Abstract
The carbamate group is a key structural motif in many approved drugs and prodrugs. There is an increasing use of carbamates in medicinal chemistry and many derivatives are specifically designed to make drug–target interactions through their carbamate moiety. In this Perspective, we present properties and stabilities of carbamates, reagents and chemical methodologies for the synthesis of carbamates, and recent applications of carbamates in drug design and medicinal chemistry.
1. Introduction
Carbamate-bearing molecules play an important role in modern drug discovery and medicinal chemistry. Organic carbamates (or urethanes) are structural elements of many approved therapeutic agents. Structurally, the carbamate functionality is related to amide-ester hybrid features and, in general, displays very good chemical and proteolytic stabilities. Carbamates are widely utilized as a peptide bond surrogate in medicinal chemistry. This is mainly due to their chemical stability and capability to permeate cell membranes. Another unique feature of carbamates is their ability to modulate inter- and intramolecular interactions with the target enzymes or receptors. The carbamate functionality imposes a degree of conformational restriction due to the delocalization of nonbonded electrons on nitrogen into the carboxyl moiety. In addition, the carbamate functionality participates in hydrogen bonding through the carboxyl group and the backbone NH. Therefore, substitution on the O- and N-termini of a carbamate offers opportunities for modulation of biological properties and improvement in stability and pharmacokinetic properties.
Carbamates have been manipulated for use in the design of prodrugs as a means of achieving first-pass and systemic hydrolytic stability. Carbamate derivatives are widely represented in agricultural chemicals, such as pesticides, fungicides, and herbicides. They play a major role in the chemical and paint industry as starting materials, intermediates, and solvents. Furthermore, organic carbamates serve a very important role as optimum protecting groups for amines and amino acids in organic synthesis and peptide chemistry.
In recent years, carbamate derivatives have received much attention due to their application in drug design and discovery. However, there are hardly any reviews on this subject in the literature. In the present Perspective, we plan to provide an overview of the leading role of organic carbamates in medicinal chemistry, with particular focus on therapeutic carbamates and carbamate-based prodrugs. In this context, we will highlight the chemical methodologies adopted for the synthesis of these carbamate derivatives. Also, we will outline successful designs of organic carbamates, including a variety of cyclic ether-derived carbamates, as suitable amide bond surrogates leading to a wide range of novel organic carbamates as potent HIV-1 protease, β-secretase, serine protease, and cysteine protease inhibitors. This information may be useful in further design of carbamate-based molecules as drugs or prodrugs.
2. Organic Carbamates: Applications and Chemical and Metabolic Stabilities
Peptide-based molecules are an important starting point for drug discovery, especially in the design of enzyme inhibitors. Because of their high affinity and specificity toward biological functions, peptide-based molecules also serve as valuable research tools. However, the poor in vivo stability, inadequate pharmacokinetic properties, and low bioavailability have generally limited their broader utility. Hence, a variety of peptide mimics are being developed to improve drug-like character along with increased potency, target specificity, and longer duration of action.1−3 To this end, several classes of peptidomimetics are tailored by replacing the native amide bond with unnatural linkages4−6 such as retro-amide,7 urea,8−12 carbamate,13 and heterocycles14,15 as peptide bond surrogates. These functionalities confer metabolic stability toward aminopeptidases, the enzymes involved in the metabolism of peptide-like drugs. The carbamate’s emerging role in medicinal chemistry is also due to its chemical stability and to its capability to increase permeability across cellular membranes. These attributes of organic carbamates have been exploited in drug design. As a result, the carbamate motif is becoming the choice for peptide bond surrogates.
Other uses of carbamates are well-known. Particularly, the employment of carbamates in various industries as agrochemicals, in the polymer industry, and also in peptide syntheses.16−18 In addition, among the various amine-protecting groups, carbamates are commonly used to enhance their chemical stability toward acids, bases, and hydrogenation.19
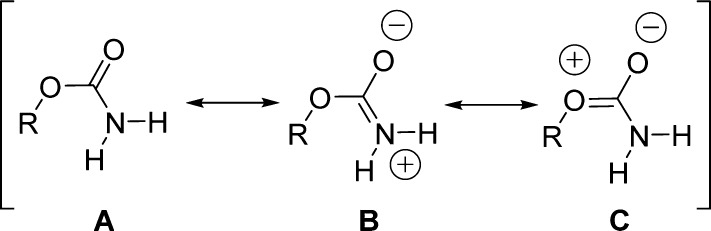
One important feature of organic carbamates is represented by the amide resonance. The amide resonance in carbamates has been studied in detail employing both experimental and theoretical methods by estimating the C–N bond rotational barriers.20−25 The amide resonance in carbamates has been shown to be about 3–4 kcal mol–1 lower than those of amides, owing to the steric and electronic perturbations due to the additional oxygen.26 Three possible resonance structures (A, B, and C, Figure 1) contribute to the stabilization of the carbamate moiety.
Figure 1.
Possible resonance structures for the carbamate moiety.
Carbamate motifs are characterized by a pseudo double bond. This implies the potential deconjugation of the heteroatom-(σ-bond)-carbon-(π-bond)-heteroatom system that restricts the free rotation about the formal single σ-bond. Therefore, two isomers, syn and anti, may coexist in carbamates (Figure 2).23,27
Figure 2.
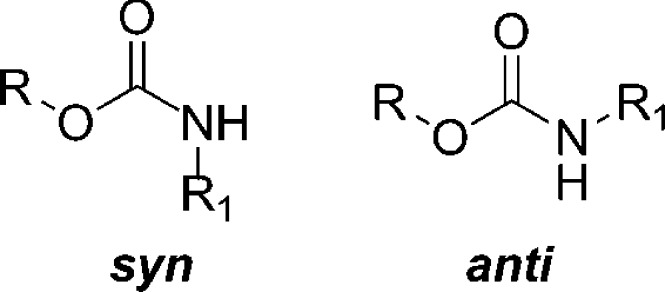
Syn and anti conformations of carbamates.
Although carbamates display close similarity to amides, they show preference for the anti-isomer conformation.28 The anti rotamer is usually favored by 1.0–1.5 kcal mol–1 for steric and electrostatic reasons with respect to the syn counterpart.22 In many cases, the energy difference may be close to zero. As a result, those carbamates are found as an approximately 50:50 mixture of syn and anti isomers, as in the case of a number of Boc-protected amino acid derivatives. This issue is of key importance since this balanced rotamer equilibria and the low activation energies render carbamates as optimal conformational switches in molecular devices.23
The influence of the R and R1 substituents on the free-energy difference between the two conformations has been investigated. Beyond steric effects, electronegativity of R1 must be considered since it may affect the conformation in many ways, including changes in the dipole moment and bond angles.28 Only the anti conformation would be expected in five-, six-, and seven-membered cyclic carbamates. Calculations of the dipole moment for the carbamate group support this expectation.29 Solvent, concentration, salts, and pH strongly influence the free energy difference of the syn and anti isomers of carbamates as well. Intra- and intermolecular hydrogen bonding may also perturb the syn–anti isomer equilibrium of carbamates.22,25,30
A representative example of hydrogen bonding and concentration dependence was provided by Gottlieb, Nudelman, and collaborators.28 The authors took into consideration N-Boc-amino acids and their corresponding methyl esters. An unusual abundance of syn-rotamer for N-Boc-amino acids was detected. N-Boc-amino acid esters give the expected spectra, consistent with previous reports of only a single species being observed at room temperature. Concentration-dependent 1H NMR spectra indicate that the proportion of the syn-rotamers increases with concentration, supporting the existence of an aggregation process.28
Since decreasing temperature is another method for stabilizing oligomerization, NMR experiments were also performed at different temperatures. As expected, when the temperature increases, the favored rotamer switches from syn to anti. Overall, the collected data strongly supports the concept that the syn rotamers of N-carbamoylated amino acids form intermolecularly H-bonded species and the OH of the carboxylic acid must be involved in this process, as the corresponding esters do not behave similarly. To explain this phenomenon, the formation of a dimer was suggested (Figure 3).
Figure 3.
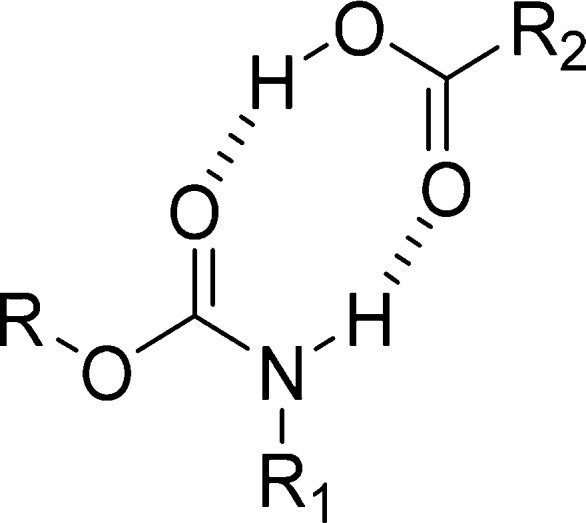
Possible dimer between a syn-carbamate and an acid group.
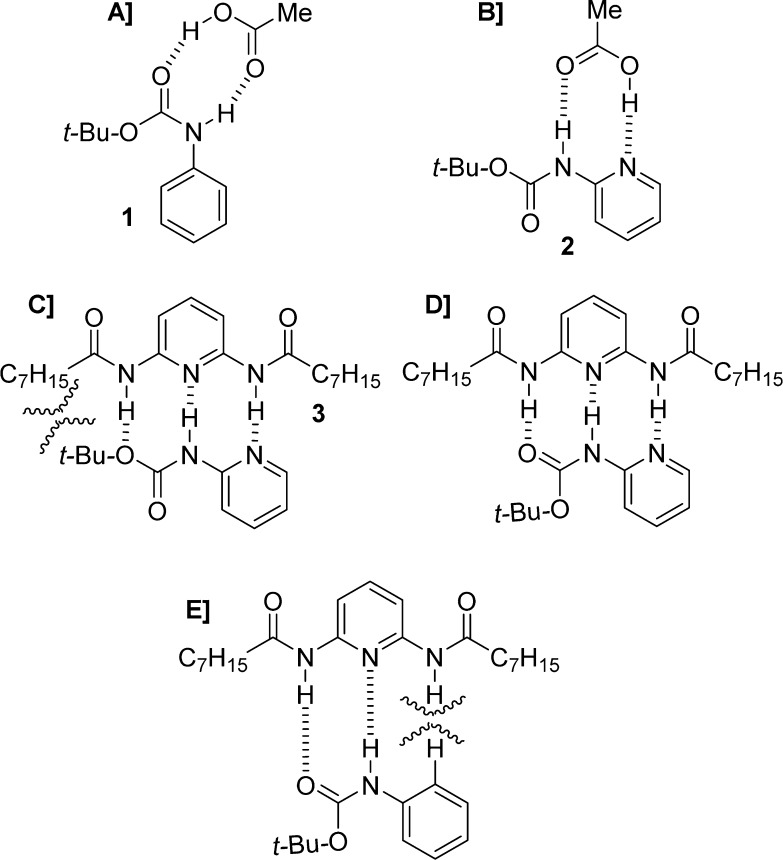
Support of this hypothesis was provided by adding increasing amounts of acetic acid to a solution of a carbamoylated amino acid ester. As expected, the syn rotamer appeared, and its concentration increased as a function of the amount of acid added. In contrast, addition of acetic acid to a solution of the corresponding carbamoylated amino acid did not affect the anti/syn ratio. In this context, Moraczewski and co-workers designed a more effective hydrogen-bonding system that selectively perturbs the syn/anti rotamer equilibrium of a target carbamate group.22 The authors examined the abilities of acetic acid and 2,6-bis(octylamido)pyridine (3) to perturb the syn/anti ratio of carbamates 1 and 2 (Figure 4).22
Figure 4.
(A) Syn-carbamate of 1 is stabilized by hydrogen bonding with acetic acid; (B) acetic acid is associated with the anti rotamer of 2; (C) association of 3 with anti-rotamer of 2; (D) association of 3 with the syn-rotamer (preferred); (E) association of 3 with the syn rotamer of 1 is disfavored.
In a CDCl3 solution, acetic acid moderately stabilizes double hydrogen bonding of the syn rotamer of phenyl carbamate 1 (Figure 4A), with no relevant effect on the syn/anti ratio for 2-pyridyl carbamate 2 (Figure 4B). In the second case, the carboxylic acid favors donation of a hydrogen bond to the more basic pyridyl nitrogen and forms the complex shown in Figure 4B. On the contrary, in the case of the donor–acceptor–donor triad 3, it strongly stabilizes the syn rotamer of 2 (Figure 4D) over the anti rotamer (Figure 4C). There is no effect on the syn/anti ratio for 1, presumably because of a steric deterrent to the formation of a hydrogen-bonded complex (Figure 4E).
The carbamate moiety plays a noteworthy role in medicinal chemistry, not only because it is found in drugs but also for its presence in a number of prodrugs.31 The rate and level of their hydrolysis is a key issue for the duration and intensity of their pharmacological activity. Fast hydrolysis of carbamate-bearing drugs may result in weak or shortened activity. On the contrary, carbamate-based prodrugs must undergo extensive hydrolysis at a suitable rate for releasing an active drug and obtaining the expected activity profile.
Vacondio et al. recently proposed an interesting study in which they compiled a large number of reliable literature data on the metabolic hydrolysis of therapeutic carbamates.32 The authors were able to exploit the collected data to gain a qualitative relationship between molecular structure and lability to metabolic hydrolysis. A trend was extrapolated, according to which the metabolic lability of carbamates decreased in the following series: aryl-OCO-NHalkyl ≫ alkyl-OCO-NHalkyl ∼ alkyl-OCO-N(alkyl)2 ≥ alkyl-OCO-N(endocyclic) ≥ aryl-OCO-N(alkyl)2 ∼ aryl-OCO-N(endocyclic) ≥ alkyl-OCO-NHAryl ∼ alkyl-OCO-NHacyl ≫ alkyl-OCO-NH2 > cyclic carbamates.32 Therefore, carbamates derived from ammonia or aliphatic amines are sufficiently long-lived. An example is represented by cefoxitin (4), a second-generation cephalosporin antibiotic (Figure 5). Cyclic five- or six-membered carbamates are quite stable and do not usually undergo metabolic ring opening. The antibacterial agent linezolid (5) is a representative example of this class (Figure 5). For these drugs, carbamate hydrolysis is not necessarily the half-life-determining metabolic reaction. On the contrary, fatty acid amide hydrolase (FAAH) inhibitor 6 (URB524)33 showed significant hydrolysis in buffer at physiological pH after 24 h (Figure 5). Other representative therapeutic carbamate drugs and prodrugs will be discussed in Sections 4 and 5, respectively.
Figure 5.
Example of carbamate drugs displaying different metabolic stability.
3. Methods for the Synthesis of Carbamates
Organic carbamates play an important role in organic synthesis, especially as subunits of biologically active compounds. Accordingly, simple and efficient methods for the synthesis of carbamates are of great interest. A number of methods have been developed for the synthesis of carbamates.
3.1. Carbamate Synthesis via Traditional Methods
Over the years, a variety of carabamates have been prepared by utilizing the Hofmann rearrangement of amides,34−36 the Curtius rearrangement of acyl azides,37,38 the reductive carbonylation of nitroaromatics,39 the carbonylation of amines,40 the reaction of alcohols with isocyanates,41 and carbon dioxide alkylation.42−45
The Hofmann rearrangement (Method I, Scheme 1) is well-recognized as a useful method to convert primary carboxamides to amines or carbamates, characterized by the reduction of one carbon in the structure.46 Much effort has been devoted to the development of modified reagents to optimize the Hofmann rearrangement since the classical method for this transformation, involving the use of an alkaline solution of bromine, is unsatisfactory and unreliable.35 A variety of oxidants and bases have been proposed as modified agents, e.g., iodine(III) reagents such as PhI(OAc)2,47 MeOBr,48 NBS-CH3ONa,49 NBS-KOH,46 lead tetraacetate,50 and benzyltrimethylammonium tribromide.51 These modified methods, however, require more than 1 equiv or an excess amount of the oxidizing reagent, which is not very convenient.
Scheme 1. Traditional Synthetic Methodologies Adopted for the Synthesis of Carbamates.
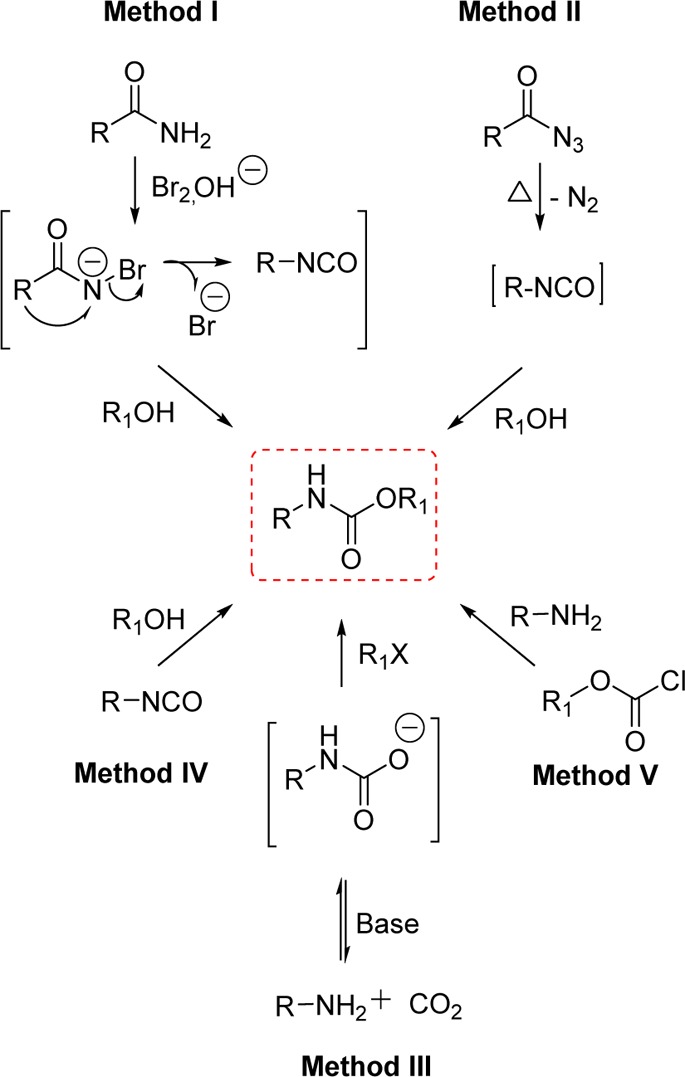
The Curtius rearrangement (Method II, Scheme 1) is the thermal decomposition of acyl azides into the isocyanate intermediate. This method is widely employed in the transformation of carboxylic acids into carbamates and ureas. Acyl azides are usually prepared from carboxylic acid derivatives such as acyl chlorides,52,53 mixed anhydrides,54,55 and hydrazides.56,57 Subsequent isocyanate intermediates can be trapped by a variety of nucleophiles to provide the carbamate derivatives. The acid chloride method is not suitable for acid-sensitive functionalities. One-pot transformations of carboxylic acids into carbamates avoids the isolation of unstable acyl azides. However, protocols involving the use of diphenylphosphoryl azide (DPPA) for the one-pot Curtius reaction are also characterized by issues related to toxicity and the high boiling point of DPPA, which creates difficulties during workup and purification.58−60 Other general methods for carbamate preparation involve the use of the highly toxic phosgene,61 phosgene derivatives,62,63 or isocyanates.64
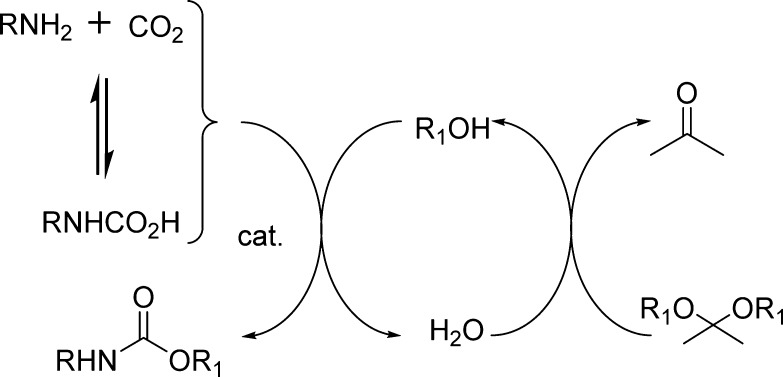
Significant efforts have been made to find an alternative to the phosgene process. A very attractive substitute for phosgene is carbon dioxide because it is a classic renewable resource (Method III, Scheme 1). In addition, its use is also very attractive due to its environmentally benign nature (nontoxic, noncorrosive, and nonflammable).65 Carbon dioxide is well-known to react rapidly with amines to form carbamic acid ammonium salts. The majority of the approaches in this context rely on the creation of the carbamate anion via the reaction of carbon dioxide and amines, followed by the reaction with electrophiles. Nevertheless, since the nucleophilicity of the carbamate anion is lower than that of the amine formed in the equilibrium of the salt formation, the subsequent reaction of the carbamate salts with alkyl halides does not selectively provide urethanes.44,66
The formation of carbamates from isocyanates (Method IV, Scheme 1) is fundamentally important to polyurethane industries. Synthetic limitations and toxicity issues, however, are associated with the use of phosgene, the most common route to obtain isocyanates.64 The readily available alkyl chloroformates are the most frequently used reagents for the preparation of carbamates (Method V, Scheme 1). However, these reagents display major drawbacks, as a large excess of base and a long reaction time are required in order to gain acceptable reaction efficiency. Moreover, excess reagents are not suitable for the synthesis of molecules bearing multiple functionalities in which the chemoselectivity is critical.67
3.2. Carbamate Synthesis via Activated Mixed Carbonates
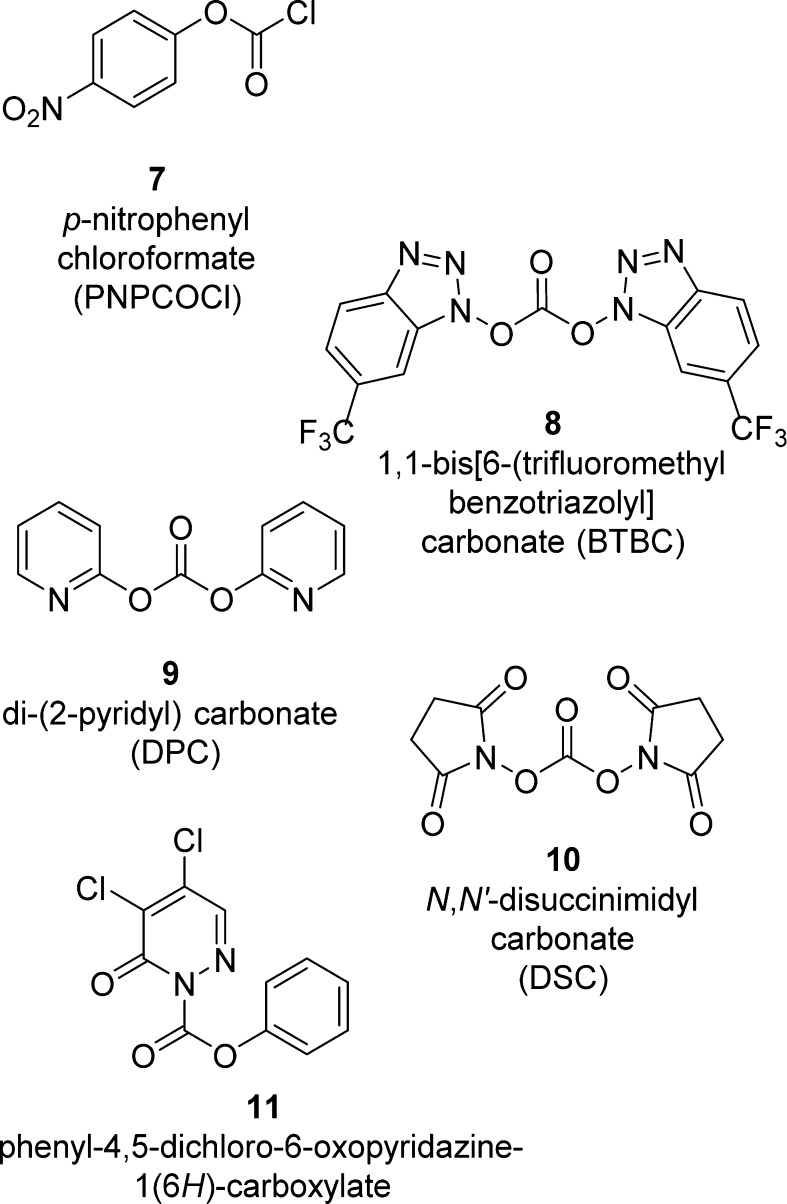
A number of organic carbonates have been developed as low-cost and benign alternatives to the phosgene-based routes for the synthesis of organic carbamates. In this context, several new alkoxycarbonylating agents (7–11) based on mixed carbonates have been developed (Figure 6). These methods are often used for the synthesis of carbamates in drug design.68−72
Figure 6.
Most commonly employed carbonate reagents for carbamate synthesis.
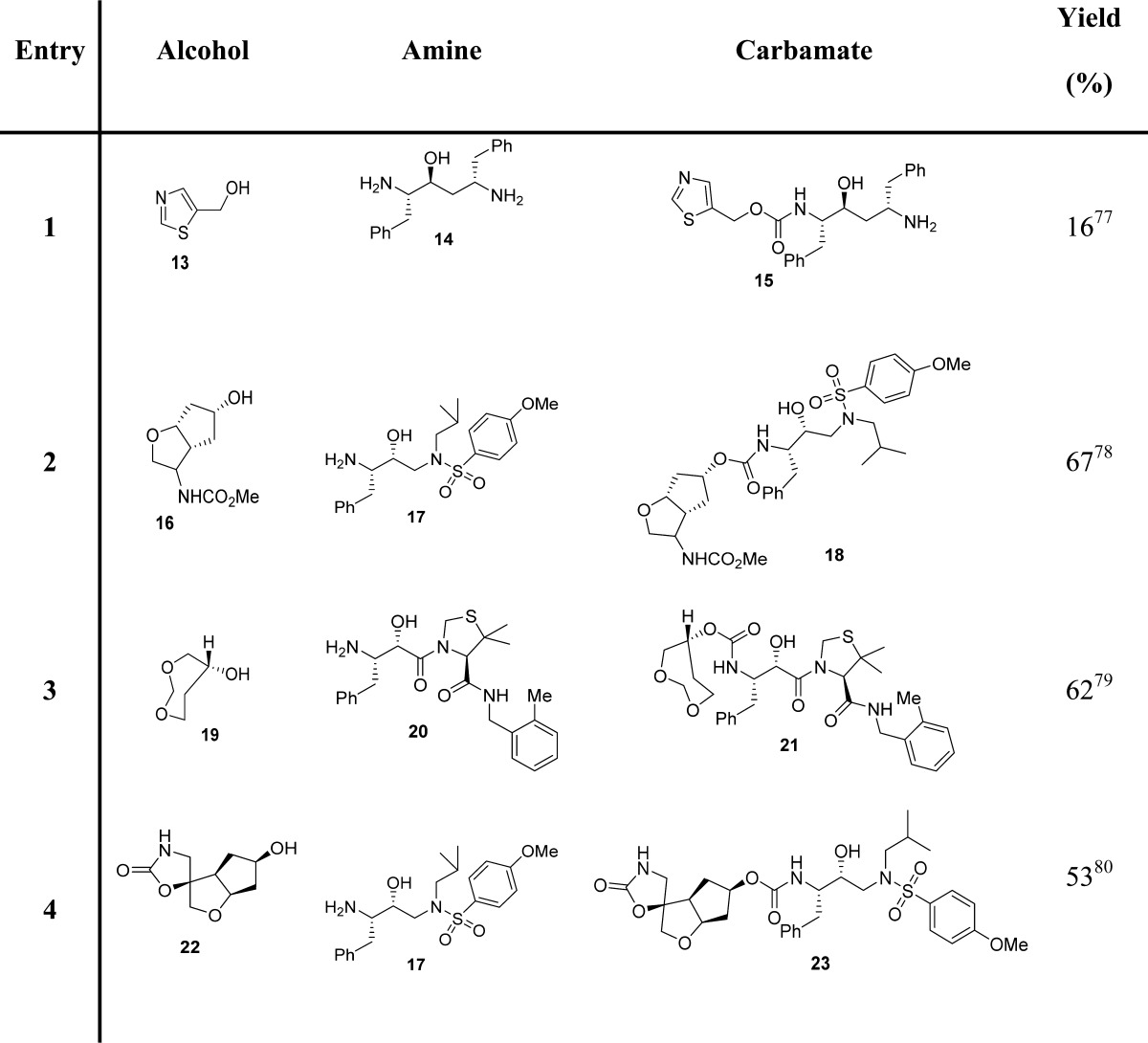
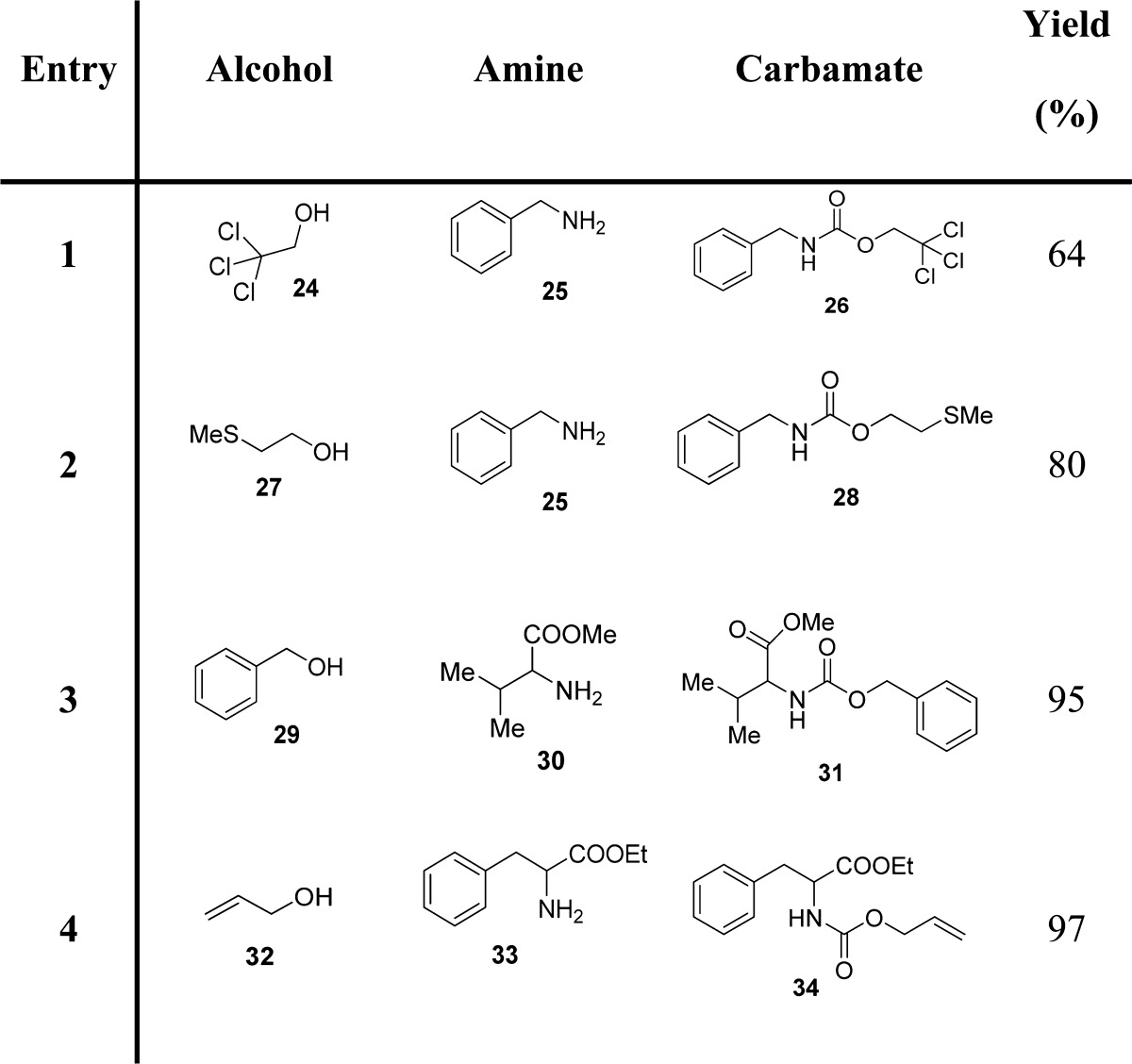
Mixed carbonates with a p-nitrophenyl moiety are frequently used for the preparation of a large range of carbamates.73−76 For this, p-nitrophenyl chloroformate (7, PNPCOCl), when treated with the suitable alcohol in the presence of base, furnishes the corresponding activated carbonates, which have been shown to be useful and effective alkoxycarbonylating reagents for suitable amines (Scheme 2). Examples of carbamate derivatives are shown in Table 1.
Scheme 2. Carbamate Synthesis via Activated Mixed Carbonates (Highlighted in the Red Box).
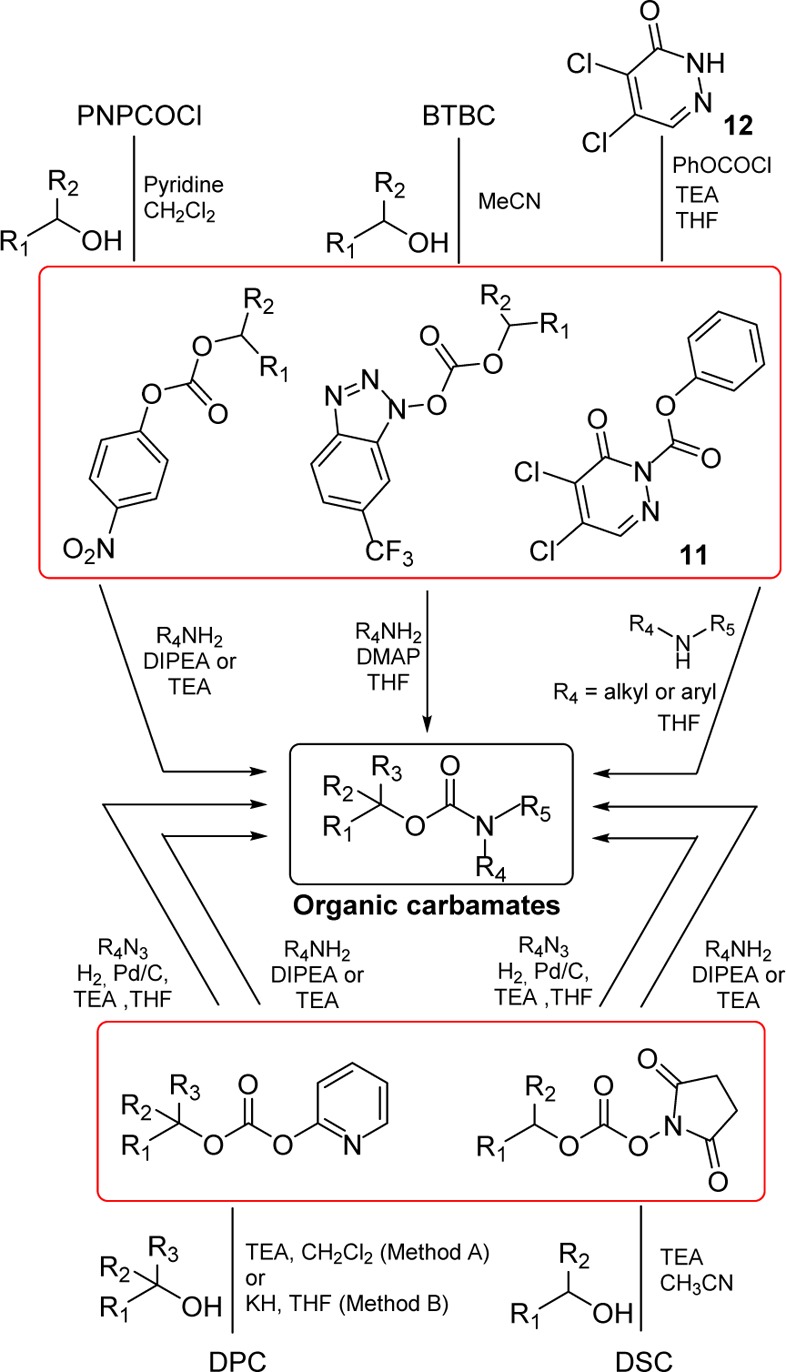
Table 1. Examples of Carbamate Formation from p-Nitrophenyl-Based Mixed Carbonates.
Several alkoxycarbonylating reagents for amino groups having heterocyclic groups, such as N-hydroxyimide, have been reported. Moreover, the utility and versatility of carbonates and oxalates containing an electron-withdrawing group, such as N-hydroxyimide and benzotriazole derivatives as reagents for various tranformations, have been described.72,81,82
Takeda et al. reported that 1-alkoxy[6-(trifluoromethyl)benzotriazolyl]carbonates easily derived from 1,1-bis[6-(trifluoromethyl)benzotriazolyl]carbonate (8, BTBC) showed high acylating reactivity toward alcohols as well as amino groups.83 BTBC was prepared from 6-trifluoromethyl-1-hydroxybenzotriazole and trichloromethyl chloroformate and purified by washing with dry ether. Moreover, it can be stored for several months in a freezer. BTBC was allowed to react with primary alcohols in acetonitrile at room temperature to give stable activated carbonates. The carbonates were treated with amines in the presence of 4-dimethylaminopyridine (DMAP), providing the corresponding carbamates (Scheme 2 and Table 2).83
Table 2. Examples of Carbamate Formation from 1,1-Bis[6-(trifluoromethyl)benzotriazolyl] Mixed Carbonates83.
In connection with our research work aimed at synthesizing biologically active polyfunctional molecules for probing enzyme active sites, we required a more general and synthetically reliable method for the synthesis of various carbamate derivatives. In 1991, we described the utility of di(2-pyridyl) carbonate (9, DPC)84 as an efficient, high-yielding, and convenient alkoxycarbonylation reagent for amines overcoming many of the limitations of existing methodologies.85 DPC was readily prepared from commercially available 2-hydroxypyridine and triphosgene in the presence of triethylamine and subsequently reacted with the suitable primary or secondary alcohol (e.g., (+)-menthol) to provide a mixed carbonate. Alkoxycarbonylation of primary and secondary amines with the mixed carbonates was carried out in the presence of triethylamine and furnished the corresponding carbamates in good yields (Scheme 2, Method A, and Table 3). Potassium hydride was used in the place of triethylamine in the preparation of the mixed carbonates containing tertiary alcohols (Scheme 2 and Table 3).85
Table 3. Examples of Carbamate Formation from 2-Pyridyl-Based Mixed Carbonates85.
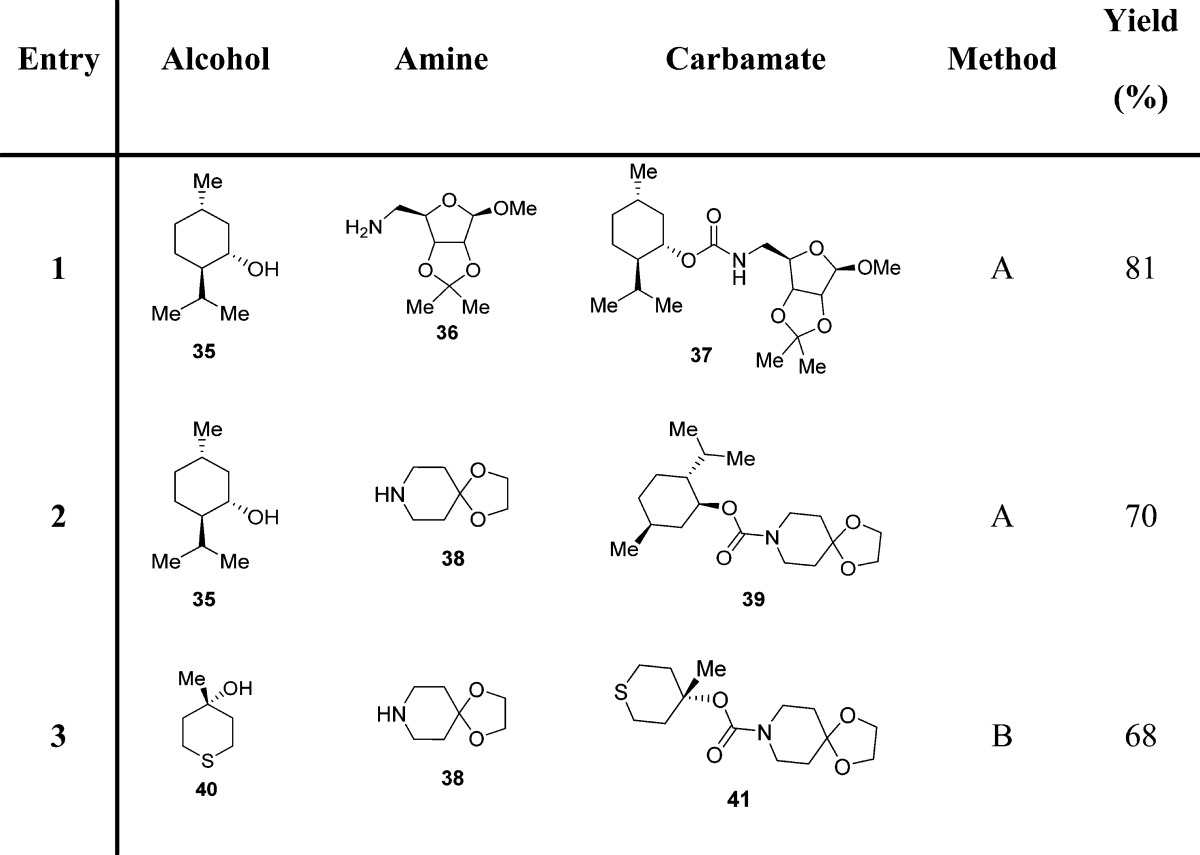
Subsequently, we investigated the scope of N,N′-disuccinimidyl carbonate (10, DSC)81 promoted alkoxycarbonylation of amines with a host of alcohols under mild conditions.86 Rich and co-workers highlighted the convenience of succinimidyl-based mixed carbonates for the high-yielding introduction of a 2-(trimethylsilyl)ethoxycarbonyl (Teoc) protecting group to amino acids, without oligopeptide byproduct formation.87 DSC was found to be a highly effective alkoxycarbonylating reagent for a variety of primary and sterically hindered secondary alcohols. DSC is commercially available, or it can be conveniently prepared from N-hydroxysuccinimide following a procedure tracing out the synthesis of DPC.85 The ready availability of DSC, the stability of the mixed carbonates, and the mildness of the reaction procedure render this method a reliable route to organic carbamates (Scheme 2 and Table 4).86
Table 4. Examples of Carbamate Formation from N,N′-Disuccinimidyl-Based Mixed Carbonates.
