Abstract
It is known that there is a significant interplay of insulin resistance, oxidative stress, dyslipidemia, and inflammation in type 2 diabetes mellitus (T2DM). The study was undertaken to investigate the effect of turmeric as an adjuvant to anti-diabetic therapy. Sixty diabetic subjects on metformin therapy were recruited and randomized into two groups (30 each). Group I received standard metformin treatment while group II was on standard metformin therapy with turmeric (2 g) supplements for 4 weeks. The biochemical parameters were assessed at the time of recruitment for study and after 4 weeks of treatment. Turmeric supplementation in metformin treated type 2 diabetic patient significantly decreased fasting glucose (95 ± 11.4 mg/dl, P < 0.001) and HbA1c levels (7.4 ± 0.9 %, P < 0.05). Turmeric administered group showed reduction in lipid peroxidation, MDA (0.51 ± 0.11 µmol/l, P < 0.05) and enhanced total antioxidant status (511 ± 70 µmol/l, P < 0.05). Turmeric also exhibited beneficial effects on dyslipidemia LDL cholesterol (113.2 ± 15.3 mg/dl, P < 0.01), non HDL cholesterol (138.3 ± 12.1 mg/dl, P < 0.05) and LDL/HDL ratio (3.01 ± 0.61, P < 0.01) and reduced inflammatory marker, hsCRP (3.4 ± 2.0 mg/dl, P < 0.05). Turmeric supplementation as an adjuvant to T2DM on metformin treatment had a beneficial effect on blood glucose, oxidative stress and inflammation.
Keywords: Diabetes, Turmeric, Metformin, Malondialdehyde, Dyslipidemia, Inflammation
Introduction
Recent trends in lifestyle have increased the incidence of diabetes mellitus and its complications worldwide. It has been projected that about 300 million and more people will be affected in the world by 2025 [1]. Insulin resistance is an early indication of T2DM and also linked to many conditions like obesity, hypertension and cardiovascular disease [2].
Free radicals formed during metabolic reactions, if not scavenged, can cause altered forms of proteins, lipids and carbohydrates. Oxidative stress as a consequence of hyperglycemia is associated with changes in inflammatory mediators and energy metabolism and can play an important role in the pathophysiology events of diabetes mellitus [3].
Maintenance of normoglycemic state still remains a medical challenge. Therapies are primarily aimed at maintaining optimum blood glucose, improve insulin sensitivity and decreasing oxidative stress. In order to delay the progression of disease numerous therapeutic remedies have been tried. Several plant products have been found to possess hypoglycemic effects, with antioxidant property and are being used in Indian traditional medicine for the management of diabetes [4].
Turmeric is a commonly used additive that gives flavor, color and adds spice to food preparations in Southeast Asian countries especially in India. It is a traditional medicine used in Ayurvedha, Unani and Siddha medicine as home remedy for various diseases. It has been documented that turmeric comprises 2–8 % of curcumin (diferuloylmethane). This is known to have potent anti-oxidant [5], anti-inflammatory [6], and anti-carcinogenic properties [7]. Previous studies in mouse models have shown that oral ingestion of curcumin reverses many of the inflammatory complications and a metabolic derangement associated with obesity and improves glycemic control in T2DM [8].
A recent study demonstrated that ingestion of 6 g of turmeric increased postprandial insulin levels. The increased insulin response resulting from Curcuma longa is probably due to the stimulation of beta-cell function by curcumin [9]. Till date no previous reports are available on the beneficial effects of turmeric as an adjuvant in T2DM and this is the first study to explore the same. The present study was aimed at analyzing the efficacy of turmeric in modulating diabetes, and to investigate the effect of turmeric on insulin resistance, oxidative stress, inflammation, and blood glucose levels.
Materials and Methods
2–Thiobarbituric acid (TBA), l-glutathione reduced (GSH), hydrogen peroxide (H2O2), 5,5′-dithiobis 2-nitrobenzoic acid (DTNB), sodium sulphate, 2-4-dinitrophenylhydrazine (DNPH) 2,4,6-tri- (2-pyridyl)-5-triazine (TPTZ) were purchased from Sigma Aldrich (USA). All other chemicals were of analytical grade obtained from Merck (India) and SRL (India). Turmeric rhizomes were obtained from the market, sun-dried, and gelatin encapsulated by M/s Acumen Pharmaceutical Ltd, Pondicherry, a certified pharmaceutical firm. Curcumalonga (Turmeric) is a member of ginger family (Zingiberaceae). Curcuma longa used in the study was authenticated in the French Institute of Pondicherry (HIPF 26738). Curcumin concentration from turmeric powder was measured by liquid chromatography–mass spectrometry (LC–MS) method. LC–MS analysis showed 2.3 % of curcumin is present in our turmeric powder. 2 g of turmeric contains 46 mg of curcumin in our analysis.
