Abstract
Chemotherapy drugs, used for prevention of uncontrolled cell proliferation in certain tissues as well as inducing apoptosis in tumor cells, are important candidates for treatment of cancer. The synthesized 2-amino-4H-chromene-3-carbonitrile derivatives effective on cancerous cells resistant to other drugs such as Paclitaxel were used due to their ability in induction of apoptosis. The growth inhibitory and inducing apoptosis activities were determined. In order to make it target-oriented, the best compound was conjugated with gold nanoparticles (NPs) by aspartic acid with chemical reduction method. Cytotoxicity effect of 2-amino-4H-chromene-3-carbonitrile derivatives against the T47D breast cancer cell line was determined by MTT assay. The synthesis of gold NPs was confirmed by transmission electron microscopy, UV–Vis and dynamic light scattering. To assess the effects of compounds on the process of apoptosis, staining methods with acridine orange–ethidium bromide and Hoechst staining by fluorescence microscopy and DNA fragmentation by the diphenylamine method were used. The synthesized compounds containing two NH2 groups on benzene rings, demonstrated more cytotoxicity effect. The effect of conjugation with gold NPs and the induction of apoptosis were studied with the best compound. The cytotoxicity effects of the synthesized 2-amino-4H-chromene-3-carbonitrile compounds were changed by replacement of NO2 group on thiol ring with different chemical groups on the benzene ring. Analyses of treated cell lines by conjugated and non-conjugated forms of compounds verified their ability in inducing apoptosis while conjugated form demonstrated higher apoptosis.
Keywords: 2-Amino-4H-chromene-3-carbonitrile derivatives, Gold nanoparticles, Conjugating, T47D, Apoptosis, Beast cancer
Introduction
Cancer is the second cause of death in human societies. Cancer is caused by cells unable to remove themselves with uncontrolled proliferation. Cancer begins when cells are mutated in gene which controls the growth [1]. Breast cancer is the fifth leading cancer after lung, stomach, liver and colon cancers throughout the world. Breast cancer is a complex multifactorial disease in which many genetic and environmental factors are involved. Any factor that increases the risk of cancer is called a risk factor. Risk factors include age, gender, family history, obesity, diet, race, age at menopause, smoking and alcohol. Although several risk factors for breast cancer have been deciphered, the etiology is not exactly clear [2, 3].
Cancer treatments include surgery, radiotherapy, chemotherapy, hormone therapy and biological therapy [4]. Although using drugs in chemotherapy is currently one of the most effective methods for treating cancer, but the cytotoxicity effects of chemotherapy drugs cause many side effects. These effects are liver and kidney damage, immune system suppression, vomiting, hair loss, anemia and gastrointestinal diseases. Thus, the injury to normal tissue by anti-tumor drugs is one of the most important limitations for cancerous patients [5, 6]. Emergence of tumor cells-resistant in conventional chemotherapy drugs has led to the use of newer drugs.
The benzopyran (chromene) from Flavonoids family (or bioflavonoid) (from the Latin word flavus meaning yellow, their color in nature) are natural compounds with numerous biological activities such as anti-tumor, anti-Leishmania and anti-bacterial properties [7, 8].
Also these compounds inhibit cell proliferation and bound to the colchicine site of β-tubulin, which result in polymerization of microtubule stopping cell cycle and eventual apoptosis. In addition, they are effective against cancerous cells resistant to other drugs and can be helpful in curing patients’ refractory to anti-tumor agents like taxons [9].
Since the mechanism of these compounds has not been reported completely, further studies on the mechanisms and effects are essential to find stronger anticancer compounds. Since apoptosis induction effect of anti-cancer compounds has been proved, several researchers have attempted to synthesize various derivatives of chromene [10, 11], although it has not been extensively studied for breast cancer.
Apoptosis or programmed cell death is a normal cellular process which is regulated to maintain the number of cells and remove unwanted cells. Right balance between apoptosis and inhibition of apoptosis plays an important role in maintaining the homeostasis of tissue and organ morphogenesis [10, 12]. Cytological and biochemical changes happen in certain cells during apoptosis, including condensation of nucleoplasm and cytoplasm, DNA fragmentation, and formation of apoptotic bounded membrane bodies that are identified and eliminated by adjacent cells [13, 14]. Any fault in this process causes pathologic conditions such as deviations from apoptosis, which causes the tumor separation, metastasis and inducing apoptosis which leads to neurological diseases such as Alzheimer’s [15, 16].
