Abstract
There exists a strong link between oxidative stress, renal dopaminergic system and hypertension. It is reported that reactive oxygen species attenuate renal proximal tubular dopamine receptor (D1R) function which disrupts sodium regulation and leads to hypertension. The mechanisms for renal D1R dysfunction however are not clear. We investigated the role of redox sensitive transcription factors AP1 and SP3 in transcriptional suppression of D1R gene and subsequent D1R signaling. Human kidney (HK2) proximal tubular cells were treated with a pro-oxidant L-buthionine sulfoximine (BSO) with and without an antioxidant tempol. In HK2 cells BSO caused oxidative stress and reduced D1R mRNA and membrane receptor expression. Incubation of HK2 cells with SKF38393, a D1R agonist, caused a concentration dependent inhibition of Na/K-ATPase. However, SKF38393 failed to inhibit Na/K-ATPase in BSO-treated cells. BSO increased AP1 and SP3 nuclear expression. Transfection with AP1 or SP3 specific siRNA abolished BSO-induced D1R down-regulation. Treatment of rats with BSO for 4 weeks increased oxidative stress and SP3—AP1 expression and reduced D1R numbers in renal proximal tubules. These rats exhibited high blood pressure and SKF38393 failed to inhibit proximal tubular Na/K-ATPase activity. Control rats were kept on tap water. Tempol per se had no effect on D1R expression or other signaling molecules but prevented BSO-induced oxidative stress, SP3—AP1 up-regulation and D1R dysfunction in both HK2 cells and rats. These data shows that oxidative stress via AP1—SP3 activation suppresses D1R transcription and function. Tempol mitigates oxidative stress, blocks AP1—SP3 activation and prevents D1R dysfunction and hypertension.
Keywords: Antioxidants, G Proteins, Hypertension, Kidney, Na+/K+-ATPase, Proximal Tubules
Introduction
Kidneys play an important role in the regulation of blood pressure primarily by maintaining sodium homeostasis.1–5 The sodium metabolism is chiefly controlled by a complex interaction among natriuretic and antinatriuretic factors.1–5 During sodium replete conditions, natriuretic factor dopamine plays a major role in maintaining sodium balance as more than 50% of excreted sodium is attributed to renal D1R activation and subsequent inhibition of proximal tubular Na/K-ATPase.6, 7
Like other G protein coupled rectors, D1R is susceptible to oxidative stress.8 It has been shown that in animal models which exhibit oxidative stress, renal D1R function is significantly reduced.9, 10 The detrimental link between oxidative stress and D1R function has been reproduced in proximal tubular cell cultures.11 Interestingly, animals with oxidative stress and renal D1R defect exhibit hypertension thus highlighting the importance of renal D1R in blood pressure regulation and a potential link between oxidative stress and hypertension.9, 10 Despite a significant amount of work on the mechanisms of oxidative stress –mediated D1R dysfunction, the role and mechanism of D1R down-regulation is not clearly understood. Various studies in animal models of hypertension show that a defect in D1R signaling is independent of receptor expression levels.12, 13 In these animals, the failure of D1R to activate downstream pathways is largely due to receptor G-protein uncoupling caused by increased membrane translocation of G protein receptor kinases (GRK), especially GRK 2 and 4.12–15 The over-stimulation of GRKs leads to receptor hyper-phosphorylation and subsequent uncoupling from G proteins.12 However, there are reports which show that receptor dysfunction due to G protein uncoupling is caused by a decrease in D1R expression.16–18 Therefore, this study was undertaken to investigate the mechanism of D1R down-regulation during oxidative stress.
ROS modulate a plethora of signaling molecules that could ultimately affect gene expression and/or protein synthesis.19, 20 This occurs, at least in part, by activation or suppression of transcription factors such as AP1 and SP3.19–24 These transcription factors can regulate genes that control protein synthesis, apoptosis, inflammation, and cell division individually or by interacting with other transcription factors.22–25 The SP family of transcription factors is characterized by the presence of three zinc fingers that form their DNA-binding domain and allow them to bind the GC and GT boxes for the regulation of basal/constitutive expression of a diverse range of viral and cellular genes both under physiological and pathophysiological conditions.22, 23 It is reported that SP3 promoter has a consensus sequence for AP1-binding site.26 AP1 family proteins are basic leucine zipper transcription factors which represent sequence-specific DNA-binding transcription factors and consist of homo- or heterodimers, which are formed by jun (c-jun, junB, junD), fos (c-fos, fra-1, fra-2), or atf proteins, in mammalian cells.27 There are reports that oxidative stress activates AP1 directly or via MAP kinase pathways and D1R promoter possesses binding sequences for AP1.25, 28, 29 Therefore, we aimed to investigate the role of AP1 and SP3 in renal D1R gene repression and function during oxidative stress.
Methods
HK2 cells, a human kidney proximal tubular cell line, were obtained from American Tissue Culture Collection (ATCC® CRL-2190™) and maintained and subcultured in ATCC recommended complete media (Invitrogen, 17005-042). Cells were grown to 85% confluency, starved overnight in DMEM-F12 and incubated with 50 mmol/L L-buthionine sulfoximine (BSO), tempol (1mM) and BSO plus tempol for 72 hr. Cells maintained in DMEM-F12 alone served as control. Malondialdehyde levels were determined by the method of Mihara and Uchiyama30 and a commercially assay kit (cat # STA-330, Cell Biolabs Inc).
D1R mRNA and ligand binding
Total RNA was isolated from HK2 cells by a RNeasy mini kit (QIAGEN) and used for cDNA synthesis and amplification of D1R and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, used as an internal control) using Advantage cDNA PCR Kit (BD Biosciences, Clonetech) as described previously.31 The PCR for D1R and GAPDH was performed with commercially available taqman® primers (Life Technologies).
For ligand binding assay membranes were prepared by harvesting the cells in ice-cold PBS followed by differential centrifugation as detailed previously.12 Membranes (50 µg protein) were suspended in 50 mmol/L TrisHCl (pH 7.4, 120 mmol/L MgCl2) and saturation assay was performed at different concentrations of [3H]SCH23390 using unlabeled SCH23390 (10 µmol/L) to obtain specific binding. Binding was stopped by rapid filtration through Whatman GF/B filters.
Na/K-ATPase Assay
HK2 Cells were grown in 24-well plates to measure Na/K-ATPase activity as 86Rb+ uptake.32 Following the treatment of cells with BSO and/or tempol, the plates were washed with PBS and incubated with varying concentrations of SKF38393 for 10 minutes at 37°C. 86Rb+ uptake was initiated by addition of 0.5 mL DMEM-F12 containing 3 µCi/mL 86Rb+ in the presence and absence of ouabain (1 mmol/L). Cells were lysed with 0.5 N NaOH (0.5 mL/well) and radioactivity was measured directly in cell lysate with a gamma counter. Protein was measured in each well to normalize 86Rb+ uptake.
ELISA
As detailed before,33 nuclear fractions were isolated using commercially available NE-PER nuclear and cytosolic protein extraction kit (Pierce). SP3 and AP1 (c-fos) nuclear translocation was quantitated by commercially available species specific ELISA kits (Antibodies-online Inc and Mybioscource). SP3 and AP1 specific optical density was measured at 450 nm. To correct for an optical imperfections, the plate was also read at 540 or 570 nm and these optical densities were subtracted from the reading recorded at 450 nM.