Study Design
The study protocol was approved by JIPMER Institute Ethics Committee (Reg. No. EC/2010/1/3) and written informed consent was obtained from all the participants. The Clinical trial was registered in CTRI (CTRI/2012/02/002442). Sixty male diabetic patients on metformin (500 mg twice a day), 35–55 years were recruited for this study. This study was a open label randomized clinical trial. Patients known to be diabetic for <2 years were randomly divided into two groups (30 each) and a simple random number table was used for treatment allocation.
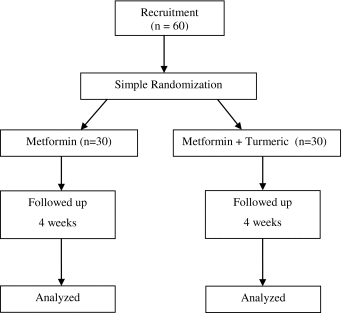
- Group I:
Diabetic patients on metformin.
- Group II:
Diabetic patients on metformin administered turmeric.
Diabetic patients in group I received only metformin (500 mg) twice a day. The patients in group II was on standard metformin therapy with turmeric powder (2 g per day, 4 capsules, 500 mg each). The turmeric capsules were ingested 2 h after metformin administration for 4 weeks. Both treatment groups were regularly monitored to measure the drug compliance. No adverse effect was reported during the study.
Sample Collection
Overnight fasting blood samples were collected at the beginning of the study and after 4 weeks with or without turmeric supplementation as per protocol. Blood samples were collected in EDTA vials. Plasma/serum was separated by centrifugation at 3,000 rpm for 10 min. Fasting Glucose, insulin level and other biochemical parameters were measured in plasma. hsCRP was measured in serum. Whole blood was used for the estimation of glycated hemoglobin, reduced glutathione (GSH). Glutathione peroxidase (GPx) and catalase were measured in erythrocytes.
Estimation of Biochemical Parameters
Plasma glucose was measured by glucose oxidase–peroxidase (GOD–POD) method. Total protein and albumin concentrations were estimated by direct Biuret method and Bromocresol green (BCG) method respectively. Glucose, total protein and albumin were estimated using reagent kits from Agappe Diagnostics (Kerala, India). AST and ALT activities in plasma were measured by IFCC method using reagent kits from ERBA diagnostics Mannheim Gmbh (Mannheim, Germany).
Total cholesterol (TC) in plasma was estimated using the cholesterol oxidase–peroxidase method (Genuine Biosystems, Chennai, India), triglyceride (TG) using an enzymatic glycerol phosphate oxidase peroxidase method (Agappe Diagnostics, Kerala, India), and high-density lipoprotein (HDL) cholesterol by the cholesterol oxidase–peroxidase method (Lab-Care Diagnostics, Mumbai, India). Very low density lipoprotein (VLDL) cholesterol and low-density lipoprotein (LDL) cholesterol were calculated using the Friedwald formula [10]. HbA1c was estimated by an immunoturbidometry method using reagent kits from Biosystem (Spain). All the above mentioned parameters were analysed using Olympus AU 400 (Siemens, Japan) clinical chemistry analyser.
Fasting plasma insulin level was determined by direct chemiluminescent technology using ADVIA Centaur, CP (Siemens Healthcare Diagnostics, Camberley, UK). The hsCRP in serum was analyzed using ELISA kit (Diagnostics Biochem. Canada Inc.). The homeostasis model assessment (HOMA) index was calculated as fasting blood glucose level (mmol/l) multiplied by fasting insulin level [(µU/ml) divided by 22.5] [11].
Estimation of Oxidant–Antioxidant Parameters
The Glutathione content was measured by the method of Beutler et al. [12]. Catalase enzyme activity in erythrocytes was estimated by the method of Aebi [13]. The plasma MDA was measured by the method of Yagi [14]. Plasma antioxidant potential was determined by ferric reducing antioxidant power (FRAP) assay [15]. The carbonylation of plasma proteins was estimated by DNPH method [16]. The GPx activity in erythrocyte was determined by method of Wendel et al. [17].