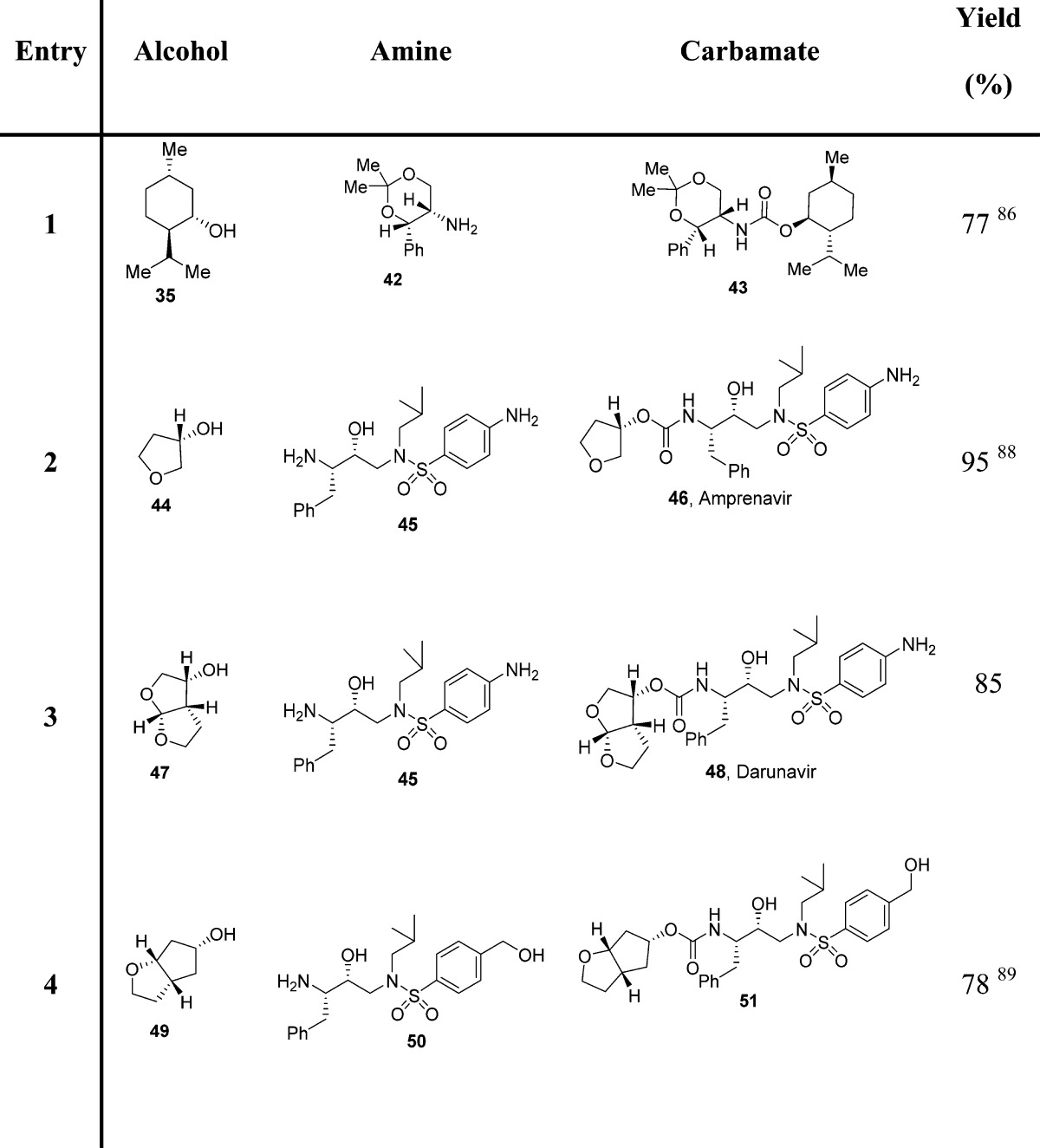
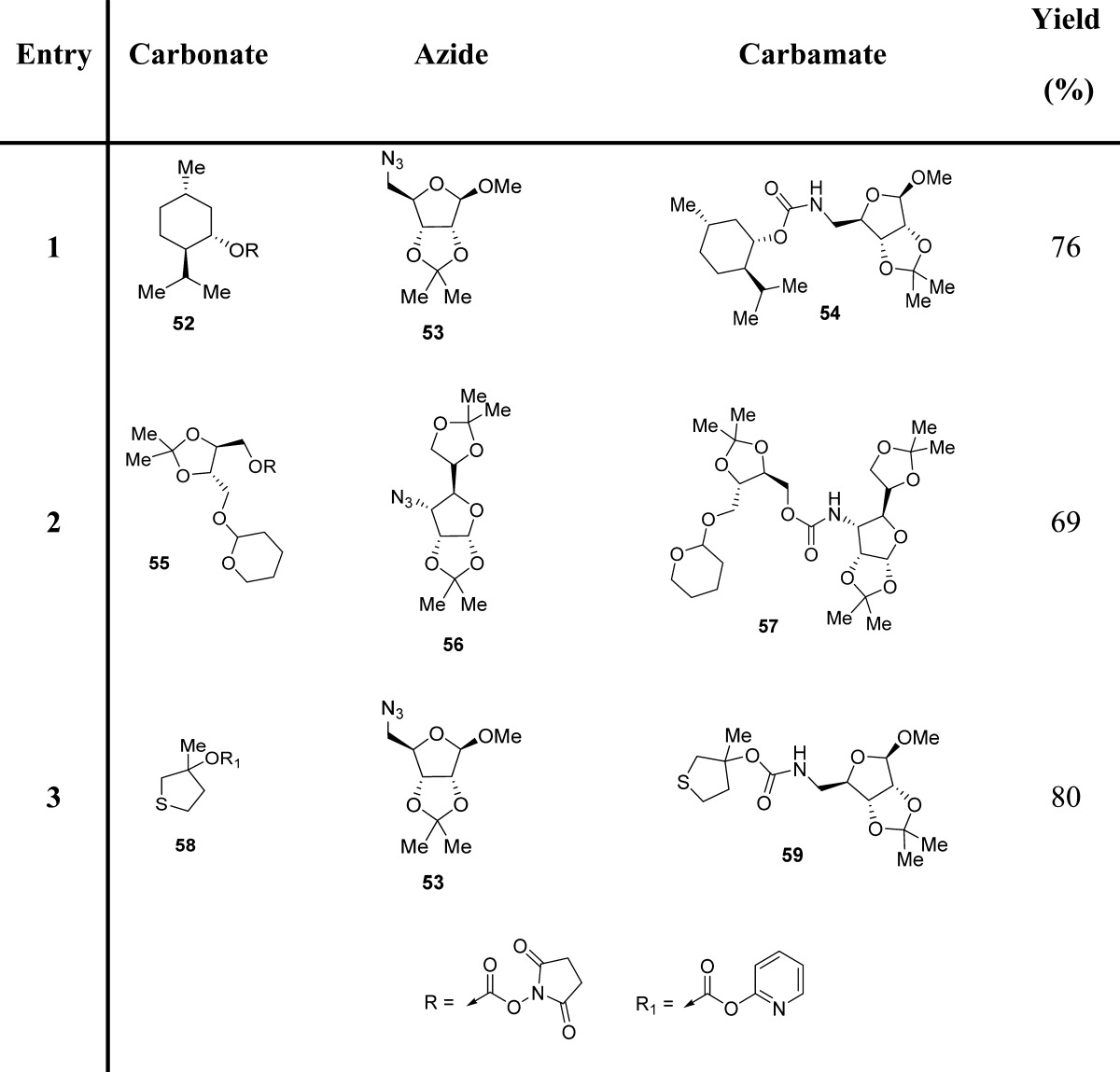
Since azides were extensively employed as incipient amines in the context of amino sugar and amino acid syntheses, their conversion into the corresponding carbamate derivatives could provide a novel, effective route for medicinal chemistry applications. In this context, a facile synthetic protocol to transform various azides into the corresponding functionalized urethanes in high yields has been developed.90 In general, mixed carbonates of variously protected alcohols were prepared by reaction of excess DSC or DPC, as described previously. Exposure of mixed carbonates to catalytic hydrogenation conditions with azides in the presence of 10% palladium on charcoal in tetrahydrofuran furnished the corresponding carbamates. Interestingly, the use of triethylamine as a promoter has a notable effect on the yield and the rate of the alkoxycarbonylation process (Scheme 2 and Table 5).90
Table 5. Examples of Carbamate Formation from Mixed Carbonates and Azides90.
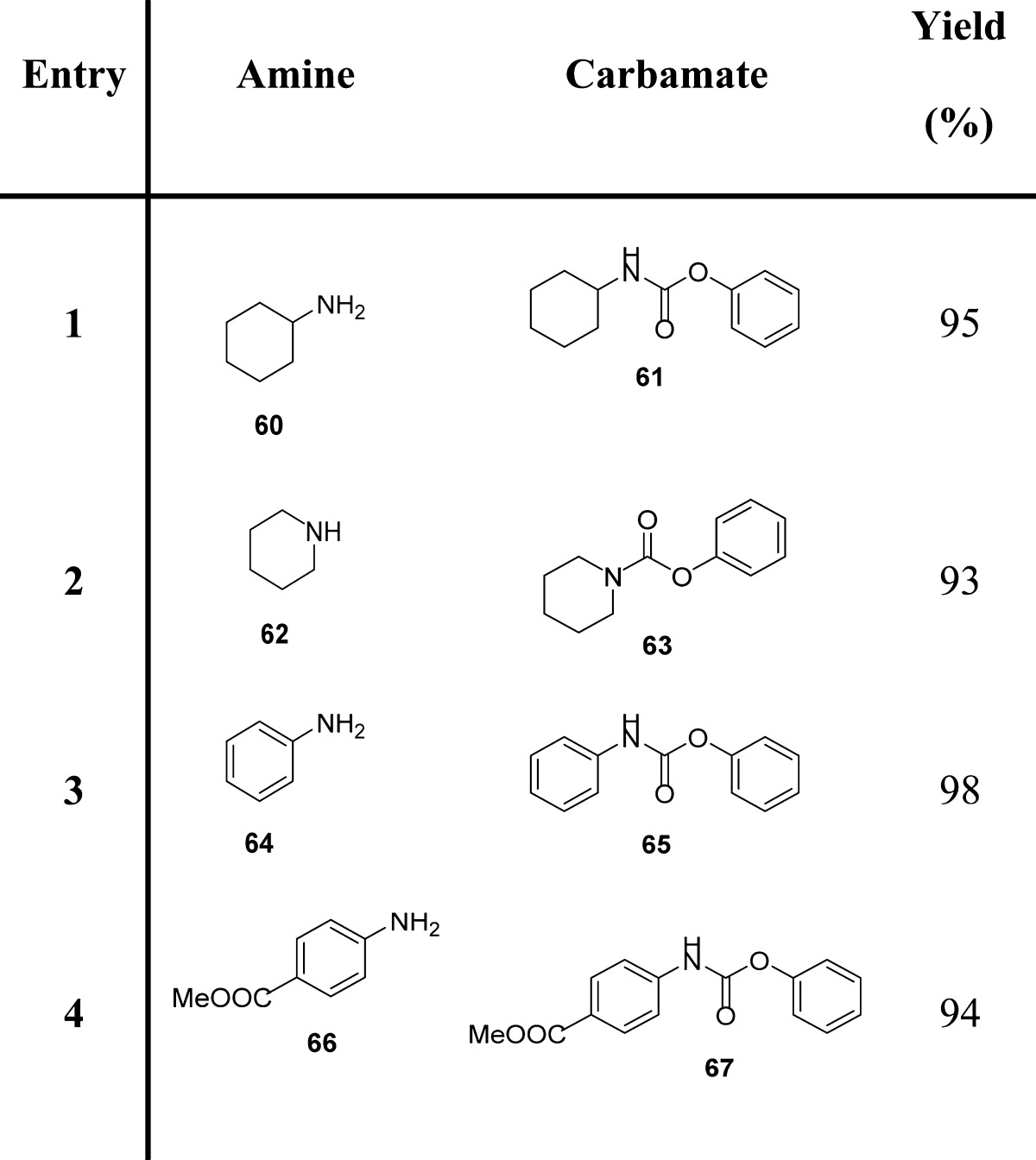
More recently, Yoon and co-workers exploited 2-substituted-pyridazin-3(2H)-ones as electrophilic transfer reagents.91,92 In particular, the authors investigated the carbonylation potency of phenyl 4,5-dichloro-6-oxopyridazine-1(6H)-carboxylate (11) to amines for the preparation of phenylcarbamates (Scheme 2 and Table 6). Compound 11 is stable in air and in organic solvents at high temperature and is prepared easily from cheap and commercially available 4,5-dichloropyridazin-3(2H)-one (12) in the presence of phenylchloroformate and triethylamine (Scheme 2).
Table 6. Examples of Carbamate Formation from Phenyl 4,5-Dichloro-6-oxopyridazine-1(6H)-carboxylate.
3.3. Recent Methodologies for Carbamate Synthesis
The application of carbon dioxide in organic synthesis has recently attracted much interest. Most of the approaches rely on the generation of the carbamate anion via the reaction of carbon dioxide and amines, followed by the reaction with electrophiles, usually alkyl halides.93−95
In this context, a mild and efficient preparation of alkyl carbamates on solid supports was described by Jung et al.96 Amines and anilines were coupled with Merrifield’s resin through a CO2 linker in the presence of cesium carbonate and tetrabutylammonium iodide (TBAI). Carbon dioxide was supplied by bubbling it into the reaction suspension, where N,N-dimethylformamide (DMF) was the solvent of choice (Scheme 3).96
Scheme 3. Solid-Phase Synthesis of Carbamates Using Aromatic Amines and Merrifield Resin.
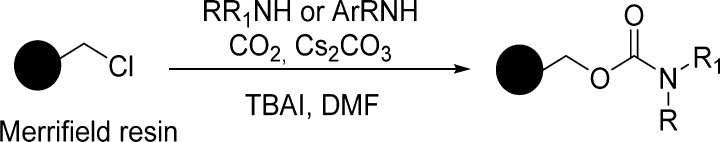
The reaction conditions are convenient for purification, and the reactions undergo complete conversions. The method is convenient for the generation of large combinatorial libraries for rapid screening of bioactive molecules. Chiral substrates susceptible to racemization have survived the conditions (Table 7).
Table 7. Solid-Phase Synthesis of Carbamates Using Merrifield Resin with Primary and Secondary Amines and Anilines96.
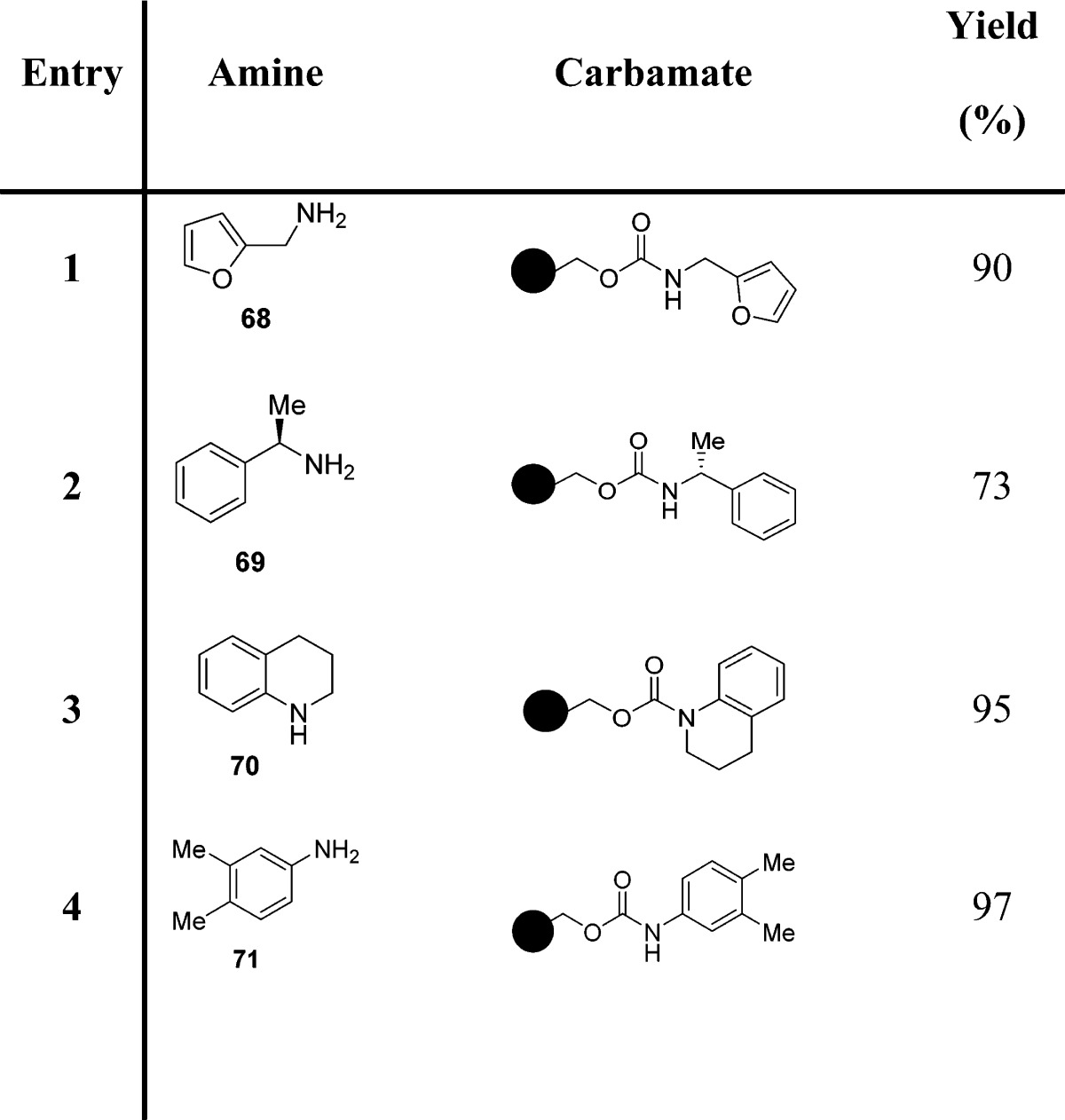
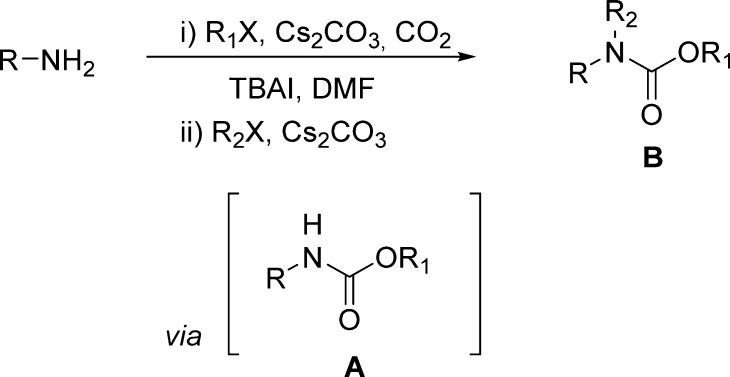
Later, these authors reported a one-pot synthesis of N-alkyl carbamates starting from primary amines (Scheme 4).96 Carbamates were generated via a three-component coupling of primary amines, CO2, and an alkyl halide in the presence of cesium carbonate and TBAI in anhydrous DMF (Scheme 4 and Table 8).
Scheme 4. Synthesis of N-Alkyl Carbamates by a Three-Component Coupling of Primary Amines, CO2, and an Alkyl Halide in the Presence of Cesium Carbonate and TBAI.
Table 8. One-Pot Synthesis of N-Alkyl Carbamates Starting from Primary Amines.
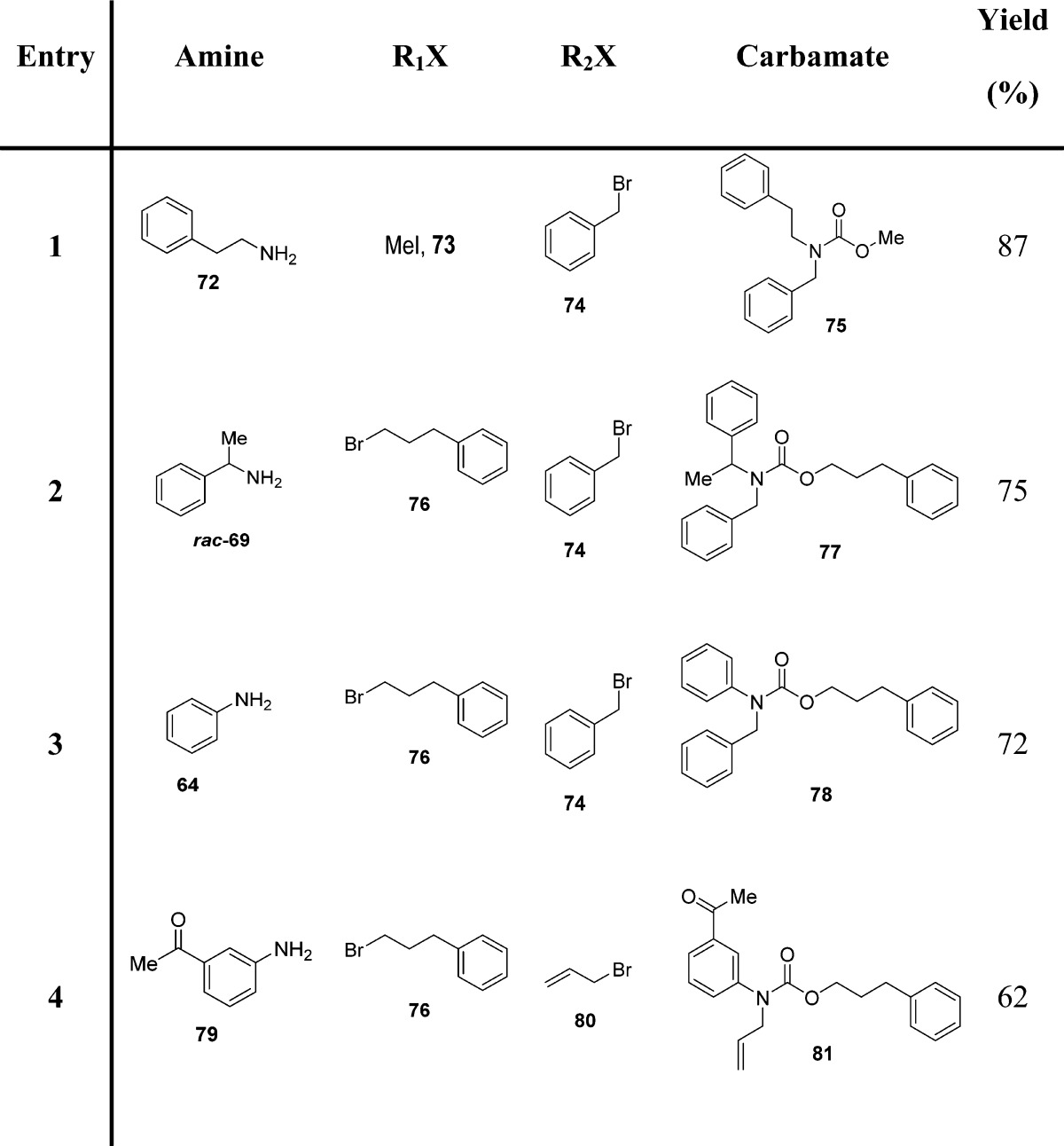
Direct N-alkylation of the intermediate carbamate A in the presence of additional cesium carbonate by using a different alkyl halide gave rise to the desired N-alkyl carbamate B (Scheme 4). Isolation of the intermediate A proved to be unnecessary, offering shortened synthetic sequences.40 It is interesting to note that TBAI helps to minimize the overalkylation of the produced carbamate, presumably by enhancing the rate of CO2 incorporation and/or stabilizing the incipient carbamate anion through conjugation with the tetrabutylammonium cation.97
Sakakura and co-workers reported urethane synthesis by the reaction of dense carbon dioxide with amines and alcohols by a procedure that is not only phosgene-free but also completely halogen-free (Scheme 5).98 Dialkyl carbonate synthesis from an alcohol and CO2 is catalyzed by metal complexes such as dialkyl(oxo)tin and dialkyl(dichloro)tin. However, the alcohol conversion is very poor. Similarly, the direct reaction of an amine, an alcohol, and carbon dioxide in the presence of dialkyltin compounds produced urethane only in a poor yield.
Scheme 5. Halogen-Free Carbamate Synthesis Employing Dense Carbon Dioxide in the Presence of Amines and Alcohols.
The low conversion observed was attributed by the authors to thermodynamic limitations and catalyst deactivation by coproduced water. In order to overcome this issue, a new reaction system utilizing acetals as a chemical dehydrating agent, with subsequent alcohol regeneration (Scheme 5), was developed.
In order to obtain urethane in good yields, dense-phase CO2 under high pressure was necessary to lower the major side reactions, namely imine formation from acetone and alkylation of amines by alcohols.
However, developing less toxic and more active catalysts based on metals other than tin was required. Later, these authors reported novel nickel-based catalytic systems for dehydrative urethane formation from carbon dioxide, amines, and alcohols (Scheme 6).99 Interestingly, adding nitrogen-based bidentate ligands efficiently improved the catalytic activity of Ni(OAc)2-based catalysts (Scheme 6 and Table 9). Bipyridines and phenanthrolines with strong coordinating abilities (low steric hindrance and high electron densities) were the better choice for obtaining urethanes in high yields. It is important to note that the Ni-phenanthroline system is more active and less toxic than dialkyl(oxo)tin under the same reaction conditions. It is also noteworthy that the catalytic activity of the Ni(OAc)2-(4,4′-dimethylbipyridine) system is highly dependent on the ligand/metal ratio (Table 9).
Scheme 6. Ni-Based Catalytic Systems for Dehydrative Urethane Formation from Carbon Dioxide, Amine, and Alcohol.
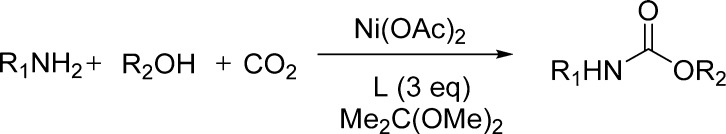
Table 9. Nickel-Catalyzed Urethane Synthesis from CO299.
Conversion of amine.
Urethane/consumed amide × 100.
Ni/L = 1:1.
Ni/L = 1:5.
Peterson and co-workers proposed a method for rapid SAR development of compounds bearing urea or carbamate functionalities (Scheme 7).100 For carbamate formation, an amine, in principle, could proceed through the carbamic acid–isocyanate reaction, and subsequent reaction with an alcohol may provide a carbamate product.
Scheme 7. DBU-Catalyzed Carbamate Formation in the Presence of Gaseous Carbon Dioxide.
While this is precedented by an intramolecular reaction variant to produce cyclic carbamates,101 the desired intermolecular coupling was not fruitful under the proposed reaction conditions. Carbamic acids produced from secondary amines, however, did react with alcohols under Mitsunobu conditions (dibenzyl azodicarboxylate, DBAD, and tributylphosphine) in a DBU-catalyzed reaction with gaseous carbon dioxide, providing the corresponding carbamates (Scheme 7 and Table 10). This reaction did not proceed through the isocyanate intermediate but rather through an SN2 displacement of the activated alcohol. This hypothesis is supported by the observed inversion of stereochemistry upon conversion of a chiral secondary alcohol to the corresponding carbamate (Table 10).100
Table 10. Carbamates from Secondary Carbamic Acids100.
Very recently, Jiao and co-workers reported a practical, PdCl2-catalyzed efficient assembly of organic azides, carbon monoxide, and alcohols for the direct synthesis of carbamates via isocyanate formation and application in situ (Scheme 8).102
Scheme 8. Carbamate Formation by a PdCl2-Catalyzed Efficient Assembly of Organic Azides, Carbon Monoxide, and Alcohols.
Mild and neutral reaction conditions and generation of harmless N2 as the byproduct render this protocol very useful, particularly for the synthesis of bioactive compounds. Moreover, the employment of CO at atmospheric pressure and the use of a small amount of PdCl2 catalyst (2 mol %) in the absence of any ligand represent a real alternative to customary carbamate synthetic methods (Table 11).102
Table 11. Carbamates from Organoazides, CO (1 atm), and Alcohols102.
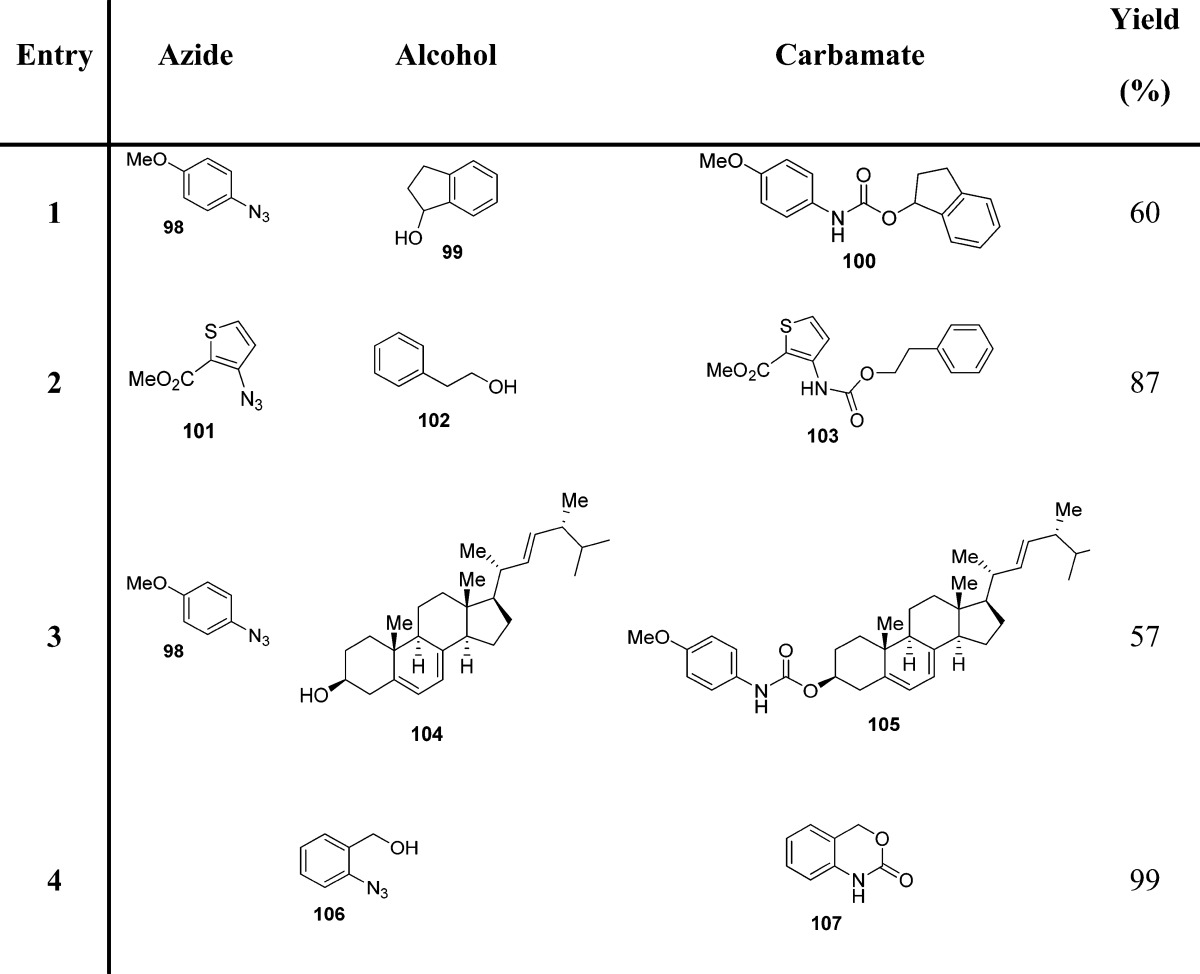
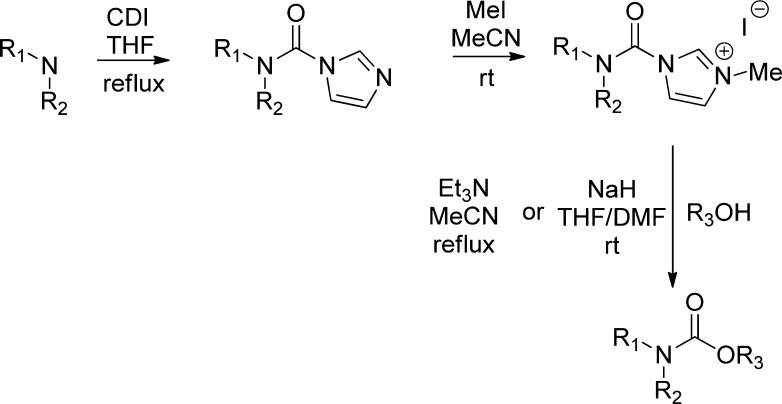
The synthesis of carbamates through the generation of carbamoyl chlorides is not convenient because of the requirement of the toxic phosgene. Also, such carbamoyl chlorides are highly reactive, prone to hydrolysis, unstable, and not suitable for long-term storage. For these problems, Batey and co-workers identified the use of carbamoylimidazolium salts as convenient N,N′-disubstituted carbamoyl transfer reagents, showing increased reactivity over carbamoylimidazoles as a result of the imidazolium effect (Scheme 9).103−105
Scheme 9. Carbamate Synthesis by the Use of Carbamoylimidazolium Salts.
These salts are readily prepared by the sequential treatment of secondary amines with N,N′-carbonyldiimidazole (CDI) and iodomethane (Scheme 9). Authors envisaged that the carbamoylimidazolium salts, while relatively unreactive with alcohols, would react with nucleophlic alkoxides to produce the corresponding carbamates (Table 12). In the case of phenols, tertiary amines are appropriate bases for the in situ generation of the reactive phenoxides. The lower acidity of aliphatic alcohols presumably prevents the formation of the alkoxide anion, which would serve as the reactive nucleophile. Less acidic alcohols react with carbamoylimidazolium after their conversion into more nucleophilic sodium alkoxides (Scheme 9).106
Table 12. Carbamates from Carbamoylimidazolium Salts and Phenols or Alcohols106.
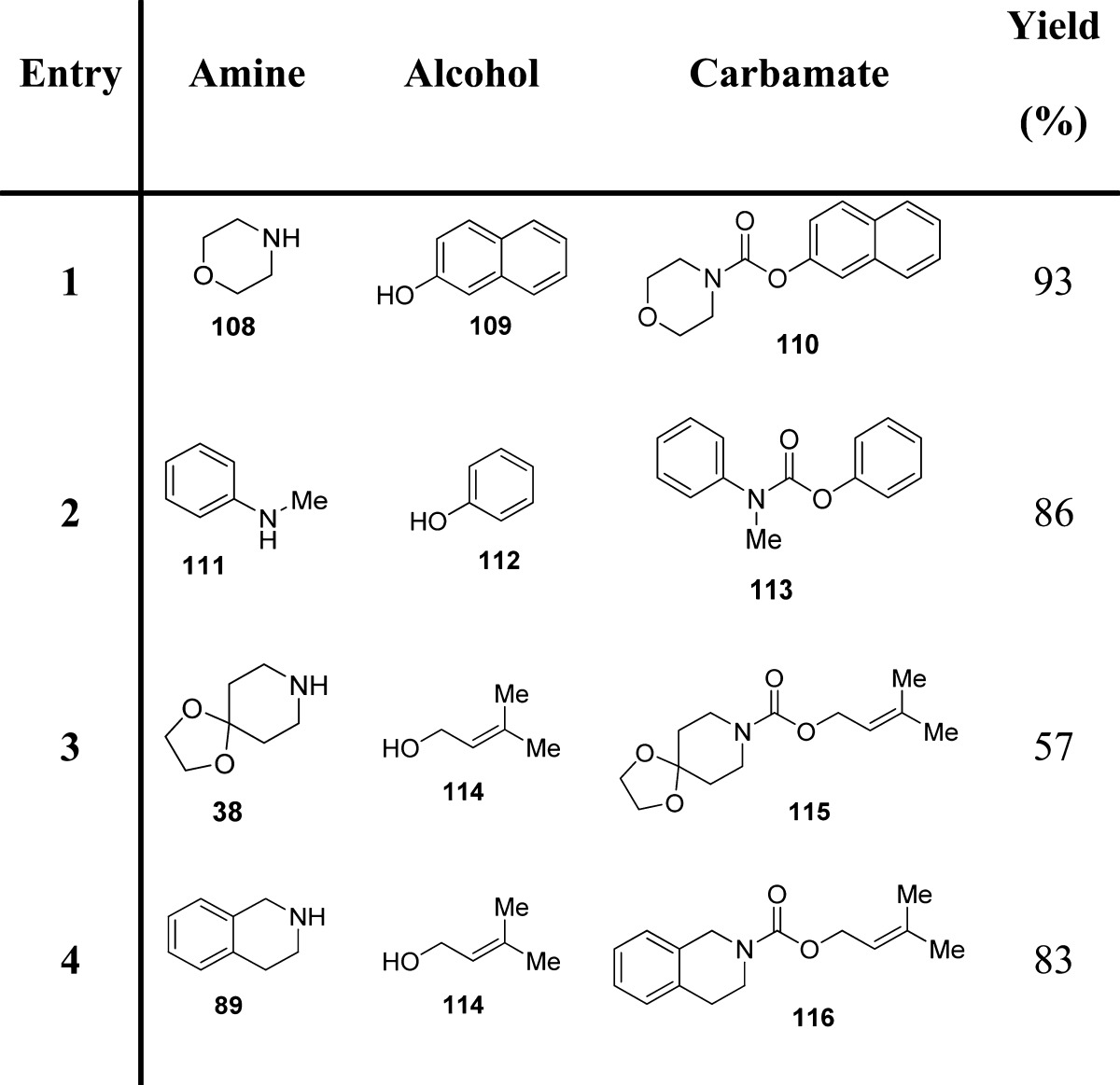
The use of solid-supported reagents has become ubiquitous due to enhanced reactivity and selectivity, milder reaction conditions, convenient work-ups, and decreased solvent waste. The modified Hofmann rearrangement, proposed by Gogoi et al., is operationally simple, inexpensive and applicable to a variety of aliphatic and aromatic amides for the synthesis of methyl carbamates (Scheme 10).36
Scheme 10. Synthesis of Methyl Carbamates by a Modified Hofmann Rearrangement.
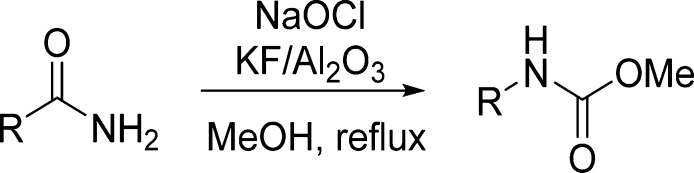
KF/Al2O3 represents a useful and interesting solid-supported strong base, which replaces organic bases in a variety of reactions.107 Sodium hypochlorite is an inexpensive, convenient, and safe alternative to the currently employed oxidants.108 This prompted the authors to investigate KF/Al2O3 along with NaOCl as an efficient reagent system for Hofmann rearrangement. KF/Al2O3 basicity stems from the formation of KOH in the initial preparation of the solid-supported material by the reaction of KF with alumina supports. Under these highly basic reaction conditions, hypochlorite ion is the predominant form of chlorine, reacting with the amide to form an N-chloroamide, which later undergoes rearrangement to the isocyanate. In the presence of methanol, the isocyanate is rapidly converted into the corresponding methyl carbamate (Table 13).36
Table 13. Carbamates from Modified Hofmann Rearrangement36.
