that the figure display the model within an individual, the full model also includes associations between twin pairs.
Footnote: Time-points are numbere 1 to 4 representing ages 8-9, 13-14, 16-17 and 19-20. ACE indicates that the latent variables are decomposed into additive genetic-, shared environmental-, and non-shared environmental- parts. AP, attention problems; Ext, externalizing/disruptive behavior; P, parent-rating; S, self-rating; ACE at time-point 2-4 represent residual variance and covariance, and ε represent residual variance. The path diagram is not a complete representation of the model, more complete path diagrams are found in eFigure 1a and b.
The cross-lagged model constrains associations across age, after adjustment for rater and time-specific bias, to take the form of regression coefficients. The cross-age stability paths (β1121, β1222, β2131, β2232, β3141, and β3242 in Figure 1) estimate the stability of AP and Ext, when controlling for the preexisting association between the two phenotypes. The cross-lagged paths estimate the independent contribution of AP at the earlier time-point on Ext at the consecutive time-point (β1122, β2132 and β3142) and the independent contribution of Ext at the earlier time-point on AP at the consecutive time-point (β1221, β2231 and β3241), while controlling for the stability in the two phenotypes. The variance and covariance of the latent constructs AP1 and Ext1 at time-point 8-9 was partitioned into A, C and E factors (Neale & Cardon, 1992). Similarly, at subsequent time-points, the residual (unexplained) variance and covariance of the latent constructs AP and Ext were decomposed into A, C and E. In eAppendix B the model is described in greater detail.
The cross-lagged model allowed us to simultaneously explore the direction and etiology of the longitudinal relationship between AP and Ext:
Direction of effect: The longitudinal direction of effects was inferred from the cross-age stability and cross-lagged paths.
Stability and innovation: Estimates for A, C, and E at each time-point, and cross-age stability and cross-lagged paths, were used to estimate covariation between AP and Ext and to identify the relative contribution of innovation and stability for maintained covariation.
Genetic and environmental sources of covariance: The estimates were also used to calculate the contributions from genetic and environmental sources in innovation and stability for maintained covariation.
eAppendix B describes how relevant parameters were calculated. For point 2 and 3 above we followed an approach suggested in Greven, Rijsdijk, Asherson, and Plomin (2012) to analyze the covariance rather than each variance separately, because we focused on the co-development of AP and Ext. All analyses were performed using the package OpenMx (S. Boker et al., 2011; S. M. Boker et al., 2012) in the software R (R Development Core Team, 2013), where full information maximum likelihood was employed to handle missing data. Confidence intervals were calculated as 95% profile likelihood confidence intervals.
Results
Descriptive
Twin correlations (i.e., within-twin pair maximum likelihood correlations) are reported in Table 1. MZ-twin correlations were generally twice that of DZ-twins, for example, for parent-rating of Ext at age 8-9 MZ twins had a correlation of 0.67 while DZ twins had a correlation of 0.37. For each phenotype at each time-point, the correlations between raters were moderate; ranging from 0.34 to 0.42 (eTable 2). The correlations between the phenotypes, within rater, at each time-point were higher; between 0.54 and 0.61 (eTable 2). The phenotypic stability (within rater) was highest between two adjacent time-points, and declined as the time-points were further apart (eTable 2). Correlations between twins in pairs for different raters and/or time-points and/or traits were higher in MZ compared to DZ twins in all but two instances, indicating that genetic effects are important also for the comorbidity and over development (eTable 3).
Table 1. Intra-class (within twin pair) correlations.
| Attention problems | MZ | DZ | |
| Age 8-9 | Parent report | 0.44 | 0.19 |
| Age 13-14 | Parent report | 0.44 | 0.25 |
| Self report | 0.38 | 0.22 | |
| Age 16-17 | Parent report | 0.49 | 0.21 |
| Self report | 0.34 | 0.16 | |
| Age 19-20 | Parent report | 0.52 | 0.19 |
| Self report | 0.30 | 0.18 | |
| Externalizing behavior | |||
| Age 8-9 | Parent report | 0.67 | 0.37 |
| Age 13-14 | Parent report | 0.73 | 0.48 |
| Self report | 0.34 | 0.18 | |
| Age 16-17 | Parent report | 0.79 | 0.53 |
| Self report | 0.32 | 0.17 | |
| Age 19-20 | Parent report | 0.54 | 0.28 |
| Self report | 0.35 | 0.14 |
Footnote: Values as estimated in Model 5, eTable 1.