Nanotechnology is the latest trend in treatment of cancer with targeted action on cancerous cells and minimal side effects on normal ones recent techniques include delivery systems, passive targeting, active targeting, intracellular transport, the placement of nanoparticle (NP) agents in intracellular organelles and various techniques for cancer diagnosis such as carbon nanotubes and biological sensors [17]. Nanotechnology has been established to change the scale of drug delivery methods. Diagnostic and therapeutic agents can be in capsule form, or are absorbed by covalently connections to NPs.
This approach can easily overcome the consequences of drug solubility, which has important functions. More than 40 % of active substances which had been identified through screening programs were compounds with poor solubility in water such as 4H-chromene [18].
According to the stage and type of cancer, designing of carrier and targeting strategies may vary [19, 20]. One of the most widely used NPs is gold NPs. Gold at nanoscale shows such features that make it an important metal in nanotechnology processes. Such NPs have many applications in biotechnology and medical fields [21].
In this work, we show that both chromene derivatives and conjugated compounds are effective in inducing apoptosis in T47D breast cancer cells although conjugated compounds are more effective.
Materials and Methods
Materials
T47D cell line was purchased from Pasteur Institute of Iran; RPMI-1640, fetal bovine serum (FBS) were obtained from Gibco; MTT, HAuCl4, aspartic acid and Hoechst 33258 were purchased from Sigma-Aldrich; acridine orange (AO), ethidium bromide (EB) and diphenylamine were obtained from Merck. Novel 2-amino-4H-chromene-3-carbonitrile derivatives were designed and synthesized in Pharmaceutical Sciences Research Center, http://psrc.tums.ac.ir.
Preparation of Compounds
IUPAC nomenclature and the structure of compounds 1–6 used in this study have been listed in Table 1.
Table 1.
IUPAC nomenclature and the structure of 2-amino-4H-chromene-3-carbonitrile derivatives
| Compounds | IUPAC | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|
| 1 | 3-Amino-9-dimethylamino-5-(5-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C16H14N4O3S | 342.37 |
|
| 2 | 3,9-Diamino-5-(5-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C14H10N4O3S | 314.32 |
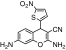
|
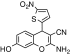
| 3 | 3-Amino-9-hydroxy-5-(5-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C14H9N3O4S | 315.30 |
|
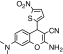
| 4 | 3,9,10-Triamino-5-(5-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C14H9N3O4S | 329.33 |
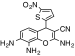
|
| 5 | 3-Amino-9-dimethylamino-5-(4-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C14H10N4O3S | 342.37 |
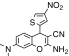
|
| 6 | 3,9-Diamino-5-(4-nitro-thiophen-2-yl)-2-oxa-bicyclo[4.4.0]deca-1(10),3,6,8-tetraene-4-carbonitrile | C14H10N4O3S | 314.32 |
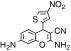
|
Cell Culture
T47D cells were used to evaluate the cytotoxicity effect of new compounds of Chromene. The cells (NCBI C203) were cultivated in RPMI-1640 medium containing 10 % FBS at 37 °C and 5 % CO2. Cell counting was performed with Hemocytometer. Percentages of viable cells were determined by trypan blue staining.
Cytotoxicity Effect of 2-Amino-4H-chromene-3-carbonitrile Derivatives
Cytotoxicity was determined by MTT assay [22]. Compounds were maintained in DMSO solution. The final concentration of DMSO in culture medium was 0.1 %. Cell cultures were used in growth phase. Ten thousand cells per well were cultivated in plates (96 wells) and incubated for 24 h (5 % CO2 and 37 °C). The medium containing dilutions of each derivative was added to wells, and incubated for 48 h at mentioned conditions. Then MTT solution (5 mg in 10 ml PBS) was added to all wells and the plates were incubated for 3–5 h. Finally, the deposit crystals of Formazan were dissolved in isopropanol. Absorbance was measured by ELISA Reader at 540 nm.
Synthesis of Gold NPs
Aspartic acid (1/5 ml) was added to 25 ml of distilled water, and then heated up to boiling point. Afterwards, HAuCl4 (5 mM, 1 ml) was added and heated until the color of solution was changed to red. After completing the reaction, it was placed in ice bath to cool down completely [23].
