Abstract
Background
Enhancing the capability of community health centers to implement best practices may mitigate health disparities. We investigated the association of Practice Adaptive Reserve (PAR) to implementation of Patient Centered Medical Home (PCMH) colorectal cancer (CRC) screening best practices (BPs) at community health center clinics in seven states.
Methods
A convenience sample of clinic staff participated in a self-administered online survey. We scored eight PCMH CRC screening BPs as a composite ranging from 0–32. The PAR composite score was scaled from 0 to 1 then categorized into three levels. Multilevel analyses examined the relationship between PAR and self-reported implementation of the PCMH BPs.
Results
Out of 296 respondents, 59% reported 6 or more PCMH BPs at their clinics. The mean PAR score was 0.66 (s.d. 0.18) and PCMH BP mean scores were significantly higher for respondents who reported higher clinic PAR categories. Compared to the lowest PAR level, adjusted PCMH BP means were 25.0 percent higher at the middle PAR level (Difference = 3.2, SE = 1.3, t = 2.44, p = 0.015) and 63.2 percent higher at the highest PAR level (Difference = 8.0, SE = 1.9, t = 4.86, p < 0.0001).
Conclusion
Higher Adaptive Reserve, as measured by the PAR score, is positively associated with self-reported implementation of PCMH CRC screening BPs by clinic staff. Future research is needed to determine PAR levels most conducive to implementing CRC screening and to develop interventions that enhance PAR in primary care settings.
Keywords: Adaptive reserve, primary care, implementation, best practices, disparities
In 2014, an estimated 50,310 people in the United States (US) will die from colorectal cancer (CRC), the second leading cause of cancer-related deaths.1 When CRC is detected at an early stage, five-year survival rates exceed 90% for those with localized disease.2 The US Preventive Services Task Force (USPSTF) recommends CRC screening for average-risk individuals 50 to 75 years old using: annual high-sensitivity fecal occult blood test (FOBT), sigmoidoscopy every 5 years combined with FOBT every 3 years, or colonoscopy every 10 years.3 However, according to the 2010 National Health Interview Survey, CRC screening rates were 58.6%, well below the Healthy People 2020 goal of 70.5%.4
Because of advances in screening and treatment, CRC incidence and mortality have been declining over the last 25 years.5,6 Unfortunately, this decline has not been shared equally, resulting in a growing racial and ethnic survival gap over the same 25-year period.6–8 CRC screening rates for Whites (59.8%) are consistently higher than those of minority populations: African Americans (55%); American Indians and Alaskan Natives (49.5%); Asian Americans (46.9%); and Hispanics (46.5%).4
Community health centers are vanguard providers of primary care for vulnerable populations, serving 20 million Americans across the US.9–12 Located in areas where care is needed but scarce, community health centers improve access to care for Americans regardless of their insurance status or ability to pay.13,14 With health care reform, community health centers are critical to the expansion of access through a primary care portal.13
Ample literature however has identified the challenges of time constraints to implementing changes in primary care practices.15–26 Among the conceptual frameworks assessing organizational change,27–31 the Practice Change and Development model was developed from studies of primary care practices.30,32,33 A comparison of high-improvement practices with those of low-improvement practices identified the four domains of the Practice Change and Development model and their reciprocal relationships: Inside Motivators, Capability for Development, Outside Motivators, and Opportunities for Development.30
The Capability for Development domain includes the qualities and resources that allow a practice to alter its operations and its beliefs/values. Within this domain, Practice Adaptive Reserve (PAR) comprises the intangible elements that provide flexibility and resilience in times of change.33 As illustrated in Figure 1, PAR centers around seven characteristics of successful work relationships. Under inquiry-centered leadership and a learning culture, these characteristics promote action and reflection34 that lead to teamwork, improvisation and sensemaking,35 as well as the accumulation of stories that enhance positive change.
Figure 1.