Animal studies
Animal studies were performed essentially as detailed in our previous publication.9, 12 The protocol was approved by IACUC. Briefly, Adult male Sprague-Dawley (SD) rats were divided in four groups and provided with L-buthionine sulfoximine (BSO) 30mmol/L, tempol (T) 1mmol/L and BSO plus T for 4–5 weeks in drinking water. Control rats were kept on drinking water. Conscious blood pressure was measure by radio-telemetry (DSI, Minneapolis, MN). Since change in blood pressure was maximum after 4 weeks of treatment, this time point was selected for further biochemical studies which were performed as detailed previously.9, 12
Statistical analysis
Differences between the means were evaluated using unpaired Student’s t-test or one-way ANOVA with post hoc Newman-Keuls multiple test, as appropriate. P<0.05 was considered statistically significant.
Results
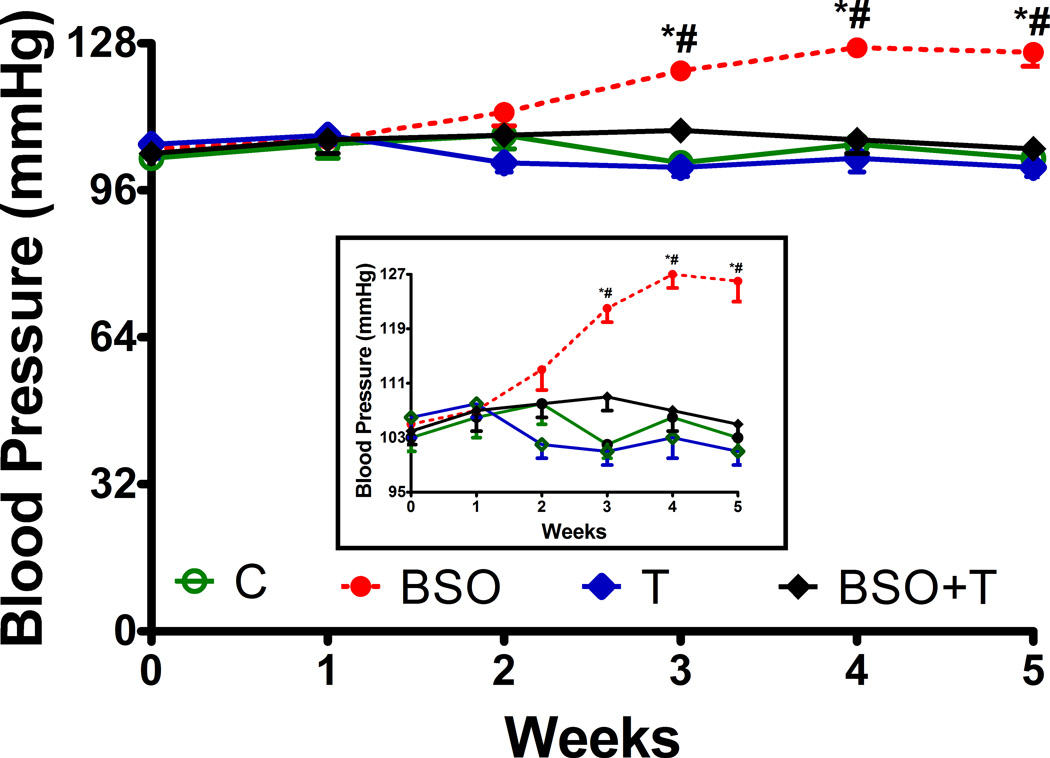
Treatment of HK2 cells with BSO or tempol for 72 hr had no significant effect on cell viability as measured by trypan blue uptake (% uptake, control: 4.01 ± 0.30, BSO: 4.91 ± 0.41, T: 3.81 ± 0.37, BSO+T: 4.46 ± 0.43) or LDH release, (µmol/mg protein/hr; control: 0.15 ± 0.01, BSO: 0.19 ± .011, T: 0.12 ± .013, BSO+T: 0.14 ± .012) and did not change cell morphology (data not shown). However, BSO treatment caused oxidative stress as evidenced by a significant increase in malondialdehyde level, a lipid peroxidation marker (nmol/mg protein; control: 53.55 ± 2.05, BSO: 86.39 ± 4.22, P< 0.05 vs. control). Tempol had no effect on basal malondialdehyde level (T: 49.05 ± 5.10) but rescued cells from BSO-induced lipid peroxidation (BSO+T: 59.3 ± 4.04, P< 0.05 vs. BSO). Treatment of SD rats with BSO also increased renal malondialdehyde (pmol/mg protein, control: 75.1 ± 6.5; BSO: 129.2 ± 8.3, P< 0.05 vs. control) and urinary 8-isoprostane excretion (pg/mg creatinine, control: 0.95 ± 0.07; BSO: 1.43 ± 0.1, P< 0.05 vs. control). An increase in both malondialdehyde and 8-isoprostane was prevented by concurrent tempol supplementation (malondialdehyde: 85.2 ± 7.6; 8-isoprostane 1.07 ± 0.6, P< 0.05 vs. BSO). Rats treated with BSO exhibited time depend increase in blood pressure, with maximum increase at 4 weeks of treatment (fig. 1). The rise in blood pressure was mitigated by tempol treatment (fig. 1). In the absence of BSO tempol had no effect on oxidative stress (malondialdehyde: 70.1 ± 3.2; 8-isoprostane: 0.89 ± .06) or blood pressure (fig. 1). BSO and tempol did not affect the food or water intake (data not shown). Tubule viability, as measured by trypan blue uptake or LDH release, was similar in all experimental groups (data not shown).
Figure 1.
Effect of L-buthionine-sulfoximine (BSO) and tempol (T) on blood pressure in rats. The insert magnifies the increase in blood pressure in BSO-treated rats. Data represents Mean ± SE from 8–10 rats in each group. *P<0.05 vs baseline (at the beginning of the treatment) and #P<0.05 vs. control at corresponding time point.