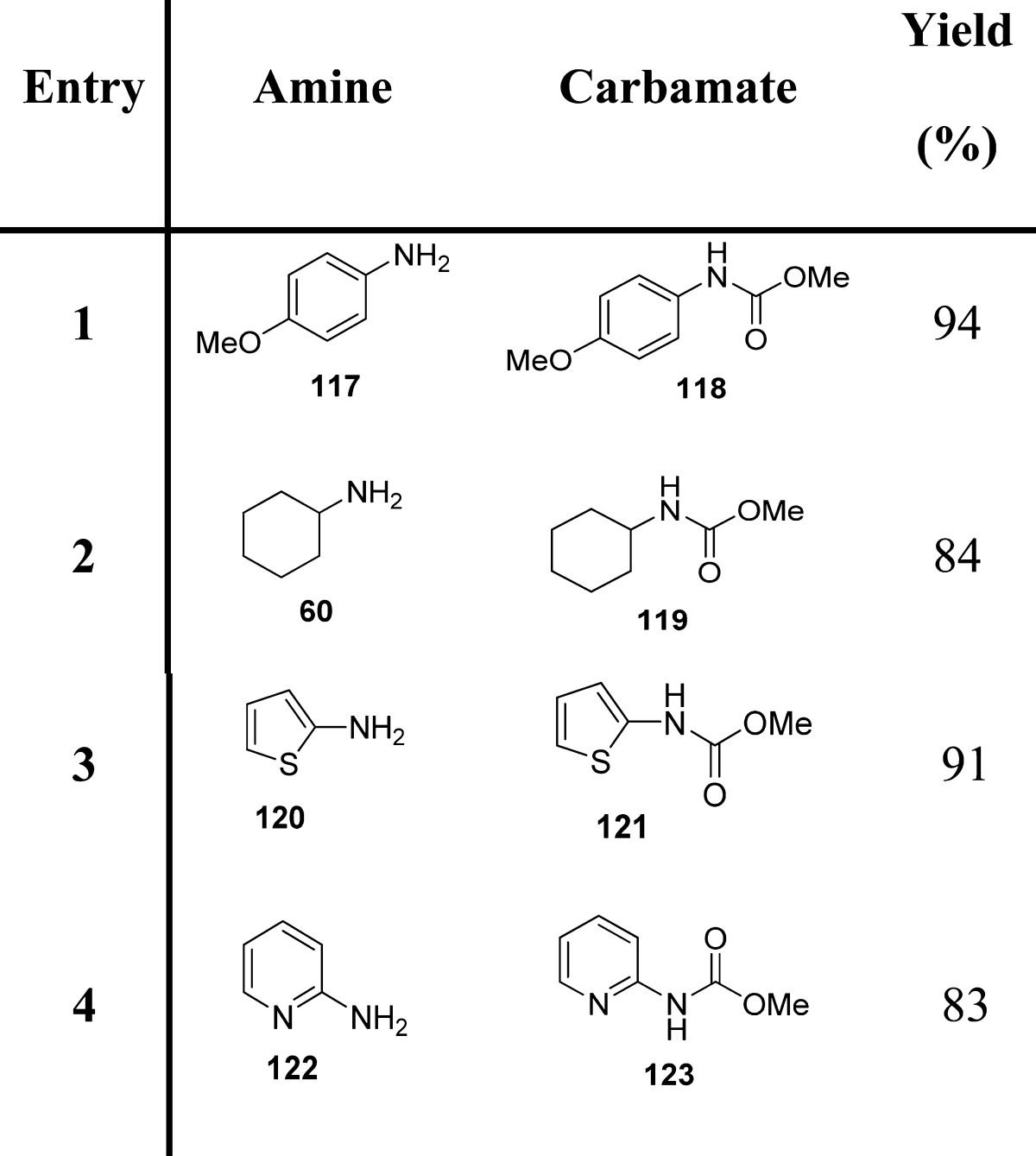
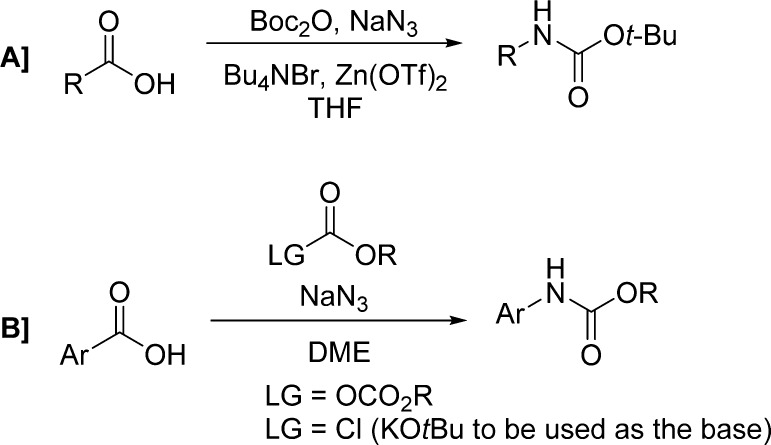
Modifications of the Curtius rearrangement have also been explored. Lebel and co-workers have reported a useful protocol for the preparation of tert-butyl carbamates from the corresponding carboxylic acids.109 Their reaction with di-tert-butyl dicarbonate and sodium azide led to the formation of the corresponding acyl azides, which then undergo a Curtius rearrangement, in the presence of tetrabutylammonium bromide and zinc(II) triflate, providing carbamates through trapping of the isocyanate intermediate (Scheme 11A and Table 14).
Scheme 11. Synthesis of Carbamates by Modified Curtius Rearrangement.
Table 14. Carbamates from Modified Curtius Rearrangement.
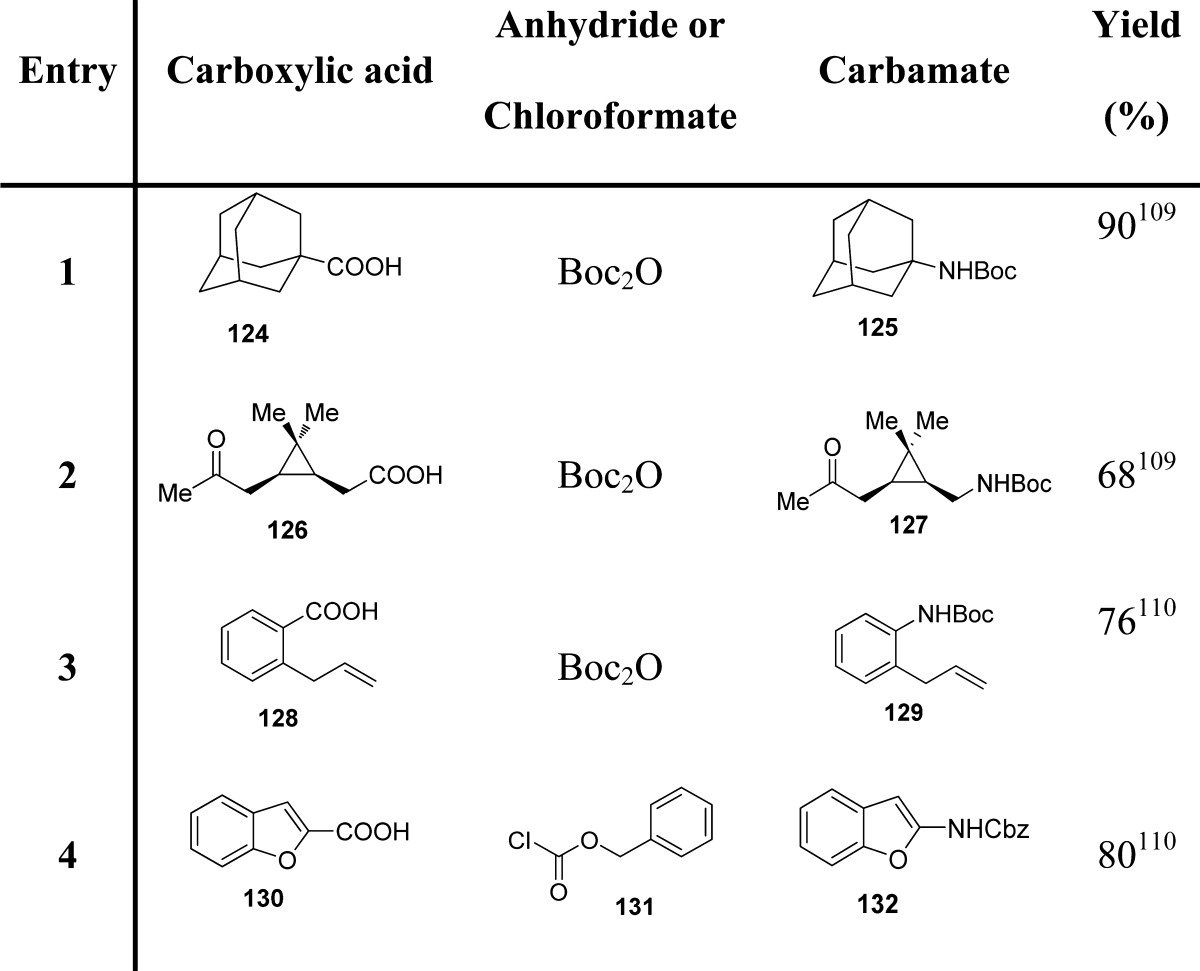
These authors extended the same methodology to the direct synthesis of carbamates of aromatic amines using aromatic carboxylic acids (Scheme 11B and Table 14).110 In particular, the reaction of a chloroformate or di-tert-butyl dicarbonate and sodium azide with an aromatic carboxylic acid produced the corresponding acyl azide, presumably through the formation of an azidoformate. In contrast to what was observed with aliphatic carboxylic acids, using similar reaction conditions, aromatic carboxylic acids led mainly to the formation of the corresponding tert-butyl ester, likely via the displacement of an azide leaving group with tert-butoxide. This may be ascribed to the higher stability of aromatic acyl azides with respect to their aliphatic counterparts. Therefore, for these substrates, the Curtius rearrangement can be promoted only at higher temperatures (40 vs 75 °C).110,111
As mentioned, alkyl chloroformates are the most frequently used reagents for the preparation of carbamates, although the need of an excess amount limits their usefulness. A promising method for preparing carbamates involves the use of a catalytic promoter.112−115 Lately, indium-mediated reactions have gained significant consideration due to the high reactivity and unique properties of indium reagents, among them nontoxicity and inertness toward air and water.116−118 Moreover, pretreatment is not required for activating indium metal. In this context, Jang and co-workers developed a simple, efficient, and selective method for synthesizing carbamates from amines, employing a catalytic amount of indium and only an equimolar amount of alkyl chloroformate (Scheme 12).67
Scheme 12. Indium-Catalyzed Carbamate Formation.
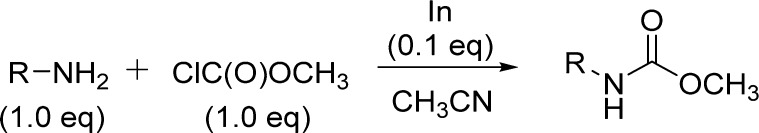
The method shows the generality for a wide variety of sterically diverse amines and alcohols and can also be applicable for the selective protection of amino groups under mild conditions (Table 15).
Table 15. Carbamates from Indium-Catalyzed Reaction of Amines and Chloroformates67.
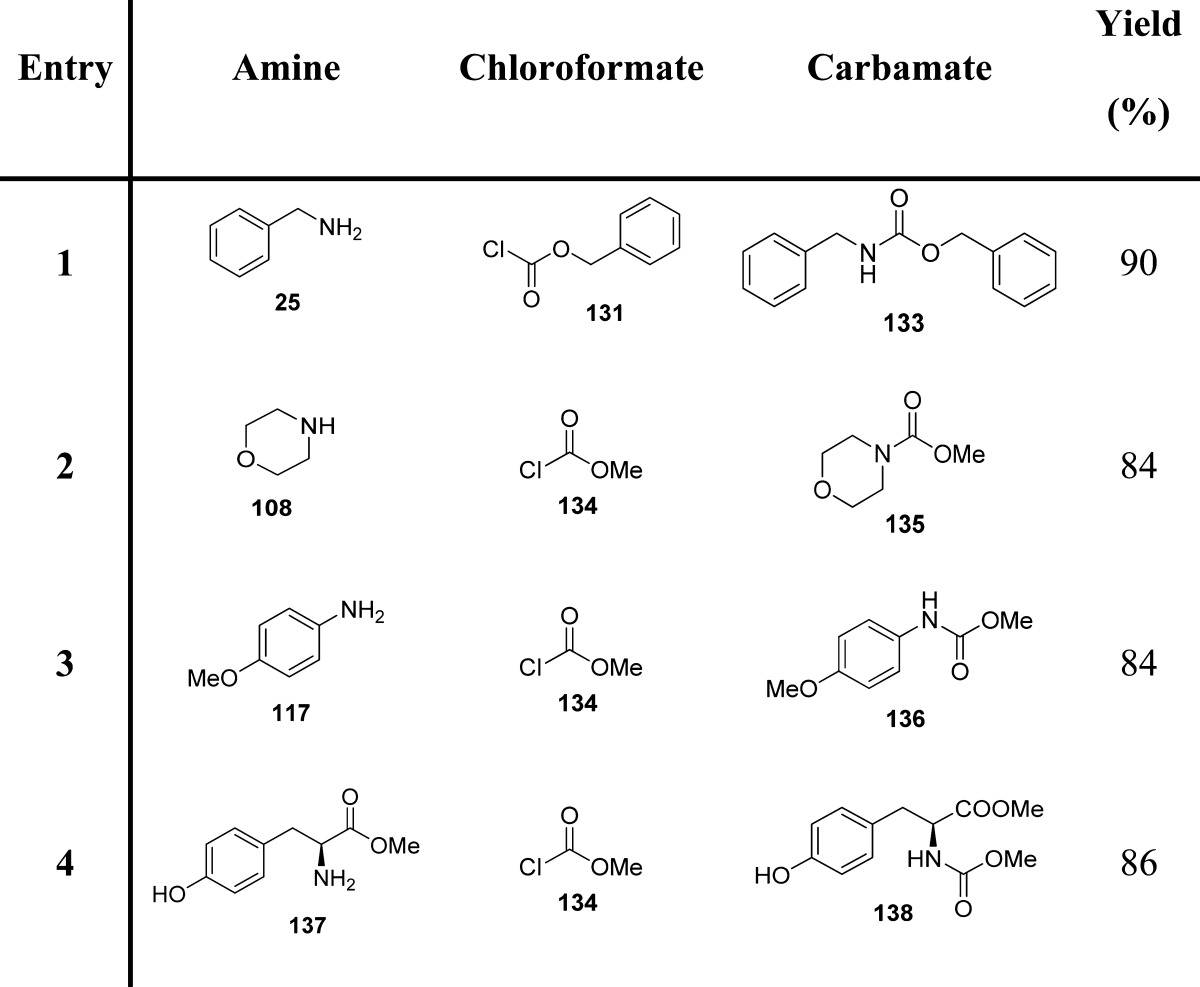
Arndtsen et al. proposed another application of indium-based reagents for the generation of N-protected amines in a single step (Scheme 13 and Table 16).119
Scheme 13. Coupling of Organoindium Reagents with Imines via Copper Catalysis.
Table 16. Carbamates from Imines and Organoindium Reagents119.
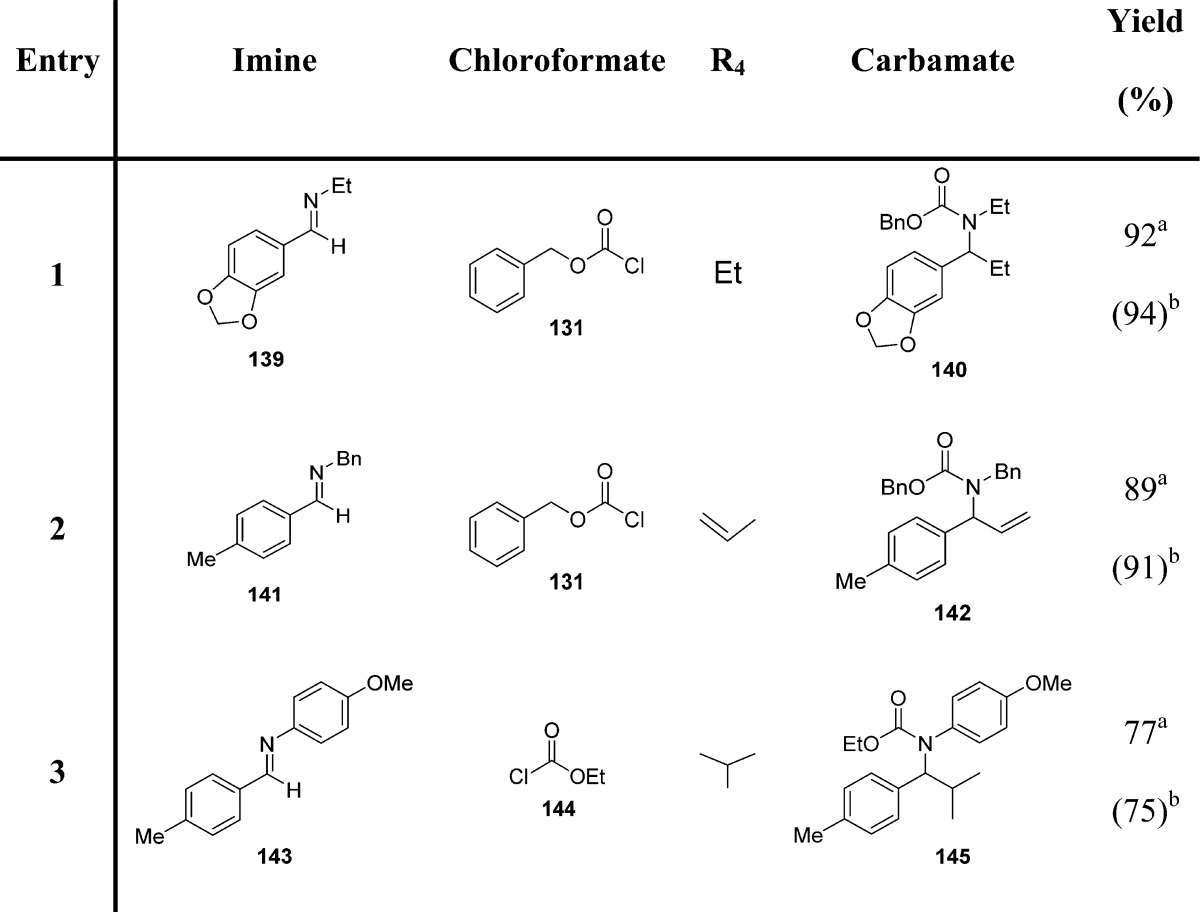
Yields refer to the use of triorganoindium reagents.
Yields refer to the use of tetraorganoindates.
Since organoindium reagents readily transfer their organic groups to an imine carbon, only one-third of an equivalent is required, and the only byproduct is represented by indium trichloride. Tetraorganoindium reagents can also be employed in a similar fashion for transferring all four organic groups. Therefore, one-fourth of an equivalent of indium is necessary for their reaction with imines. Copper(I) chloride (10%) was found to be the most efficient catalyst.
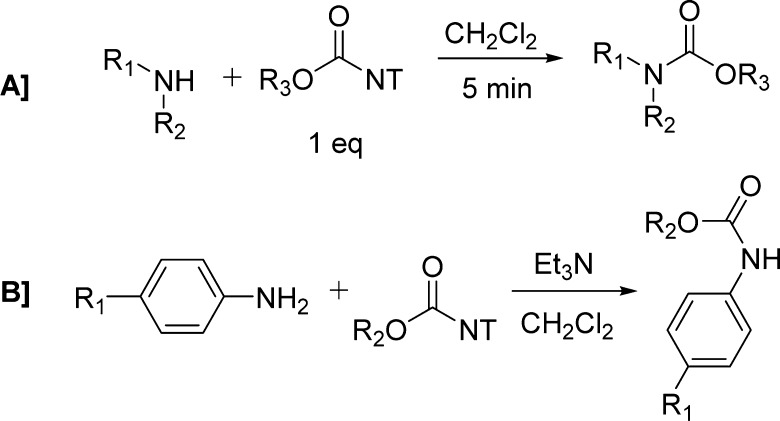
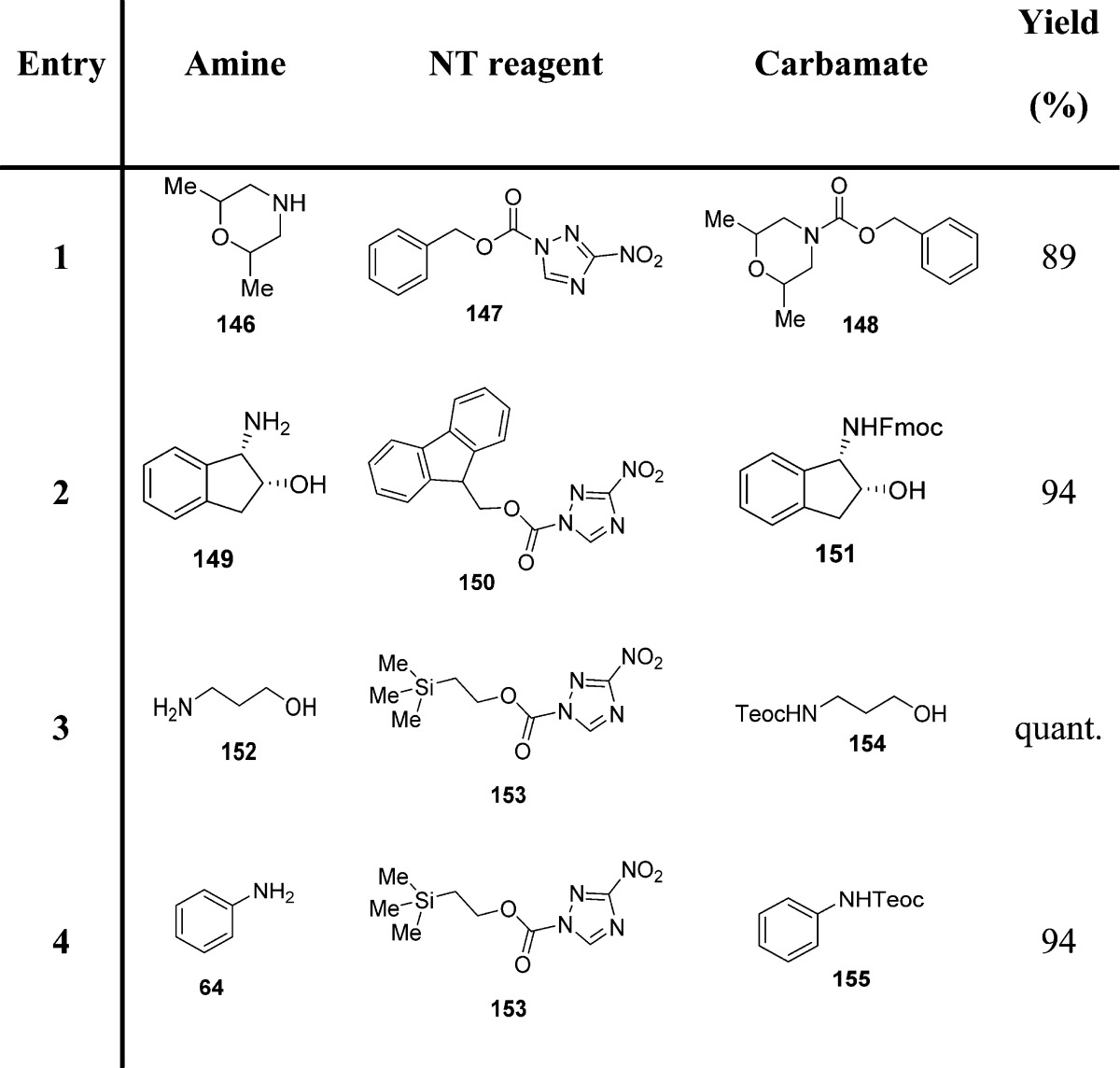
Sodeoka and colleagues reported the use of 1-alkoxycarbonyl-3-nitro-1,2,4-triazole reagents as useful intermediates for the preparation of carbamates (Scheme 14).121 To achieve a rapid and clean reaction, the features of the leaving group have a key role. An ideal leaving group should have a highly electron-withdrawing element in order to increase the electrophilicity of the carbonyl carbon, and the nucleophilicity should be low to avoid side reactions. It should also be easily separated from the reaction product. 3-Nitro-1,2,4-triazole (NT),120 although showing nucleophilicity, could be easily removed from the reaction due to its insolubility in dichloromethane or chloroform.
Scheme 14. Carbamate Synthesis Employing 1-Alkoxycarbonyl-3-nitro-1,2,4-triazole Reagents.
NT-based reagents have a series of benefits such as high stability, since they can be stored for long periods without decomposition. Reactions of these NT reagents with primary and secondary amines proceeded quickly to give the corresponding carbamates in >95% yield (Scheme 14A and Table 17). In contrast to aliphatic amines, aromatic amines were less reactive. However, the addition of triethylamine was found to be effective in promoting the reactions (Scheme 14B and Table 17).121
Table 17. Carbamates from NT-Based Reagents and Amines or Anilines121.
The reductive carbonylation of aromatic nitro compounds to the corresponding carbamates has remained a subject of great interest both from mechanistic and application standpoints (Scheme 15). In this section, we will briefly mention the methodologies involving the use of an alcohol, although other procedures employing chloroformates have also been recently reported.122,123
Scheme 15. Carbamate Preparation by Reductive Carbonylation of Aromatic Nitro Compounds.

Cheng and collaborators report the use of Ru(CO)4– and Ru3(CO)12 complexes for the catalysis of this reaction and highlighted the key effect of alcohol on the selectivity of carbamates (Table 18).124 The results clearly indicate that low selectivity of carbamate is closely related to the ability of the alcohol to reduce nitroarenes to amino derivatives. Therefore, the employment of an alcohol that cannot reduce nitroarene greatly increases the selectivity of carbamate. Later, the binuclear rhodium complex [(Ph3P)4Rh2(μ-OH)2]·2C6H6 was employed as an effective catalyst for the reductive carbonylation of nitrobenzenes to carbamate esters (Table 18).125 Palladium-based catalysts have also been explored (Table 18).126−128
Table 18. Carbamates from Reductive Carbonylation of Nitro Compounds.
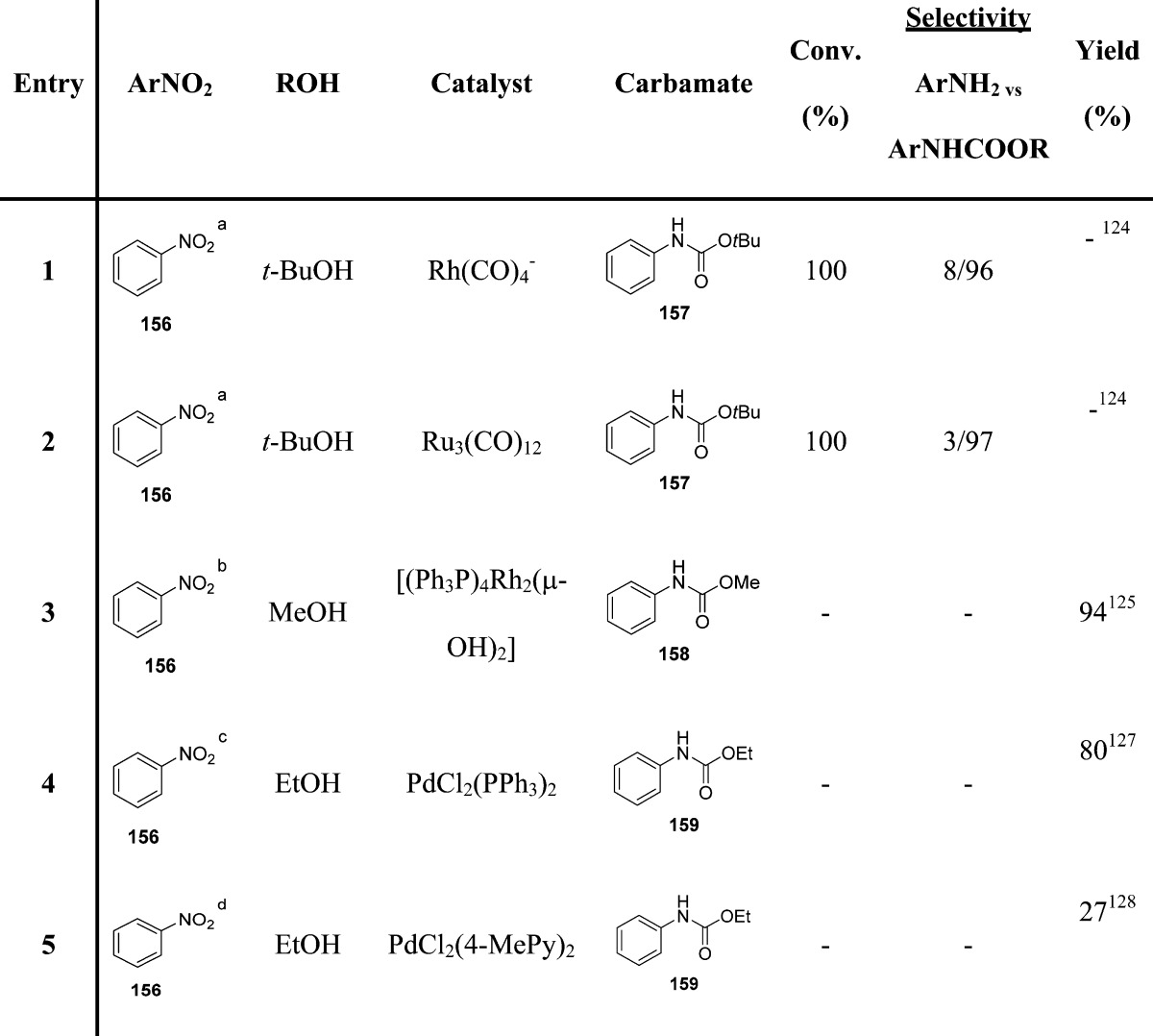
Rh(CO)4(PPN) or Ru(CO)12 0.2 mmol, alcohol, 30 mL, PhNO2 (10.0 mmol), CO, 400 psi, 140 °C.
PhNO2 (2 mmol), [(Ph3P)4Rh2(μ-OH)2]·2C6H6 (0.01 mmol), 2,2′-bipyridyl (0.2 mmol) and alcohol (30 mmol) in dry benzene (12 mL), CO 1000 psi, 180 °C.
PhNO2 (0.10 mol), ethanol (0.17 mol), 0.046 g PdCl2(PPh3)2, CO, 425 psi, 180 °C.
PhNO2 (27 mmol), ethanol (20 mL), 180 °C, CO = 580 psi; Py = pyridine.
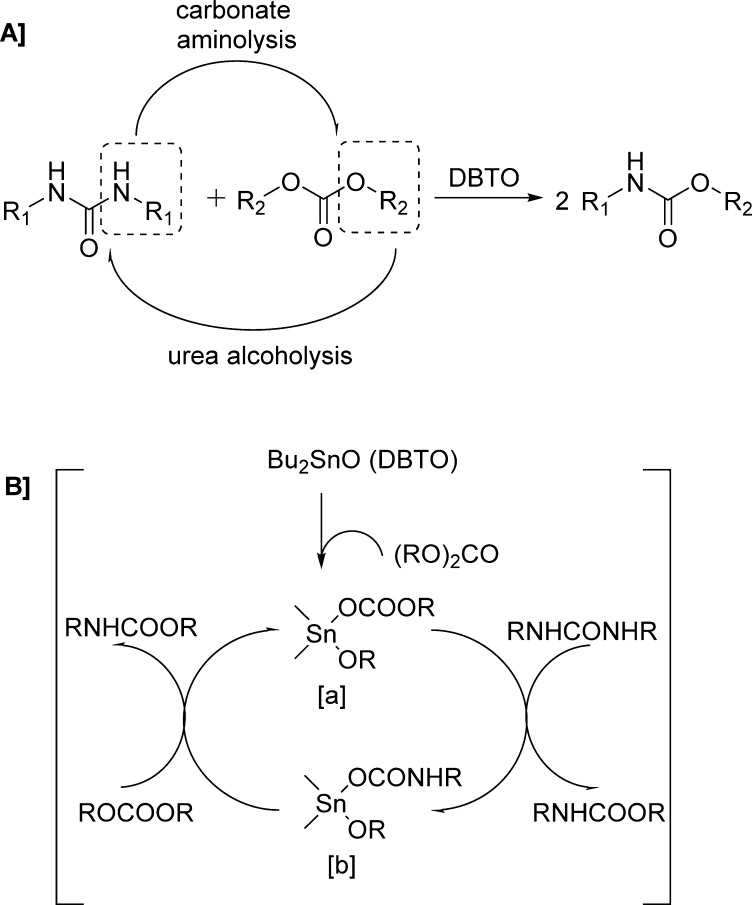
Carbamate synthesis via transfunctionalization of substituted ureas and carbonates in the presence of di-n-butyltin oxide (DBTO) as the catalyst was reported by Chaudhari and colleagues (Scheme 16A and Table 19).129
Scheme 16. Carbamate Synthesis via Transfunctionalization of Substituted Ureas and Carbonates in the Presence of DBTO.
Table 19. Carbamates Formed via Transfunctionalization of Substituted Ureas and Carbonates Using DBTO Catalyst129.

The carbonate reactivity pattern seems to be driven by the leaving group ability of the alkoxides and phenoxide to form the carbamate observed in aminolysis of carbonates. It has been shown that basicity of reacting urea plays a vital role in the catalytic activity of this reaction. Indeed, aliphatic ureas show higher reactivity compared to aromatic ureas due to their higher basicity. The basic DBTO is supposed to work as a nucleophile by attacking the carbonyl carbon of the carbonate, thus generating the catalytically active species dibutyl alkoxy carbonato tin [a].130 As shown, species [a] interacts with substituted urea to eliminate one molecule of carbamate, forming dibutyl alkoxy carbamato tin [b].131 A further reaction of species [b] with a carbonate results in the formation of one more molecule of carbamate with regeneration of the active species [a] (Scheme 16B).
Use of dialkyl carbonates as environmentally friendly and nontoxic phosgene substitutes in alkoxycarbonylation reactions has also been exploited by Porco et al. (Scheme 17).132
Scheme 17. Zr(IV)-Catalyzed Carbonate–Carbamate Exchange.
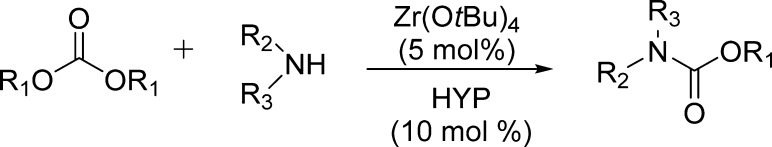
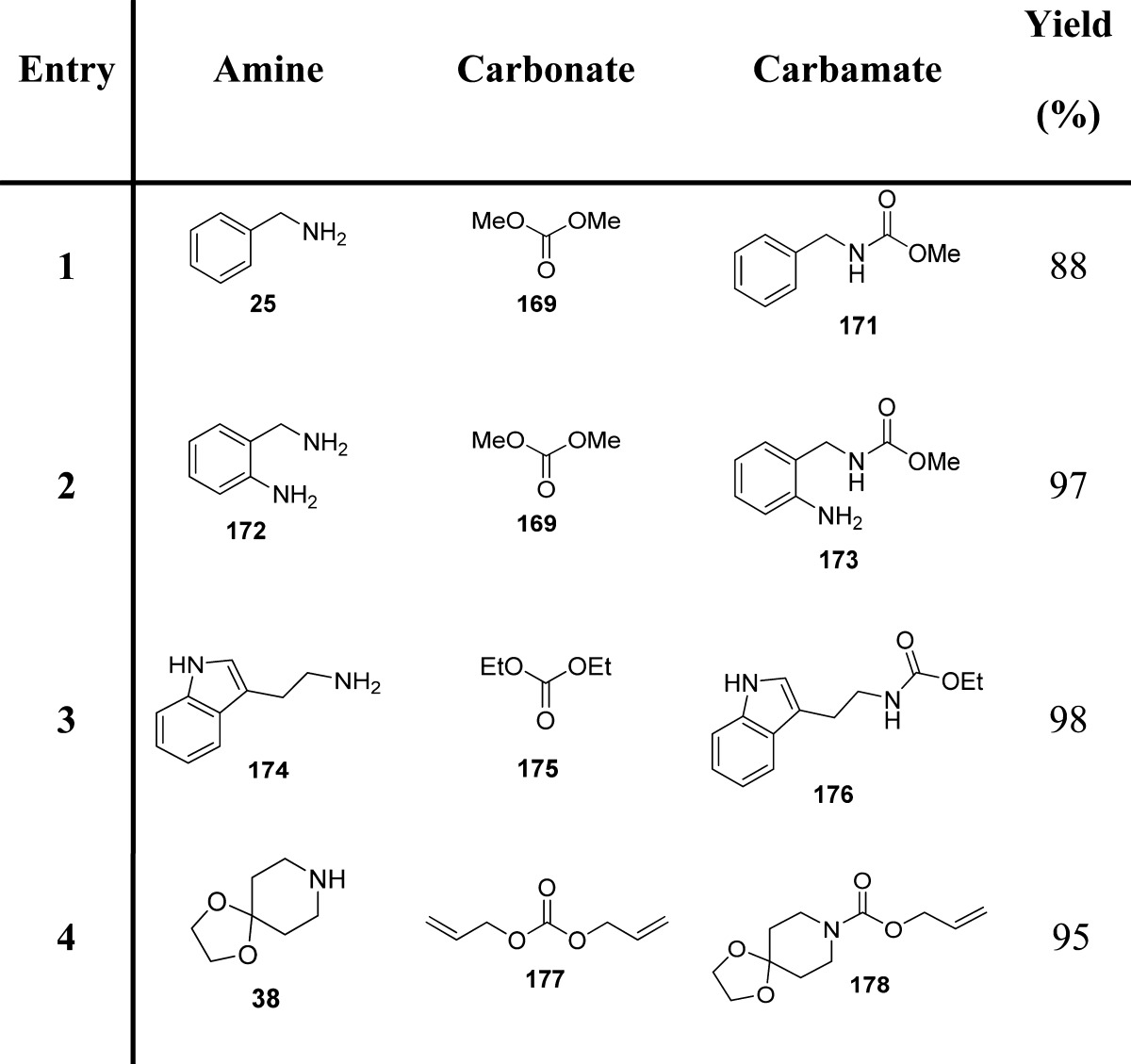
Particularly, the authors examined the scope of Zr(IV)-catalyzed carbonate–carbamate exchange processes to prepare carbamates from dialkyl carbonates employing 2-hydroxypyridine (HYP) as a catalytic additive (Table 20).
Table 20. Carbamates Formed via Zr(IV)-Catalyzed Exchange Process132.
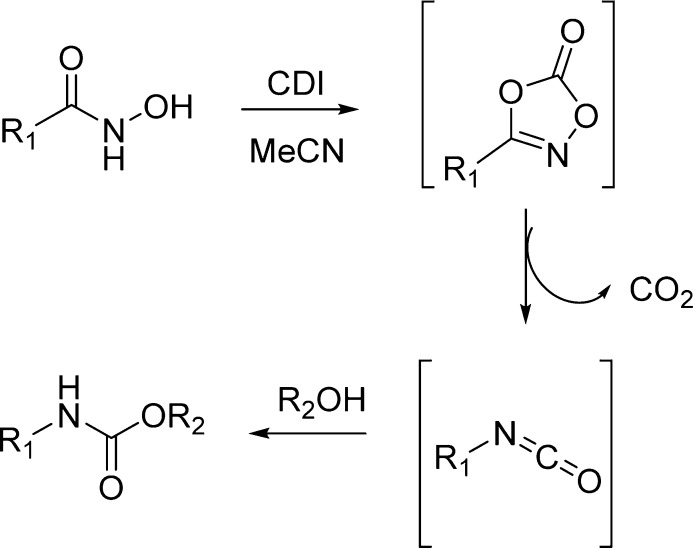
Recently, Padiya and co-workers reported a useful method for preparing carbamates in an aqueous media (Scheme 18).133
Scheme 18. Carbamates Synthesis in Aqueous Media by the Use of CDI.
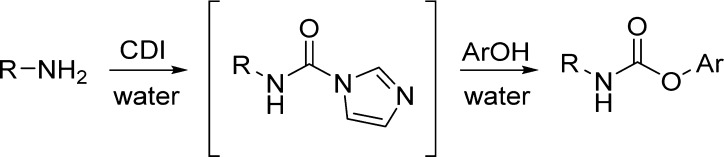
Interestingly, they found that 1,1′-carbonyldiimidazole (CDI), although unstable in water, rapidly reacts in aqueous media with amine to give good yields of the corresponding N-substituted carbonylimidazolide. Carbonylimidazolide derived from the primary amine reacts in situ with a nucleophile such as phenol, providing the corresponding carbamate. The product precipitates out from the reaction mixture and can be obtained in high purity by filtration, making the method simple and scalable (Table 21).133
Table 21. Carbamates from in Situ Generation of Carbonylimidazole in Water133.
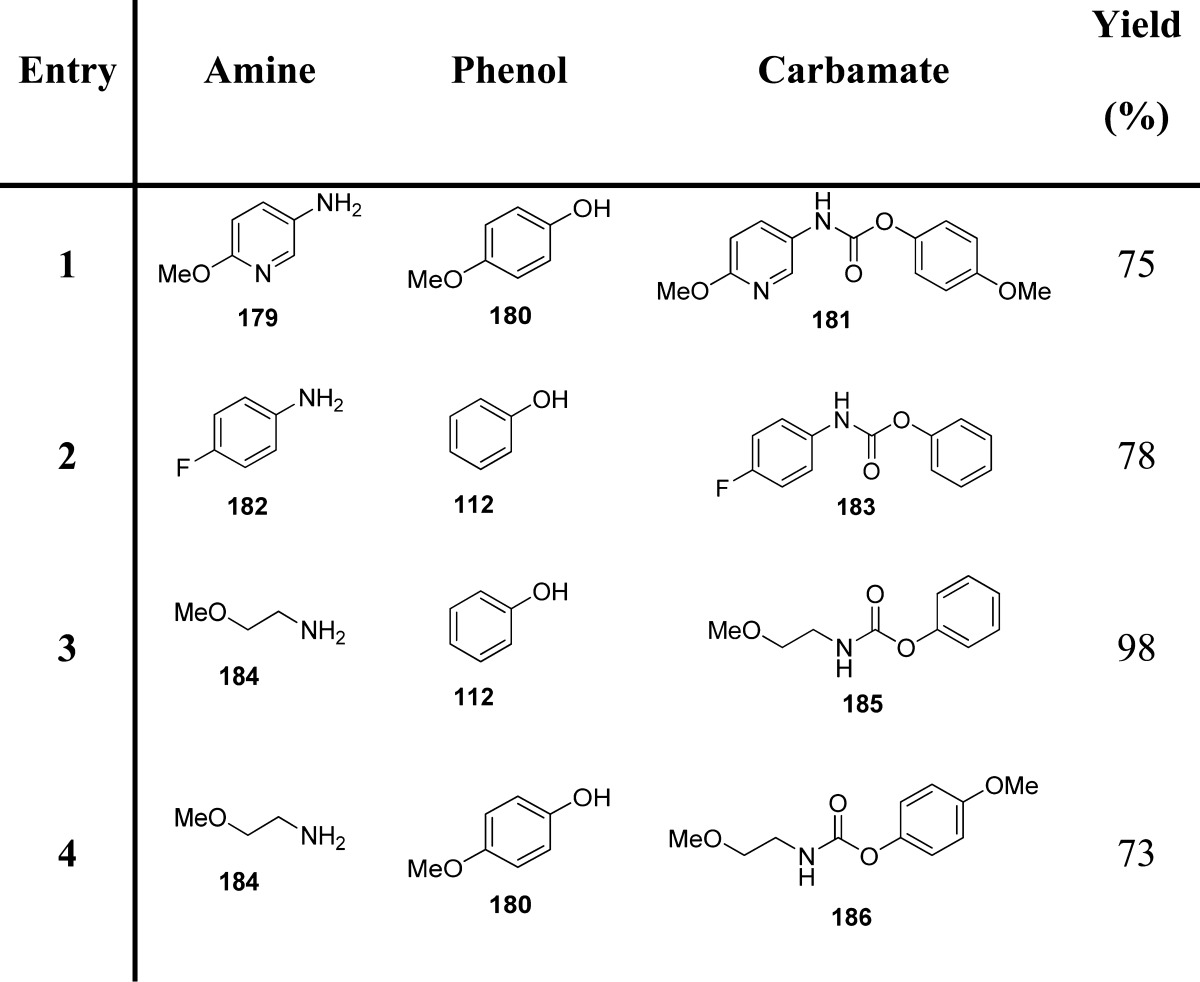
CDI was also found to mediate the Lossen rearrangement, which occurs in the transformation of an activated hydroxamic acid into the corresponding isocyanate (Scheme 19).134
Scheme 19. CDI-Mediated Lossen Rearrangement for Carbamate Synthesis.