The cross-lagged model
We fitted the cross-lagged model; the most important results are presented in Figure 2 and 3, the standardized genetic and environmental variance components are found in eTable 4, whereas all parameters estimates from the model are presented in eTable 5.
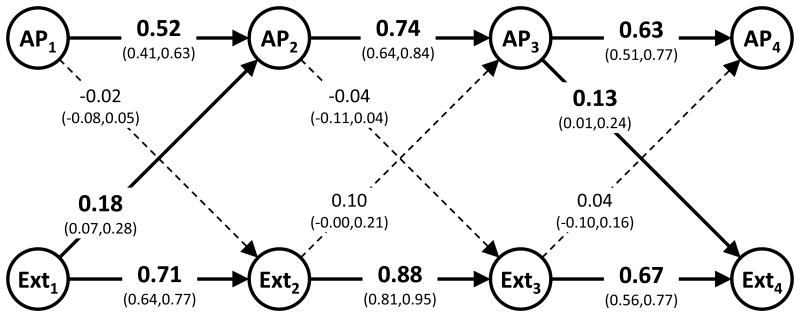
Figure 2.
Standardized cross-age stability paths and cross-lagged paths.
Footnote: Bold figures and solid lines represent estimates where the confidence interval does not overlap the zero. Time-points are numbere 1 to 4 representing ages 8-9, 13-14, 16-17 and 19-20. AP, attention problems. Ext, externalizing behavior.
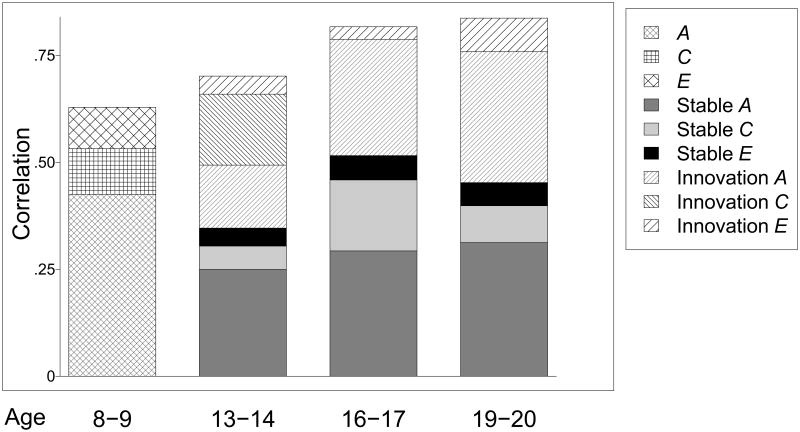
Figure 3.
Genetic and environmental effects in the co-development of attention problems and externalizing behavior as expressed by the correlation between constructs at each time-point.
Footnote: “Stable” refers to correlation explained by earlier time-points, “Innovation” refers to correlation explained by effect that are new at the time-point. A, additive genetic effects. C, shared environmental effects. E, non-shared environmental effects.
Direction of effects
All standardized stability paths were large (0.52-0.88) and significant, that is, each phenotype at one time-point was predicted by the same phenotype at the previous time-point, while adjusting for the other phenotype at the previous time-point (Figure 2). Only two out of the potential six cross-lagged paths were significantly different from zero; Ext at age 8-9 predicted AP at age 13-14, with a standardized regression coefficient of 0.18, and AP at age 16-17 predicted Ext at age 19-20, with a standardized regression coefficient of 0.13 (Figure 2). To quantify the effect sizes of the cross-lagged paths we may compare them to the effect sizes of the corresponding stability paths; one standard deviation (SD) increase in AP at age 8-9 predicted a 0.52 SD increase in AP at age 13-14 while one SD increase in Ext at age 8-9 predicted a 0.18 SD increase in AP at age 13-14. At age 16-17 one SD increase in AP and Ext predicted 0.13 and 0.67 SD increase in Ext at age 19-20, respectively.
Stability and innovation
Figure 3 shows the phenotypic correlation between AP and Ext, as well as the relative contribution of innovation and stability to the observed correlations. The phenotypic correlations (i.e., the correlations between the latent AP and Ext constructs are shown on Y-axis of Figure 3) were 0.63, 0.70, 0.82, and 0.84 at the consecutive time-points. To test whether the correlations were significantly different across time-points we fitted a model where they were constrained to be equal. The model fitted poorer regardless if all four time-points were assumed equal (likelihood ratio test (LRT): χ2 = 36.30, df = 3, p-value < 0.001) or just the last three (LRT: χ2 = 20.78, df = 2, p-value < 0.001), suggesting that the covariation between AP and Ext increase over time. We did not observe any increasing or decreasing pattern of covariance explained by innovation and stability; 49%, 63% and 54% of the covariance was accounted for by innovation sources at the last three time-points (Figure 3).
