Abstract
This work was designated to monitor the coagulation abnormalities associated with the gradual progression of liver diseases. The study included fifty patients; forty were diagnosed with liver cirrhosis with different stages categorized according to the Childs-Pugh classification and another ten patients were diagnosed with hepatocellular carcinoma (HCC). Haemostatic variables including fibrinogen (FI), calcium (FIV), transglutaminase (FXIII), prothrombin time (PT) and platelet count were estimated in patients and compared with the baseline levels of healthy subjects (n = 10). The results demonstrated that the fibrinogen level was progressively decreased, whereas PT was progressively prolonged in Child A, Child B and Child C groups. The maximum deterioration was observed in HCC patients. Calcium significantly increased in mild (Child A) and moderate (Child B) but not in Child C cirrhosis and HCC patients. FXIII level did not show any significant changes in cirrhotic patients compared to healthy group. Some of the haemostatic variables we investigated were correlated with serum albumin and bilirubin but not with aminotransferases (ALT and AST). The results indicated that the haemostatic abnormalities in fibrinogen, calcium and PT (but not FXIII) were deteriorated in parallel with the gradual regression of the constitutional function of liver.
Keywords: Liver cirrhosis, HCC, Coagulation factors, Prothrombin time, FXIII, FIV, Fibrinogen
Introduction
Blood coagulation is a cascade of processes involving a set of proteins in addition to calcium (FIV). The liver plays a central role in clotting process, where it synthesizes the majority of proteins involved in fibrinolysis in addition to thrombopoietin, which is responsible for platelet production from megakaryocytes. Consequently, patients with liver disease may have a disturbed balance of procoagulant and anti-coagulant factors which deviate from the normal coagulation cascade [1]. Also, the hepatic reticuloendothelial system plays an important role in disposing activated coagulation and fibrinolysis-related factors and inhibitors. Mild fibrotic changes in liver tissue may progress into liver cirrhosis. Aging causes the normal liver architecture to gradually deteriorate and this interferes with blood flow and functions [2]. The severity of liver cirrhosis is classified according to Child-Pugh score [3], based upon the level of bilirubin, albumin, prothrombin time (PT), presence of ascites and encephalopathy. Moreover, some cirrhotic cases are known to progress into hepatocellular carcinoma (HCC).
A wide spectrum of hematological disturbances is known to be observed in patients with chronic liver disease. The most commonly encountered abnormalities are anemia and bleeding [4]. Also, acute and chronic liver diseases are invariably associated with coagulation disorders due to multiple causes such as decreased synthesis of clotting and coagulation inhibitory factors, decreased clearance of activated factors, quantitative and qualitative platelet defects, hyperfibrinolysis and accelerated intravascular coagulation [5]. Previous reports have shown that hepatocellular diseases may display a decrease in vitamin K-dependent factors including FII, FVII, FIX and FX, whereas other parameters remain normal. Except for FVIII and vWF, all procoagulant and inhibitory factors are decreased, which reflects an impaired protein synthesis. Vitamin K deficiency leads to the production of abnormal vitamin K-dependent factors. The factors lack gamma-carboxy glutamic acid residues in the N-terminal part of their molecules [6]. In addition to vitamin K-dependent coagulation factors, fibrinogen (FI) and FV are variably decreased in patients with liver disease [7]. Calcium (FIV), on the other hand, decreases with the progression of cirrhosis from compensative (Child A and B) to uncompensative stage [8]. Also, some of the components of the fibrinolytic system are altered in the direction of hyperfibrinolysis (high plasma level of tissue plasminogen activator and low level of α2-plasmin inhibitor), but others are altered in the direction of hypofibrinolysis (low plasminogen and high plasminogen activation inhibitor type1) [9]. FXIII deficiency, however, was found to be rare in patients with liver cirrhosis, but it is associated with a clinical bleeding tendency and an unfavorable prognosis for future hemorrhages and survival [10].