Statistical Analysis
All results are presented as mean ± S.D and compared using the non-parametric Mann–Whitney test for two groups. The data were analyzed using SPSS. 19 software. Data variations before and after administration of turmeric were analysed by non-parametric paired t test. Data variations between the groups were analyzed by independent ‘t’ test. A P value of <0.05 was considered as statistically significant.
Results
Table 1 depicts the baseline characteristics of the two groups.
Table 1.
Basic characteristic of subjects
| Parameter | Diabetic patients on metformin (n = 30) | Diabetic patients on metformin (turmeric group) (n = 30) |
|---|---|---|
| Age (years) | 46.8 ± 6.1 | 47 ± 7.17 |
| BMI (kg/m2) | 24.1 ± 3.26 | 23.4 ± 3.03 |
| Duration of diabetes (years) | <2 | <2 |
Table 2 shows the effect of turmeric powder supplementation on biochemical parameters of diabetic subjects on metformin treatment. There was significant decrease in fasting plasma glucose in both groups but the decrease was comparatively more in turmeric supplemented group. The reduction was 6 % in group I and 15 % in group II when compared to baseline level respectively. There was no statistically significant difference in post prandial glucose levels, when compared between the groups and within the groups. A statistically significant decrease of HbA1C (5 %) was observed in group II on comparison to basal level of the same group (P < 0.01). There was no significant difference in the fasting plasma insulin, glucose/insulin ratio, HOMA-IR, liver and kidney profile.
Table 2.
Effect of turmeric powder supplementation on biochemical parameters of diabetic subject on metformin treatment
| Parameters | Patients on metformin treatment (n = 30) | Patients on metformin + turmeric powder treatment (n = 30) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 month | P value | Baseline | 1 month | P value | |
| Fasting plasma glucose (mg/dl) | 111 ± 24 | 102 ± 18a | 0.008 | 116 ± 23 | 95 ± 11.4*** | 0.001 |
| Post prandial glucose (mg/dl) | 203 ± 36 | 196 ± 26 | 0.058 | 208 ± 31 | 197 ± 28 | 0.104 |
| Fasting plasma insulin (µU/ml) | 23 ± 16.4 | 19 ± 13 | 0.162 | 18 ± 9.9 | 22 ± 12 | 0.475 |
| Glucose/insulin ratio | 8.4 ± 7.5 | 7.6 ± 4.8 | 0.654 | 7.7 ± 3.3 | 7.8 ± 5.3 | 0.836 |
| HOMA-IR | 6.4 ± 4 | 4.7 ± 2.2 | 0.308 | 5.1 ± 2.7 | 4 ± 2.3 | 0.295 |
| HbA1c (%) | 7.8 ± 0.5 | 7.5 ± 0.7 | 0.054 | 7.9 ± 1.3 | 7.4 ± 0.9* | 0.044 |
| Urea (mg/dl) | 20 ± 7.4 | 24 ± 3.7 | 0.167 | 21.2 ± 4.2 | 22.1 ± 4.3 | 0.656 |
| Creatinine (mg/dl) | 1 ± 0.1 | 1 ± 0.1 | 0.545 | 1 ± 0.1 | 1 ± 0.1 | 0.062 |
| AST (IU/l) | 25 ± 0.1 | 25 ± 0.13 | 0.831 | 24 ± 6.0 | 26 ± 6.4 | 0.176 |
| ALT (IU/l) | 23 ± 7.2 | 25 ± 6.0 | 0.137 | 26.6 ± 11 | 23 ± 7.0 | 0.122 |
| Total protein (g/dl) | 7.3 ± 0.3 | 7.4 ± 0.3 | 0.518 | 7.3 ± 0.9 | 7.5 ± 0.4 | 0.309 |
| Albumin (g/dl) | 4.3 ± 0.3 | 4.4 ± 0.3 | 0.088 | 4.3 ± 0.2 | 4.5 ± 0.4 | 0.264 |
Data are expressed as mean ± S.D
* P < 0.05, *** P < 0.001
aComparsion of basal versus 1 month value within group
Table 3 shows the effect of turmeric powder supplementation on oxidative stress parameters of diabetic patients on metformin treatment. There was significant increase in the reduced glutathione level in both groups but the increase was more in group II when compared to group I. There was significant decrease in MDA (13.6 %) (P < 0.01) and hsCRP (16.6 %) (P < 0.05) when compared to basal level respectively. Total antioxidant capacity was increased significantly up to 26.4 % in group II (P < 0.05) when compared to group I in which 16.2 % was noticed. No significant changes were observed in GPx, catalase and protein carbonylation levels in both groups.