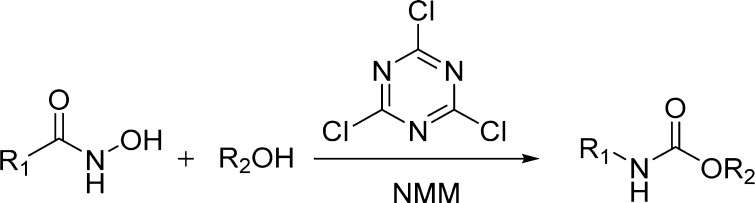
The proposed methodology is experimentally efficient and mild, being characterized by imidazole and CO2 as the only stoichiometric byproducts. This method is a green and unconventional alternative to the Curtius and Hofmann rearrangements (Table 22).135 Another method based on the Lossen rearrangement was recently proposed.136 The methodology envisaged the reaction of a hydroxamic acid with an alcohol, promoted by 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride; TCT) in the presence of an excess of N-methyl morpholine (NMM) (Scheme 20 and Table 22).
Table 22. Carbamates from CDI- and TCT-Mediated Lossen Rearrangement135,136.
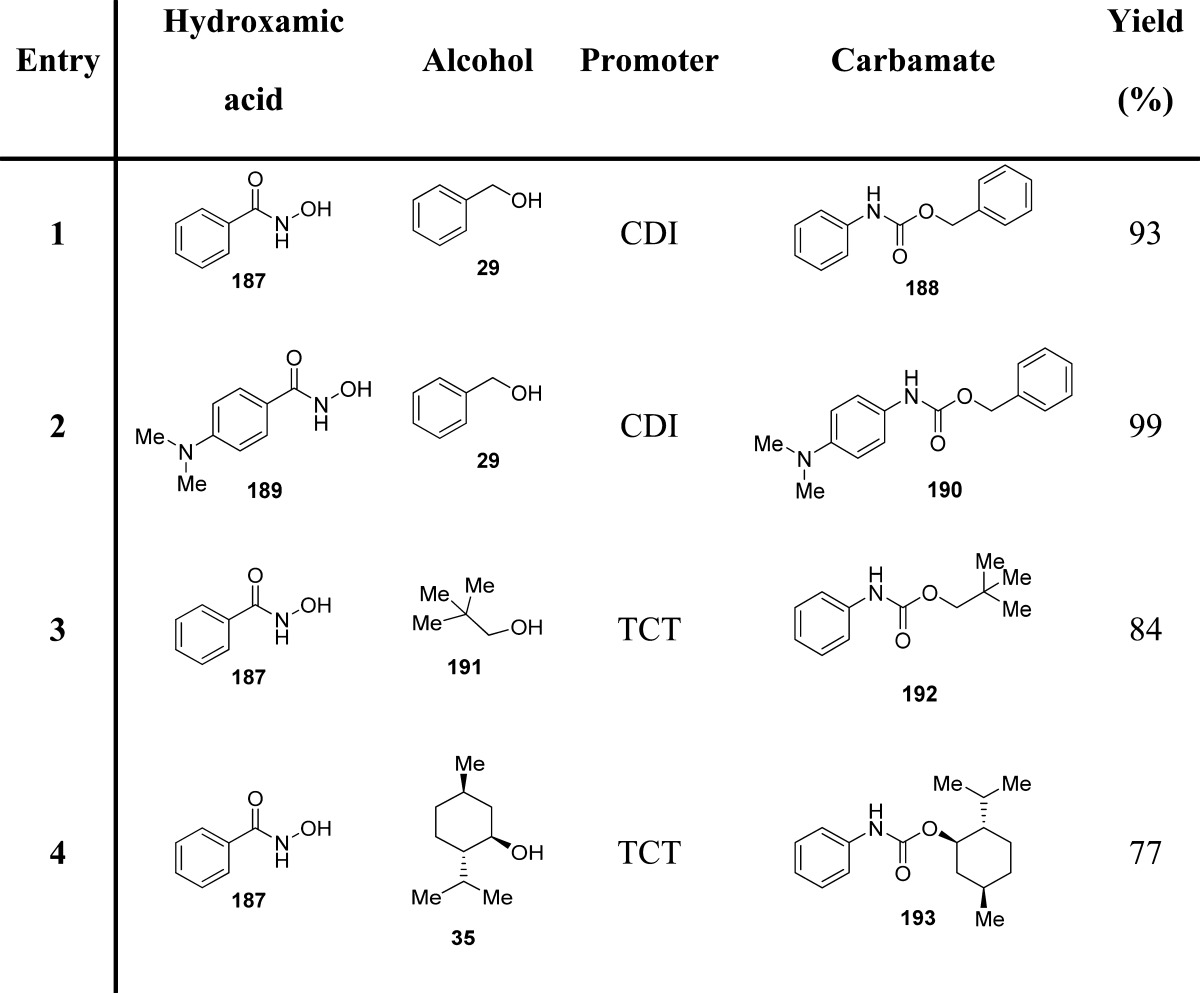
Scheme 20. TCT-Mediated Lossen Rearrangement for Carbamate Synthesis.
4. Carbamates with Clinical Potential
Carbamates are inherent to many FDA approved drugs. This structural motif is also a key functionality in numerous medicinal agents with clinical potential. In this section, a series of therapeutic carbamates with a variety of applications is outlined.
4.1. Miscellaneous Carbamates with Clinical Relevance
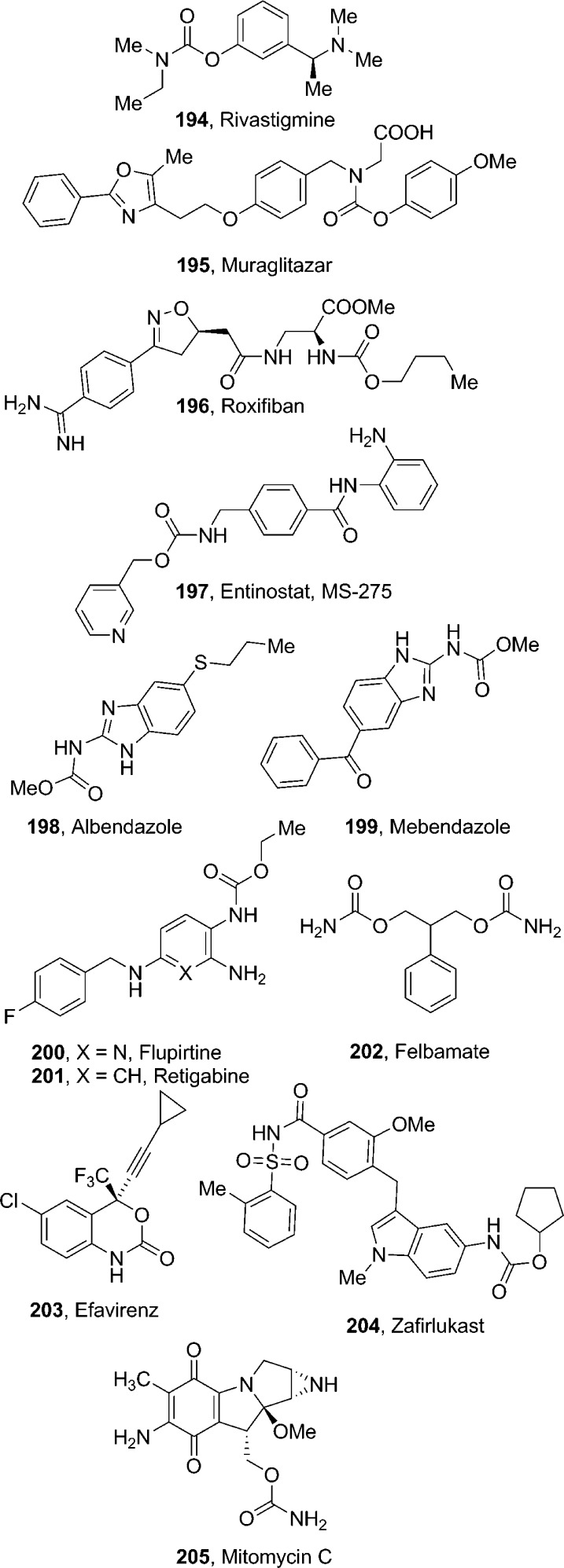
4.1.1. Rivastigmine
Rivastigmine (194, Figure 7) tartrate (Exelon, Novartis Pharma) is a carbamate derivative that reversibly inhibits the metabolism of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) preferentially in the central nervous system (CNS). It is used for the treatment of mild-to-moderate Alzheimer’s disease (AD) dementia and dementia due to Parkinson’s disease.137,138 The drug can be administered orally or via a transdermal patch. The transdermal patch reduces side effects such as nausea and vomiting. Rivastigmine undergoes extensive metabolism by ChE-mediated hydrolysis to the decarbamylated metabolite, without involvement of the major cytochrome P450 (CYP450) isozymes. The metabolite may undergo N-demethylation as well as conjugation. The pharmacokinetic half-life of rivastigmine in AD patients is around 1.5 h. When given orally, rivastigmine is well-absorbed, with a bioavailability of about 40% administered as a 3 mg dose.137,139
Figure 7.
Carbamates with clinical potential.
4.1.2. Muraglitazar
Muraglitazar (195) contains a carbamate functionality. It is a potent, novel nonthiazolidindione peroxisome proliferator-activated receptor dual agonist (PPARα/γ) that demonstrated highly efficacious glucose and lipid lowering activities in vivo, along with an excellent ADME profile.140 In a double-blind randomized clinical trial, muraglitazar resulted in a statistically significant improvement in plasma triglyceride, HDL cholesterol, apoB, and non-HDL cholesterol concentrations at week 12. Muraglitazar reduced triglyceride concentrations to a larger extent than did pioglitazone, regardless of baseline triglyceride levels. Muraglitazar and pioglitazone treatment was associated with slight (3–4%) increases in LDL cholesterol. However, muraglitazar development was discontinued due to major adverse cardiovascular side effects.141
4.1.3. Roxifiban
Roxifiban (196) is a carbamate derivative with a methyl ester prodrug. It is a potent, nonpeptide antagonist of the glycoprotein IIb/IIIa receptor.142,143 The free acid resulting from roxifiban hydrolysis blocks the binding of fibrinogen to the receptor, thereby inhibiting platelet aggregation and providing a mechanism for antithrombotic therapy. However, clinical development of roxifiban was discontinued in October 2001.
4.1.4. Entinostat
Entinostat (197, MS-275) contains a pyridylmethyl carbamate functionality.144 It is undergoing clinical trials for the treatment of various cancers. Entinostat preferentially inhibits HDAC1 (IC50 = 300 nM) over HDAC3 (IC50 = 8 μM) and is reported to have no inhibitory activity toward HDAC8 (IC50 > 100 μM). This drug induces cyclin-dependent kinase inhibitor 1A (p21/CIP1/WAF1), thereby slowing cell growth, differentiation, and tumor development in vivo. Recent studies suggest that 197 may be particularly useful as an antineoplastic agent when combined with other drugs like adriamycin.144−146
4.1.5. Albendazole and Mebendazole
Albendazole (198, Albenza, Teva Pharmaceuticals) is a broad-spectrum anthelmintic carbamate drug. It undergoes rapid hepatic oxidation by liver microsomal enzymes, producing the active metabolite albendazole sulfoxide, which is then oxidized to the inactive metabolites albendazole sulfone and albendazole-2-amino sulfone.147
Mebendazole (199) is a methyl carbamate derivative showing broad-spectrum anthelmintic properties. It demonstrated efficacy in the oral treatment of ascariasis, uncinariasis, oxyuriasis, and trichuriasis. Like other benzimidazole anthelmintics, mebendazole’s primary mechanism of action is consistent with tubulin binding.148 Mebendazole was discontinued in 2011.149
4.1.6. Flupirtine and Retigabine
Flupirtine (200) and retigabine (201) are ethyl carbamate derivatives. Flupirtine is a centrally acting nonopioid analgesic150 that was identified within an antiepileptic drug discovery program by the U.S. National Institutes of Health. The doses used in a small clinical trial exceeded those established for analgesic activity.151 On the basis of this data, subsequent structural optimization resulted in retigabine.152 Retigabine has anticonvulsant properties that appear to be mediated by opening or activating neuronal voltage-gated potassium channels. Flupirtine showed N-methyl-d-aspartate (NMDA) receptor antagonist properties.
4.1.7. Felbamate
Felbamate (202, Felbatol, Meda Pharmaceuticals) is an alkyl carbamate derivative. It is an antiepileptic drug. The mechanism of action of felbamate involves a dual mechanism involving inhibition of N-methyl-d-aspartate (NMDA) receptor response and positive modulation of γ-amino butyric acid subtype A (GABAA) receptor, thus decreasing neuronal excitation.153 Felbamate is rapidly absorbed (tmax = 2–6 h) with an oral bioavailability > 90%.154 Felbamate undergoes moderate metabolism via CYP3A4 and CYP2E1 isoenzymes, which are amenable to inhibition and induction effects.155,156 The clinical use of felbamate has declined in recent years due to its serious adverse side effects.
4.1.8. Efavirenz
Efavirenz (203, Sustiva or Stocrin, Bristol-Myers Squibb) is a cyclic carbamate derivative. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). The drug is used as part of highly active antiretroviral therapy (HAART).157,158 However, its use is associated with variable treatment response and adverse effects, in most part because of the large differences in pharmacokinetics.159 CYP2B6 is the main enzyme catalyzing the major clearance mechanism of efavirenz (8-hydroxylation to 8-hydroxyefavirenz) in vivo.160,161
4.1.9. Zafirlukast
Zafirlukast (204, Accolate, AstraZeneca) is a cyclopentyl N-aryl carbamate derivative. It is a selective and competitive receptor antagonist of the cysteinyl leukotrienes D-4 and E-4, which is indicated for the prophylaxis and treatment of mild-to-moderate persistent and chronic asthma.162 Both O → CH2 and O → NH bioisosteric analogues of Zafirlukast were found to be potent. The carbamate moiety present in zafirlukast provided an excellent in vitro and in vivo profile and high oral bioavailability.163 Zafirlukast undergoes hepatic metabolism, where hydroxylation by cytochrome CYP2C9 is the major biotransformation pathway. The metabolites of zafirlukast do not significantly contribute to its overall activity.164
4.1.10. Mitomycin C
Mitomycin C (205, MMC, Mutamycin) is a complex carbamate derivative. It is an antitumor antibiotic that was identified in the 1950s in fermentation cultures of the Gram-negative bacteria Streptomyces caespitosus.165 MMC is a site-specific, nondistorting DNA cross-linking agent.166,167 However, recent reports suggest that DNA may not be the primary target of the drug. In particular, interaction of MMC with rRNA and subsequent inhibition of protein translation has been proposed.168 MMC is customarily used as a chemotherapeutic agent in the treatment of several types of cancer, such as bladder, colon, and breast cancers.169
4.2. Therapeutic Carbamates as HIV Protease Inhibitors
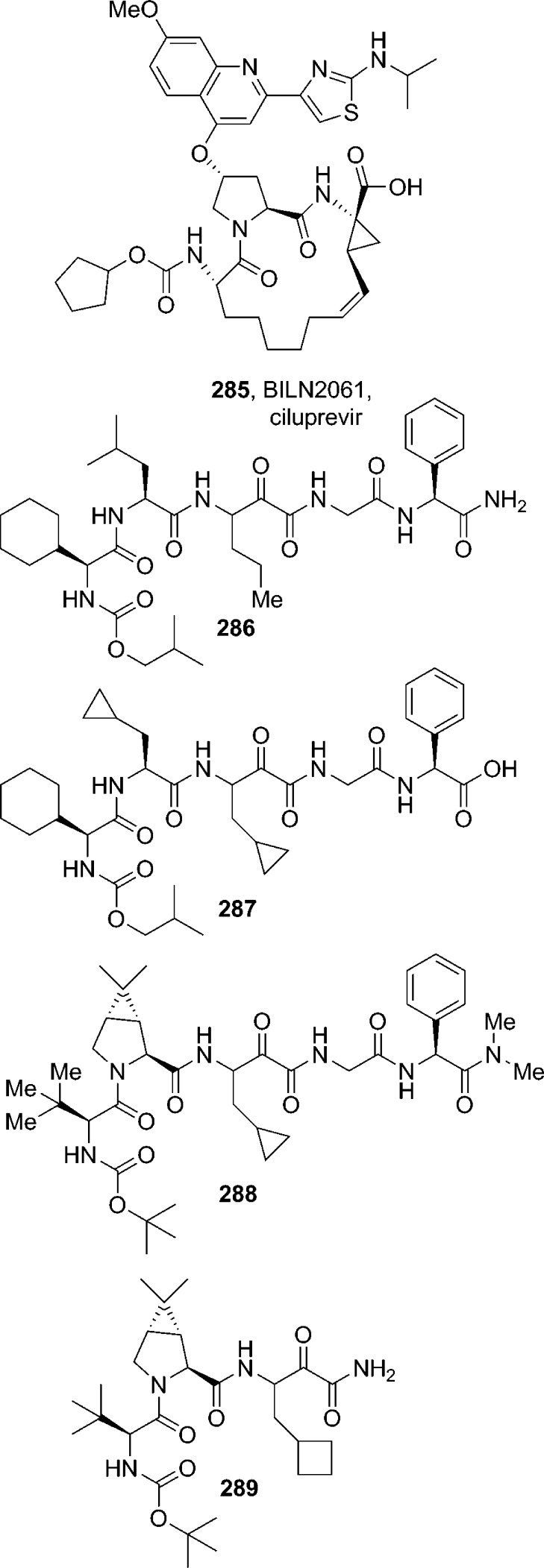
HIV protease is an aspartic acid protease responsible for the cleavage of the Gag–pol polyprotein into functional proteins essential for the production of infections progeny virus. Inactivation of HIV-1 protease either by site-directed mutagenesis or by chemical inhibition results in the formation of immature, noninfections virus particles. As a consequence, HIV-1 protease is an attractive target in antiviral therapy. HIV protease is a C2-symmetric, 198-amino acid homodimeric aspartyl protease in which each protein subunit contributes one Asp-Thr-Gly motif to the single active site.170 The X-ray crystallographic analysis of the native protein and subsequent protein–ligand complexes and extensive research programs on other aspartyl proteases, including human renin,171 provided a path toward accelerated drug discovery programs targeting HIV protease.172−174 A number of FDA-approved HIV protease inhibitor drugs contain an important carbamate functionality. In this section, currently approved protease inhibitor drugs are discussed (Figure 8).
Figure 8.
Representative carbamate-containing therapeutic HIV protease inhibitors.
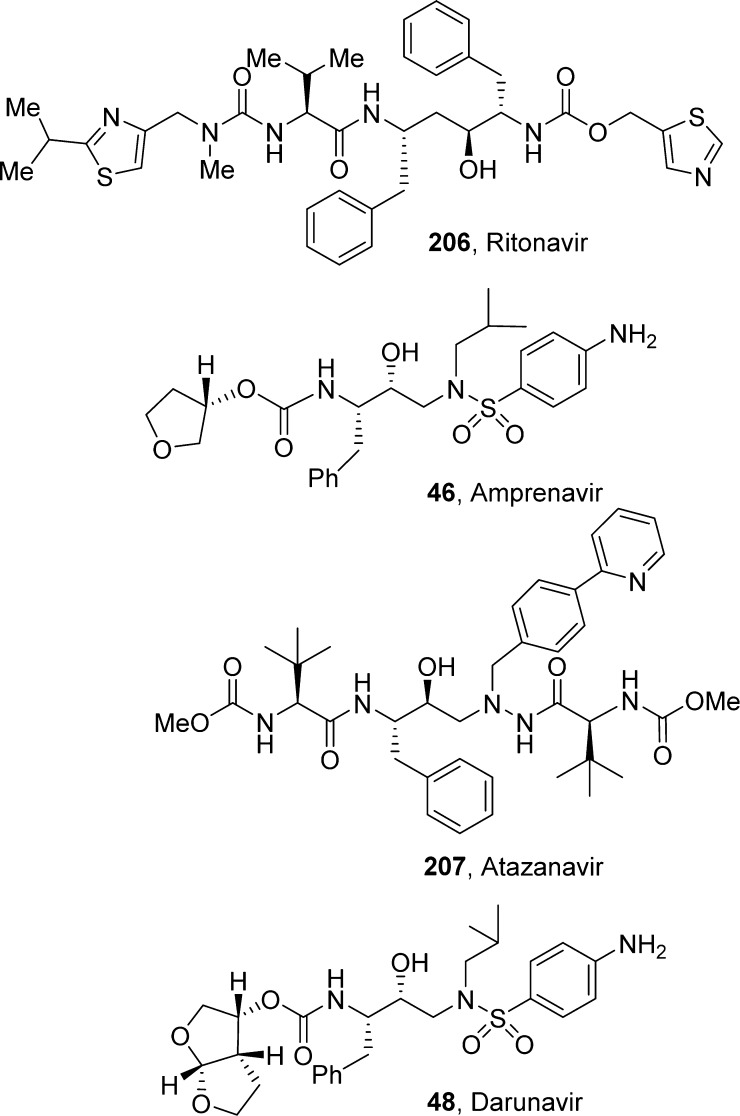
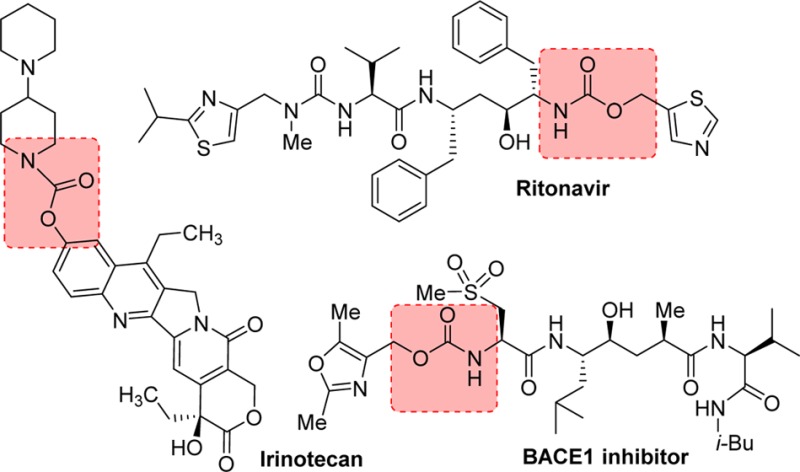
4.2.1. Ritonavir
Ritonavir (206, Norvir, ABT-538, A-84538, AbbVie, Inc.) structure possesses a thiazolyl methyl carbamate functionality. It is a peptidomimetic inhibitor of both the HIV-1 and HIV-2 proteases and was approved by the FDA in March 1996.175 This first-generation protease inhibitor was developed at Abbott Laboratories. The discovery of ritonavir was based on studies with C2-symmetric diamine subunits. Ritonavir showed EC50 of 0.025 μM, bioavailability of 78%, and a plasma half-life of 1.2 h. Ritonavir has a high molecular weight; however, it showed excellent pharmacokinetic properties. This is possibly due to the increased stability of the thiazole groups to oxidative metabolism and also due to its effect on cytochrome P450 oxidative enzymes. Ritonavir is a type II heme ligand that fits into the CYP3A4 active site cavity and irreversibly binds to the heme iron via the thiazole nitrogen.176 Inhibiting CYP3A4, ritonavir increases plasma concentrations of other anti-HIV drugs oxidized by CYP3A4, thereby improving their clinical efficacy.
4.2.2. Amprenavir
Amprenavir (46, Agenerase, VX-478, GlaxoSmithKline, Vertex) is a tetrahydrofuranyl carbamate derivative. It was approved by the FDA in April 1999. Amprenavir was identified as a potent, orally bioavailable HIV-1 protease inhibitor with a low molecular weight and a mean IC50 of 12 nM.177 It is marketed with a twice-a-day dosing format. Amprenavir structure bears a stereochemically defined tetrahydrofuranylcarbamate engaging in a weak backbone interaction with the protease.178,179In vitro and in vivo studies have shown that amprenavir is primarily metabolized by CYP3A4, and the two major metabolites result from oxidation of the tetrahydrofuran and aniline moieties.180
4.2.3. Atazanavir
Atazanavir (207, ATV, Reyataz (ATV sulfate), BMS-232632, Bristol-Myers Squibb) is a methyl carbamate derivative. It is a hydroxyethylene hydrazide-based second-generation HIV-protease inhibitor developed in the late 1990s and approved by the FDA in June 2003.181 ATV contains two methylcarbamate functionalities. It showed potent enzyme inhibitory activity (Ki = 2.66 nM), and its antiviral IC50 in HIVMN-infected MT-2 cells was 26 nM.182,183 ATV displayed excellent bioavailability. The favorable pharmacological profile for ATV raised the possibility of once-daily dosing.182,184
4.2.4. Darunavir
Darunavir (48, DRV TMC-114) possesses a structure-based designed bis-tetrahydrofuranyl (bis-THF) carbamate functionality. It is a new generation HIV-1 protease inhibitor with improved bioavailability, potency, and drug properties. DRV also maintains high potency against multidrug-resistant HIV-1 strains. The design of DRV originated from the backbone binding concept envisaging that an effective protease inhibitor maximizes rich networks of hydrogen-bonding interactions with the backbone atoms throughout the active site of the protease.185 The bis-THF moiety present in DRV was designed based on the X-ray structure of inhibitor-HIV-1 protease complexes. The bis-THF carbamate moiety of DRV was found to be essential for enzyme affinity (see Figure 14 for details). DRV demonstrated exceptional potency against both wild-type HIV isolates and a wide range of resistant variants.186,187 DRV received FDA approval in 2006 for the treatment of HIV/AIDS patients harboring multidrug-resistant HIV-1 variants. In 2008, DRV received full approval for the treatment of therapy-naive adults and children.188 DRV is metabolized by the isoenzyme CYP3A4.189,190 However, in the presence of a low dose of ritonavir, DRV exhibits very good pharmacokinetic properties in patients.191
Figure 14.
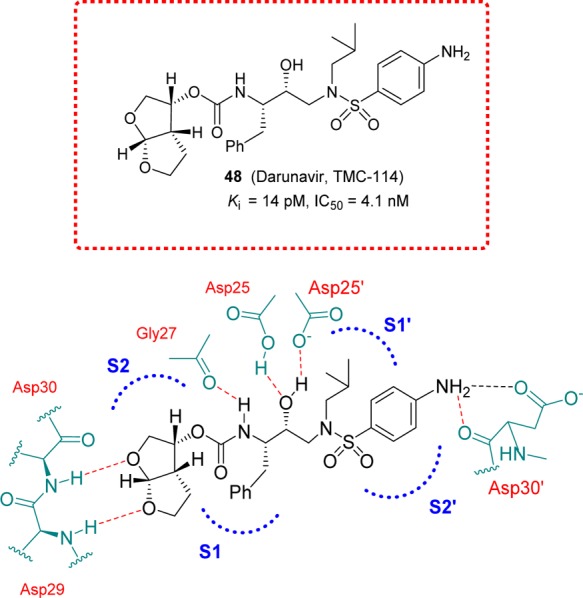
Darunavir and highlight of the X-ray structure of darunavir-bound HIV-1 protease showing the main interactions.
5. Carbamate Prodrugs and Their Metabolism
Prodrugs are chemically modified forms of the actual pharmacologically active drug that undergo in vivo transformation to release the active drug molecule. This is a well-established strategy to improve drug disposition properties (physicochemical, biopharmaceutical, or pharmacokinetic properties) of pharmacologically relevant compounds and thereby increase their drug-like profile.192,193 A prodrug strategy helps to overcome a variety of hurdles in drug formulation and delivery such as (i) poor oral absorption and aqueous solubility, (ii) poor lipid solubility, (iii) chemical instability, (iv) rapid presystemic metabolism, (v) toxicity and local irritation, and (vi) lack of site-selective delivery.193
A functional group on the parent drug may be used to form a chemical bond with the promoiety. Generally, the linker should be self-removing or cleavable so that the parent drug can be released spontaneously or under a certain triggering condition, such as the presence of an enzyme or a change in pH. The promoiety coupled to the parent drug provides the ability to improve the drug-like properties or overcome the barriers in delivering the drug to its target cells.194
Carbamates are the esters of carbamic acid, preferentially used in the design of prodrugs as a means of achieving first-pass and systemic hydrolytic stability. Carbamates are typically enzymatically more stable than the corresponding esters. They are, in general, more susceptible to hydrolysis than amides.195 Thus, bioconversion of carbamate prodrugs requires esterases for the release of the parent drug. Upon hydrolysis, carbamate esters release the parent phenol or alcohol drug and carbamic acid, which, due to its chemical instability, breaks down to the corresponding amine and carbon dioxide. Carbamates of primary amines can also fragment into isocyanates and alcohols on treatment with bases, a further potential pathway for metabolic degradation.192,195 The OH-catalyzed hydrolysis of these carbamate esters (R′-NHCO-OR) is strongly dependent on both the pKa of the proton on the leaving group (ROH) and the degree of substitution on the nitrogen of the carbamate ester.196 Since phenols have a lower pKa with respect to alcohols, carbamate esters of phenols are generally more chemically labile than those of alcohols. In the case of alcohols, both the N-monosubstituted and N,N-disubstituted carbamates are chemically stable toward hydrolysis. In phenols, N,N-disubstituted carbamates are chemically stable, whereas N-monosubstituted carbamates are the most labile toward chemical hydrolysis. Short-lived carbamates have also been used as prodrugs of heteroaromatic amines (e.g., capecitabine, 217) and amidines (lefradafiban (221), dabigatran).195
5.1. Alcohol and Phenol Carbamate Prodrugs
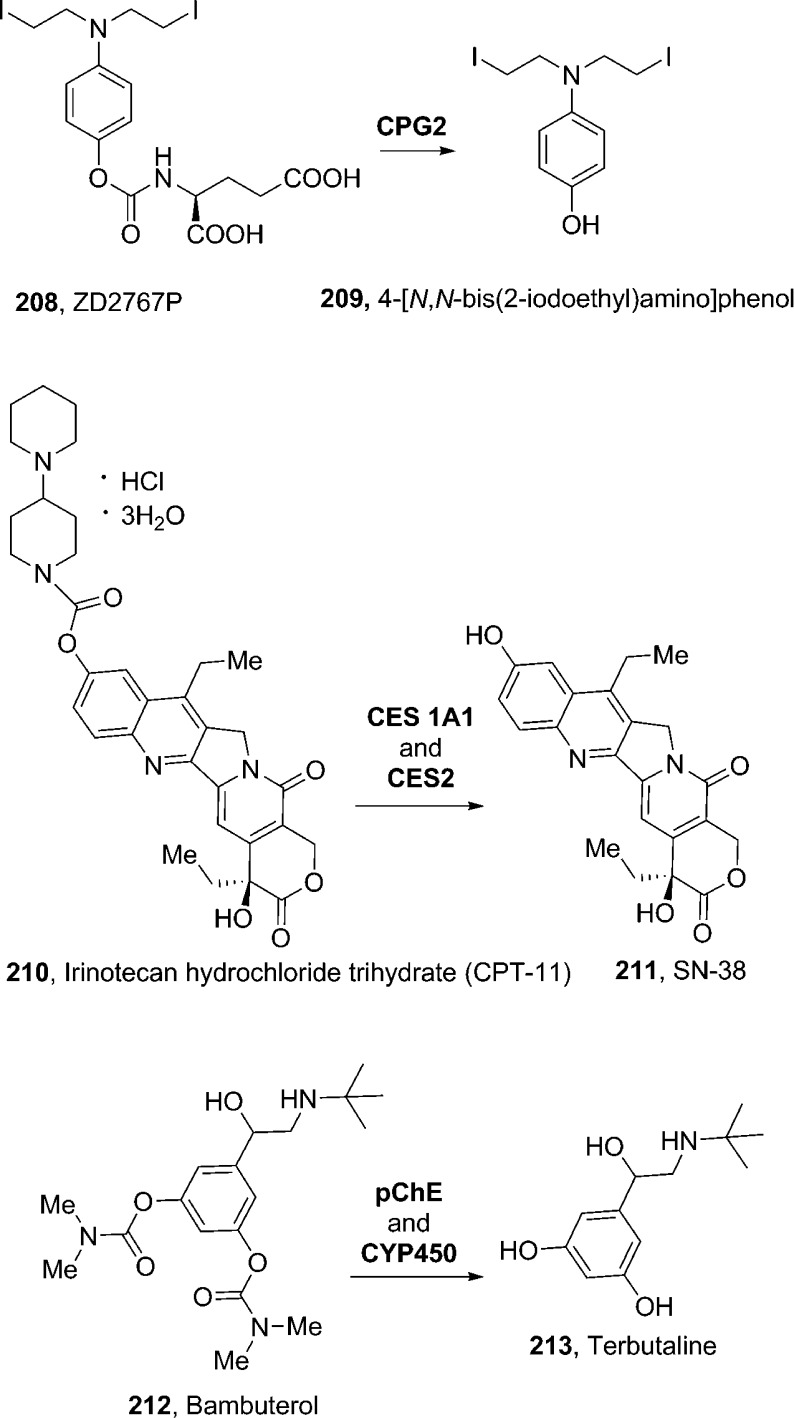
Most of the therapeutically relevant carbamate prodrugs have been designed as substrates of specific enzymes. Antibody-directed enzyme prodrug therapy (ADEPT)197,198 and gene-directed enzyme prodrug therapy (GDEPT)199 are new strategies for targeting tumors. Carboxypeptidase G2 (CPG2), an enzyme of bacterial origin, has been shown to catalyze the cleavage of an amide, carbamate, or urea linkage between glutamic acid and an aromatic group. On the basis of this specificity, a large number of prodrugs have been designed and synthesized for CPG2. As shown in Figure 9, the prodrug 208 (ZD2767P)200 is activated by hydrolysis at the carbamate bond by CPG2 to the corresponding potent di-iodophenol mustard (209).201208 was found to possess the best profile in terms of enzymatic kinetics, cytotoxicity, and in vivo efficacy. It was selected for clinical development. The half-life (t1/2) of the drug is approximately 2 min, which is enough for diffusion into the tumor cell from the local release site and to minimize peripheral toxicity.192,202
Figure 9.
Examples of phenol carbamate prodrugs and their metabolic activation.
Irinotecan was designed to deliver camptothecin as a predominant topoisomerase I inhibitor for anticancer therapy. Irinotecan hydrochloride salt 210 (CPT-11, Camptosar; Pfizer) is a parenteral aqueous soluble carbamate prodrug of antineoplastic topoisomerase I inhibitor 211 (SN-38, 7-ethyl-10-hydroxy-camptothecin). The potent antitumor activity of irinotecan is due to rapid formation of active metabolite 211in vivo (Figure 9). In this molecule, a dipiperidino ionizable promoiety is linked to the phenol functionality by a carbamate bond, thus improving the overall aqueous solubility.203−205 The bioconversion back to 211 occurs primarily by human liver microsomal carboxylesterases, CES 1A1 and CES2, which release the ionizable piperidinopiperidine promoiety and 211, the active form of the drug.205
Beyond minimizing the rate of enzymatic hydrolysis of its prodrug, sustained drug action can also be provided by decreasing the rate of drug metabolism. This is the case of bambuterol (212, Bambec, AstraZeneca), a bis-dimethyl carbamate prodrug of the β2-agonist terbutaline (213), which is used as a bronchodilator in the treatment of asthma. The phenolic moiety of terbutaline is subjected to rapid presystemic metabolism. In bambuterol, protection of this functionality also avoids first-pass intestinal and hepatic metabolism. This prodrug is inactive, however, after oral administration; it is slowly converted to terbutaline, mainly outside the lungs, by a series of hydrolysis and oxidation reactions (maily catalyzed by plasma cholinesterase, pChE, and by CYP450, Figure 9).206,207 This allows a once-daily bambuterol treatment with respect to the three daily terbutaline administrations.208
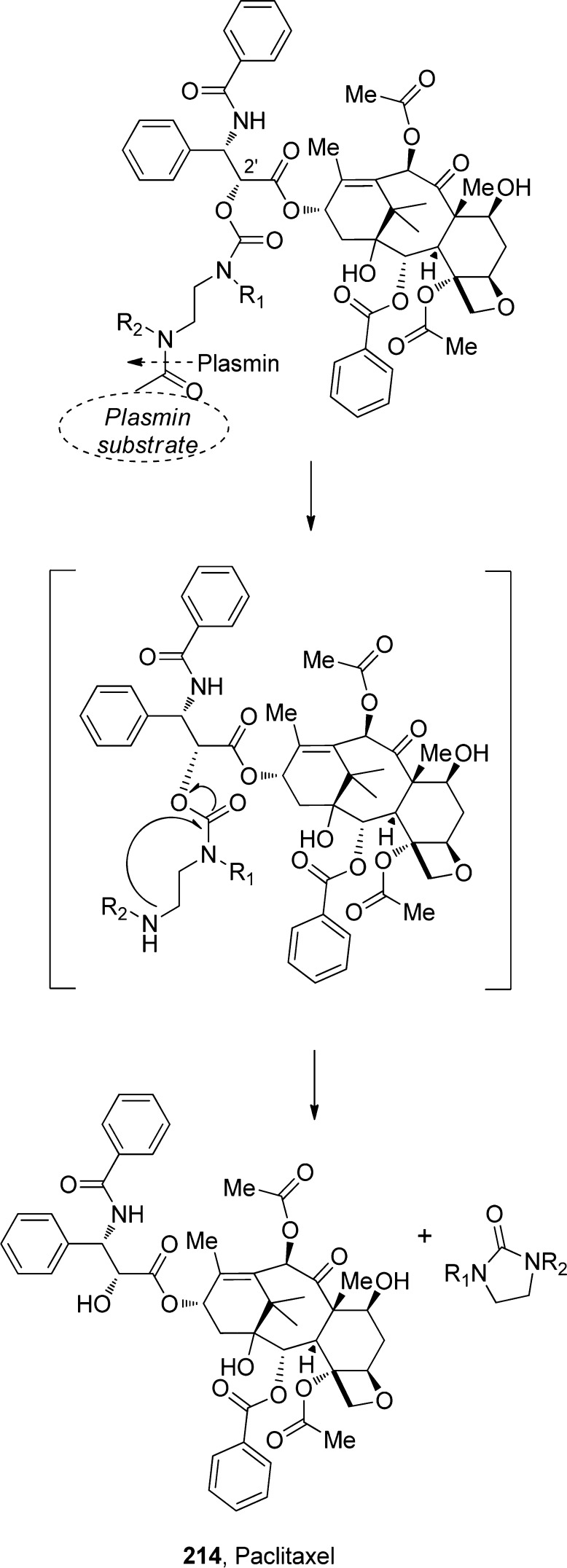
An N,N′-dimethyl ethylenediamine spacer, used for the evaluation of cyclization-elimination-based prodrugs of phenols209 and alcohols,210 has been used for the development of prodrugs as a part of the ADEPT activation strategy. When activated by a specific enzyme, the terminal amino group on the spacer activates and initiates an intramolecular cyclization reaction to eliminate a phenol211 or alcohol212 parent drug with parallel release of the cyclized spacer. In one such application, Scherren et al.213 explored paclitaxel-2′-carbamates. This is particularly interesting because a free 2′-hydroxyl group is important for biological activity. In general, carbamate linkages are more stable in vivo than esters and carbonates. Since the proteolytic active form of plasmin is located in the tumor, linking a cytotoxic drug to a plasmin substrate may result in tumor-selective delivery. On the basis of this rationale, following plasmin hydrolysis, the spacer is expected to undergo spontaneous cyclization to yield a cyclic urea derivative (imidazolidinone), thereby releasing paclitaxel (214), as illustrated in Scheme 21.192,214
Scheme 21. Plasmin Hydrolysis and Subsequent Spontaneous Cyclization of the N,N′-Dimethyl Ethylenediamine Spacer and Release of Paclitaxel.