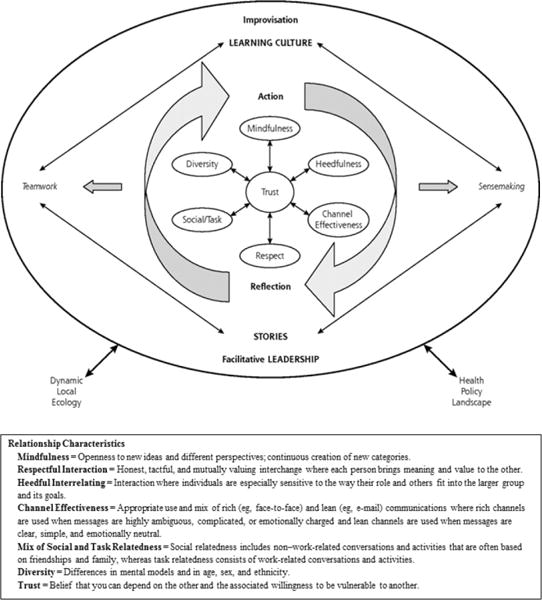
Relationship-centered Practice Adaptive Reserve Model
A model of primary care transformation, the Patient-Centered Medical Home (PCMH) seeks to improve patient and staff experiences, outcomes, safety and system efficiency.36–38 The National Demonstration Project (NDP) evaluated implementation of the PCMH model in 36 highly motivated primary care practices and found that PAR was essential to practices’ ability to manage change.39 To our knowledge, PAR has not been studied at community health centers where it is especially important to understand because of high personnel turnover and the demanding work environment.40,41
The Cancer Prevention and Control Research Network (CPCRN) is a national network of academic, public health, and community partners who work together to reduce the burden of cancer. In this report we describe our novel research, a collaboration with community health centers in seven states, examining the association of PAR with PCMH CRC screening best practices (BPs).
This study’s CRC screening BPs were guided by the 2011 National Committee for Quality Assurance PCMH standards.42 We selected standards that enhance access and continuity, specifically PCMH 1G that focuses on the practice team: a) holding regular team meetings and communication processes; and b) using standing orders for services. We also selected tracking and follow-up of tests (PCMH 5A) as well as referrals (PCMH 5B). Although our outcome measure has not been validated and not all of the BPs shown to improve CRC screening rates or quality, our community health center partners were interested to collaborate on a project consistent with national efforts to transform primary care through the PCMH model.
METHODS
A convenience sample of providers and staff at participating clinics completed our CPCRN survey between January through May 2013. All study procedures were approved by each site’s Institutional Review Boards, the Coordinating Center at University of North Carolina at Chapel Hill, and the Centers for Disease Control and Prevention.
Recruitment
CPCRN sites in WA, SC, TX, GA, and CO partnered with their Primary Care Association (PCA) to identify potential sites with four PCAs directly emailing their community health centers to explore their interest. Five CPCRN sites (TX, GA, CA, CO, MO) contacted community health centers directly via email, telephone calls, or in person meetings. In most cases, one individual from each community health center served as the main contact and sent an introductory email to eligible staff members encouraging their participation. One PCA (SC) also directly recruited participants at a meeting of staff members.
At two, four, six and eight weeks post-invitation, reminder emails were sent to potential participants. Some sites offered $25 gift cards to participants, other sites offered incentives to participating community health centers, and one site declined any incentives.
Based on the available funds for incentives and to ensure broad representation across clinical roles, our online survey was programmed to allow a maximum of 10 staff from each clinic to complete the survey: up to three providers (physicians, nurse practitioners, and physician assistants), three nurses or quality improvement staff, and four medical assistants.
Survey content
We designed the survey to be completed in 20 minutes. Section A consisted of the 23 item PAR Scale43,44 with the word “practice” changed to “clinic”, additional items from the Clinician Staff Questionnaire, and another key informant study.45 Section B assessed the primary CRC screening modality recommended at the clinic. Section C covered four evidence-based approaches to increase CRC screening. Section D inquired whether the clinic had eight CRC screening BPs and how often the respondent performed the CRC screening best practices in the past month. To examine regular team meetings and communication processes, our survey inquired about daily “huddles”, a strategy borrowed from football and increasingly adopted in primary care.46 Section E included demographic questions and work history (number of hours and years worked at the clinic).