Table 3.
Effect of turmeric powder supplementation on oxidative stress parameters of diabetic patients on metformin treatment
| Parameters | Patients on metformin treatment (n = 30) | Patients on metformin + turmeric powder treatment (n = 30) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 month | P value | Baseline | 1 month | P value | |
| Glutathione (mg/gHb) | 2.4 ± 0.8 | 2.8 ± 0.6a | 0.035 | 2.5 ± 0.64 | 3.0 ± 0.68** | 0.003 |
| GPX (U/gHb) | 17.3 ± 12 | 15 ± 5 | 0.293 | 13 ± 6.0 | 14 ± 8.4 | 0.524 |
| Catalase (K/ml) | 103 ± 37 | 93 ± 16 | 0.167 | 106 ± 29 | 98 ± 25 | 0.758 |
| Protein carbonylation (nmol/g protein) | 2.7 ± 1.0 | 2.5 ± 1.0 | 0.423 | 2.6 ± 1.0 | 2.4 ± 0.8 | 0.131 |
| MDA (µmol/l) | 0.63 ± 0.3 | 0.53 ± 0.2 | 0.67 | 0.67 ± 0.16 | 0.51 ± 0.11* | 0.029 |
| Total antioxidant status (µmol/l) | 444 ± 124 | 478 ± 133 | 0.333 | 434 ± 126 | 511 ± 70* | 0.048 |
| hsCRP (mg/dl) | 5.3 ± 2.1 | 4.8 ± 2.0 | 0.346 | 4.9 ± 2.6 | 3.4 ± 2.0* | 0.03 |
Data are expressed as mean ± S.D
* P < 0.05, ** P < 0.01
aComparsion of basal versus 1 month value within group
Table 4 shows the effect of turmeric powder supplementation on lipid profile of diabetic patients on metformin treatment. There was a significant decrease in the LDL cholesterol level in group II when compared to group I. The mean percentage of changes for LDL cholesterol level in turmeric treated group was 7.42 % to metformin treated group. Non HDL cholesterol and LDL/HDL cholesterol ratios were also significantly lower in group II when compared to group I. There was no significant change in the TC, TG, HDL-cholesterol and VLDL-cholesterol levels.
Table 4.
Turmeric supplementation on lipid profile in diabetic patients on metformin treatment
| Parameters | Patients on metformin treatment (n = 30) | Patients on metformin + turmeric powder treatment (n = 30) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 month | P value | Baseline | 1 month | P value | |
| Total cholesterol (mg/d) | 181.5 ± 22.3 | 178.1 ± 24.8 | 0.597 | 184.6 ± 14.6 | 176.6 ± 14.32 | 0.082 |
| Triglycerides (mg/dl) | 127.3 ± 33.3 | 123.4 ± 23.8 | 0.593 | 120.56 ± 37.1 | 125.7 ± 29.21 | 0.55 |
| HDL cholesterol (mg/dl) | 34.3 ± 7.1 | 36.7 ± 6.9 | 0.106 | 36.02 ± 8.1 | 38.33 ± 5.04 | 0.185 |
| LDL cholesterol (mg/dl) | 121.7 ± 26.3 | 116.8 ± 27 | 0.481 | 124.4 ± 17 | 113.2 ± 15.3** | 0.01 |
| VLDL cholesterol (mg/dl) | 25.5 ± 6.7 | 23.9 ± 5.6 | 0.593 | 24.1 ± 7.4 | 25.1 ± 5.84 | 0.55 |
| Non HDL cholesterol (mg/dl) | 147.2 ± 23.9 | 141.2 ± 27.4 | 0.375 | 148.5 ± 17.5 | 138.3 ± 12.1* | 0.031 |
| LDL/HDL cholesterol | 3.74 ± 1.29 | 3.33 ± 1.06 | 0.124 | 3.6 ± 1.03 | 3.01 ± 0.61** | 0.005 |
Data are expressed as mean ± S.D
* P < 0.05, ** P < 0.01
Discussion
In developing countries, ~80 % of individuals depend primarily on traditional medicine to meet their healthcare needs. Although various treatment regimens are available, the importance and beneficial effects of dietary phytochemicals is being explored. Turmeric rhizome (C. longa) is commonly used in Indian households for treating common cold, sore throat, fever, biliary disorders, anorexia, wounds and sinusitis. The average content of curcumin in turmeric powder is 2–8 % and thus on an average 500 mg of turmeric contains 22 mg of curcumin [18]. Turmeric has been shown to have beneficial effects in several diseases including diabetes mellitus. Therefore turmeric has been shown to exhibit antioxidant, antidiabetic and antiinflammatory properties and hence may be used as a good adjuvant.
