Abstract
Despite the importance of juxtaglomerular (JG) cell recruitment in the pathophysiology of cardiovascular diseases, the mechanisms that underlie renin production under conditions of chronic stimulation remain elusive. We have previously shown that CD44+ mesenchymal-like cells (CD44+ cells) exist in the adult kidney. Under chronic sodium deprivation these cells are recruited to the JG area and differentiate to new renin-expressing cells. Given the proximity of macula densa (MD) to the JG area and the importance of MD released prostanoids in renin synthesis and release, we hypothesized that chronic sodium deprivation induces MD release of prostanoids; stimulating renal CD44+ cell activation and differentiation. CD44+ cells were isolated from adult kidneys and co-cultured with the MD cell line, MMDD1, in normal or low sodium medium. Low sodium stimulated PGE2 production by MMDD1 and induced migration of CD44+ cells. These effects were inhibited by addition of a Cox-2 inhibitor (NS398) or an EP4 receptor antagonist (AH23848) to MMDD1 or CD44+ cells respectively. Addition of PGE2 to CD44+ cells increased cell migration and induced renin expression. In vivo activation of renal CD44+ cells during JG recruitment was attenuated in wild type mice subjected to salt restriction in the presence of Cox-2 inhibitor Rofecoxib. Similar results were observed in EP4 receptor knockout mice subjected to salt restriction. These results show that the PGE2/ EP4 pathway plays a key role in the activation of renal CD44+ MSC-like cells during conditions of JG recruitment; highlighting the importance of this pathway as a key regulatory mechanism of JG recruitment.
Keywords: sodium, renal physiology, mesenchymal stem cell, Prostaglandin, Cox-2
INTRODUCTION
The renin-angiotensin aldosterone system plays a key physiological role in the regulation of blood pressure, electrolyte homeostasis and kidney development. Renin is an aspartyl protease that catalyzes the first and rate limiting step in the activation of the Renin Angiotensin System (RAS). Factors regulating renin expression have the potential to significantly impact overall RAS activity and potentially can lead to novel therapeutic targets for RAS and cardiovascular/renal disease.
The primary source of renin in the circulation is the kidney. In the adult kidney renin expression is restricted to the terminal portion of the afferent arterioles in the juxtaglomerular (JG) area and is expressed in specialized cells termed JG cells. Despite this restricted expression of renin in the adult kidney, environmental stimuli, such as chronic ischemia, prolonged adrenergic activation, volume and sodium depletion, produce an increase of the number of cells expressing renin along the afferent arteriole, in the interstitium and inside the glomerulus, in a pattern partially recapitulating embryonic distribution of renin expression1, 2. This process, known as JG recruitment 2, involves re-expression of renin in renal smooth muscle cells (VSMCs) along the arteriole 2 and differentiation of pericytes 3 and adult CD44+ mesenchymal-like cell populations to renin expressing cells 4. Despite the importance of this mechanism in regulating renin, the exact cellular processes, as well as the identities of local mediators and cellular receptors involved, are not fully understood.
The macula densa (MD) is an area of highly specialized cells at the thick ascending limb of the loop of Henle that are located adjacent to the afferent arterioles where they are in a unique position to affect changes within the JG area. Indeed, it is well established that in response to salt restriction, MD cells secrete molecules that directly regulate renin expression and release 5-7. Among them, PGE2 derived from the Cox-2/ prostaglandin synthase cascade plays a central role 8, 9. PGE2 acts via a class of EP1-4 receptors. From those, EP4 is of prime importance for renin regulation 10-13.
Several studies have shown that stem cell/progenitor like cells exist in the adult kidney and that these cells are activated under conditions of injury of physiological stress14, 15. Our laboratory has recently shown that CD44+ mesenchymal-like cells (CD44+ cells) exist in the adult kidney4. Under conditions of salt restriction these cells are activated, accumulate in the JG area and differentiate to renin expressing cells4. Here, we tested the hypothesis that MD-derived prostanoids by stimulating the receptors on renal CD44+ cells are crucial for their activation and differentiation to renin expressing cells under conditions of salt restriction.
METHODS
Reagents
PGE2, EP4 receptor antagonist (AH23848), PGI2 and IP receptor antagonist (CAY10441) were purchased from Cayman Chemical; Forskolin (FSK), 1-Methyl-3-isobutylxanthine (IBMX), N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS398) and Lipopolysaccharide (LPS) from Sigma. Cell DMEM was purchased from Sigma and the MesenCult® Proliferation Kit from Stem Cell Technology.
MMDD1 cells
MMDD1, a renal epithelial cell line with properties of macula densa cells (e.g., expression of cyclooxygenase 2 (Cox-2), and NKCC2), were kindly supplied by Dr. J. Schnermann (National Institutes of Health)16. The cells were routinely maintained in DMEM/nutrient mixture Ham's F-12, supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 mg/mL), incubated at 37 °C in the presence of 5% CO2. The medium was changed every 2 days. Where necessary, MMDD1 cells were exposed to a reduced extracellular NaCl concentration. Here, confluent MMDD1 monolayers were exposed to serum-free Dulbecco's modified Eagle's medium in a 1:1 mixture with isotonic saline (control) and 300 mM mannitol to reduce NaCl concentration to one-half as described in Yang et al16.