Statistical analysis
To understand the relationship between PAR and staff implementation of PCMH CRC screening BPs, we examined differences in the PCMH BP (our dependent variable) means at three different PAR levels. The PAR composite score (an independent variable) was scaled from 0 to 1 with higher values representing greater agreement with PAR items (i.e., 0.00 = complete disagreement and 1.00 = perfect agreement) and categorized into three levels (0.00–<0.60, 0.60–<0.80, and 0.80–1.00). These categories represent respondents in the lowest, highest and middle 2 PAR quartiles. With eight PCMH CRC screening BPs each scored from 0 to 4 (never/rarely/occasionally/usually/always), the PCMH BPs score ranged from 0 to 32.
We examined unadjusted PCMH BP means and means adjusted for these independent variables or fixed effects: state, staff role, age group, number of years employed at the clinic, and number of hours worked per week. The PCMH BP means were calculated using multilevel general linear mixed models.47,48 The random variable was clinic. Given an intraclass correlation of 0.18, we included clinic-specific random intercepts to account for interdependencies of survey responses due to clinic staff nested within clinics. Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Out of 327 staff members who took the survey, 31 did not complete the demographic section yielding 296 respondents from 75 clinics for this analysis. Providers, quality improvement/operations/clinic managers, nurses, and medical assistants were all represented on the survey, with 59% reporting 6 or more PCMH CRC screening BPs for age eligible patients at their clinics. Table 1 notes the majority of respondents was non-Hispanic, female, had a college degree, and provided services in a language(s) other than English.
Table 1.
Characteristics of Community Health Center Clinic Staff Respondents
| Respondents (n=296) | n (%) |
|---|---|
|
| |
| Female | 234 (79.0) |
|
| |
| Race* | |
| White | 189 (70.3) |
| Black, African, African-American | 30 (10.1) |
| Asian | 34 (11.4) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) |
| Native American, American Indian or Alaska Native | 9 (3.0) |
| Other | 41 (13.9) |
|
| |
| Ethnicity | |
| Non-Hispanic | 189 (63.8) |
|
| |
| Staff role | |
| Provider-Physician | 33 (11.2) |
| Provider-Nurse Practicioner/Physician Assistant | 43 (14.5) |
| Quality Improvement/ Operations/Clinic Manager | 26 (8.8) |
| Nurse | 103 (34.8) |
| Medical assistant | 107 (36.1) |
|
| |
| Age (years) | |
| 20–29 | 52 (17.6) |
| 30–39 | 96 (32.4) |
| 40–49 | 71 (24.0) |
| 50 plus | 77 (26.0) |
|
| |
| Highest level of education completed | |
| High school or less/GED | 13 (4.4) |
| Associates degree/some college or trade school | 136 (45.9) |
| Bachelor’s degree | 37 (12.5) |
| Graduate degree | 110 (37.2) |
|
| |
| Years employed at clinic | |
| 0 – 2 | 129 (43.6) |
| 3 – 4 | 52 (17.6) |
| 5 – 9 | 71 (24.0) |
| 10 plus | 44 (14.8) |
|
| |
| Number of hours worked each week | |
| Less than 40 hours | 55 (18.6) |
| 40 hours | 179 (60.5) |
| Greater than 40 hours | 62 (20.9) |
|
| |
| Provide services in language(s) other than English | |
| Yes* | 177 (59.8) |
| Spanish | 158 (53.4) |
| Chinese (e.g., Cantonese, Mandarin, or other dialects) | 115 (38.9) |
| Vietnamese | 8 (2.6) |
| Other | 28 (9.1) |
Total exceeds 100% as respondents were allowed to specify more than category.
The mean PAR score was 0.66, ranging from 0.02 to 1.00 (Table 2). Table 3 shows the responses to each PCMH BPs. Of interest, less than half of the participants reported these two BPs: 1) daily huddles, huddle sheets or checklists, and 2) standing orders/orders prepared by the nurses/medical assistants for providers to sign. Over 40% reported that they did not track CRC screening orders and completion of CRC screening.
Table 2.