Our study demonstrated a significant decrease in fasting plasma glucose levels in both groups. The decrease in glucose levels in group I (without turmeric supplementation) may be due to better drug compliance and health education of patients on recruitment of subjects. Comparatively there was a greater reduction in diabetic patients supplemented with turmeric powder. A previous study reported that curcumin increases glycogen storage in liver and suppresses activities of gluconeogenic enzymes thereby reducing blood glucose in db/db mice [19]. This could be a plausible explanation in human as well.
Subjects who were administered with turmeric showed decreased glycated hemoglobin levels when compared with subjects treated with metformin alone. Glycated hemoglobin reflects average blood glucose fluctuations over a period of 60–90 days and thus is an index of long term glucose homeostasis. Hyperglycemia leads to increased production of free radicals due to glucose auto-oxidation, which in turn enhances the glycation of proteins. Previous studies have shown that the antioxidant property of curcumin may reduce the glycosylation of hemoglobin in the presence of high glucose concentration [20].
Oxidative stress plays an important role in the development of diabetes and cardiovascular diseases. Hyperglycemia leads to increased production of free radicals which increases lipid peroxidation products (MDA) in T2DM [21, 22]. Increased generations of harmful free radicals are known to cause disruption of membranes, lipids and other cellular components. In the present study we found that MDA, the end product of lipid peroxidation was significantly elevated in both groups at the time of recruitment. With concomitant use of metformin and turmeric as an adjuvant, there was a significant decrease in MDA level in group II. Curcumin, the principal constituent in turmeric has a unique conjugated structure, which includes two methoxylated phenols and an enol form of b-diketone, possesses typical radical-trapping ability as a chain-breaking antioxidant [23, 24] thereby prevents MDA generation.
In our study, diabetic patients of both groups showed lower levels of GSH at the time of recruitment. After 4 weeks of the study, we found a significant increase in the GSH level in both groups. In comparison with group I there was a marginal increase in the GSH level in group 2. Supplementation with turmeric ameliorates hyperglycemia by reducing the influx of glucose through the polyol pathway, thus maintaining GSH at optimal concentrations [25]. Addition of turmeric in therapy resulted in improvement of the antioxidant status in group II when compared to group I who were under metformin treatment alone. This enhancement may be attributed to the antioxidant property of curcumin present in turmeric powder.
In diabetes, an increase in TC, LDL-cholesterol, VLDL-cholesterol, TG and decrease in HDL-cholesterol levels are likely to increase the risk of cardiovascular complications. Lipoprotein abnormalities have been identified as one of the several risk factors that could account for increase in atherosclerosis in diabetes [26]. In the present study administration of 2 g of turmeric powder to diabetic subjects lowered LDL cholesterol, non HDL cholesterol and LDL/HDL cholesterol ratio with no change in the remaining lipid profile parameters. A previous study showed that 10 mg of curcumin given twice a day for 28 days significantly lowered the serum LDL levels and increased the serum HDL levels in patients with atherosclerosis [27]. It has been reported that administration of turmeric extract inhibited the oxidation of low density lipoprotein (LDL) and was also found to have hypocholesterolemic effect in rabbits with experimental atherosclerosis [28]. Hence turmeric administration may aid in the prevention of cardiovascular risk.
Inflammation plays a pivotal role in the development of micro-vascular and macro-vascular complications in diabetes. Several studies have shown that diabetes is accompanied by elevated CRP levels [29–31]. In our study hsCRP was decreased markedly after 4 weeks of turmeric supplementation on comparison with baseline level at the time of recruitment. This may be due to the potent anti-inflammatory effect exerted by curcumin. Its anti-inflammatory effect is mediated through inhibition of NF-kB activation which in turn, suppresses the expression of a number of proinflammatory cytokines tumor necrotic factor-α (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6) and downregulates the mRNA expression of several proinflammatory enzymes (e.g., COX-2, LOX-5, MMPs, and iNOS) which are under direct regulation of NF-kB [32–34].
Thus curcumin present in turmeric powder exhibits potent antidiabetic, antioxidant, and anti-inflammatory properties, which will be beneficial in alleviating the complications occurring in diabetes.