These studies and others did not stepwisely monitored the coagulation factors abnormalities during the gradual deterioration of liver disease. This triggers our interest to follow the changing pattern of some coagulation factors in patients with different cirrhotic stages and HCC; and to test whether there are correlation between some haemostatic variables and the commonly used hepatic markers.
Materials and Methods
Patients and Grouping
The study included 40 patients (30 males and 10 females, aged 35–70 years) admitted to the National Institute of Liver, Monofia University, Egypt. The initial presentation proved post-hepatitis cirrhosis in 30 patients and the development of HCC in ten patients. According to Child’s classification [3], cirrhotic patients were divided into three grades (ten patients each): mild cirrhosis (Child A) (group IIA), moderate cirrhosis (Child B) (group IIB) and advanced cirrhosis (Child C) (group IIC), whereas HCC patients (n = 10) were included in group III. During the study period, patients did not receive anticoagulant treatment and those with active bleeding were not included. In addition, ten healthy subjects were voluntarily taken as a normal control. After the study protocol was approved by the ethical committee, patients were informed and blood samples were collected with anticoagulant (3.8 % sodium citrate (1:10 ratio) for determination of fibrinogen level or with heparin for the determination of blood calcium. Plasma was immediately collected by centrifugation and used to measure the haemostatic parameters. Another portion of blood was left to clot and serum was recovered by centrifugation and used for other biochemical investigations.
Investigations
Haemostatic Variables
Plasma fibrinogen level (FI) was measured based on Clauss method [11] using the commercially available kit (Technoclone, GmbH, Austria) and following the manufacturer instructions. PT was estimated by thromboplastin with calcium (ThromboMax, DiaMed, Schweiz). FXIII was measured according to Flckenscher and Stüber [12] using Berichrom FXIII reagents. Heparinzed plasma was used to determine the concentration of serum calcium according Faulkner and Meites method [13] (Diamond Diagnostics kits). Platelets were counted by coulter counter (S-plus STKR, counter electronic Co., Florida, USA).
Hepatic Variables
Liver transaminases (ALT, AST), serum albumin and bilirubin were determined using the commercially available kits following the manufacturer’s instructions designated for each variable.
Statistical Analysis
Results were presented as mean (±standard deviation), comparison between groups was performed by ANOVA test and Pearson correlation coefficients between variables were assessed by Graphpad software (Graphpad, USA). P Value of <0.05 was considered statistically significant.
Results
Hepatic Variables
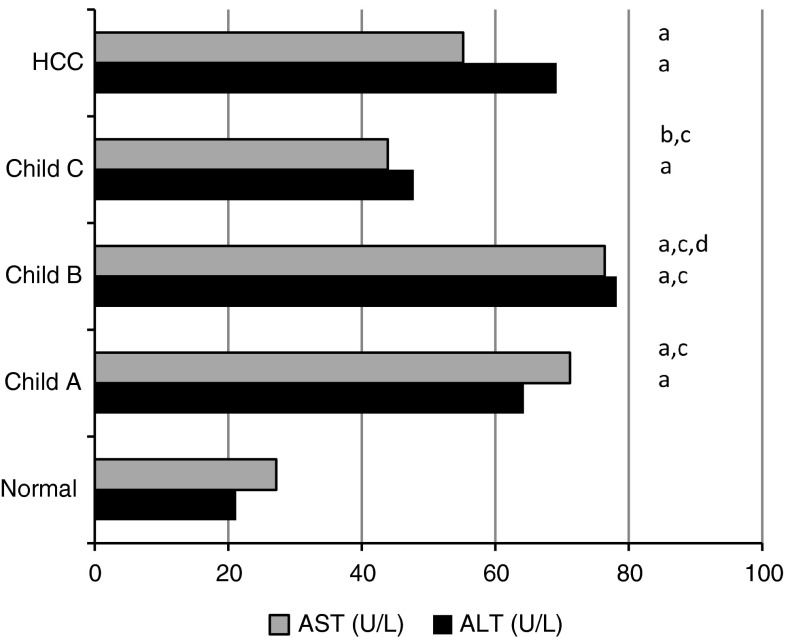