Practice Adaptive Reserve (PAR) Scores by State
| State | N | Mean | SD | Min | Q1 | Q2 | Q3 | Max |
|---|---|---|---|---|---|---|---|---|
| California | 28 | 0.60 | 0.23 | 0.02 | 0.46 | 0.65 | 0.78 | 0.96 |
| Colorado | 52 | 0.66 | 0.18 | 0.26 | 0.52 | 0.66 | 0.78 | 1.00 |
| Georgia | 25 | 0.71 | 0.19 | 0.24 | 0.63 | 0.73 | 0.83 | 1.00 |
| Missouri | 4 | 0.65 | 0.06 | 0.58 | 0.61 | 0.65 | 0.69 | 0.73 |
| South Carolina | 19 | 0.68 | 0.17 | 0.21 | 0.60 | 0.65 | 0.77 | 1.00 |
| Texas | 79 | 0.66 | 0.18 | 0.07 | 0.54 | 0.70 | 0.79 | 0.98 |
| Washington | 89 | 0.66 | 0.15 | 0.21 | 0.57 | 0.68 | 0.75 | 0.95 |
| Combined | 296 | 0.66 | 0.18 | 0.02 | 0.55 | 0.67 | 0.77 | 1.00 |
Scores are scaled from 0.00 to 1.00, with 1.00 being a perfect score of agreement; Q = Quartiles
Table 3.
PCMH Colorectal Cancer Screening Best Practices Reported by Survey Respondents
| Never n (%) |
Rarely n (%) |
Occasionally n (%) |
Usually n (%) |
Always n (%) |
|
|---|---|---|---|---|---|
| Daily huddles, huddle sheets or checklists to go over scheduled patients who need CRC screening. | 175 (59.1) | 8 (2.7) | 16 (5.4) | 54 (18.3) | 43 (14.5) |
| Standing CRC screening orders or orders prepared by nurses/medical assistants then signed by providers. | 167 (56.4) | 3 (1.0) | 17 (5.7) | 62 (21.0) | 47 (15.9) |
| Tracking of patients who had CRC screening orders. | 140 (47.3) | 20 (6.8) | 22 (7.4) | 59 (19.9) | 55 (18.6) |
| Tracking of patients who completed CRC screening tests. | 129 (43.6) | 15 (5.1) | 23 (7.8) | 64 (21.6) | 65 (21.9) |
| Tracking of abnormal CRC screening tests. | 104 (35.1) | 12 (4.0) | 13 (4.4) | 68 (23.0) | 99 (33.5) |
| Referrals for diagnostic work-up of abnormal CRC screening tests. | 57 (19.3) | 6 (2.0) | 23 (7.8) | 66 (22.3) | 144 (48.6) |
| Tracking of diagnostic work-up completed by patients with abnormal CRC screening tests. | 96 (32.4) | 9 (3.1) | 21 (7.1) | 69 (23.3) | 101 (34.1) |
| Referrals to specialists* for patients with abnormal colonoscopies. | 52 (17.5) | 10 (3.4) | 26 (8.8) | 55 (18.6) | 153 (51.7) |
PCMH = Patient Centered Medical Home; CRC = colorectal cancer screening
Referrals may range from follow-up with gastroenterologists, evaluation and treatment by surgeons and/or oncologists, to consultation from palliative care specialists.
Table 4 demonstrates that higher PAR scores were associated with greater implementation of PCMH BPs: respondents in the lowest PAR category had lower mean PCMH BPs scores and respondents in the highest PAR category had higher mean PCMH BPs scores. Additionally, PCMH BP means in higher PAR categories were significantly higher than means in lower PAR categories (p < 0.03). This relationship persisted after statistically controlling for other independent variables that were related to the outcome.
Table 4.
PCMH Best Practices Score Means by PAR Level
| PAR Score | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|
| Score (0–32) | Score (0–32) | Difference** | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | p value | |
| 0.00 – <0.60 | 12.85 | 10.70 – 14.99 | 12.67 | 9.90, 15.44 | NA | NA | NA |
| 0.60 – <0.80 | 15.79 | 14.04 – 17.54 | 15.84 | 13.31, 18.36 | 3.16 | 0.61, 5.72 | 0.015 |
| 0.80 – 1.00 | 20.25 | 17.61 – 22.88 | 20.68 | 17.51, 23.86 | 8.01 | 4.76, 11.26 | < 0.0001 |
PCMH = Patient Centered Medical Home
PAR = Practice Adaptive Reserve
Adjusted for state, age, staff role, years worked at the clinic, hours worked each week
Difference between mean and lowest category mean
Evidence our model fits the data is supported by the pseudo r-square of 36% with a proportional reduction in error of 18% and a proportional reduction in variance of 9% at level 1 relative to the unconditional model.48,49
DISCUSSION
Findings from this multi-state survey suggest that higher Adaptive Reserve, as measured by the PAR score, is associated with greater implementation of PCMH CRC screening BPs. Our results are consistent with the NDP findings that practices with strong Adaptive Reserve were able to make the most far reaching changes.39,50 Wagner et al. also underscored that meaningful practice change is unlikely unless an organization has Adaptive Reserve.51
To our knowledge, this is one of the first studies to examine PAR at community health center clinics, which are expected to serve 20 million new patients under the Affordable Care Act.13,52 Our sample of community health center clinics had a PAR score (mean 0.66, s.d. 0.18) comparable to the NDP practices (mean baseline PAR score 0.69; s.d. 0.35), selected for being highly motivated and having significant capability for change.