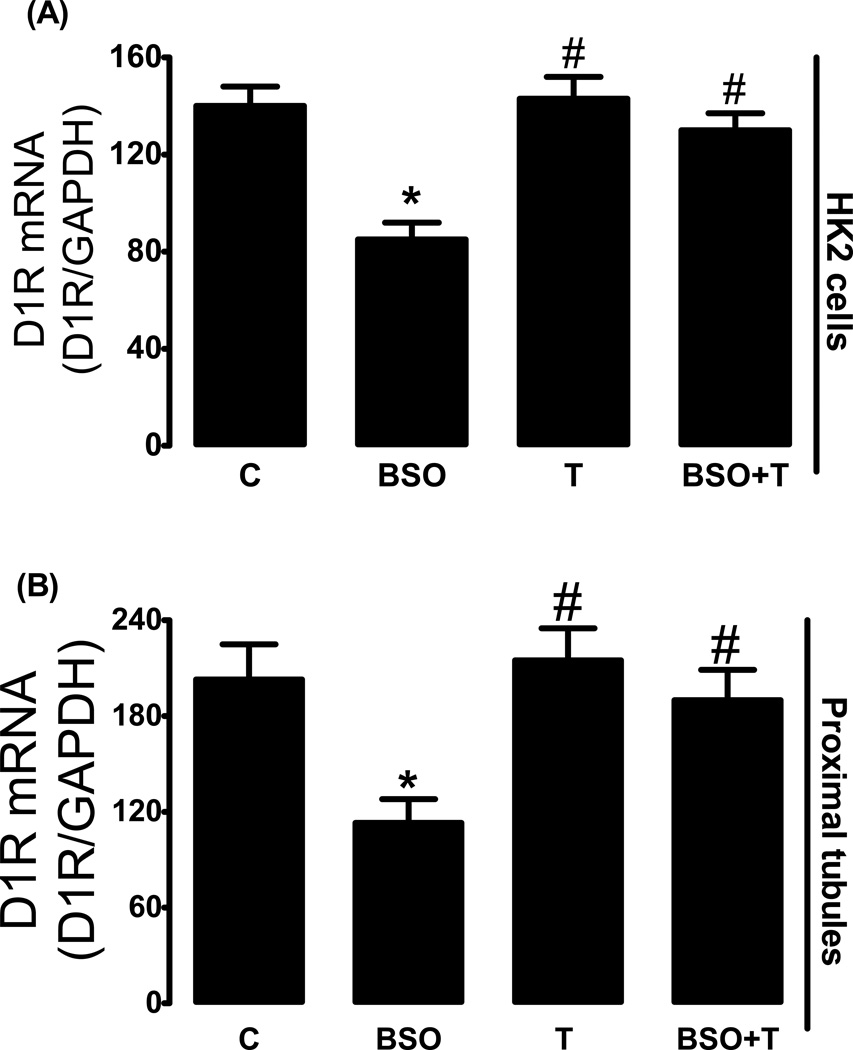
Effect of BSO on D1R mRNA and ligand binding
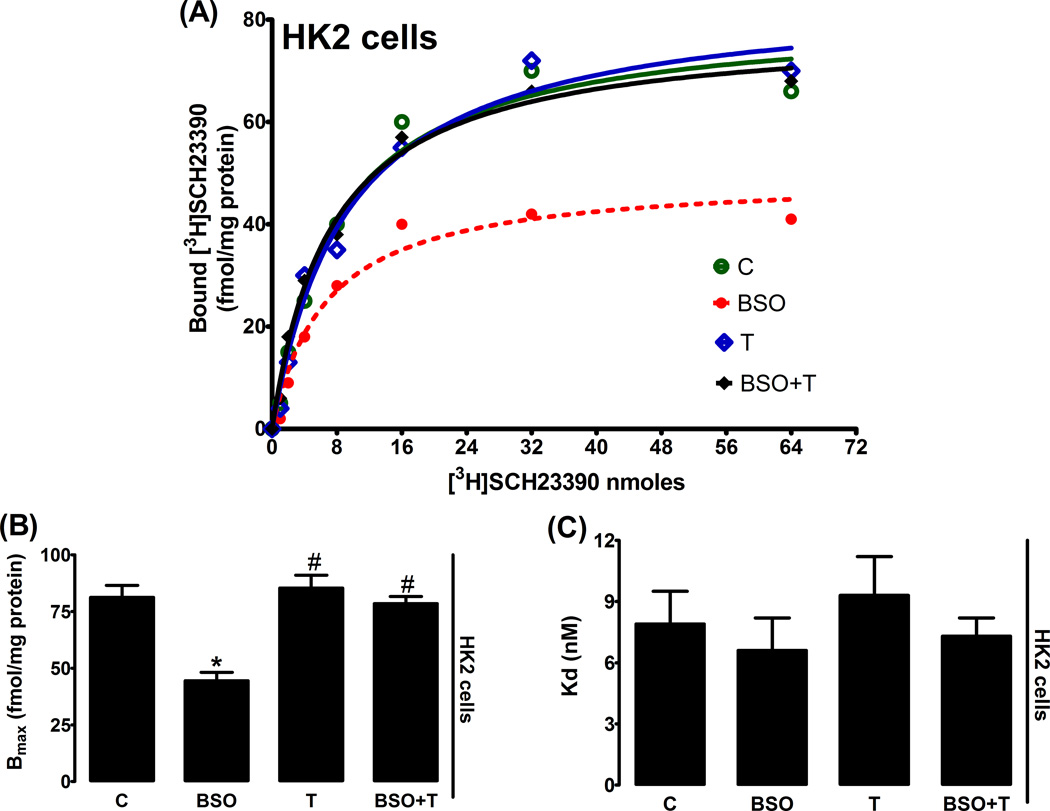
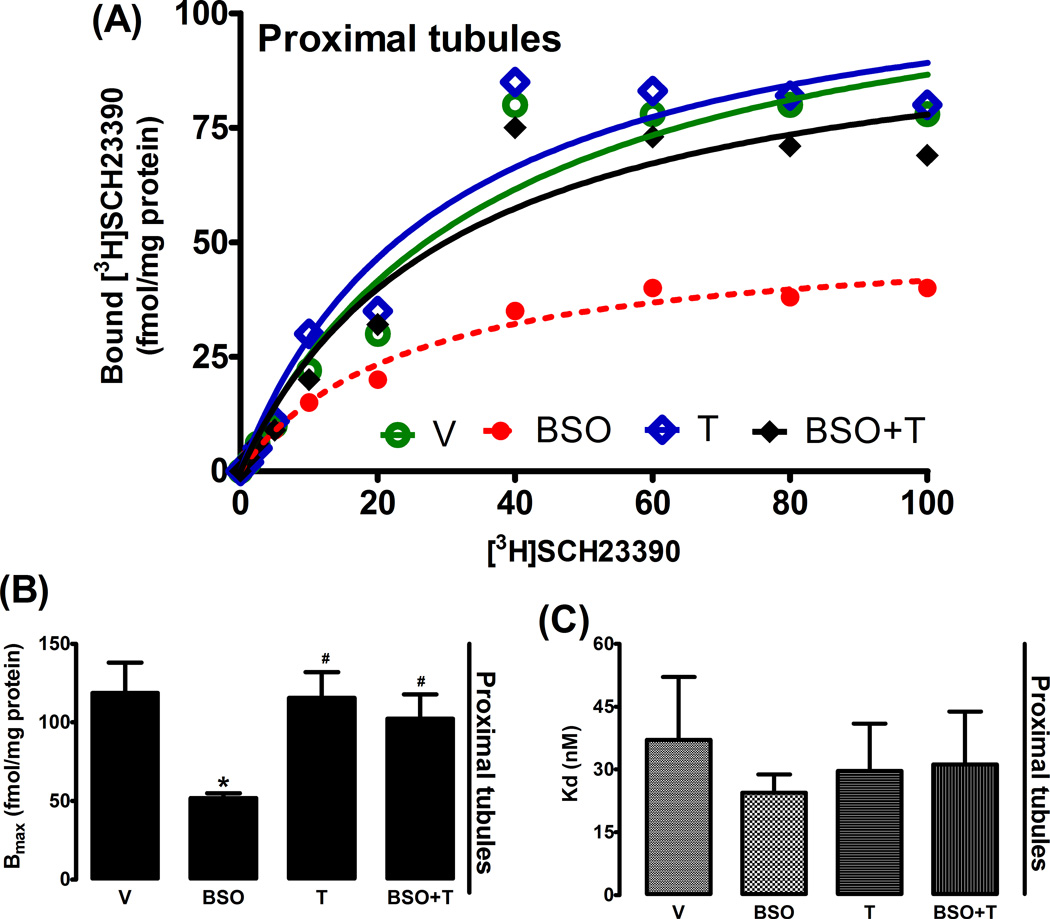
Incubation of HK2 with BSO reduced D1R mRNA level as compared to control (fig. 2Α). Treatment of SD rats with BSO also decreased D1R mRNA level in renal proximal tubules (fig. 2B). In both BSO-treated HK2 cells and renal proximal tubules from BSO-treated rats, the decrease in message level was paralleled by a marked reduction in membrane D1R numbers while the affinity of D1R remained unchanged (fig. 3A–C, 4A–C). Antioxidant tempol prevented the decrease in D1R mRNA level and receptor number (fig. 2–4).