To assess the integrity of liver function, serum levels of transaminases (ALT and AST), albumin and bilirubin were investigated. Also, abdominal ultrasonography was performed to detect cirrhosis, presence or absence of ascites and/or tumors. Accordingly, patients were categorized into three cirrhotic stages (according to the calculated score of Child’s system) or HCC. Patients with Child A (group IIa) were encephalopathy none and have no ascites. The levels of both serum bilirubin and albumin were normal (0.68 ± 0.22 mg/dl and 4.1 ± 0.48 mg/dl, respectively) (Table 1). Higher levels (but <twofold increase) of transaminases (ALT and AST) were observed in this group (Fig. 1). Patients in group IIb were encephalopathy none, or with grade 1 with or without ascites. Their serum bilirubin level (2.13 ± 0.63 mg/dl) was significantly higher than normal (P < 0.001), whereas serum albumin (3.84 ± 0.26 mg/dl) was significantly lower than both normal control (P < 0.01) but insignificantly lower than patients in Child A groups (P > 0.05). Also, these patients had an elevated ALT and AST (<twofold increase). Cirrhotic patients with Child C (group IIC) had an advanced grade of encephalopathy (grades 2, 3 or 4) and ascites. Their serum bilirubin was about fourfold the normal level (4.25 ± 1.17 mg/dl) and showed marked decrease in serum albumin (2.6 ± 0.35 mg/dl) (P < 0.001). The average Child’s scores of cirrhotic patients in subgroups IIa, IIb and IIc were 5, 7 and 10, respectively. Patients with HCC (group III) had advanced grade of encephalopathy, ascites, and the levels of both serum bilirubin and albumin were dramatically deteriorated (3.74 ± 0.4, 2.72 ± 0.24 mg/dl, respectively) similar to the pattern of patients with cirrhosis Child C.
Table 1.
Serum bilirubin, albumin, ascites and encephalopathy grade in cirrhotic and HCC patients
| Group (Stage) | Bilirubin (mg/dl) | Albumin (mg/dl) | Ascites | Encephalopathy grade |
|---|---|---|---|---|
| I (Normal) | 0.73 ± 0.20 | 4.42 ± 0.38 | No | None |
| Cirrhosis | ||||
| IIa (Child A) | 0.68 ± 0.22 | 4.1 ± 0.48 | No | None |
| IIb (Child B) | 2.13 ± 0.63a,b | 3.84 ± 0.26a | No to mild | 0–1 |
| IIc (Child C) | 4.25 ± 1.17a,b,c | 2.6 ± 0.35a | Moderate | 1–2 |
| III (HCC) | 3.74 ± 0.4a,b,c | 2.72 ± 0.24a | Severe | 2–4 |
aSignificant difference of the corresponding group versus the normal group (P < 0.001)
bSignificant difference of the corresponding group versus child A group (P < 0.001)
cSignificant difference of the corresponding group versus child B group (P < 0.001)
Fig. 1.
Mean values of serum aminotransaminases (ALT and AST) in normal, cirrhotic and HCC patients
Haemostatic Variables
Platelet count was normal in Child A cirrhotic patients (195,500 ± 31,200 cells/ml). Patients with Child B, C and HCC (groups: IIb, IIc and III), however revealed a significant thrmpocytopenia, compared to both normal and child A cirrhotic patients, where the platelets counts were: 86,000 ± 23,100, 82,500 ± 14,900 and 81,200 ± 12,300 cell/μl, respectively (Table 2). The range of PT was 12.40–29.10 s in all patients investigated. The mean levels of Child A, Child B, Child C, and HCC patients were 14.48 ± 2.36, 17.92 ± 3.30, 21.84 ± 6.70, and 20.60 ± 3.85 s, respectively. Compared to normal group, PT was significantly prolonged in patients with Child C and HCC, Compared to patients with Child A, PT was significantly increased only in cirrhotic patients with Child C.
Table 2.