Genetic and environmental sources of covariance
Figure 3 also shows the genetic and environmental contribution to the correlation between AP and Ext. At the last three time-points A, C and E was partitioned into stable and innovation effects. The fraction of the covariation explained by genetic effects (stability and innovation) could be assumed to be the same across the different time-points; that is, a model where the fractions of genetic effects were assumed equal at 67% across time-points did not yield a poorer fit (LRT: χ2= 5.50, df = 3, p-value = 0.138). Forty-six per cent of the genetic effects were from innovation sources (i.e., the remaining 54% of the genetic covariance was due to genetic stability); a model constraining the relative contribution of innovation A to be constant at 46% over the last three time-points did not fit the data worse (LRT: χ2 = 2.02, df = 2, p-value = 0.364).
Discussion
Contrary to the established view that ADHD is preceding externalizing behaviors, we found that externalizing traits in middle childhood influenced ADHD-like traits in early adolescence. However, ADHD-like traits in late adolescence influenced externalizing traits in early adulthood, which is consistent with the notion that childhood ADHD contributes independently to antisocial personality disorder, criminality and substance abuse in adulthood (Barkley et al., 2004; Elkins et al., 2007; Lee et al., 2011; Satterfield et al., 2007). The correlation between the traits increased across age, thus the correlation between ADHD-like and externalizing traits in adulthood is not only due to pre-existing associations between the traits (Lynam, 1996). Interestingly, genetic innovation explained a significant part of this overlap, indicating that change in the etiologic factors is the rule, rather than the exception. The combination of these findings indicates that both clinicians and researchers need to consider complex etiologic and developmental models for the comorbidity of ADHD and externalizing behaviors. For instance, our finding that childhood externalizing traits predicts elevated levels of ADHD symptoms in adolescence may challenge the validity of the DSM age-at-onset criterion for ADHD.
Our study extends previous findings by suggesting that the pattern of co-development change across time. One study specifically exploring the potential importance of a dynamic co-development of ADHD-like and externalizing traits suggest that childhood externalizing problems predicts subsequent levels of ADHD (Lahey et al., 2002); we replicate this finding. Our finding of no direct longitudinal association between ADHD-like traits in mid-childhood and externalizing traits in early adolescence, adjusting for pre-existing overlaps between the traits, is also in line with some of the previous research (Lahey et al., 2002; Lee et al., 2011; van Lier et al., 2007). We observed non-significant cross-lagged effects from early adolescence to late adolescence. This finding is novel and may suggest that the co-occurrence of ADHD-like and externalizing traits is stable during this developmental period. In contrast to the developmental relationship observed from childhood to early adolescence (i.e., externalizing traits predict ADHD-like traits), the reverse association was observed from mid-adolescence to early adulthood, indicating that ADHD-like traits may exacerbate externalizing tendencies in the transition from adolescence into adult life.
As expected, we found high correlations between ADHD-like and externalizing traits at all time-point from childhood to adulthood (Angold et al., 1999; Biederman et al., 1991; Costello et al., 2003; Singh, 2008; Young & Thome, 2011). Our data indicate that the correlations between ADHD-like and externalizing traits in adolescence and adulthood was partly due to stable sources of covariation, which is consistent with the view that the comorbidity originates early and remains stable over time. This is in line with research showing that children with ADHD plus externalizing problems are at increased risk for a similar behavioral profile in adulthood (Lynam, 1996). Here we demonstrate that the magnitude of the covariation increase over time and that innovative effects were equally important as stable sources. Clearly, future attempts to understand the development of the comorbidity between ADHD and externalizing traits needs to use longitudinal data and consider both stable and time-varying factors.