NPs formation was confirmed by techniques such as transmission electron microscopy (TEM), UV–Vis and dynamic light scattering (DLS; zeta-synthesizer).
Conjugation with AuNPs
Compound number 4 which presented the best results in MTT assay was used for conjugation with gold NPs. Process was as follows:
Prepared colloidal solutions of gold NPs (proper dose) were solved with compound number 4 (1:1) in PBS buffer [PBS (pH 7.4) = 8 g NaCl, 2.82 g Na2HPO4·12H2O, 1.42 g NaH2PO4·2H2O, 1 l DDW)], and the mixture was incubated while shaking at 4 °C for 48 h [23]. Samples were analyzed by UV–Vis and DLS.
Apoptosis Induction
The effects of compounds on the process of apoptosis were assessed by staining methods such as AO–EB and Hoechst 33258 staining [24, 25], and DNA fragmentation by diphenylamine method [26].
AO–EB Staining
T47D cells were cultivated in culture flasks. After sufficient growth and adding compounds, they were incubated for 24 h at 37 °C and 5 % CO2. Cells were collected with trypsin. The solution was centrifuged 200 g for 3 min. Then the supernatant was removed and cells were diluted in PBS buffer. Cell suspension was mixed with dye solution (1:1 of each color), and the mixture was incubated for 5 min. The samples were placed on a slide and then examined by the fluorescent microscope to determine the percentage of apoptotic cells by counting 400–500 cells (Ziess, Germany).
Hoechst 33258 Staining
Cells were cultured in plates (six wells). After incubating and adding IC50 concentration of compounds, cells were rinsed with PBS (twice) and fixed with paraformaldehyde 4 % for 20 min at 4 °C. Then cells were rinsed with PBS again and covered with Hoechst 33258 for 15 min in the dark place at room temperature. The solution was removed and cells were washed with PBS again (once). The photos of cells were taken with the inverted fluorescent microscope (Motic AE31, USA/Canada).
DNA Fragmentation
DNA fragmentation was performed based on previous procedures [26]. Cells were incubated for 24 h, and then were treated with compounds (at IC50) for 24 h. After incubation, cells were collected and centrifuged at 200 g for 10 min at 4 °C. Supernatant was discarded, and cell sediment was lysed with lysis buffer (Tris base + EDTA + Triton X-100) and then shaked. For separating DNA fragment from intact chromatin, the solution was centrifuged again. Supernatant was removed and put in tube A. Lysis buffer (0/5 ml) was added to the sediment again (tube B). Then trichloroacetic acid (25 %) was added to both tubes (A and B), and after shaking for a few seconds, they were incubated for 24 h at 4 °C. DNA was precipitated and separated by centrifugation. Supernatant of both tubes were carefully discarded and then 80 μl of trichloroacetic acid (5 %) was added to the pellets. Samples were heated at 83 °C for 20 min for DNA hydrolyzing. Finally 160 μl of fresh reagent diphenylamine was added and vertexed. The samples were kept for 24 h at room temperature. Absorption of samples was measured at 600 nm by ELISA Reader, and the percentage of DNA fragmentation was calculated by the following equation.
Statistical Analysis
Each experiment was repeated three times and data were reported as mean ± SE. Comparative test was used included t tests and P value of results were significant (p ≤ 0.05).
IC50 (concentration of a compound reducing by 50 % cell growth compared to control) of all compounds accurately were calculated by %viability and using the Pharm-PCS statistical package version 5.1.2600 (Springer-Verlag, New York).
Results
Cytotoxic Studies: MTT Assay
The cytotoxicity of 2-amino-4H-chromene-3-carbonitrile derivatives was assayed against T47D cells. The cells (1 × 104 cell/well) were cultured in RPMI-1640 medium at 37 °C overnight in the presence of the derivatives at different concentrations (1–650 μM) for 48 h. The percentage of cell viability was estimated by the MTT assay (Fig. 1). IC50 values were calculated from the semi-logarithmic dose–response plots using the Pharm-PCS statistical package. Result of cytotoxicity effects of 2-amino-4H-chromene-3-carbonitrile derivatives is illustrated in Table 2. The viability of cells treated with compounds (1–650 μM) compared to cells without treatment, greatly reduced. All compounds had cytotoxicity effect with IC50 less than 200 μM. According to the results shown in Fig. 1 and Table 1 the relative strength of compounds can be compared. It is evident that compound 2 showed the highest amount (181.26 μM) and compound 4 had the best result (81.42 μM). So the compound 4 was the most active compound in this series and selected for further studies.