5.2. Amine and Amidine Carbamate Prodrugs
The amine group is one of the most common functional groups in many approved drugs. Amines in drugs can cause physicochemical hurdles that have the potential to limit their safety and effective delivery to desired sites of action. Therefore, a variety of prodrugs of amines have been designed to overcome formulation and delivery barriers. The carbamate functionality has been utilized in many prodrug strategies designed for amines. Short-lived carbamates are also used as prodrugs of heteroaromatic amines and amidines.192
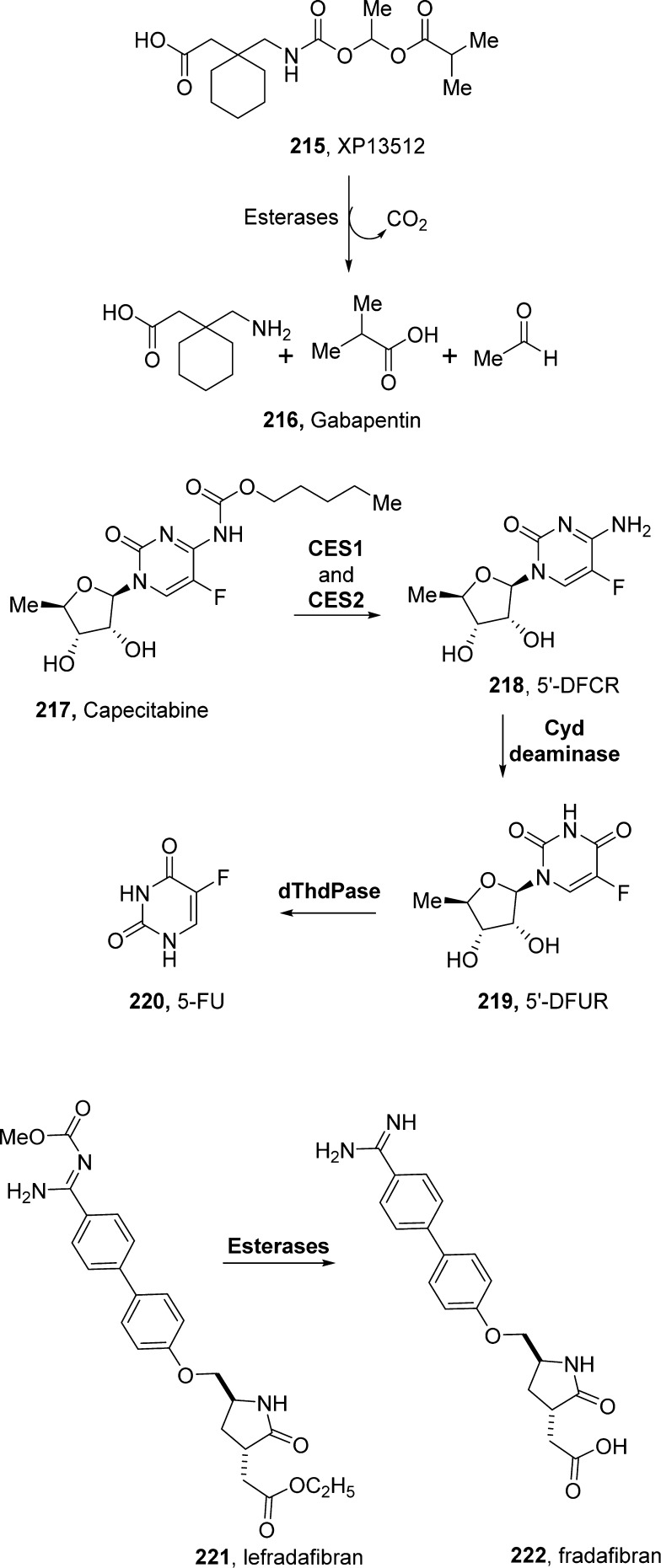
Gabapentin (216, Neurontin; Pfizer, Figure 10) is a structural analogue of γ-aminobutyric acid (GABA). It is marketed as an anticonvulsant and an analgesic agent. Gabapentin shows a number of limitations, including saturable absorption, high interpatient variability, lack of dose proportionality, and a short half-life. Gabapentin enacarbil (215, Horizant, previously known as XP13512) is a carbamate prodrug of gabapentin. The prodrug is benefited by a monocarboxylate transporter type 1 (MCT1). MCT1 is expressed in all segments of the colon and upper gastrointestinal tract. The prodrug also helps the sodium-dependent multivitamin transporter (SMV T), responsible for absorption of multiple essential nutrients.215,216 Following absorption via these pathways, the prodrug is rapidly converted to gabapentin by nonspecific esterases, mainly in enterocytes and to a lesser extent in the liver. During conversion to gabapentin, each molecule of 215 also generates carbon dioxide, acetaldehyde, and isobutyrate (Figure 10).217 The oral bioavailability of 215 was improved from 25 to 84% in monkeys. It showed dose-proportional gabapentin exposure in humans.193 In 2011, Xenoport received FDA approval (Horizant) for the treatment of moderate-to-severe restless legs syndrome. In 2012, Horizant was also approved for the management of postherpetic neuralgia (PHN) in adults.218
Figure 10.
Examples of amine and amidine prodrugs and their metabolic activation.
Capecitabine (217, Xeloda, Roche) was designed to achieve greater selectivity than its active form, 5-fluorouracil (220, 5-FU).219 It is an orally administered carbamate prodrug of 5-FU, belonging to the fluoropyrimidine carbamate class. It requires a cascade of three enzymes for the bioconversion to the active drug.220 As shown in Figure 10, the enzymatic bioconversion starts in the liver, where human carboxylesterases 1 and 2 (CES1 and CES2) cleave the carbamate ester bond.219 Intact capecitabine is absorbed in the intestine, and its bioconversion in the liver releases the parent drug. To some extent, its bioconversion proceeds in tumors, thus avoiding any systemic toxicity. In particular, the remaining transformations to 5-FU are catalyzed by cytidine deaminase and thymidine phosphorylase. The latter enzyme is highly enriched in tumors, thus providing selective release of 5-FU in cancer cells.220,221 The absorption of capecitabine is evident since 95% of an orally administered dose is recovered in urine and the Tmax of 5-FU is reached in approximately 1.5–2 h.193 Capecitabine is currently approved as a first line of therapy for colorectal and breast cancers and is also approved for use in combination with other anticancer drugs.192,222
Alkoxycarbonyl derivatives can serve as useful prodrugs for benzamidines. For example, the methoxycarbonyl methyl ester lefradafiban (221, BIBU104, Boehringer Ingelheim, Germany) is effectively converted to the active platelet aggregation inhibitor fradafiban (222, BIBU 52) after oral administration. This was revealed by monitoring the plasma concentrations of 222 and by ex vivo platelet aggregation studies. Lefradafiban is the orally active prodrug of fradafiban, a glycoprotein IIb/IIIa receptor antagonist.192 Esterases, but not CYP450-dependent enzymes, are involved in the conversion of lefradafiban to fradafiban in vivo (Figure 10).223
6. Cyclic Ether-Derived Carbamates as HIV-1 Protease Inhibitors
Over the years, we have developed a series of novel HIV-1 protease inhibitors incorporating cyclic ether-derived carbamates designed based on the X-ray structures of inhibitor-HIV-1 protease complexes.224,225 In this endeavor, we have specifically developed stereochemically defined cyclic ether templates, where the cyclic ether oxygen could effectively replace a peptide carbonyl oxygen. The advantage of such replacement is to reduce peptidic features and improve metabolic stability of compounds. These cyclic ligands have been incorporated as carbamate derivatives. The evolution of the carbamate structural template is shown in Figure 11. On the basis of the X-ray crystal structure of saquinavir (223)-bound HIV-1 protease, we first investigated 3-(R)-tetrahydrofuranylglycine so that the 3-(R)-THF ring oxygen would interact with the Asp30 NH, similar to the asparagine side chain carbonyl oxygen of saquinavir (compound 224).226−228 In an effort to reduce molecular weight, the P3 quinoline was removed, and the amide bond was replaced with a carbamate to provide inhibitor 225 with significant reduction of molecular weight (515 Da from 670 Da). The X-ray crystal structure of 225-bound HIV-1 protease revealed that the ring oxygen of the 3-(S)-tetrahydrofuran (3-(S)-THF) is within proximity to form a hydrogen bond with the Asp29 NH bond in the S2 subsite. The importance of the carbamate moiety is evident. The carbamate NH forms a hydrogen bond with the backbone carbonyl of Gly27, and the carbamate carbonyl functionality makes a tightly bound water-mediated hydrogen bond with the backbone NH’s of the flap Ile50 and Ile50′ in the active site.
Figure 11.
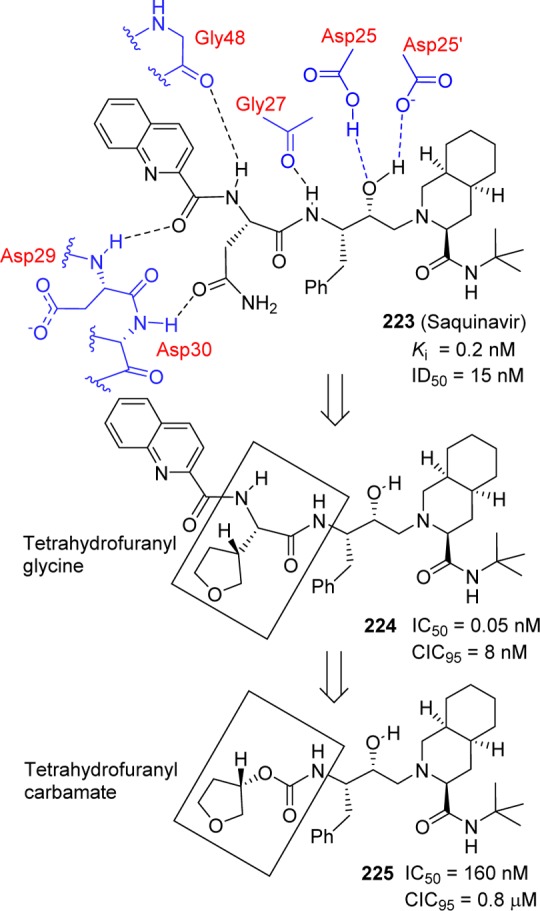
Evolution of 3-tetrahydrofuranyl carbamate as an HIV-1 protease inhibitor.
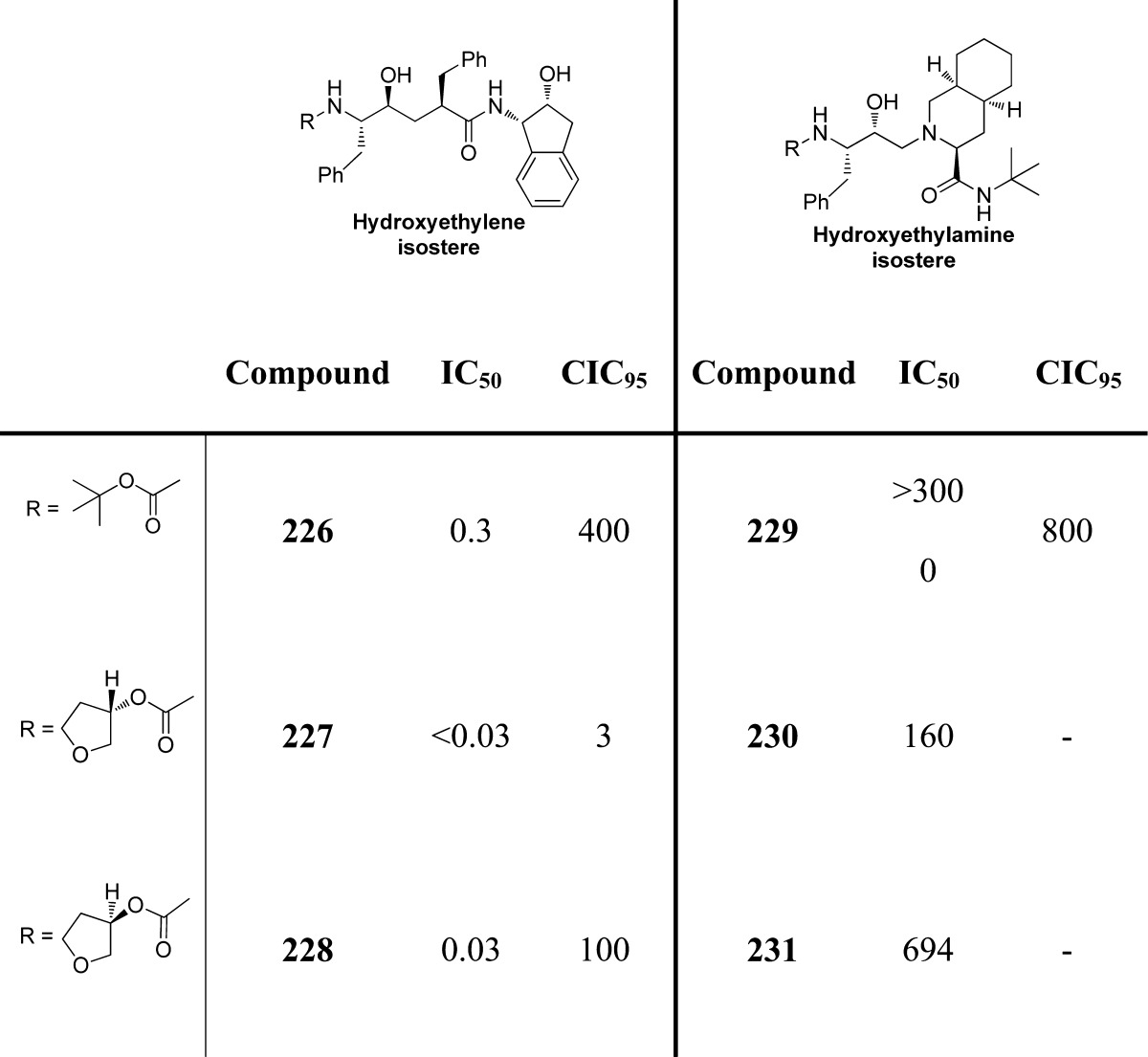
Our further investigation of the 3-(S)-THF in inhibitors containing a hydroxyethylene isostere led to a series of exceptionally potent inhibitors.178 As shown in Table 23, 3-(S)-THF-containing carbamate drivatives (compounds 226–231) provided very potent inhibitors in antiviral assays. The potency enhancing effect of 3-(S)-THF carbamate was subsequently demonstrated in inhibitors containing the (R)-(hydroxyethyl)sulfonamide isostere.229 Clinical development of inhibitor 46 (VX476) led to FDA approval of amprenavir for the treatment of HIV/AIDS patients.179,230
Table 23. Exploration of 3-Tetrahydrofuranyl Urethanes.
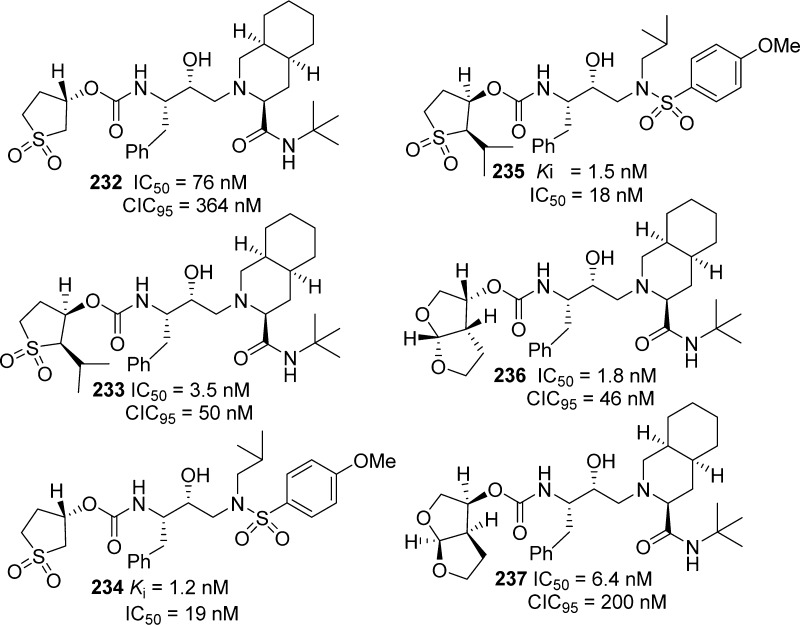
Further development of carbamate-derived novel HIV-1 protease inhibitors is shown in Figure 12. We have designed a variety of inhibitors incorporating cyclic sulfones and bicyclic ligands (Figure 12, compounds 232–237).230,231 These ligands were conceived in order to maximize hydrogen-bonding interactions with the protease backbone as well as to fill in the hydrophobic pocket in the S2 subsite. On the basis of the X-ray structure of saquinavir-bound HIV-1 protease, we then designed a fused bicyclic tetrahydrofuran (bis-THF) ligand to form hydrogen bonds with backbone aspartates in the S2 subsite as well as to fill in the hydrophobic site adjacent to the P3-quinoline ring of saquinavir (Figure 12).185,232 An X-ray structural analysis of 236-bound HIV-1 protease revealed that the bis-THF carbamate mimics the majority of P2–P3-amide bonds of saquinavir. A detailed structure–activity study also established that the stereochemistry of the bis-THF ring, and the position of the ring oxygens is critical to potency.
Figure 12.
Cyclic sulfolane and bicyclic ligand-derived carbamates as HIV-1 protease inhibitors.
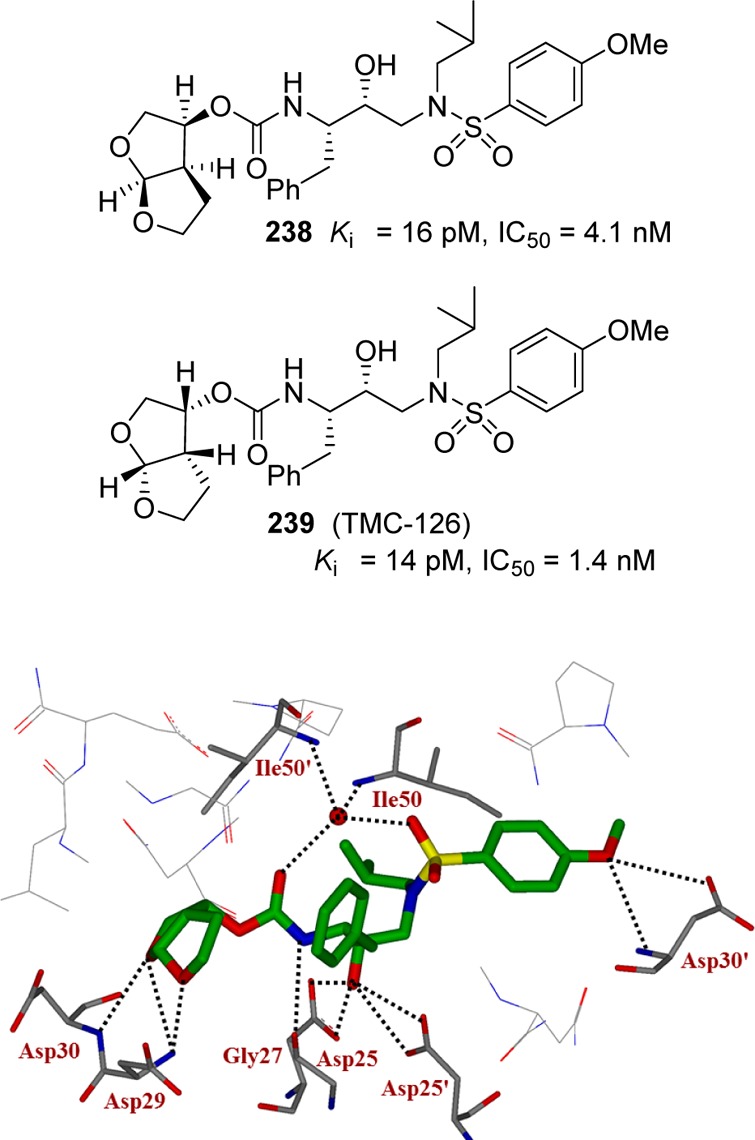
With the development of a bis-THF carbamate that could form a network of hydrogen bonds in the S2 subsite of HIV-1 protease, we investigated transition state isosteres that can be functionalized to form hydrogen bonds in the S2′ subsite. Our basic hypothesis was to design inhibitors that form a network of hydrogen bonds with the protease backbone atoms throughout the active site of HIV-1 protease, from S2 to S2′ subsites. This backbone binding strategy to combat drug resistance led to the development of a series of very potent carbamate-derived protease inhibitors.185,225,226 As shown in Figure 13, we incorporated the bis-THF ligand in the (R)-hydroxyethylsulfonamide isostere bearing p-methoxysulfonamide as the P2′ ligand so that the methoxy oxygen can interact with aspartate backbone atoms in the S2′ subsite. The resulting inhibitors exhibited notable potency.233,234 Inhibitor 239 with a (3R,3aS,6aR)-bis-THF as the P2 ligand is significantly more potent in an antiviral assay than corresponding inhibitor 238 with an enantiomeric bis-THF ligand. An X-ray structure of 239-bound HIV-1 protease revealed that the carbamate NH formed a hydrogen bond with the backbone Gly27 carbonyl group and that carbamate carbonyl of 239 is involved in an interesting tetra-coordinated hydrogen-bonding interaction with the structural water molecule, inhibitor sulfonamide oxygen, and the flap Ile 50 NH residues. Also, the structure revealed interactions with the backbone atoms in both the S2 and S2′ subsites.235,236
Figure 13.
Design of bicyclic carbamate and inhibitor 239-bound HIV-1 protease X-ray structure.
Further replacement of the p-methoxy group at the S2′ to a p-amino group led to inhibitor 48 (Figure 14). This inhibitor showed marked enzyme inhibitory activity as well as antiviral activity. An in-depth antiviral study revealed that 48 maintained excellent antiviral activity against multidrug-resistant HIV-1 variants.237−239 The X-ray structural studies of darunavir-bound HIV-1 protease showed extensive active site interactions (Figure 14). Particularly, it formed a network of hydrogen bonds with the protein backbone throughout the active site. Darunavir also exhibited favorable pharmacokinetic properties. Subsequently, clinical development led to its FDA approval as darunavir for the treatment of HIV/AIDS patients.240,241
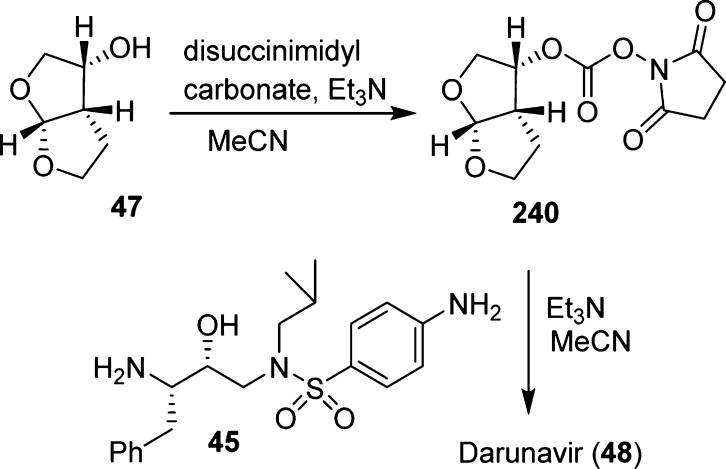
The carbamate functionality of darunavir (48) was assembled as shown in Scheme 22. (3R,3aS,6aR)-3-Hydroxyhexahydrofuro[2,3-b]furan (bis-THF) 47 was treated with disuccinimidyl carbonate to provide activated mixed carbonate 240. Reaction of this activated carbonate with hydroxyethylsulfonamide isostere 45 provided darunavir.242,243
Scheme 22. Assembly of Carbamate Functionality of Darunavir .
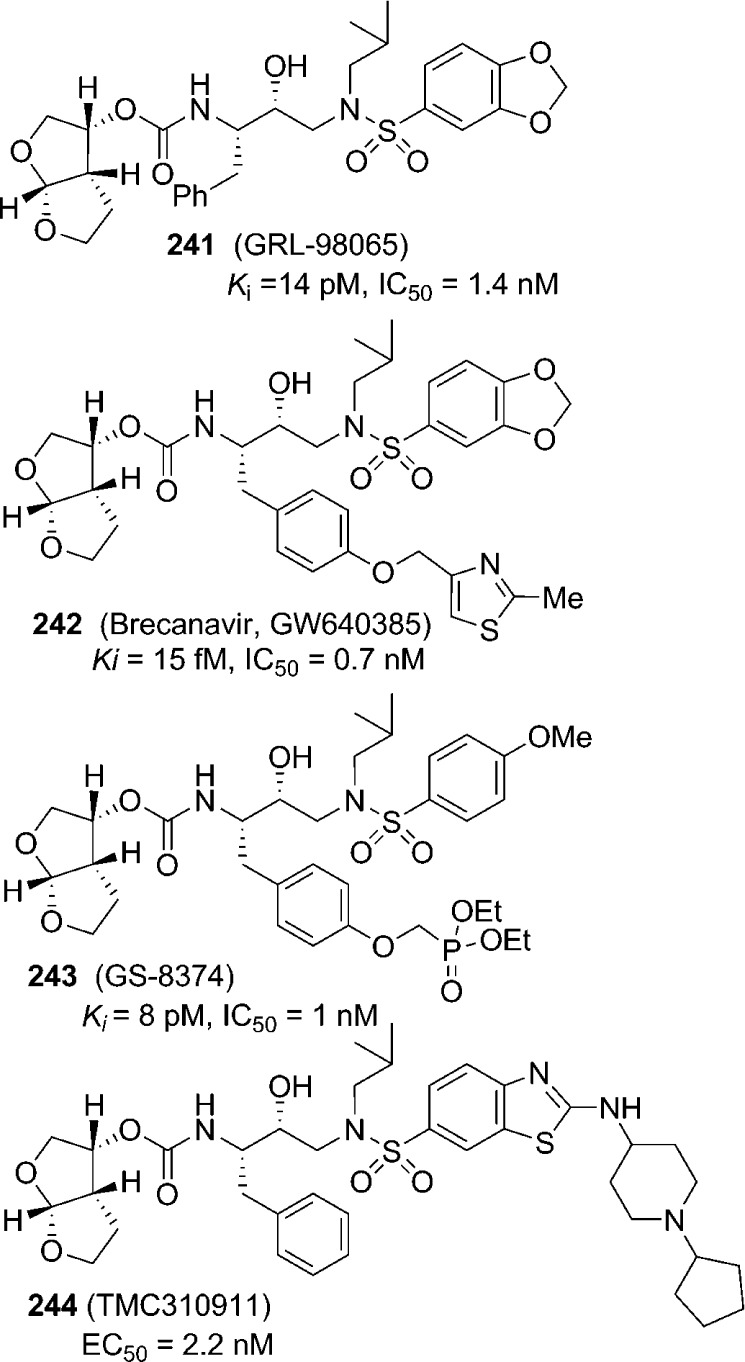
The backbone binding inhibitor design strategies to combat drug resistance have been further utilized by us and others to advance a number of other preclinical and clinical inhibitors with carbamates.185,225 Figure 15 shows selected bis-THF-derived carbamates (241–244) with marked enzyme and antiviral activities.244−247 Like darunavir, inhibitor-bound X-ray structures of these inhibitors showed a network of hydrogen bonds in both S2 and S2′ subsites of HIV-1 protease. The inhibitor side chains as well as the bis-THF bicyclic framework also effectively filled the hydrophobic pockets in the active site.
Figure 15.
Bis-THF-derived protease inhibitors for preclinical and clinical development.
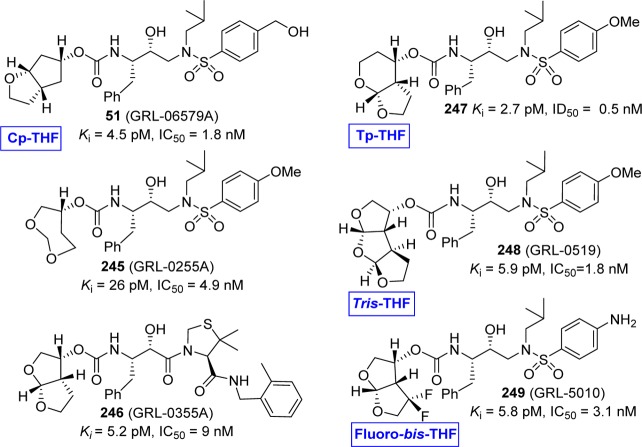
We have outlined a selected number of cyclic ether-derived carbamates that have been developed based on the backbone binding concept in Figure 16.185,225 Particularly, incorporation of these stereochemically defined oxacyclic ligands such as Cp-THF, Tp-THF, Tris-THF, and fluoro-bis-THF provided exceptionally potent inhibitors (51 and 245–249) with clinical potential.247−252 The importance of the carbamate functionality in these inhibitors is particularly worthy of note. X-ray crystal structures of these inhibitors in complex with HIV-1 protease provided the ligand-binding site interactions responsible for their respective antiviral potency against wild-type and multidrug-resistant viruses. In general, inhibitors are involved in hydrogen-bonding interactions with Asp29, Asp30, Gly27, Asp25, Asp25′, and Asp30′ in the HIV-1 protease active site. Furthermore, the ring cycles adequately fill the hydrophobic pockets in the active site.251
Figure 16.
Cyclic ether carbamate-derived novel protease inhibitors.
7. Carbamates as β- and γ-Secretase inhibitors
The search for an effective treatment for Alzheimer’s disease (AD) remains a major challenge in medicine. One of the pathological hallmarks of AD is the formation of β-amyloid (Aβ) peptides in the cortex of AD patients. Aβ-peptides are generated from β-amyloid precursor protein (APP) by sequential cleavage by β-secretase (also known as BACE1 or memapsin 2) and γ-secretase. Due to this central role of Aβ-production, both β-secretase and γ-secretase have been implicated as important therapeutic targets for AD intervention.253,254 As a result, design and synthesis of selective β-secretase and γ-secretase inhibitors have become an intense area of research over the years.
7.1. Development of β-Secretase Inhibitors
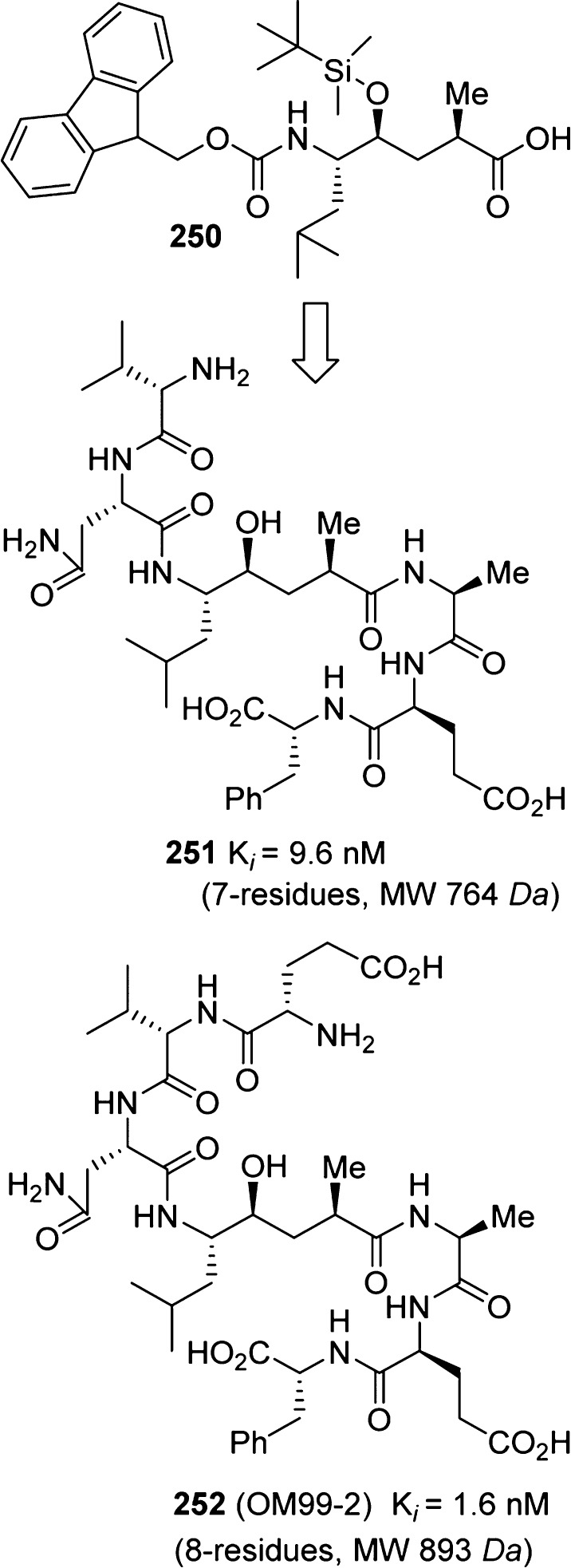
Following the discovery of β-secretase, the first-generation β-secretase inhibitors were designed and synthesized by Ghosh, Tang, and co-workers.255 As shown in Figure 17, utilizing a carbamate derivative of the Leu–Ala isostere 250, potent pseudopeptide inhibitors 251 and 252 were identified. The X-ray crystal structure of 252-bound β-secretase was determined to provide molecular insight into the ligand binding site interactions.256 The in-depth structural analysis thus provided critical drug design templates and led to the beginning of structure-based design approaches to peptidomimetic/nonpeptide β-secretase inhibitors.254,255
Figure 17.
Design of pseudopeptide BACE1 inhibitors.
The X-ray structure of 252-bound β-secretase revealed that the P2 asparagine side chain carboxamide nitrogen formed an intermolecular hydrogen bond with the P4 glutamic acid carbonyl group.
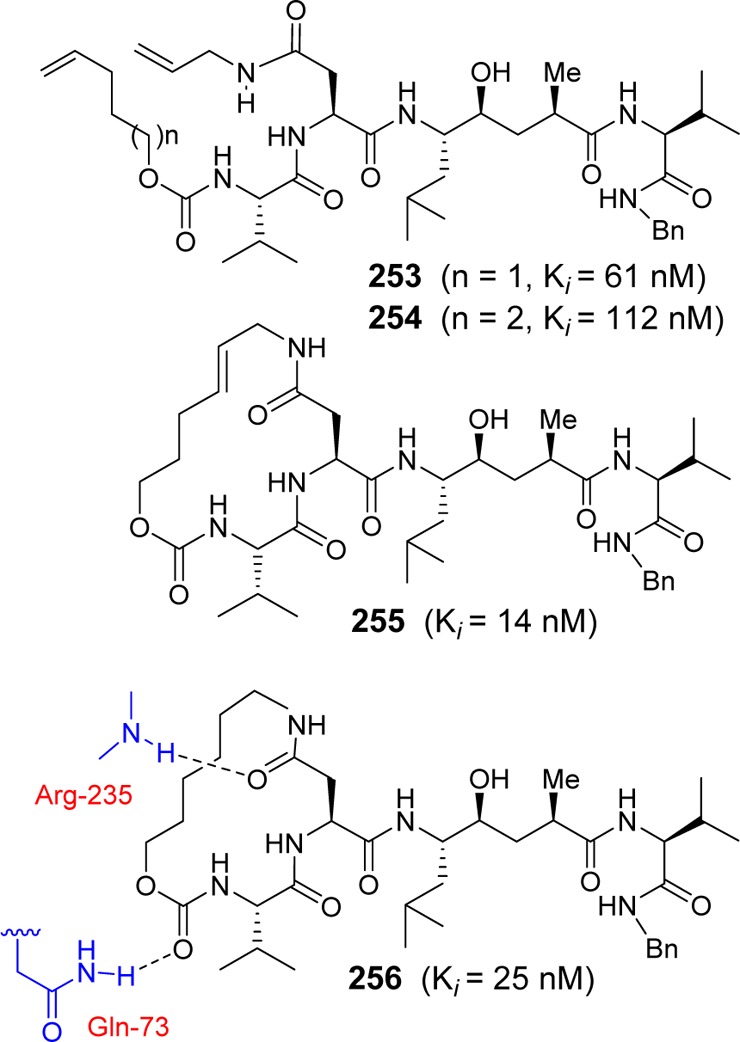
On the basis of this molecular insight, a number of 14–16-membered cycloamide-carbamate-based macrocyclic inhibitors were designed and synthesized.257 As shown in Figure 18, acyclic carbamate derivatives (253 and 254) were less potent than their corresponding cyclic inhibitors. Inhibitor 255, with a 16-membered macrocycle containing a trans-olefin, amide and carbamate functionalities within the macrocycle, showed good β-secretase inhibitory activity. Saturated inhibitor 256 is less potent against BACE1, but it showed enhanced potency for BACE2. X-ray structural studies of inhibitor 256-bound secretase revealed that the carbamate carbonyl forms a hydrogen bond with the Gln73 side chain carboxamide residue. Interestingly, unsaturated inhibitor 255 showed slight selectivity against memapsin 1 (Ki = 31 nM). The design of a selective inhibitor is important for reducing toxicity through off-target effects. Particularly, selectivity over other aspartic proteases, such as BACE2, pepsin, renin, cathepsin D (Cat-D), and cathepsin E, may be important for the reduction of side effects and drug efficiency.258
Figure 18.
Carbamate-based macrocyclic BACE1 inhibitors.
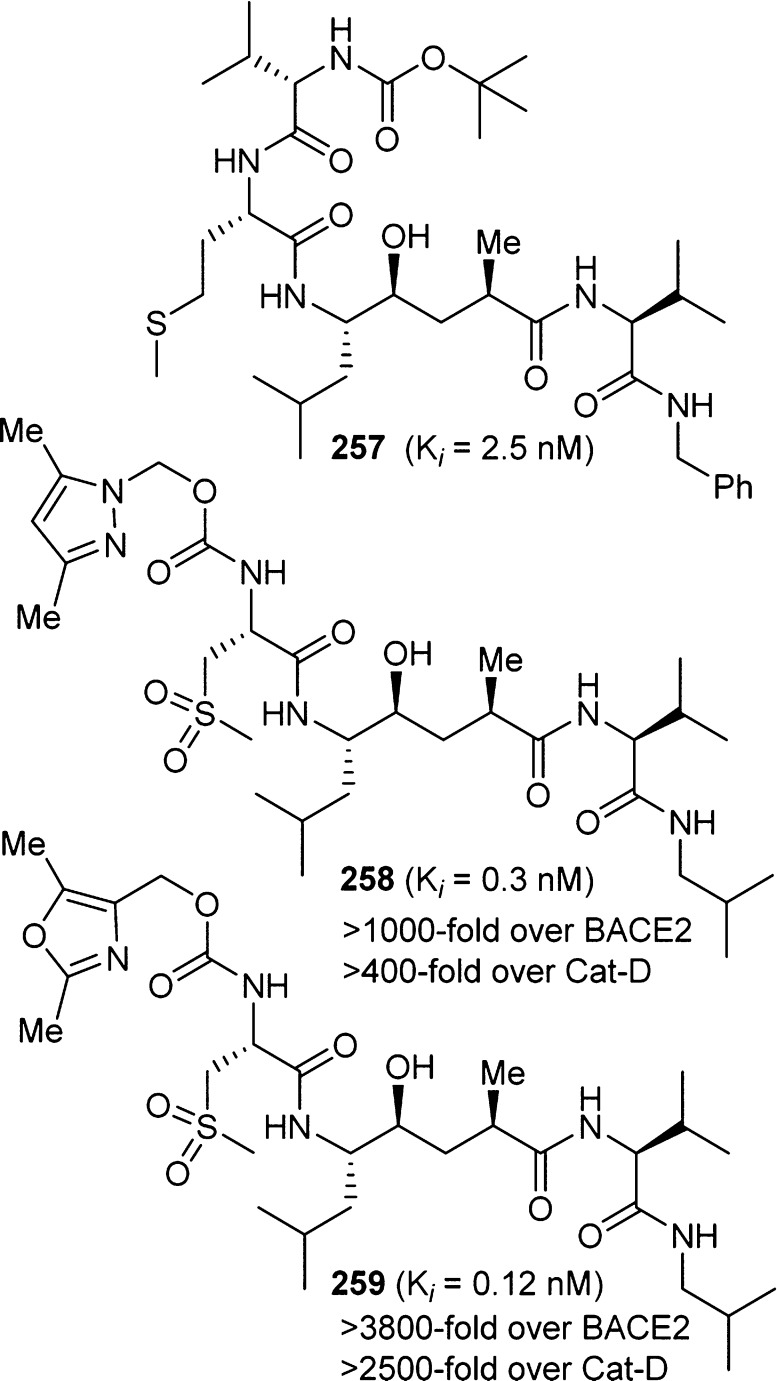
On the basis of our detailed structure–activity studies and X-ray structural analysis, we have designed a variety of highly selective and potent BACE1 inhibitors. In this Perspective, we will highlight only the development of BACE1 inhibitors bearing carbamate functionalities. As shown in Figure 19, inhibitor 257 is a potent BACE1 inhibitor. However, it did not show selectivity against BACE2 or Cat-D. Subsequent structure-based design led to the development of selective inhibitors 258 and 259, which contain a pyrazolylmethyl and oxazolymethyl carbamate at the P3 position, respectively.259 Inhibitor 258 showed excellent BACE1 potency and selectivity over BACE2 and cathepsin D. The X-ray crystal structure of 258-bound β-secretase revealed that the carbamate carbonyl formed a hydrogen bond with the Thr-232 backbone NH. Also, the pyrazole nitrogen formed a strong hydrogen bond with the Thr-232 side chain hydroxyl group. The P2-sulfonyl functionality formed a number of hydrogen bonds in the S2 subsite as well. On the basis of this molecular insight, oxazole-derived 259 was designed to provide a more stable and selective inhibitor.
Figure 19.
Carbamate-derived selective BACE1 inhibitors.
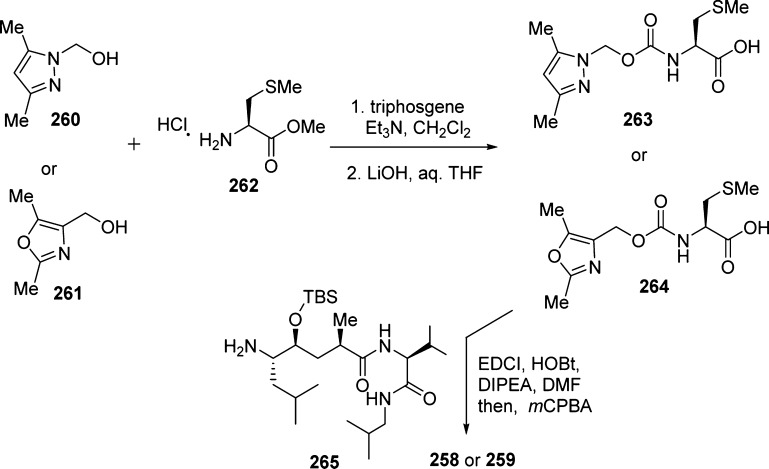
The synthesis of inhibitors 258 and 259 is outlined in Scheme 23. Urethanes 263 and 264 were prepared by treatment of 2,5-dimethylpyrazolylmethanol (260) or 2,5-dimethyl-4-oxazolemethanol (261) with triphosgene in the presence of triethylamine, followed by l-methionine methyl ester hydrochloride (262).
Scheme 23. Synthesis of BACE1 Inhibitors 258 and 259.