Figure 2.
Dopamine D1 receptor (D1R) mRNA level in HK2 cells and rat renal proximal tubules. (A) D1R mRNA levels in control (C, DMEM-F12), L-buthionine sulfoximine (BSO), tempol (T) and BSO + tempol treated HK2 cells. (B) Renal proximal tubular D1R mRNA levels in control (C, tape water), BSO, T and BSO + tempol treated SD rats. Experiments were performed in triplicate and data represents Mean ± SE from 4–6 cell passages (cells from passage 2–8) or 5–7 rats in each group. *P<0.05 vs C and #P<0.05 vs BSO.
Figure 3.
Membrane dopamine D1 receptor (D1R) expression in HK2 cells. A representative dose response curve of D1R antagonist [3H]SCH23390 in HK2 membranes (A). Bmax and Kd obtained from Scatchard plot (B and C). Experiments were performed in triplicate and data represents Mean ± SE from 4–6 cell passages (cells from passage 2–8). *P<0.05 vs C and #P<0.05 vs BSO.
Figure 4.
Membrane dopamine D1 receptor (D1R) expression in rat renal proximal tubules. A representative dose response curve of D1R antagonist [3H]SCH23390 in proximal tubule membranes (A). Bmax and Kd obtained from Scatchard plot (B and C). Experiments were performed in triplicate and data represents Mean ± SE from 5–7 rats in each group. *P<0.05 vs C and #P<0.05 vs BSO.
Effect of BSO on Na/K-ATPase regulation
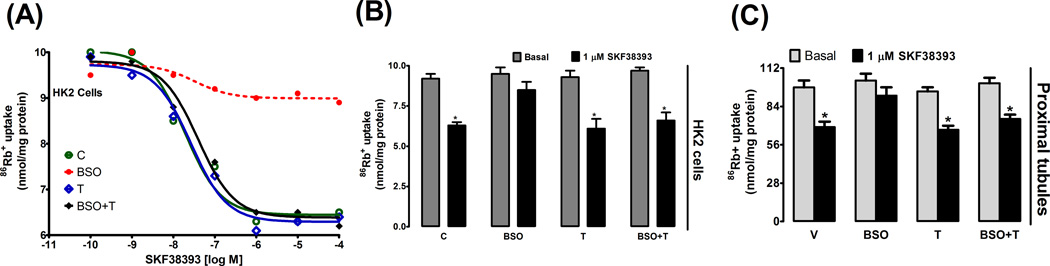
In control HK2 cells SKF38393, a D1R agonist, caused dose dependent inhibition of Na/K-ATPase activity which peaked at 1 µmol/L concentration (fig. 5A). However, in cells treated with BSO, SKF38393 failed to significantly inhibit Na/K-ATPase activity (fig. 5A, B). SKF38393 also failed to inhibit Na/K-ATPase activity in renal proximal tubules from BSO-treated rats when compared to control (fig. 5C). Tempol treatment mitigated BSO effect, as SKF38393-induced Na/K-ATPase inhibition was comparable in control vs. BSO plus tempol-treated HK2 cells (fig. 5A–B) and in proximal tubules from control vs BSO plus tempol treated rats (fig. 5C). Tempol or BSO did not affect basal Na/K-ATPase activity (fig. 5B, C).
Figure 5.
SKF38393-induced inhibition of Na/K-ATPase activity in HK2 cells and rat renal proximal tubules. A representative dose response curve of SKF38393-induced inhibition of Na/K-ATPase activity in control (C), L-buthionine sulfoximine (BSO), tempol (T) and BSO + tempol treated HK2 cells (A). SKF3893-induced (1 µmol/L) inhibition of Na/K-ATPase activity in HK2 cells (B) and renal proximal tubules (C). Experiments were performed in triplicate and data represents Mean ± SE from 4–6 cell passages (cells from passage 2–8) or 5–7 rats in each group. *P<0.05 vs. basal.
Effect of BSO on SP3—D1R Interaction
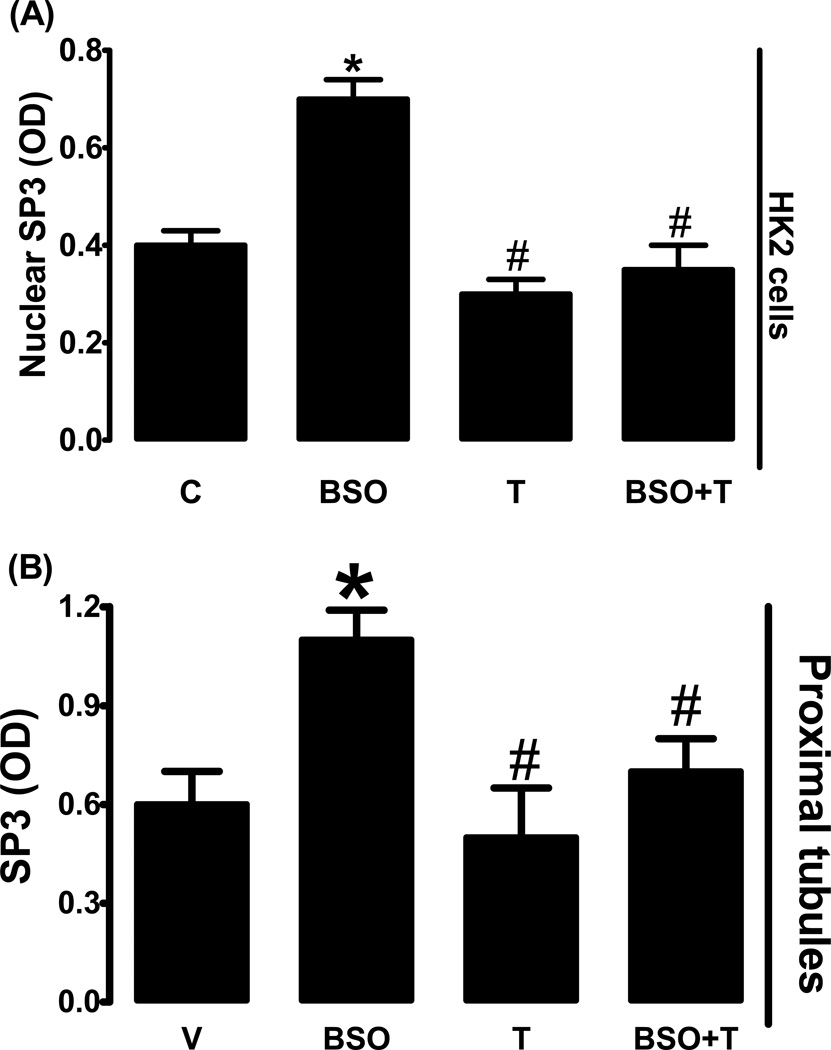
BSO treatment in HK2 cells increased nuclear SP3 expression which was blocked by tempol (fig. 6A). Renal proximal tubules from BSO-treated rats also exhibited increased nuclear SP3 expression when compared to control and tempol blocked BSO-induced SP3 up-regulation (fig. 6B). Tempol had no effect on SP3 expression in the absence of BSO in HK2 cells or rat renal tubules (fig 6A, B).