Isolation and culture of renal progenitor cells
Renal CD44+ MSCs were isolated from mice kidneys as described 4. Briefly, kidneys were perfused in vivo with saline and then harvested, minced, and digested with 0.1% collagenase type I for 30 min at 37°C. The cell suspensions were washed and filtered through 70-μm and 40-μm mesh filters, and residual red blood cells removed by treatment with cold ACK buffer (0.15 M potassium-ammonium chloride). CD44+ cells were isolated by two cycles of FACS sorting via specific gates. Dead cells were excluded with 7AAD (7-Aminoactinomycin D), doublets were excluded on the basis of three hierarchical gates (forward/side scatter area, forward scatter height/width, and side scatter height/width). Renal CD44+ cells collected by FACS were cultured in growth medium MesenCult® Proliferation Kit (stem cell technology) at 37 °C in the presence of 5% CO2. Medium was changed every 2-3 days. Cells were used for experiments during passages 3-5.
RT-PCR and quantitative RT- PCR
The mRNA levels of all the genes checked in this study were quantified by RTPCR and quantitative RT-PCR. Total RNA was isolated from tissues or cells using Trizol reagent according to manufacturer's recommendations (Invitrogen). First strand cDNA was synthesized from 2 μg of total renal RNA using the Omniscript RT kit (Qiagen), and oligo-dT as the primer. 2 μL per reaction of cDNAs were used as the template for real-time PCR amplification. Quantitative RT-PCR was carried out using ABI Prism 7700 Applied Biosystems Sequence Detection System and SYBR Green PCR kit (Qiagen) or TaqMan probe set and TaqMan PCR kit (Applied Biosystems).
In vitro cell differentiation
The differentiation assay was performed as described 17. Briefly, 8-Bromo adenosine 3’, 5’-cyclic monophosphate cAMP (1 mM), 3-Isobutyl-1-Methylxanthine (IBMX) (0.1 mM), or vehicle control (DMSO) were added to culture media daily during the treatment period. In differentiated C57BL/6 Ren1c-YFP renal CD44+ cells, the renin expression was determined by fluorescence microscopy, using YFP expression as a surrogate for renin expression.
Immunofluorescence or immunohistochemical staining
Immunohistochemistry of kidney sections (5 microns thick) was performed using standard procedures. Kidney tissue sections were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. After blocking with 5% serum/PBS for 1 h, sections were incubated with primary antibodies diluted in 5% serum/PBS overnight at 4°C. Slides subsequently were washed in PBS and incubated with secondary fluorochrome-conjugated antibodies for 45 min. The following primary antibodies were used: anti-CD44 (immunohistochemistry: BioLegend, #103001, 1/50 dilution, immunofluorescence: Abcam #ab6124, 1/100dilution), sheep anti-renin (immunohistochemistry: Innovative Res 1206, 1/100 dilution) or rabbit anti-renin (immunofluorescence: 1/10000 dilution, kindly provided by Dr. Tadashi Inagami, Vanderbilt University. The following secondary antibodies were used at a 1:500 dilution for 45 minutes-1h at room temperature: Alexa 488 goat anti-rabbit IgG (A-11008), Alexa 594 goat anti-rabbit IgG (A-11012), Alexa 594 goat anti-rat IgG (A-11007), Alexa 633 donkey anti-sheep IgG (A-21100). Secondary antibodies were purchased from Invitrogen. Nuclei were counterstained with DAPI. Kidneys were embedded into OCT compound (Optimal Cutting Temperature compound), coronal sectioned, and 5micron slices cut. Confocal images were taken in the cortex and acquired with a LSM 510 Meta DuoScan microscope (Zeiss) and processed using LSM 5 software, version 4.2. Images were acquired and analyzed by a blinded investigator. N=3-4 animals per group, 3-4 tissue slices per kidney, 3-4 images per tissue slice. Quantification was performed in Image J.
Cell Migration Assay
Migration of CD44+ cells was assessed using 24-well plates with Transwell inserts (8.0 um pore; Costar), as described18. MMDD1 cells were seeded in 24 well plates at 25×105 cells/ well. Once the MMDD1 cells had attached to the plastic, the MMDD1 cells were the serum starved for 18h. The MMDD1 cells were then primed for Cox-2 expression by overnight incubation with low salt medium. Where appropriate the Cox-2 inhibitor was added during the overnight incubation in low salt medium. The following day Transwell inserts containing CD44+ MSCs were set up for migration assays in serum-free normal-salt DMEM media supplemented 1x Penicillin-Streptomycin. Renal CD44+ MSCs were pretreated with various inhibitors as indicated.