Medical care is a complex, highly interdependent process influenced by relationships and motivation between and among individual professionals, group level microsystem team processes, culture, leadership, decision-support systems, and incentives.53 Efforts to conceptualize the implementation of evidence-based interventions have converged to a set of multi-level factors.54,55 A systematic review yielded 33 measures that assessed one or more of these levels: structural, organizational, provider, patient, and innovation.56 Of interest, the PAR scale was not included in this systematic review56 because practice-based research and PCMH39 were not among the common keywords identified in the implementation science literature.57
Systematic reviews also suggest that educational or knowledge-based interventions targeting individual providers to improve quality of care have been largely unsuccessful.58–61 Instead, system-level changes that address the complexities of health care delivery show greater promise. A clinic-level, population-based PCMH redesign resulted in slightly better clinical outcomes of coronary heart disease, fewer ambulatory-care sensitive hospitalizations, fewer total inpatient admissions, 17% lower inpatient costs, and 7% lower total health care costs.62 Liss et al. attributed these positive results to the effective provision of whole-person care facilitated by: 1) enhanced care team staffing (including reductions in physicians’ patient panels from an average of 2327 to 1800 patients); 2) pairing longer office visits (lengthened from 20 to 30 minutes) with promotion of virtual medicine use; and 3) outreach for patients’ chronic and acute needs. The impact of enhanced care team staffing and longer office visits on Adaptive Reserve warrants further study. Conversely, identification of Adaptive Reserve levels most conducive to implementing and sustaining change is also needed.
Given the limited resources and personnel time, we surveyed a convenience sample of clinics and clinic staff and we did not standardize incentives across all the participating clinics. These potential limitations may lead to selection bias and higher PAR scores. The community health center clinics we studied were also geographically limited and cannot be generalized to other clinics in the same states or in other states. Our survey analyses focused on Adaptive Reserve, only one aspect of the Practice Change and Development domains. We also conducted individual level analyses (i.e., staff-reported scores) instead of clinic level analyses (operations/clinic manager reported scores). Lastly, prospective studies are needed to determine causality.
CONCLUSIONS
With health care reform, expansion of primary care through community health centers will be dramatic. As the primary care portal to vulnerable populations, community health centers serve a critical role in promoting CRC screening to populations that are disproportionately under-screened. Findings from this study inform the implementation of CRC screening BPs as well as the many foreseen and unforeseen changes necessitated by the expansion of primary care through community health centers. Given the complexities of health care delivery, interventions need to move beyond individual providers and address the practice team as well as the systems that these teams deliver care in. Future research is needed to determine PAR levels most conducive to implementing change and to develop interventions that enhance PAR in the clinical setting.
Acknowledgments
We thank the participating PCAs and community health centers’ site directors and staff. We are also grateful to Jim Hotz, MD and Kathleen Clark for their guidance of this study.
Support: This research was supported by Centers for Disease Control and Prevention U48DP001911, U48DP001949-02, U48DP001936, U48DP0010909, U48DP001938, U48DP001934, U48DP001903, U48DP001944, U48DP001946, U48DP001924, U48DP001938, and National Cancer Institute grants R01CA124397 and R21CA136460. Contents of this manuscript are solely the responsibility of the authors and do not represent the official view of the Centers for Disease Control and Prevention, the National Cancer Institute, the Department of Health and Human Services, or the U.S. Government.
Footnotes
Conflict of Interest.
None of the authors have any financial disclosures to make, and all authors declare that they have no competing interests of any kind.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014 Mar;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. 2011 http://www.cancer.org/Research/CancerFactsFigures/index. Accessed September 23, 2011.