Saponification of the resulting methyl esters provided the corresponding acids. Coupling of amine 265 with acids 263 and 264, as described previously, and subsequent oxidation of the sulfides with m-chloroperbenzoic acid furnished inhibitors 258 and 259.259
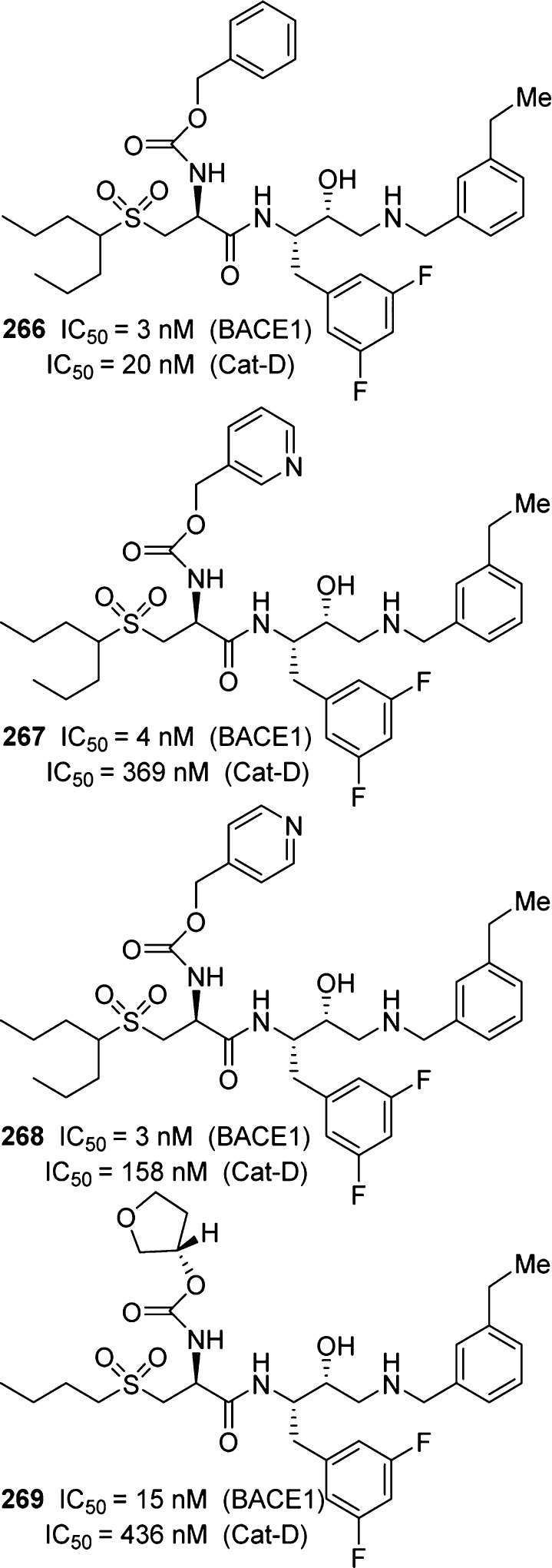
Freskos and co-workers have reported a series of β-secretase inhibitors that incorporated polar carbamate derivatives as the P2 ligand.260,261 This strategy led to improve the Cat-D selectivity. It was hypothesized that the S2 subsite of Cat-D is more lipophilic and less tolerant of polar groups. As can be seen in Figure 20, benzyl carbamate derivative 266 displayed 6-fold selectivity over Cat-D. However, polar 3-pyridylmethyl derivative 267 improved selectivity nearly 90-fold. The corresponding 4-pyridyl methyl compound 268 provided a reduction in selectivity (∼50-fold). 3-(S)-Tetrahydrofuranyl carbamate 269 showed a nearly 30-fold selectivity over Cat-D. These inhibitors have also shown good to excellent IC50 values in HEK cells.
Figure 20.
Butyl sulfone carbamate-derived selective BACE1 inhibitors.
7.2. Development of γ-Secretase Inhibitors
Over the years, many structural classes of potent and selective γ-secretase inhibitors have been reported. A number of inhibitors displayed drug-like properties and also inhibited Aβ production in animal models. In this section, we will review inhibitors with carbamate functionality. On the basis of γ-secretase inhibitor 270 (LY-411575), Peters and co-workers designed a series of carbamate derivatives of dibenzazepinone as potent and metabolically stable γ-secretase inhibitors.265 As shown in Figure 21, carbamate derivative 271 was prepared based on 270.262−265 Subsequently, carbamate 272 emerged as a potent γ-secretase inhibitor.
Figure 21.
Carbamate-derived potent γ-secretase inhibitors.
Researchers at Pharmacopeia and Schering-Plough Research Institute developed a series of potent γ-secretase inhibitors containing tetrahydroquinoline sulfonamide and piperidine sulfonamide carbamates.266−268 As shown in Figure 22, a number of representative carbamate derivatives showed IC50 values in the low nanomolar range. Racemic carbamate 273 first showed a good IC50 value. Enantiomers were then separated by HPLC. One of the enantiomers showed an IC50 value of 39 nM, whereas the other enantiomer displayed an IC50 > 1000 nM. Absolute stereochemistry of the active enantiomer was not determined. Piperidine carbamate 274 also showed good potency. Carbamate derivative 275 displayed a good membrane Aβ IC50 value; however, it showed poor CYP properties. Further modification led to compound 276 with good inhibitory activity and improved CYP properties.
Figure 22.
Tetrahydroquinoline and piperidine sulfonamide carbamate-derived γ-secretase inhibitors.
Bergstrom and co-workers reported a series of carbamate-appended N-alkyl sulfonamides as γ-secretase inhibitors.269,270 Figure 23 depicts selected examples that show potent Aβ inhibitory activity. Sulfonamide derivative 277 was identified as a potent γ-secretase inhibitor. Exploration of carbamate-appended N-alkylsulfonamides resulted in potent inhibitors such as 278–280. Tertiary carbamate 280 showed significant reduction of brain Aβ in transgenic mice compared to that of its benzyl derivative. This compound also showed improved brain-to-plasma ratio and good absolute brain concentration.
Figure 23.
Carbamate-appended sulfonamides as γ-secretase inhibitors.
8. Carbamate-Based HCV Therapeutics
Hepatitis C virus (HCV) is a bloodborne virus that is found worldwide. There are multiple strains or genotypes of the HCV virus. HCV infections lead to progressive liver damage, cirrhosis, and liver cancer. In recent years, there have been a number of new and effective antiviral drugs developed for the treatment of hepatitis C. These include the development of HCV NS3/4A protease inhibitors and inhibitors HCV NS5A. In this section, carbamate-derived therapeutics will be discussed.
8.1. Carbamate-Derived Serine Protease Inhibitors
Serine proteases are a large family of proteolytic enzymes that play a variety of critical roles in many physiological processes.271,272 Deregulation of serine proteases has been related to the pathogenesis of diseases such as stroke, inflammation, Alzheimer’s disease, cancer, and arthritis. Therefore, significant research efforts have been focused in the discovery of serine protease inhibitors. The active site of all serine proteases consists of a catalytic triad of Ser, His, and Asp. The nucleophilic attack by the hydroxyl group of serine at the carbonyl carbon of the scissile bond of the substrate, via general base catalysis by histidine, leads to the tetrahedral transition state. The tetrahedral intermediate ultimately collapses, leading to cleavage products.273−275 These key active residues are conserved in all serine proteases. X-ray structural studies revealed that these residues are superimposable in the majority of serine proteases.273,276 Therefore, selectivity over other serine proteases represents a key issue to be taken into consideration during inhibitor design. Most early inhibitors acted via a covalent mechanism in which an electrophilic group formed a covalent bond with the serine hydroxyl of the catalytic triad. The electrophilic groups are commonly referred to as serine traps or warheads. However, covalent inhibitors lack selectivity and specificity against other proteases in the same class or clan. The rational design of covalent serine protease inhibitors usually involves the selection of a good substrate to be linked to a serine trap/warhead. Chloromethyl ketones, diphenyl phosphonate esters, trifluoromethyl ketones, peptidyl boronic acids, α-ketoheterocycles, and β-lactam derivatives are usually employed as warheads. On the basis of these warheads, a variety of irreversible and reversible covalent serine protease inhibitors were designed.275 In this section, representative serine protease inhibitors containing a carbamate functionality will be outlined.
Carbamate derivative 281 (Figure 24), a diphenyl phosphonate ester containing a Cbz group and bearing a single amino acid side chain, showed very good inhibitory activity against human plasma kallikrein, useful for the treatment of hereditary angioedema.277,278 Thrombin is an attractive therapeutic target for drug development against pulmonary embolism, thrombosis, and related diseases.279,280 Compound 282 showed good potency and selectivity against human thrombin. It is stable and displayed no activity against acetylcholinesterase and no selectivity over cysteine proteases. Peptidyl boronic acid-based thrombin inhibitors were developed by DuPont-Merck. In particular, N-Boc derived inhibitor 283 is a potent inhibitor (Ki = 0.004 nM).281,282 Imperiali and co-workers introduced trifluoromethyl ketones as specific serine protease inhibitors, particularly for chymotrypsin and elastase.283 Researchers at AstraZeneca designed numerous peptidyl trifluoromethyl ketone derivatives as potent human elastase inhibitors.284−286 Further optimization of features resulted in the development of a number of orally active inhibitors. In particular, methyl carbamate derivative inhibitor 284 was shown to be a very potent inhibitor (Ki = 13 nM) with excellent oral bioavailability in laboratory animals.286,287 Optically pure compound 284 with an (S)-configuration at the P1 isopropyl side chain became a candidate for clinical development for potential treatment of elastase-implicated respiratory diseases.
Figure 24.
Structures of representative carbamate-containing kallikrein, thrombin, and elastase inhibitors.
Peptidomimetic boronic acid-based hepatitis C virus (HCV) NS3/4A protease inhibitors were designed and synthesized for the treatment of chronic HCV infections. HCV infections can lead to progressive liver damage, cirrhosis, and liver cancer.288 The NS3/4A serine protease plays a critical role in virus replication and has become an antiviral drug development target.289,290 The first specific and potent HCV protease inhibitor with good oral bioavailability was ciluprevir, which contains a carbamate functionality (285, BILN 2061, Figure 25). This noncovalent macrocyclic peptidic inhibitor was the result of a substrate-based approach for the design of active site inhibitors. This inhibitor is very active in enzymatic (IC50 = 3 nM) and cell-based replicon assays (IC50 = 1.2 nM) of HCV genotype 1.291 Ciluprevir was later discontinued due to cardiac toxicity in animal models, but its development paved the way to boceprevir (Victrelis, Schering-Plough, approved by FDA in May 2011) and telaprevir (VX-950, Vertex Pharmaceuticals and Johnson & Johnson).292,293 In particular, for the development of boceprevir, the introduction of a ketoamide moiety, together with P2 and P3 optimization, led to inhibitor 286 showing a Ki of 66 nM.294 Its X-ray crystal structure in complex with the enzyme also provided insight for further optimization. Indeed, a cyclopropylalanine residue was found to be optimal at P1, and the resulting carbamate derivative 287 showed a Ki of 15 nM. Although inhibitors 286 and 287 displayed good enzyme inhibitory potency, they did not display cellular activity in a subgenomic HCV replicon assay, possibly because of their strong peptidic character.295 The discovery that an N-methylated leucine at P2 was critical for both enzymatic potency and cellular activity led to the potential of cyclopropyl-fused proline being envisaged as an optimum, conformationally constrained surrogate for this part of the inhibitor. Combination of the P2-optimized ligand with previously optimized P1 and P3 residues provided carbamate derivative 288 with a Ki = 3.8 nM and IC90 = 100 nM.296 Finally, truncation and P1 optimization, by the employment of a cyclobutyl moiety, led to compound 289 (Ki = 76 nM), the direct boceprevir ancestor.297
Figure 25.
Structural evolution of carbamate-containing HCV NS3/4A protease inhibitors from ciluprevir to the discovery of boceprevir.
Subsequently, compounds 290 and 291 (Figure 26) with a carbamate containing P2 proline core showed very potent inhibitory activity (IC50 = 2 nM for 290 and 23 nM for 291).298 Similarly, macrocyclic inhibitor 292 with an α-amino cyclic boronate showed good potency (IC50 = 43 nM).299
Figure 26.
Structure of carbamate-containing HCV NS3/4A protease inhibitors.
The electron-withdrawing effect of the ester and amide functionalities was also utilized in the design of α-ketoester- or α-ketoamide-derived transition state inhibitors.300 A range of HCV NS3/4A protease inhibitors were designed and synthesized, incorporating α-ketoamide templates at the scissile site. Structure-based design led to a variety of potent acyclic and cyclic inhibitors with ketoamide templates, as exemplified in compounds 293 (IC50 = 3.8 nM) and 294 (IC50 = 30 nM) (Figure 27).297,301−303 Edwards et al. developed peptidyl α-ketoheterocycles as a new template for inactivation of elastase. Tripeptidyl α-ketobenzoxazole 295 inhibited human neutrophil elastase (HNE) with an IC50 of 3 nM. The ketooxazoline-derived inhibitor 296 displayed very potent activity against HNE (IC50 = 0.6 nM).304
Figure 27.
Carbamate-containing α-ketoamide inhibitors of HCV NS3/4A protease and α-ketoheterocycle inhibitors of HNE.
8.2. HCV NS5A Inhibitors
Carbamate derivatives also play a key role as inhibitors of HCV NS5A, which represents a new and promising target for HCV therapy. HCV NS5A is a zinc-binding phosphoprotein, and its role in the HCV virus life cycle is still not clear.305 However, it plays a critical role in HCV RNA replication. Also, it is involved in virion morphogenesis.306 Due to the lack of enzymatic function, inhibitors of this viral-encoded protein have been pursued.307 Researchers at Bristol-Myers Squibb screened a library of compounds for their ability to inhibit HCV RNA replication. This led to the identification of a lead compound specifically interfering with RNA replication and later proving to inhibit the activity of NS5A protein. Subsequent optimization was focused on broadening the genotype specificity and improving pharmacokinetic properties of compounds. Symmetry of the molecule played an important role in inhibitory potency. This finally led to the discovery of daclatasvir (297, BMS-790052, Figure 28) a first-in-class inhibitor of the HCV NS5A replication complex.308 Daclatasvir was approved in Europe in August, 2014. Ledipasvir (298, GS-5885, Gilead Sciences, Figure 28) is another carbamate-containing HCV NS5A inhibitor with potent antiviral activity against HCV genotypes 1a and 1b.309 Harvoni, a combination of ledipasvir and sofosbuvir (a nucleotide polymerase inhibitor), was approved by the FDA in October 2014 for the treatment of chronic HCV genotype 1 infection. This also represents the first approved regimen that does not require administration with interferon or ribavirin.310,311
Figure 28.
Carbamate-containing HCV NS5A inhibitors daclatasvir and ledipasvir.
9. Carbamates as Cysteine Protease Inhibitors
Cysteine proteases, also known as thiol proteases, are proteolytic enzymes responsible for the degradation of proteins.312 These enzymes are divided into three classes based on their sequence homology: the papain, caspase, and picornaviridae families. The papain family of proteases is the most known and studied.313−315
Cysteine proteases have been identified in a variety of diverse organisms, such as bacteria, eukaryotic micro-organisms, plants, and animals and are divided into the clans CA, CD, CE, CF, and CH in the MEROPS peptidase database.
The largest subfamily among the class of cysteine proteases is the papain-like cysteine proteases, originating from papain as the archetype of the cysteine proteases. Clan CA proteases utilize catalytic Cys, His, and Asn residues that are invariably in this order in the primary sequence of the protease. Clan CA, Family C1 (papain-family) cysteine proteases are well-characterized for many eukaryotic organisms. Also, the best characterized Plasmodium cysteine proteases, namely, the falcipains, belong to papain-family (clan CA) enzymes.
Clan CD presents two catalytic residues, His and Cys, in sequence; Clan CE has a triad formed by His, Glu, or Asp and Cys at the C-terminus; in clan CF, the asparagine residue of the catalytic triad is replaced by a glutamate residue and the catalytic triad is ordered as Glu, Cys, and His; clan CG has a dyad of two cysteine residues, and Clan CH presents a Cys, Thr, and His triad with the catalytic cysteine at the N-terminus.
The proteolytic mechanism involves the formation of a thiolate–imidazolium ion pair, which provides a highly nucleophilic cysteine thiol. Over the years, many cysteine protease inhibitors have been designed by appropriately linking electrophilic warheads to the specific recognition sequence of peptide substrates. Reversible inhibitor warheads include aldehydes, α-ketoamides, α-ketoesters, and α-ketoacids. These inhibitors interact with the protease active site, forming the tetrahedral intermediate, but are eventually hydrolyzed, regenerating both the enzyme and the inhibitor in an equilibrium reaction. Irreversible inhibitors of cysteine proteases include epoxides, aziridines, haloketones, vinyl sulfones, and acyloxymethylketones. These inhibitors inactivate the target through alkylation of the active site cysteine thiol, permanently disabling enzyme function.273,313,316−318
The occurrence of severe acute respiratory syndrome (SARS) in 2003 and the subsequent identification of a novel coronavirus as the etiological agent recognized cysteine proteases SARS-CoV 3CLpro and SARS-CoV PLpro (papain-like protease) as possible targets for drug design.319,320 Subsequent structure-based design based on a previous inhibitor’s X-ray co-crystal structure with the enzyme321 provided carbamate derivative 299 (Figure 29) as a potent SARS-CoV 3CLpro inhibitor (IC50 = 80 μM).322
Figure 29.
Representative carbamate-containing cysteine protease inhibitors.
A wide variety of human rhinovirus 3C (HRV 3C) protease inhibitors were developed by the incorporation of α,β-unsaturated carbonyl moieties as warheads. Hanzlik et al. reported the first HRV 3C protease inhibitors containing a peptide portion and incorporating α,β-unsaturated esters.323 The peptide parts were selected based on the substrate cleavage site. The representative carbamate-containing inhibitor 300 (Figure 29) showed an IC50 value of 130 nM.
Human cathepsin K plays a critical role in bone resorption. In an effort to block bone resorption, noncovalent cathepsin K inhibitors were developed. Kim et al. provided carbamate derivative 301 (Figure 29) as a noncovalent and reversible cathepsin K (IC50 = 0.01 μM) and L inhibitor (IC50 = 0.002 μM).324 GlaxoWellcome scientists developed carbamate-containing ketoamide-based cathepsin K inhibitors such as 302 (Figure 29) (IC50 = 0.072 nM).325 Starting from a potent ketone-based inhibitor with unsatisfactory drug-like properties,326,327 incorporation of P2–P3 elements from the ketoamide-based inhibitor 302 led to a hybrid series of ketone-based cathepsin K inhibitors with improved bioavailability, as exemplified in inhibitor 303 (Figure 29) (IC50 = 4 nM).328
Cathepsin S has been suggested for the development of agents against a range of immune disorders. A new class of nonpeptidic and noncovalent cathepsin S inhibitors was reported in 2007.329 Subsequent structural optimization resulted in a very potent and competitive noncovalent carbamate-containing inhibitor 304 (Figure 29) (IC50 = 20 nM).
10. Carbamates as Endocannabinoid Metabolizing Enzyme Inhibitors
Carbamates have been employed in the design of serine hydrolase inhibitiors. In this section, we will focus on inhibitors of endocannabinoid metabolizing enzymes, in which the carbamate functionality plays an important role.
The endocannabinoid system is known to be a ubiquitous neuromodulatory system with a wide range of action that can be found in every primitive organism. It is composed of cannabinoid receptors (CBRs), endogenous cannabinoids (endocannabinoids, ECs), and the enzymes responsible for their production, transport, and degradation.330,331 ECs are a class of signaling lipids, such as N-arachidonoyl ethanolamine (anandamide, AEA), oleamide, and 2-arachidonoyl glycerol (2-AG), that exert their biological actions through the interaction with two G-protein coupled receptors, CB1 and CB2. They modulate a range of responses and processes including pain, inflammation, appetite, motility, sleep, thermoregulation, and cognitive and emotional states.332 The actions of these signaling lipids are rapidly terminated by cellular reuptake and subsequent hydrolysis operated by a number of enzymes. An attractive approach involved the modulation of the EC system and aimed at eliciting the desirable effects of CBRs activation through the pharmacological inactivation of the main endocannabinoid metabolizing enzymes, namely, monoacylglycerol lipase (MAGL) and α/β-hydrolase domain containing 6 and 12 (ABHD6 and ABHD12). These three serine hydrolases account for approximately 99% of 2-AG hydrolysis in the CNS,333 whereas fatty acid amide hydrolase (FAAH) is responsible for AEA inactivation.330 Inactivation of these enzymes would elevate the endogenous concentrations of all of its substrate and consequently prolong and potentiate their beneficial effects on pain and anxiety without evoking the classical CB1R agonists side effects (hypomotility, hypothermia, and catalepsy).
Monoacylglycerol lipase (MAGL) is the primary enzyme responsible for the hydrolysis of 2-AG in the CNS.333 About 85% of the total 2-AG hydrolysis in the brain is ascribed to MAGL. MAGL is a 33 kDa membrane enzyme belonging to the superfamily of the serine hydrolases with a catalytic triad represented by Ser122, His269, and Asp239.334 It is ubiquitously present in the brain (cortex, hippocampus, cerebellum, thalamu, and striatum), where it localizes to presynaptic terminals, even if lower levels are found in the brainstem and hypothalamus. A concomitant distribution in membranes as well as in the cytosol has been reported. MAGL shares a common folding motif called the α/β-hydrolase fold. Studies in recent years have shown that MAGL inhibitors elicit antinociceptive, anxiolytic, and antiemetic responses. MAGL inhibitors have also been shown to exert anti-inflammatory action in the brain and protect against neurodegeneration through lowering eicosanoid production.335 Recently, the potential of MAGL inhibitors for the therapy of Fragile X syndrome has been reported.336 The early discovered MAGL inhibitors were molecules able to target the cysteine residues present in the active site of the enzyme. Later, the research has been focused on the synthesis of compounds covalently binding to Ser241 of the catalytic triad. Among them, carbamate 305 (URB602, Figure 30) was the first selective inhibitor of 2-AG degradation, although its potency remained limited (IC50 = 28 μM on rat brain).337 Selective MAGL inhibitors bearing a carbamate scaffold were developed by Cravatt and co-workers.338 Inhibitor 306 (JZL184, Figure 30) exhibited selectivity toward FAAH in vitro (IC50 = 3.9 nM and 4 μM for human recombinant MAGL and FAAH, respectively).338 More recently, Cravatt and co-workers reported a distinct class of O-hexafluoroisopropyl (HFIP) carbamates bearing a reactive group that is bioisosteric with endocannabinoid substrates.339 The representative compound, 307 (KML29, Figure 30, IC50 = 5.9 nM, human MAGL), displays excellent potency and in vivo. In comparison to previously described O-aryl carbamates, inhibitor showed enhanced selectivity over FAAH and other serine hydrolases.339
Figure 30.
Representative carbamate-containing inhibitors of MAGL, ABHD6, and FAAH.
ABHD6 gene encodes a ∼35 kDa protein containing an N-terminal transmembrane region followed by a catalytic domain that includes the canonical GXSXG active-site motif of serine hydrolases. ABHD6 is a unique and highly conserved enzyme in mammals and is mainly expressed in the brain, liver, kidney, and brown adipose tissue. As a member of the serine hydrolase class, ABHD6 is predicted to hydrolyze esters, amides, or thioester bonds in substrates that could include small molecules, lipids, or peptides. Although, the full range of substrates regulated by this enzyme in vivo is currently unknown.340 Recent studies have also shown that ABHD6 carbamate inhibitors produce anti-inflammatory and neuroprotective effects in a mouse model of traumatic brain injury.341 Among them, optimized inhibitor 308 (Figure 30) displayed an IC50 value of 70 nM and notable selectivity.342
FAAH is a membrane-bound enzyme belonging to the amidase family. The analysis of its crystal structure revealed a core composed of a characteristic Ser-Ser-Lys catalytic triad.343 The catalytic residues of FAAH are buried deep within the enzyme and are accessible by two narrow channels. The importance of FAAH was demonstrated by the generation of FAAH knockout mice. FAAH–/– mice showed an elevated resting brain concentration of AEA and manifested (i) an analgesic phenotype in both the carrageenan model of inflammatory pain and in the formalin model of spontaneous pain,344 (ii) a reduction in inflammatory responses,345 and (iii) improvements in slow wave sleep and memory acquisition.346,347 The URB class of compounds was the first class of inhibitors identified for FAAH, and it is well-represented by 309 (URB597, Figure 30, IC50 = 4.6 nM).348 The N-(6-phenyl)hexylcarbamate analogue 310 (JP83, Figure 30, IC50 = 14 nM) is another very potent compound representative of the biphenyl series of inhibitors.349 Gattinoni and co-workers developed a series of oxime carbamate inhibitors. Compound 311 (Figure 30, IC50 = 8 nM) displayed good affinity and selectivity toward FAAH.350 More recently, Butini et al. developed a new class of potent and selective FAAH reversible carbamate inhibitors.351 Among them, compound 312 (NF1245, Figure 30, Ki = 0.16 nM on mouse brain FAAH) showed excellent activity. The compound showed impressive selectivity toward all the enzymes and receptors of the endocannabinoid system.351
11. Conclusions
In this Perspective, the role of carbamates in drug design and medicinal chemistry has been highlighted. In particular, the Perspective covers physical properties of carbamates and the development of novel chemical methodologies overcoming the historical safety and toxicity issues related to their preparation. Furthermore, the importance of carbamate-derived compounds in medicinal chemistry and their widespread employment as drugs and prodrugs have been discussed. Also showcased is the exploitation of organic carbamates in the development of numerous aspartic acid, serine, and cysteine protease inhibitors. We hope that this Perspective will stimulate further use of organic carbamate as a structural motif in drug design and medicinal chemistry.
Acknowledgments
Financial support of this research by the National Institutes of Health (GM53386) and Purdue University is gratefully acknowledged. We would like to thank our colleagues Anthony Tomaine, Heather Osswald, and Anindya Sarkar (Purdue University) for helpful discussions.
Glossary
Abbreviations Used
- AD
Alzheimer’s disease
- Cat-D
cathespsin D
- FAAH
fatty acid amide hydrolase
- PPAR
peroxisome proliferator-activated receptor
- GABA
γ-amino butyric acid
- HAART
highly active antiretroviral therapy
- bis-THF
bis-tetrahydrofuran
- BACE1
beta-site amyloid precursor protein cleaving enzyme 1
- MDR
multidrug resistant
- NNRTI
non-nucleoside reverse transcriptase inhibitors
- ADEPT
antibody-directed enzyme prodrug therapy
- GDEPT
gene-directed enzyme prodrug therapy
- MCT1
monocarboxylate transporter type 1
- SMVT
sodium-dependent multivitamin transporter
- CES1
carboxylesterases 1
- MAGL
monoacylglycerol lipase
Biographies
Arun K. Ghosh obtained his BS and MS in Chemistry from the University of Calcutta and Indian Institute of Technology, Kanpur, India, respectively. He received his Ph.D. degree in chemistry from the University of Pittsburgh (1985). He then carried out postdoctoral research in Professor E. J. Corey’s laboratory at Harvard University (1985–1988). He was a research fellow at Merck Research Laboratories prior to joining the chemistry faculty at the University of Illinois, Chicago, as an assistant Professor in 1994. In 2005, he joined the department of Chemistry and the Department of Medicinal Chemistry at Purdue University, West Lafayette. In 2009, he became the Ian P. Rothwell Distinguished Professor of Chemistry and Medicinal Chemistry. His research interests are in the areas of synthetic organic, bioorganic, and medicinal chemistry.
Margherita Brindisi graduated cum laude in Medicinal Chemistry at the University of Siena, Italy (2004), where she also received her Ph.D. degree in Pharmaceutical Sciences (2008). She was a postdoctoral fellow at Purdue University, West Lafayette (2010–2011), working in the research group of Professor Ghosh in the design and synthesis of novel β-secretase inhibitors for the treatment of Alzheimer’s disease. She subsequently moved back to the University of Siena, where she became a researcher in the Department of Biotechnology, Chemistry, and Pharmacy (2012–present). Her current research interests include design and synthesis of novel potential therapeutics for parasitic diseases, cancer, and neurodegenerative disorders.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Conradi R. A.; Hilgers A. R.; Ho N. F.; Burton P. S. The influence of peptide structure on transport across Caco-2 cells. II. Peptide bond modification which results in improved permeability. Pharm. Res. 1992, 9, 435–439. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E.Pseudo-peptides in Drug Discovery; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Sewald N.; Jakubke H.-D.. Peptides: Chemistry and Biology; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Vagner J.; Qu H.; Hruby V. J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowick J. S. What I have learned by using chemical model systems to study biomolecular structure and interactions. Org. Biomol. Chem. 2006, 4, 3869–3885. [DOI] [PubMed] [Google Scholar]
- Liskamp R. M.; Rijkers D. T.; Kruijtzer J. A.; Kemmink J. Peptides and proteins as a continuing exciting source of inspiration for peptidomimetics. ChemBioChem 2011, 12, 1626–1653. [DOI] [PubMed] [Google Scholar]
- Fletcher M. D.; Campbell M. M. Partially modified retro-inverso peptides: development, synthesis, and conformational behavior. Chem. Rev. 1998, 98, 763–796. [DOI] [PubMed] [Google Scholar]
- Patil B. S.; Vasanthakumar G. R.; Suresh Babu V. V. Isocyanates of N alpha-[(9-fluorenylmethyl)oxy]carbonyl amino acids: synthesis, isolation, characterization, and application to the efficient synthesis of urea peptidomimetics. J. Org. Chem. 2003, 68, 7274–7280. [DOI] [PubMed] [Google Scholar]
- Sureshbabu V. V.; Patil B. S.; Venkataramanarao R. Preparation, isolation, and characterization of Nalpha-Fmoc-peptide isocyanates: solution synthesis of oligo-alpha-peptidyl ureas. J. Org. Chem. 2006, 71, 7697–7705. [DOI] [PubMed] [Google Scholar]
- Semetey V.; Hemmerlin C.; Didierjean C.; Schaffner A. P.; Giner A. G.; Aubry A.; Briand J. P.; Marraud M.; Guichard G. Unexpected stability of the urea cis–trans isomer in urea-containing model pseudopeptides. Org. Lett. 2001, 3, 3843–3846. [DOI] [PubMed] [Google Scholar]
- Myers A. C.; Kowalski J. A.; Lipton M. A. Facile incorporation of urea pseudopeptides into protease substrate analogue inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 5219–5222. [DOI] [PubMed] [Google Scholar]
- Fischer L.; Didierjean C.; Jolibois F.; Semetey V.; Manuel Lozano J.; Briand J. P.; Marraud M.; Poteau R.; Guichard G. Propensity for local folding induced by the urea fragment in short-chain oligomers. Org. Biomol. Chem. 2008, 6, 2596–2610. [DOI] [PubMed] [Google Scholar]
- Cho C. Y.; Moran E. J.; Cherry S. R.; Stephans J. C.; Fodor S. P.; Adams C. L.; Sundaram A.; Jacobs J. W.; Schultz P. G. An unnatural biopolymer. Science 1993, 261, 1303–1305. [DOI] [PubMed] [Google Scholar]
- Sureshbabu V. V.; Venkataramanarao R.; Naik S. A.; Chennakrishnareddy G. Synthesis of tetrazole analogues of amino acids using Fmoc chemistry: isolation of amino free tetrazoles and their incorporation into peptides. Tetrahedron Lett. 2007, 48, 7038–7041. [Google Scholar]
- Sureshbabu V. V.; Hemantha H. P.; Naik S. A. Synthesis of 1,2,4-oxadiazole-linked orthogonally urethane-protected dipeptide mimetics. Tetrahedron Lett. 2008, 49, 5133–5136. [Google Scholar]
- Dibenedetto A.; Aresta M.; Fragale C.; Narracci M. Reaction of silylalkylmono- and silylalkyldi-amines with carbon dioxide: evidence of formation of inter- and intra-molecular ammonium carbamates and their conversion into organic carbamates of industrial interest under carbon dioxide catalysis. Green Chem. 2002, 4, 439–443. [Google Scholar]
- Gupte S. P.; Shivarkar A. B.; Chaudhari R. V. Carbamate synthesis by solid-base catalyzed reaction of disubstituted ureas and carbonates. Chem. Commun. 2001, 2620–2621. [Google Scholar]
- Martin L. L.; Davis L.; Klein J. T.; Nemoto P.; Olsen G. E.; Bores G. M.; Camacho F.; Petko W. W.; Rush D. K.; Selk D.; Smith C. P.; Vargas H. M.; Winslow J. T.; Effland R. C.; Fink D. M. Synthesis and preliminary structure–activity relationships of 1-[(3-fluoro-4-pyridinyl)amino]-3-methyl-1H-indol-5-yl methyl carbamate (P10358), a novel acetylcholinesterase inhibitor. Bioorg. Med. Chem. Lett. 1997, 7, 157–162. [Google Scholar]
- Wuts P. G. M.; Greene T. W.. Protective Groups in Organic Synthesis, 4th ed.; Wiley: Hoboken, NJ, 2006. [Google Scholar]
- Cox C.; Lectka T. Solvent effects on the barrier to rotation in carbamates. J. Org. Chem. 1998, 63, 2426–2427. [DOI] [PubMed] [Google Scholar]
- Rablen P. R. Computational analysis of the solvent effect on the barrier to rotation about the conjugated C–N bond in methyl N,N-dimethylcarbamate. J. Org. Chem. 2000, 65, 7930–7937. [DOI] [PubMed] [Google Scholar]
- Moraczewski A. L.; Banaszynski L. A.; From A. M.; White C. E.; Smith B. D. Using hydrogen bonding to control carbamate C–N rotamer equilibria. J. Org. Chem. 1998, 63, 7258–7262. [DOI] [PubMed] [Google Scholar]
- Dugave C.; Demange L. Cis–trans isomerization of organic molecules and biomolecules: implications and applications. Chem. Rev. 2003, 103, 2475–2532. [DOI] [PubMed] [Google Scholar]
- Wiberg K. B.; Bailey W. F. Dipole-stabilized carbanions: a computational study of N-methylformamide anion and methyl N-methylcarbamate anion. J. Org. Chem. 2002, 67, 5365–5368. [DOI] [PubMed] [Google Scholar]
- Deetz M. J.; Forbes C. C.; Jonas M.; Malerich J. P.; Smith B. D.; Wiest O. Unusually low barrier to carbamate C–N rotation. J. Org. Chem. 2002, 67, 3949–3952. [DOI] [PubMed] [Google Scholar]
- Kaur D.; Sharma P.; Bharatam P. V. Amide resonance in thio- and seleno- carbamates: a theoretical study. J. Mol. Struct.: THEOCHEM 2005, 757, 149–153. [Google Scholar]
- Lauvergnat D.; Hiberty P. C. Role of conjugation in the stabilities and rotational barriers of formamide and thioformamide. An ab initio valence-bond study. J. Am. Chem. Soc. 1997, 119, 9478–9482. [Google Scholar]
- Marcovici-Mizrahi D.; Gottlieb H. E.; Marks V.; Nudelman A. On the stabilization of the syn-rotamer of amino acid carbamate derivatives by hydrogen bonding. J. Org. Chem. 1996, 61, 8402–8406. [Google Scholar]
- Lee C. M.; Kumler W. D. The dipole moment and structure of the carbamate group. J. Am. Chem. Soc. 1961, 83, 4596–4600. [Google Scholar]
- Dugave C.Chemical aspects of the restricted rotation of esters, amides, and related compounds. In Cis–trans Isomerization in Biochemistry; Dugave C., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp 143–166. [Google Scholar]
- Testa B.; Mayer J. M.. Hydrolysis in Drug and Prodrug Metabolism—Chemistry, Biochemistry, and Enzymology; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Vacondio F.; Silva C.; Mor M.; Testa B. Qualitative structure-metabolism relationships in the hydrolysis of carbamates. Drug Metab. Rev. 2010, 42, 551–589. [DOI] [PubMed] [Google Scholar]
- Tarzia G.; Duranti A.; Tontini A.; Piersanti G.; Mor M.; Rivara S.; Plazzi P. V.; Park C.; Kathuria S.; Piomelli D. Design, synthesis, and structure–activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J. Med. Chem. 2003, 46, 2352–2360. [DOI] [PubMed] [Google Scholar]
- Burk M. J.; Allen J. G. A mild amide to carbamate transformation. J. Org. Chem. 1997, 62, 7054–7057. [Google Scholar]
- Matsumura Y.; Maki T.; Satoh Y. Electrochemically induced Hofmann rearrangement. Tetrahedron Lett. 1997, 38, 8879–8882. [Google Scholar]
- Gogoi P.; Konwar D. An efficient modification of the Hofmann rearrangement: synthesis of methyl carbamates. Tetrahedron Lett. 2007, 48, 531–533. [Google Scholar]
- Curtius T. Hydrazide und azide organischer säuren I. Abhandlung. J. Prakt. Chem. 1894, 50, 275–294. [Google Scholar]
- Scriven E. F. V.; Turnbull K. Azides: their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar]
- Cenini S.; Crotti C.; Pizzotti M.; Porta F. Ruthenium carbonyl catalyzed reductive carbonylation of aromatic nitro-compounds—a selective route to carbamates. J. Org. Chem. 1988, 53, 1243–1250. [Google Scholar]
- Salvatore R. N.; Ledger J. A.; Jung K. W. An efficient one-pot synthesis of N-alkyl carbamates from primary amines using Cs2CO3. Tetrahedron Lett. 2001, 42, 6023–6025. [Google Scholar]
- Waldman T. E.; Mcghee W. D. Isocyanates from primary amines and carbon-dioxide—dehydration of carbamate anions. J. Chem. Soc., Chem. Commun. 1994, 957–958. [Google Scholar]
- Darensbourg D. J.; Niezgoda S. A.; Draper J. D.; Reibenspies J. H. Mechanistic aspects of the copolymerization of CO2 and epoxides by soluble zinc bis(phenoxide) catalysts as revealed by their cadmium analogues. J. Am. Chem. Soc. 1998, 120, 4690–4698. [Google Scholar]
- Sakakura T.; Saito Y.; Okano M.; Choi J. C.; Sako T. Selective conversion of carbon dioxide to dimethyl carbonate by molecular catalysis. J. Org. Chem. 1998, 63, 7095–7096. [DOI] [PubMed] [Google Scholar]
- Yoshida M.; Hara N.; Okuyama S. Catalytic production of urethanes from amines and alkyl halides in supercritical carbon dioxide. Chem. Commun. 2000, 151–152. [Google Scholar]
- Salvatore R. N.; Chu F. X.; Nagle A. S.; Kapxhiu E. A.; Cross R. M.; Jung K. W. Efficient Cs2CO3-promoted solution and solid phase synthesis of carbonates and carbamates in the presence of TBAI. Tetrahedron 2002, 58, 3329–3347. [Google Scholar]
- Senanayake C. H.; Fredenburgh L. E.; Reamer R. A.; Larsen R. D.; Verhoeven T. R.; Reider P. J. Nature of N-bromosuccinimide in basic-media—the true oxidizing species in the Hofmann rearrangement. J. Am. Chem. Soc. 1994, 116, 7947–7948. [Google Scholar]
- Moriarty R. M.; Chany C. J.; Vaid R. K.; Prakash O.; Tuladhar S. M. Preparation of methyl carbamates from primary alkylcarboxamides and arylcarboxamides using hypervalent iodine. J. Org. Chem. 1993, 58, 2478–2482. [Google Scholar]
- Radlick P.; Brown L. R. A versatile modification of the Hofmann rearrangement. Synthesis 1974, 4, 290–292. [Google Scholar]
- Huang X. C.; Keillor J. W. Preparation of methyl carbamates via a modified Hofmann rearrangement. Tetrahedron Lett. 1997, 38, 313–316. [Google Scholar]