Prothrombin time, INR and platelets
| Group (Stage) | PT (s) | INR | Platelets (cell/μl) |
|---|---|---|---|
| I (Normal) | 12.83 ± 0.36 | 1.32 ± 0.06 | 232.4 ± 28.5 |
| Cirrhosis | |||
| IIa (Child A) | 14.48 ± 2.36 | 1.43 ± 0.15 | 195.5 ± 31.2a |
| IIb (Child B) | 17.92 ± 3.30a, | 2.2 ± 0.80 | 86.0 ± 23.1a,b |
| IIc (Child C) | 21.84 ± 6.70a,b | 3.38 ± 2.05 | 82.5 ± 14.9a,b |
| V (HCC) | 20.60 ± 3.85a,b | 3.29 ± 1.30 | 81.2 ± 12.3a,b |
aSignificant difference of the corresponding group versus the normal group
bSignificant difference of the corresponding group versus Child A group
Coagulation Factors
Plasma fibrinogen concentration ranged from 0.36 to 3.46 g/l. The variation in fibrinogen level in the patients with liver cirrhosis and HCC is shown in Table 3. Compared to healthy subjects (2.74 ± 0.44 g/l), fibrinogen significantly and gradually decreased, in parallel to the severity of liver disease. In cirrhotic patients (Childs A, B and C) the fibrinogen concentrations were 1.91 ± 0.43, 1.76 ± 0.55 and 0.94 ± 0.30 g/l, respectively. Also, HCC patients had lower plasma fibrinogen compared to all stages of cirrhosis (0.99 ± 0.28 g/l). Plasma calcium (FIV) was above the baseline of normal subjects in cirrhotic patients with Childs A and B (14.48 ± 3.1 and 14.88 ± 2.84 mg/dl). Child C and HCC patients, however, showed normal calcium levels (9.41 ± 1.83 and 8.32 ± 1.78 mg/dl, respectively) (Table 3). The range of FXIII was 70–140 %, and the average values of normal, Child A, Child B, Child C and HCC patients were: 100.8 ± 16.6, 107.6 ± 13.8, 110.4 ± 17.2, 98.9 ± 18.08, and 86.80 ± 13.5, respectively.
Table 3.
Fibrinogen, calcium and FXIII levels in cirrhotic and HCC patients
| Group (Stage) | Fibrinogen | Calcium | FXIII |
|---|---|---|---|
| (g/l) | (mg/dl) | (Anilide/μmol/mg protein/min) | |
| I (Normal) | 2.74 ± 0.44 | 9.33 ± 0.73 | 100.8 ± 16.6 |
| Cirrhosis | |||
| IIa (Child A) | 1.91 ± 0.43a | 14.48 ± 3.1a | 107.6 ± 13.8 |
| IIb (Child B) | 1.76 ± 0.55a | 14.88 ± 2.84a | 110.4 ± 17.2 |
| IIc (Child C) | 0.94 ± 0.3a,b,c | 9.41 ± 1.83b,c | 98.90 ± 18.1 |
| III (HCC) | 0.99 ± 0.28a,b,c | 8.32 ± 1.78b,c | 86.80 ± 13.5 |
aSignificant difference of the corresponding group versus the normal group
bSignificant difference of the corresponding group versus Child A group
cSignificant difference of the corresponding group versus Child B group
Also, hepatic variables (albumin and bilirubin) were correlated well with fibrinogen, (r = −0.721 and −0.747, respectively), while PT and platelet count were weakly correlated with calcium. Transaminases, however did not show any correlation with all haemostatic variables investigated (Table 4).
Table 4.