- 3.US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41–45. [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012 Aug;27(8):1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayanian JZ. Racial disparities in outcomes of colorectal cancer screening: biology or barriers to optimal care? J Natl Cancer Inst. 2010 Apr 21;102(8):511–513. doi: 10.1093/jnci/djq089. [DOI] [PubMed] [Google Scholar]
- 8.SEER*Stat Database: Incidence – SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases. National Cancer Institute, DCCPS, Surveillance Research Program. 2008 www.seer.cancer.gov. Accessed October 1, 2012.
- 9.Lefkowitz B. The health center story: forty years of commitment. J Ambul Care Manage. 2005 Oct-Dec;28(4):295–303. doi: 10.1097/00004479-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Proser M. Deserving the spotlight: health centers provide high-quality and cost-effective care. J Ambul Care Manage. 2005 Oct-Dec;28(4):321–330. doi: 10.1097/00004479-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Chin MH. Quality improvement implementation and disparities: the case of the health disparities collaboratives. Medical care. 2010 Aug;48(8):668–675. doi: 10.1097/MLR.0b013e3181e3585c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Association of Community Health Centers. The Need. http://www.nachc.org/health-center-need.cfm. Accessed July 28, 2010.
- 13.Adashi EY, Geiger HJ, Fine MD. Health care reform and primary care–the growing importance of the community health center. N Engl J Med. 2010 Jun 3;362(22):2047–2050. doi: 10.1056/NEJMp1003729. [DOI] [PubMed] [Google Scholar]
- 14.National Association of Community Health Centers. Official Web Site of the National Association of Community Health Centers. http://www.nachc.org/about-our-health-centers.cfm. Accessed July 28, 2010.
- 15.Ka‘ano’i ME, Braun KL, Gotay CC. Primary care physicians’ knowledge, attitudes and practices related to cancer screening and cancer prevention clinical trials. Pac Health Dialog. 2004 Sep;11(2):160–165. [PubMed] [Google Scholar]
- 16.Meredith LS, Mendel P, Pearson M, et al. Implementation and maintenance of quality improvement for treating depression in primary care. Psychiatr Serv. 2006 Jan;57(1):48–55. doi: 10.1176/appi.ps.57.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Graham IM, Stewart M, Hertog MG. Factors impeding the implementation of cardiovascular prevention guidelines: findings from a survey conducted by the European Society of Cardiology. Eur J Cardiovasc Prev Rehabil. 2006 Oct;13(5):839–845. doi: 10.1097/01.hjr.0000219112.02544.24. [DOI] [PubMed] [Google Scholar]
- 18.Miller PM, Stockdell R, Nemeth L, et al. Initial steps taken by nine primary care practices to implement alcohol screening guidelines with hypertensive patients. Subst Abus. 2006 Jun;27(1–2):61–70. doi: 10.1300/J465v27n01_08. [DOI] [PubMed] [Google Scholar]
- 19.MacGregor K, Handley M, Wong S, et al. Behavior-change action plans in primary care: a feasibility study of clinicians. J Am Board Fam Med. 2006 May-Jun;19(3):215–223. doi: 10.3122/jabfm.19.3.215. [DOI] [PubMed] [Google Scholar]
- 20.Mattocks K, Lalime K, Tate JP, et al. The state of physician office-based health information technology in Connecticut: current use, barriers and future plans. Conn Med. 2007 Jan;71(1):27–31. [PubMed] [Google Scholar]
- 21.Helfrich CD, Weiner BJ, McKinney MM, Minasian L. Determinants of implementation effectiveness: adapting a framework for complex innovations. Medical care research and review. 2007 Jun;64(3):279–303. doi: 10.1177/1077558707299887. [DOI] [PubMed] [Google Scholar]
- 22.Wolfson D, Bernabeo E, Leas B, et al. Quality improvement in small office settings: an examination of successful practices. BMC Fam Pract. 2009;10:14. doi: 10.1186/1471-2296-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolford SJ, Clark SJ, Ahmed S, Davis MM. Feasibility and acceptability of a 1-page tool to help physicians assess and discuss obesity with parents of preschoolers. Clin Pediatr. 2009 Nov;48(9):954–959. doi: 10.1177/0009922809338060. [DOI] [PubMed] [Google Scholar]