Figure 6.
SP3 nuclear expression in HK2 cells and rat renal proximal tubules. Effect of BSO on SP3 nuclear expression in L-buthionine sulfoximine (BSO), tempol (T) and BSO + tempol treated HK2 cells (A) and renal proximal tubules from BSO and/or tempol treated rats (B). Experiments were performed in triplicate and data represents Mean ± SE from 4–6 cell passages (cells from passage 2–8) or 5–7 rats in each group. *P<0.05 vs C and #P<0.05 vs BSO.
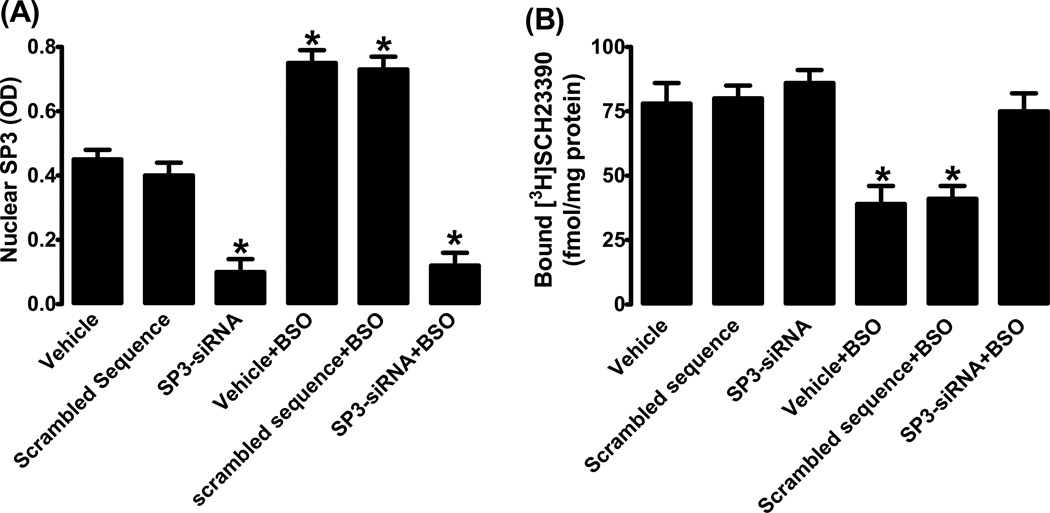
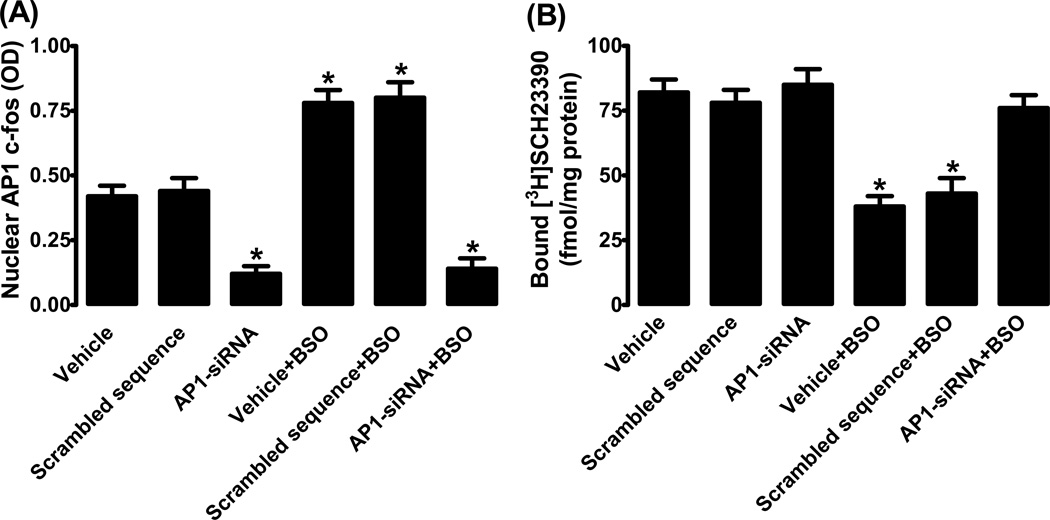
As shown in figure 7A, siRNA down-regulated SP3 by ~80% as compared to cells incubated with vehicle (transfection reagent alone) or scrambled DNA. As expected BSO failed to up-regulate SP3 in siRNA transfected cells (fig. 7A). We found that BSO failed to down-regulate D1R in SP3 siRNA transfected cells (fig. 7B). Basal membrane D1R expression was not affected by transfection reagent, scrambled DNA or SP3 siRNA (fig. 7B). As shown in supplement figure S1, scrambled sequence had no effect on basal or BSO-induced decrease in membrane D1R protein expression.
Figure 7.
SP3 and D1 receptor interaction. (A) SP3 nuclear expression in HK2 cells transfected with SP3 specific siRNA or scrambled DNA. (B) D1 receptor ligand [3H]SCH23390 binding. Mean ± SE from 4–6 experiments (cells from passage 2–8) performed in triplicate. *P<0.05 vs. vehicle.
Effect of BSO on AP1 (c-fos) —D1R Interaction
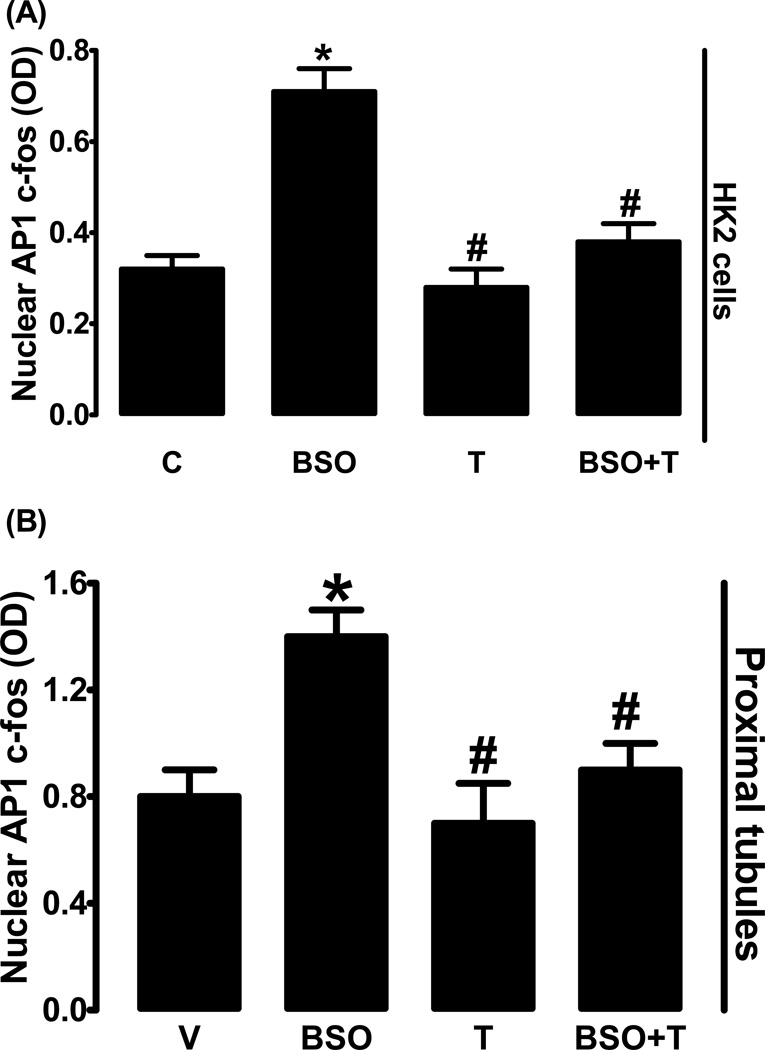
Treatment of HK2 cells with BSO significantly increased nuclear AP1 (c-fos) expression compared to control (fig. 8A). In HK2 cells tempol treatment did not affect AP1 expression, however tempol abolished BOS-induced AP1 up-regulation (fig. 8A). BSO-treatment also increased AP1 expression in proximal tubules and tempol blocked this increase while having no effect on AP1 in the absence of BSO (fig. 8B).