Fig. 1.
The effect of compounds 1–6 on T47D. Cells were treated by different doses of compounds for 48 h at 37 °C; 5 % CO2. Data are means of three or more experiments and are reported as mean ± SD (p ≤ 0.05)
Table 2.
Comparison of the biological activity of 2-amino-4H-chromene-3-carbonitrile derivatives on T47D human breast cancer cell line
| Compounds | T47D IC50 (μM) ± SE |
|---|---|
| 1 | 107.64 ± 8.5 |
| 2 | 181.26 ± 18.4 |
| 3 | 89.33 ± 5.3 |
| 4 | 81.42 ± 6.1 |
| 5 | 123.93 ± 7.7 |
| 6 | 158.93 ± 11.6 |
Data are means of three experiments and are reported as mean ± SE
Preparation of AuNP-Conjugated Compound
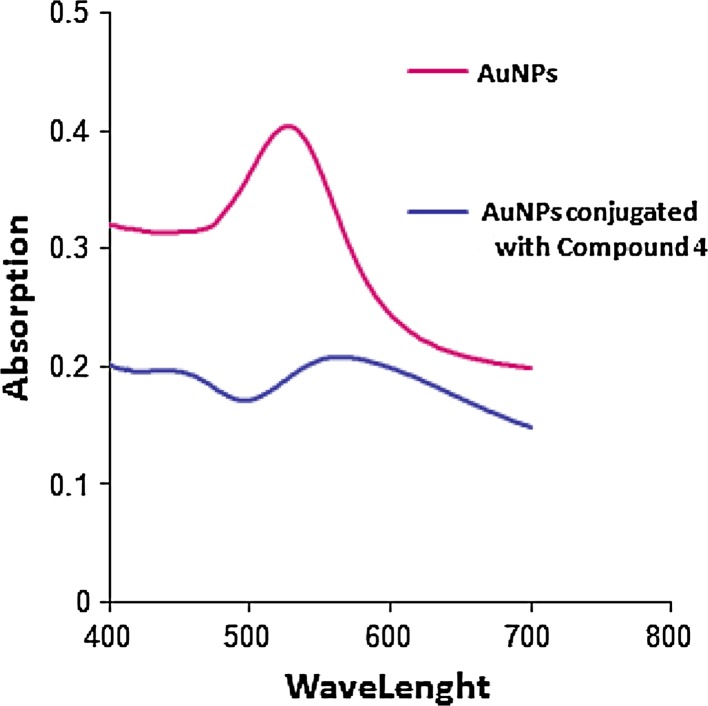
In order to make it functionally specific and target-oriented, compound 4 was chosen to be conjugated with synthesized gold NPs by aspartic acid with chemical reduction. The result of TEM analysis is shown in Fig. 2. The sizes of AuNPs were reported to be 18 ± 10 nm. The synthesis of gold NPs was confirmed by techniques such as UV–Vis and DLS. UV-spectra of AuNPs and its conjugation with compound 4 can be seen in Fig. 4. As indicated, the red shift in the spectrum was caused by conjugation (Fig. 3).
Fig. 2.

The image of gold nanoparticle taken by TEM
Fig. 4.
a Size distribution curve of AuNPs synthesized with aspartic acid, and b zeta potential of AuNPs synthesized with aspartic acid
Fig. 3.
UV–Vis spectra of AuNPs and its conjugation with compound 4
According to the figures, particle size and zeta potential increase from 33.32 to 187.9 nm and −8.99 to −5.55 mV (determined by DLS), respectively, which confirmed the conjugation (Figs. 4, 5).
Fig. 5.
a Size distribution curve of AuNPs conjugated with compound 4, and b zeta potential of AuNPs conjugated with compound 4
Effect of AuNP-Conjugated Compound on Cell Viability
The cytotoxicity effect of non-conjugated compound and its conjugated form with gold NPs were examined and compared (Fig. 6). Consequently, IC50 value was decreased by conjugation from 81.42 to 49.87 μM.
Fig. 6.