- 24.Lemay CA, Beagan BM, Ferguson WJ, Hargraves JL. Lessons learned from a collaborative to improve care for patients with diabetes in 17 community health centers, Massachusetts, 2006. Prev Chronic Dis. 2010 Jul;7(4):A83. [PMC free article] [PubMed] [Google Scholar]
- 25.Abbo ED, Zhang Q, Zelder M, Huang ES. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008 Dec;23(12):2058–2065. doi: 10.1007/s11606-008-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.To T, McLimont S, Wang C, Cicutto L. How much do health care providers value a community-based asthma care program? A survey to collect their opinions on the utilities of and barriers to its uptake. BMC health services research. 2009;9:77. doi: 10.1186/1472-6963-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation science. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999 Sep;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner BJ. A theory of organizational readiness for change. Implementation science: IS. 2009;4:67. doi: 10.1186/1748-5908-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen D, McDaniel RR, Jr, Crabtree BF, et al. A practice change model for quality improvement in primary care practice. J Healthc Manag. 2004 May-Jun;49(3):155–168. discussion 169–170. [PubMed] [Google Scholar]
- 31.Holt DT, Helfrich CD, Hall CG, Weiner BJ. Are you ready? How health professionals can comprehensively conceptualize readiness for change. J Gen Intern Med. 2010 Jan;25(Suppl 1):50–55. doi: 10.1007/s11606-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin MA, Zyzanski SJ, Zronek S, et al. A clinical trial of tailored office systems for preventive service delivery. The Study to Enhance Prevention by Understanding Practice. Am J Prev Med. 2001 Jul;21(1):20–28. doi: 10.1016/s0749-3797(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 33.Miller WL, Crabtree BF, Nutting PA, et al. Primary care practice development: a relationship-centered approach. Annals of family medicine. 2010;8(Suppl 1):S68–79. doi: 10.1370/afm.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safran DG, Miller W, Beckman H. Organizational dimensions of relationship-centered care. Theory, evidence, and practice. Journal of general internal medicine. 2006 Jan;21(Suppl 1):S9–15. doi: 10.1111/j.1525-1497.2006.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan Z. Magnet recognition and practice development: two journeys towards practice improvement in health care. Int J Nurs Pract. 2009 Dec;15(6):495–501. doi: 10.1111/j.1440-172X.2009.01798.x. [DOI] [PubMed] [Google Scholar]
- 36.Fillmore H, Dubard CA, Ritter GA, Jackson CT. Health Care Savings with the Patient-Centered Medical Home: Community Care of North Carolina’s Experience. Population health management. 2013 Sep 21; doi: 10.1089/pop.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birnberg JM, Drum ML, Huang ES, et al. Development of a safety net medical home scale for clinics. J Gen Intern Med. 2011 Dec;26(12):1418–1425. doi: 10.1007/s11606-011-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stange KC, Miller WL, Nutting PA, et al. Context for understanding the National Demonstration Project and the patient-centered medical home. Annals of family medicine. 2010;8(Suppl 1):S2–8. doi: 10.1370/afm.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nutting PA, Crabtree BF, Stewart EE, et al. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Annals of family medicine. 2010;8(Suppl 1):S33–44. doi: 10.1370/afm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi AS, Selia E, McDonnell K. Stress and provider retention in underserved communities. Journal of health care for the poor and underserved. 2009 Aug;20(3):597–604. doi: 10.1353/hpu.0.0163. [DOI] [PubMed] [Google Scholar]
- 41.Rosenblatt RA, Andrilla CH, Curtin T, Hart LG. Shortages of medical personnel at community health centers: implications for planned expansion. JAMA. 2006 Mar 1;295(9):1042–1049. doi: 10.1001/jama.295.9.1042. [DOI] [PubMed] [Google Scholar]
- 42.NCQA PCMH 2011 standards, elements and factors: documentation guideline/data sources. http://www.ncqa.org/portals/0/Programs/Recognition/PCMH_2011_Data_Sources_6.6.12.pdf. Accessed 2014 August 21.