Figure 8.
AP1 (c-fos) nuclear expression in HK2 cells and rat renal proximal tubules. Effect of BSO on AP1 nuclear expression in L-buthionine sulfoximine (BSO), tempol (T) and BSO + tempol treated HK2 cells (A) and renal proximal tubules from BSO and/or tempol treated rats (B). Experiments were performed in triplicate and data represents Mean ± SE from 4–6 cell passages (cells from passage 2–8) or 5–7 rats in each group. *P<0.05 vs C and #P<0.05 vs BSO.
Transfection of HK2 cells with AP1 specific siRNA reduced AP1 expression and BSO did not up-regulate it (fig. 9A). As seen with SP3 down-regulation, AP1 down-regulation also mitigated BSO-induced reduction of membrane D1R expression (fig 9B). AP1 specific siRNA had no effect on basal D1R expression (fig. 9B).
Figure 9.
AP1 (c-fos) and D1 receptor interaction. (A) AP1 nuclear expression in HK2 cells transfected with SP3 specific siRNA or scrambled DNA. (B) D1 receptor ligand [3H]SCH23390 binding. Mean ± SE from 4–6 experiments (cells from passage 2–8) performed in triplicate. *P<0.05 vs. vehicle.
Effect of BSO on SP3—AP1 interaction
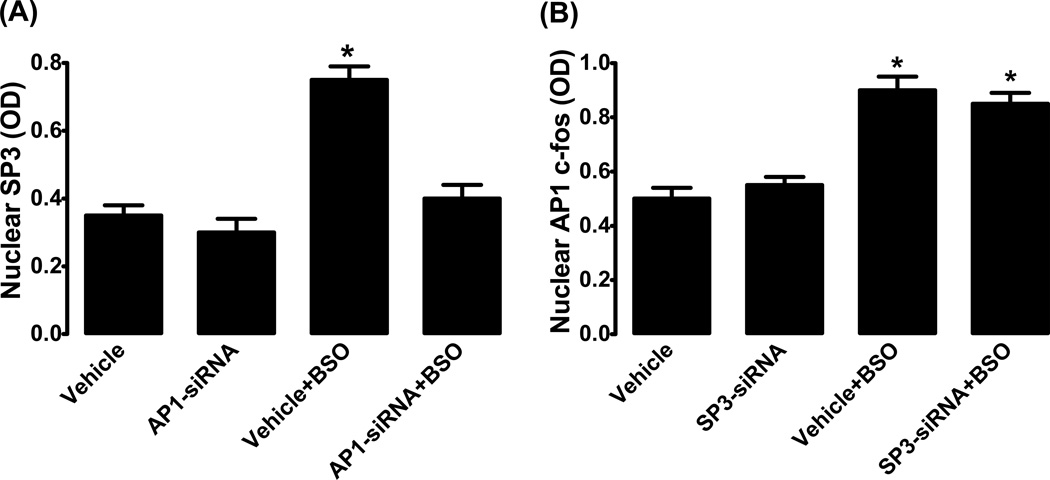
Transfection of HK2 cells with AP1 siRNA had no effect on basal SP3 expression (fig. 10A). However, AP1 siRNA abolished BSO-induced SP3 up-regulation (fig. 10A). On the other hand, transfection of cells with SP3 siRNA failed to block BSO-induced AP1 up-regulation (fig. 10B). SP3 siRNA had no effect on basal AP1 expression (fig. 10B).
Figure 10.
AP1 (c-fos) and SP3 interaction. (A) Nuclear expression of SP3 in the absence and presence of AP1 siRNA in HK2 cells treated with L-buthionine sulfoximine (BSO). (B) Nuclear expression of AP1 (c-fos) in the absence and presence of SP3 siRNA in BSO treated HK2 cells. Mean ± SE from 4–6 experiments (cells from passage 2–8) performed in triplicate. *P<0.05 vs. vehicle.
Since SP3 and AP1 specific siRNA were able to mitigate the BSO effect suggesting an increased protein synthesis, we wanted to see the effect of BSO and tempol on nuclear to cytosolic ratio of these transcription factors. As shown in figure S2A,B, BSO, tempol or BSO plus tempol treatment had no effect on nuclear to cytosolic ratio of SP3 or AP1.
Discussion
The present study shows that oxidative stress mediated activation of transcription factors AP1 and SP3 leads to transcriptional D1R down-regulation resulting in receptor dysfunction and hypertension. BSO-induced oxidative stress decreased D1R message level and membrane receptor expression which led to failure of SKF38393, a D1R agonist, to inhibit Na/K-ATPase activity. BSO treatment activated AP1 and SP3 which was blocked by transfecting the HK2 cells with AP1 and SP3 siRNA respectively. BSO-induced up-regulation of SP3 was blocked by AP1 siRNA while SP3 siRNA had no effect on AP1 activation. Additionally, transfection of HK2 cells with AP1 and SP3 siRNA prevented BSO-mediated D1R dysfunction. Supplementation of tempol to BSO-treated HK2 cells mitigated oxidative stress and abolished AP1 and SP3 activation and rescued D1R function. Additionally, tempol prevented oxidative and AP3—AP1 upregulation, D1R dysfunction and development of hypertension in BSO-treated SD rats.
BSO treatment induced oxidative stress as evidenced by increased malondialdehyde and 8-isoprostane levels and as expected tempol prevented this phenomenon. Both, BSO and tempol did not affect proximal tubular or HK2 cell viability or morphology suggesting that these compounds are not toxic at the dose employed and duration of this study. We and others have shown that oxidative stress reduces D1R receptor function in various animal models and cell cultures.8, 11, 33 In agreement, we also found that the BSO treatment reduced D1R message level and membrane receptor expression. The decrease in D1R expression was reflected by the loss of receptor function as SKF38393 failed to inhibit Na/K-ATPase. It is well established that renal dopamine system contributes to more than 50% of sodium excretion during sodium replete condition thus playing a pivotal role in renal sodium metabolism and blood pressure regulation.6, 34 Therefore, the loss of renal D1R function due to oxidative stress could be a significant risk factor for oxidative stress-associated hypertension. Here in, we also found that oxidative stress was paralleled by a marked increase in blood pressure in BSO-treated rats. The involvement of oxidative stress is further substantiated by the findings that tempol which mitigated oxidative stress was able to prevent D1R down-regulation, restore SKF38393-induced Na/K-ATPase inhibition and maintain normal blood pressure.