- Baumgarten H. E.; Smith H. L.; Staklis A. Reactions of amines. XVIII. Oxidative rearrangement of amides with lead tetraacetate. J. Org. Chem. 1975, 40, 3554–3561. [Google Scholar]
- Kajigaeshi S.; Asano K.; Fujisaki S.; Kakinami T.; Okamoto T. Oxidation using quaternary ammonium polyhalides 0.1. An efficient method for the Hofmann degradation of amides by use of benzyltrimethylammonium tribromide. Chem. Lett. 1989, 3, 463–464. [Google Scholar]
- Erhardt P. W. Curtius conversion of acids to amines under neutral conditions via an anthrylmethyl carbamate. J. Org. Chem. 1979, 44, 883–884. [Google Scholar]
- Yamatsugu K.; Kamijo S.; Suto Y.; Kanai M.; Shibasaki M. A concise synthesis of Tamiflu: third generation route via the Diels–Alder reaction and the Curtius rearrangement. Tetrahedron Lett. 2007, 48, 1403–1406. [Google Scholar]
- Overman L. E.; Taylor G. F.; Petty C. B.; Jessup P. J. trans-1-N-Acylamino-1,3-dienes: preparation from dienoic acids. J. Org. Chem. 1978, 43, 2164–2167. [Google Scholar]
- Dussault P. H.; Xu C. Curtius rearrangement and Wolff homologation of functionalized peroxides. Tetrahedron Lett. 2004, 45, 7455–7457. [Google Scholar]
- Jean L.; Baglin I.; Rouden J.; Maddaluno J.; Lasne M. C. A convenient route to 1-benzyl 3-aminopyrrolidine and 3-aminopiperidine. Tetrahedron Lett. 2001, 42, 5645–5649. [Google Scholar]
- Koza G.; Ozcan S.; Sahin E.; Balci M. Regioselective synthesis of the 3,4-dihydrofuro[3,2-d]pyrimidin-2(1H)-one skeleton: a new class of compound. Tetrahedron 2009, 65, 5973–5976. [Google Scholar]
- Ma D.; Sun H. General route to 2,4,5-trisubstituted piperidines from enantiopure β-amino esters. Total synthesis of Pseudodistomin B triacetate and Pseudodistomin F. J. Org. Chem. 2000, 65, 6009–6016. [DOI] [PubMed] [Google Scholar]
- Migawa M. T.; Swayze E. E. A solid-phase synthesis of N,N′-disubstituted ureas and perhydroimidazo[1,5-a]pyrazines via the Curtius rearrangement. Org. Lett. 2000, 2, 3309–3311. [DOI] [PubMed] [Google Scholar]
- Wolff O.; Waldvogel S. R. Reliable protocol for the large scale synthesis of diphenylphosphoryl azide (DPPA). Synthesis 2004, 1303–1305. [Google Scholar]
- Nowick J. S.; Powell N. A.; Nguyen T. M.; Noronha G. An improved method for the synthesis of enantiomerically pure amino-acid ester isocyanates. J. Org. Chem. 1992, 57, 7364–7366. [Google Scholar]
- Majer P.; Randad R. S. A safe and efficient method for preparation of N,N′-unsymmetrically disubstituted ureas utilizing triphosgene. J. Org. Chem. 1994, 59, 1937–1938. [Google Scholar]
- Batey R. A.; Santhakumar V.; Yoshina-Ishii C.; Taylor S. D. An efficient new protocol for the formation of unsymmetrical tri- and tetrasubstituted ureas. Tetrahedron Lett. 1998, 39, 6267–6270. [Google Scholar]
- Ozaki S. Recent advances in isocyanate chemistry. Chem. Rev. 1972, 72, 457–496. [Google Scholar]
- Abla M.; Chol J. C.; Sakakura T. Halogen-free process for the conversion of carbon dioxide to urethanes by homogeneous catalysis. Chem. Commun. 2001, 2238–2239. [DOI] [PubMed] [Google Scholar]
- Yoshida Y.; Ishii S.; Yamashita T. A direct synthesis of carbamate ester from carbon dioxide, amine and alkyl halide. Chem. Lett. 1984, 13, 1571–1572. [Google Scholar]
- Kim J. G.; Jang D. O. Indium-catalyzed reaction for the synthesis of carbamates and carbonates: selective protection of amino groups. Tetrahedron Lett. 2009, 50, 2688–2692. [Google Scholar]
- Matassa V. G.; Maduskuie T. P. Jr.; Shapiro H. S.; Hesp B.; Snyder D. W.; Aharony D.; Krell R. D.; Keith R. A. Evolution of a series of peptidoleukotriene antagonists: synthesis and structure/activity relationships of 1,3,5-substituted indoles and indazoles. J. Med. Chem. 1990, 33, 1781–1790. [DOI] [PubMed] [Google Scholar]
- Roberts N. A.; Martin J. A.; Kinchington D.; Broadhurst A. V.; Craig J. C.; Duncan I. B.; Galpin S. A.; Handa B. K.; Kay J.; Krohn A.; Lambert R. W.; Merrett J. H.; Mills J. S.; Parkes K. E. B.; Redshaw S.; Ritchie A. J.; Taylor D. L.; Thomas G. J.; Machin P. J. Rational design of peptide-based HIV proteinase inhibitors. Science 1990, 248, 358–361. [DOI] [PubMed] [Google Scholar]
- Eckert H.; Forster B. Triphosgene, a crystalline phosgene substitute. Angew. Chem., Int. Ed. Engl. 1987, 26, 894–895. [Google Scholar]
- Denarié M.; Grehouillat D.; Malfroot T.; Senet J. P.; Sennyey G.; Wolf P. 2,2′-Carbonyl-bis(3,5-dioxo-4-methyl-1,2,4-oxadiazolidine): a new reagent for the preparation of carbamates and amides, application to the synthesis of dipeptides. Tetrahedron Lett. 1987, 28, 5823–5826. [Google Scholar]
- Takeda K.; Tsuboyama K.; Yamaguchi K.; Ogura H. 1,1′-Bis[6-(trifluoromethyl)benzotriazolyl] oxalate (BTBO): a new reactive coupling reagent for the synthesis of dipeptides, esters, and thio esters. J. Org. Chem. 1985, 50, 273–275. [Google Scholar]
- Kornblum N.; Scott A. A new method for protecting amines. J. Org. Chem. 1977, 42, 399–400. [Google Scholar]
- Rosowsky A.; Wright J. E. N.omega.-Alkoxycarbonylation of .alpha.,.omega.-diamino acids with 2-(trimethylsilyl)ethyl 4-nitrophenyl carbonate. J. Org. Chem. 1983, 48, 1539–1541. [Google Scholar]
- Dressman B. A.; Spangle L. A.; Kaldor S. W. Solid phase synthesis of hydantoins using a carbamate linker and a novel cyclization/cleavage step. Tetrahedron Lett. 1996, 37, 937–940. [Google Scholar]
- Rahmathullah S. M.; Hall J. E.; Bender B. C.; McCurdy D. R.; Tidwell R. R.; Boykin D. W. Prodrugs for amidines: synthesis and anti-Pneumocystis carinii activity of carbamates of 2,5-bis(4-amidinophenyl)furan. J. Med. Chem. 1999, 42, 3994–4000. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Bilcer G.; Schiltz G. Syntheses of FDA approved HIV protease inhibitors. Synthesis 2001, 2203–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.; Steffey M.; Agniswamy J.; Wang Y. F.; Amano M.; Weber I. T.; Mitsuya H. Substituent effects on P2-cyclopentyltetrahydrofuranyl urethanes: design, synthesis, and X-ray studies of potent HIV-1 protease inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2308–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Gemma S.; Simoni E.; Baldridge A.; Walters D. E.; Ide K.; Tojo Y.; Koh Y.; Amano M.; Mitsuya H. Synthesis and biological evaluation of novel allophenylnorstatine-based HIV-1 protease inhibitors incorporating high affinity P2-ligands. Bioorg. Med. Chem. Lett. 2010, 20, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.; Baldridge A.; Ide K.; Koh Y.; Mitsuya H. Design and synthesis of stereochemically defined novel spirocyclic P2-ligands for HIV-1 protease inhibitors. Org. Lett. 2008, 10, 5135–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H.; Kobayashi T.; Shimizu K.; Kawabe K.; Takeda K. A novel active ester synthesis reagent (N,N′-disuccinimidyl carbonate). Tetrahedron Lett. 1979, 20, 4745–4746. [Google Scholar]
- Ogura H.; Sato O.; Takeda K. β-Elimination of β-hydroxyamino acids with disuccinimido carbonate. Tetrahedron Lett. 1981, 22, 4817–4818. [Google Scholar]
- Takeda K.; Tsuboyama K.; Hoshino M.; Kishino M.; Ogura H. A synthesis of a new type of alkoxycarbonylating reagents from 1,1-bis[6-(trifluoromethyl)benzotriazolyl] carbonate (BTBC) and their reactions. Synthesis 1987, 557–560. [Google Scholar]
- Sunggak K.; Jae I. L.; Young K. K. Di-2-pyridyl carbonate: a new efficient coupling agent for the direct esterification of carboxylic acids. Tetrahedron Lett. 1984, 25, 4943–4946. [Google Scholar]
- Ghosh A. K.; Duong T. T.; McKee S. P. Di(2-pyridyl) carbonate promoted alkoxycarbonylation of amines: a convenient synthesis of functionalized carbamates. Tetrahedron Lett. 1991, 32, 4251–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Doung T. T.; McKee S. P.; Thompson W. J. N,N′-Dissuccinimidyl carbonate: a useful reagent for alkoxycarbonylation of amines. Tetrahedron Lett. 1992, 33, 2781–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute R. E.; Rich D. H. Synthesis and evaluation of novel activated mixed carbonate reagents for the introduction of the 2-(trimethylsilyl)ethoxycarbonyl(Teoc)-protecting group. Synthesis 1987, 346–349. [Google Scholar]
- Corey E. J.; Zhang F. Y. re- and si-face-selective nitroaldol reactions catalyzed by a rigid chiral quaternary ammonium salt: a highly stereoselective synthesis of the HIV protease inhibitor Amprenavir (Vertex 478). Angew. Chem., Int. Ed. Engl. 1999, 38, 1931–1934. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Takayama J. Enantioselective synthesis of cyclopentyltetrahydrofuran (Cp-THF), an important high-affinity P2-ligand for HIV-1 protease inhibitors. Tetrahedron Lett. 2008, 49, 3409–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Mckee S. P.; Duong T. T.; Thompson W. J. An efficient synthesis of functionalized urethanes from azides. J. Chem. Soc., Chem. Commun. 1992, 1308–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. D.; Kim H. K.; Kim J. J.; Cho S. D.; Kim S. K.; Shiro M.; Yoon Y. J. N-Nitration of secondary amines with 4-chloro-5-methoxy-2-nitropyridazin-3-one. J. Org. Chem. 2003, 68, 9113–9115. [DOI] [PubMed] [Google Scholar]
- Lee H. G.; Kim M. J.; Park S. E.; Kim J. J.; Kim B. R.; Lee S. G.; Yoon Y. J. Phenyl 4,5-dichloro-6-oxopyridazine-1(6H)-carboxylate as carbonyl source: facile and selective synthesis of carbamates and ureas under mild conditions. Synlett 2009, 2809–2814. [Google Scholar]
- Tsuda T.; Watanabe K.; Miyata K.; Yamamoto H.; Saegusa T. Preparation and characterization of copper(I) amides. Inorg. Chem. 1981, 20, 2728–2730. [Google Scholar]
- Inesi A.; Mucciante V.; Rossi L. A convenient method for the synthesis of carbamate esters from amines and tetraethylammonium hydrogen carbonate. J. Org. Chem. 1998, 63, 1337–1338. [Google Scholar]
- McGhee W. D.; Riley D. P.; Christ M. E.; Christ K. M. Palladium-catalyzed generation of O-allylic urethanes and carbonates from amines/alcohols, carbon dioxide, and allylic chlorides. Organometallics 1993, 12, 1429–1433. [Google Scholar]
- Salvatore R. N.; Flanders V. L.; Ha D.; Jung K. W. Cs2CO3-promoted efficient carbonate and carbamate synthesis on solid phase. Org. Lett. 2000, 2, 2797–2800. [DOI] [PubMed] [Google Scholar]
- Salvatore R. N.; Chu F.; Nagle A. S.; Kapxhiu E. A.; Cross R. M.; Jung K. W. Efficient Cs2CO3-promoted solution and solid phase synthesis of carbonates and carbamates in the presence of TBAI. Tetrahedron 2002, 58, 3329–3347. [Google Scholar]
- Abla M.; Choi J. C.; Sakakura T. Halogen-free process for the conversion of carbon dioxide to urethanes by homogeneous catalysis. Chem. Commun. 2001, 2238–2239. [DOI] [PubMed] [Google Scholar]
- Abla M.; Choi J. C.; Sakakura T. Nickel-catalyzed dehydrative transformation of CO2 to urethanes. Green Chem. 2004, 6, 524–525. [Google Scholar]
- Peterson S. L.; Stucka S. M.; Dinsmore C. J. Parallel synthesis of ureas and carbamates from amines and CO2 under mild conditions. Org. Lett. 2010, 12, 1340–1343. [DOI] [PubMed] [Google Scholar]
- Kodaka M.; Tomohiro T.; Okuno H. The mechanism of the Mitsunobu reaction and its application to CO2 fixation. J. Chem. Soc., Chem. Commun. 1993, 81–82. [Google Scholar]
- Ren L.; Jiao N. PdCl2 catalyzed efficient assembly of organic azides, CO, and alcohols under mild conditions: a direct approach to synthesize carbamates. Chem. Commun. 2014, 50, 3706–3709. [DOI] [PubMed] [Google Scholar]
- Batey R. A.; Santhakumar V.; Yoshina-Ishii C.; Taylor S. D. An efficient new protocol for the formation of unsymmetrical tri- and tetrasubstituted ureas. Tetrahedron Lett. 1998, 39, 6267–6270. [Google Scholar]
- Batey R. A.; Yoshina-Ishii C.; Taylor S. D.; Santhakumar V. A new protocol for the formation of carbamates and thiocarbamates using carbamoyl imidazolium salts. Tetrahedron Lett. 1999, 40, 2669–2672. [Google Scholar]
- Grzyb J. A.; Batey R. A. Carbamoylimidazolium salts as diversification reagents: an application to the synthesis of tertiary amides from carboxylic acids. Tetrahedron Lett. 2003, 44, 7485–7488. [Google Scholar]
- Grzyb J. A.; Shen M.; Yoshina-Ishii C.; Chi W.; Brown R. S.; Batey R. A. Carbamoylimidazolium and thiocarbamoylimidazolium salts: novel reagents for the synthesis of ureas, thioureas, carbamates, thiocarbamates and amides. Tetrahedron 2005, 61, 7153–7175. [Google Scholar]
- Blass B. E. KF/Al2O3 mediated organic synthesis. Tetrahedron 2002, 58, 9301–9320. [Google Scholar]
- Bright Z. R.; Luyeye C. R.; Morton A. S.; Sedenko M.; Landolt R. G.; Bronzi M. J.; Bohovic K. M.; Gonser M. W.; Lapainis T. E.; Hendrickson W. H. Competing reactions of secondary alcohols with sodium hypochlorite promoted by phase-transfer catalysis. J. Org. Chem. 2005, 70, 684–687. [DOI] [PubMed] [Google Scholar]
- Lebel H.; Leogane O. Boc-protected amines via a mild and efficient one-pot Curtius rearrangement. Org. Lett. 2005, 7, 4107–4110. [DOI] [PubMed] [Google Scholar]
- Lebel H.; Leogane O. Curtius rearrangement of aromatic carboxylic acids to access protected anilines and aromatic ureas. Org. Lett. 2006, 8, 5717–5720. [DOI] [PubMed] [Google Scholar]
- Leogane O.; Lebel H. One-pot Curtius rearrangement processes from carboxylic acids. Synthesis 2009, 1935–1940. [Google Scholar]
- Vauthey I.; Valot F.; Gozzi C.; Fache F.; Lemaire M. An environmentally benign access to carbamates and ureas. Tetrahedron Lett. 2000, 41, 6347–6350. [Google Scholar]
- Pandeya R. K.; Dagadeb S. P.; Dongareb M. K.; Kumara P. Synthesis of carbamates using Yttria–Zirconia based Lewis acid catalyst. Synth. Commun. 2003, 33, 4019–4027. [Google Scholar]
- Distaso M.; Quaranta E. Group 3 metal (Sc, La) triflates as catalysts for the carbomethoxylation of aliphatic amines with dimethylcarbonate under mild conditions. Tetrahedron 2004, 60, 1531–1539. [Google Scholar]
- Zhou H.; Shi F.; Tian X.; Zhang Q.; Deng Y. Synthesis of carbamates from aliphatic amines and dimethyl carbonate catalyzed by acid functional ionic liquids. J. Mol. Catal. A: Chem. 2007, 271, 89–92. [Google Scholar]
- Podlech J.; Maier T. C. Indium in organic synthesis. Synthesis 2003, 633–655. [Google Scholar]
- Nair V.; Ros S.; Jayan C. N.; Pillai B. S. Indium- and gallium-mediated carbon–carbon bond-forming reactions in organic synthesis. Tetrahedron 2004, 60, 1959–1982. [Google Scholar]
- Augé J.; Lubin-Germain N.; Uziel J. Recent advances in indium-promoted organic reactions. Synthesis 2007, 1739–1764. [Google Scholar]
- Black D. A.; Arndtsen B. A. General approach to the coupling of organoindium reagents with imines via copper catalysis. Org. Lett. 2006, 8, 1991–1993. [DOI] [PubMed] [Google Scholar]
- Browne E. J. 1,2,4-Triazoles containing nitro groups. Aust. J. Chem. 1969, 22, 2251–2254. [Google Scholar]
- Shimizu M.; Sodeoka M. Convenient method for the preparation of carbamates, carbonates, and thiocarbonates. Org. Lett. 2007, 9, 5231–5234. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S.; Narsihmulu C.; Jagadeshwar V. Ultrasound promoted ‘one pot’ conversion of nitrocompounds to carbamates. Synlett 2002, 771–772. [Google Scholar]
- Porzelle A.; Woodrow M. D.; Tomkinson N. C. O. Facile procedure for the synthesis of N-aryl-N-hydroxy carbamates. Synlett 2009, 798–802. [Google Scholar]
- Liu C. H.; Cheng C. H. The role of alcohol in the catalytic reductive carbonylation of nitrobenzenes to carbamates in the presence of Rh(CO)4– or Ru3(CO)12. J. Organomet. Chem. 1991, 420, 119–123. [Google Scholar]
- Mizuno T.; Alper H. Reductive carbonylation of nitrobenzenes catalyzed by a new binuclear rhodium complex. J. Mol. Catal. A: Chem. 1997, 121, 119–122. [Google Scholar]
- Wehman P.; Kamer P. C. J.; van Leeuwen P. W. N. M. Reductive carbonylation of aromatic dinitro compounds with a palladium(phenanthroline)2(triflate)2 catalyst and an aromatic carboxylic acid as cocatalyst. Chem. Commun. 1996, 217–218. [Google Scholar]
- Chuang S. S. C.; Chen B. J. P.. The synthesis of carbamate from the reductive carbonylation of nitrobenzene over Pd-based catalysts. In Catalysis of Organic Reactions; Sowa J. R., Jr., Ed.; Taylor & Francis: Boca Raton, FL, 2005; Chapter 52. [Google Scholar]
- Krogul A.; Skupińska J.; Litwinienko G. Catalytic activity of PdCl2 complexes with pyridines in nitrobenzene carbonylation. J. Mol. Catal. A: Chem. 2011, 337, 9–16. [Google Scholar]
- Shivarkar A. B.; Gupte S. P.; Chaudhari R. V. Carbamate synthesis via transfunctionalization of substituted ureas and carbonates. J. Mol. Catal. A: Chem. 2004, 223, 85–92. [Google Scholar]
- Shaikh A. A. G.; Sivaram S. Dialkyl and diaryl carbonates by carbonate interchange reaction with dimethyl carbonate. Ind. Eng. Chem. Res. 1992, 31, 1167–1170. [Google Scholar]
- Suciu E. N.; Kuhlmann B.; Knudsen G. A.; Michaelson R. C. Investigation of dialkyltin compounds as catalysts for the synthesis of dialkyl carbonates from alkyl carbamates. J. Organomet. Chem. 1998, 556, 41–54. [Google Scholar]
- Han C.; Porco J. R. Synthesis of carbamates and ureas using Zr(IV)-catalyzed exchange processes. Org. Lett. 2007, 9, 1517–1520. [DOI] [PubMed] [Google Scholar]
- Padiya K. J.; Gavade S.; Kardile B.; Tiwari M.; Bajare S.; Mane M.; Gaware V.; Varghese S.; Harel D.; Kurhade S. Unprecedented “in water” imidazole carbonylation: paradigm shift for preparation of urea and carbamate. Org. Lett. 2012, 14, 2814–2817. [DOI] [PubMed] [Google Scholar]
- Kreye O.; Wald S.; Meiera M. A. R. Introducing catalytic Lossen rearrangements: sustainable access to carbamates and amines. Adv. Synth. Catal. 2013, 355, 81–86. [Google Scholar]
- Dube P.; Nathel N. F.; Vetelino M.; Couturier M.; Aboussafy C. L.; Pichette S.; Jorgensen M. L.; Hardink M. Carbonyldiimidazole-mediated Lossen rearrangement. Org. Lett. 2009, 11, 5622–5625. [DOI] [PubMed] [Google Scholar]
- Hamon F.; Prié G.; Lecornué F.; Papot S. Cyanuric chloride: an efficient reagent for the Lossen rearrangement. Tetrahedron Lett. 2009, 50, 6800–6802. [Google Scholar]
- Williams B. R.; Nazarians A.; Gill M. A. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin. Ther. 2003, 25, 1634–1653. [DOI] [PubMed] [Google Scholar]
- Emre M.; Aarsland D.; Albanese A.; Byrne E. J.; Deuschl G.; De Deyn P. P.; Durif F.; Kulisevsky J.; van Laar T.; Lees A.; Poewe W.; Robillard A.; Rosa M. M.; Wolters E.; Quarg P.; Tekin S.; Lane R. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2509–2518. [DOI] [PubMed] [Google Scholar]
- Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract., Suppl. 2002, 45–63. [PubMed] [Google Scholar]
- Devasthale P. V.; Chen S.; Jeon Y.; Qu F.; Shao C.; Wang W.; Zhang H.; Cap M.; Farrelly D.; Golla R.; Grover G.; Harrity T.; Ma Z.; Moore L.; Ren J.; Seethala R.; Cheng L.; Sleph P.; Sun W.; Tieman A.; Wetterau J. R.; Doweyko A.; Chandrasena G.; Chang S. Y.; Humphreys W. G.; Sasseville V. G.; Biller S. A.; Ryono D. E.; Selan F.; Hariharan N.; Cheng P. T. Design and synthesis of N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5- methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine [Muraglitazar/BMS-298585], a novel peroxisome proliferator-activated receptor alpha/gamma dual agonist with efficacious glucose and lipid-lowering activities. J. Med. Chem. 2005, 48, 2248–2250. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb announces discontinuation of development of investigational oral treatment for type 2 diabetes. http://www.chemeurope.com/en/news/54972/bristol-myers-squibb-announces-discontinuation-of-development-of-investigational-oral-treatment-for-type-2-diabetes.html (accessed November 15, 2014).
- Mousa S. A.; DeGrado W. F.; Mu D. X.; Kapil R. P.; Lucchesi B. R.; Reilly T. M. Oral antiplatelet, antithrombotic efficacy of DMP 728, a novel platelet GPIIb/IIIa antagonist. Circulation 1996, 93, 537–543. [DOI] [PubMed] [Google Scholar]
- Xue C. B.; Wityak J.; Sielecki T. M.; Pinto D. J.; Batt D. G.; Cain G. A.; Sworin M.; Rockwell A. L.; Roderick J. J.; Wang S.; Orwat M. J.; Frietze W. E.; Bostrom L. L.; Liu J.; Higley C. A.; Rankin F. W.; Tobin A. E.; Emmett G.; Lalka G. K.; Sze J. Y.; Di Meo S. V.; Mousa S. A.; Thoolen M. J.; Racanelli A. L.; Hausner E. A.; Reilly T. M.; DeGrado W. F.; Wexler R. R.; Olson R. E. Discovery of an orally active series of isoxazoline glycoprotein IIb/IIIa antagonists. J. Med. Chem. 1997, 40, 2064–2084. [DOI] [PubMed] [Google Scholar]
- Saito A.; Yamashita T.; Mariko Y.; Nosaka Y.; Tsuchiya K.; Ando T.; Suzuki T.; Tsuruo T.; Nakanishi O. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaboin J.; Wild J.; Hamidi H.; Khanna C.; Kim C. J.; Robey R.; Bates S. E.; Thiele C. J. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002, 62, 6108–6115. [PubMed] [Google Scholar]
- Rosato R. R.; Almenara J. A.; Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003, 63, 3637–3645. [PubMed] [Google Scholar]
- Lanchote V. L.; Takayanagui O. M.; Mateus F. H. Enantioselective renal excretion of albendazole metabolites in patients with neurocysticercosis. Chirality 2004, 16, 520–525. [DOI] [PubMed] [Google Scholar]
- Luder P. J.; Siffert B.; Witassek F.; Meister F.; Bircher J. Treatment of hydatid disease with high oral doses of mebendazole. Long-term follow-up of plasma mebendazole levels and drug interactions. Eur. J. Clin. Pharmacol. 1986, 31, 443–448. [DOI] [PubMed] [Google Scholar]
- Teva Pharmaceuticals product line changes: mebendazole tablets. http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM277319.pdf (accessed September 1, 2014).
- Jakovlev V.; Achterrath-Tuckermann U.; von Schlichtegroll A.; Stroman F.; Thiemer K. [General pharmacologic studies on the analgesic flupirtine]. Arzneimittelforschung 1985, 35, 44–55. [PubMed] [Google Scholar]
- Seaman C. A.; Sheridan P. H.; Engel J.; Molliere M.; Narang P. K.; Nice F. J.. Flupirtine. In New Anticonvulsant Drugs; Meldrum B. S., Porter R. J., Eds.; Libbey: London, 1986. [Google Scholar]
- Seydel J. K.; Schaper K. J.; Coats E. A.; Cordes H. P.; Emig P.; Engel J.; Kutscher B.; Polymeropoulos E. E. Synthesis and quantitative structure-activity relationships of anticonvulsant 2,3,6-triaminopyridines. J. Med. Chem. 1994, 37, 3016–3022. [DOI] [PubMed] [Google Scholar]
- White H. S.; Wolf H. H.; Swinyard E. A.; Skeen G. A.; Sofia R. D. A neuropharmacological evaluation of felbamate as a novel anticonvulsant. Epilepsia 1992, 33, 564–572. [DOI] [PubMed] [Google Scholar]
- Patsalos P. N. Drug interactions with the newer antiepileptic drugs (AEDs)—part 1: pharmacokinetic and pharmacodynamic interactions between AEDs. Clin. Pharmacokinet. 2013, 52, 927–966. [DOI] [PubMed] [Google Scholar]
- Wagner M. L.; Graves N. M.; Marienau K.; Holmes G. B.; Remmel R. P.; Leppik I. E. Discontinuation of phenytoin and carbamazepine in patients receiving felbamate. Epilepsia 1991, 32, 398–406. [DOI] [PubMed] [Google Scholar]
- Reidenberg P.; Glue P.; Banfield C. R.; Colucci R. D.; Meehan J. W.; Radwanski E.; Mojavarian P.; Lin C. C.; Nezamis J.; Guillaume M.; Affrime M. B. Effects of felbamate on the pharmacokinetics of phenobarbital. Clin. Pharmacol. Ther. 1995, 58, 279–287. [DOI] [PubMed] [Google Scholar]
- Best B. M.; Goicoechea M. Efavirenz—still first-line king?. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Madero J.; Villasis-Keever A.; Mendez P.; Mosqueda-Gomez J. L.; Torres-Escobar I.; Gutierrez-Escolano F.; Juarez-Kasusky I.; Magana-Aquino M.; Ramos-Santos C.; Perez-Saleme L.; Rangel-Frausto S.; Antuna-Puente B.; Soto-Ramirez L. E.; Lima V.; Belaunzaran-Zamudio F.; Crabtree-Ramirez B.; Montaner J. Prospective, randomized, open label trial of Efavirenz vs Lopinavir/Ritonavir in HIV+ treatment-naive subjects with CD4+<200 cell/mm3 in Mexico. J. Acquired Immune Defic. Syndr. 2010, 53, 582–588. [DOI] [PubMed] [Google Scholar]
- Ogburn E. T.; Jones D. R.; Masters A. R.; Xu C.; Guo Y.; Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug. Metab. Dispos. 2010, 38, 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. A.; Gorski J. C.; Jones D. R.; Hall S. D.; Flockhart D. A.; Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 2003, 306, 287–300. [DOI] [PubMed] [Google Scholar]
- Desta Z.; Saussele T.; Ward B.; Blievernicht J.; Li L.; Klein K.; Flockhart D. A.; Zanger U. M. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007, 8, 547–558. [DOI] [PubMed] [Google Scholar]
- Phipatanakul W.; Eggleston P. A.; Conover-Walker M. K.; Kesavanathan J.; Sweitzer D.; Wood R. A. A randomized, double-blind, placebo-controlled trial of the effect of zafirlukast on upper and lower respiratory responses to cat challenge. J. Allergy Clin. Immunol. 2000, 105, 704–710. [DOI] [PubMed] [Google Scholar]
- Matassa V. G.; Maduskuie T. P. Jr.; Shapiro H. S.; Hesp B.; Snyder D. W.; Aharony D.; Krell R. D.; Keith R. A. Evolution of a series of peptidoleukotriene antagonists: synthesis and structure/activity relationships of 1,3,5-substituted indoles and indazoles. J. Med. Chem. 1990, 33, 1781–1790. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen P. N.; Koopmans P. P. Pharmacokinetic profile of zafirlukast. Clin. Pharmacokinet. 2002, 41, 105–114. [DOI] [PubMed] [Google Scholar]
- Szybalski W.; Iyer V. N. Crosslinking of DNA by enzymatically or chemically activated mitomycins and porfiromycins, bifunctionally “alkylating” antibiotics. Fed. Proc. 1964, 23, 946–957. [PubMed] [Google Scholar]
- Hopkins P. B.; Millard J. T.; Woo J.; Weidner M. F.; Kirchner J. J.; Sigurdsson S. T.; Raucher S. Sequence preferences of DNA interstrand cross-linking agents—importance of minimal DNA structural reorganization in the cross-linking reactions of mechlorethamine, cisplatin, and mitomycin-C. Tetrahedron 1991, 47, 2475–2489. [Google Scholar]
- Alley S. C.; Brameld K. A.; Hopkins P. B. DNA interstrand cross-linking by 2,5-bis(1-aziridinyl)-1,6-benzoquinone—nucleotide-sequence preferences and covalent structure of the Dg-to-Dg cross-links at 5′-D(Gn(N)C) in synthetic oligonucleotide duplexes. J. Am. Chem. Soc. 1994, 116, 2734–2741. [Google Scholar]
- Snodgrass R. G.; Collier A. C.; Coon A. E.; Pritsos C. A. Mitomycin C inhibits ribosomal RNA: a novel cytotoxic mechanism for bioreductive drugs. J. Biol. Chem. 2010, 285, 19068–19075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J.; Pinedo H. M. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs 1990, 1, 5–13. [PubMed] [Google Scholar]
- Kohl N. E.; Emini E. A.; Schleif W. A.; Davis L. J.; Heimbach J. C.; Dixon R. A.; Scolnick E. M.; Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. U.S.A. 1988, 85, 4686–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney E. A.; Humblet C.. Structure-based design: from renin to HIV protease. Structure-Based Ligand Design; Gubernator K., Böhm H.-J., Eds.; Wiley-VCH Verlag GmbH: New York, 1998. [Google Scholar]
- Navia M. A.; Fitzgerald P. M.; McKeever B. M.; Leu C. T.; Heimbach J. C.; Herber W. K.; Sigal I. S.; Darke P. L.; Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature 1989, 337, 615–620. [DOI] [PubMed] [Google Scholar]
- Wlodawer A.; Miller M.; Jaskolski M.; Sathyanarayana B. K.; Baldwin E.; Weber I. T.; Selk L. M.; Clawson L.; Schneider J.; Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science 1989, 245, 616–621. [DOI] [PubMed] [Google Scholar]
- Lapatto R.; Blundell T.; Hemmings A.; Overington J.; Wilderspin A.; Wood S.; Merson J. R.; Whittle P. J.; Danley D. E.; Geoghegan K. F.; Hawrylik S. J.; Lee S. E.; Scheld K. G.; Hobart P. M. X-ray analysis of HIV-1 proteinase at 2.7 A resolution confirms structural homology among retroviral enzymes. Nature 1989, 342, 299–302. [DOI] [PubMed] [Google Scholar]
- New drugs—reports of new drugs recently approved by the FDA. Ritonavir. Bioorg. Med. Chem. 1997, 5, 461–462. [PubMed] [Google Scholar]
- Sevrioukova I. F.; Poulos T. L. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 18422–18427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H.; Millard J.; Rooney J.; Tisdale M.; Parry N.; Sadler B. M.; Blum M. R.; Painter G. In vitro antiviral activity of 141W94 (VX-478) in combination with other antiretroviral agents. Antiviral Res. 1996, 29, 53–56. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Thompson W. J.; McKee S. P.; Duong T. T.; Lyle T. A.; Chen J. C.; Darke P. L.; Zugay J. A.; Emini E. A.; Schleif W. A.; Huff J. R.; Anderson P. F. 3-Tetrahydrofuran and pyran urethanes as high-affinity P2-ligands for HIV-1 protease inhibitors. J. Med. Chem. 1993, 36, 292–294. [DOI] [PubMed] [Google Scholar]
- Kim E. E.; Baker C. T.; Dwyer M. D.; Murcko M. A.; Rao B. G.; Tung R. D.; Navia M. A. Crystal structure of HIV-1 protease in complex with VX-478, a potent and orally bioavailable inhibitor of the enzyme. J. Am. Chem. Soc. 1995, 117, 1181–1182. [Google Scholar]
- Sadler B. M.; Hanson C. D.; Chittick G. E.; Symonds W. T.; Roskell N. S. Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob. Agents Chemother. 1999, 43, 1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm125077.htm (accessed September 1, 2014).
- Bold G.; Fassler A.; Capraro H. G.; Cozens R.; Klimkait T.; Lazdins J.; Mestan J.; Poncioni B.; Rosel J.; Stover D.; Tintelnot-Blomley M.; Acemoglu F.; Beck W.; Boss E.; Eschbach M.; Hurlimann T.; Masso E.; Roussel S.; Ucci-Stoll K.; Wyss D.; Lang M. New aza-dipeptide analogues as potent and orally absorbed HIV-1 protease inhibitors: candidates for clinical development. J. Med. Chem. 1998, 41, 3387–3401. [DOI] [PubMed] [Google Scholar]
- Robinson B. S.; Riccardi K. A.; Gong Y. F.; Guo Q.; Stock D. A.; Blair W. S.; Terry B. J.; Deminie C. A.; Djang F.; Colonno R. J.; Lin P. F. BMS-232632, a highly potent human immunodeficiency virus protease inhibitor that can be used in combination with other available antiretroviral agents. Antimicrob. Agents Chemother. 2000, 44, 2093–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tiec C.; Barrail A.; Goujard C.; Taburet A. M. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 2005, 44, 1035–1050. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.; Weber I. T.; Mitsuya H. Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance. Acc. Chem. Res. 2008, 41, 78–86. [DOI] [PubMed] [Google Scholar]
- Koh Y.; Nakata H.; Maeda K.; Ogata H.; Bilcer G.; Devasamudram T.; Kincaid J. F.; Boross P.; Wang Y. F.; Tie Y.; Volarath P.; Gaddis L.; Harrison R. W.; Weber I. T.; Ghosh A. K.; Mitsuya H. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 2003, 47, 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer S.; Azijn H.; Surleraux D.; Jochmans D.; Tahri A.; Pauwels R.; Wigerinck P.; de Bethune M. P. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 2005, 49, 2314–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA approves new HIV treatment for patients who do not respond to existing drugs. http://www.fda.gov/newsEvents/Newsroom/PressAnnouncements/2006/ucm108676.htm (accessed September 10, 2014).
- Rittweger M.; Arasteh K. Clinical pharmacokinetics of darunavir. Clin. Pharmacokinet. 2007, 46, 739–756. [DOI] [PubMed] [Google Scholar]
- Fujimoto H.; Higuchi M.; Watanabe H.; Koh Y.; Ghosh A. K.; Mitsuya H.; Tanoue N.; Hamada A.; Saito H. P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol. Pharm. Bull. 2009, 32, 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeir M.; Lachau-Durand S.; Mannens G.; Cuyckens F.; van Hoof B.; Raoof A. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 2009, 37, 809–820. [DOI] [PubMed] [Google Scholar]
- Stella V. J.Prodrugs: Challenges and Rewards. Springer:, New York, 2007. [Google Scholar]
- Rautio J.; Kumpulainen H.; Heimbach T.; Oliyai R.; Oh D.; Jarvinen T.; Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discovery 2008, 7, 255–270. [DOI] [PubMed] [Google Scholar]
- Mahato R.; Tai W.; Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug. Delivery Rev. 2011, 63, 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio Zaragoza D.Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. 1st ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Bundgaard H.; Buur A.; Lee V. H. Timolol prodrugs: preparation and hydrolysis kinetics of N-benzoyl carbamate esters of timolol and related compounds. Acta Pharm. Suec. 1988, 25, 293–306. [PubMed] [Google Scholar]
- Springer C. J.; Dowell R.; Burke P. J.; Hadley E.; Davis D. H.; Blakey D. C.; Melton R. G.; Niculescu-Duvaz I. Optimization of alkylating agent prodrugs derived from phenol and aniline mustards: a new clinical candidate prodrug (ZD2767) for antibody-directed enzyme prodrug therapy (ADEPT). J. Med. Chem. 1995, 38, 5051–5065. [DOI] [PubMed] [Google Scholar]
- Springer C. J.; Niculescu-Duvaz I. I. Antibody-directed enzyme prodrug therapy (ADEPT): a review. Adv. Drug. Delivery Rev. 1997, 26, 151–172. [DOI] [PubMed] [Google Scholar]
- Niculescu-Duvaz I.; Springer C. J. Introduction to the background, principles, and state of the art in suicide gene therapy. Methods Mol. Med. 2004, 90, 1–27. [DOI] [PubMed] [Google Scholar]
- Francis R. J.; Sharma S. K.; Springer C.; Green A. J.; Hope-Stone L. D.; Sena L.; Martin J.; Adamson K. L.; Robbins A.; Gumbrell L.; O’Malley D.; Tsiompanou E.; Shahbakhti H.; Webley S.; Hochhauser D.; Hilson A. J.; Blakey D.; Begent R. H. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br. J. Cancer 2002, 87, 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey D. C.; Burke P. J.; Davies D. H.; Dowell R. I.; East S. J.; Eckersley K. P.; Fitton J. E.; McDaid J.; Melton R. G.; Niculescu-Duvaz I. A.; Pinder P. E.; Sharma S. K.; Wright A. F.; Springer C. J. ZD2767, an improved system for antibody-directed enzyme prodrug therapy that results in tumor regressions in colorectal tumor xenografts. Cancer Res. 1996, 56, 3287–3292. [PubMed] [Google Scholar]
- Niculescu-Duvaz D.; Scanlon I.; Niculescu-Duvaz I.; Springer C. J. A higher yielding synthesis of the clinical prodrug ZD2767P using di-protected 4-[N,N-bis(2-hydroxyethyl)amino]phenyl chloroformate. Tetrahedron Lett. 2005, 46, 6919–6922. [Google Scholar]
- Bencharit S.; Morton C. L.; Howard-Williams E. L.; Danks M. K.; Potter P. M.; Redinbo M. R. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat. Struct. Biol. 2002, 9, 337–342. [DOI] [PubMed] [Google Scholar]
- Sanghani S. P.; Quinney S. K.; Fredenburg T. B.; Davis W. I.; Murry D. J.; Bosron W. F. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug. Metab. Dispos. 2004, 32, 505–511. [DOI] [PubMed] [Google Scholar]
- Slatter J. G.; Su P.; Sams J. P.; Schaaf L. J.; Wienkers L. C. Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab. Dispos. 1997, 25, 1157–1164. [PubMed] [Google Scholar]
- Svensson L. A.; Tunek A. The design and bioactivation of presystemically stable prodrugs. Drug. Metab. Rev. 1988, 19, 165–194. [DOI] [PubMed] [Google Scholar]
- Tunek A.; Levin E.; Svensson L. A. Hydrolysis of 3H-bambuterol, a carbamate prodrug of terbutaline, in blood from humans and laboratory animals in vitro. Biochem. Pharmacol. 1988, 37, 3867–3876. [DOI] [PubMed] [Google Scholar]
- Persson G.; Pahlm O.; Gnosspelius Y. Oral Bambuterol versus Terbutaline in patients with asthma. Curr. Ther. Res. Clin. E 1995, 56, 457–465. [Google Scholar]
- Saari W. S.; Schwering J. E.; Lyle P. A.; Smith S. J.; Engelhardt E. L. Cyclization-activated prodrugs. Basic carbamates of 4-hydroxyanisole. J. Med. Chem. 1990, 33, 97–101. [DOI] [PubMed] [Google Scholar]
- Saari W. S.; Schwering J. E.; Lyle P. A.; Smith S. J.; Engelhardt E. L. Cyclization-activated prodrugs. Basic esters of 5-bromo-2′-deoxyuridine. J. Med. Chem. 1990, 33, 2590–2595. [DOI] [PubMed] [Google Scholar]
- Lougerstay-Madec R.; Florent J. C.; Monneret C.; Nemati F.; Poupon M. F. Synthesis of self-immolative glucuronide-based prodrugs of a phenol mustard. Anti-Cancer Drug Des. 1998, 13, 995–1007. [PubMed] [Google Scholar]
- Zhang Z.; Tanabe K.; Hatta H.; Nishimoto S. Bioreduction activated prodrugs of camptothecin: molecular design, synthesis, activation mechanism and hypoxia selective cytotoxicity. Org. Biomol. Chem. 2005, 3, 1905–1910. [DOI] [PubMed] [Google Scholar]
- de Groot F. M. H.; van Berkom L. W. A.; Scheeren H. W. Synthesis and biological evaluation of 2′-carbamate-linked and 2′-carbonate-linked prodrugs of paclitaxel: selective activation by the tumor-associated protease plasmin. J. Med. Chem. 2000, 43, 3093–3102. [DOI] [PubMed] [Google Scholar]
- de Groot F. M. H.; Loos W. J.; Koekkoek R.; van Berkom L. W. A.; Busscher G. F.; Seelen A. E.; Albrecht C.; de Bruijn P.; Scheeren H. W. Elongated multiple electronic cascade and cyclization spacer systems in activatible anticancer prodrugs for enhanced drug release. J. Org. Chem. 2001, 66, 8815–8830. [DOI] [PubMed] [Google Scholar]
- Cundy K. C.; Annamalai T.; Bu L.; De Vera J.; Estrela J.; Luo W.; Shirsat P.; Torneros A.; Yao F.; Zou J.; Barrett R. W.; Gallop M. A. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J. Pharmacol. Exp. Ther. 2004, 311, 324–333. [DOI] [PubMed] [Google Scholar]
- Cundy K. C.; Branch R.; Chernov-Rogan T.; Dias T.; Estrada T.; Hold K.; Koller K.; Liu X.; Mann A.; Panuwat M.; Raillard S. P.; Upadhyay S.; Wu Q. Q.; Xiang J. N.; Yan H.; Zerangue N.; Zhou C. X.; Barrett R. W.; Gallop M. A. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J. Pharmacol. Exp. Ther. 2004, 311, 315–323. [DOI] [PubMed] [Google Scholar]
- Cundy K. C.; Sastry S.; Luo W.; Zou J.; Moors T. L.; Canafax D. M. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J. Clin. Pharmacol. 2008, 48, 1378–1388. [DOI] [PubMed] [Google Scholar]
- HORIZANT: NDA 022399—FDA approved labeling text. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022399s006,s007lbl.pdf (accessed September 1, 2014).