Pearson correlations between hepatic and haemostatic variables in cirrhotic and HCC patients
| Variable | FI | FIV(Ca2+) | FXIII | PT | Platelet |
|---|---|---|---|---|---|
| Albumin | 0.721 | 0.366 | 0.115 | −0.643 | 0.731 |
| Bilirubin | −0.747 | −0.375 | −0.112 | 0.636 | −0.727 |
| AST | −0.168 | 0.427 | 0.067 | 0.016 | −0.271 |
| ALT | −0.389 | 0.333 | −0.114 | 0.236 | −0.499 |
Correlation coefficient estimated by Pearson correlation
Bold numbers indicate good correlation between variables
Discussion
Fibrosis represents the initial stage of histological abnormalities of the liver tissue where it develops next to liver inflammation. This inflammation activates the hepatic stellate cells (HSC) and triggers the over production and deposition of the extracellular matrix (ECM) proteins, particularly collagen. These events lead to the loss of the constitutional blood and oxygen infusion to the liver cells, and subsequently they are converted into myofibroblasts [14]. This represents the initial scenario that affects the synthetic capabilities of liver cells. The literature has provided a long list of factors that trigger liver fibrogenesis including chronic infection with hepatitis viruses (HBV and HCV) [15, 16]. The later (HCV) is considered the most common cause of liver disease in Egypt [17]. Untreated patients may progress into cirrhosis and a small percentage of them may develop HCC. This scenario takes years (15–20 years) during which many complications such as ascites, renal failure, hepatic encephalopathy and variceal bleeding may develop. Although the deterioration of the coagulation system in liver disease is well reported, the gradual monitoring of haemostatic variables may help to follow the prognosis of liver disease.
Chronic infection with HCV was the underlying factor of the liver failure of the patients we investigated. To ensure that, patients were screened for the absence of HBV, HCV and schistosomal infections and the involvement of HCV infection was confirmed with RT-PCR. The mechanism through which HCV leads to hepatic dysfunction was repeatedly reported, where the viral proteins usually transactivate many of the host cell genes [18]. Thus HCV-induced transformation of infected cells reflects the phenotypical changes (seen in chronically infected liver), which progressed gradually over years.
Pugh et al. and Truscott and Child, [3, 19] had introduced a modified method to assess the operative risk in cirrhotic patients. According to this classification, patients were classified into three grades (Child A, Child B and Child C), which respectively indicated mild, moderate or severe conditions of cirrhosis. This classification based on PT and serum level of both albumin and bilirubin, in addition to ascites and/or encephalopathy. Accordingly, the average scores of cirrhotic patients included in this work were 5, 7 and 10. The initial presentation depicted the progressive deterioration of the liver function which was accompanied with some haemostatic abnormalities. Platelets count progressively decreased from normal (groups IIa and IIb) to marked thrombocytopenia in cirrhotic patients in groups IIc and HCC patients. Thrombocytopenia is usually due to hyperslpnism in addition to other mechanisms that negatively alter platelet function [20]. Good correlations were observed between platelet count and serum albumin (r = 0.7) and bilirubin (r = −0.73).
The formation of fibrin is a central event in the process of coagulation, initially involving the cleavage of four small peptides to produce fibrin monomers [21], which spontaneously polymerize [22]. This polymer is susceptible to the fibrinolytic enzyme plasmin and requires the enzymatic action of FXIIIa to produce insoluble fibrin. This process reflects the integration between the coagulation factors we investigated (FI, FIV and FXIII). Also, fibrinogen (FI) is an important diagnostic index to follow the dynamics of the disease and may be helpful in diagnosing the haemorrhagic tendencies before they are clinically manifested. In consistence with previous studies [23], the data we obtained revealed a significant reduction in fibrinogen level in cirrhotic and HCC patients compared to normal subjects. The reduction was progressive, where the fibrinogen decreased by 30, 35.8, 65.7 and 63.9 % (of the corresponding normal level) in patients with cirrhosis Childs A, B, C and HCC, respectively (Table 2). This decrease may occur due to the increased fibrinogen degradation products. Violi et al. [24] have demonstrated that patients with higher fibrinogen degradation products have recorded higher levels of serum bilirubin indicating the association between the severity of liver disease and the low fibrinogen level. Similarly, the data revealed a negative correlation between fibrinogen and serum bilirubin (r = −0.75) and positive correlation with albumin (r = 0.72). No correlation, however, was seen between fibrinogen and aminotransaminases (ATL and AST). The variable fibrinogen levels in cirrhotic patients encouraged some investigators to monitor the response of cirrhotic patients, with hyperfibrinolytic activity, to epsilon-aminocaproic acid treatment [25]. Other investigators have used plasma fibrinogen to differentiate liver failure with and without malignancy [26]. Herein, the level of fibrinogen is quite similar in patients with cirrhosis (Child C) or HCC, which minimizes the chance of accurate discrimination among such cases. Liver diseases not only alter the concentration of circulating fibrinogen, but also make it functionally abnormal [27]. The functional abnormality of the circulating fibrinogen does not necessarily mean that the molecule secreted by the diseased liver is abnormal. It is conceivable that a normal fibrinogen is secreted by the abnormal liver and it undergoes rapid alteration in circulation due to abnormal plasma environment [28].