The effect of oxidative stress on D1R receptor expression is not clear. Various reports including from our own lab show that oxidative stress mediated D1R dysfunction in obese animals and SHR is confined to receptor G protein uncoupling without any significant change in receptor expression.12, 15 The failure of D1R receptors to communicate with cognate G proteins is reportedly due to serine hyper-phosphorylation. Although the mechanism for receptor hyper-phosphorylation could be attributed to a plethora of protein kinases, it is widely perceived that activation of GRKs (serine/threonine kinases) play a major role in D1R function. GRKs are distributed throughout the cell and upon activation these kinases are recruited to cell membrane. Upon translocation to membranes, GRKs have been shown to phosphorylate serine residues of GPCRs which leads to receptor desensitization. Although this is a well-recognized mechanism for D1R desensitization, there are reports that animal models which exhibit oxidative stress and moderate hypertension and fail to produce natriuresis in response to dopamine, a decrease in receptor number alone could contribute to D1R dysfunction.16–18 To identify the role and mechanism of D1R down-regulation, we focused on redox sensitive transcription factors which possess putative binding affinity for D1R promoter. In here we found that BSO reduced both the D1R mRNA level and membrane receptor numbers suggesting a transcriptional suppression. In agreement to earlier reports21, 22 that both SP3 and AP1 are redox sensitive, we found that treatment of HK2 cells and SD rats with BSO activated SP3 and AP1. In HK2 cells SP3 and AP1 siRNA rescued D1R function suggesting that activation of these transcriptional factors contributes to D1R receptor dysfunction. Treatment of cells and rats with tempol blocked BSO-mediated activation of these transcription factors. It is worth mentioning that tempol had no effect on basal AP1 and SP3 expression, suggesting that the prevention of BSO-induced activation of AP1 and SP3 could be due to its ability to prevent oxidative stress. However, a direct effect on these transcription factors cannot be ruled out. Nevertheless, the data show that oxidative stress via AP1-SP3 signaling abolished D1R function which can be rescued by tempol, thus signifying the therapeutic potential of antioxidants in preventing the development of hypertension.
D1R promoter is a cis-acting element located between nucleotides −1154 and −1136 related to the translation start site.29 Although it is reported that the D1R promoter possesses binding sequences for both SP3 and AP129 and down-regulation of AP1 and/or SP3 mitigated detrimental effects of BSO as it relates to D1R function, it is unclear whether one or both of these factors are involved in D1R transcriptional suppression. To identify an exact link between AP1, SP3 and D1R expression we again down-regulated SP3 to see its effect on BSO-induced AP1 activation. Next we used AP1 siRNA and measured BSO-induced SP3 up-regulation. We found that SP3 down-regulation failed to block BSO-induced AP1 activation. On the other hand, AP1 siRNA abolished BSO dependent SP3 up-regulation. These data show that BSO via AP1 activation up-regulates SP3 which in turn contributes to D1R down-regulation. It is reported that SP3 promoter has binding sequences for AP1 and an activated SP3 could lead to transcriptional activation or suppression of target genes. Collectively, these findings provide a strong support to our hypothesis that oxidative stress via AP1 activates SP3. Once activated, SP3 transcriptionally down-regulates renal D1R causing failure of SKF38393 to inhibit Na/K-ATPase activity. Antioxidant tempol mitigates oxidative stress, abolishes AP1—SP3 activation and prevents D1R dysfunction.
Limitations
BSO by inhibiting glutamate-cysteine ligase activity causes a robust decrease in glutathione levels, both GSH and GSSG, which could increase a plethora of ROS.35 We did not measure ROS in this study, however, tempol can metabolize both H2O2 and superoxide which are commonly associated with BSO treatment.36, 37 It has been previously shown that AP1 activation causes transcriptional up-regulation of glutamate-cysteine ligase which could lead to mitigation of oxidative stress.38 However, BSO is a potent inhibitor of glutamate-cysteine ligase and we have previously shown that glutathione levels are significantly low in rats treated with BSO.33, 39 We did not investigate whether tempol mediated normalization of AP1—SP3 signaling in BSO-treated rats is due to general reduction of oxidative stress or a more direct effect of tempol on these transcription factors. However, It is worth mentioning that tempol, in the absence of BSO, had no effect on AP1—SP3 activation, indicating that the effect of tempol could be confined to mitigation of oxidative stress. We found that BSO in the absence or presence of tempol did not affect nuclear to cytosolic ratio of SP3 or AP1. It seems that oxidative stress increased the protein content of these transcription factors which are then translocated to nucleus. We did not study the mechanisms for these phenomena as it was beyond the scope of our current study.
Perspective
This study identifies a novel molecular mechanism for D1R down-regulation during oxidative stress. We show that redox sensitive transcription factors involving AP1—SP3 cascade can suppress D1R transcription which leads to receptor down-regulation and dysfunction and hypertension. Although oxidative stress could activate both AP1 and SP3 signaling, here in we show that oxidative stress up-regulates AP1 which in turn activates SP3. It has been shown that D1R promoter has a binding sequence for SP3 which can act both as a transcriptional activator and a suppressor. Therefore, upon activation SP3 binds to D1R promoter which leads to receptor down-regulation and loss of receptor function. Antioxidant tempol while mitigating oxidative stress maintains normal AP1—SP3 signaling and prevents D1R dysfunction and hypertension. These data underline the role of oxidative stress in renal D1R defect which could be a contributing factor to the development of hypertension given the importance of renal dopamine system in sodium and blood pressure regulation.
Supplementary Material
Novelty and Significance.
What is New?
Redox sensitive transcriptional pathway AP1—SP3 suppresses D1R transcription leading to receptor down-regulation and dysfunction.
Antioxidant can prevent activation of redox sensitive transcription factors and thus protect GPCR function during oxidative stress
What is Relevant?
The data reinforces the role of oxidative stress in abrogating renal D1R function and the potential implication in hypertension.
The study signifies the translational potential as a more direct therapeutic approach can be focused toward the specific transcription factors in oxidative stress-associated hypertension.
Summary
Oxidative stress activated AP1 which up-regulates SP3 in HK2 cells and rat renal proximal tubules. The enhanced AP1—SP3 signaling suppresses D1R transcription causing receptor down-regulation which leads to the failure of SKF38393 to inhibit Na/K-ATPase activity. Antioxidant tempol mitigates oxidative stress, normalizes AP—SP3 signaling and prevents D1R down-regulation and hypertension.