Immunoblotting
Immunoblotting was performed as described 19. Protein lysates were prepared with radioimmunoprecipitation assay (RIPA) buffer (50mM Tris pH 7.8, 150mM NaCl, 0.5% Na deoxycholate, 1% Triton X-100) containing protease inhibitors (Roche). Denatured proteins (20-40μg) were separated by SDS-polyacrylamide gel electrophoresis, and transferred onto PVDF. Blocking and antibody incubation was performed with 5% nonfat dry milk in TBS-Tween (62.5mM Tris pH7.5, 150mM NaCl, 0.1% Tween-20). Rabbit anti-murine polyclonal antibody to EP4 was used at a dilution of 1:1000. Visualization of the bands was performed with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Cell Signaling, 1:1000) and ECL-Prime(GE Healthcare).
Animal Studies
Male C57BL/6 wild type mice 6-8 weeks of age (Charles River Laboratories), male C57BL/6 Ren1c-YFP mice 6-8 weeks of age (kindly provided by Dr. Gomez at University of Virginia Medical Center), and 6-8 week recombinant inbred (RI) background EP4−/− and their co-isogenic controls kindly provided by Dr. Coffman at Duke University and Dr. Beverly Koller at University of North Carolina) were used for the mouse studies. The generation and maintenance of EP4−/− mice has been reported previously 16. On a C57BL/6 background EP4−/− die postnatally as a result of patent ductus arteriosus. Therefore, a RI strain was generated by >35 generations of successive intercross of EP4 +/− mice of mixed genetic background composed of 129P2C57BL/6, and DBA/2 alleles. EP4−/− and their congenic wild type controls were identified at weaning and co-housed for the duration of the experiment. All the animals were maintained on a 12-hour light/12-hour dark cycle at an ambient temperature of 24°C and 60% humidity. Food and water were provided as indicated in each experiment. For low salt diet plus Furosemide administration (in drinking water - 2.28 mmol/L), animals were placed on a low salt diet (0.02% NaCl) for 10 days. Control animals received normal chow (0.4% NaCl). All animal procedures were approved by the Institutional Animal Care and Use Committees at Duke University. For low salt diet plus Cox-2 inhibitor administration, animals were placed on a low sodium diet (0.02% NaCl) with Cox-2 inhibitor 62.5mg/kg and furosemide in drinking water for 10 days.
Statistical Analysis
All the results are presented as the mean ± SEM. All of the experiments were performed in triplicate and repeated 3 to 4 times. ANOVA with Bonferroni correction was used to determine significance. Graphs are displayed as mean ± SEM
RESULTS
PGE2 promotes CD44+ MSC migration and renin expression
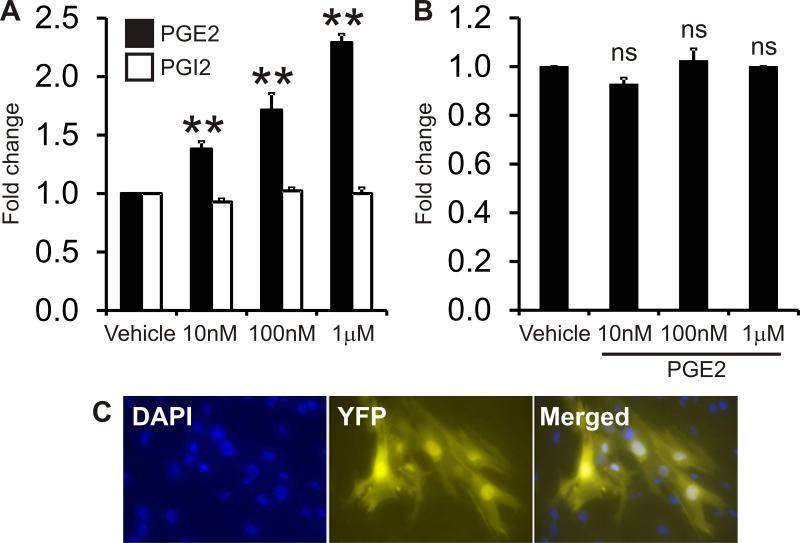
To investigate the effects of prostanoids on adult renal mesenchymal-like cells, we treated renal CD44+ mesenchymal-like cells (CD44+ cells) directly with PGE2 or PGI2 and tested effects on cell proliferation, migration and differentiation to renin expressing cells. CD44+ cells were isolated from kidneys of adult male C57BL/6 wild type mice by Fluorescence Activated Cell Sorting (FACS) and cells were passaged 3-5 times before use. To study the effects of PGE2 on migration we performed transwell migration assays using a Boyden chamber. Renal CD44+ cells were seeded on the upper layer of the cell permeable membrane and treated overnight with different concentrations of PGE2 or PGI2 (1-1000 nM). 24 hours later, the number of cells which had migrated through the membrane to the bottom well was quantified. Treatment with PGE2, but not PGI2, resulted in significantly increased migration of CD44+ cells I (Figure 1A). PGE2 did not affect CD44+ cell proliferation at the concentrations tested (Figure 1B). CD44+ cells isolated from Ren1c-YFP were treated with PGE2 for 10 days and then examined under immunofluorescence microscopy for YFP expression. We found that prolonged treatment of CD44+ cells with high doses of PGE2 (10μM) induced renin expression (Figure 1C). No YFP was detected in vehicle treated cells. YFP and renin were found to co-express in the same cells validating the YFP model (Figure S1A). Moreover, PGE2 increased renin expression in wild-type CD44+ cells (Figure S1B). Collectively, these data demonstrate that PGE2 enhances renal CD44+ cell migration and differentiation to renin expressing cells.