Comparison of %viability of compound 4 and its conjugation with gold nanoparticles in T47D cell line. Each experiment was repeated three times (**p < 0.01; ***p < 0.001)
Study of Apoptosis
The first and most noticeable effect following exposure of cells to toxic compounds is the alteration in cell nucleus morphology in a medium culture. To assess the effects of compounds on the process of apoptosis, staining methods with AO–EB and Hoechst staining by fluorescence microscope and DNA fragmentation by the diphenylamine method were used. Fluorescence light microscopy with differential uptake of fluorescent dyes (AO/EB staining) is the method that recognizes the apoptosis and necrosis cells. AO penetrates the nucleus of all cells and makes it looks green and EB taken by cells when cytoplasmic membrane integrity is lost, makes nuclei looks red. EB also dominates over AO. Thus normal cells display normal green nucleus; early apoptotic cells have bright green nucleus with condensed and fragmented chromatin; late apoptotic cells display condensed and fragmented orange chromatin; while the cells that have died due to necrosis, have a normal orange nucleus with condensed chromatin [27]. Also, Hoechst is a fluorescence dye that binds specially to DNA structure and makes nuclei looks blue. Analyses of treated T47D cell line by conjugated and non-conjugated forms verified that these compounds are able to induce apoptosis (Figs. 7, 8).
Fig. 7.
Fluorescent microscope images of T47D cells stained by acridine orange–ethidium bromide. a Control, b treated sample with compound 4, c AuNPs, and d treated sample with compound 4 conjugated with AuNPs
Fig. 8.
Induction of morphological changes in the nucleus of T47D cells in the presence and absence of AuNPs stained by Hoechst 33258 and detected by inverted fluorescent microscope. a Control, b treated sample with compound 4, c AuNPs, and d treated sample with compound 4 conjugated with AuNPs (no treatment as control)
All these results showed the increase of DNA fragmentation and apoptotic cells percentage while treated with compound 4 conjugated with AuNPs (Tables 3, 4).
Table 3.
Comparison of apoptotic cells percentage treated with compound 4 and its conjugation with AuNPs
| Compounds | Compound 4 | Conjugated compound 4 |
|---|---|---|
| %Apoptotic cells | 35 | 43 |
Table 4.
Comparison of %DNA fragmentation of T47D cells treated with compound 4 and its conjugation with AuNPs
| Compounds | Control | Compound 4 | AuNPs | Conjugated compound 4 |
|---|---|---|---|---|
| %DNA fragmentation | 1.1 | 34.3 | 1.2 | 41.8 |
Discussion
Cancer is a disease in which cells are divided and multiplied abnormally and metastasized to healthy tissues. Most of the chemotherapy agents may apply their therapeutic effects by inducing apoptosis. Apoptosis is an important metabolic step in the regulation of cell number and growth. If apoptosis is failed, metabolism impairs and tumors form and grow [28].
In this study, cytotoxicity effects and apoptosis induction of synthesized 4H-chromene derivatives were investigated. According to previous studies, other derivatives of these compounds would induce apoptosis in tumor cells by interaction of tubulin formation or inhibition in tubulin polymerization. The T47D cell line containing mutated P53 was used since the chromene derivatives have especially better effect on it [10, 11, 13].
In this study, MTT assay was used to evaluate viability and survival (%) and toxicity of compounds; this was the most applicable method for evaluating cell survival in the similar investigation.
Based on the results from the cytotoxicity assay of 2-amino-4H-chromene-3-carbonitrile compounds in T47D cell line shown in Table 2, there was an obvious distinction according to structure and functional groups (Table 1). Both compounds had a similar composition and different functional groups.
Difference between compounds 3 and 4 is in R group attached to the benzene ring, results in a significant effect on cytotoxicity since compound 4 with NH2 displayed the best result.
Compounds 1 and 5 are in the next category. They both have N(CH3)2 attached to the benzene ring and the difference is in the position of NO2 groups attached to the thiol ring. NO2 in compound 1 is in positions 5 while it is located on position 4 in compound 5, thus the NO2 group in position 5 showed a better outcome.
IC50 of compounds 2 and 6 were above 150 mM that had lower effect than the other compounds.
Their functional group NH2 has been attached to the benzene ring and the difference was in the position of NO2 groups attached to the thiol ring. NO2 in compound 2 is in positions 5 while it is located on position 4 in compound 6, thus the NO2 group in position 4 showed a better outcome.
According to the obtained information, existence of two NH2 groups as well as OH and N(CH3)2 on the benzene ring, would increase the cytotoxicity effect of the compounds. Whereas, existence of one NH2 group would reduce the effect of compounds.