In line with previous research, we found that the overlap between ADHD-like and externalizing traits was largely explained by shared genetic factors (Dick et al., 2005; Knopik et al., 2013; Nadder et al., 2002; Tuvblad et al., 2009). Particularly, one study has shown that the covariation between the two traits across time is governed by genetics (Nadder et al., 2002). Our results confirms these findings and moreover suggests that stable shared environment factors may contribute to the covariation between ADHD-like and externalizing traits in early adolescence. This finding needs to be interpreted carefully as most (Dick et al., 2005; Knopik et al., 2013; Nadder et al., 2002; Tuvblad et al., 2009), but not all (Burt et al., 2001), studies suggest that shared environmental sources of variation has a limited impact on ADHD-like traits. More importantly, the present study extends previous research by showing that the increased correlation between ADHD-like and externalizing traits from childhood to early adulthood was largely due to new genetic factors. This is in line with previous results where support for the “developmentally dynamic” hypothesis (i.e., genetic innovation explain significant fractions of the variation throughout development) was found when ADHD-like (Chang et al. 2013) and externalizing (Wichers et al. 2013) traits were studied separately. There are examples of widespread pleiotropic effects of genetic risk variants for psychiatric disorders (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013). Our finding of genetic innovation does not rule out pleiotropic effects for ADHD-like and externalizing traits, but suggests that at least part of these influences are developmental specific.
Strength and limitations
One of the strengths of this study is that is uses a representative sample; all twins born during approximately 18 months in Sweden were invited to participate. The data used have been collected prospectively in four waves from mid-childhood to young adulthood. We have utilized as much information as possible by including multiple informants; this allowed us to try and isolate a shared view of the traits measured, and remove rater-bias. Researchers have previously argued that this approach produces more valid inferences since it includes subjective and objective views of the constructs and accounts for rater-specific changes over development (Chang et al., 2013; Kendler et al., 2008; Wichers et al., 2013).
A limitation of this study is that the associations across time-points are forced to go through the cross-age stability and cross-lagged paths. This puts a constraint on how genetic and environmental factors influences subsequent time-points, i.e. the stability contributions to covariance. This is in contrast to the “Cholesky decomposition” approach, used in for example previous studies of the two traits in this sample (Chang et al., 2013; Wichers et al., 2013), where genetic and environmental factors are allowed to more freely influence the measures at later time-points.
The fact that we are using a population-based sample and quantitative measures of ADHD and externalizing problems, rather than clinically diagnosed ADHD, may limit the generalizability to more extreme forms of ADHD. However, we have recently observed a strong genetic link between the extreme and the sub-threshold variation of ADHD symptoms, suggesting that the same etiologic factors are involved in the full range of symptoms of inattention, hyperactivity and impulsivity (Larsson, Chang, D'Onofrio, & Lichtenstein, 2013).
Although the sample has relatively high response-rates in the younger ages, the attrition is not insignificant, with the lowest response-rate for the twins in young adulthood of 59%. If the responders are not representative of the cohort, the parameter estimates (especially in the older ages) might be biased.
Conclusions
This study challenges a simplistic view of ADHD as a stable condition that co-occurs with externalizing behavior because of early emerging stable factors. Our findings provide an empirical foundation for more developmentally-dynamic theories of the comorbidity. Future research that aims to enhance our understanding of the mechanisms underlying the co-development of ADHD and externalizing disorders needs to take bi-directional relationships and time-varying etiologic factors (i.e., genetic and environmental innovation) into consideration.
Key points.
ADHD and externalizing/anti-social behaviors frequently co-occur; the etiology of this comorbidity is not well investigated.
Using a longitudinal assessed twin cohort we disentangled stable and emerging sources of covariation between the traits.
Externalizing traits predicted ADHD-like traits from middle childhood to early adolescence while ADHD-like traits predicted externalizing traits from late adolescence to young adulthood.
About 50% of the covariation between the traits was attributable to previous measures of the traits, and 50% to newly emerging sources; a majority of which were genetic.
The development of comorbidity of ADHD and externalizing behavior seems to be more dynamic than generally considered, future research and clinical practitioners need to take bi-directionality and emerging factors into consideration.
Acknowledgments
Dr Kuja-Halkola had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/Support: This study was supported by the Swedish Research Council and Swedish Research Council for Health, Working Life and Welfare; the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no 340-2013-5867; National Institute of Child Health and Human Development (HD061817).