Figure 1. PGE2 promotes CD44+ cell migration and differentiation in vitro.
(A) Renal CD44+ MSC-like cell migration was measured by transwell Boyden Chamber assay. Cells were treated with 0, 10nM, 100nM, or 1μM of PGE2 or PGI2. The data is shown as a fold change where the number of migrated CD44+ cells in the vehicle control was taken to be 1. N=3. Comparisons are made to the vehicle control **P<0.01.
(B) CD44+ MSC-like cell proliferation was measured by MTS assay. Cells were seeded in 96 wells plates and treated with 0, 10nM, 100nM or 1 μM of PGE2 for 3 days. The data is shown as a fold change, with MTS absorbance in the vehicle control taken to be 1. N=3. Comparisons made to the vehicle control, ns: not significant.
(C) C57BL/6 renin 1c YFP mouse renal CD44+ cells were treated with 10μM PGE2 for 10 days. YFP was used as a surrogated marker for renin expression. Representative experiment shown.
PGE2 promotes CD44+ MSC migration and renin expression via the EP4 receptor
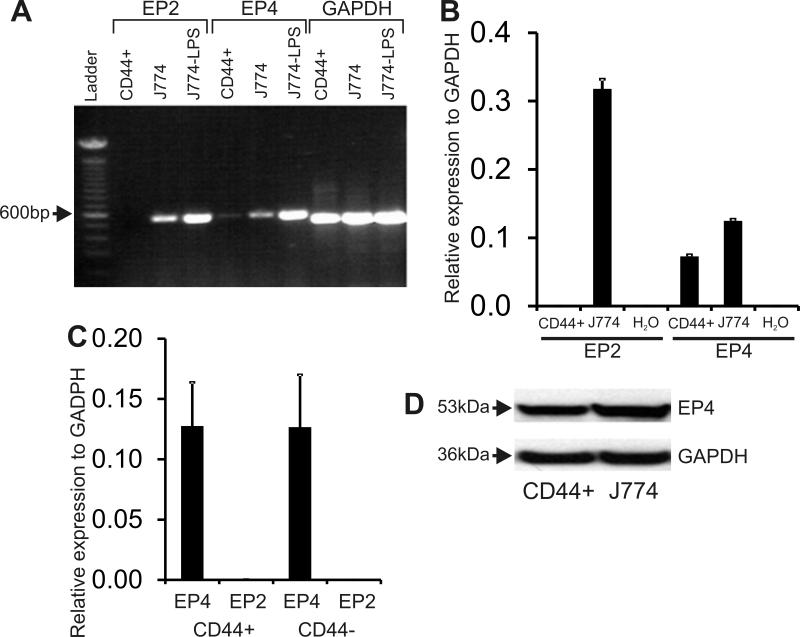
The above results suggested that PGE2, but not PGI2, induces migration and differentiation of CD44+ cells. Since PGE2 has been shown to stimulate renin via activation of E-Prostanoid receptor 2 and 4 (EP2 and EP4) 20, we first examined the expression of these genes in renal CD44+ cells. CD44+ cells were isolated from kidneys of adult male C57BL/6 wild type mice by FACS and mRNA was isolated. As a control, mRNA was isolated from vehicle or LPS treated J774 macrophage cell line. CD44+ cells were found to express the EP4 but not the EP2 receptor as determined by RT-PCR (Figure 2A). This result was subsequently validated by qRT-PCR (Figure 2B, 2C) and expression of EP4 protein was confirmed by immunoblotting (Figure 2D). EP2 expression was either very low or not detectable in CD44+ and CD44- cells (Figures 2A, 2B, 2C).
Figure 2. Expression of EP2 and EP4 receptors in renal CD44+ cells.
(A) RT-PCR for EP2 or EP4 receptor expression. GAPDH was used as a loading control. EP2, EP4 and GAPDH bands were 554, 560 and 516bp in size respectively. CD44+: CD44+ cells; J774: macrophage cell line. LPS treatment was used as a positive control to up-regulate expression of EP2 and EP4 receptors in the J774 cell line. Representative experiment shown.
(B) Expression of EP2 and EP4 mRNA in CD44+ cells detected by qRT-PCR. Gene expression data is shown relative to GAPDH. MSC: renal CD44+ MSC-like cells; J774: macrophage cell line; H2O: Negative control.
(C) Expression of EP2 and EP4 mRNA in CD44+ and CD44- cells as determined by qPCR. Gene expression data is shown relative to GAPDH. N=3.