New technologies have been used to increase the efficiency of chemotherapeutic agents and reduce their side effects. One of these methods is applying nanotechnology in medical field [29]. Due to the unique properties of biodegradable NPs, they can be applied for new therapeutic methods such as targeted drug delivery and controlled release [30]. NPs loaded with anti-cancer drugs, can easily reach to the cell membrane and increase the drug concentration at the cell surface. One of the most applicable NPs is gold NP [21, 31, 32].
Since the compound 4 was the strongest compound, it was conjugated with gold NPs synthesized with aspartic acid by chemical reduction method. Before conjugation, formation of gold NPs was confirmed and then they were incubated for 48 h with compound 4 for conjugation. Morphology and structure of gold NPs were investigated by TEM that showed the size of 18 ± 10 nm.
The UV–Vis spectroscopy and DLS were used for investigation of synthesized gold NPs and their conjugation with compounds. Study of UV–Vis spectra of gold NPs and conjugated form illustrated that the conjugated form had longer wavelength than the gold NPs.
Another method was DLS, which was used to check the size of the particles. In this method, the speed of the particles depends on particle size and also the surface structure and concentration of ions in the environment. Thus, the reported size was the hydrodynamic diameter. Molecules attached to the surface of the particles increased their diameter. Hydrodynamic diameter of the gold NPs was 33.23 nm while the real core size determined by TEM was 18 ± 10 nm which confirmed the existence of mentioned subjects. The size of conjugated NPs determined by this method was 187.6 nm and by comparing with the gold NPs (32.23 nm) conjugation was confirmed. Surface potential was investigated by DLS, too. It was increased from −8.99 mV in AuNPs to −5.25 mV in the conjugated form. This indicated the connection of gold NPs synthesized with aspartic acid (containing carboxylic acid groups) to compound 4 with the NH2 as the functional groups. The result of cytotoxicity effect investigation suggested that the compound 4 conjugated with AuNPs was more efficient to achieve to desired result (Fig. 6). IC50 reduced from 81.42 ± 6.1 to 49.87 ± 4.5 μM by conjugation (Table 3).
Induction of apoptosis is one of the most important procedures to destroy cancer cells without any side effect [28]. To assess the effects of compounds on the process of apoptosis, staining methods with AO–EB and Hoechst staining by fluorescence microscopy and DNA fragmentation by diphenylamine method were used.
Cells were treated with compound 4 and its conjugated form (at IC50) for 24 h and then harvested and stained. The results showed in Figs. 7 and 8 evidently demonstrate the induction of apoptosis by compounds in compare with controls (nontreated; AuNPs). Percentage of apoptotic cells were calculated by AO–EB staining (Table 4) and showed an increase from 35 to 43 %.
DNA fragmentation is an end point of apoptosis which takes place after caspase activation. Cells were treated with compound 4 and conjugated form for 24 h. The results showed the rising percentage of DNA fragmentation from 34.3 to 41.8 after conjugation. This probably comes from the decrease in the expression of BCl-2 in T47D cell line; there was an inverse relation between the expression of this factor and releasing of cytochrome C. By the decrease of BCl-2 expression in T47D cell line the release of cytochrome C would increase (cytochrome C is a necessary factor for the initiation of apoptosis), which would lead to the formation of apoptosome, acting of caspases 9 and 3 and DNA fragmentation as a result. Consequently, apoptosis would be caused by such agents [33, 34].