Conclusion
Along with antihyperglycemic therapy, turmeric may be supplemented as an adjuvant to prevent or retard molecular complications of T2DM. Thus turmeric aids in maintaining normoglycemic status, improves redox imbalance and impedes the development of further complications. Further studies are needed to ascertain the molecular effects of long term administration of turmeric in T2DM.
Acknowledgments
We thank the Indian Council of Medical Research (ICMR), New Delhi, India for providing financial support in the form of Junior Research Fellowship to Mrs. Maithili karpaga selvi. N. The authors thank Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) for providing the necessary infrastructure for the study. We also thank C-CAMP, NCBS, Bangalore for quantification of curcumin in turmeric extract.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Wild S, Roglic G, Green A. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Nicholas SB. Lipid disorders in obesity. Curr Hypertens Rep. 1999;1:131–136. doi: 10.1007/s11906-999-0007-8. [DOI] [PubMed] [Google Scholar]
- 3.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 4.Rowais NA. Herbal medicine in the treatment of diabetes mellitus. Saudi Med J. 2002;23:1327–1331. [PubMed] [Google Scholar]
- 5.Shishodia S, Sethi G, Aggarwal B. Curcumin: getting back to the roots. Ann NY Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 6.Khopde SM, Priyadarsini P, Venkatesan, et al. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys Chem. 1999;80:85–89. doi: 10.1016/S0301-4622(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Kumar A, Harti ACB. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 8.Stuart P. Dietary circumin significantly improves obesity associated inflammation and diabetes in mouse models of diabesity. J Endocrinol. 2008;149(7):3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickenberg J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9:43. doi: 10.1186/1475-2891-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, Duron OM, Kelly B. Improved method for determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Yagi K. Assay for blood, plasma or serum lipid peroxides. Methods Enzymol. 1984;105:28–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 15.Iris F, Benzie F. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 16.Reznick AZ, Packer L. Oxidative damage to protein: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 17.Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/S0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- 18.Parviz K. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-b and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara H. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res Clin Pract. 2000;80:185–190. doi: 10.1016/j.diabres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Sushil K, Justin R, Kimberly J. Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic Biol Med. 2006;41(1):92–96. doi: 10.1016/j.freeradbiomed.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Griesmacher A, Kindhauser M, Andert SE. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:475–496. doi: 10.1016/S0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- 22.Sekeroglu H, Sahin H. The effect of dietary treatment on erythrocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase, and serum lipid peroxidation in patients with type 2 diabetes mellitus. Clin Biochem. 2000;33:669–674. doi: 10.1016/S0009-9120(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 23.Sreejayan Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 24.Masuda T, Maekawa T. Chemical studies on antioxidant mechanisms of curcumin: analysis of oxidative coupling products from curcumin and linoleate. J Agric Food Chem. 2001;49:2539–2547. doi: 10.1021/jf001442x. [DOI] [PubMed] [Google Scholar]
- 25.Arun N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr. 2002;57:41–52. doi: 10.1023/A:1013106527829. [DOI] [PubMed] [Google Scholar]
- 26.Assmann G, Schulte H. The prospective cardiovascular munster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am Heart J. 1988;116:1713–1724. doi: 10.1016/0002-8703(88)90220-7. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez Bosca A, Soler A, Carrion-Gutierrez MA. An hydroalcoholic extract of Curcuma longa lowers the abnormally high values of human-plasma fibrinogen. Mech Ageing Dev. 2000;114(3):207–210. doi: 10.1016/S0047-6374(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 28.Tortosa MC, Mesa MD, Aguilera MC. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 29.Corrado E, Rizzo M, Muratori I, Coppola G, Novo S. Association of elevated fibrinogen and C-reactive protein levels with carotid lesions in patients with newly diagnosed hypertension or type II diabetes. Arch Med Res. 2006;37:1004–1009. doi: 10.1016/j.arcmed.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad J, Ahmed F, Siddiqui MA. Inflammatory markers, insulin resistance and carotid intima-media thickness in North-Indian type 2 diabetic subjects. J Assoc Physicians India. 2007;55:693–699. [PubMed] [Google Scholar]
- 31.Farah R, Shurtz-Swirski R, Lapin O. Intensification of oxidative stress and inflammation in type 2 diabetes despite antihyperglycemic treatment. Cardiovasc Diabetol. 2008;22:7–20. doi: 10.1186/1475-2840-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang MT, Lysz T, Ferraro T. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 33.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 34.Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced upregulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid Redox Signal. 2005;7:1612–1620. doi: 10.1089/ars.2005.7.1612. [DOI] [PubMed] [Google Scholar]