(D) Immunoblot for EP4. Representative images are shown.
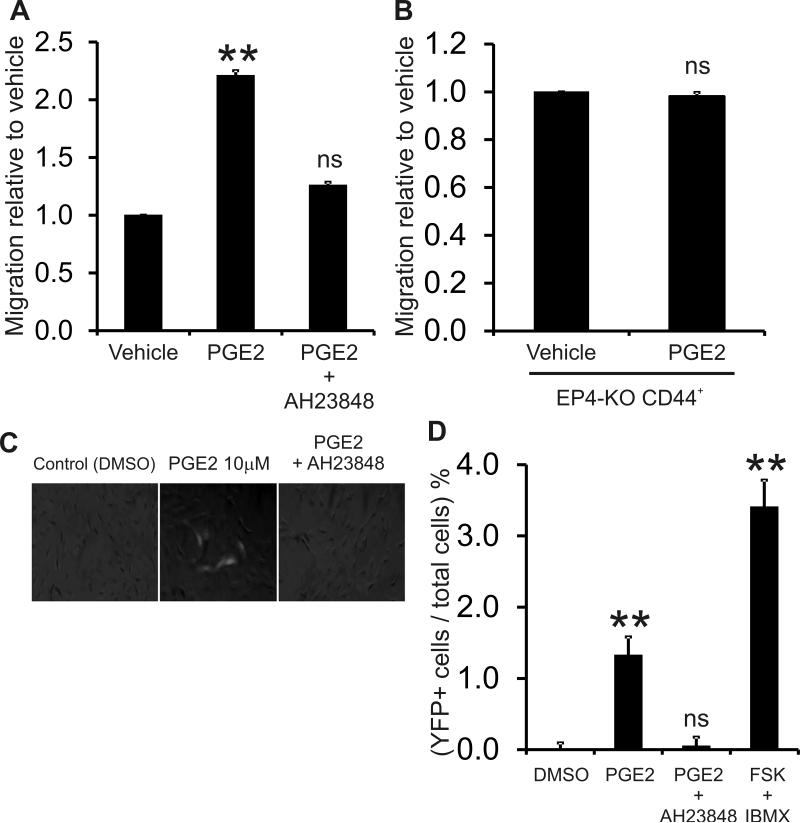
To determine the importance of EP4 receptor in CD44+ cell biology, we pretreated renal CD44+ cells with an EP4 antagonist prior to PGE2 treatment. Migration was examined using the transwell Boyden chamber. Similar to the data in Figure 1, PGE2 induced a 2-fold increase in migration while the EP4 antagonist AH23848 significantly attenuated PGE2 induced renal CD44+ cell migration (Figure 3A). Moreover, when renal CD44+ cells were isolated from mice lacking the EP4 receptor 13, the effect on PGE2 on CD44+ cell migration was abrogated (Figure 3B). Similarly, pretreatment of the CD44+ cells with AH23848 abolished the effects of PGE2 in renin induction of these cells (Figures 3C and 3D). The results show that PGE2 induces renal CD44 + cell migration and differentiation to renin expressing cells through the EP4 receptor.
Figure 3. PGE2 promotes CD44+ cell migration and differentiation via EP4 receptor.
(A) Renal CD44+ cell migration was measured by transwell Boyden Chamber assay. Cells were treated with PGE2 (1μM) in the presence or absence of 10μM AH23848, an EP4 receptor inhibitor. The data is shown as a fold change where the number of migrated CD44+ cells in the vehicle control was taken to be 1. N=3. Comparisons made to the vehicle control **P<0.01.
(B) Renal CD44 cells were isolated form EP4 −/− mice. The EP4 −/− renal CD44+ MSCs were treated with PGE2 1μM overnight and migration was measured by transwell Boyden Chamber assay. The data is shown as a fold change where the number of migrated CD44+ MSC-like cells in the vehicle control was taken to be 1. N=3. P=0.52 ns, not significant.
(C) Renal CD44+ cells isolated from Ren1c-YFP were treated with PGE2 (10μM) for 10 days in the presence or absence 10μM AH23848, and then examined under immunofluorescence microscopy for YFP expression as a surrogate marker for renin expression. Representative merged bright field and fluorescent images are shown. Forskolin (FSK) and IBMX treatment were used as positive control for renin induction.
(D) Quantitative analysis of (C). Data are presented as mean + SE of 3 experiments each with at least three samples per condition. Comparisons made to DMSO, **P<0.01.