References
- 1.Blagosklonny MV. A node between proliferation, apoptosis and growth arrest. BioEssays. 1999;21(8):704–709. doi: 10.1002/(SICI)1521-1878(199908)21:8<704::AID-BIES10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med. 2000;342(8):564–571. doi: 10.1056/NEJM200002243420807. [DOI] [PubMed] [Google Scholar]
- 3.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34(4):378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Larsen ME, Rowntree J, Young AM, Pearson S, Smith J, Gibson OJ, et al. Chemotherapy side-effect management using mobile phones. In: Conference proceedings of the IEEE Engineering in Medicine and Biology Society, 2008, pp. 5152–5. [DOI] [PubMed]
- 6.Wang J, Chen B, Chen J, Cai X, Xia G, Liu R, et al. Synthesis and antitumor efficacy of daunorubicin-loaded magnetic nanoparticles. Int J Nanomed. 2011;6:203–211. doi: 10.2147/IJN.S16165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KY, Nam DH, Moon CS, Seo SH, Lee JY, Lee YS. Synthesis and anticancer activity of lavendustin a derivatives containing arylethenylchromone substituent’s. Eur J Med Chem. 2006;41(8):991–996. doi: 10.1016/j.ejmech.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Sairafianpour M, Kayser O, Christensen J, Asfa M, Witt M, Staerk D, et al. Leishmanicidal and antiplasmodial activity of constituents of Smirnowia iranica. J Nat Prod. 2002;65(12):1754–1758. doi: 10.1021/np020244s. [DOI] [PubMed] [Google Scholar]
- 9.Gourdeau H, Leblond L, Hamelin B, Desputeau C, Dong K, et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4-5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol Cancer Ther. 2004;3(11):1375–1383. [PubMed] [Google Scholar]
- 10.Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure–activity relationships of the 4-aryl group. J Med Chem. 2004;47(25):6299–6310. doi: 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]
- 11.Kemnitzer W, Drewe J, Jiang S, Zhang H, Zhao J, Crogan-Grundy C, et al. Discovery of 4-aryl-4H-chromenes as a new series of Apoptosis Inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure–activity relationships of fused rings at the 7, 8-positions. J Med Chem. 2007;50(12):2858–2864. doi: 10.1021/jm070216c. [DOI] [PubMed] [Google Scholar]
- 12.O’Driscoll L, Linehan R, Clynes M. Survivin: role in normal cells and in pathological conditions. Curr Cancer Drug Targets. 2003;3(2):131–152. doi: 10.2174/1568009033482038. [DOI] [PubMed] [Google Scholar]
- 13.Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high throughput screening assay. 2. Structure–activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg Med Chem Lett. 2005;15(21):4745–4751. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 14.Reed JC, Tomaselli KJ. Drug discovery opportunities from apoptosis research. Curr Opin Biotechnol. 2000;11(6):586–592. doi: 10.1016/S0958-1669(00)00148-8. [DOI] [PubMed] [Google Scholar]
- 15.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17(9):2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 16.Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10(2):283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thrall JH. Nanotechnology and medicine. Radiology. 2004;230:315–318. doi: 10.1148/radiol.2302031698. [DOI] [PubMed] [Google Scholar]
- 18.Merisko LE, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur Pharm Sci. 2003;18(2):113–120. doi: 10.1016/S0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 19.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle based bio-bar-codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 20.Nam JM, Stoeva SI, Mirkin CA. Bio-bar-code-based DNA detection with PCR-like sensitivity. J Am Chem Soc. 2004;126(19):5932–5933. doi: 10.1021/ja049384+. [DOI] [PubMed] [Google Scholar]
- 21.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, Quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 22.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 23.Zarabi FM, Farhangi A, Mazdeh KS, Ansarian Z, Zare D, Akbarzadeh A, et al. Synthesis of gold nanoparticles coated with aspartic acid and their conjugation with FVIII protein and FVIII antibody. Indian J Clin Biochem. 2013. doi:10.1007/s12291-013-0323-2. [DOI] [PMC free article] [PubMed]
- 24.Byung KC, Chang HC, Hyun LO, Yong KK. Role of ERK activation in cisplatin-induced apoptosis in A172 human glioma cells. Neurotoxicology. 2004;25:915–924. doi: 10.1016/j.neuro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 26.Gibb RK, Gercel-Talor C. Use of diphenylamine in the detection of apoptosis. Methods Mol Med. 2001;39:679–680. doi: 10.1385/1-59259-071-3:679. [DOI] [PubMed] [Google Scholar]
- 27.Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. doi: 10.1016/S0169-409X(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 30.Song M, Zhang R, Dai Y, Gao F, Chi H, Lv G, et al. The in vitro inhibition of multidrug resistance by combined nanoparticulate titanium dioxide and UV irradiation. Biomaterials. 2006;27(23):4230–4238. doi: 10.1016/j.biomaterials.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Soma C, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21(1):1–7. doi: 10.1016/S0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 32.Hu YP, Jarillon S, Dubernet C, Couvreur P, Robert J. On the mechanism of action of doxorubicin encapsulation in nanospheres for the reversal of multidrug resistance. Cancer Chemother Pharmacol. 1996;37:556–560. doi: 10.1007/s002800050428. [DOI] [PubMed] [Google Scholar]
- 33.Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PRM. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87:909–917. doi: 10.1038/sj.bjc.6600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cia J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]