Measurement of PT usually reflects the integrity of other haemostatic factors such as FXa, FVa and FIV [29]. As it was anticipated, PT concomitantly increased with the progression of liver failure. The longest PT was recorded in Child C cirrhotic and HCC patients (21.8 and 20.6 s, respectively) compared to 14.4 and 17.9 s in patients of Child A and Child B, respectively. Since PT value is related to hepatic synthesis of proteins, it is widely used as a surrogate marker of liver function. Also, PT is one of the five parameters used to calculate Child’s classes, the widely used system to assess the severity of liver cirrhosis [30]. Also, PT is crucially dependent on FVII, whose blood level is influenced by the liver functional mass. This may explain the prolongation of PT in patients with progressive cirrhosis and HCC [31].
The role of trasnglutaminase (FXIII) in the coagulation process is limited to the covalent crosslinking of specific Glutamine residues in one fibrin molecule to Lysine residues in another. These isopeptide bonds stabilize the clot against proteolytic insult [32]. In previous work conducted in our laboratory we observed that, FXIII was found with higher activity in fibrotic liver indicating its involvement in cross-linking process during the early inflammatory stage of fibrosis [33]. Although FXIII is synthesized in liver, and in contrast to fibrinogen, it did not show a significant variation among patients with mild or moderate cirrhosis. A similar finding was previously reported [34]. Patients with Child C cirrhosis and HCC, however, had slightly (statistically insignificant) lower concentrations of trasnglutaminase. The constitutive existence of FXIII in the ECM [35], fulfills its involvement in matrix assembly [36]. FXIII activity and the level of its substrates, particularly collagen, in the ECM are the limiting factors in the development of hepatic scar. In liver the conditions favor the stability of ECM proteins and the development of fibrosis, where the high FXIII activity may be explained by the increased binding of the nuclear factor-kappa-B (NF-κB) to the NF-κB motif of the FXIII promoter [37].
Thrombocytopenia was not observed in patients with Child A cirrhosis, where the platelet count was insignificantly decrease in this stage compared with normal control. Marked decrease in platelet count was seen in patients with progressive cirrhosis (Child B and C) and HCC, where it decreased to 37, 35.3 and 34.9 %, respectively relative to the normal control. Thrombocytopenia in cirrhosis is mainly explained by the accelerated platelet destruction/sequestration as a result of hypersplenism or due to decrease of thrombopoietin level. Excessive platelet consumption because of cirrhosis-related hypercoagulability has also been assumed to be an etiopathologic factor of thrombocytopenia [38].
Abnormalities of calcium homeostasis are caused by some metabolic disorders. Liver disease, however significantly affects the normal calcium level. Herein plasma calcium was significantly increased in patients with mild and moderate cirrhosis. In Child C patients, its level was restored to the normal values and slightly decreased in HCC patients. Earlier studies have reported the deficiency of calcium with the progression of cirrhosis from compensative (Child A and B) to uncompensative cirrhosis [8]. The change of calcium levels in early and moderate cirrhosis was explained by the probable decrease of vitamin D and the reduction of calcium absorption. This decrease induces the secretion of PTH (secondary hyperparathyroidism), which may increase bone resorption and subsequent increase of blood calcium, which in turn feedback inhibits PTH release. This scenario is expected in healthy liver. In early stages of cirrhosis and due to the low clearance efficiency of the liver, PTH remains high and maintains bone resorption. In advanced stages (Child C and HCC), in contrast, the decrease of vitamin D3 leads to a decrease of calcium absorption.