Macula densa derived PGE2 activates adult renal CD44+ in vitro via the EP4 receptor
The macula densa is an important source of PGE2 and plays a major role in the regulation of JG renin expression and release during changes in salt intake5. To study the macular densa mediated effects on the CD44+ cell in vitro we used the MMDD1 cell line. To enhance secretion of PGE2, the MMDD1 cells were incubated with DMEM medium with reduced sodium chloride (67mM)16 where appropriate. Serum-starved renal CD44+ MSCs were plated on transwell inserts and the inserts placed into the wells containing the MMDD1 cells. The co-culture was conducted in normal-salt DMEM without serum and CD44+ cells were allowed to migrate through the filters at 37°C overnight. Overnight co-culture of renal CD44+ cells with MMDD1 cells exposed to low salt conditions, increased CD44+ cell migration by two-fold as measured using transwell Boyden chamber migration assays when compared to co-culture of renal CD44+ cells with MMDD1 cells exposed to normal salt conditions (Figure 4). This effect on migration was abrogated by addition of a Cox-2 inhibitor (NS398), which blocks PGE2 production in MMDD1 cells, or by addition of the EP4 receptor antagonist AH23848 to the CD44+ cells (upper chamber) (Figure 4). Treatment of the CD44+ cells with a PGI2 receptor antagonist (CAY 10441) had no effect on this migratory response (Figure 4), demonstrating that PGE2 secreted from the MMDD1 cells is the prostanoid affecting the CD44+ cell migration and confirming that these effects are mediated via the EP4 receptor.
Figure 4. Cox-2 Inhibition abrogates MD cell induced CD44+ cell migration In Vitro.
MMDD1 cells (25×104 cells/ well) were seeded in a 24 well plate and cultured overnight in normal or low sodium medium (to induce Cox-2 expression) in the presence or absence of Cox-2 inhibitor NS398 10μM. Low salt medium was used to induce Cox-2 expression in the MMDD1 cells. Serum-starved renal CD44+ cells (1×105) were plated on the transwell inserts and inserts were placed in the wells containing the MMDD1 cells. The co-culture was conducted in normal-salt DMEM without serum. CD44+ cells were allowed to migrate through the filters at 37°C overnight. AH23848 (EP4 receptor antagonist) 10μM and CAY10441 (PGI2 receptor antagonist) 10μM were added where appropriate. N=3. Comparisons made to the vehicle control in normal sodium media * p<0.05.
Cox-2 inhibition blocked renal CD44+ cell activation in vivo after low salt and Furosemide treatment
Next, to investigate whether the Cox-2 and PGE2 mechanisms played a role in renal CD44+ RPC activation in vivo, wild type C57BL/6 mice were subjected to salt restriction by low salt diet and furosemide treatment 4 (2.28 mmol/L, drinking water) for 10 days in the presence or absence of the Cox-2 inhibitor Rofecoxib 21 (62.5mg/kg). Low salt diet increased plasma renin concentration (Figure S2). This increase in plasma renin concentration was significantly inhibited by Rofecoxib (Figure S1). Kidneys were harvested and processed for immunofluorescent microscopy using CD44 and renin specific antibodies. As anticipated from previous studies 4, salt restriction resulted in increased renin expression and JG cell number, as well as an increase in the number of CD44+ cells (Figure 5A, with quantification provided in Figures 5B and 5C). However, in Rofecoxib treated mice, both renin expression and the number of CD44+ cells were significantly attenuated (Figure 5A, with quantification provided in Figures 5B and 5C). These results suggest that PGE2 is important for the activation of renal CD44+ cells during salt restriction-induced JG cell recruitment.
Figure 5. Pharmacological inhibition of Cox-2 blocked renal CD44+ cell activation in vivo after low salt/Furosemide treatment.
Wild Type C57BL/6 mice were administered a normal salt diet with vehicle or low salt diet accompanied by furosemide treatment for 10 days with or without the Cox-2 inhibitor Rofecoxib.
(A) Representative confocal images of kidney sections immunostained for CD44 [red] and renin [green], and nuclei [DAPI, blue] from low sodium and furosemide treated and control untreated mice. Scale bar 100 microns.
(B) The number of CD44+ cells and glomeruli were counted. The total number of glomeruli-associated CD44+ cells was divided by the total number of glomeruli. The value in the normal diet vehicle control group was taken to be 1. Comparisons made to either the normal diet vehicle control group * p<0.05, or between the vehicle and Rofecoxib groups treated with low sodium and furosemide ‡ p<0.05. N=3-4 per group.
(C) The area of renin immunostaining was quantified by Image J. The value in the normal diet vehicle control group was taken to be 1. Comparisons made to either the normal diet vehicle control group * p<0.05, or between the vehicle and Rofecoxib groups treated with low sodium and furosemide ‡ p<0.05. N=3-4 per group.
EP4 receptor is essential for renal CD44+ cell activation in vivo after low salt and Furosemide treatment
To investigate the importance of EP4 receptor in the CD44+ cell activation after salt restriction in vivo, we employed EP4 knockout mice 13. As described in the previous section, mice were treated with a low salt diet and furosemide for 10 days. At the end of treatment, kidneys were harvested and CD44 and renin expression examined by immunofluorescent microscopy. As shown in Figures 6 A-C, in wild type mice, low salt and furosemide treatment resulted in an increase in both renin expressing cells and the number of CD44+ cells. These responses were significantly attenuated in the EP4 knockout mouse kidney from EP4−/− mice (Figure 6 A-C). These results demonstrate the importance of EP4 receptor in PGE2 induced CD44+ activation in vivo.