- 43.Nutting PA, Crabtree BF, Miller WL, et al. Journey to the patient-centered medical home: a qualitative analysis of the experiences of practices in the National Demonstration Project. Annals of family medicine. 2010;8(Suppl 1):S45–56. doi: 10.1370/afm.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaen CR, Crabtree BF, Palmer RF, et al. Methods for evaluating practice change toward a patient-centered medical home. Annals of family medicine. 2010;8(Suppl 1):S9–20. doi: 10.1370/afm.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohng H, Kuniyuki A, Edelson J, et al. Capability for Change at Community Health Centers: An Exploratory Study on the Implementation of an Evidence-based Intervention. Asian Pacific Journal of Cancer Prevention. 2014 doi: 10.7314/apjcp.2013.14.12.7451. [DOI] [PubMed] [Google Scholar]
- 46.Stewart EE, Johnson BC. Huddles: Improve Office Efficiency in Mere Minutes. Daily gatherings of your care team can help you meet daily challenges. Family Practice Manangement. 2007;14(6):27–29. [PubMed] [Google Scholar]
- 47.Dickinson LM, Basu A. Multilevel modeling and practice-based research. Annals of family medicine. 2005 May-Jun;3(Suppl 1):S52–60. doi: 10.1370/afm.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2. Sage Publications; 2001. [Google Scholar]
- 49.Snijders T, Bosker R. Multilevel Analysis: An Introduction to basic and Advanced Multilevel Modeling. 2. SAGE Publications; 2004. [Google Scholar]
- 50.Crabtree BF, Nutting PA, Miller WL, et al. Primary care practice transformation is hard work: insights from a 15-year developmental program of research. Medical care. 2011 Dec;49(Suppl):S28–35. doi: 10.1097/MLR.0b013e3181cad65c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner EH, Coleman K, Reid RJ, et al. The changes involved in patient-centered medical home transformation. Prim Care. 2012 Jun;39(2):241–259. doi: 10.1016/j.pop.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.National Association of Community Health Care Centers. The Challenges. 2010 www.nachc.org/health-center-challenges.cfm. Accessed July 28, 2010.
- 53.Shortell SM, Jones RH, Rademaker AW, et al. Assessing the impact of total quality management and organizational culture on multiple outcomes of care for coronary artery bypass graft surgery patients. Medical care. 2000 Feb;38(2):207–217. doi: 10.1097/00005650-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. American journal of community psychology. 2008 Jun;41(3–4):327–350. doi: 10.1007/s10464-008-9165-0. [DOI] [PubMed] [Google Scholar]
- 55.Proctor EK, Landsverk J, Aarons G, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Administration and policy in mental health. 2009 Jan;36(1):24–34. doi: 10.1007/s10488-008-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudoir SR, Dugan AG, Barr CH. Measuring factors affecting implementation of health innovations: a systematic review of structural, organizational, provider, patient, and innovation level measures. Implementation science: IS. 2013;8:22–42. doi: 10.1186/1748-5908-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabin BA, Brownson RC, Haire-Joshu D, et al. A glossary for dissemination and implementation research in health. J Public Health Manag Pract. 2008 Mar-Apr;14(2):117–123. doi: 10.1097/01.PHH.0000311888.06252.bb. [DOI] [PubMed] [Google Scholar]
- 58.Leykum LK, Palmer R, Lanham H, et al. Reciprocal learning and chronic care model implementation in primary care: results from a new scale of learning in primary care. BMC health services research. 2011;11:44. doi: 10.1186/1472-6963-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Evaluation & the health professions. 2006 Mar;29(1):126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 60.Grimshaw JM, Eccles MP. Is evidence-based implementation of evidence-based care possible? The Medical journal of Australia. 2004 Mar 15;180(6 Suppl):S50–51. doi: 10.5694/j.1326-5377.2004.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 61.Weiner BJ, Amick H, Lee SY. Conceptualization and measurement of organizational readiness for change: a review of the literature in health services research and other fields. Medical care research and review: MCRR. 2008 Aug;65(4):379–436. doi: 10.1177/1077558708317802. [DOI] [PubMed] [Google Scholar]
- 62.Liss DT, Fishman PA, Rutter CM, et al. Outcomes among chronically ill adults in a medical home prototype. The American journal of managed care. 2013;19(10):e348–358. [PMC free article] [PubMed] [Google Scholar]