In conclusion the data emphasize the gradual deterioration of the coagulation factors investigated along with the progression of liver disease. Further studies may be undertaken to involve coagulation factors as a key markers of noninvasive monitoring of liver fibrogenesis.
References
- 1.Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 2013;12(5):713–724. [PubMed] [Google Scholar]
- 2.Páramo JA, Rocha E. Hemostasis in advanced liver disease. Semin Thromb Hemost. 1993;19(3):184–90. [DOI] [PubMed]
- 3.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 4.Solomon L. Hematologic complications of liver disease and alcoholism. In: Hoffman R, Benz E, editors. Basic principles and practice of hematology. New York: Churchill Livingstone; 1994. [Google Scholar]
- 5.Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22(1):83–96. doi: 10.1055/s-2002-23205. [DOI] [PubMed] [Google Scholar]
- 6.Mammen EF. Coagulation abnormalities in liver disease. Hematol Oncol Clin North Am. 1992;6(6):1247–1257. [PubMed] [Google Scholar]
- 7.Tripodi A. Hemostasis in chronic liver disease. Thromb Haemost J. 2006;4(9):2064–2065. doi: 10.1111/j.1538-7836.2006.02078.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang FJ, Cao J, Ma LP, Jin ZX. Study on cellular and serum concentration of calcium and magnesium in peripheral blood cells of cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2004;12(3):144–147. [PubMed] [Google Scholar]
- 9.Lisman T, Leebeek FW, Mosnier LO, Bouma BN, Meijers JC, Janssen HL, et al. Thrombin-activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121:131–139. doi: 10.1053/gast.2001.25481. [DOI] [PubMed] [Google Scholar]
- 10.Tacke F, Fiedler K, Von Depka M, Luedde T, Hecker H, Manns MP, et al. Clinical and prognostic role of plasma coagulation factor XIII activity for bleeding disorders and 6-year survival in patients with chronic liver disease. Liver Int. 2006;26(2):173–181. doi: 10.1111/j.1478-3231.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 11.Clauss A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haemat. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 12.Flckensher K, Aab A, Stüber W. A photometric assay for blood coagulation factor XIII Thromb. Haemostasis. 1991;65:353–540. [PubMed] [Google Scholar]
- 13.Faulkner WR, Meites S. Selected methods of clinical chemistry. Washington DC: American Association for Clinical Chemistry; 1982. [Google Scholar]
- 14.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27(4):413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, Ratziu V, Benhamou Y, Opolon P, Cacoub P, Bedossa P. Natural history of HCV infection. Bailliere’s Best Pract Res Clin Gastroenterol. 2000;14(2):211–228. doi: 10.1053/bega.1999.0071. [DOI] [PubMed] [Google Scholar]
- 16.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factor of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739. doi: 10.1016/S0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 17.Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci USA. 2010;107:14757–14762. doi: 10.1073/pnas.1008877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Zhhang SL, Li K, Hong Y, Wang Q, Li Y, et al. NS5ATP9, a gene up regulated by HCV NS5A protein. Cancer Lett. 2008;259(2):192–197. doi: 10.1016/j.canlet.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Turcotte J, Child C. The liver and portal hypertension. In: Child CI, editor. Surgery and portal hypertension. Philadelphia: WB Saunders; 1964. pp. 50–58. [Google Scholar]
- 20.Ordinas A, Escolar G, Cirera I, Viñas M, Cobo F, Bosch J, et al. Existence of a platelet–adhesion defect in patients with cirrhosis independent of hematocrit: studies under flow conditions. Hepatology. 1996;24(5):1137–1142. doi: 10.1002/hep.510240526. [DOI] [PubMed] [Google Scholar]