Figure 6. Renal CD44+ cell activation is attenuated in EP4 knockout mice treated with low salt /Furosemide.
EP4 knockout mice were administered a normal salt diet with vehicle or low salt diet accompanied by furosemide treatment for 10 days.
(A) Representative confocal images of kidney sections immunostained for CD44 [red] and renin [green], and nuclei [DAPI, blue] from low sodium and furosemide treated and control untreated mice. Scale bar 100 microns.
(B) The number of CD44+ cells and glomeruli were counted. The total number of glomeruli-associated CD44+ cells was divided by the total number of glomeruli. The value in the normal diet vehicle control group was taken to be 1. Comparisons made to either the normal diet vehicle control group * p<0.05, or between the vehicle and Rofecoxib groups treated with low sodium and furosemide ‡ p<0.05. N=3-4 per group.
(C) The area of renin immunostaining was quantified by Image J. The value in the normal diet vehicle control group was taken to be 1. Comparisons made to either the normal diet vehicle control group * p<0.05, or between the vehicle and Rofecoxib groups treated with low sodium and furosemide ‡ p< 0.05. N=3-4 per group.
DISCUSSION
Several studies have shown that stem cell/progenitor cells exist in the adult kidney and are activated following sustained stress or injury 14, 15. Our laboratory has recently shown that adult renal CD44+ MSC-like cells act as renin progenitor cells 4. These CD44+ cells are activated during conditions of sodium constriction and contribute to the increased renin response by accumulating in the JG area and differentiating to renin producing cells4.
In the current study we extend these findings and illustrate that adult renal CD44+ cells express EP4 receptors and that co-culture of MMDD1 cells with adult renal CD44+ cells stimulate migration of CD44+ cells; the effect was attenuated with treatment of MMDD1 cells with Cox-2 inhibitor or treatment of the CD44+ cells with an EP4 antagonist. Direct exposure to PGE2 increased CD44+ cell migration in an EP4 dependent manner. In addition, our results indicated that treatment of CD44+ cells with higher doses of PGE2 induced renin expression in vitro. Finally, both the activation of renal CD44+ and the increase in renin expression in response to salt restriction are diminished in mice lacking the EP4 receptor gene. Since CD44+ cells loose CD44 expression concurrent with an increase in renin expression, it is difficult to find CD44/Renin double positive cells in vivo, however, previous lineage tracing studies have shown that the CD44+ cells are a precursor to the renin+ cells7. Thus, taken together, our data supports the novel concept that renal stem/progenitor cells contribute to the process of JG recruitment and suggest that MD, via the secretion of PGE2 and activation of the EP4 receptor on the renal progenitor cells, are involved in this process.
It is well established that MD derived prostaglandin E2 (PGE2) is one of the principal mediators of local control of renin release in the kidney 9. According to the classic paradigm, MD cells sense salt and/or other biochemical signals that induce the production of PGE2 9. PGE2 acts on EP2 or EP4 receptors in JG cells and stimulates renin release20; although more recent evidence supports the notion that EP4 is the receptor responsible in mediating the PGE2 induced renin release under conditions of salt restriction in vivo 13. Our data suggests that the MD/PGE2/EP4 axis plays a broader role in renin regulation by promoting renin expression in CD44+ cells in the adult kidney. Indeed, PGE2 has recently gained much attention as a mediator of in vitro and in vivo migration and differentiation of hematopoietic stem cells, endothelial progenitor cells and mesenchymal stem cells 22-24. Our studies provides additional insights into the role of PGE2 in stem cell biology and, to our knowledge, provides the first such evidence for PGE2 affecting the fate of renal stem cells. Future experiments to discern how a PGE2 dose gradient affects renin expression during salt restriction in adult or during renal development, as well as elucidating the downstream pathways involved, would be of high interest. For example, Len Zon and colleagues have demonstrated that PGE2 affects hematopoietic stem cell biology by the cAMP dependent activation of the Wnt signaling pathway 24. Moreover, cAMP is a known regulator of transcription and an examination of the transcription factors and transcripts regulated by cAMP in CD44+ cells following exposure to PGE2 may identify targets for future study.
Although our studies highlight the role of MD/PGE2/EP4 axis we cannot exclude that other molecules or receptors such as PGI2, nitric oxide, IP receptor receptors play a role in vivo 10 and might be involved in the CD44+ cell response during salt restriction. Indeed, although PGE2 treatment did not affect CD44+ cell proliferation in vitro, Cox-2 inhibition was sufficient to abrogate the CD44+ cell expansion after low sodium furosemide treatment in vivo. Similar results were obtained from with EP4 receptor knock out mice. Similarly, we cannot exclude that the MD/PGE2/EP4 mechanisms affect other cellular processes involved in JG recruitment. JG recruitment involves several mechanisms such as proliferation of the existing renin-expressing JG cells, induction of renin expression in vascular smooth muscle cells of the afferent arteriole 2, 25, and transdifferentiation of pericytes 3. Studying the effects of MD/PGE2/EP4 upon these pathways would be of great interest.