- Quinney S. K.; Sanghani S. P.; Davis W. I.; Hurley T. D.; Sun Z.; Murry D. J.; Bosron W. F. Hydrolysis of capecitabine to 5′-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J. Pharmacol. Exp. Ther. 2005, 313, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Miwa M.; Ura M.; Nishida M.; Sawada N.; Ishikawa T.; Mori K.; Shimma N.; Umeda I.; Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer. 1998, 34, 1274–1281. [DOI] [PubMed] [Google Scholar]
- Venturini M. Rational development of capecitabine. Eur. J. Cancer 2002, 38, 3–9. [DOI] [PubMed] [Google Scholar]
- Walko C. M.; Lindley C. Capecitabine: a review. Clin. Ther. 2005, 27, 23–44. [DOI] [PubMed] [Google Scholar]
- Muller T. H.; Weisenberger H.; Brickl R.; Narjes H.; Himmelsbach F.; Krause J. Profound and sustained inhibition of platelet aggregation by Fradafiban, a nonpeptide platelet glycoprotein IIb/IIIa antagonist, and its orally active prodrug, Lefradafiban, in men. Circulation 1997, 96, 1130–1138. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Ramu Sridhar P.; Kumaragurubaran N.; Koh Y.; Weber I. T.; Mitsuya H. Bis-tetrahydrofuran: a privileged ligand for darunavir and a new generation of hiv protease inhibitors that combat drug resistance. ChemMedChem 2006, 1, 939–950. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Anderson D. D.; Weber I. T.; Mitsuya H. Enhancing protein backbone binding—a fruitful concept for combating drug-resistant HIV. Angew. Chem., Int. Ed. 2012, 51, 1778–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L.; Zhang X. C.; Hartsuck J. A.; Tang J. Crystal structure of an in vivo HIV-1 protease mutant in complex with saquinavir: insights into the mechanisms of drug resistance. Protein Sci. 2000, 9, 1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Thompson W. J.; Holloway M. K.; McKee S. P.; Duong T. T.; Lee H. Y.; Munson P. M.; Smith A. M.; Wai J. M.; Darke P. L.; Zugay J. A.; Emini E. A.; Schleif W. A.; Huff J. R.; Anderson P. F. Potent HIV protease inhibitors: the development of tetrahydrofuranylglycines as novel P2-ligands and pyrazine amides as P3-ligands. J. Med. Chem. 1993, 36, 2300–2310. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.. Anti-HIV protease inhibitor Darunavir. In Introduction to Biopharmaceuticals and Small Molecule Drug Research and Development: Theory and Case Studies; Ganellin R., Jefferis R., Roberts S., Eds.; Elsevier: Waltham, MA, 2013; pp 355–384. [Google Scholar]
- Vazquez M. L.; Bryant M. L.; Clare M.; DeCrescenzo G. A.; Doherty E. M.; Freskos J. N.; Getman D. P.; Houseman K. A.; Julien J. A.; Kocan G. P.; Mueller R. A.; Shieh H.-S.; Stallings W. C.; Stegeman R. A.; Talley J. J. Inhibitors of HIV-1 protease containing the novel and potent (R)-(hydroxyethyl)sulfonamide isostere. J. Med. Chem. 1995, 38, 581–584. [DOI] [PubMed] [Google Scholar]
- Tung R. D.; Livingston D. J.; Rao B. G.; Kim E. E.; Baker C. T.; Boger J. S.; Chambers S. P.; Deininger D. D.; Dwyer M.; Elsayed L.; Fulghum J.; Li B.; Murcko M. A.; Navia M. A.; Novak P.; Pazhanisamy S.; Stuver C.; Thomson J. A., Design and synthesis of Amprenavir, a novel HIV protease inhibitor. In Protease Inihitors in AIDS Therapy; Ogden R. C., Flexner C. W., Eds.; Marcel Dekker Inc.: New York, 2001; pp 101–137. [Google Scholar]
- Ghosh A. K.; Lee H. Y.; Thompson W. J.; Culberson C.; Holloway M. K.; McKee S. P.; Munson P. M.; Duong T. T.; Smith A. M.; Darke P. L.; Zugay J. A.; Emini E. A.; Schleif W. A.; Huff J. R.; Anderson P. F. The development of cyclic sulfolanes as novel and high-affinity P2 ligands for HIV-1 protease inhibitors. J. Med. Chem. 1994, 37, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Kincaid J. F.; Walters D. E.; Chen Y.; Chaudhuri N. C.; Thompson W. J.; Culberson C.; Fitzgerald P. M.; Lee H. Y.; McKee S. P.; Munson P. M.; Duong T. T.; Darke P. L.; Zugay J. A.; Schleif W. A.; Axel M. G.; Lin J.; Huff J. R. Nonpeptidal P2 ligands for HIV protease inhibitors: structure-based design, synthesis, and biological evaluation. J. Med. Chem. 1996, 39, 3278–3290. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Shin D.; Swanson L.; Krishnan K.; Cho H.; Hussain K. A.; Walters D. E.; Holland L.; Buthod J. Structure-based design of non-peptide HIV protease inhibitors. Farmaco 2001, 56, 29–32. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Kincaid J. F.; Cho W.; Walters D. E.; Krishnan K.; Hussain K. A.; Koo Y.; Cho H.; Rudall C.; Holland L.; Buthod J. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 1998, 8, 687–690. [DOI] [PubMed] [Google Scholar]
- Tie Y.; Boross P. I.; Wang Y. F.; Gaddis L.; Hussain A. K.; Leshchenko S.; Ghosh A. K.; Louis J. M.; Harrison R. W.; Weber I. T. High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor (UIC-94017) active against multi-drug-resistant clinical strains. J. Mol. Biol. 2004, 338, 341–352. [DOI] [PubMed] [Google Scholar]
- Kovalevsky A. Y.; Liu F.; Leshchenko S.; Ghosh A. K.; Louis J. M.; Harrison R. W.; Weber I. T. Ultra-high resolution crystal structure of HIV-1 protease mutant reveals two binding sites for clinical inhibitor TMC114. J. Mol. Biol. 2006, 363, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K.; Kato R.; Kavlick M. F.; Nguyen A.; Maroun V.; Maeda K.; Hussain K. A.; Ghosh A. K.; Gulnik S. V.; Erickson J. W.; Mitsuya H. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC-126), and selection of a novel (A28S) mutation in the protease active site. J. Virol. 2002, 76, 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y.; Matsumi S.; Das D.; Amano M.; Davis D. A.; Li J.; Leschenko S.; Baldridge A.; Shioda T.; Yarchoan R.; Ghosh A. K.; Mitsuya H. Potent inhibition of HIV-1 replication by novel non-peptidyl small molecule inhibitors of protease dimerization. J. Biol. Chem. 2007, 282, 28709–28720. [DOI] [PubMed] [Google Scholar]
- Surleraux D. L.; de Kock H. A.; Verschueren W. G.; Pille G. M.; Maes L. J.; Peeters A.; Vendeville S.; De Meyer S.; Azijn H.; Pauwels R.; de Bethune M. P.; King N. M.; Prabu-Jeyabalan M.; Schiffer C. A.; Wigerinck P. B. Design of HIV-1 protease inhibitors active on multidrug-resistant virus. J. Med. Chem. 2005, 48, 1965–1973. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Dawson Z. L.; Mitsuya H. Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorg. Med. Chem. 2007, 15, 7576–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.; Mitsuya H., Darunavir, a new PI with dual mechanism: from a novel drug design concept to new hope against drug-resistant HIV. In Aspartic Acid Proteases as Therapeutic Targets; Ghosh A. K., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Vol. 45, pp 205–243. [Google Scholar]
- Ghosh A. K.; Leshchenko S.; Noetzel M. Stereoselective photochemical 1,3-dioxolane addition to 5-alkoxymethyl-2(5H)-furanone: synthesis of bis-tetrahydrofuranyl ligand for HIV protease inhibitor UIC-94017 (TMC-114). J. Org. Chem. 2004, 69, 7822–7829. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Chen Y. Synthesis and optical resolution of high affinity P2-ligands for HIV-1 protease inhibitors. Tetrahedron Lett. 1995, 36, 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M.; Koh Y.; Das D.; Li J.; Leschenko S.; Wang Y. F.; Boross P. I.; Weber I. T.; Ghosh A. K.; Mitsuya H. A novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI), GRL-98065, is potent against multiple-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 2007, 51, 2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F.; Andrews C. W.; Brieger M.; Furfine E. S.; Hale M. R.; Hanlon M. H.; Hazen R. J.; Kaldor I.; McLean E. W.; Reynolds D.; Sammond D. M.; Spaltenstein A.; Tung R.; Turner E. M.; Xu R. X.; Sherrill R. G. Ultra-potent P1 modified arylsulfonamide HIV protease inhibitors: the discovery of GW0385. Bioorg. Med. Chem. Lett. 2006, 16, 1788–1794. [DOI] [PubMed] [Google Scholar]
- Cihlar T.; He G. X.; Liu X.; Chen J. M.; Hatada M.; Swaminathan S.; McDermott M. J.; Yang Z. Y.; Mulato A. S.; Chen X.; Leavitt S. A.; Stray K. M.; Lee W. A. Suppression of HIV-1 protease inhibitor resistance by phosphonate-mediated solvent anchoring. J. Mol. Biol. 2006, 363, 635–647. [DOI] [PubMed] [Google Scholar]
- Dierynck I.; Van Marck H.; Van Ginderen M.; Jonckers T. H.; Nalam M. N.; Schiffer C. A.; Raoof A.; Kraus G.; Picchio G. TMC310911, a novel human immunodeficiency virus type 1 protease inhibitor, shows in vitro an improved resistance profile and higher genetic barrier to resistance compared with current protease inhibitors. Antimicrob. Agents Chemother. 2011, 55, 5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Sridhar P. R.; Leshchenko S.; Hussain A. K.; Li J.; Kovalevsky A. Y.; Walters D. E.; Wedekind J. E.; Grum-Tokars V.; Das D.; Koh Y.; Maeda K.; Gatanaga H.; Weber I. T.; Mitsuya H. Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance. J. Med. Chem. 2006, 49, 5252–5261. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Gemma S.; Baldridge A.; Wang Y. F.; Kovalevsky A. Y.; Koh Y.; Weber I. T.; Mitsuya H. Flexible cyclic ethers/polyethers as novel P2-ligands for HIV-1 protease inhibitors: design, synthesis, biological evaluation, and protein-ligand X-ray studies. J. Med. Chem. 2008, 51, 6021–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Chapsal B. D.; Baldridge A.; Steffey M. P.; Walters D. E.; Koh Y.; Amano M.; Mitsuya H. Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation. J. Med. Chem. 2011, 54, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Xu C. X.; Rao K. V.; Baldridge A.; Agniswamy J.; Wang Y. F.; Weber I. T.; Aoki M.; Miguel S. G.; Amano M.; Mitsuya H. Probing multidrug-resistance and protein-ligand interactions with oxatricyclic designed ligands in HIV-1 protease inhibitors. ChemMedChem 2010, 5, 1850–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo Gomez P. M.; Amano M.; Yashchuk S.; Mizuno A.; Das D.; Ghosh A. K.; Mitsuya H. GRL-04810 and GRL-05010, difluoride-containing nonpeptidic HIV-1 protease inhibitors (PIs) that inhibit the replication of multi-PI-resistant HIV-1 in vitro and possess favorable lipophilicity that may allow blood-brain barrier penetration. Antimicrob. Agents Chemother. 2013, 57, 6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Osswald H. L. BACE1 (beta-secretase) inhibitors for the treatment of Alzheimer’s disease. Chem. Soc. Rev. 2014, 7, 6765–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Hong L.; Ghosh A. K.. The discovery of β-secretase and development toward a clinical inhibitor for AD: an exciting academic collaboration. In Aspartic Acid Proteases as Therapeutic Targets; Ghosh A. K., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Vol. 45, pp 413–440. [Google Scholar]
- Ghosh A. K.; Shin D. W.; Koelsch G.; Lin X.; Ermolieff J.; Tang J. Design of potent inhibitors for human brain memapsin 2 (β-secretase). J. Am. Chem. Soc. 2000, 122, 3522–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L.; Koelsch G.; Lin X.; Wu S.; Terzyan S.; Ghosh A. K.; Zhang X. C.; Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 2000, 290, 150–153. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Devasamudram T.; Hong L.; DeZutter C.; Xu X.; Weerasena V.; Koelsch G.; Bilcer G.; Tang J. Structure-based design of cycloamide-urethane-derived novel inhibitors of human brain memapsin 2 (beta-secretase). Bioorg. Med. Chem. Lett. 2005, 15, 15–20. [DOI] [PubMed] [Google Scholar]
- Diment S.; Leech M. S.; Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J. Biol. Chem. 1988, 263, 6901–6907. [PubMed] [Google Scholar]
- Ghosh A. K.; Kumaragurubaran N.; Hong L.; Lei H.; Hussain K. A.; Liu C. F.; Devasamudram T.; Weerasena V.; Turner R.; Koelsch G.; Bilcer G.; Tang J. Design, synthesis and X-ray structure of protein-ligand complexes: important insight into selectivity of memapsin 2 (beta-secretase) inhibitors. J. Am. Chem. Soc. 2006, 128, 5310–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freskos J. N.; Fobian Y. M.; Benson T. E.; Bienkowski M. J.; Brown D. L.; Emmons T. L.; Heintz R.; Laborde A.; McDonald J. J.; Mischke B. V.; Molyneaux J. M.; Moon J. B.; Mullins P. B.; Bryan Prince D.; Paddock D. J.; Tomasselli A. G.; Winterrowd G. Design of potent inhibitors of human beta-secretase. Part 1. Bioorg. Med. Chem. Lett. 2007, 17, 73–77. [DOI] [PubMed] [Google Scholar]
- Freskos J. N.; Fobian Y. M.; Benson T. E.; Moon J. B.; Bienkowski M. J.; Brown D. L.; Emmons T. L.; Heintz R.; Laborde A.; McDonald J. J.; Mischke B. V.; Molyneaux J. M.; Mullins P. B.; Bryan Prince D.; Paddock D. J.; Tomasselli A. G.; Winterrowd G. Design of potent inhibitors of human beta-secretase. Part 2. Bioorg. Med. Chem. Lett. 2007, 17, 78–81. [DOI] [PubMed] [Google Scholar]
- Wong G. T.; Manfra D.; Poulet F. M.; Zhang Q.; Josien H.; Bara T.; Engstrom L.; Pinzon-Ortiz M.; Fine J. S.; Lee H. J.; Zhang L.; Higgins G. A.; Parker E. M. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 2004, 279, 12876–12882. [DOI] [PubMed] [Google Scholar]
- Lanz T. A.; Hosley J. D.; Adams W. J.; Merchant K. M. Studies of Abeta pharmacodynamics in the brain, cerebrospinal fluid, and plasma in young (plaque-free) Tg2576 mice using the gamma-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-l-alaninamide (LY-411575). J. Pharmacol. Exp. Ther. 2004, 309, 49–55. [DOI] [PubMed] [Google Scholar]
- Best J. D.; Jay M. T.; Otu F.; Ma J.; Nadin A.; Ellis S.; Lewis H. D.; Pattison C.; Reilly M.; Harrison T.; Shearman M. S.; Williamson T. L.; Atack J. R. Quantitative measurement of changes in amyloid-beta(40) in the rat brain and cerebrospinal fluid following treatment with the gamma-secretase inhibitor LY-411575 [N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-l-alaninamide]. J. Pharmacol. Exp. Ther. 2005, 313, 902–908. [DOI] [PubMed] [Google Scholar]
- Peters J. U.; Galley G.; Jacobsen H.; Czech C.; David-Pierson P.; Kitas E. A.; Ozmen L. Novel orally active, dibenzazepinone-based gamma-secretase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5918–5923. [DOI] [PubMed] [Google Scholar]
- Guo T.; Gu H.; Hobbs D. W.; Rokosz L. L.; Stauffer T. M.; Jacob B.; Clader J. W. Design, synthesis, and evaluation of tetrahydroquinoline and pyrrolidine sulfonamide carbamates as gamma-secretase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 3010–3013. [DOI] [PubMed] [Google Scholar]
- McBriar M. D.; Clader J. W.; Chu I.; Del Vecchio R. A.; Favreau L.; Greenlee W. J.; Hyde L. A.; Nomeir A. A.; Parker E. M.; Pissarnitski D. A.; Song L.; Zhang L.; Zhao Z. Discovery of amide and heteroaryl isosteres as carbamate replacements in a series of orally active gamma-secretase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 215–219. [DOI] [PubMed] [Google Scholar]
- Pissarnitski D. A.; Asberom T.; Bara T. A.; Buevich A. V.; Clader J. W.; Greenlee W. J.; Guzik H. S.; Josien H. B.; Li W.; McEwan M.; McKittrick B. A.; Nechuta T. L.; Parker E. M.; Sinning L.; Smith E. M.; Song L.; Vaccaro H. A.; Voigt J. H.; Zhang L.; Zhang Q.; Zhao Z. 2,6-Disubstituted N-arylsulfonyl piperidines as gamma-secretase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 57–62. [DOI] [PubMed] [Google Scholar]
- Bergstrom C. P.; Sloan C. P.; Wang H. H.; Parker M. F.; Smith D. W.; Zheng M.; Hansel S. B.; Polson C. T.; Barber L. E.; Bursuker I.; Guss V. L.; Corsa J. A.; Barten D. M.; Felsenstein K. M.; Roberts S. B. Nitrogen-appended N-alkylsulfonamides as inhibitors of gamma-secretase. Bioorg. Med. Chem. Lett. 2008, 18, 175–178. [DOI] [PubMed] [Google Scholar]
- Bergstrom C. P.; Sloan C. P.; Lau W. Y.; Smith D. W.; Zheng M.; Hansel S. B.; Polson C. T.; Corsa J. A.; Barten D. M.; Felsenstein K. M.; Roberts S. B. Carbamate-appended N-alkylsulfonamides as inhibitors of gamma-secretase. Bioorg. Med. Chem. Lett. 2008, 18, 464–468. [DOI] [PubMed] [Google Scholar]
- Heutinck K. M.; ten Berge I. J.; Hack C. E.; Hamann J.; Rowshani A. T. Serine proteases of the human immune system in health and disease. Mol. Immunol. 2010, 47, 1943–1955. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C.; Bond J. S. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C.; Asgian J. L.; Ekici O. D.; James K. E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002, 102, 4639–4750. [DOI] [PubMed] [Google Scholar]
- Walker B.; Lynas J. F. Strategies for the inhibition of serine proteases. Cell. Mol. Life Sci. 2001, 58, 596–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.; Abbenante G.; Fairlie D. P. Protease inhibitors: current status and future prospects. J. Med. Chem. 2000, 43, 305–341. [DOI] [PubMed] [Google Scholar]
- Perona J. J.; Craik C. S. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995, 4, 337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksyszyn J.; Boduszek B.; Kam C. M.; Powers J. C. Novel amidine-containing peptidyl phosphonates as irreversible inhibitors for blood coagulation and related serine proteases. J. Med. Chem. 1994, 37, 226–231. [DOI] [PubMed] [Google Scholar]
- Abuelyaman A. S.; Jackson D. S.; Hudig D.; Woodard S. L.; Powers J. C. Synthesis and kinetic studies of diphenyl 1-(N-peptidylamino)alkanephosphonate esters and their biotinylated derivatives as inhibitors of serine proteases and probes for lymphocyte granzymes. Arch. Biochem. Biophys. 1997, 344, 271–280. [DOI] [PubMed] [Google Scholar]
- Stone S. R. Thrombin inhibitors: a new generation of antithrombotics. Trends Cardiovasc. Med. 1995, 5, 134–140. [DOI] [PubMed] [Google Scholar]
- Luchtman-Jones L.; Broze G. J. Jr. The current status of coagulation. Ann. Med. 1995, 27, 47–52. [DOI] [PubMed] [Google Scholar]
- Lim M. S.; Johnston E. R.; Kettner C. A. The solution conformation of (D)Phe-Pro-containing peptides: implications on the activity of Ac-(D)Phe-Pro-boroArg-OH, a potent thrombin inhibitor. J. Med. Chem. 1993, 36, 1831–1838. [DOI] [PubMed] [Google Scholar]
- Cacciola J.; Fevig J. M.; Alexander R. S.; Brittelli D. R.; Kettner C. A.; Knabb R. M.; Weber P. C. Synthesis of conformationally-restricted boropeptide thrombin inhibitors. Bioorg. Med. Chem. Lett. 1996, 6, 301–306. [Google Scholar]
- Imperiali B.; Abeles R. H. Inhibition of serine proteases by peptidyl fluoromethyl ketones. Biochemistry 1986, 25, 3760–3767. [DOI] [PubMed] [Google Scholar]
- Veale C. A.; Bernstein P. R.; Bryant C.; Ceccarelli C.; Damewood J. R. Jr.; Earley R.; Feeney S. W.; Gomes B.; Kosmider B. J.; Steelman G. B.; Thomas R. M.; Vacek E. P.; Williams J. C.; Wolanin D. J.; Woolson S. Nonpeptidic inhibitors of human leukocyte elastase. 5. Design, synthesis, and X-ray crystallography of a series of orally active 5-aminopyrimidin-6-one-containing trifluoromethyl ketones. J. Med. Chem. 1995, 38, 98–108. [DOI] [PubMed] [Google Scholar]
- Bernstein P. R.; Gomes B. C.; Kosmider B. J.; Vacek E. P.; Williams J. C. Nonpeptidic inhibitors of human leukocyte elastase. 6. Design of a potent, intratracheally active, pyridone-based trifluoromethyl ketone. J. Med. Chem. 1995, 38, 212–215. [DOI] [PubMed] [Google Scholar]
- Edwards P. D.; Andisik D. W.; Bryant C. A.; Ewing B.; Gomes B.; Lewis J. J.; Rakiewicz D.; Steelman G.; Strimpler A.; Trainor D. A.; Tuthill P. A.; Mauger R. C.; Veale C. A.; Wildonger R. A.; Williams J. C.; Wolanin D. J.; Zottola M. Discovery and biological activity of orally active peptidyl trifluoromethyl ketone inhibitors of human neutrophil elastase. J. Med. Chem. 1997, 40, 1876–1885. [DOI] [PubMed] [Google Scholar]
- Veale C. A.; Bernstein P. R.; Bohnert C. M.; Brown F. J.; Bryant C.; Damewood J. R. Jr.; Earley R.; Feeney S. W.; Edwards P. D.; Gomes B.; Hulsizer J. M.; Kosmider B. J.; Krell R. D.; Moore G.; Salcedo T. W.; Shaw A.; Silberstein D. S.; Steelman G. B.; Stein M.; Strimpler A.; Thomas R. M.; Vacek E. P.; Williams J. C.; Wolanin D. J.; Woolson S. Orally active trifluoromethyl ketone inhibitors of human leukocyte elastase. J. Med. Chem. 1997, 40, 3173–3181. [DOI] [PubMed] [Google Scholar]
- Zoulim F.; Chevallier M.; Maynard M.; Trepo C. Clinical consequences of hepatitis C virus infection. Rev. Med. Virol. 2003, 13, 57–68. [DOI] [PubMed] [Google Scholar]
- Njoroge F. G.; Chen K. X.; Shih N. Y.; Piwinski J. J. Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection. Acc. Chem. Res. 2008, 41, 50–59. [DOI] [PubMed] [Google Scholar]
- Thomson J. A.; Perni R. B. Hepatitis C virus NS3-4A protease inhibitors: countering viral subversion in vitro and showing promise in the clinic. Curr. Opin. Drug Discovery Dev. 2006, 9, 606–617. [PubMed] [Google Scholar]
- Lamarre D.; Anderson P. C.; Bailey M.; Beaulieu P.; Bolger G.; Bonneau P.; Bos M.; Cameron D. R.; Cartier M.; Cordingley M. G.; Faucher A. M.; Goudreau N.; Kawai S. H.; Kukolj G.; Lagace L.; LaPlante S. R.; Narjes H.; Poupart M. A.; Rancourt J.; Sentjens R. E.; St George R.; Simoneau B.; Steinmann G.; Thibeault D.; Tsantrizos Y. S.; Weldon S. M.; Yong C. L.; Llinas-Brunet M. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 2003, 426, 186–189. [DOI] [PubMed] [Google Scholar]
- Venkatraman S.; Bogen S. L.; Arasappan A.; Bennett F.; Chen K.; Jao E.; Liu Y. T.; Lovey R.; Hendrata S.; Huang Y.; Pan W.; Parekh T.; Pinto P.; Popov V.; Pike R.; Ruan S.; Santhanam B.; Vibulbhan B.; Wu W.; Yang W.; Kong J.; Liang X.; Wong J.; Liu R.; Butkiewicz N.; Chase R.; Hart A.; Agrawal S.; Ingravallo P.; Pichardo J.; Kong R.; Baroudy B.; Malcolm B.; Guo Z.; Prongay A.; Madison V.; Broske L.; Cui X.; Cheng K. C.; Hsieh Y.; Brisson J. M.; Prelusky D.; Korfmacher W.; White R.; Bogdanowich-Knipp S.; Pavlovsky A.; Bradley P.; Saksena A. K.; Ganguly A.; Piwinski J.; Girijavallabhan V.; Njoroge F. G. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: a potential therapeutic agent for the treatment of hepatitis C infection. J. Med. Chem. 2006, 49, 6074–6086. [DOI] [PubMed] [Google Scholar]
- Malcolm B. A.; Liu R.; Lahser F.; Agrawal S.; Belanger B.; Butkiewicz N.; Chase R.; Gheyas F.; Hart A.; Hesk D.; Ingravallo P.; Jiang C.; Kong R.; Lu J.; Pichardo J.; Prongay A.; Skelton A.; Tong X.; Venkatraman S.; Xia E.; Girijavallabhan V.; Njoroge F. G. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob. Agents Chemother. 2006, 50, 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasappan A.; Njoroge F. G.; Chan T. Y.; Bennett F.; Bogen S. L.; Chen K.; Gu H.; Hong L.; Jao E.; Liu Y. T.; Lovey R. G.; Parekh T.; Pike R. E.; Pinto P.; Santhanam B.; Venkatraman S.; Vaccaro H.; Wang H.; Yang X.; Zhu Z.; McKittrick B.; Saksena A. K.; Girijavallabhan V.; Pichardo J.; Butkiewicz N.; Ingram R.; Malcolm B.; Prongay A.; Yao N.; Marten B.; Madison V.; Kemp S.; Levy O.; Lim-Wilby M.; Tamura S.; Ganguly A. K. Hepatitis C virus NS3-4A serine protease inhibitors: SAR of P′2 moiety with improved potency. Bioorg. Med. Chem. Lett. 2005, 15, 4180–4184. [DOI] [PubMed] [Google Scholar]
- Bogen S. L.; Ruan S.; Liu R.; Agrawal S.; Pichardo J.; Prongay A.; Baroudy B.; Saksena A. K.; Girijavallabhan V.; Njoroge F. G. Depeptidization efforts on P3-P2′ alpha-ketoamide inhibitors of HCV NS3-4A serine protease: effect on HCV replicon activity. Bioorg. Med. Chem. Lett. 2006, 16, 1621–1627. [DOI] [PubMed] [Google Scholar]
- Bogen S. L.; Arasappan A.; Bennett F.; Chen K.; Jao E.; Liu Y. T.; Lovey R. G.; Venkatraman S.; Pan W.; Parekh T.; Pike R. E.; Ruan S.; Liu R.; Baroudy B.; Agrawal S.; Chase R.; Ingravallo P.; Pichardo J.; Prongay A.; Brisson J. M.; Hsieh T. Y.; Cheng K. C.; Kemp S. J.; Levy O. E.; Lim-Wilby M.; Tamura S. Y.; Saksena A. K.; Girijavallabhan V.; Njoroge F. G. Discovery of SCH446211 (SCH6): a new ketoamide inhibitor of the HCV NS3 serine protease and HCV subgenomic RNA replication. J. Med. Chem. 2006, 49, 2750–2757. [DOI] [PubMed] [Google Scholar]
- Venkatraman S. Discovery of boceprevir, a direct-acting NS3/4A protease inhibitor for treatment of chronic hepatitis C infections. Trends Pharmacol. Sci. 2012, 33, 289–294. [DOI] [PubMed] [Google Scholar]
- Boloor A.; Hanway D.; Joshi M.; Winn D. T.; Mendez G.; Walls M.; Wei P.; Qian F.; Zhang X.; Zhang Y.; Hepperle M. E.; Li X.; Campbell D. A.; Betancort J. M. Synthesis and antiviral activity of HCV NS3/4A peptidomimetic boronic acid inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5708–5711. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang Y. K.; Liu Y.; Ding C. Z.; Zhou Y.; Li Q.; Plattner J. J.; Baker S. J.; Zhang S.; Kazmierski W. M.; Wright L. L.; Smith G. K.; Grimes R. M.; Crosby R. M.; Creech K. L.; Carballo L. H.; Slater M. J.; Jarvest R. L.; Thommes P.; Hubbard J. A.; Convery M. A.; Nassau P. M.; McDowell W.; Skarzynski T. J.; Qian X.; Fan D.; Liao L.; Ni Z. J.; Pennicott L. E.; Zou W.; Wright J. Novel macrocyclic HCV NS3 protease inhibitors derived from alpha-amino cyclic boronates. Bioorg. Med. Chem. Lett. 2010, 20, 5695–5700. [DOI] [PubMed] [Google Scholar]
- Hu L. Y.; Abeles R. H. Inhibition of cathepsin B and papain by peptidyl alpha-keto esters, alpha-keto amides, alpha-diketones, and alpha-keto acids. Arch. Biochem. Biophys. 1990, 281, 271–274. [DOI] [PubMed] [Google Scholar]
- Chen K. X.; Njoroge F. G.; Pichardo J.; Prongay A.; Butkiewicz N.; Yao N.; Madison V.; Girijavallabhan V. Design, synthesis, and biological activity of m-tyrosine-based 16- and 17-membered macrocyclic inhibitors of hepatitis C virus NS3 serine protease. J. Med. Chem. 2005, 48, 6229–6235. [DOI] [PubMed] [Google Scholar]
- Liu R.; Abid K.; Pichardo J.; Pazienza V.; Ingravallo P.; Kong R.; Agrawal S.; Bogen S.; Saksena A.; Cheng K. C.; Prongay A.; Njoroge F. G.; Baroudy B. M.; Negro F. In vitro antiviral activity of SCH446211 (SCH6), a novel inhibitor of the hepatitis C virus NS3 serine protease. J. Antimicrob. Chemother. 2007, 59, 51–58. [DOI] [PubMed] [Google Scholar]
- Venkatraman S.; Velazquez F.; Wu W.; Blackman M.; Chen K. X.; Bogen S.; Nair L.; Tong X.; Chase R.; Hart A.; Agrawal S.; Pichardo J.; Prongay A.; Cheng K. C.; Girijavallabhan V.; Piwinski J.; Shih N. Y.; Njoroge F. G. Discovery and structure–activity relationship of P1–P3 ketoamide derived macrocyclic inhibitors of hepatitis C virus NS3 protease. J. Med. Chem. 2009, 52, 336–346. [DOI] [PubMed] [Google Scholar]
- Edwards P. D.; Bernstein P. R. Synthetic inhibitors of elastase. Med. Res. Rev. 1994, 14, 127–194. [DOI] [PubMed] [Google Scholar]
- Gemma S.; Brogi S.; Novellino E.; Campiani G.; Maga G.; Brindisi M.; Butini S. HCV-targeted antivirals: current status and future challenges. Curr. Pharm. Des. 2014, 20, 3445–3464. [DOI] [PubMed] [Google Scholar]
- Belda O.; Targett-Adams P. Small molecule inhibitors of the hepatitis C virus-encoded NS5A protein. Virus Res. 2012, 170, 1–14. [DOI] [PubMed] [Google Scholar]
- Gao M.; Nettles R. E.; Belema M.; Snyder L. B.; Nguyen V. N.; Fridell R. A.; Serrano-Wu M. H.; Langley D. R.; Sun J. H.; O’Boyle D. R. II; Lemm J. A.; Wang C.; Knipe J. O.; Chien C.; Colonno R. J.; Grasela D. M.; Meanwell N. A.; Hamann L. G. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010, 465, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj J.; Dahari H.; Rong L.; Sansone N. D.; Nettles R. E.; Cotler S. J.; Layden T. J.; Uprichard S. L.; Perelson A. S. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 3991–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawitz E. J.; Gruener D.; Hill J. M.; Marbury T.; Moorehead L.; Mathias A.; Cheng G.; Link J. O.; Wong K. A.; Mo H.; McHutchison J. G.; Brainard D. M. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J. Hepatol. 2012, 57, 24–31. [DOI] [PubMed] [Google Scholar]
- Afdhal N.; Zeuzem S.; Kwo P.; Chojkier M.; Gitlin N.; Puoti M.; Romero-Gomez M.; Zarski J. P.; Agarwal K.; Buggisch P.; Foster G. R.; Brau N.; Buti M.; Jacobson I. M.; Subramanian G. M.; Ding X.; Mo H.; Yang J. C.; Pang P. S.; Symonds W. T.; McHutchison J. G.; Muir A. J.; Mangia A.; Marcellin P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [DOI] [PubMed] [Google Scholar]
- FDA approves first combination pill to treat hepatitis C. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm418365.htm (accessed November 15, 2014).
- Barrett A. J.; Rawlings N. D. Evolutionary lines of cysteine peptidases. Biol. Chem. 2001, 382, 727–733. [DOI] [PubMed] [Google Scholar]
- Leung-Toung R.; Zhao Y.; Li W.; Tam T. F.; Karimian K.; Spino M. Thiol proteases: inhibitors and potential therapeutic targets. Curr. Med. Chem. 2006, 13, 547–581. [DOI] [PubMed] [Google Scholar]
- Buhling F.; Fengler A.; Brandt W.; Welte T.; Ansorge S.; Nagler D. K. Review: novel cysteine proteases of the papain family. Adv. Exp. Med. Biol. 2000, 477, 241–254. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv. Enzymol. Relat. Areas Mol. Biol. 1990, 63, 271–347. [DOI] [PubMed] [Google Scholar]
- Santos M. M.; Moreira R. Michael acceptors as cysteine protease inhibitors. Mini-Rev. Med. Chem. 2007, 7, 1040–1050. [DOI] [PubMed] [Google Scholar]
- Vicik R.; Busemann M.; Baumann K.; Schirmeister T. Inhibitors of cysteine proteases. Curr. Top. Med. Chem. 2006, 6, 331–353. [DOI] [PubMed] [Google Scholar]
- Nicoll-Griffith D. A. Use of cysteine-reactive small molecules in drug discovery for trypanosomal disease. Expert Opin. Drug. Discovery 2012, 7, 353–366. [DOI] [PubMed] [Google Scholar]
- Drosten C.; Gunther S.; Preiser W.; van der Werf S.; Brodt H. R.; Becker S.; Rabenau H.; Panning M.; Kolesnikova L.; Fouchier R. A.; Berger A.; Burguiere A. M.; Cinatl J.; Eickmann M.; Escriou N.; Grywna K.; Kramme S.; Manuguerra J. C.; Muller S.; Rickerts V.; Sturmer M.; Vieth S.; Klenk H. D.; Osterhaus A. D.; Schmitz H.; Doerr H. W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [DOI] [PubMed] [Google Scholar]
- Ksiazek T. G.; Erdman D.; Goldsmith C. S.; Zaki S. R.; Peret T.; Emery S.; Tong S.; Urbani C.; Comer J. A.; Lim W.; Rollin P. E.; Dowell S. F.; Ling A. E.; Humphrey C. D.; Shieh W. J.; Guarner J.; Paddock C. D.; Rota P.; Fields B.; DeRisi J.; Yang J. Y.; Cox N.; Hughes J. M.; LeDuc J. W.; Bellini W. J.; Anderson L. J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Xi K.; Ratia K.; Santarsiero B. D.; Fu W.; Harcourt B. H.; Rota P. A.; Baker S. C.; Johnson M. E.; Mesecar A. D. Design and synthesis of peptidomimetic severe acute respiratory syndrome chymotrypsin-like protease inhibitors. J. Med. Chem. 2005, 48, 6767–6771. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Xi K.; Grum-Tokars V.; Xu X.; Ratia K.; Fu W.; Houser K. V.; Baker S. C.; Johnson M. E.; Mesecar A. D. Structure-based design, synthesis, and biological evaluation of peptidomimetic SARS-CoV 3CLpro inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5876–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J. S.; Venkatraman S.; Furness K.; Nimkar S.; Shepherd T. A.; Wang Q. M.; Aube J.; Hanzlik R. P. Synthesis and evaluation of peptidyl Michael acceptors that inactivate human rhinovirus 3C protease and inhibit virus replication. J. Med. Chem. 1998, 41, 2579–2587. [DOI] [PubMed] [Google Scholar]
- Kim T. S.; Hague A. B.; Lee T. I.; Lian B.; Tegley C. M.; Wang X.; Burgess T. L.; Qian Y. X.; Ross S.; Tagari P.; Lin C. H.; Mayeda C.; Dao J.; Jordan S.; Mohr C.; Cheetham J.; Viswanadhan V.; Tasker A. S. 4-Piperidinylphenyl)aminoethyl amides as a novel class of non-covalent cathepsin K inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 87–90. [DOI] [PubMed] [Google Scholar]
- Barrett D. G.; Boncek V. M.; Catalano J. G.; Deaton D. N.; Hassell A. M.; Jurgensen C. H.; Long S. T.; McFadyen R. B.; Miller A. B.; Miller L. R.; Payne J. A.; Ray J. A.; Samano V.; Shewchuk L. M.; Tavares F. X.; Wells-Knecht K. J.; Willard D. H. Jr.; Wright L. L.; Zhou H. Q. P2–P3 conformationally constrained ketoamide-based inhibitors of cathepsin K. Bioorg. Med. Chem. Lett. 2005, 15, 3540–3546. [DOI] [PubMed] [Google Scholar]
- Marquis R. W.; Ru Y.; LoCastro S. M.; Zeng J.; Yamashita D. S.; Oh H. J.; Erhard K. F.; Davis L. D.; Tomaszek T. A.; Tew D.; Salyers K.; Proksch J.; Ward K.; Smith B.; Levy M.; Cummings M. D.; Haltiwanger R. C.; Trescher G.; Wang B.; Hemling M. E.; Quinn C. J.; Cheng H. Y.; Lin F.; Smith W. W.; Janson C. A.; Zhao B.; McQueney M. S.; D’Alessio K.; Lee C. P.; Marzulli A.; Dodds R. A.; Blake S.; Hwang S. M.; James I. E.; Gress C. J.; Bradley B. R.; Lark M. W.; Gowen M.; Veber D. F. Azepanone-based inhibitors of human and rat cathepsin K. J. Med. Chem. 2001, 44, 1380–1395. [DOI] [PubMed] [Google Scholar]
- Marquis R. W.; Ward K. W.; Roethke T.; Smith B. R.; Ru Y.; Yamashita D. S.; Tomaszek T. A.; Gorycki P. D.; Cheng H. Y.; James I. E.; Stroup G. B.; Lark M. W.; Gowen M.; Veber D. F. An azepanone-based inhibitor of human cathepsin K with improved oral bioavailability in the rat and the monkey. Mol. Pharmaceutics 2004, 1, 97–100. [DOI] [PubMed] [Google Scholar]
- Barrett D. G.; Catalano J. G.; Deaton D. N.; Long S. T.; McFadyen R. B.; Miller A. B.; Miller L. R.; Samano V.; Tavares F. X.; Wells-Knecht K. J.; Wright L. L.; Zhou H. Q. Acyclic, orally bioavailable ketone-based cathepsin K inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 22–27. [DOI] [PubMed] [Google Scholar]
- Thurmond R. L.; Beavers M. P.; Cai H.; Meduna S. P.; Gustin D. J.; Sun S.; Almond H. J.; Karlsson L.; Edwards J. P. Nonpeptidic, noncovalent inhibitors of the cysteine protease cathepsin S. J. Med. Chem. 2004, 47, 4799–4801. [DOI] [PubMed] [Google Scholar]
- Seierstad M.; Breitenbucher J. G. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J. Med. Chem. 2008, 51, 7327–7343. [DOI] [PubMed] [Google Scholar]
- Lambert D. M.; Fowler C. J. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005, 48, 5059–5087. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [DOI] [PubMed] [Google Scholar]
- Blankman J. L.; Simon G. M.; Cravatt B. F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labar G.; Bauvois C.; Borel F.; Ferrer J. L.; Wouters J.; Lambert D. M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem 2010, 11, 218–227. [DOI] [PubMed] [Google Scholar]
- Mulvihill M. M.; Nomura D. K. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013, 92, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. M.; Sepers M.; Henstridge C. M.; Lassalle O.; Neuhofer D.; Martin H.; Ginger M.; Frick A.; DiPatrizio N. V.; Mackie K.; Katona I.; Piomelli D.; Manzoni O. J. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun. 2012, 3, 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. R.; Duranti A.; Tontini A.; Rivara S.; Rosengarth A.; Clapper J. R.; Astarita G.; Geaga J. A.; Luecke H.; Mor M.; Tarzia G.; Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem. Biol. 2007, 14, 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. Z.; Li W.; Booker L.; Burston J. J.; Kinsey S. G.; Schlosburg J. E.; Pavon F. J.; Serrano A. M.; Selley D. E.; Parsons L. H.; Lichtman A. H.; Cravatt B. F. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. W.; Niphakis M. J.; Lum K. M.; Cognetta A. B. III; Wang C.; Matthews M. L.; Niessen S.; Buczynski M. W.; Parsons L. H.; Cravatt B. F. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem. Biol. 2012, 19, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K. L.; Tsuboi K.; Chang J. W.; Whitby L. R.; Speers A. E.; Pugh H.; Cravatt B. F. Discovery and optimization of piperidyl-1,2,3-triazole ureas as potent, selective, and in vivo-active inhibitors of alpha/beta-hydrolase domain containing 6 (ABHD6). J. Med. Chem. 2013, 56, 8270–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F.; Zhang Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 2013, 30, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Blankman J. L.; Cravatt B. F. A functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. J. Am. Chem. Soc. 2007, 129, 9594–9595. [DOI] [PubMed] [Google Scholar]
- Bracey M. H.; Hanson M. A.; Masuda K. R.; Stevens R. C.; Cravatt B. F. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science 2002, 298, 1793–1796. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H.; Shelton C. C.; Advani T.; Cravatt B. F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 2004, 109, 319–327. [DOI] [PubMed] [Google Scholar]
- Massa F.; Marsicano G.; Hermann H.; Cannich A.; Monory K.; Cravatt B. F.; Ferri G. L.; Sibaev A.; Storr M.; Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Invest. 2004, 113, 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S.; Sanchez-Alavez M.; Wills D. N.; Cravatt B. F.; Henriksen S. J. Characterization of the sleep-wake patterns in mice lacking fatty acid amide hydrolase. Sleep 2004, 27, 857–865. [DOI] [PubMed] [Google Scholar]
- Varvel S. A.; Wise L. E.; Niyuhire F.; Cravatt B. F.; Lichtman A. H. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 2007, 32, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Tarzia G.; Duranti A.; Tontini A.; Piersanti G.; Mor M.; Rivara S.; Plazzi P. V.; Park C.; Kathuria S.; Piomelli D. Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J. Med. Chem. 2003, 46, 2352–2360. [DOI] [PubMed] [Google Scholar]
- Alexander J. P.; Cravatt B. F. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem. Biol. 2005, 12, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni S.; Simone C. D.; Dallavalle S.; Fezza F.; Nannei R.; Battista N.; Minetti P.; Quattrociocchi G.; Caprioli A.; Borsini F.; Cabri W.; Penco S.; Merlini L.; Maccarrone M. A new group of oxime carbamates as reversible inhibitors of fatty acid amide hydrolase. Bioorg. Med. Chem. Lett. 2010, 20, 4406–4411. [DOI] [PubMed] [Google Scholar]
- Butini S.; Brindisi M.; Gemma S.; Minetti P.; Cabri W.; Gallo G.; Vincenti S.; Talamonti E.; Borsini F.; Caprioli A.; Stasi M. A.; Di Serio S.; Ros S.; Borrelli G.; Maramai S.; Fezza F.; Campiani G.; Maccarrone M. Discovery of potent inhibitors of human and mouse fatty acid amide hydrolases. J. Med. Chem. 2012, 55, 6898–6915. [DOI] [PubMed] [Google Scholar]