Acknowledgements
None
Source(s) of Funding
National Institutes of Health, NIDDK (DK098509)
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Guyton AC. Dominant role of the kidneys and accessory role of whole-body autoregulation in the pathogenesis of hypertension. American journal of hypertension. 1989;2:575–585. doi: 10.1093/ajh/2.7.575. [DOI] [PubMed] [Google Scholar]
- 2.Guyton AC. The surprising kidney-fluid mechanism for pressure control--its infinite gain! Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science (New York, N.Y.) 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension. 1992;19:I2–I8. doi: 10.1161/01.hyp.19.1_suppl.i2. [DOI] [PubMed] [Google Scholar]
- 5.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney international. Supplement. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 6.Chen C, Lokhandwala MF. Dopaminergic receptors in hypertension. Pharmacology & toxicology. 1992;70:S11–S16. doi: 10.1111/j.1600-0773.1992.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 7.Jose PA, Raymond JR, Bates MD, Aperia A, Felder RA, Carey RM. The renal dopamine receptors. Journal of the American Society of Nephrology : JASN. 1992;2:1265–1278. doi: 10.1681/ASN.V281265. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. International journal of molecular sciences. 2013;14:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banday AA, Lokhandwala MF. Transcription factor nrf2 protects renal dopamine d1 receptor function during oxidative stress. Hypertension. 2013;62:512–517. doi: 10.1161/HYPERTENSIONAHA.113.01358. [DOI] [PubMed] [Google Scholar]
- 10.Umrani DN, Banday AA, Hussain T, Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese zucker rats. Hypertension. 2002;40:880–885. doi: 10.1161/01.hyp.0000039963.01288.d3. [DOI] [PubMed] [Google Scholar]
- 11.Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine d1-like receptors from g proteins via a mechanism involving protein kinase c and g-protein-coupled receptor kinase 2. Free radical biology & medicine. 2006;40:13–20. doi: 10.1016/j.freeradbiomed.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine d1 receptor hyperphosphorylation, and restores d1 receptor-g-protein coupling and function in obese zucker rats. Diabetes. 2005;54:2219–2226. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- 13.Jose PA, Eisner GM, Drago J, Carey RM, Felder RA. Dopamine receptor signaling defects in spontaneous hypertension. American journal of hypertension. 1996;9:400–405. doi: 10.1016/0895-7061(95)00351-7. [DOI] [PubMed] [Google Scholar]
- 14.Jose PA, Eisner GM, Felder RA. Regulation of d1 receptor function in spontaneous hypertension. Advances in pharmacology (San Diego, Calif.) 1998;42:525–528. doi: 10.1016/s1054-3589(08)60805-4. [DOI] [PubMed] [Google Scholar]
- 15.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of d1 dopamine and at1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 16.Contreras F, Fouillioux C, Bolivar A, Simonovis N, Hernandez-Hernandez R, Armas-Hernandez MJ, Velasco M. Dopamine, hypertension and obesity. Journal of human hypertension. 2002;16(Suppl 1):S13–S17. doi: 10.1038/sj.jhh.1001334. [DOI] [PubMed] [Google Scholar]
- 17.Lokhandwala MF, Hussain T. Defective renal dopamine d1-like receptor signal transduction in obese hypertensive rats. Acta physiologica Scandinavica. 2000;168:251–255. doi: 10.1046/j.1365-201x.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Li F, Jose PA, Ecelbarger CM. Reduction of renal dopamine receptor expression in obese zucker rats: Role of sex and angiotensin ii. American journal of physiology. Renal physiology. 2010;299:F1164–F1170. doi: 10.1152/ajprenal.00604.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagi R. Oxidative stress in cardiovascular disease: A new avenue toward future therapeutic approaches. Recent patents on cardiovascular drug discovery. 2006;1:151–159. doi: 10.2174/157489006777442450. [DOI] [PubMed] [Google Scholar]
- 20.Heyman SN, Rosen S, Rosenberger C. A role for oxidative stress. Contributions to nephrology. 2011;174:138–148. doi: 10.1159/000329383. [DOI] [PubMed] [Google Scholar]
- 21.Kaur J, Bansal MP. Effect of vitamin e on alcohol-induced changes in oxidative stress and expression of transcription factors nfkappab and ap-1 in mice brain cerebral hemispheres. Indian journal of experimental biology. 2008;46:562–567. [PubMed] [Google Scholar]
- 22.Li L, Davie JR. The role of sp1 and sp3 in normal and cancer cell biology. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2010;192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Li L, He S, Sun JM, Davie JR. Gene regulation by sp1 and sp3. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2004;82:460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- 24.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, Kenner L, Tschachler E, Wagner EF. Activator protein 1 (fos/jun) functions in inflammatory bone and skin disease. Arthritis research & therapy. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin FL, Chang CI, Chuang KP, Wang CY, Liu HJ. Advanced glycation end products down-regulate gap junctions in human hepatoma skhep 1 cells via the activation of src-dependent erk1/2 and jnk/sapk/ap1 signaling pathways. Journal of agricultural and food chemistry. 2010;58:8636–8642. doi: 10.1021/jf904240c. [DOI] [PubMed] [Google Scholar]
- 26.Tapias A, Monasterio P, Ciudad CJ, Noe V. Characterization of the 5'-flanking region of the human transcription factor sp3 gene. Biochimica et biophysica acta. 2005;1730:126–136. doi: 10.1016/j.bbaexp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Vesely PW, Staber PB, Hoefler G, Kenner L. Translational regulation mechanisms of ap-1 proteins. Mutation research. 2009;682:7–12. doi: 10.1016/j.mrrev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara Y, Ito F, Shimamoto N. Increased expression of c-fos by extracellular signal-regulated kinase activation under sustained oxidative stress elicits bimel upregulation and hepatocyte apoptosis. The FEBS journal. 2011;278:1873–1881. doi: 10.1111/j.1742-4658.2011.08105.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Minowa MT, Mouradian MM. Two distinct promoters drive transcription of the human d1a dopamine receptor gene. The Journal of biological chemistry. 1996;271:25292–25299. doi: 10.1074/jbc.271.41.25292. [DOI] [PubMed] [Google Scholar]
- 30.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical biochemistry. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 31.Banday AA, Siddiqui AH, Menezes MM, Hussain T. Insulin treatment enhances at1 receptor function in ok cells. American journal of physiology. Renal physiology. 2005;288:F1213–F1219. doi: 10.1152/ajprenal.00361.2003. [DOI] [PubMed] [Google Scholar]
- 32.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal na,k-atpase is reduced by insulin. Hypertension. 2003;41:1353–1358. doi: 10.1161/01.HYP.0000069260.11830.CD. [DOI] [PubMed] [Google Scholar]
- 33.Banday AA, Fazili FR, Lokhandwala MF. Oxidative stress causes renal dopamine d1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappab and protein kinase c. Journal of the American Society of Nephrology : JASN. 2007;18:1446–1457. doi: 10.1681/ASN.2006121373. [DOI] [PubMed] [Google Scholar]
- 34.Bek MJ, Eisner GM, Felder RA, Jose PA. Dopamine receptors in hypertension. The Mount Sinai journal of medicine, New York. 2001;68:362–369. [PubMed] [Google Scholar]
- 35.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (s-n-butyl homocysteine sulfoximine) The Journal of biological chemistry. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 36.Cuzzocrea S, McDonald MC, Mazzon E, Filipe HM, Centorrino T, Lepore V, Terranova ML, Ciccolo A, Caputi AP, Thiemermann C. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Critical care medicine. 2001;29:102–111. doi: 10.1097/00003246-200101000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Thiemermann C, McDonald MC, Cuzzocrea S. The stable nitroxide, tempol, attenuates the effects of peroxynitrite and oxygen-derived free radicals. Critical care medicine. 2001;29:223–224. doi: 10.1097/00003246-200101000-00055. [DOI] [PubMed] [Google Scholar]
- 38.Rahman I, Smith CA, Antonicelli F, MacNee W. Characterisation of gamma-glutamylcysteine synthetase-heavy subunit promoter: A critical role for ap-1. FEBS letters. 1998;427:129–133. doi: 10.1016/s0014-5793(98)00410-4. [DOI] [PubMed] [Google Scholar]
- 39.Banday AA, Muhammad AB, Fazili FR, Lokhandwala M. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in sprague-dawley rats. Hypertension. 2007;49:664–671. doi: 10.1161/01.HYP.0000255233.56410.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.