Footnotes
All authors declare no conflict of interest.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991a. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991b. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms and Profiles. Burlington: University of Vermont, Research Center for Children, Youth and Families; 2003. [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of child psychology and psychiatry, and allied disciplines. 1999;40(1):57–87. [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. Journal of child psychology and psychiatry, and allied disciplines. 2004;45(2):195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. The American journal of psychiatry. 1991;148(5):564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker Steven M, Neale Michael C, Maes Hermine H, Wilde Michael J, Spiegel Michael, Brick Timothy R, Estabrook Ryne, Bates Timothy C, Mehta Paras, von Oertzen Timo, Gore Ross J, Hunter Michael D, Hackett Daniel C, Karch Julian, Brandmaier Andreas M. OpenMx User Guide, Release 1.3 2012 [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychological bulletin. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono WG. Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: the importance of shared environment. Journal of abnormal psychology. 2001;110(4):516–525. doi: 10.1037/0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and psychopathology. 2005;17(1):145–165. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA psychiatry. 2013;70(3):311–318. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of general psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. Journal of abnormal child psychology. 2005;33(2):219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of general psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Greven CU, Rijsdijk FV, Asherson P, Plomin R. A longitudinal twin study on the association between ADHD symptoms and reading. Journal of child psychology and psychiatry, and allied disciplines. 2012;53(3):234–242. doi: 10.1111/j.1469-7610.2011.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychological medicine. 2008;38(11):1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Archives of general psychiatry. 2012;69(12):1295–1303. doi: 10.1001/archgenpsychiatry.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Bidwell LC, Flessner C, Nugent N, Swenson L, Bucholz KK, Madden PA, Heath AC. DSM-IV defined conduct disorder and oppositional defiant disorder: an investigation of shared liability in female twins. Psychological medicine. 2013:1–12. doi: 10.1017/S0033291713001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Burke J, Rathouz PJ, McBurnett K. Waxing and waning in concert: dynamic comorbidity of conduct disorder with other disruptive and emotional problems over 7 years among clinic-referred boys. Journal of abnormal psychology. 2002;111(4):556–567. doi: 10.1037//0021-843x.111.4.556. [DOI] [PubMed] [Google Scholar]
- Larsson H, Chang Z, D'Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychological medicine. 2013:1–7. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clinical psychology review. 2011;31(3):328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-study. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2007;10(1):67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Lynam DR. Early identification of chronic offenders: who is the fledgling psychopath? Psychological bulletin. 1996;120(2):209–234. doi: 10.1037/0033-2909.120.2.209. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Abikoff H, Moulton JL., 3rd Significance of childhood conduct problems to later development of conduct disorder among children with ADHD: a prospective follow-up study. Journal of abnormal child psychology. 2004;32(5):565–573. doi: 10.1023/b:jacp.0000037784.80885.1a. [DOI] [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychological medicine. 2002;32(1):39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Neale Michael C, Cardon Lon R. Methodology for genetic studies of twins and families. Dordrecht ; Boston: Kluwer Academic Publishers; 1992. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing, [computer software] 2013 [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological bulletin. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Satterfield JH, Faller KJ, Crinella FM, Schell AM, Swanson JM, Homer LD. A 30-year prospective follow-up study of hyperactive boys with conduct problems: adult criminality. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(5):601–610. doi: 10.1097/chi.0b013e318033ff59. [DOI] [PubMed] [Google Scholar]
- Singh I. Beyond polemics: science and ethics of ADHD. Nat Rev Neurosci. 2008;9(12):957–964. doi: 10.1038/nrn2514. [DOI] [PubMed] [Google Scholar]
- Taylor E, Chadwick O, Heptinstall E, Danckaerts M. Hyperactivity and conduct problems as risk factors for adolescent development. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(9):1213–1226. doi: 10.1097/00004583-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Zheng M, Raine A, Baker LA. A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9-10 year old boys and girls. Journal of abnormal child psychology. 2009;37(2):153–167. doi: 10.1007/s10802-008-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier PA, van der Ende J, Koot HM, Verhulst FC. Which better predicts conduct problems? The relationship of trajectories of conduct problems with ODD and ADHD symptoms from childhood into adolescence. Journal of child psychology and psychiatry, and allied disciplines. 2007;48(6):601–608. doi: 10.1111/j.1469-7610.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Wichers M, Gardner C, Maes HH, Lichtenstein P, Larsson H, Kendler KS. Genetic innovation and stability in externalizing problem behavior across development: a multi-informant twin study. Behavior genetics. 2013;43(3):191–201. doi: 10.1007/s10519-013-9586-x. [DOI] [PubMed] [Google Scholar]
- Young S, Thome J. ADHD and offenders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011;12(Suppl 1):124–128. doi: 10.3109/15622975.2011.600319. [DOI] [PubMed] [Google Scholar]