- 21.Repke D, Gemmell CH, Guha A, Turitto VT, Broze GJJ, Nemerson Y. Hemophilia as a defect of the tissue factor pathway of blood coagulation: effect of factors VIII and IX on factor X activation in a continuous-flow reactor. Proc Natl Acad Sci USA. 1990;87:7623–7627. doi: 10.1073/pnas.87.19.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blombäck B, Blombäck M. The molecular structure of fibrinogen. Ann NY Acad Sci. 1972;202:77–97. doi: 10.1111/j.1749-6632.1972.tb16323.x. [DOI] [PubMed] [Google Scholar]
- 23.Arif S, Khan AS, Khan AR. Changes in fibrinogen level in liver cirrhosis. J Ayub Med Coll Abbottabad. 2002;14(2):19–21. [PubMed] [Google Scholar]
- 24.Violi F, Ferro D, Basili S, Quintarelli C, Saliola M, Alessandri C, et al. Hyperfibrinolysis increases the risk of gastrointestinal hemorrhage in patients with advanced cirrhosis. Hepatology. 1992;15(4):672–676. doi: 10.1002/hep.1840150420. [DOI] [PubMed] [Google Scholar]
- 25.Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96(5):1581–1586. doi: 10.1111/j.1572-0241.2001.03781.x. [DOI] [PubMed] [Google Scholar]
- 26.Miatto O, Casaril M, Gabrielli GB, Nicoli N, Bellisola G, Corrocher R. Diagnostic and prognostic value of serum copper and plasma fibrinogen in hepatic carcinoma. Cancer. 1985;55(4):774–778. doi: 10.1002/1097-0142(19850215)55:4<774::AID-CNCR2820550415>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Martinz J, Palascak J, Kawasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61(2):535–538. doi: 10.1172/JCI108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnoff OD, Forman WB. Criteria for the differentiation of dysfibrinogenemia states. Semin Hematol. 1976;13:141–157. [PubMed] [Google Scholar]
- 29.Davi EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance and regulation. Biochemistry. 1991;30(43):10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 30.Butt AK, Khan AA, Alam A, Shah SW, Shafqat F, Naqvi AB. Predicting hospital mortality in cirrhotic patients: comparison of child and acute physiology, age and chronic health evaluation (APACHE 111) scoring systems. Am J Gastroenterol. 1998;93:2469–2475. doi: 10.1111/j.1572-0241.1998.00706.x. [DOI] [PubMed] [Google Scholar]
- 31.Grimaudo S, Craxi A, Gentile S, Di Paolantonio T, Vaccaro A, Venezia G, et al. Prolonged prothrombin time, Factor VII and activated FVII levels in chronic liver disease are partly dependent on Factor VII gene polymorphisms. Dig Liver Dis. 2005;37:446–450. doi: 10.1016/j.dld.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 33.El Borai MS, Ibrahim WM, Hessien M, El-keey M. Effect of alpha-tocopherol on tissue transglutaminase and reversibility of thioacetamide-induced liver fibrosis in rats. Turkish J of Biochem. 2005;31(1):13–20. [Google Scholar]
- 34.Klingemann HG, Brunswig D, Liehr H. Fibrinogen and fibrin structure in patients with cirrhosis of the liver. Z Gastroenterol. 1978;16(9):564–573. [PubMed] [Google Scholar]
- 35.Knittel T, Fellmer P, Ramadori G. Gene expression and regulation of plasminogen activator inhibitor type I in hepatic stellate cells of rat liver. Gastroenterology. 1996;111:745–754. doi: 10.1053/gast.1996.v111.pm8780581. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Aziz G, Lebeau G, Rescan PY. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137:1333–1342. [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, Schwarting R, Norton P, Zern MA, et al. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am J Physiol. 1997;272(2):281–288. doi: 10.1152/ajpgi.1997.272.2.G281. [DOI] [PubMed] [Google Scholar]
- 38.Ikura Y, Ohsawa M, Okada M, Iwai Y, Wakasa K. The significance of platelet consumption in the development of thrombocytopenia in patients with cirrhosis. Am J Med Sci. 2013;346(3):199–203. doi: 10.1097/MAJ.0b013e31826e364d. [DOI] [PubMed] [Google Scholar]