PERSPECTIVES
Our data shows the activation of renal stem/progenitor cells in response to salt restriction requires PGE2/EP4. These studies provide new insights about role of renal stem cells in renal physiology and diseases as well as advancing our understanding regarding JG recruitment and regulation of the renin angiotensin system.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
- What is New?
- PGE2 released by a macula densa cell line under conditions of low sodium induced CD44+ cell migration; an effect inhibited by an EP4 receptor antagonist.
- In vivo, genetic ablation of EP4 or inhibition of Cox-2 by Rofecoxib attentuated the ability of low sodium to induce both renal CD44+ cell activation during JG recruitment and the production of renin.
- What is relevant?
- Juxtaglomerular (JG) cell recruitment is important in the pathophysiology of hypertension; however mechanisms that underlie renin production under conditions of chronic stimulation are relatively unknown.
- We have previously shown that CD44+ mesenchymal-like cells exist in the adult kidney. Under chronic sodium deprivation these cells are recruited to the JG area where they differentiate into new renin-expressing cells.
- Summary
- The PGE2/EP4 pathway is a key regulatory mechanism of JG recruitment.
ACKNOWLEDGEMENTS
We are highly appreciative of a number of people. Hui Mu provided excellent technical support. Conrad Hodgkinson helped with the preparation of the figures and the text.
SOURCE OF FUNDING
Research conducted in these studies was supported by National Heart, Lung, and Blood Institute grants RO1 HL81744, HL72010, and HL73219 (to V.J.D.); and the Edna and Fred L. Mandel Jr. Foundation (to V.J.D. and M.M.). M.M. was also supported by an American Heart Association National Scientist Development Award (10SDG4280011).
Footnotes
CONFLICT OF INTERESTS
None.
REFERENCES
- 1.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y. Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney. An ultrastructural-cytochemical and autoradiographic study. Am J Pathol. 1977;87:581–602. [PMC free article] [PubMed] [Google Scholar]
- 2.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 3.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez ML. Pericytes synthesize renin. World J Nephrol. 2013;2:11–16. doi: 10.5527/wjn.v2.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Gomez JA, Klein S, Zhang Z, Seidler B, Yang Y, Schmeckpeper J, Zhang L, Muramoto GG, Chute J, Pratt RE, Saur D, Mirotsou M, Dzau VJ. Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. Journal of the American Society of Nephrology : JASN. 2013;24:1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtz A. Renin release: sites, mechanisms, and control. Annual review of physiology. 2011;73:377–399. doi: 10.1146/annurev-physiol-012110-142238. [DOI] [PubMed] [Google Scholar]
- 6.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen BL, Stubbe J, Hansen PB, Andreasen D, Skott O. Localization of prostaglandin E(2) EP2 and EP4 receptors in the rat kidney. Am J Physiol Renal Physiol. 2001;280:F1001–1009. doi: 10.1152/ajprenal.2001.280.6.F1001. [DOI] [PubMed] [Google Scholar]
- 8.Komhoff M, Jeck ND, Seyberth HW, Grone HJ, Nusing RM, Breyer MD. Cyclooxygenase-2 expression is associated with the renal macula densa of patients with Bartter-like syndrome. Kidney Int. 2000;58:2420–2424. doi: 10.1046/j.1523-1755.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 9.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 11.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol. 2000;279:F819–F825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 12.Nusing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol. 2005;16:2354–2362. doi: 10.1681/ASN.2004070556. [DOI] [PubMed] [Google Scholar]
- 13.Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Kim HS, Koller BH, Coffman TM. A major role for the EP4 receptor in regulation of renin. Am J Physiol Renal Physiol. 2011;301:F1035–F1041. doi: 10.1152/ajprenal.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nature reviews Nephrology. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 15.Herrera M, Mirotsou M. Stem cells: potential and challenges for kidney repair. American journal of physiology Renal physiology. 2014;306:F12–F23. doi: 10.1152/ajprenal.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Gomez JA, Klein S, Zhang Z, Seidler B, Yang Y, Schmeckpeper J, Zhang L, Muramoto GG, Chute J, Pratt RE, Saur D, Mirotsou M, Dzau VJ. Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. Journal of the American Society of Nephrology : JASN. 2013;24:1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA. beta-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am J Physiol Endocrinol Metab. 2011;300:E468–E477. doi: 10.1152/ajpendo.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Parsons KK, Chi L, Malakauskas SM, Le TH. Glutathione S-transferase-micro1 regulates vascular smooth muscle cell proliferation, migration, and oxidative stress. Hypertension. 2009;54:1360–1368. doi: 10.1161/HYPERTENSIONAHA.109.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol. 2004;287:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 21.Harris RC. Interactions between COX-2 and the renin-angiotensin system in the kidney. Acta physiologica Scandinavica. 2003;177:423–427. doi: 10.1046/j.1365-201X.2003.01101.x. [DOI] [PubMed] [Google Scholar]
- 22.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200:400–406. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 23.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.